
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 22 February 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1047554
This article is part of the Research TopicMeasurable Residual Disease in Hematologic MalignanciesView all 12 articles
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) offers a survival benefit to adult patients affected by acute lymphoblastic leukemia (ALL). However, to avoid an overt disease relapse, patients with pre or post transplant persistence or occurrence of measurable residual disease (MRD) may require cellular or pharmacological interventions with eventual side effects. While the significance of multiparametric flow cytometry (MFC) in the guidance of ALL treatment in both adult and pediatric patients is undebated, fewer data are available regarding the impact of MRD monitoring, as assessed by MFC analysis, in the allo-HSCT settings. Aim of this article is to summarize and discuss currently available information on the role of MFC detection of MRD in adult ALL patients undergoing allo-HSCT. The significance of MFC-based MRD according to sensitivity level, timing, and in relation to molecular techniques of MRD and chimerism assessment will be also discussed.
In acute leukemia of either lymphoid or myeloid lineage, measurable residual disease (MRD) is defined as the presence of residual malignant cells in bone marrow (BM) or peripheral blood (PB) of patients who achieved morphologic complete remission (CR) after treatment interventions (1). The methods currently available for MRD detection are multiparameter flow cytometry (MFC), and/or molecular biology techniques including real-time quantitative polymerase chain reaction (RQ-PCR), digital droplet PCR, and next-generation sequencing (NGS) (2). Importantly, the different sensitivity limits of these techniques, ranging from 1x10-4 (MFC) to 1x10-6 (NGS), and the occurrence of disease relapse in otherwise MRD negative patients, have recently prompted the replacement of the adjective “minimal” with that of “measurable” in reference to residual disease (3). For an in-depth review of molecular techniques of MRD analysis the reader is referred to recent reviews (2, 4).
In acute lymphoblastic leukemia (ALL), the most used techniques for MRD monitoring are MFC, which relies on the identification of aberrantly expressed antigens by leukemic cells, and RQ-PCR analysis, which detects rearranged immunoglobulin (Ig)/T-cell receptor (TCR) genes, or recurrent gene fusions such as BCR-ABL1 in chromosome Philadelphia (Ph) positive patients (1–3). Although both MFC and RQ-PCR are applicable to most ALL cases (i.e., 90% and 90-95%, respectively) the two techniques differ in terms of sensitivity, with RQ-PCR being generally more sensitive than MFC (i.e., 1x10-4 to 1x10-5 vs 1x10-4) (4, 5). Nonetheless, MFC is widely used in many countries, including United States (6) where the consensus from North American experts recommends using RQ-PCR over MFC for Ph-positive ALL patients only (7). On the contrary, European countries use more frequently standardized RQ-PCR for MRD testing (5). Accordingly, a recent survey on 95 European Society for Bone Marrow Transplantation (EBMT)-affiliated centers has reported that in Europe ALL MRD monitoring is mainly performed by RQ-PCR, either alone or in conjunction with MFC (8).
Over the years MRD monitoring has been introduced in clinical trials and disease-specific guidelines as measure of treatment efficacy and predictor of relapse, thus informing response-adapted therapies in pediatric (9) and adult ALL patients (10, 11). Although there is no consensus on which sensitivity threshold should be reached to define MRD positivity, it is now generally accepted to use methods detecting at least 1 leukemic cell out of 10,000 nucleated cells (≥1x10-4) (7).
Despite rigorous indications regarding MRD monitoring (by either MFC or RQ-PCR) throughout induction and consolidation therapies (10, 11), limited information is available about MRD assessment in adult ALL patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT), which in turn is a potentially life-saving treatment for selected patients with high-risk features or MRD positivity following induction and consolidation (11, 12).
Based on these premises, aim of this review is to explore the role of MFC MRD monitoring in adult ALL patients undergoing allo-HSCT, highlighting both advantages and pitfalls of the MFC technique even in relation to RQ-PCR. Eventual correlations with analysis aimed at evaluating patient chimerism status throughout immune reconstitution will be also discussed.
MFC rapidly analyzes single cells or particles as they flow past single or multiple lasers while suspended in a buffered salt-based solution. Each particle or cell is analyzed for visible light scatter and one or multiple fluorescence parameters detected as a result of emission by fluorochrome-conjugated specific monoclonal antibodies against surface, cytoplasm or nuclear antigens that are differently expressed by leukemic vs normal cells (13, 14). A key feature of MFC, which remains an indispensable tool for the immunophenotypic characterization of leukemic cells at diagnosis, is the capability to distinguish cellular subpopulation via multiparametric assessment of quantitative differences in antigen expression on single cells, and to enumerate the relative size of the resulting subpopulation (14). Importantly, the possibility to discriminate and enumerate different subpopulations within complex mixtures of cells such as BM, PB, or cerebral fluid has made MFC a highly suitable technique for MRD detection and quantification (14). ALL is a heterogeneous malignancy that originates from B- and T-lineage lymphoid precursors and is driven by a spectrum of genetic aberrations including mutations, chromosome translocations and aneuploidy in genes involved in the development of lymphoid cells and regulation of cell cycle progression (15). The most common markers used to identify leukemic B cells and to differentiate them from normal progenitor B cells (hematogones) are CD10, CD19, CD34, CD38, and CD45. In B-ALL, CD10 and CD45 usually show abnormally low levels, although, in some cases, CD10 expression is higher, which helps in the distinction from hematogones, or absent (16). Further markers include CD58, that is usually overexpressed in ALL cases (17), and antigens associated with genetic lesions such as CD123 (hyperdiploidy), CD66c (hyperdiploidy and BCR/ABL), NG2 (MLL-rearrangements), CRLF2 (CRLF2-rearrangements), and lack of CD44 positivity (TEL/AML1 and B-ALL with MYC-translocation) (18). Worthy of note, MRD analysis in B-ALL patients treated with CD19-targeted therapies may require an alternative gating strategy without the use of CD19 as B-cell-specific marker (19). As for T-ALL the most common markers used to identify leukemic blasts include the down-modulation of surface CD3 expression and the cytoplasmic CD3 positivity, with the expression of terminal deoxynucleotidyl transferase (TdT) and CD34 suggesting an immature T-lymphoid process (16). The positive expression or variations in intensity of CD2, CD4, CD5, CD7, and CD8 levels are frequently used as a gating strategy for MRD (16). CD1a can show a positive or negative expression and may be a useful target for MRD evaluation (16).
MFC MRD can be tracked by two methods of analysis: i) through the identification of the immunophenotypic pattern of leukemic cells at the diagnosis (i.e., leukemia-associated immunophenotype/LAIP) that can be followed over time; ii) by discriminating the differences between the immunophenotype of leukemia cells in the MRD sample compared to normal B-lymphoid progenitors (i.e., hematogones) or normal T-lymphoid progenitors (i.e., thymocytes), through a “different from normal” (DFN) approach (20, 21). As both LAIP- and DFN-methods present potential pittfalls due respectively to immunophenotypic shifts of leukemic blasts and post-chemotherapy changes of hematopoiesis, it is generally suggested to maximize the accuracy of MRD analysis through a comprehensive integrated LAIP-based DFN approach (21–23). The latter define a set of aberrancies including (a) the abnormal expression of antigens not typically expressed by the particular cell type, (b) the over/under- expression of normally expressed antigens, and/or (c) the asynchronous expression of normally expressed antigens (21).
As previously stated, MFC has generally a lower sensitivity than molecular biology techniques, however the use of standardized protocols allows to reach a similar sensitivity to RQ-PCR provided the acquisition of an adequate number of cells (preferably more of 4 x 106) from a first-aspirate, fresh, viable sample (24). MFC MRD monitoring has some advantages over other methods. These include the rapidity of execution, the relatively low cost, the ability to quantify antigens for targeting agents, and the possibility to analyze samples without knowing the immunophenotypic characteristics of leukemic cells at diagnosis, which is an added value for referral transplant centers (7, 20). Disadvantages include the risk of false negative results due to immunophenotypic shifts throughout treatment, the difficulty to discriminate leukemic B cells from hematogones in a regenerating/reconstituting BM, the large dependence of the analysis on the operator skill, and lack of standardization (7, 20). With the latter regard, protocols aimed at standardizing MFC analysis among laboratories in terms of harmonization and alignment of the technical aspects are currently ongoing (24, 25).
