
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 March 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1039670
 Yoon Ju Bang1†
Yoon Ju Bang1† Hee Jun Choi1†
Hee Jun Choi1† Isaac Kim2
Isaac Kim2 Moo-Hyun Lee3
Moo-Hyun Lee3 Seeyoun Lee4
Seeyoun Lee4 Hyuk Jai Shin5
Hyuk Jai Shin5 Seok Jin Nam6
Seok Jin Nam6 Jeong Eon Lee6
Jeong Eon Lee6 Byung-Joo Chae6
Byung-Joo Chae6 Se Kyung Lee6
Se Kyung Lee6 Jai Min Ryu6
Jai Min Ryu6 Seok Won Kim6*
Seok Won Kim6*Purpose: The incidence of early tumor detection is increasing due to popularization of breast cancer screening and the development of imaging techniques. Thus, suitable preoperative localization is required for proper diagnosis and treatment of non-palpable breast lesions. The purpose of this study was to evaluate the efficacy and safety of indocyanine green (ICG)-hyaluronic acid (HA) mixture for lesion localization compared to activated charcoal.
Methods: This was a multicenter, randomized, open-label, parallel phase 3 clinical trial performed at four centers in Korea. Female patients scheduled for surgery to remove non-palpable breast lesions were enrolled. One hundred and nine patients were randomly assigned to a control group (activated charcoal: 0.3. – 1 mL) or a study group (ICG-HA mixture, 0.2 mL) for the localization of a breast lesion. The primary endpoint was the accuracy of resection. Secondary endpoints included the technical success rate, histopathological accuracy, skin pigmentation rate, and adverse event rate.
Results: A total of 104 patients were eligible for per-protocol analysis (control group, n = 51; study group, n = 53). The accuracy of resection in the study group was not inferior to that of the control group (90.57% vs. 98.04%, 95% confidence interval (CI): -2.31 – 18.91, p = 0.21). There was no statistically significant difference in technical success rate between the two groups (marking on breast skin: p = 0.11, marking on the excised specimen: p = 0.12). However, there were statistically significant differences in histopathological accuracy (0.26 ± 0.13 vs. 0.33 ± 0.17, p = 0.01) and skin pigmentation rate (0.00% vs. 30.77%, p< 0.01). Adverse events were not reported in either group.
Conclusions: When localization was performed using ICG-HA, the accuracy of resection was not inferior to that of activated charcoal. However, skin pigmentation rate was significantly lower. In conclusion, ICG-HA is effective and safe for localizing of non-palpable breast lesions.
The detection of early tumor which is non-palpable and can only be found using precise localization has increased with the popularization of breast ultrasound (US) screening and the advent of imaging techniques. Preoperative localization for non-palpable breast lesions is very important for minimal but accurate excision of non-palpable breast lesions for proper diagnosis and treatment (1). Various procedures, including injection of a bioavailable dye, wire localization, radioactive seed localization and skin marking with an oil-based or water-based pen to pre-mark the non-palpable lesion, have been used so that lesions could be distinguished during surgeries (2–5).
Wire localization is a classic and widely used technique with several disadvantages. It should be performed on the day of surgery because of its disadvantages such as risk of wire migration or withdrawal, pain to patients, and interference with surgical approaches (2, 6, 7). US-guided localization using bioavailable dye for visualizing non-palpable breast lesions is rapid and easy to perform without using mammography (MMG). It has been shown to decrease positive cancer margin rates and re-operation rates (8). Charcoal tattooing is a widely used method without the risk of fast dye dispersion. Surgery can be planned after a few days. However, it has disadvantages of skin pigmentation and foreign body reaction (9).
