
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 09 February 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1047388
This article is part of the Research Topic Rising PSA in Post-Therapy Prostate Cancer Patients View all 4 articles
Background: Androgen-deprivation therapy (ADT) is used for the treatment of prostate cancer. However, the specific risk factors for the development of castration-resistant disease are still unclear. The present study sought to identify predictors of patient prognostic outcomes through analyses of clinical findings in large numbers of prostate cancer patients following ADT treatment.
Methods: Data pertaining to 163 prostate cancer patients treated at the Second Affiliated Hospital of Bengbu Medical University and Maoming People’s Hospital from January 1, 2015, to December 30, 2020, were retrospectively analyzed. Dynamic changes in prostate-specific antigen (PSA) levels were regularly assessed, including both time to nadir (TTN) and nadir PSA (nPSA). Univariate and multivariate analyses were performed with Cox risk proportional regression models, while differences in biochemical progression-free survival (bPFS) were compared among groups with Kaplan-Meier curves and log-rank tests.
Results: The bPFS values over the median 43.5-month follow-up period differed significantly between patients with nPSA levels < 0.2 ng/mL and ≥ 0.2 ng/mL, being 27.6 months and 13.5 months, respectively (log-rank P < 0.001). A significant difference in median bPFS was also observed when comparing patients with a TTN ≥ 9 months (27.8 months) to those with a TTN < 9 months (13.5 months) (log-rank P < 0.001).
Conclusions: TTN and nPSA are valuable predictors of prognosis in prostate cancer patients after ADT treatment, with better outcomes evident in patients with nPSA < 0.2 ng/mL and TTN > 9 months.
Prostate cancer is an increasingly common cause of human morbidity and mortality. In China, the incidence and mortality of prostate cancer in China account for 8.2% and 13.6% of the global estimates, respectively, according to the GLOBOCAN2020 data (1). As of 2015, prostate cancer was estimated to affect 10 out of every 100,000 people in mainland China, making it the most common urological malignancy (2). Approximately 68% of prostate cancer patients in mainland China have metastatic tumors at the time of diagnosis and androgen deprivation is the standard treatment modality for this type of prostate cancer (3). However, following an initial period during which patients respond well to ADT (median duration of 18-24 months) (4), the levels of prostate-specific antigen (PSA) tend to rise along with disease progression due to the emergence of castration-resistant prostate cancer (CRPC).
The identification of accurate and robust predictive biomarkers associated with prostate cancer patient outcomes following ADT remains an active research hotspot. Since the initial establishment of PSA as a relevant biomarker three decades ago, PSA levels are usually monitored during prostate cancer screening and diagnosis, and also offer value in the context of patient prognostic evaluation (4–6). After the initiation of ADT in patients with prostate cancer, the PSA levels undergo dynamic changes and monitoring these changes can provide insight into the patient outcomes. However, the actual prognostic value of parameters such as the nadir PSA (nPSA) and time to nPSA (TTN) levels remains the subject of substantial debate and further clinical analyses will be critical to fully clarify the relevance of these variables in different patient cohorts (7–9).
Several recent reports have indicated the importance of both nPSA and TNN values as prognostic indicators in the context of ADT treatment. For example, Matsubara et al. reported that an nPSA > 0.1 ng/mL and a TTN of > 6 months were consistent with favorable long-term prognosis (10). Several studies have also demonstrated relationships between these two parameters and the overall survival (OS) or progression-free survival (rPFS) of prostate cancer patients (10–12). However, to our knowledge, there is little information on the value of the PSA halving time in prognostic prediction. Here, a retrospective analysis of patients with prostate cancer patients who underwent ADT treatment was performed to more fully understand the associations between nPSA, TTN, PSA halving time, and patient survival outcomes.
The study conducted a retrospective analysis of 163 patients with prostate cancer ≥ 50 years of age who were treated between January 1, 2015, and December 30, 2020, at two medical centers in China, namely, the Second Affiliated Hospital of Bengbu Medical College and Maoming People’s Hospital. Patients were diagnosed with metastatic or locally advanced prostate cancer based on prostate biopsy analyses and treated with androgen deprivation in the form of total androgen blockade (i.e., surgical/pharmacological castration + anti-androgen drugs). Patients who had been diagnosed with another type of cancer or who had previously undergone radical surgery, chemotherapy, and/or radiotherapy were excluded from the study.
Patient follow-up data were gathered from hospital medical record systems. Traditional computed tomography (CT) scans and bone imaging were used exclusively for baseline staging and subsequent follow-up. Monthly measurements of serum testosterone and PSA levels were performed for the first two years, with testing performed every three months on average after the first two years. The baseline PSA levels at the initiation of ADT, as well as the nPSA, TTN, and PSA half-lives were analyzed. Serum testosterone concentrations were also analyzed to confirm that patients had successfully achieved a castration-like state (< 50 ng/dL). The nPSA was defined as the lowest measured PSA concentration over the course of ADT while TTN was defined as the interval between ADT initiation and nPSA. The PSA halving time indicated the rate of PSA decline following the initiation of ADT.