MFC MRD monitoring has been demonstrated as a valuable tool for assessment of response to treatment and prognostic evaluation not only in pediatric (26, 27) but even in adult ALL patients after induction and consolidation. For instance, in a retrospective study analyzing 323 adult patients affected by B-ALL and monitored by MFC (4-6 color panel, sensitivity 10-4), Ravandi and colleagues found that a negative post induction MRD status was associated with a significantly higher disease-free survival (DFS) according to multivariable analysis (28). In a prospective multicenter trial monitoring 179 adolescent and adult high-risk Ph-negative ALL patients by MFC MRD (4 color panel, sensitivity 5 x 10-4), undetectable levels of early post consolidation MRD were associated to a quite favorable prognosis even in the absence of allo-HSCT (29). In a multicenter series of 1487 pediatric and adult patients affected by B-cell precursor (BCP) ALL, positive MFC MRD (6 color panel, sensitivity 10-4) on days 15, 29 and 79 was significantly associated with hazard of relapse in multivariable analysis (30). Finally, according to a very recent report on 134 Ph negative pediatric and adult B-ALL patients, integrated dynamic MFC MRD assessed on days 14, 25 and 45 (8 color panel, sensitivity 10-4) was an independent factor for overall survival (OS) at multivariate analysis, also defining risk-classification criteria leading to effective allo-HSCT in high-risk, but not in low and intermediate risk patients (31). Concerning T-ALL, a multicenter study regarding 274 pediatric and adult patients showed that a negative MFC MRD assessment (6 color panel, sensitivity 6 x 10-5), on day 15 might be useful for an early and accurate identification of patients with a very low risk of relapse (32). Similarly, a retrospective study on 94 adult patients affected by T-ALL showed that MFC MRD (6-8 color, sensitivity 10-4) positivity at the end of induction was an independent prognostic factor for cumulative incidence risk, relapse-free survival, and OS (33).
Adult ALL remains an aggressive disease. In fact, despite dose-intensification strategies leading to high response rate to induction chemotherapy, and the availability of highly active targeted immunotherapies for resistant or relapsed disease (34), only 30-40% of adult ALL patients will achieve long-term remission (35). In this scenario allo-HSCT still represents an effective therapeutic treatment and is currently part of adult ALL standard clinical care (36, 37). However, a significant percentage (~40%) of patients will relapse after allo-HSCT, while other (15 to 26%) will die due to non-relapse mortality (NRM) (37–39) despite recent advances in transplant management (38, 39). In keeping with these premises, in young adult and adult patients allo-HSCT is currently part of post-consolidative therapy in case of high-risk features such as Ph-positivity, Ph-like disease, and persistent MRD as assessed by either MFC or RQ-PCR (11, 12). In MRD regard, prospective and retrospective multicenter studies have demonstrated that allo-HSCT improves the outcome of adult ALL patients who are MRD positive after induction (33, 40) or consolidation therapy (41, 42).
Although less explored, MRD testing has been shown to have a prognostic significance even with respect to allo-HSCT outcome. For instance, a retrospective EBMT registry study on 2780 adult ALL patients undergoing myeloablative allo-HSCT in first complete remission (CR) and evaluated by MFC and/or RQ-PCR techniques (threshold >10-4) demonstrated by multivariate analysis that MRD positivity at transplant was a significant independent factor for lower OS, leukemia free survival (LFS), and for higher relapse incidence (RI) (43). Similar data have emerged from a recent meta-analysis on 21 published reports according with a positive MFC or RQ-PCR MRD at allo-HSCT is associated with lower OS, event free survival (EFS) and relapse-free survival (RFS) (44). Overall, this evidence underlines the leading role played by MRD regarding the best timing of allo-HSCT, mostly in light of the availability of new drugs such as inotuzumab ozogamicin and blinatumumab, potentially able to obtain pre-transplant MRD clearance with mechanisms of action different from chemotherapy (45). Interestingly, a deep MRD negativity may also question the advisability of allo-HSCT. In fact, a recent trial assessing MRD with a high sensitivity (limit of detection 0.2x10-6) and standardized technology (2 tube 8 color MFC panels for BCP-ALL and T-ALL, respectively) (24) has shown that adults with high-risk features, Ph− ALL, and deep MRD clearance after induction and early consolidation have favorable outcomes without allo-HSCT (46).
A few studies have specifically analyzed the role of MFC MRD monitoring in ALL prior to and following allo-HSCT. As shown in Table 1 (33, 47–55), data related to adult patients mostly derive from retrospective and heterogeneous series, sometimes including children, and using different sensitivity levels [10-3 to 10-5].
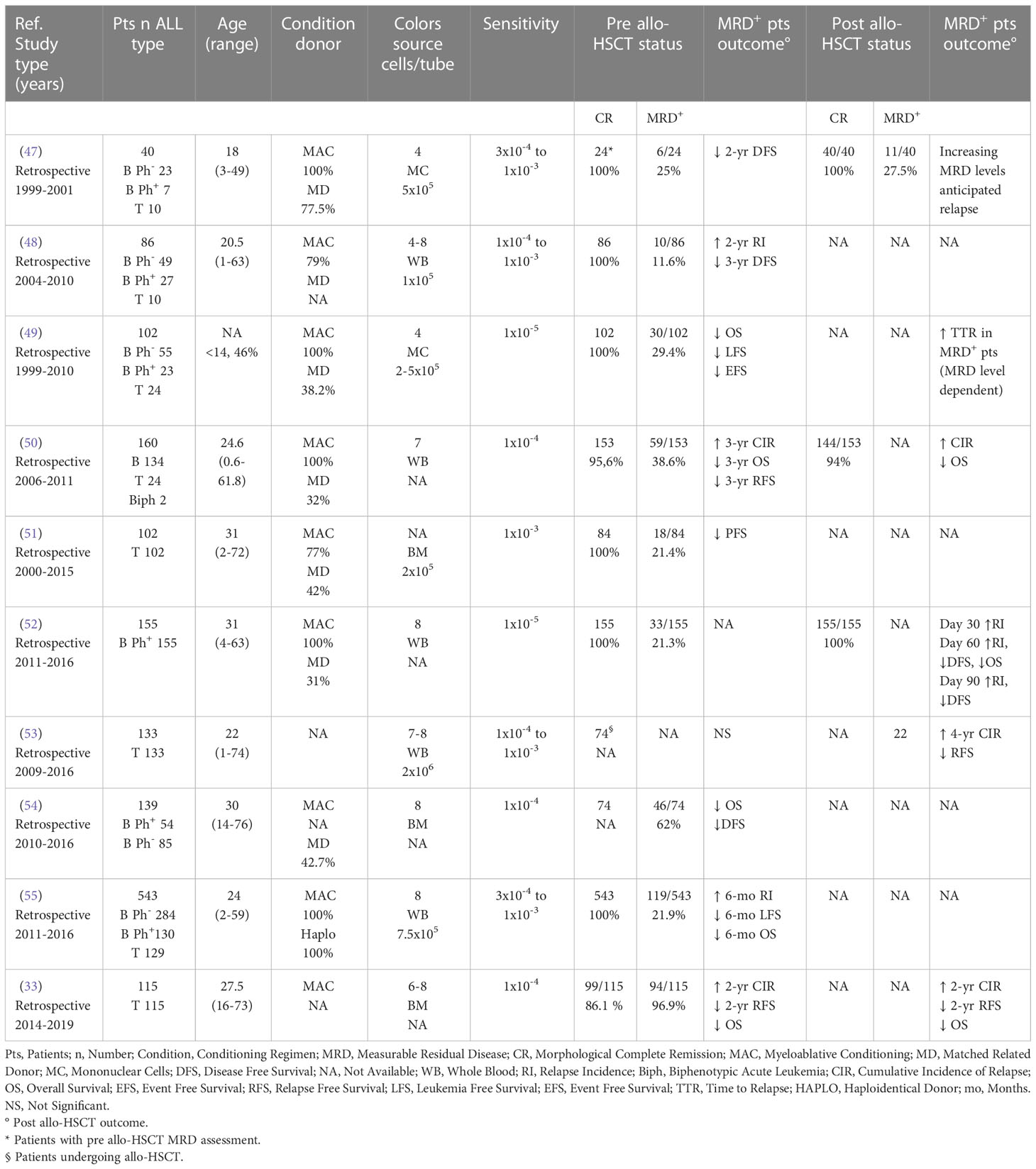
Table 1 Impact of pre and/or post allo-HSCT MFC MRD according to ALL series published in the last 20 years and including adult patients.
