
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 07 February 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1044327
This article is part of the Research TopicResearch and Development of Small Molecule Anti-cancer Drugs Targeting Immune CheckpointsView all 8 articles
 Beibei Yang1†
Beibei Yang1† Bing Wang2,3†
Bing Wang2,3† Yongbang Chen2,4†
Yongbang Chen2,4† Ning Wan2,3,5*
Ning Wan2,3,5* Fei Xie2,5
Fei Xie2,5 Ning Yang6
Ning Yang6 Liqing Lu2,5
Liqing Lu2,5 Weibin Xiao2,5
Weibin Xiao2,5 Jin Yuan2,3,5
Jin Yuan2,3,5 Jian Li2,5
Jian Li2,5 Bo Xie6*
Bo Xie6* Bo Ji2,5*
Bo Ji2,5*Background: Several randomized controlled trials (RCTs) have confirmed the favorable clinical benefit of pembrolizumab in advanced non-small cell lung cancer (NSCLC). However, considering the strict inclusion and exclusion criteria in clinical research, there are certain differences between patients in the real-world, it is unclear whether the findings of clinical trials are fully representative of the treatment efficacy in patients who will eventually use it. Therefore, to further comprehensively assess the efficacy and safety of pembrolizumab in NSCLC, we conducted a systematic review and meta-analysis based on the latest RCTs and real-world studies (RWSs).
Methods: We systematically searched PubMed, Embase, The Cochrane Library, The Web of Science, and clinical trials.gov as of December 2021. RCTs and RWSs of patients receiving pembrolizumab monotherapy or in combination with chemotherapy for advanced NSCLC were included.
Results: The meta-analysis ultimately included 11 RCTs and 26 RWSs with a total of 10,695 patients. The primary outcomes of this study were overall survival (OS), progression-free survival (PFS), objective response rate (ORR), serious adverse events (SAEs), the incidence of severe pneumonia reactions, and drug-related mortality. Direct meta-analysis results showed that in RCTs, pembrolizumab in combination with chemotherapy was superior to chemotherapy in terms of OS (HR=0.60, 95%CI:0.50-0.73), PFS (HR=0.47, 95%CI:0.38-0.58) and ORR (OR=3.22, 95%CI:2.57-4.03); pembrolizumab monotherapy was superior to chemotherapy in terms of OS (HR=0.73, 95%CI:0.66-0.80) and ORR (OR=1.90, 95%CI:1.17-3.09), but comparable to chemotherapy in terms of PFS (HR=0.83, 95%CI:0.66-1.04). The ORR values in retrospective single-arm studies were 45% (40%-51%).
Conclusion: In RCTs, pembrolizumab monotherapy or in combination with chemotherapy is more effective and safer than chemotherapy for advanced NSCLC. In RWSs, ECOG PS 0-1 was shown to correlate with PFS and OS for patients with NSCLC.
According to the Global Cancer Statistics 2020, lung cancer remains the leading cause of cancer deaths with an incidence and mortality rate of 11.4% and 18.0%, respectively, with 2,206,771 and 1,796,144 new diagnoses and deaths, respectively (1). About 1,918,030 new cancer cases are expected to occur in the United States in 2022, with lung cancer having the third highest incidence rate in the United States, but still the highest mortality rate (2). The latest statistical report on cancer in China shows that lung cancer has the highest incidence and mortality rate (3). In addition, the 5-year survival rate for lung cancer ranges from approximately 10%~20% in most countries (4). Lung cancer includes small cell lung cancer and non-small cell lung cancer (NSCLC), of which NSCLC accounts for about 85% of all lung cancers, and most patients have locally advanced or metastatic disease at the time of diagnosis (5, 6). The traditional treatment of NSCLC is usually based on surgical resection, radiotherapy, and targeted therapy, but its therapeutic effect is poor (7). In recent years, immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 pathway have made breakthroughs in the treatment of NSCLC, which are more effective and safer compared with conventional treatments (8–10).
Pembrolizumab is a highly selective humanized anti-PD-1 IgG4 kappa isotype monoclonal antibody that disrupts the interaction of PD-1 with its ligand, thus showing better antitumor activity (11). In 2016, pembrolizumab was approved by the US Food and Drug Administration (FDA) for the treatment of patients with metastatic NSCLC (12). There have also been numerous clinical studies showing that pembrolizumab can provide a large clinical benefit for patients with advanced NSCLC. For example, in the KEYNOTE-024 (13) and KEYNOTE-042 (14) studies, the results of which showed improved overall survival (OS) with pembrolizumab alone in first-line untreated patients with advanced NSCLC. In addition, the results of the KEYNOTE-189 study (15) showed that pembrolizumab in combination with chemotherapy in first-line treatment of patients with metastatic NSCLC significantly prolonged OS and progression-free survival (PFS) compared with chemotherapy. In addition to the randomized controlled trials (RCTs) mentioned above, relevant studies from real-world data have also been published, and these studies likewise confirm that the actual treatment effects of pembrolizumab in a real-world setting are consistent with the comparative efficacy in RCTs (16–18).
Although a meta-analysis of pembrolizumab for advanced NSCLC has been reported currently, the sample sizes included in these studies (19–21) are small and the data from relevant RCTs have been updated over time; therefore, in this paper, the latest reported RCTs will be included, relevant data will be combined and analyzed by extraction. The results of published studies based on real-world data will be summarized on time, aiming to synthesize the available evidence on the efficacy and safety of pembrolizumab monotherapy or in combination with chemotherapy for patients with advanced NSCLC.
This meta-analysis was conducted according to the Preferred Reporting Initiative for Systematic Reviews and Meta-analyses (PRISMA) guidelines. We searched the PubMed, Cochrane Library, Web of Science, and Embase databases through December 2021 for RCTs and retrospective studies involving pembrolizumab monotherapy or in combination with chemotherapy for patients with advanced NSCLC. The keywords were “Non-small Cell Lung Cancer”, “Lung neoplasm” and “Non-small Cell Lung”. We also reviewed abstracts from the American Society of Clinical Oncology (ASCO), the World Conference on Lung Cancer (WCLC), and the European Society of Medical Oncology (ESMO). We also searched the ClinicalTrials.gov website (https://clinicaltrials.gov) to find ongoing studies and unpublished data.
Inclusion criteria: 1) Population: patients with advanced NSCLC diagnosed by histology; 2) Intervention: pembrolizumab monotherapy or in combination with chemotherapy, regardless of dose and duration; 3) Control: chemotherapy; 4) Outcome: overall survival (OS) and progression-free survival (PFS) (measured as hazard ratio(HR)), objective response rate (ORR), serious adverse events (SAEs); 5) Studies published in English. If studies were followed multiple times over time, we only report the most recent relevant data. Studies that did not meet the inclusion criteria were excluded.
Data were extracted and evaluated independently by two different authors, and differences were further discussed with the third author to reach a consensus. Each clinical trial recorded the first author, year of publication, number of patients, ORR, PFS, OS, and safety outcomes, including the incidence of serious adverse events (SAEs), pneumonia (≥Grade 3), and drug-related death rate.