Biochemical progression was defined as three consecutively elevated PSA test results one week apart with two test results exceeding the 50% nadir. Biochemical progression-free survival (bPFS) was calculated as the interval between the start of ADT and the first measurement of elevated PSA levels associated with biochemical progression.
Tumor load was determined by the presence of at least four bone metastases and at least one metastasis outside the median bone or in the pelvis, or the presence of visceral metastases, as defined by the Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer (CHAARTED) study (13).
Continuous data are presented as means ± standard deviation while categorical data including Gleason scores are presented as medians. Continuous data were compared using ANOVA and categorical data with Fisher’s exact test. Univariate and multivariate analyses of patient bPFS were performed using the Cox proportional hazards model, examining the association between this endpoint and variables including age, BMI, Gleason score, staging, post-treatment PSA halving time, metastatic load, nPSA, and TTN values. Cut-off values were determined using receiver operating characteristic (ROC) curves, and bPFS was assessed using Kaplan-Meier curves and log-rank tests. P-values < 0.05 were considered statistically significant. SPSS 27.0.1.0 (IBM, NY, USA) was used for all data analyses.
The median age of the 163 enrolled patients was 72 years (range:53-89 years; Table 1). The majority of the patients (55.2%) had Gleason scores between 8 and 10 at time of diagnosis with 73.0% of the patients having metastatic (M1) disease. Surgical castration was performed in nine cases (5.5%)while the remaining 94.5% of patients instead underwent ADT (3.6 mg once/28 days) in the form of subcutaneous goserelin acetate treatment. All patients received orally administered 50 mg/day of bicalutamide in parallel with the castration treatment. At the start of ADT, the median PSA level of these patients was 98.59 ng/mL while the mean nPSA value was 2.36 ng/mL.
To define the risk factors associated with bPFS in patients with metastatic prostate cancer, the data of 199 patients were analyzed using univariate and multivariate Cox regression. A decline in the PSA level to ≤ 2 ng/mL (hazard ratio [HR] 0.462, P = 0.001) and a TTN < 9 months (HR 1.736, P = 0.021) were found to be independently associated with the risk of bPFS (Table 2). PSA levels < 0.2 ng/ml were associated with favorable prognosis, and the risk of disease progression declined as the time taken to reach the PSA nadir increased.
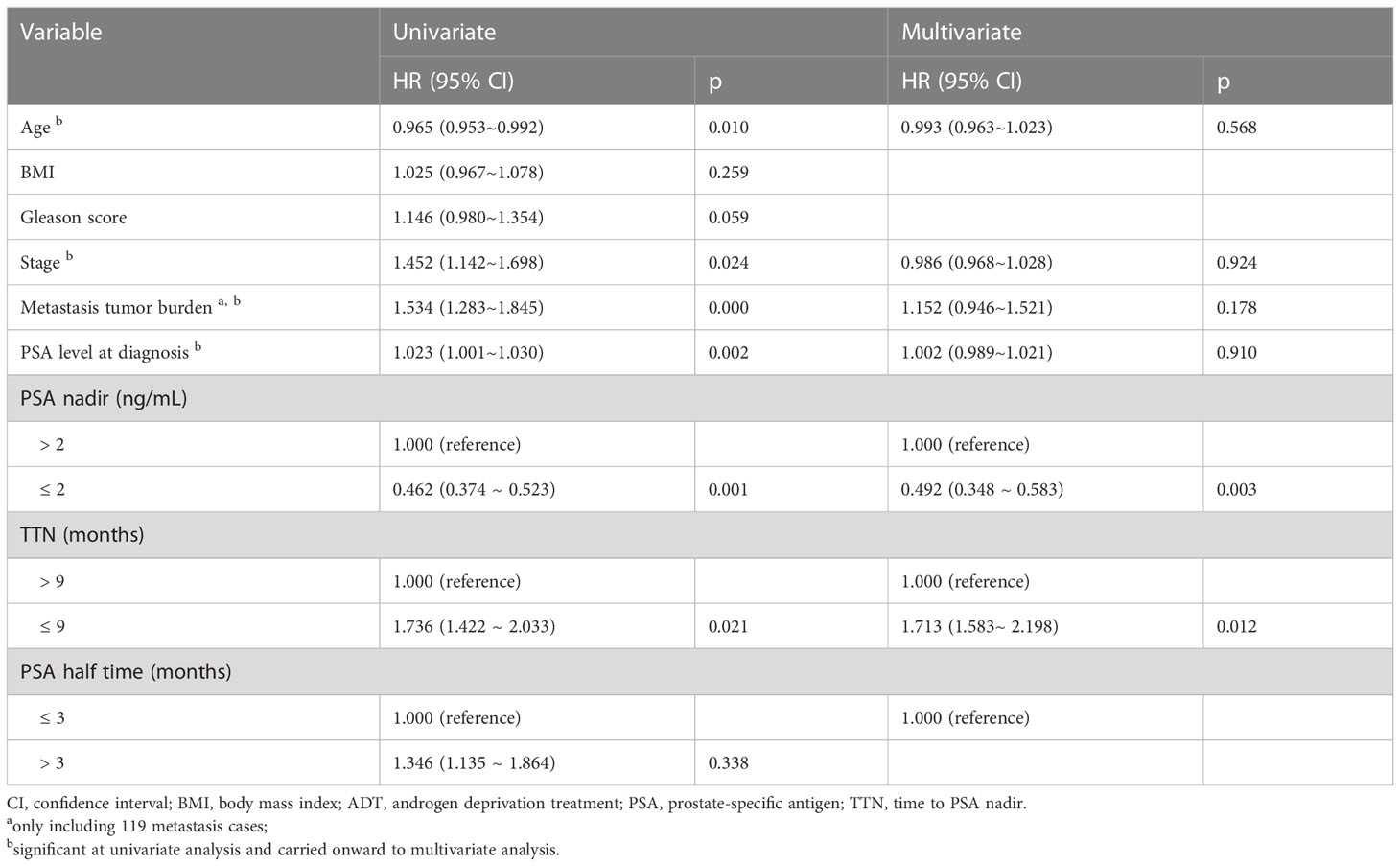
Table 2 Univariate and multivariable analysis of potential risk factors for biochemical progression-free survival.