Eight (89%) out of 9 studies including a total of 1180 patients showed a predictive role of positive pre allo-HSCT MRD towards DFS/LFS (33, 47–51, 54, 55), cumulative incidence of relapse (CIR) or risk (33, 48, 50, 55), and OS (33, 49, 50, 54, 55), while only 1 study did not find any impact on transplant outcome (53) (Table 1). Similar data were observed in the pediatric setting. In fact, according to a retrospective study on 64 children with ALL, low (10-4 to <10-3) and high (≥10-3) pre allo-HSCT MFC MRD levels were predictive of a proportionally increasing 5-year CIR (56). Similarly, in a retrospective study on 36 children, MFC MRD levels ≥ 10-4 were associated to a higher CIR (57). According to a prospective study on 105 children, patients with MFC MRD ≥ 10-3 had a higher CIR than subjects with MRD < 10-3 or negative (58). Finally, in a retrospective study on 69 children evaluated by either MFC or RQ-PCR, a positive pre transplant MRD was associated to a higher CIR (59).
As reported in Table 1, post allo-HSCT MFC MRD was evaluated only in 6 series including adults, and accounting approximatively for 355 ALL patients. According to all studies a positive MRD was associated to a reduced DFS/RFS (33, 52, 53), OS (33, 50, 52), time to relapse (47, 49), and to a higher CIR or risk (33, 50, 52, 53). Few data on post allo-HSCT MFC MRD monitoring are available in the pediatric setting. A multinational study on 616 pediatric and young adult ALL patients evaluating pre and post allo-HSCT MRD levels by either MFC or RQ-PCR, showed by univariate analysis that low (<10-4) to very high (≥10-3) post-transplant MRD levels were associated to a progressively higher relapse hazard (60). Moreover, patients undergoing allo-HSCT with detectable MRD and showing high or very high post transplant MRD had increasingly higher chances of relapse according to Cox regression model (60).
Interestingly, recent evidences support the usefulness of dynamic peri-transplant (i.e., serial pre and post allo-HSCT) MFC MRD monitoring. For instance, a retrospective study on 271 T-ALL adult and pediatric patients has recently shown that dynamic peri-transplant MFC MRD monitoring could be better in discriminating the risk of relapse than single time point pre or post allo-HSCT assessments (61). Similarly, in a pediatric series of 166 ALL patients undergoing haploidentical unmanipulated transplant and dynamic peri-transplant MFC MRD assessments, increasing MRD levels were associated to lower LFS and OS, and higher CIR (62).
Overall, regardless technical differences and the relatively low series number, the studies summarized in Table 1 indicate that in adult ALL patients undergoing allo-HSCT MFC can be a reliable MRD assessment technique. Moreover, studies in adult and pediatric patients indicate that MFC may have an increasing predictivity depending on MRD positivity levels (47, 49, 60) and/or peri-transplant trend (61, 62). Unfortunately, no data are available regarding the predictive impact of post over pre allo-HSCT MFC MRD monitoring. However, in a large multicenter study including 616 children, post transplant MRD (evaluated by either MFC or RQ-PCR) resulted more predictive than pre transplant MRD with respect to allo-HSCT outcome (60).
The paucity of studies on MFC MRD monitoring in adult (and even pediatric) ALL patients prior to and after allo-HSCT is somehow surprising considering the wide use of MFC MRD assessment of the same patients while undergoing induction and consolidation therapies (10, 11, 63). Worthy of note, several authors have recently shown the feasibility and predictive significance of MFC MRD positivity prior and/or following allo-HSCT even in adult AML patients (64–66). For instance, in a series of 279 patients receiving myeloablative conditioning in first or second CR, a positive MFC (10 color panel, sensitivity ≤10-3) MRD prior to allo-HSCT was associated with inferior OS and higher risk of relapse in a multivariable analysis (65). Furthermore, in a study on 810 adult AML patients who underwent MFC MRD monitoring before and 20 to 40 days after allografting, peri-allo-HSCT MRD dynamics improved accuracy of risk over pre- and post-allo-HSCT assessment across conditioning intensities (66).
Previous data from ALL studies have shown that MFC and RQ-PCR amplification of antigen-receptor genes yield remarkably similar measurements if MRD is present at a ≥ 10-4 level (67). Although most information on RQ-PCR MRD monitoring in adult allo-HSCT setting derives from a limited number of studies, often focused on Ph positive patients (Table 2), based on our literature revision the prognostic significance of MFC and RQ-PCR towards allo-HSCT outcome seems quite comparable. Accordingly, 5 (71.4%) out of 7 studies including 2267 patients evidenced a predictive role of detectable RQ-PCR MRD levels towards DFS/RFS (70, 72), CIR (68, 73), and OS (68, 70, 72, 73) (Table 2). The significance of post allo-HSCT RQ-PCR MRD was evaluated by 4 studies on more than 612 patients, all evidencing the impact of RQ-PCR MRD monitoring towards DFS/RFS (52, 70, 71), CIR (52, 68, 71) and OS (52, 70, 71) (Table 2). Of note, similar data were observed in the pediatric setting (74–76).
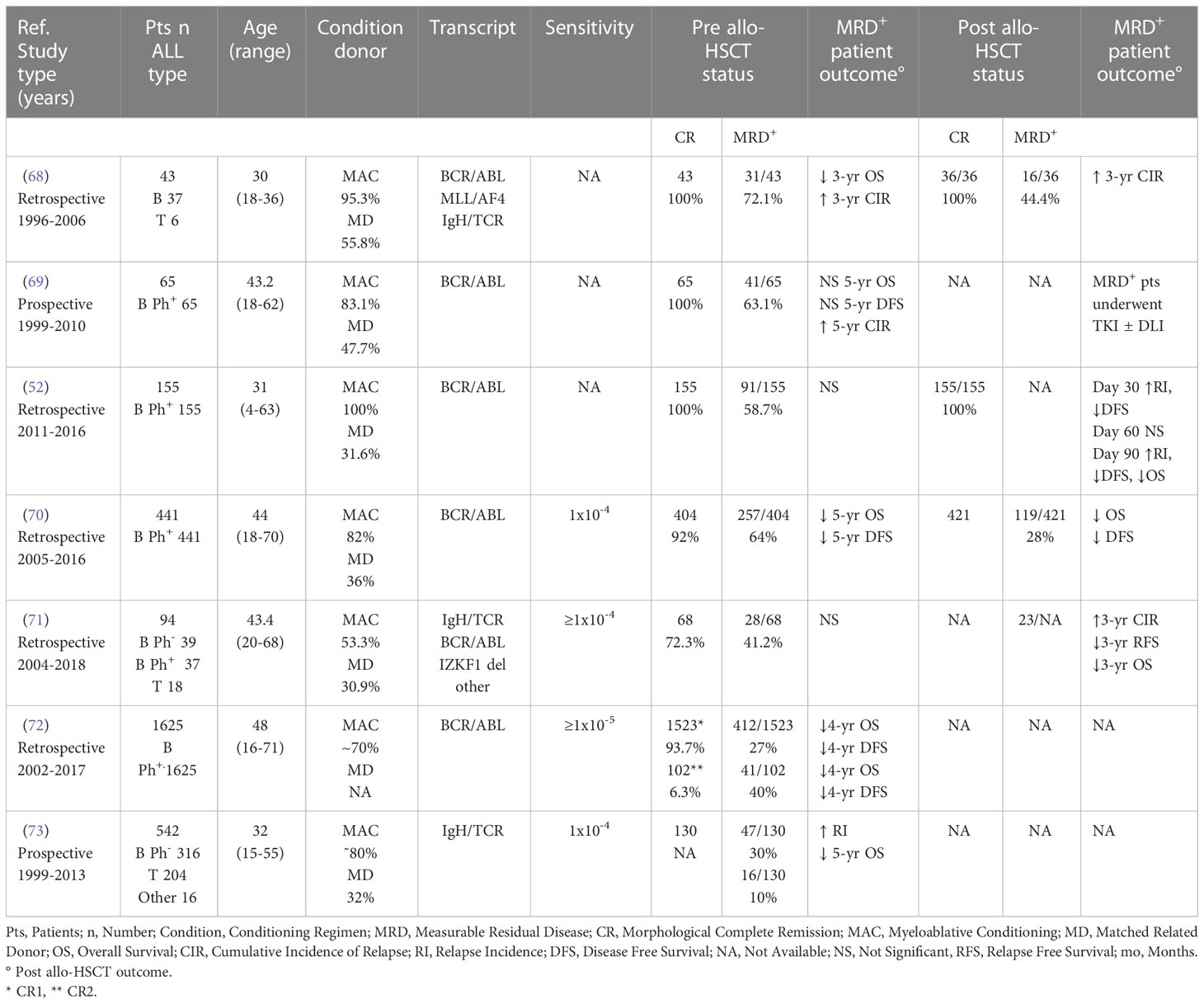
Table 2 Impact of pre and/or post allo-HSCT RQ-PCR MRD according to ALL series published in the last 20 years and including adult patients.