The risk of bias in uncontrolled studies was assessed using the non-randomized methodological item MINORS (22). RCTs were assessed using the Cochrane Risk of Bias tool (23). The quality of this study was independently assessed by two reviewers and validated by a third reviewer.
Stata version 15.0 was obtained separately for ORR, 6-month progression-free survival (6m PFSr), 6-month overall survival (6m OSr), 1-year progression-free survival (1y PFSr), 1-year overall survival (1y OSr), 36-month overall survival (36m OSr), serious advanced events (SAEs), pneumonia (≥Grade 3), and drug-related mortality of the pooled data. In addition, ORR was further stratified according to tumor PD-L1 expression status, histology type and so on. Heterogeneity of the extracted data was assessed by I2 statistic and chi-square Q test, where I2≥50% (I2 statistic) or P ≤ 0.05 (Q test) was considered as significant heterogeneity. In the case of high potential heterogeneity, a random-effects model was used to avoid underestimating the standard error of the combined data.
A total of 12,035 articles were generated by the search strategy, of which 1,118 articles were retrieved in PubMed, 7,155 articles in EMBASE, 640 articles in the Cochrane Library, 3,118 articles in the Web of Science database, 4 articles from other sources. Finally, 11 RCTs and 26 retrospective studies were selected for combined analysis based on inclusion and exclusion criteria, respectively (The flow chart of literature screening is shown in Figure 1).
Of the 11 RCTs (17 articles) included, 6 studies (7 articles) reported the efficacy and adverse effects of pembrolizumab in combination with chemotherapy for the treatment of advanced NSCLC, enrolling a total of 1,541 patients. 5 studies (10 articles) reported pembrolizumab monotherapy for the treatment of advanced NSCLC, enrolling a total of 3,299 patients. In addition, of the 26 retrospective studies included, only 3 studies were pembrolizumab in combination with chemotherapy versus chemotherapy, enrolling a total of 222 patients. The other 23 studies were pembrolizumab single-arm studies. The detailed characteristics of the included studies are shown in Tables 1, 2.
The risk of bias for the 11 RCTs and 26 retrospective studies included in this study are summarized in Supplementary Tables S1, S2.
A combined analysis of relevant data extracted from RCTs showed that the ORR for treatment with pembrolizumab monotherapy or in combination with chemotherapy was significantly better than chemotherapy (OR=2.52, 95%CI:1.75-3.61) (Figure 2A). Pembrolizumab in combination with chemotheray was obviously better than chemotherapy in terms of ORR (OR=3.22, 95%CI:2.57-4.03). And for pembrolizumab monotherapy versus chemotherapy, the pooled ORR was OR=1.90, 95%CI:1.17-3.09 (Figure 2A). In addition, of the included studies of pembrolizumab monotherapy, only Martin Reck (31) had a population with PD-L1≥50%, while the remaining four studies (33, 36–38) had a population with PD-L1≥1%.
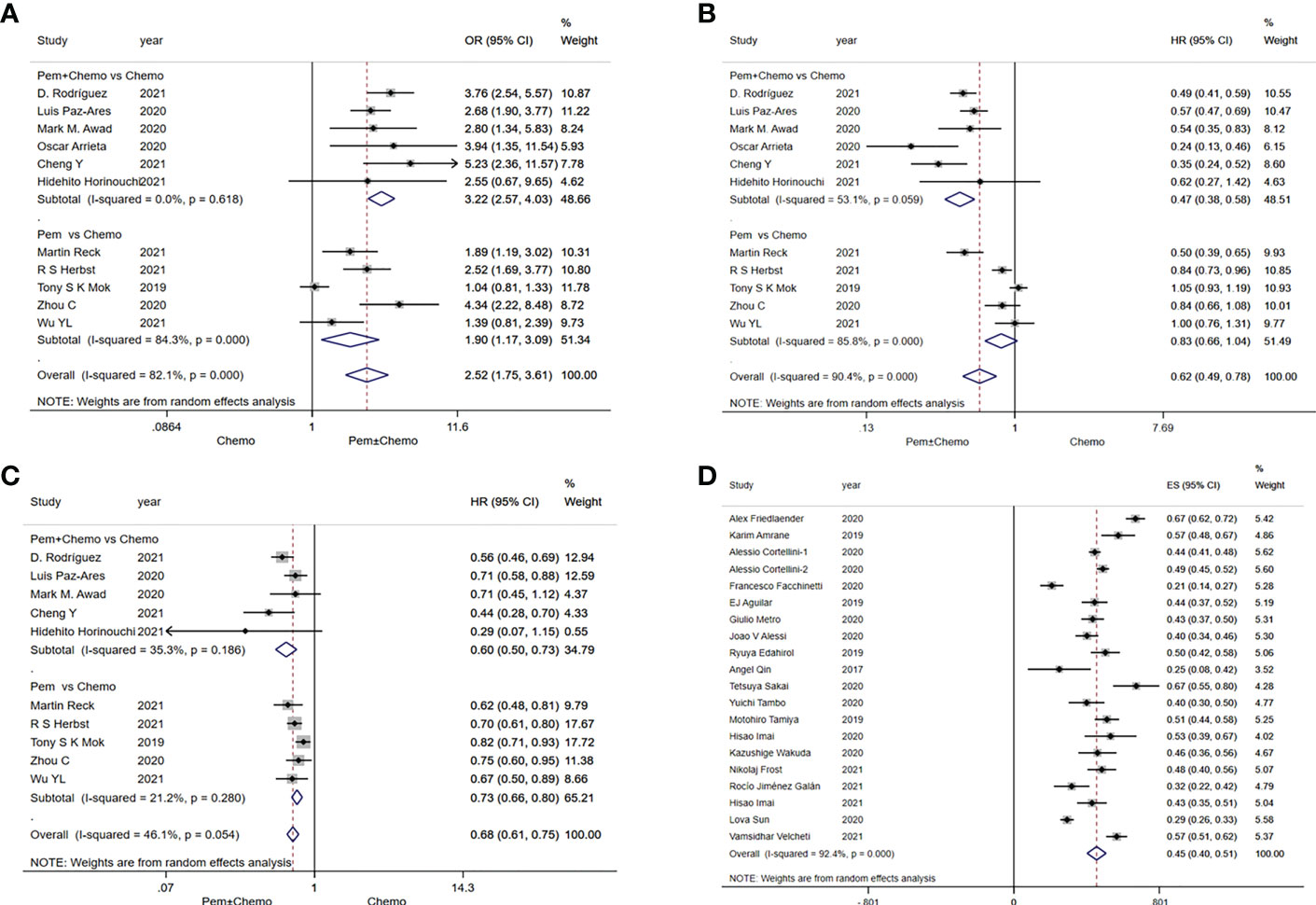
Figure 2 Forest plots of survival outcomes in randomized clinical trials (RCTs) and real-world studies (RWSs). (A) objective response rate in RCTs, (B) progression-free survival in RCTs, (C) overall survival in RCTs, (D) objective response rate in RWSs.