Lower nPSA levels were found to be associated with a lower risk of bPFS (Table 3), and this predictive relationship remained true when controlling for variables including age, BMI, tumor load, baseline PSA level, stage, and Gleason score. For patients with PSA levels < 4 ng/mL (defined as normalization), the odds of PFS were reduced with shorter times to normalization, although no significant association with the emergence of castration resistance was detected (Table 4). When intergroup comparisons were performed for patients with normalization times of 8-12, 12-24, or > 24 weeks, it was found that better prognosis was associated with shorter normalization times, with a normalization time > 24 weeks found to be an independent predictor of patient outcome (HR 0.695, P = 0.001). These data indicate that PSA levels that fall more rapidly to within the reference ranges following ADT initiation are associated with better patient outcomes. Unlike the PSA normalization time, TTN remained a significant predictor of patient bPFS even after controlling for other risk factors and using the cut-off values of 6, 10, or 12 months.
Regarding the PSA halving time, a shorter halving time was found to be associated with worse prognosis when comparing groups using the cut-off values of 0.5, 1, 1.5, and 3 months. Although a trend towards a worse prognosis with lower PSA halving time wa observed, but it the the group comparisons were not statistically significant. From this we conclude that PSA halving time is not a good predictor of ADT initiation.
The area under the curve (AUC) values for nPSA and TTN were 0.804 and 0.833, respectively (Figure 1); as these values fall above 0.7 but below 0.9, this suggests that these variables offer some level of prognostic value. The nPSA value was associated with a sensitivity and specificity of 65.7% and 73.6%, respectively, when selecting 0.2 ng/mL as the optimal cut-off value, indicating that nPSA values > 0.2 ng/mL are suggestive of a poorer prognosis. Similarly, 9 months was selected as the optimal TTN cut-off value, yielding a sensitivity and specificity of 71.6% and 73.9%, respectively. Thus, patients with a TTN > 9 months showed better prognostic outcomes. The AUC for the PSA halving time was only 0.563, indicating that this parameter was insufficiently accurate for the reliable assessment of patient bPFS risk.
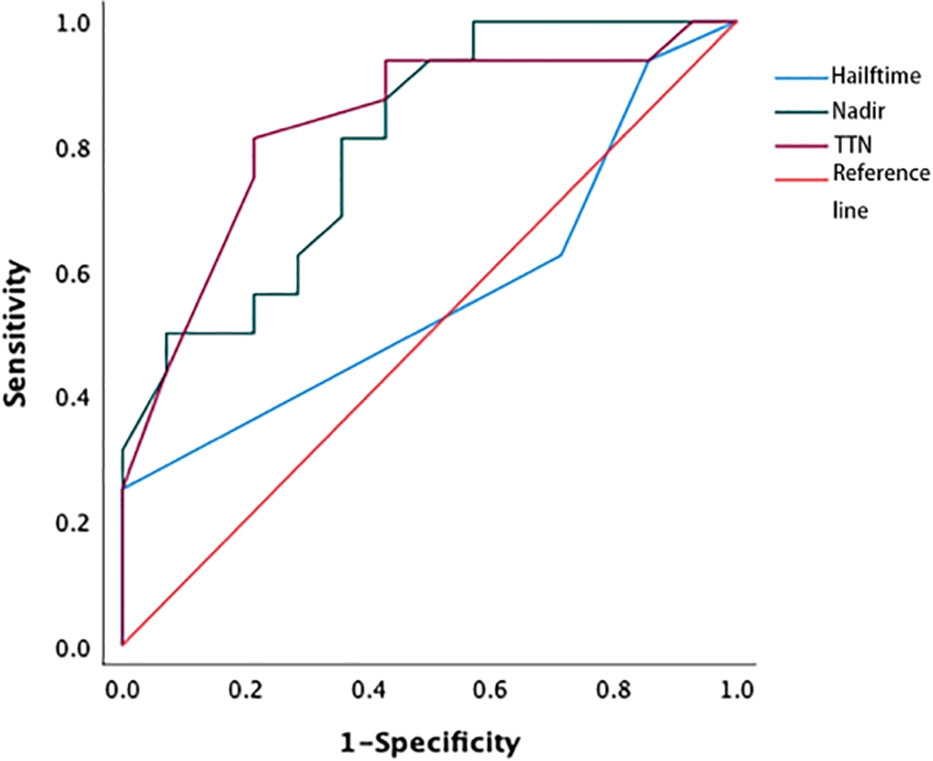
Figure 1 ROC curve of nPSA, TTN, and PSA halving time. The areas under the curve for the PSA nadir and PSA time to nadir were 0.804 and 0.833, respectively, while AUC for the PSA halving time was 0.563. ROC, receiver operating characteristic; TTN, time to PSA nadir.