Chimerism analysis, the investigation of the genotype origin of post-allografting hematopoiesis, has been historically considered a well-established method for monitoring the outcome of allo-HSCT in terms of engraftment and eventual risk of relapse (77). About chimerism the term “complete donor chimerism” refers to a hematopoiesis that is fully genetically derived from donor, whereas the term “mixed chimerism” refers to a hematopoiesis with genetic origins from both donor and patient (78). Over the years, several methods for chimerism analysis have been progressively introduced in clinics, including assessing short tandem repeats (STR), fluorescent PCR, RQ-PCR of single nucleotide polimorphism, and fluorescence in situ hybridization in gender-mismatched allo-HSCT (77). Chimerism can be defined on several levels, but PB and BM are the most frequently used sources. Notably, the degree of chimerism can be analyzed in these tissues without any further manipulation (i.e., overall chimerism) or within certain cellular fractions, such as T cells, B cells, CD34+ or myeloid cells (i.e., subset chimerism) (78). Currently, there is no general agreement on the preferred source/subpopulation of assessment (79, 80), which in turn is dependent on the technique used.
The American Society for Transplantation and Cellular Therapy recommends chimerism evaluations at specific time points during the first year post allo-HSCT (e.g., days +30, +90, +180, and +365) and whenever required according to disease characteristics (81), while the EMBT generally suggests serial and quantitative analysis of chimerism given the short time interval between mixed chimerism detection and relapse (82). Chimerism is in fact a dynamic process, and patients with increasing levels of recipient chimerism have been traditionally retained at risk of relapse and therefore treated with preemptive immune therapy (i.e., immunosuppressive drug tapering, DLI) (79, 83).
Little data are available on the clinical impact of chimerism with respect to MRD monitoring as determined by MFC and/or molecular biology techniques (83, 84). A retrospective study analyzing 101 adult allo-HSCT ALL patients undergoing chimerism monitoring by multiplex STR assay (sensitivity 10-2), showed that an increasing mixed chimerism in CD34+ BM cells was an independent negative prognostic factor for OS and relapse in multivariable analysis (84). However, in a subgroup of 22 patients undergoing RQ-PCR MRD monitoring, MRD assessment was much more sensitive (86%) and specific (95%) than chimerism (84). In a retrospective study regarding a small series of adult patients affected by AML and ALL, MFC (6 color panel) and RQ-PCR (WT-1) showed a moderate concordance with chimerism analysis (assessed by STR-PCR), suggesting the usefulness of MRD monitoring over chimerism in stratifying patients with respect to relapse risk (85). Recently, Pincez and colleagues have demonstrated in a pediatric series of 72 patients, mostly affected by AML and ALL, that an increasing mixed chimerism (assessed by STR-PCR) was never the first evidence of relapsing leukemia, that in turn was detected by more sensitive techniques of MRD analysis (i.e., RQ-PCR and only partially MFC with a sensitivity ranging from 2 to 10 x 10-4) (86). Interestingly, Semchenkova and colleagues have recently demonstrated that in doubtful MRD positive cases, RQ-PCR chimerism testing in questionable MRD+ sorted cells can be useful for approval or disapproval of MRD presence (87).
In the absence of large studies, clear indications about assessment schedules, and due to the lack of reference methods among the increasing number of different strategies of chimerism analysis, it is difficult to establish the role of MRD and hence, MFC MRD monitoring, with respect to chimerism. Therefore, any comparison between chimerism and post allo-HSCT MRD monitoring should consider the sensitivities and specificities of the techniques available in each center. As shown in Table 3, most of the techniques currently used for MRD evaluation (88–98) including MFC (88–90) display a higher sensitivity than the majority of chimerism detection methods.
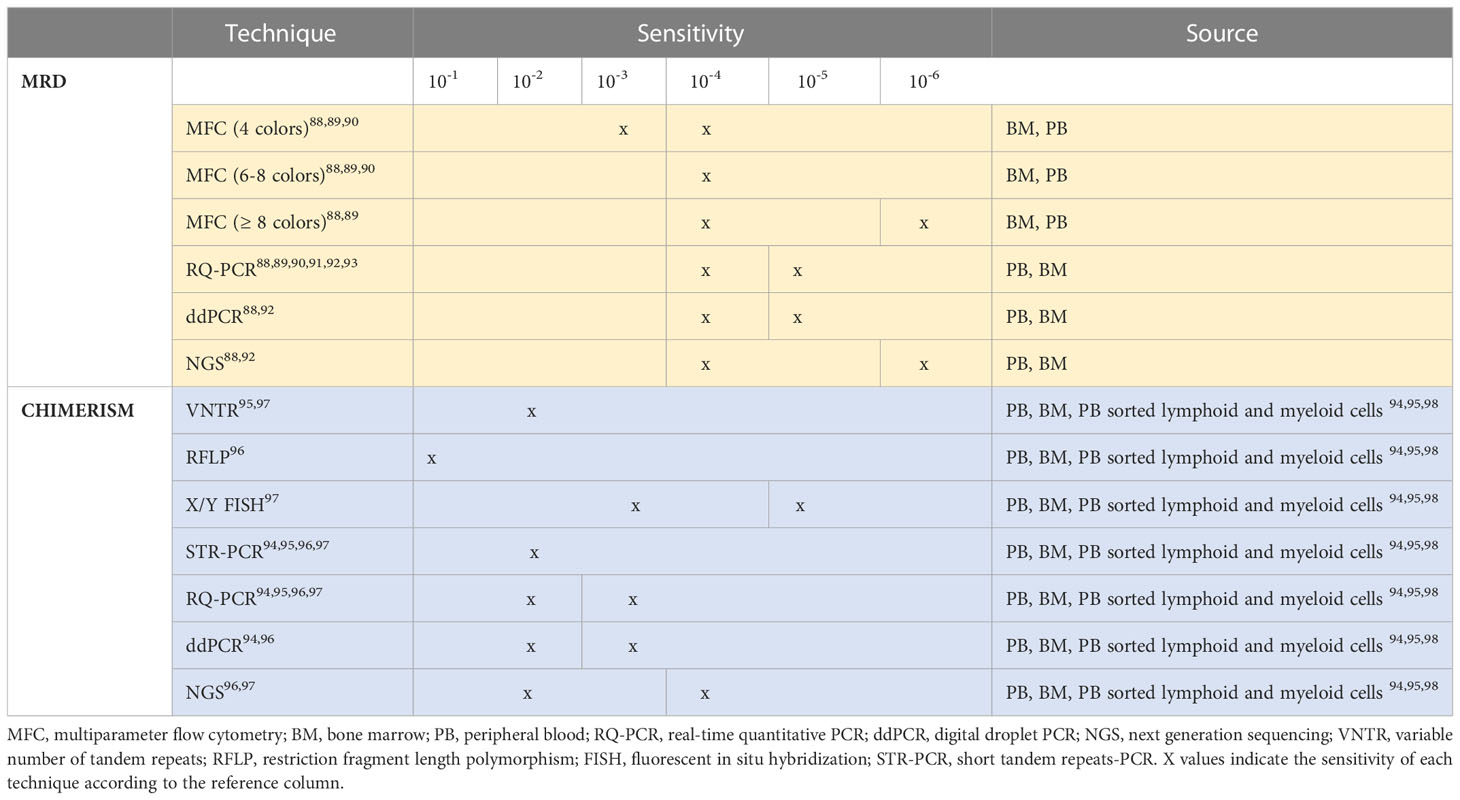
Table 3 MRD and chimerism assessment techniques according to sensitivity and preferable source of analysis.