In RWSs, data were extracted from 20 included single-arm studies with a combined analysis ORR value of 45% (40%-51%) (Figure 2D). Analysis based on PD-L1 expression status showed a combined ORR value of 47% (42%-52%) in patients with PD-L1≥50%, 43% (36%-51%) in patients with PD-L1 50%-89%, and 53% (45%-62%) in patients with PD-L1≥90%. In addition, we also differentiated between histological types, with an ORR value of 46% (42%-51%) in non-squamous patients and 48% (43%-53%) in squamous patients (Supplementary Figure S1).
In RCTs, pembrolizumab monotherapy or in combination with chemotherapy had a significant advantage over chemotherapy in terms of PFS (HR=0.62, 95%CI:0.49-0.78) (Figure 2B). Significant difference of PFS was observed in favor of pembrolizumab in combination with chemotherapy versus chemotherapy (HR=0.47, 95%CI:0.38-0.58). But pembrolizumab monotherapy was comparable to chemotherapy (HR=0.83, 95%CI:0.66-1.04) (Figure 2B). In terms of OS, pembrolizumab monotherapy or in combination with chemotherapy also has advantages over chemotherapy. Both pembrolizumab in combination with chemotherapy (HR=0.60, 95%CI:0.50-0.73) and pembrolizumab monotherapy (HR=0.73, 95%CI:0.66-0.80) significantly prolonged overall survival of patients (Figure 2C).
In RWSs, in univariate analysis, PS 0-1 was significantly correlated with prolongation of PFS and OS. Not using steroid (or baseline steroid<10mg) was significantly related to a prolonged PFS in univariate analysis. In multivariate analysis, PS 0-1 was identified as an independent predictor of OS prolongation. In addition, baseline steroid use was identified as an independent predictor of OS shortening in multivariate analysis (Table 3).
In RCTs, combined analysis results of 12m PFSr and 24m PFSr were 3.02 (95%CI:2.31-3.95) and 4.43 (95%CI:2.50-7.87), respectively (Figure 3A). Relevant data were extracted from the RWSs, and the values for the combined analyses 6m PFSr and 12m PFSr were 35% (24%-47%) and 27% (21%-33%), respectively (Figure 3C).
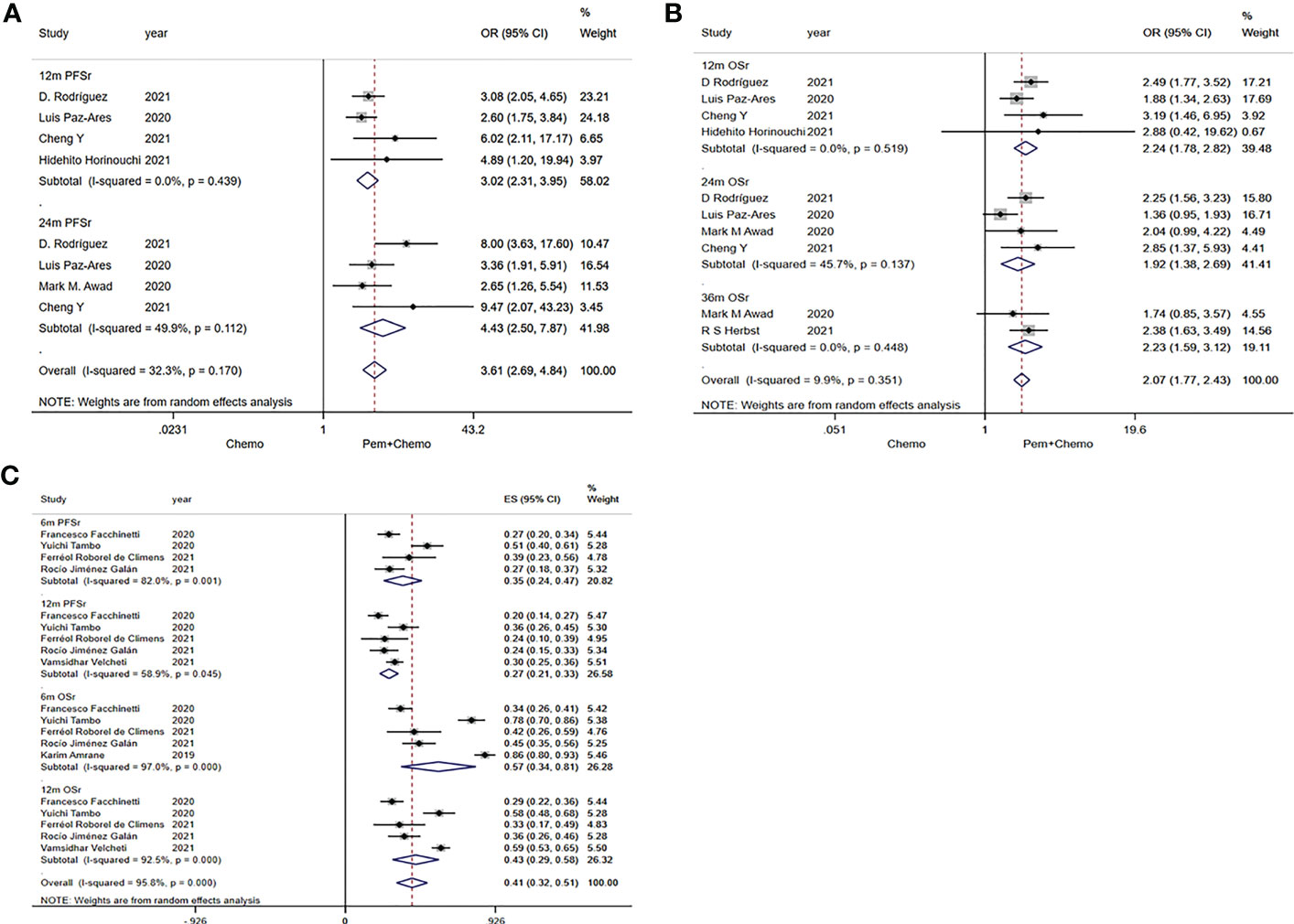
Figure 3 Forest plots of PFSr and OSr in randomized clinical trials (RCTs) and real-world studies (RWSs). (A) 12m and 24m PFSr in RCTs, (B) 12m, 24m, 36m OSr in RCTs, (C) 6m and 12m PFSr, 6m and 12m OSr in RWSs.
The pooled values of 12m OSr, 24m OSr and 36m OSr were 2.24 (95%CI:1.78-2.82), 1.92 (95%CI:1.38-2.69), 2.23 (95%CI:1.59-3.12), respectively (Figure 3B). Relevant data were extracted from the four RWSs, and the combined values of 6m OSr and 12m OSr were 57% (34%-81%) and 43% (29%-58%), respectively (Figure 3C).