In patients with an nPSA < 0.2 and ≥ 0.2 ng/mL, the respective median bPFS values were 27.6 months and 13.5 months, with a significant difference between these groups (Log-rank P < 0.001). Similarly, patients with a TTN ≥ 9 months showed a better median bPFS (27.8 months) relative to patients with a TTN < 9 months (13.5 months; Log-rank P < 0.001)(Figure 2).
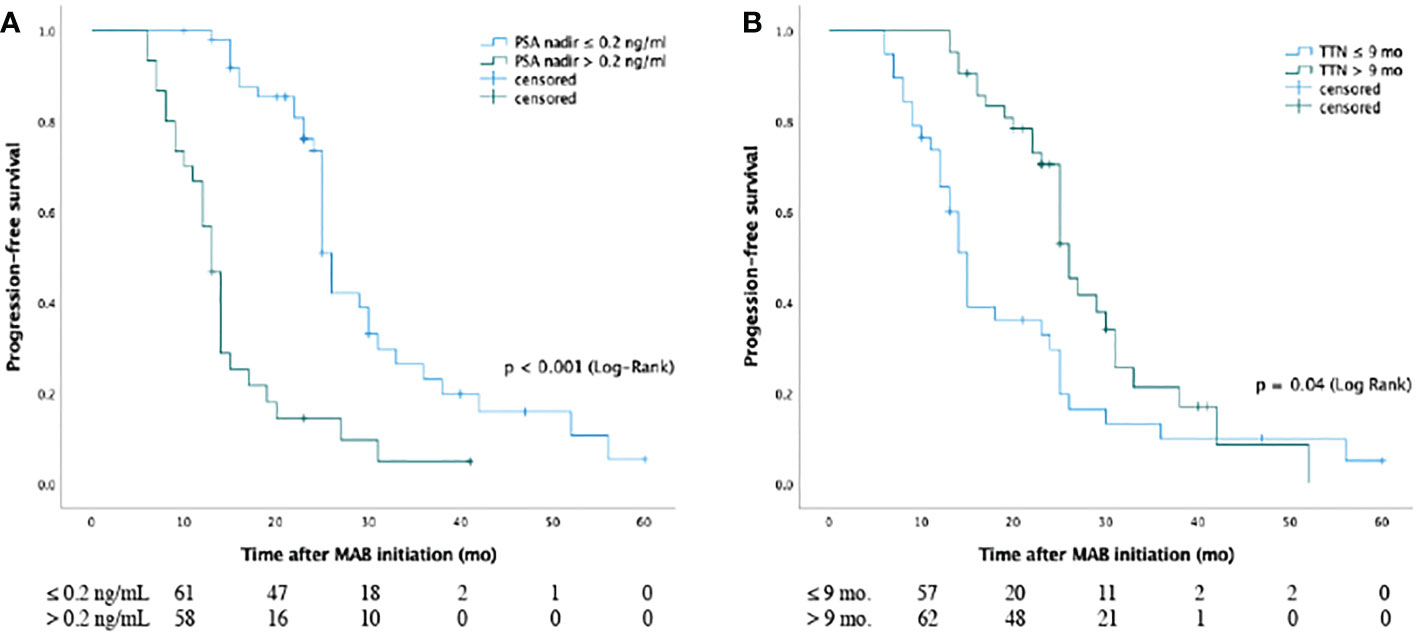
Figure 2 Kaplan-Meier curves for biochemical progression-free survival of nPSA (A) and TTN (B) for patients with prostate cancer from MAB initiation. The median bPFS values (A) were 27.6 months and 13.5 months for patients in the two groups with PSA nadir < 0.2 ng/ml and ≥ 0.2 ng/ml, respectively, and there was significant difference between groups (P < 0.001 [log-rank]). The median bPFS values (B) were 27.8 months and 13.5 months for cases in two groups with TTN ≥ 9 months and < 9 months, respectively, and there was significant difference between the two groups (P < 0.001 [log rank]). Abbreviations: PSA: Prostate-specific antigen; TTN: time to PSA nadir; MAB: maximal androgen blockade; nPSA: PSA nadir.