Allo-HSCT is a complex therapeutic procedure whose outcome depends on several patient-, disease- and transplant-related cofactors. Although the prognostic role of pre-transplant MRD (as assessed by either MFC or RQ-PCR) is generally accepted (44, 99), few data are available on the post-transplant setting, which is characterized by a delicate balance between the graft-versus-leukemia effects, that in turn depend on graft-versus-host disease (GVHD) prophylaxis, occurrence and treatment, and the eventual residual disease. Moreover, no definite guidelines regarding MRD time-point assessments or levels for preemptive interventions are currently available.
In agreement with previous literature analysis (44, 99), 89% of the studies here retrieved reported a negative impact of pre allo-HSCT MFC MRD on the post-transplant outcome of adult ALL patients (Table 1). Although the extent to which the intensity of conditioning may affect MRD clearance remains debated (1), patients from most of these series underwent myeloablative regimens that resulted ineffective. Importantly, newly available drugs such inotuzumab ozogamicin and blinatumumab are currently used to obtain pre-transplant MRD clearance (45).
The role of MRD monitoring after allo-HSCT has been traditionally poorly explored. In addition to the previous lack of effective relapse-preventing interventions outside immunosuppressive drug tapering and donor lymphocyte infusion (DLI), or tyrosine kinase inhibitors in Ph positive ALL patients (100, 101), this was mainly due to the use of chimerism analysis as MRD surrogate. Nowdays, the availability of potential premptive and therapeutic post allo-HSCT interventions in either pediatric or adult ALL patients (102–107) highlights the need of highly specific and sensitive measures of MRD. However, post-transplant MRD monitoring may be troublesome for referral centers, mostly due to a difficult access to diagnostic samples, whose availability is critical in case of LAIP-based MFC and RQ-PCR Ig/TCR gene techniques (6). MFC can be a valuable tool for post allo-HSCT MRD monitoring as it is fast, applicable to most ALL cases, and somehow independent from diagnostic samples when a DFN approach is used (20, 21). According to our literature revision, all studies specifically addressing the role of post-transplant MFC MRD monitoring reported an adverse outcome for MRD positive patients (Table 1). Yet, transplant clinicians should be aware that the sensitivity and reliability of MFC MRD monitoring is dependent on sample type (BM) and quality (adequate cell number and vitality), provided rigorous technical assumptions (at least 6-8 color panel, acquisition of at least 4x106 cells), standardization, and operator expertise (24, 25). As BM samples from patients with concurrent GVHD or herpetic infections can be inadequate for MFC assessment due to a low cellularity, some transplant centers evaluate MRD by both MFC and molecular methods, though with economic burden (8). In fact, in case of inadequate BM samples, MFC MRD should be interpreted with caution and integrated, if possible, with data obtained by RQ-PCR. Whatever the technique used, an additional issue for transplant physician is the need to combine MRD and chimerism data, as they may give contrasting results based on different sample sources and method sensitivities. Moreover, standards for measurement intervals for MRD and chimerism and definitions of thresholds for initiating therapy are still missing (84).
Overall, many questions remain to be addressed regarding MFC MRD monitoring in adult ALL patients undergoing allo-HSCT, mostly in the post-transplant setting. Although MFC can be a reliable tool for MRD assessment, potentially reaching RQ-PCR sensitivity levels, a close interaction between transplant clinicians and reference laboratory is recommended in order to select the optimal method for MRD evaluation in each patient and to obtain clinically useful data.
CT and AR performed literature search and wrote the manuscript. MK supervised the manuscript. All authors contributed to the article and approved the submitted version.
This work has been supported by: Alessandro Moretti Foundation, Verona, Italy. We apologize to those authors whose work has not been cited.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Czyz A, Nagler A. The role of measurable residual disease (MRD) in hematopoietic stem cell transplantation for hematological malignancies focusing on acute leukemia. Int J Mol Sci (2019) 20(21):5362. doi: 10.3390/ijms20215362
2. Muffly L. Measurable residual disease in acute lymphoblastic leukemia: Techniques and therapeutic utility. Clin Adv Hematol Oncol (2022) 7):419–21.
3. Kim IS. Minimal residual disease in acute lymphoblastic leukemia: Technical aspects and implications for clinical interpretation. Blood Res (2020) 55(S1):S19–26. doi: 10.5045/br.2020.S004
4. Brüggemann M, Kotrova M. Minimal residual disease in adult ALL: Technical aspects and implications for correct clinical interpretation. Blood Adv (2017) 1(25):2456–66. doi: 10.1182/bloodadvances.2017009845
5. van Dongen JJ, van der Velden VH, Brüggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: Need for sensitive, fast, and standardized technologies. Blood (2015) 125(26):3996–4009. doi: 10.1182/blood-2015-03-580027
6. Abou Dalle I, Jabbour E, Short NJ. Evaluation and management of measurable residual disease in acute lymphoblastic leukemia. Ther Adv Hematol (2020) 11:2040620720910023. doi: 10.1177/2040620720910023
7. Short NJ, Jabbour E, Albitar M, de Lima M, Gore L, Jorgensen J, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: A consensus of north American experts. Am J Hematol (2019) 94(2):257–65. doi: 10.1002/ajh.25338
8. Nagler A, Baron F, Labopin M, Polge E, Esteve J, Bazarbachi A, et al. Measurable residual disease (MRD) testing for acute leukemia in EBMT transplant centers: A survey on behalf of the ALWP of the EBMT. Bone Marrow Transplant (2021) 56(1):218–24. doi: 10.1038/s41409-020-01005-y
9. Pui CH, Pei D, Raimondi SC, Coustan-Smith E, Jeha S, Cheng C, et al. Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with response-adapted therapy. Leukemia (2017) 31(2):333–9. doi: 10.1038/leu.2016.234
10. Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C, et al. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27(suppl 5):v69–82. doi: 10.1093/annonc/mdw025
11. NCCN. Clinical practice guidelines. acute lymphoblastic leukemia, version 1.2022 (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/all.pdf.