Safety including the rate of Grade 3-5 Treatment Related Adversed Events (TRAEs), Grade 3-5 Immune Related Adversed Events (IRAEs), pneumonitis (≥Grade 3) and drug-related deaths were displayed in Table 4. For Grade 3-5 TRAEs and Grade 3-5 IRAEs, the incidence of pembrolizumab monotherapy and in combination with chemotherapy were 20% (15%-26%) and 64% (49%-78%) in RCTs, In the 26 retrospective studies, 2 retrospective controlled studies reported Grade 3-5 TRAEs. 3 retrospective single-arm studies mentioned grade 3-5 IRAEs. 7 retrospective studies mentioned the incidence of pneumonia (≥Grade 3), and 3 retrospective studies reported drug-related mortality. 2 retrospective controlled studies reported a Grade 3-5 TRAE rate of 51% (40%-61%) in patients with NSCLC treated with pembrolizumab in combination with chemotherapy. 3 retrospective single-arm studies reported a grade 3-5 IRAE rate of 17% (14%-21%) in patients receiving pembrolizumab monotherapy for NSCLC.
In addition, for pneumonitis (≥Grade 3), a combined analysis of 9 RCTs showed an incidence of 4% (2%-6%) in patients treated with pembrolizumab in combination with chemotherapy and 3% (2%-4%) in patients treated with pembrolizumab monotherapy, respectively. In retrospective studies, the incidence were 2% (-2%-6%) in patients treated with pembrolizumab in combination with chemotherapy. And the incidence was 3% (1%-5%) in retrospective single-arm studies.
As for drug-related death, 9 RCTs mentioned the incidence of drug-related death in patients treated with pembrolizumab, and the combined analysis incidences were 2% (1%-4%) and 2% (0%-3%) in patients treated with pembrolizumab in combination with chemotherapy and pembrolizumab monotherapy, respectively. In addition, combined data from 2 retrospective controlled studies showed that drug-related mortality occurred in patients with NSCLC treated with pembrolizumab in combination with chemotherapy at 2% (-2%-6%), while in 1 retrospective single-arm study the results showed that drug-related mortality occurred in pembrolizumab monotherapy treatment at 4% (-2%-10%). Table 5 shows the safety results after combining the data from the RCTs and the retrospective studies, respectively.
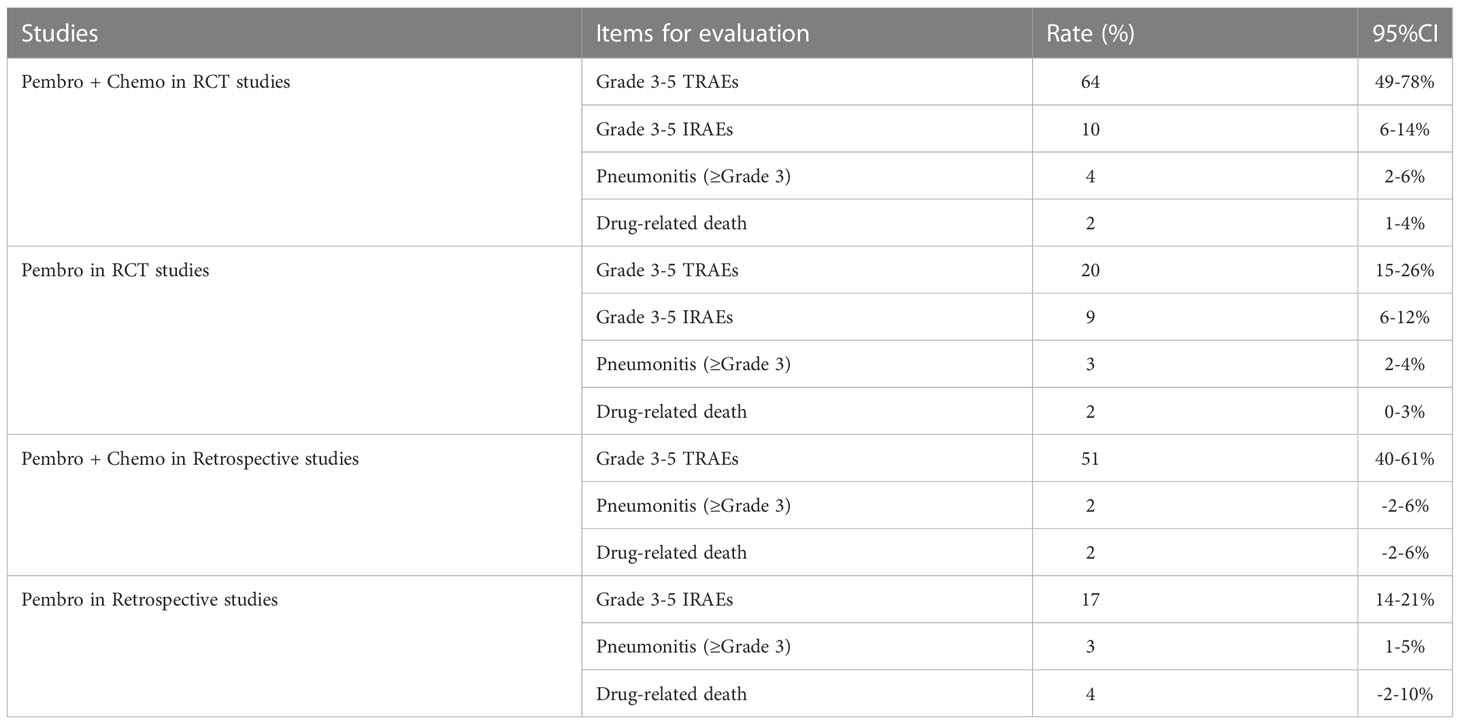
Table 5 Meta-analysis of the rate of SAEs, pneumonitis, and drug-related death in combination therapy.
Results of subgroup analyses are shown in Supplementary Figures S2–S4. In the subgroup with PD-L1<1%, pembrolizumab in combination with chemotherapy showed significant ORR, PFS, and OS advantages over chemotherapy. In the subgroup with PD-L1≥1%, pembrolizumab monotherapy or in combination with chemotherapy showed the best ORR, PFS and OS advantage over chemotherapy. In the subgroup with PD-L1 = 1-49%, pembrolizumab in combination with chemotherapy showed the best ORR, PFS and OS advantage. pembrolizumab monotherapy was superior in OS, however, pembrolizumab monotherapy was comparable to chemotherapy in PFS. In patients with PD-L1 ≥50%, pembrolizumab monotherapy or in combination with chemotherapy had a significant ORR, PFS and OS advantage over chemotherapy.
Pembrolizumab in combination with chemotherapy had a significant ORR, PFS and OS advantage over chemotherapy in both squamous and non-squamous NSCLC. Among patients receiving first-line therapy, pembrolizumab monotherapy or in combination with chemotherapy had a greater ORR, PFS and OS advantage over chemotherapy. In patients treated with second-line or more line therapy, ORR and PFS of pembrolizumab monotherapy or in combiantion with chemotherapy was higher than that of chemotherapy. In addition, among patients receiving second-line or more line regimens, pembrolizumab monotherapy was significantly better than chemotherapy in terms of OS (HR=0.71, 95%CI: 0.63-0.80).
A sensitivity analysis was performed in this study to examine the effect of uncertainty on the final results. The results of the sensitivity analysis showed the included studies did not significantly affect the outcome of pembrolizumab monotherapy or in combination with chemotherapy (Supplementary Figure S5).