Given the high rates of advanced prostate cancer in China, ADT remains an important focus of clinical research interest (9). In addition to aiding the screening and diagnosis of prostate cancer, the PSA level is an invaluable tool to monitor and evaluate the prognosis of patients after the initiation of ADT treatment. Dynamic shifts in PSA concentrations over the course of the treatment can provide insight into patient outcomes, with several variables including nPSA, TTN, and the PSA halving time shown to offer prognostic utility in certain patient populations (12, 14, 15). However, these findings are not universal, and the clinical significance of these PSA dynamics as biomarkers of CRPC development thus remains controversial (8, 16).
Here, it was found that lower nPSA and longer TTN values were significantly associated with better outcomes in patients with prostate cancer. This is consistent with data from several reports demonstrating the prognostic utility of these two variables in patients treated with ADT (17, 18). Shi et al., for example, performed a retrospective analysis of data from 153 individuals with metastatic CRPC undergoing combination ADT and docetaxel treatment, revealing a reduced response to chemotherapy and poorer prognosis in patients with higher nPSA levels and shorter TTN (8). A double-blind randomized trial in which the efficacy of abiraterone acetate and prednisone (AAP) + ADT was compared to placebo + ADT showed that patients with lower nPSA values (0.1 ng/ml) six months after treatment had better prognosis (10). In addition, Choueiri et al. reported that TTN <6 months and an nPSA value of 0.2 ng/mL were independently predictive of shorter OS (19). Harshman et al. suggested that a PSA level of 0.2 ng/mL after seven months of treatment extends the OS of patients with metastatic hormone-sensitive prostate cancer (20). Matsubara et al. found that low PSA levels (0.1 ng/ml) after six months may indicate a good long-term response to treatment (10) while a PSA level of 4 ng/mL or less after seven months was found to be a strong predictor of survival (20). While these prior studies have emphasized the utility of nPSA and TTN values as predictors of OS and PFS in patients with advanced prostate cancer, the optimal predictors differed (14, 19, 21). Our analysis indicated that a PSA < 0.2 ng/mL and a TTN of > 9 months were optimal predictors of bPFS in patients with metastatic prostate.
The present multicenter retrospective case review approach revealed a significant association between nPSA levels < 0.2 ng/mL of nPSA and better median bPFS outcomes, relative to patients with nPSA levels ≥ 0.2 ng/mL of nPSA(27.6 vs 13.5 months, respectively), with these patients showing an overall 67.7% lower progression risk (HR, 0.323). Other studies have similarly reported lower nPSA levels to be related to a better prognosis (9, 22), although the actual cut-off values for nPSA have varied among studies (8, 9, 22–24). Several studies have used a cut-off value of 0.2 ng/mL (8, 23, 25), although some reports suggest that an undetectable nPSA level is related to better prognostic outcomes (26) and other reports have used a higher cut-off of 4.0 ng/mL (22). These variations may be attributable to different follow-up approaches and study population characteristics.
It is generally believed that a more rapid decline in the PSA level is associated with a greater likelihood of eliminating the proportion of reactive prostate cancer cells, leading to increased patient survival. However, it was found that while rapidly declining PSA levels and the transcriptional outcome of ADT were related, they were not associated with cancer cell death. Alternatively, a rapid decrease in PSA levels may indicate the downregulation of PSA expression in hormone-sensitive prostate cancer cells as PSA expression is regulated by androgen through the androgen receptor pathway (6, 12). In this study, TTN was found to be associated with prognosis in prostate cancer patients, with a longer TTN being similarly associated with a longer bPFS. Specifically, patients with a TTN > 9 months exhibited a longer median survival than did patients with a TTN < 9 months (27.8 vs 13.5 months; P = 0.004), in line with prior reports (23, 24, 27–29). Different studies have used TTN cut-off values from 6-12 months, with a cut-off of 9 months being the most common (24, 27).
Here, lower nPSA values were associated with lower odds of bPFS (P < 0.001), consistent with prior reports (30). This study performed subgroup analyses by treating nPSA and TTN as continuous variables. The results showed that the likelihood of bPFS increased 1.794-fold in the subgroup with nPSA levels between 0.2 and 4 ng/mL, and 5.332-fold in the subgroup with nPSA levels > 4 ng/mL, compared to the subgroup with TTN > 12 months, and that the risk increased by 1.245-fold at TTN < 12 months and 3.408-fold at TTN < 6 months compared to the subgroup with TTN > 12 months (30).