12. Giebel S, Marks DI, Boissel N, Baron F, Chiaretti S, Ciceri F, et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: A position statement of the European working group for adult acute lymphoblastic leukemia (EWALL) and the acute leukemia working party of the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant (2019) 54(6):798–809. doi: 10.1038/s41409-018-0373-4
13. McKinnon KM. Flow cytometry: An overview. Curr Protoc Immunol (2018) 120:5.1.1–5.1.11. doi: 10.1002/cpim.40
14. Wood BL. Principles of minimal residual disease detection for hematopoietic neoplasms by flow cytometry. Cytometry B Clin Cytom (2016) 90(1):47–53. doi: 10.1002/cyto.b.21239
15. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med (2015) 373(16):1541–52. doi: 10.1056/NEJMra1400972
16. Correia RP, Bento LC, de Sousa FA, Barroso RS, Campregher PV, Bacal NS. How I investigate minimal residual disease in acute lymphoblastic leukemia. Int J Lab Hematol (2021) 43(3):354–63. doi: 10.1111/ijlh.13463
17. Chen JS, Coustan-Smith E, Suzuki T, Neale GA, Mihara K, Pui CH, et al. Identification of novel markers for monitoring minimal residual disease in acute lymphoblastic leukemia. Blood (2001) 97(7):2115–20. doi: 10.1182/blood
18. Dworzak MN, Buldini B, Gaipa G, Ratei R, Hrusak O, Luria D, et al. International-BFM-FLOW-network. AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom (2018) 94(1):82–93. doi: 10.1002/cyto.b.21518
19. Verbeek MWC, Buracchi C, Laqua A, Nierkens S, Sedek L, Flores-Montero J, et al. Flow cytometric minimal residual disease assessment in b-cell precursor acute lymphoblastic leukaemia patients treated with CD19-targeted therapies - a EuroFlow study. Br J Haematol (2022) 197(1):76–81. doi: 10.1111/bjh.17992
20. Chen X, Wood BL. Monitoring minimal residual disease in acute leukemia: Technical challenges and interpretive complexities. Blood Rev (2017) 31(2):63–75. doi: 10.1016/j.blre.2016.09.006
21. Fuda F, Chen W. Minimal/Measurable residual disease detection in acute leukemias by multiparameter flow cytometry. Curr Hematol Malig Rep (2018) 13(6):455–66. doi: 10.1007/s11899-018-0479-1
22. Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, Ngai LL, et al. Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD working party. Blood (2021) 138(26):2753–67. doi: 10.1182/blood.2021013626
23. Röhnert MA, Kramer M, Schadt J, Ensel P, Thiede C, Krause SW, et al. Reproducible measurable residual disease detection by multiparametric flow cytometry in acute myeloid leukemia. Leukemia (2022) 36:2208–17. doi: 10.1038/s41375-022-01647-5
24. Theunissen P, Mejstrikova E, Sedek L, van der Sluijs-Gelling AJ, Gaipa G, Bartels M, et al. EuroFlow consortium. Standardized flow cytometry for highly sensitive MRD measurements in b-cell acute lymphoblastic leukemia. Blood (2017) 129(3):347–57. doi: 10.1182/blood-2016-07-726307
25. Maurer-Granofszky M, Schumich A, Buldini B, Gaipa G, Kappelmayer J, Mejstrikova E, et al. An extensive quality control and quality assurance (QC/QA) program significantly improves inter-laboratory concordance rates of flow-cytometric minimal residual disease assessment in acute lymphoblastic leukemia: An I-BFM-FLOW-Network report. Cancers (Basel) (2021) 13(23):6148. doi: 10.3390/cancers13236148
26. Coustan-Smith E, Sancho J, Behm FG, Hancock ML, Razzouk BI, Ribeiro RC, et al. Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood (2002) 100(1):52–8. doi: 10.1182/blood-2002-01-0006
27. Mussolin L, Buldini B, Lovisa F, Carraro E, Disarò S, Lo Nigro L, et al. Detection and role of minimal disseminated disease in children with lymphoblastic lymphoma: The AIEOP experience. Pediatr Blood Cancer (2015) 62(11):1906–13. doi: 10.1002/pbc.25607
28. Ravandi F, Jorgensen JL, O'Brien SM, Jabbour E, Thomas DA, Borthakur G, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol (2016) 172(3):392–400. doi: 10.1111/bjh.13834
29. Ribera JM, Oriol A, Morgades M, Montesinos P, Sarrà J, González-Campos J, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: Final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol (2014) 32(15):1595–604. doi: 10.1200/JCO.2013.52.2425
30. Modvig S, Hallböök H, Madsen HO, Siitonen S, Rosthøj S, Tierens A, et al. Value of flow cytometry for MRD-based relapse prediction in b-cell precursor ALL in a multicenter setting. Leukemia (2021) 35(7):1894–906. doi: 10.1038/s41375-020-01100-5
31. Cai Z, Liu Y, Tang B, Wu Z, Wang Z, Lin R, et al. Dynamics of minimal residual disease defines a novel risk-classification and the role of allo-HSCT in adult ph-negative b-cell acute lymphoblastic leukemia. Leuk Lymphoma (2022) 63(13):1–10. doi: 10.1080/10428194.2022.2115841
32. Modvig S, Madsen HO, Siitonen SM, Rosthøj S, Tierens A, Juvonen V, et al. Correction: Minimal residual disease quantification by flow cytometry provides reliable risk stratification in T-cell acute lymphoblastic leukemia. Leukemia (2019) 33(6):1324–36.
33. Wang H, Zhou Y, Huang X, Zhang Y, Qian J, Li J, et al. Minimal residual disease level determined by flow cytometry provides reliable risk stratification in adults with T-cell acute lymphoblastic leukaemia. Br J Haematol (2021) 193(6):1096–104. doi: 10.1111/bjh.17424
34. Shang Y, Zhou F. Current advances in immunotherapy for acute leukemia: An overview of antibody, chimeric antigen receptor, immune checkpoint, and natural killer. Front Oncol (2019) 9:917. doi: 10.3389/fonc.2019.00917
35. Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J (2017) 7(6):e577. doi: 10.1038/bcj.2017.53
36. DeFilipp Z, Advani AS, Bachanova V, Cassaday RD, Deangelo DJ, Kebriaei P, et al. Hematopoietic cell transplantation in the treatment of adult acute lymphoblastic leukemia: Updated 2019 evidence-based review from the American society for transplantation and cellular therapy. Biol Blood Marrow Transplant (2019) 25(11):2113–23. doi: 10.1016/j.bbmt.2019.08.014
37. Liang EC, Craig J, Torelli S, Cunanan K, Iglesias M, Arai S, et al. Allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia in the modern era. Transplant Cell Ther (2022) 28(8):490–5. doi: 10.1016/j.jtct.2022.05.010
38. Giebel S, Labopin M, Socié G, Beelen D, Browne P, Volin L, et al. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the acute leukemia working party of the European society for blood and marrow transplantation. Haematologica (2017) 102(1):139–49. doi: 10.3324/haematol.2016.145631
39. Nishiwaki S, Akahoshi Y, Morita-Fujita M, Shimizu H, Uchida N, Ozawa Y, et al. Improvements in allogeneic hematopoietic cell transplantation outcomes for adults with ALL over the past 3 decades. Blood Adv (2022) 6(15):4558–69. doi: 10.1182/bloodadvances.2022008032
40. Dhédin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, et al. Role of allogeneic stem cell transplantation in adult patients with ph-negative acute lymphoblastic leukemia. Blood (2015) 125(16):2486–96. doi: 10.1182/blood-2014-09-599894
41. Gökbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R, et al. German Multicenter study group for adult acute lymphoblastic leukemia. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood (2012) 120(9):1868–76. doi: 10.1182/blood-2011-09-377713
42. Nagafuji K, Miyamoto T, Eto T, Kamimura T, Taniguchi S, Okamura T, et al. Monitoring of minimal residual disease (MRD) is useful to predict prognosis of adult patients with ph-negative ALL: Results of a prospective study (ALL MRD2002 study). J Hematol Oncol (2013) 6:14. doi: 10.1186/1756-8722-6-14
43. Pavlů J, Labopin M, Niittyvuopio R, Socié G, Yakoub-Agha I, Wu D, et al. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: A retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol (2019) 12(1):108. doi: 10.1186/s13045-019-0790-x
44. Shen Z, Gu X, Mao W, Yin L, Yang L, Zhang Z, et al. Influence of pre-transplant minimal residual disease on prognosis after allo-SCT for patients with acute lymphoblastic leukemia: Systematic review and meta-analysis. BMC Cancer (2018) 18(1):755. doi: 10.1186/s12885-018-4670-5
45. Curran E, Muffly L, Luskin MR. Innovative approaches to the management of acute lymphoblastic leukemia across the age spectrum. Am Soc Clin Oncol Educ Book (2022) 42:1–11. doi: 10.1200/EDBK_349647
46. Ribera JM, Morgades M, Ciudad J, Montesinos P, Esteve J, Genescà E, et al. Chemotherapy or allogeneic transplantation in high-risk Philadelphia chromosome-negative adult lymphoblastic leukemia. Blood (2021) 137(14):1879–94. doi: 10.1182/blood.2020007311
47. Sánchez J, Serrano J, Gómez P, Martínez F, Martín C, Madero L, et al. Clinical value of immunological monitoring of minimal residual disease in acute lymphoblastic leukaemia after allogeneic transplantation. Br J Haematol (2002) 116(3):686–94. doi: 10.1111/j.1365-2141.2002.3311a.x