Funnel plot analysis of studies of pembrolizumab monotherapy or in combination with chemotherapy treatment showed a significant asymmetric distribution (Supplementary Figure S6A); the Egger linear regression test verified that there was potential publication bias (Supplementary Figure S6B). The results of the funnel plot and the Egger linear regression test show that there was potential publication bias in our current study. The possible reason for this bias is the inclusion of two types of studies in this study, both RCTs and retrospective studies.
The literature included in the published meta-analysis studies on pembrolizumab for advanced NSCLC is dominated by RCTs, Zhou et al. (20) included 5 RCTs involving 1,289 patients, direct meta-analysis showed that pembrolizumab monotherapy or in combination with chemotherapy improved clinical outcomes compared with chemotherapy in patients with NSCLC. However, this study only focused on patients with first-line therapy and PD-L1≥50%. In addition, Kim et al. (21) included 4 RCTs involving 2,754 patients, their findings showed that pembrolizumab monotherapy or in combination with chemotherapy significantly improved PFS and OS in patients with advanced or metastatic NSCLC treated in the first-line. This study was published in 2019 and included a relatively small number of RCTs and patients. In addition, a small number of meta-analyses were included in RWSs. Mencoboni M et al. (62) included 32 RWSs of programmed death-1/programmed death ligand-1 (PD-1/PD-L1) for advanced NSCLC, confirming the efficacy and safety results of PD-1/PD-L1 in real-world clinical practice were similar to those in clinical trials. However, there have been no meta-analysis or systematic reviews of the efficacy and safety of pembrolizumab for the treatment of advanced NSCLC in the real world. In order to better understand the clinical efficacy of pembrolizumab in patients with NSCLC. The main innovation of our study was to analyze the efficacy of pembrolizumab treated with NSCLC in both clinical trials and real-world clinical settings. We also performed subgroup analyses for different histological types, PD-L1 expression status and different lines of treatment.
In this meta-analysis, we included 11 RCTs (n=4,840 patients) and 26 retrospective studies (n=5,819 patients). In RCTs, Pembrolizumab in combination with chemotherapy was superior to chemotherapy in ORR, PFS, and OS, but pembrolizumab monotherapy was superior to chemotherapy in ORR and OS, and no significant difference was achieved in PFS. This is consistent with the findings of Zhou et al. (20). In RCTs, we performed a combined analysis of OS, PFS, and ORR according to PD-L1 expression. In patients with PD-L1<1%, pembrolizumab in combination with chemotherapy is superior to chemotherapy in terms of ORR, PFS and OS. Pembrolizumab monotherapy or in combination with chemotherapy had a significant advantage over chemotherapy in terms of OS, PFS, and ORR in patients with PD-L1≥1%, and PD-L1≥50%. In addition, pembrolizumab in combination with chemotherapy showed the best PFS and OS benefit compared with chemotherapy in patients with PD-L1 = 1%~49%, however pembrolizumab monotherapy was comparable to chemotherapy in terms of PFS. This is similar to the results of the subgroup analysis of Passiglia et al. (63), where PD-1 in combination with chemotherapy was associated with a significant increase in ORR and PFS in patients with high PD-L1 expression, and in patients with low PD-L1 expression, PD-1 in combination with chemotherapy significantly improved OS in this group, and in the PD-L1 negative group PD-1 in combination with chemotherapy favored an increase in ORR. The difference is that our study compared pembrolizumab monotherapy or in combination with chemotherapy compared to the chemotherapy group, and Passiglia et al. (63) included PD-L1 combination chemotherapy compared to PD-L1 combined with chemotherapy compared with PD-1 alone or PD-1/PD-L1 combined with CTLA-4. In future studies, we need head-to-head studies to analyze which immunotherapy-based strategy with different PD-L1 expression is the best choice. In this study, in terms of grade 3-5 TRAEs and IRAEs, the incidence of AE was lower with pembrolizumab monotherapy compared to pembrolizumab in combination with chemotherapy in RCTs (20% vs. 64%, 9% vs. 10%). Combining the above subgroup results and AE incidence, we can recommend that in clinical practice for patients with PD-L1 ≥ 50%, pembrolizumab monotherapy seems to be an effective treatment strategy, and for PD-L1 < 50%, pembrolizumab monotherapy should be selected with caution, unless the patient reconsiders if the AE is intolerable.
In addition, the pooled ORR rate for pembrolizumab monotherapy in the 23 retrospective single-arm studies we included was 45%, which was similar to the results of the previous KEYNOTE-024 (32). It is noteworthy that the pooled ORR for patients treated with pembrolizumab (PD-L1 ≥50%) in the clinical trial of KEYNOTE-042 (14) was 39%, compared with a pooled ORR of 47% for patients with PD-L1 ≥ 50% in the retrospective study. thus showing that the ORR for patients with PD-L1 ≥50% in the retrospective study was slightly higher than in clinical trials. One possible explanation for our data is the inclusion of more patients with an ECOG PS 0 or 1 in real-world studies. We compared the safety of both RCTs and real-world studies, which showed a higher incidence of grade 3-5 TRAEs in RCTs with pembrolizumab in combination with chemotherapy than in retrospective studies (64% vs. 51%), but a significantly lower rate of grade 3-5 IRAEs with pembrolizumab monotherapy than in retrospective studies (9% vs. 17%). In addition, indirect analysis showed that the rates of pneumonia (grade ≥3) and drug-related death in the retrospective studies were comparable to those in the RCTs. A possible reason for the difference in safety results between the RCTs and the RWSs is that the number and sample size of the combined RCTs were larger than RWSs. This analysis suggests that the results of both RCTs and RWSs suggest that patients with high PD-L1 expression do benefit from first-line monotherapy with IO, seemingly confirming the results of the KEYNOTE-598 and EMPOWER-Lung 1 trials (64, 65). However, the variability in ORR and safety at the same time suggests that we cannot simply use the findings of clinical trials to assess the effectiveness and safety in real-world patients, and it is particularly important to analyze the included populations and subgroup analyses in RCTs and RWSs separately to more accurately estimate potential efficacy.
Previously, studies have confirmed that ICI monotherapy or in combination with chemotherapy are alternative options for first or second-line treatment in patients with advanced NSCLC (34, 66–68). In this study, we included 3 RCTs for patients treated in second-line or more lines. Our results showed that pembrolizumab monotherapy or in combination with chemotherapy had an advantage over chemotherapy in terms of PFS and ORR. In addition, We found that pembrolizumab monotherapy in second-line or more line therapy prolonged OS compared to chemotherapy. It is worth noting that of the 3 included RCTs of second-line or more line therapy, 1 was a phase II study of pembrolizumab in combination with chemotherapy that included patients with PD-L1≥50% and PD-L1<50%, and the clinical benefit of this study was primarily from the overall population (regardless of PD-L1 expression). Of the other 2 studies, 1 was a 5-year follow-up study of pembrolizumab monotherapy and 1 was a phase III study of pembrolizumab that included patients with PD-L1≥1% and PD-L1≥50%, with clinical benefit data from the second-line or postline study primarily for patients with PD-L1≥1%. This results also further explained the efficacy of the pembrolizumab regimen in different lines of treatment. However, in the real world, the actual clinical effect of pembrolizumab monotherapy in patients with low PD-L1 expression or posterior line therapy has not been explored in too many studies, mainly because these patients have a heavy tumor load themselves, and for these patients, the main purpose of treatment is to rapidly reduce the tumor volume, prevent excessive disease progression, and increase progression-free survival. However, in patients with low PD-L1 expression or second-line patients, immune checkpoint inhibitor monotherapy may cause rapid disease progression, so in clinical practice, this treatment option is less in patients with low PD-L1 expression.