Here, ROC curves revealed that the PSA halving time was unrelated to prognostic outcomes in patients undergoing ADT, with an AUC of just 0.553. This may be attributable to the fact that reductions in PSA levels following the initiation of ADT do not occur at a constant rate. Indeed, previous findings suggest that the response of prostate cancer to ADT is triphasic, including an androgen-PSA response, tumor atrophy, and quiescent proliferation phases (31). Given that prostate tumors are heterogeneous, they are likely to harbor a proportion of hormone-unresponsive cells leading to inevitable differences in the rates at which the PSA levels decline and tumors adapt to ADT treatment. Accordingly, the PSA halving time cannot reliably reflect the proportions of androgen-independent cells in a given patient, rendering it unsuitable for prognostic evaluation in prostate cancer patients (14).
Early PSA responses to ADT (>30% reduction after 4 or 12 weeks) are frequently used to gauge patient prognosis (32, 33). Dynamic shifts in serum PSA concentrations have been used as tools both to explore the prognosis of hormone-sensitive prostate cancer patients and monitor CRPC patients undergoing second-line ADT (34, 35) while also providing an approach for assessing the prognosis of CRPC patients undergoing chemotherapy (36).
There are several limitations to this study. These include the relatively small sample size, retrospective design, and the potential for differences in evaluation criteria at the two medical centers. In addition, other common measures of patient survival such as OS or disease-free survival were not analyzed. Moreover, the current standard of care for patients diagnosed with metastatic hormone-sensitive prostate cancer besides ADT, including Abirateone or AR blocking (Enzalutamide/apalutamide/daralotamide+docetaxel), was not included in the statistics.
These results suggest that both TTN and nPSA offer value as predictors of prostate cancer patient outcomes following ADT, with better outcomes seen in patients with an nPSA level < 0.2 ng/mL and a TTN > 9 months. These findings provide a foundation for guiding the treatment and monitoring of prostate cancer patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conceptualization, MH, CG, ZT; methodology, MH and YM; software, YM; validation, MH, YM and ZB; investigation, YM; resources, ZB, YL and GL; data curation, YL and GL; writing—original draft preparation, YM, ZB; writing—review and editing, MH and YM; visualization, YM; supervision, MH, CG, ZT; project administration, MH, CG, ZT. All authors have read and agreed to the published version of the manuscript.
This research was funded by Guangdong Medical Science and Technology Research Fund Project (No. A2022464); High-Level Hospital Construction Research Project of Maoming People`s Hospital and Maoming Municipal Science and Technology Bureau Special Plan (No. 2020KJZX018);the Key Projects of Anhui Provincial Educational Department (No. KJ2019A0373). The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data, nor in the writing of the manuscript or the decision to publish the results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. He J, Chen WQ, Li N, Cao W, Ye D, Ma J, et al. China Guideline for the screening and early detection of prostate cancer (2022, Beijing). Zhonghua Zhong Liu Za Zhi (2022) 44:29–53. doi: 10.3760/cma.j.cn112152-20211226-00975
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Wang X, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
3. Chen R, Sjoberg DD, Huang Y, Xie L, Zhou L, He D, et al. Prostate specific antigen and prostate cancer in Chinese men undergoing initial prostate biopsies compared with Western cohorts. J Urol (2017) 197:90–6. doi: 10.1016/j.juro.2016.08.103
4. Eggener S. Hormonal therapy for prostate cancer. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, editors. Campell-Walsh-Wein urology, 12th edition. Philadelphia: Saunders Elsevier (2021). p. 16714–54.