48. Bachanova V, Burke MJ, Yohe S, Cao Q, Sandhu K, Singleton TP, et al. Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: Effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant (2012) 18(6):963–8. doi: 10.1016/j.bbmt.2012.02.012
49. Sanchez-Garcia J, Serrano J, Serrano-Lopez J, Gomez-Garcia P, Martinez F, Garcia-Castellano JM, et al. Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in ALL. Bone Marrow Transplant (2013) 48(3):396–402. doi: 10.1038/bmt.2012.147
50. Bar M, Wood BL, Radich JP, Doney KC, Woolfrey AE, Delaney C, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treat (2014) 2014:421723. doi: 10.1155/2014/421723
51. Brammer JE, Saliba RM, Jorgensen JL, Ledesma C, Gaballa S, Poon M, et al. Multi-center analysis of the effect of T-cell acute lymphoblastic leukemia subtype and minimal residual disease on allogeneic stem cell transplantation outcomes. Bone Marrow Transplant (2017) 52(1):20–7. doi: 10.1038/bmt.2016.194
52. Zhao X, Zhao X, Chen H, Qin Y, Xu L, Zhang X, et al. Comparative analysis of flow cytometry and RQ-PCR for the detection of minimal residual disease in Philadelphia chromosome-positive acute lymphoblastic leukemia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2018) 24(9):1936–43. doi: 10.1016/j.bbmt.2018.03.015
53. Wang YZ, Hao L, Chang Y, Jiang Q, Jiang H, Zhang LP, et al. A seven-color panel including CD34 and TdT could be applied in >97% patients with T cell lymphoblastic leukemia for minimal residual disease detection independent of the initial phenotype. Leuk Res (2018) 72:12–9. doi: 10.1016/j.leukres.2018.07.012
54. Huang A, Huang C, Tang G, Cheng H, Liu M, Ding J, et al. Impact of clinical utility of MRD assessment with different techniques on survival in acute b lymphoblastic leukemia. Leuk Lymphoma (2018) 59(5):1073–83. doi: 10.1080/10428194.2017.1369072
55. Zhao XS, Liu YR, Xu LP, Wang Y, Zhang XH, Chen H, et al. Minimal residual disease status determined by multiparametric flow cytometry pretransplantation predicts the outcome of patients with ALL receiving unmanipulated haploidentical allografts. Am J Hematol (2019) 94(5):512–21. doi: 10.1002/ajh.25417
56. Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood (2012) 120(2):468–72. doi: 10.1182/blood-2012-02-409813
57. Umeda K, Hiramatsu H, Kawaguchi K, Iwai A, Mikami M, Nodomi S, et al. Impact of pretransplant minimal residual disease on the post-transplant outcome of pediatric acute lymphoblastic leukemia. Pediatr Transplant (2016) 20(5):692–6. doi: 10.1111/petr.12732
58. Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 children's oncology Group/Pediatric blood and marrow transplant consortium trial. Blood (2014) 123(13):2017–25. doi: 10.1182/blood-2013-10-534297
59. Ifversen M, Turkiewicz D, Marquart HV, Winiarski J, Buechner J, Mellgren K, et al. Low burden of minimal residual disease prior to transplantation in children with very high risk acute lymphoblastic leukaemia: The NOPHO ALL2008 experience. Br J Haematol (2019) 184(6):982–93. doi: 10.1111/bjh.15761
60. Bader P, Salzmann-Manrique E, Balduzzi A, Dalle JH, Woolfrey AE, Bar M, et al. More precisely defining risk peri-HCT in pediatric ALL: Pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv (2019) 3(21):3393–405. doi: 10.1182/bloodadvances.2019000449
61. Wang ZD, Wang YW, Xu LP, Zhang XH, Wang Y, Chen H, et al. Predictive value of dynamic peri-transplantation MRD assessed by MFC either alone or in combination with other variables for outcomes of patients with T-cell acute lymphoblastic leukemia. Curr Med Sci (2021) 41(3):443–53. doi: 10.1007/s11596-021-2390-6
62. Wang XY, Fan QZ, Xu LP, Wang Y, Zhang XH, Chen H, et al. The quantification of minimal residual disease pre- and post-unmanipulated haploidentical allograft by multiparameter flow cytometry in pediatric acute lymphoblastic leukemia. Cytometry B Clin Cytom (2020) 98(1):75–87. doi: 10.1002/cyto.b.21840
63. Pemmaraju N, Kantarjian H, Jorgensen JL, Jabbour E, Jain N, Thomas D, et al. Significance of recurrence of minimal residual disease detected by multi-parameter flow cytometry in patients with acute lymphoblastic leukemia in morphological remission. Am J Hematol (2017) 92(3):279–85. doi: 10.1002/ajh.24629
64. Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood (2013) 122(10):1813–21. doi: 10.1182/blood-2013-06-506725
65. Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB, et al. Pre- and post-transplant quantification of measurable ('minimal') residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia (2016) 30(7):1456–64. doi: 10.1038/leu.2016.46
66. Paras G, Morsink LM, Othus M, Milano F, Sandmaier BM, Zarling LC, et al. Conditioning intensity and peritransplant flow cytometric MRD dynamics in adult AML. Blood (2022) 139(11):1694–706. doi: 10.1182/blood.2021014804
67. Campana D. Minimal residual disease in acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program (2010) 2010:7–12. doi: 10.1182/asheducation-2010.1.7
68. Spinelli O, Peruta B, Tosi M, Guerini V, Salvi A, Zanotti MC, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica (2007) 92(5):612–8. doi: 10.3324/haematol.10965
69. Lussana F, Intermesoli T, Gianni F, Boschini C, Masciulli A, Spinelli O, et al. Achieving molecular remission before allogeneic stem cell transplantation in adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Impact on relapse and long-term outcome. Biol Blood Marrow Transplant (2016) 22(11):1983–7. doi: 10.1016/j.bbmt.2016.07.021
70. Candoni A, Rambaldi A, Fanin R, Velardi A, Arcese W, Ciceri F, et al. Outcome of allogeneic hematopoietic stem cell transplantation in adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia in the era of tyrosine kinase inhibitors: A registry-based study of the Italian blood and marrow transplantation society (GITMO). Biol Blood Marrow Transplant (2019) 25(12):2388–97. doi: 10.1016/j.bbmt.2019.07.037
71. Wethmar K, Matern S, Eßeling E, Angenendt L, Pfeifer H, Brüggemann M, et al. Monitoring minimal residual/relapsing disease after allogeneic haematopoietic stem cell transplantation in adult patients with acute lymphoblastic leukaemia. Bone Marrow Transplant (2020) 55(7):1410–20. doi: 10.1038/s41409-020-0801-0
72. Nishiwaki S, Akahoshi Y, Mizuta S, Shinohara A, Hirabayashi S, Noguchi Y, et al. Measurable residual disease affects allogeneic hematopoietic cell transplantation in ph+ ALL during both CR1 and CR2. Blood Adv (2021) 5(2):584–92. doi: 10.1182/bloodadvances.2020003536
73. Beelen DW, Arnold R, Stelljes M, Alakel N, Brecht A, Bug G, et al. Long-term results of allogeneic stem cell transplantation in adult ph- negative high-risk acute lymphoblastic leukemia. Transplant Cell Ther (2022) 28(12):834–42. doi: 10.1016/j.jtct.2022.08.024
74. Sutton R, Shaw PJ, Venn NC, Law T, Dissanayake A, Kilo T, et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol (2015) 168(3):395–404. doi: 10.1111/bjh.13142
75. Lovisa F, Zecca M, Rossi B, Campeggio M, Magrin E, Giarin E, et al. Pre- and post-transplant minimal residual disease predicts relapse occurrence in children with acute lymphoblastic leukaemia. Br J Haematol (2018) 180(5):680–93. doi: 10.1111/bjh.15086
76. Bader P, Kreyenberg H, von Stackelberg A, Eckert C, Salzmann-Manrique E, Meisel R, et al. Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol (2015) 33(11):1275–84. doi: 10.1200/JCO.2014.58.4631
77. Tozzo P, Delicati A, Zambello R, Caenazzo L. Chimerism monitoring techniques after hematopoietic stem cell transplantation: An overview of the last 15 years of innovations. Diagnostics (Basel) (2021) 11(4):621. doi: 10.3390/diagnostics11040621
78. Preuner S, Lion T. Post-transplant monitoring of chimerism by lineage-specific analysis. In: Beksaç M, editor. Bone marrow and stem cell transplantation. methods in molecular biology, vol 1109. New York, NY: Humana Press (2014). doi: 10.1007/978-1-4614-9437-9_14
79. Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: Possible role for pre-emptive immunotherapy? J Clin Oncol (2004) 22(9):1696–705. doi: 10.1200/JCO.2004.05.198
80. Zeiser R, Spyridonidis A, Wäsch R, Ihorst G, Grüllich C, Bertz H, et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia (2005) 19(5):814–21. doi: 10.1038/sj.leu.2403719
81. Kharfan-Dabaja MA, Kumar A, Ayala E, Aljurf M, Nishihori T, Marsh R, et al. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: A report on behalf of the American society for transplantation and cellular therapy. Transplant Cell Ther (2021) 27(8):642–9. doi: 10.1016/j.jtct.2021.04.007
82. Carreras E, Dufour C, Mohty M, Kröger N eds. Bader p. documentation of engraftment and chimerism after HSCT. In: The EBMT handbook: Hematopoietic stem cell transplantation and cellular therapies, 7th ed. Cham (CH: Springer.