Numerous clinical trial studies have demonstrated the effectiveness of pembrolizumab in the first-line treatment of patients with PD-L1 expression-positive NSCLC. but its clinicopathological correlates have not been concluded in RCTs. In this study, we explored the variables of interest in terms of PFS and OS. In univariate analysis,we found that PS 0-1 was confirmed to be associated with prolonged PFS and OS, whereas gender, age (<70 or ≥70 and <65 or ≥65), smoking status, histological type, immune-related adverse effects and radiotherapy were not associated with PFS and OS. In multivariate analysis, PS 0-1 and not using steroid (or baseline steroid<10mg) were confirmed as independent relevant predictors of OS prolongation, whereas gender, brain metastasis status, age (<65 or ≥65), histological type and were not confirmed as independent predictors of PFS and OS. Although the above results explain the clinically relevant factors related to OS and PFS. However, further confirmation regarding the validity of the clinical subgroups is needed in the future with more clinical trial studies.
The strength of this meta-analysis was that we included the most recent relevant data from more comprehensive RCTs and retrospective studies, directly comparing the clinical efficacy and safety of pembrolizumab monotherapy or in combination with chemotherapy versus conventional chemotherapy. Further provides evidence-based medical evidence for the superior clinical benefit of pembrolizumab in patients with NSCLC. Previously published RCTs, although reporting better survival and response rates for pembrolizumab in advanced NSCLC, may have resulted in the exclusion of some patients from the oncology group due to the strict inclusion and exclusion criteria for screening patients in RCTs. However, retrospective studies from real-world data are beneficial in providing further evidence of clinical benefit for a broader range of oncology patients, including those excluded from RCTs. Also, by comparing the combined clinical efficacy and safety results of the respective RCTs and retrospective studies, it provides a basis for more accurate estimation of potential patient outcomes in clinical practice.
There are some limitations in the present study. First, due to the lack of data related to tumor mutation burden analysis in the included RCTs and retrospective studies, the current study did not distinguish the relationship of tumor mutation burden and the efficacy of pembrolizumab monotherapy or in combination with chemotherapy treatment regimens. Second, Most of the RWSs included in this study were single-arm studies of pembrolizumab monotherapy, and there was a lack of controlled studies of pembrolizumab monotherapy or combination chemotherapy versus chemotherapy in the real world. RWSs may have heterogeneity in design or patient selection, lack of standardization in treatment regimens, or some bias in the different data collection processes, among other factors, which may have affected the results. And most of RWSs were conducted on patients with high PD-L1 expression, but not on patients with low PD-L1 expression, so more studies are needed to further validate the benefits of pembrolizumab in this population. Third, the study has not analyzed the efficacy and safety of immune monotherapy, immune combination chemotherapy, and dual immune therapy under different PD-L1 expression scenarios to determine which immune-based strategy is the best choice.
In conclusion, this study further explains the superior clinical efficacy and acceptable toxicity of pembrolizumab monotherapy or in combination with chemotherapy regimens in patients with NSCLC, further providing evidence to support the use of pembrolizumab in clinical practice. In addition, in univariate analyses of RWSs, PS 0-1 appeared to be associated with PFS and OS.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
BY, BW and NW proposed the idea and designed the study. BW and YC performed the literature search and data extraction. FX, NY, LL, WX, and JY compiled and analyzed the data, and developed the figures and tables. BY and BW wrote the manuscript. JL, BX, BJ, and NW guided the study and the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Health and Wellness Commission Project of Shaanxi Province, China (Grant No. 2022D048), Science and Technology Program of Guangzhou, China (Grant No. 201509010012), the Natural Science Foundation of Guangdong Province, China (Grant No. 2021A1515012251) and Science and Technology Program of Guangzhou, China (Grant No. 202002030446).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1044327/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. JNCC (2022) 2(1):1–9. doi: 10.1016/j.jncc.2022.02.002
4. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (2018) 391(10125):1023–75. doi: 10.1016/S0140-6736(17)33326-3
5. Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-Small-Cell lung cancer. N Engl J Med (2017) 377(9):849–61. doi: 10.1056/NEJMra1703413
6. Crinò L, Weder W, van Meerbeeck J, Felip E. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2010) 21 Suppl 5:v103–15. doi: 10.1093/annonc/mdq207
7. Tang S, Qin C, Hu H, Liu T, He Y, Guo H, et al. Immune checkpoint inhibitors in non-small cell lung cancer: Progress, challenges, and prospects. Cells-Basel (2022) 11(3):320. doi: 10.3390/cells11030320
8. Ackermann CJ, Reck M, Paz-Ares L, Barlesi F, Califano R. First-line immune checkpoint blockade for advanced non-small-cell lung cancer: Travelling at the speed of light. Lung Cancer (2019) 134:245–53. doi: 10.1016/j.lungcan.2019.06.007
9. Ferrara R, Imbimbo M, Malouf R, Paget-Bailly S, Calais F, Marchal C, et al. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database Syst Rev (2021) 4(4):D13257. doi: 10.1002/14651858.CD013257.pub3
10. Ellis PM, Vella ET, Ung YC. Immune checkpoint inhibitors for patients with advanced non-Small-Cell lung cancer: A systematic review. Clin Lung Cancer (2017) 18(5):444–59. doi: 10.1016/j.cllc.2017.02.001
11. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med (2015) 372(21):2018–28. doi: 10.1056/NEJMoa1501824
12. Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra-Kalyani PS, He K, et al. FDA Approval summary: Pembrolizumab for treatment of metastatic non-small cell lung cancer: First-line therapy and beyond. Oncologist (2017) 22(11):1392–9. doi: 10.1634/theoncologist.2017-0078
13. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
14. Mok T, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
15. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
16. Cortellini A, Tiseo M, Banna GL, Cappuzzo F, Aerts J, Barbieri F, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother (2020) 69(11):2209–21. doi: 10.1007/s00262-020-02613-9