5. Miszczyk M, Magrowski Ł, Masri O, Jabłon'ska I, Nowicka Z, Krzysztofiak T, et al. Prostate-specific antigen kinetics and metastasis-free survival in patients treated with external beam radiotherapy combined with high-dose-rate brachytherapy boost and androgen deprivation therapy for localized prostate cancer. J Contemp Brachyther (2022) 14:15–22. doi: 10.5114/jcb.2022.113546
6. Hah YS, Lee JS, Rha KH, Hong SJ, Chung BH, Koo KC. Effect of prior local treatment and prostate-specific antigen kinetics during androgen-deprivation therapy on the survival of castration-resistant prostate cancer. Sci Rep (2019) 9(1):11899. doi: 10.1038/s41598-019-48424-6
7. Afriansyah A, Hamid ARAH, Mochtar CA, Umbas R. Prostate specific antigen(PSA) kinetic as a prognostic factor in metastatic prostate cancer receiving androgen deprivation therapy: systematic review and meta-analysis. F1000Res (2018) 7:246. doi: 10.12688/f1000research.14026.1
8. Shi X, Pei X, Fan J, Liu T, Zhang D, Yang T, et al. PSA nadir and time to PSA nadir during initial androgen deprivation therapy as prognostic factors in metastatic castration-resistance prostate cancer patients treated with docetaxel. Andrologia (2021) 53:e13916. doi: 10.1111/and.13916
9. Teoh JY, Tsu JH, Yuen SK, Chan SY, Chiu PK, Wong KW, et al. Survival outcomes of Chinese metastatic prostate cancer patients following primary androgen deprivation therapy in relation to prostate-specific antigen nadir level. Asia Pac J Clin Oncol (2017) 13:e65–71. doi: 10.1111/ajco.12313
10. Matsubara N, Chi KN, Özgüroğlu M, Rodriguez-Antolin A, Feyerabend S, Fein L, et al. Correlation of prostate-specific antigen kinetics with overall survival and radiological progression-free survival in metastatic castration-sensitive prostate cancer treated with abiraterone acetate plus prednisone or placebos added to androgen deprivation therapy: Post hoc analysis of phase 3 LATITUDE study. Eur Urol (2020) 77:494–500. doi: 10.1016/j.eururo.2019.11.021
11. Teoh JY, Tsu JH, Yuen SK, Tamura K, Motoyama D, Ito T, et al. Prognostic significance of time to prostate-specific antigen (PSA) nadir and its relationship to survival beyond time to PSA nadir for prostate cancer patients with bone metastases after primary androgen deprivation therapy. Ann Surg Oncol (2015) 22:1385–91. doi: 10.1245/s10434-014-4105-8
12. Lin YC, Lin PH, Shao IH, Chu YC, Kan HC, Liu CY, et al. Prostate-specific antigen kinetics effects on outcomes of low-volume metastatic prostate cancer patients receiving androgen deprivation therapy. J Oncol (2021) 2021:9648579. doi: 10.1155/2021/9648579
13. Gravis G, Boher JM, Chen YH, Liu G, Fizazi K, Carducci MA, et al. Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: Further analyses of CHAARTED and GETUG-AFU15 studies. Eur Urol (2018) 73:847. doi: 10.1016/j.eururo.2018.02.001
14. Sasaki T, Yoshiki S. The importance of time to prostate-specific antigen (PSA) nadir after primary androgen deprivation therapy in hormone-naïve prostate cancer patients. J Clin Med (2018) 7(12):565. doi: 10.3390/jcm7120565
15. Huang SP, Bao BY, Wu MT, Choueiri TK, Goggins WB, Liu CC, et al. Significant associations of prostate-specific antigen nadir and time to prostate-specific antigen nadir with survival in prostate cancer patients treated with androgen-deprivation therapy. Aging Male (2012) 15:34–41. doi: 10.3109/13685538.2011.580398
16. Bitterman DS, Chen MH, Wu J, Renshaw AA, Loffredo M, Kantoff PW, et al. Prostate-specific antigen nadir and testosterone level at prostate-specific antigen failure following radiation and androgen suppression therapy for unfavorable-risk prostate cancer and the risk of all-cause and prostate cancer-specific mortality. Cancer (2021) 127:2623–30. doi: 10.1002/cncr.33543
17. Pei X, Wu K, Sun Y, Gao X, Gou X, Xu J, et al. PSA time to nadir as a prognostic factor of first-line docetaxel treatment in castration-resistant prostate cancer: Multicenter validation in patients from the Chinese prostate cancer consortium. Urol Oncol (2020) 38:2.e11–7. doi: 10.1016/j.urolonc.2019.07.014
18. Wu KJ, Pei XQ, Tian G, Gao X, Gou X, Xu J, et al. PSA time to nadir as a prognostic factor of first-line docetaxel treatment in castration-resistant prostate cancer: evidence from patients in northwestern China. Asian J Androl (2018) 20:173–7. doi: 10.4103/aja.aja_34_17
19. Choueiri TK, Xie W, D’Amico AV, Ross RW, Hu JC, Pomerantz M, et al. Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy. Cancer (2009) 115:981–7. doi: 10.1002/cncr.24064
20. Harshman LC, Chen YH, Liu G, Pei XQ, Chen YH, Tian G, et al. Seven-month prostate-specific antigen is prognostic in metastatic hormone-sensitive prostate cancer treated with androgen deprivation with or without docetaxel. J Clin Oncol (2018) 36(4):376–82. doi: 10.1200/JCO.2017.75.3921
21. Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from southwest oncology group trial 9346 (INT-0162). J Clin Oncol (2006) 24:3984–90. doi: 10.1200/JCO.2006.06.4246