83. Pulsipher MA. Chimerism versus minimal residual disease monitoring after allogeneic transplantation–when do we act and will intervention improve outcomes? Biol Blood Marrow Transplant (2014) 20(10):1461–2. doi: 10.1016/j.bbmt.2014.07.026
84. Terwey TH, Hemmati PG, Nagy M, Pfeifer H, Gökbuget N, Brüggemann M, et al. Comparison of chimerism and minimal residual disease monitoring for relapse prediction after allogeneic stem cell transplantation for adult acute lymphoblastic leukemia. Biol Blood Marrow Transplant (2014) 20(10):1522–9. doi: 10.1016/j.bbmt.2014.05.026
85. Rossi G, Carella AM, Minervini MM, Savino L, Fontana A, Pellegrini F, et al. Minimal residual disease after allogeneic stem cell transplant: A comparison among multiparametric flow cytometry, wilms tumor 1 expression and chimerism status (Complete chimerism versus low level mixed chimerism) in acute leukemia. Leuk Lymphoma (2013) 54(12):2660–6. doi: 10.3109/10428194.2013.789508
86. Pincez T, Santiago R, Bittencourt H, Louis I, Bilodeau M, Rouette A, et al. Intensive monitoring of minimal residual disease and chimerism after allogeneic hematopoietic stem cell transplantation for acute leukemia in children. Bone Marrow Transplant (2021) 56(12):2981–9. doi: 10.1038/s41409-021-01408-5
87. Semchenkova A, Brilliantova V, Shelikhova L, Zhogov V, Illarionova O, Mikhailova E, et al. Chimerism evaluation in measurable residual disease-suspected cells isolated by flow cell sorting as a reliable tool for measurable residual disease verification in acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Cytometry B Clin Cytom (2021) 100(5):568–73. doi: 10.1002/cyto.b.21982
88. Denys B, van der Sluijs-Gelling AJ, Homburg C, van der Schoot CE, de Haas V, Philippé J, et al. Improved flow cytometric detection of minimal residual disease in childhood acute lymphoblastic leukemia. Leukemia (2013) 27(3):635–41. doi: 10.1038/leu.2012.231
89. van Dongen JJ, Seriu T, Panzer-Grümayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet (1998) 352(9142):1731–8. doi: 10.1016/S0140-6736(98)04058-6
90. Brüggemann M, Schrauder A, Raff T, Pfeifer H, Dworzak M, Ottmann OG, et al. Standardized MRD quantification in European ALL trials: proceedings of the second international symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia (2010) 24(3):521–35. doi: 10.1038/leu.2009.268
91. Kotrova M, van der Velden VHJ, van Dongen JJM, Formankova R, Sedlacek P, Brüggemann M, et al. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant (2017) 52(7):962–8. doi: 10.1038/bmt.2017.16
92. Ladetto M, Brüggemann M, Monitillo L, Ferrero S, Pepin F, Drandi D, et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in b-cell disorders. Leukemia (2014) 28(6):1299–307. doi: 10.1038/leu.2013.375
93. van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, et al. European Study group on MRD detection in ALL (ESG-MRD-ALL). analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia (2007) 21(4):604–11. doi: 10.1038/sj.leu.2404586
94. Stahl T, Rothe C, Böhme MU, Kohl A, Kröger N, Fehse B. Digital PCR panel for sensitive hematopoietic chimerism quantification after allogeneic stem cell transplantation. Int J Mol Sci (2016) 17(9):1515. doi: 10.3390/ijms17091515
95. Blouin AG, Ye F, Williams J, Askar M. A practical guide to chimerism analysis: Review of the literature and testing practices worldwide. Hum Immunol (2021) 82(11):838–49. doi: 10.1016/j.humimm.2021.07.013
96. Delie A, Verlinden A, Beel K, Deeren D, Mazure D, Baron F, et al. Use of chimerism analysis after allogeneic stem cell transplantation: Belgian guidelines and review of the current literature. Acta Clin Belg (2021) 76(6):500–8. doi: 10.1080/17843286.2020.1754635
97. Pettersson L, Vezzi F, Vonlanthen S, Alwegren K, Hedrum A, Hauzenberger D. Development and performance of a next generation sequencing (NGS) assay for monitoring of mixed chimerism. Clin Chim Acta (2021) 512:40–8. doi: 10.1016/j.cca.2020.10.034
98. Mountjoy L, Palmer J, Kunze KL, Khera N, Sproat LZ, Leis JF, et al. Does early chimerism testing predict outcomes after allogeneic hematopoietic stem cell transplantation? Leuk Lymphoma (2021) 62(1):252–4. doi: 10.1080/10428194.2020.1827249
99. Campana D, Leung W. Clinical significance of minimal residual disease in patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol (2013) 162(2):147–61. doi: 10.1111/bjh.12358
100. Warraich Z, Tenneti P, Thai T, Hubben A, Amin H, McBride A, et al. Relapse prevention with tyrosine kinase inhibitors after allogeneic transplantation for Philadelphia chromosome-positive acute lymphoblast leukemia: A systematic review. Biol Blood Marrow Transplant (2020) 26(3):e55–64. doi: 10.1016/j.bbmt.2019.09.022
101. Biederstädt A, Rezvani K. How I treat high-risk acute myeloid leukemia using pre-emptive adoptive cellular immunotherapy. Blood (2022) 63(141):22–38. doi: 10.1182/blood.2021012411
102. Stein AS, Kantarjian H, Gökbuget N, Bargou R, Litzow MR, Rambaldi A, et al. Blinatumomab for acute lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2019) 25(8):1498–504. doi: 10.1016/j.bbmt.2019.04.010
103. Naik S, Vasileiou S, Tzannou I, Kuvalekar M, Watanabe A, Robertson C, et al. Donor-derived multiple leukemia antigen-specific T-cell therapy to prevent relapse after transplant in patients with ALL. Blood (2022) 139(17):2706–11. doi: 10.1182/blood.2021014648
104. Izumi A, Tachibana T, Ando T, Tanaka M, Kanamori H, Nakajima H. A case series of patients treated with inotuzumab ozogamicin for acute lymphoblastic leukemia relapsed after allogeneic hematopoietic cell transplantation. Int J Hematol (2022) 115(1):69–76. doi: 10.1007/s12185-021-03217-4
105. Bazarbachi A, Labopin M, Aljurf M, Niittyvuopio R, Balsat M, Blaise D, et al. 20-year steady increase in survival of adult patients with relapsed Philadelphia-positive acute lymphoblastic leukemia post allogeneic hematopoietic cell transplantation. Clin Cancer Res (2022) 28(5):1004–12. doi: 10.1158/1078-0432.CCR-21-2675
106. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med (2014) 371(16):1507–17. doi: 10.1056/NEJMoa1407222
107. Zhao XY, Xu ZL, Mo XD, Chen YH, Lv M, Cheng YF, et al. Preemptive donor-derived anti-CD19 CAR T-cell infusion showed a promising anti-leukemia effect against relapse in MRD-positive b-ALL after allogeneic hematopoietic stem cell transplantation. Leukemia (2022) 36(1):267–70. doi: 10.1038/s41375-021-01351-w
Keywords: measurable residual disease, multiparameter flow cytometry, acute lymphoblastic leukemia, allogeneic hematopoietic stem cell transplantation, adult patients
Citation: Tecchio C, Russignan A and Krampera M (2023) Immunophenotypic measurable residual disease monitoring in adult acute lymphoblastic leukemia patients undergoing allogeneic hematopoietic stem cell transplantation. Front. Oncol. 13:1047554. doi: 10.3389/fonc.2023.1047554
Received: 18 September 2022; Accepted: 11 January 2023;
Published: 22 February 2023.
Edited by:
Sara Galimberti, University of Pisa, ItalyReviewed by:
Marina Martello, University of Bologna, ItalyCopyright © 2023 Tecchio, Russignan and Krampera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Tecchio, Y3Jpc3RpbmEudGVjY2hpb0B1bml2ci5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.