17. Velcheti V, Hu X, Piperdi B, Burke T. Real-world outcomes of first-line pembrolizumab plus pemetrexed-carboplatin for metastatic nonsquamous NSCLC at US oncology practices. Sci Rep (2021) 11(1):9222. doi: 10.1038/s41598-021-88453-8
18. Frost N, Kollmeier J, Misch D, Vollbrecht C, Grah C, Matthes B, et al. Pembrolizumab as first-line palliative therapy in PD-L1 overexpressing (≥ 50%) NSCLC: Real-world results with special focus on PS ≥ 2, brain metastases, and steroids. Clin Lung Cancer (2021) 22(5):411–22. doi: 10.1016/j.cllc.2021.02.001
19. Frederickson AM, Arndorfer S, Zhang I, Lorenzi M, Insinga R, Arunachalam A, et al. Pembrolizumab plus chemotherapy for first-line treatment of metastatic nonsquamous non-small-cell lung cancer: a network meta-analysis. Immunotherapy-Uk (2019) 11(5):407–28. doi: 10.2217/imt-2018-0193
20. Zhou Y, Lin Z, Zhang X, Chen C, Zhao H, Hong S, et al. First-line treatment for patients with advanced non-small cell lung carcinoma and high PD-L1 expression: pembrolizumab or pembrolizumab plus chemotherapy. J Immunother Cancer (2019) 7(1):120. doi: 10.1186/s40425-019-0600-6
21. Kim R, Keam B, Hahn S, Ock CY, Kim M, Kim TM, et al. First-line pembrolizumab versus pembrolizumab plus chemotherapy versus chemotherapy alone in non-small-cell lung cancer: A systematic review and network meta-analysis. Clin Lung Cancer (2019) 20(5):331–8. doi: 10.1016/j.cllc.2019.05.009
22. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. Anz J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
23. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol (2021) 32(7):881–95. doi: 10.1016/j.annonc.2021.04.008
25. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
26. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto PH, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol (2020) 15(10):1657–69. doi: 10.1016/j.jtho.2020.06.015
27. Awad MM, Gadgeel SM, Borghaei H, Patnaik A, Yang JC, Powell SF, et al. Long-term overall survival from KEYNOTE-021 cohort G: Pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous NSCLC. J Thorac Oncol (2021) 16(1):162–8. doi: 10.1016/j.jtho.2020.09.015
28. Arrieta O, Barrón F, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, Díaz-García D, et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: The PROLUNG phase 2 randomized clinical trial. JAMA Oncol (2020) 6(6):856–64. doi: 10.1001/jamaoncol.2020.0409
29. Cheng Y, Zhang L, Hu J, Wang D, Hu C, Zhou J, et al. Pembrolizumab plus chemotherapy for Chinese patients with metastatic squamous NSCLC in KEYNOTE-407. JTO Clin Res Rep (2021) 2(10):100225. doi: 10.1016/j.jtocrr.2021.100225
30. Horinouchi H, Nogami N, Saka H, Nishio M, Tokito T, Takahashi T, et al. Pembrolizumab plus pemetrexed-platinum for metastatic nonsquamous non-small-cell lung cancer: KEYNOTE-189 Japan study. Cancer Sci (2021) 112(8):3255–65. doi: 10.1111/cas.14980
31. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-Small-Cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol (2021) 39(21):2339–49. doi: 10.1200/JCO.21.00174
32. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-Small-Cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37(7):537–46. doi: 10.1200/JCO.18.00149
33. Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez-Gracia JL, et al. Five year survival update from KEYNOTE-010: Pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol (2021) 16(10):1718–32. doi: 10.1016/j.jtho.2021.05.001
34. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
35. Herbst RS, Baas P, Perez-Gracia JL, Felip E, Kim DW, Han JY, et al. Use of archival versus newly collected tumor samples for assessing PD-L1 expression and overall survival: an updated analysis of KEYNOTE-010 trial. Ann Oncol (2019) 30(2):281–9. doi: 10.1093/annonc/mdy545
36. Mok T, Wu Y, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Final analysis of the phase III KEYNOTE-042 study: pembrolizumab (Pembro) versus platinum-based chemotherapy (Chemo) as first-line therapy for patients (Pts) with PD-L1-positive locally advanced/metastatic NSCLC. Ann Onco (2019) 30(Supplement 2):ii38–68. doi: 10.1093/annonc/mdz063
37. Zhou C, Feng J, Ma S, Chen H, Ma Z, Huang C, et al. Randomized, open-label phase III study of pembrolizumab (pembro) vs docetaxel (doce) in patients (pts) with previously treated NSCLC with PD-L1 tumour proportion score (TPS) ≥1%: KEYNOTE-033. Ann Oncol (2020) 31:S816. doi: 10.1016/j.annonc.2020.08.1576
38. Wu YL, Zhang L, Fan Y, Zhou J, Zhang L, Zhou Q, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China study. Int J Cancer (2021) 148(9):2313–20. doi: 10.1002/ijc.33399
39. Afzal MZ, Dragnev K, Shirai K. A tertiary care cancer center experience with carboplatin and pemetrexed in combination with pembrolizumab in comparison with carboplatin and pemetrexed alone in non-squamous non-small cell lung cancer. J Thorac Dis (2018) 10(6):3575–84. doi: 10.21037/jtd.2018.06.08
40. Liao J, Liu C, Long Q, Wu X, Wang H, Yu H, et al. Direct comparison between the addition of pembrolizumab or bevacizumab for chemotherapy-based first-line treatment of advanced non-squamous non-small cell lung cancer lacking driver mutations. Front Oncol (2021) 11:752545. doi: 10.3389/fonc.2021.752545
41. Zhang J, Wu D, Zhang Z, Long J, Tian G, Wang Y, et al. Pembrolizumab or bevacizumab plus chemotherapy as first-line treatment of advanced nonsquamous non small cell lung cancer: A retrospective cohort study. Technol Cancer Res Treat (2021) 20:2091185732. doi: 10.1177/15330338211039676
42. Cortellini A, Friedlaender A, Banna GL, Porzio G, Bersanelli M, Cappuzzo F, et al. Immune-related adverse events of pembrolizumab in a Large real-world cohort of patients with NSCLC with a PD-L1 expression ≥ 50% and their relationship with clinical outcomes. Clin Lung Cancer (2020) 21(6):498–508. doi: 10.1016/j.cllc.2020.06.010
43. Friedlaender A, Metro G, Signorelli D, Gili A, Economopoulou P, Roila F, et al. Impact of performance status on non-small-cell lung cancer patients with a PD-L1 tumour proportion score ≥50% treated with front-line pembrolizumab. Acta Oncol (2020) 59(9):1058–63. doi: 10.1080/0284186X.2020.1781249
44. Qin A, Street L, Cease K, Viglianti BL, Warren EH, Zhao L, et al. Clinical determinants of durable clinical benefit of pembrolizumab in veterans with advanced non-Small-Cell lung cancer. Clin Lung Cancer (2017) 18(5):559–64. doi: 10.1016/j.cllc.2017.01.012
45. Ksienski D, Wai ES, Croteau N, Freeman AT, Chan A, Fiorino L, et al. Pembrolizumab for advanced nonsmall cell lung cancer: Efficacy and safety in everyday clinical practice. Lung Cancer (2019) 133:110–6. doi: 10.1016/j.lungcan.2019.05.005