22. Bello JO. Predictors of survival outcomes in native sub Saharan black men newly diagnosed with metastatic prostate cancer. BMC Urol (2017) 17:39. doi: 10.1186/s12894-017-0228-0
23. Koo KC, Park SU, Kim KH, Rha KH, Hong SJ, Yang SC, et al. Predictors of survival in prostate cancer patients with bone metastasis and extremely high prostate-specific antigen levels. Prostate Int (2015) 3:10–5. doi: 10.1016/j.prnil.2015.02.006
24. Hong SY, Cho DS, Kim SI, Ahn HS, Kim SJ. Prostate-specific antigen nadir and time to prostate-specific antigen nadir following maximal androgen blockade independently predict prognosis in patients with metastatic prostate cancer. Korean J Urol (2012) 53:607–13. doi: 10.4111/kju.2012.53.9.607
25. Lin TT, Chen YH, Wu YP, Chen SZ, Li XD, Lin Y, et al. Risk factors for progression to castration-resistant prostate cancer in metastatic prostate cancer patients. J Cancer (2019) 10:5608–13. doi: 10.7150/jca.30731
26. Keto CJ, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL, et al. Detectable prostate-specific antigen nadir during androgen-deprivation therapy predicts adverse prostate cancer-specific outcomes: results from the SEARCH database. Eur Urol (2014) 65:620–7. doi: 10.1016/j.eururo.2012.11.052
27. Ji G, Song G, Huang C, He S, Zhou L. Rapidly decreasing level of prostate-specific antigen during initial androgen deprivation therapy is a risk factor for early progression to castration-resistant prostate cancer: A retrospective study. Med (Baltimore) (2017) 96:e7823. doi: 10.1093/annonc/mdx662.001
28. Moul JW. Hormone naïve prostate cancer: Predicting and maximizing response intervals. Asian J Androl (2015) 17:929–35. doi: 10.4103/1008-682X.152821
29. Hamano I, Hatakeyama S, Narita S, Takahashi M, Sakurai T, Kawamura S, et al. Impact of nadir PSA level and time to nadir during initial androgen deprivation therapy on prognosis in patients with metastatic castration-resistant prostate cancer. World J Urol (2019) 37:2365–73. doi: 10.1007/s00345-019-02664-3
30. Tomioka A, Tanaka N, Yoshikawa M, Miyake M, Anai S, Chihara Y, et al. Nadir PSA level and time to nadir PSA are prognostic factors in patients with metastatic prostate cancer. BMC Urol (2014) 14:33. doi: 10.1186/1471-2490-14-33
31. Mizokami A, Izumi K, Konaka H, Kitagawa Y, Kadono Y, Narimoto K, et al. Understanding prostate-specific antigen dynamics in monitoring metastatic castration-resistant prostate cancer: implications for clinical practice. Asian J Androl (2017) 19:143–8. doi: 10.4103/1008-682X.179159
32. Uchimoto T, Komura K, Fukuokaya W, Kimura T, Takahashi K, Nishimura K, et al. Early prostate-specific antigen (PSA) change at four weeks of the first-line treatment using abiraterone and enzalutamide could predict Early/Primary resistance in metastatic castration-resistant prostate cancer. Cancers (Basel) (2021) 13:526. doi: 10.3390/cancers13030526
33. Fuerea A, Baciarello G, Patrikidou A, Albigès L, Massard C, Di Palma M, et al. Early PSA response is an independent prognostic factor in patients with metastatic castration-resistant prostate cancer treated with next-generation androgen pathway inhibitors. Eur J Cancer (2016) 61:44–51. doi: 10.1016/j.ejca.2016.03.070
34. España S, Ochoa de Olza M, Sala N, Piulats JM, Ferrandiz U, Etxaniz O, et al. PSA kinetics as prognostic markers of overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate. Cancer Manag Res (2020) 12:10251–60. doi: 10.2147/CMAR.S270392
35. Kim M, Lee J, Jeong CW, Ku JH, Kim HH, Kwak C. Prostate-specific antigen kinetic profiles during androgen deprivation therapy as prognostic factors in castration-resistant prostate cancer. Urol Oncol (2015) 33(5):203. doi: 10.1016/j.urolonc.2015.01.017
36. Miyake H, Hara T, Tamura K, Sugiyama T, Furuse H, Ozono S, et al. Independent association between time to prostate-specific antigen (PSA) nadir and PSA progression-free survival in patients with docetaxel-naïve, metastatic castration-resistant prostate cancer receiving abiraterone acetate, but not enzalutamide. Urol Oncol (2017) 35:432–7. doi: 10.1016/j.urolonc.2017.01.006
Keywords: prostatic cancer, PSA changes, androgen deprivation treatment, biochemical progress-free survival, time to nadir PSA
Citation: Hu M, Mao Y, Guan C, Tang Z, Bao Z, Li Y and Liang G (2023) Dynamic changes in PSA levels predict prognostic outcomes in prostate cancer patients undergoing androgen -deprivation therapy: A multicenter retrospective analysis. Front. Oncol. 13:1047388. doi: 10.3389/fonc.2023.1047388
Received: 30 September 2022; Accepted: 30 January 2023;
Published: 09 February 2023.
Edited by:
Daniel Keizman, Tel Aviv Sourasky Medical Center, IsraelReviewed by:
Maik Werner Kschischo, Koblenz University of Applied Sciences, GermanyCopyright © 2023 Hu, Mao, Guan, Tang, Bao, Li and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingqiu Hu, aHVtaW5ncWl1QG1lLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.