46. Aguilar EJ, Ricciuti B, Gainor JF, Kehl KL, Kravets S, Dahlberg S, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol (2019) 30(10):1653–9. doi: 10.1093/annonc/mdz288
47. Facchinetti F, Mazzaschi G, Barbieri F, Passiglia F, Mazzoni F, Berardi R, et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer (2020) 130:155–67. doi: 10.1016/j.ejca.2020.02.023
48. Metro G, Banna GL, Signorelli D, Gili A, Galetta D, Galli G, et al. Efficacy of pembrolizumab monotherapy in patients with or without brain metastases from advanced non-small cell lung cancer with a PD-L1 expression ≥50%. J Immunother (2020) 43(9):299–306. doi: 10.1097/CJI.0000000000000340
49. Imai H, Wasamoto S, Yamaguchi O, Suzuki K, Sugiyama T, Uchino J, et al. Efficacy and safety of first-line pembrolizumab monotherapy in elderly patients (aged≥ 75 years) with non-small cell lung cancer. J Cancer Res Clin Oncol (2020) 146(2):457–66. doi: 10.1007/s00432-019-03072-1
50. Alessi JV, Ricciuti B, Jiménez-Aguilar E, Hong F, Wei Z, Nishino M, et al. Outcomes to first-line pembrolizumab in patients with PD-L1-high (≥50%) non-small cell lung cancer and a poor performance status. J Immunother Cancer (2020) 8(2):e001007. doi: 10.1136/jitc-2020-001007
51. Amrane K, Geier M, Corre R, Léna H, Léveiller G, Gadby F, et al. First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a multicenter real-life cohort: The PEMBREIZH study. Cancer Med (2020) 9(7):2309–16. doi: 10.1002/cam4.2806
52. Wakuda K, Yabe M, Kodama H, Nishioka N, Miyawaki T, Miyawaki E, et al. Efficacy of pembrolizumab in patients with brain metastasis caused by previously untreated non-small cell lung cancer with high tumor PD-L1 expression. Lung Cancer (2021) 151:60–8. doi: 10.1016/j.lungcan.2020.11.009
53. Tamiya M, Tamiya A, Hosoya K, Taniguchi Y, Yokoyama T, Fukuda Y, et al. Efficacy and safety of pembrolizumab as first-line therapy in advanced non-small cell lung cancer with at least 50% PD-L1 positivity: a multicenter retrospective cohort study (HOPE-001). Invest New Drugs (2019) 37(6):1266–73. doi: 10.1007/s10637-019-00843-y
54. Edahiro R, Kanazu M, Kurebe H, Mori M, Fujimoto D, Taniguchi Y, et al. Clinical outcomes in non-small cell lung cancer patients with an ultra-high expression of programmed death ligand-1 treated using pembrolizumab as a first-line therapy: A retrospective multicenter cohort study in Japan. PloS One (2019) 14(7):e220570. doi: 10.1371/journal.pone.0220570
55. Sakai T, Udagawa H, Matsumoto S, Yoh K, Nosaki K, Ikeda T, et al. Morphological, immune and genetic features in biopsy sample associated with the efficacy of pembrolizumab in patients with non-squamous non-small cell lung cancer. J Cancer Res Clin Oncol (2021) 147(4):1227–37. doi: 10.1007/s00432-020-03413-5
56. Tambo Y, Sone T, Shibata K, Nishi K, Shirasaki H, Yoneda T, et al. Real-world efficacy of first-line pembrolizumab in patients with advanced or recurrent non-Small-Cell lung cancer and high PD-L1 tumor expression. Clin Lung Cancer (2020) 21(5):e366–79. doi: 10.1016/j.cllc.2020.02.017
57. Roborel DCF, Chouaid C, Poulet C, Leroy V, Stoven L, Cortot AB, et al. Salvage immunotherapy with pembrolizumab in patients hospitalized for life-threatening complications of NSCLC. JTO Clin Res Rep (2021) 2(5):100147. doi: 10.1016/j.jtocrr.2021.100147
58. Jiménez GR, Prado-Mel E, Pérez-Moreno MA, Caballano-Infantes E, Flores MS. Influence of performance status on the effectiveness of pembrolizumab monotherapy in first-line for advanced non-Small-Cell lung cancer: Results in a real-world population. Biol (Basel) (2021) 10(9):890. doi: 10.3390/biology10090890
59. Hosoya K, Fujimoto D, Morimoto T, Kumagai T, Tamiya A, Taniguchi Y, et al. Clinical factors associated with shorter durable response, and patterns of acquired resistance to first-line pembrolizumab monotherapy in PD-L1-positive non-small-cell lung cancer patients: a retrospective multicenter study. BMC Cancer (2021) 21(1):346. doi: 10.1186/s12885-021-08048-4
60. Imai H, Kishikawa T, Minemura H, Yamada Y, Ibe T, Yamaguchi O, et al. Pretreatment Glasgow prognostic score predicts survival among patients with high PD-L1 expression administered first-line pembrolizumab monotherapy for non-small cell lung cancer. Cancer Med (2021) 10(20):6971–84. doi: 10.1002/cam4.4220
61. Sun L, Davis CW, Hwang WT, Jeffries S, Sulyok LF, Marmarelis ME, et al. Outcomes in patients with non-small-cell lung cancer with brain metastases treated with pembrolizumab-based therapy. Clin Lung Cancer (2021) 22(1):58–66. doi: 10.1016/j.cllc.2020.10.017
62. Mencoboni M, Ceppi M, Bruzzone M, Taveggia P, Cavo A, Scordamaglia F, et al. Effectiveness and safety of immune checkpoint inhibitors for patients with advanced non small-cell lung cancer in real-world: Review and meta-analysis. Cancers (Basel) (2021) 13(6):1388. doi: 10.3390/cancers13061388
63. Passiglia F, Galvano A, Gristina V, Barraco N, Castiglia M, Perez A, et al. Is there any place for PD-1/CTLA-4 inhibitors combination in the first-line treatment of advanced NSCLC?-a trial-level meta-analysis in PD-L1 selected subgroups. Transl Lung Cancer Res (2021) 10(7):3106–19. doi: 10.21037/tlcr-21-52
64. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (2021) 397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2
65. Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee D, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-Small-Cell lung cancer with PD-L1 tumor proportion Score≥50%: Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol (2021) 39(21):2327–38. doi: 10.1200/JCO.20.03579
66. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
67. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
68. Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: A systematic review and meta-analysis. JAMA Oncol (2018) 4(2):210–6. doi: 10.1001/jamaoncol.2017.4427
Keywords: pembrolizumab, meta-analysis, non-small cell lung cancer, randomized controlled trials, real-world studies
Citation: Yang B, Wang B, Chen Y, Wan N, Xie F, Yang N, Lu L, Xiao W, Yuan J, Li J, Xie B and Ji B (2023) Effectiveness and safety of pembrolizumab for patients with advanced non-small cell lung cancer in real-world studies and randomized controlled trials: A systematic review and meta-analysis. Front. Oncol. 13:1044327. doi: 10.3389/fonc.2023.1044327
Received: 14 September 2022; Accepted: 25 January 2023;
Published: 07 February 2023.
Edited by:
Jian Yu, Beihang University, ChinaReviewed by:
Valerio Gristina, University of Palermo, ItalyCopyright © 2023 Yang, Wang, Chen, Wan, Xie, Yang, Lu, Xiao, Yuan, Li, Xie and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Wan, ZGVsYTA4MTFAMTYzLmNvbQ==; Bo Ji, amJqZW5ueUBzaW5hLmNvbQ==; Bo Xie, MjkxMzIxNjkwNEBxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.