Indocyanine green (ICG) approved by Food and Drug Administration (FDA) is the most widely accepted fluorophore used in various clinical fields, including sentinel lymph node (SLN) mapping, identification of solid tumors, lymphography, angiography, and anatomical imaging during surgery (10–16). In the field of breast oncology, ICG has been used for monitoring skin perfusion in nipple-sparing mastectomies to guide the locations of mastectomy incisions and minimize ischemic complications, and for performing sentinel lymph node biopsy (17). However, there is no report of tattooing localization using ICG under US-guidance for non-palpable breast lesions. Through a phase-2 clinical trial, we have suggested that ICG-hyaluronic acid (HA) (LuminoMark™) can be used for accurate preoperative localization without skin pigmentation in benign breast diseases (18). The aim of this study was to evaluate the efficacy and safety of ICG-HA for localizing non-palpable breast lesions including breast cancer and confirm that ICG-HA is not inferior to activated charcoal, which has been widely used.
This was a multicenter, randomized, open-label, parallel phase 3 clinical trial done at four centers, Samsung Medical Center, Dongsan Medical Center, National Cancer Center, and Myongji Hospital in Korea (ClinicalTrials.gov Identifier: NCT 04606329). This study aimed to evaluate the efficacy and safety of ICG-HA as a novel mixture for localization compared to activated charcoal.
This clinical trial was designed to last for eight weeks, including screening, randomization, localization, surgery, and follow-up visits. Figure 1 shows the study flow chart. After randomization, localization was performed at visit 2 or 3. At visit 3, surgery and the first evaluation of efficacy and safety were performed. Efficacy and safety were evaluated again at visits 4 and 5.
To calculate the number of subjects to prove the non-inferiority of ICG-HA to activated charcoal, the negative resection margin rate of the study group and the control group was set at 81.1% (9). The limit of non-inferiority was set at 22.5% by referring to a previous study (19) that compared localization methods in patients who underwent surgery to remove non-palpable breast lesions. A sample size of 54 in each group achieved a power of 80% to detect a difference between group proportions of -0.225.
1) Level of significance, α= 0.025 (one-sided)
2) Power of the test, 1-β= 0.8
3) Allocation ratio= 1:1
4) Resection margin negative rate; study group, pt 0.811
5) Resection margin negative rate; control group, pc 0.811
6) Limit of non-inferiority, -δ= -0.225
In this clinical trial, 0.2 mL of ICG-HA mixture (LuminoMark™, Hanlim Pharm. Co., Ltd., Korea) injection was set as the dose of the test drug, and 0.3 – 1 mL of activated charcoal (Chacotrace®, Phebra, Australia) was set as the control according to the results of the phase 2 clinical trial (18).
The study population targeted females aged between 19 and 80 years who were scheduled for surgeries to remove non-palpable breast lesions confirmed by breast US, regardless of the type of lesion. Palpability of the breast lesion was evaluated by the surgeon at the first visit. There was no limit to the size of the lesion since palpability depended not only on the size of the lesion, but also on the breast size and the location of the lesion. Inclusion and exclusion criteria are detailed in Table 1.
Figure 2 shows a flow chart of participant enrollment. A total of 120 provided consent to participate in this clinical trial. However, 11 patients were excluded after screening. Thus, 109 patients were randomized at a 1:1 ratio to a control group (N = 56) and a study group (N = 53). Among these 109 randomized patients, 108 (52 in the study group and 56 in the control group) were included in the safety analysis after excluding one patient who missed injection of the clinical trial drug. One hundred and six patients (51 in the test group and 55 in the control group) in the safety analysis (SA) group were included in the full analysis (FA) group after excluding two patients with missing resection margin evaluations. Of these 106 patients in the FA group, 104 patients (51 in the test group, 53 in the control group) were included in the per-protocol (PP) analysis after excluding two patients for violating the inclusion/exclusion criteria and measurement timing of the primary efficacy endpoint.
After randomization, localization was performed for the target lesion by injecting 0.2 mL of Luminomak™ (study group) using a 26-gauge needle or 0.3 – 1 mL of Chacotrace® (control group) using a 18-gauge needle. Skin excision to avoid skin pigmentation was not allowed. However, it was allowed if skin excision was necessary for complete removal because the lesion was close to the skin. In the case of multiple lesions, one target lesion was selected by the investigator and the test drug was used only for the target lesion. Other lesions were also removed according to the general method of each institution. Localization with ICG-HA was visualized using near-infrared fluorescence. Intraoperative photographs were taken after skin incision and excision. Follow-up photographs were then obtained (Figure 3).
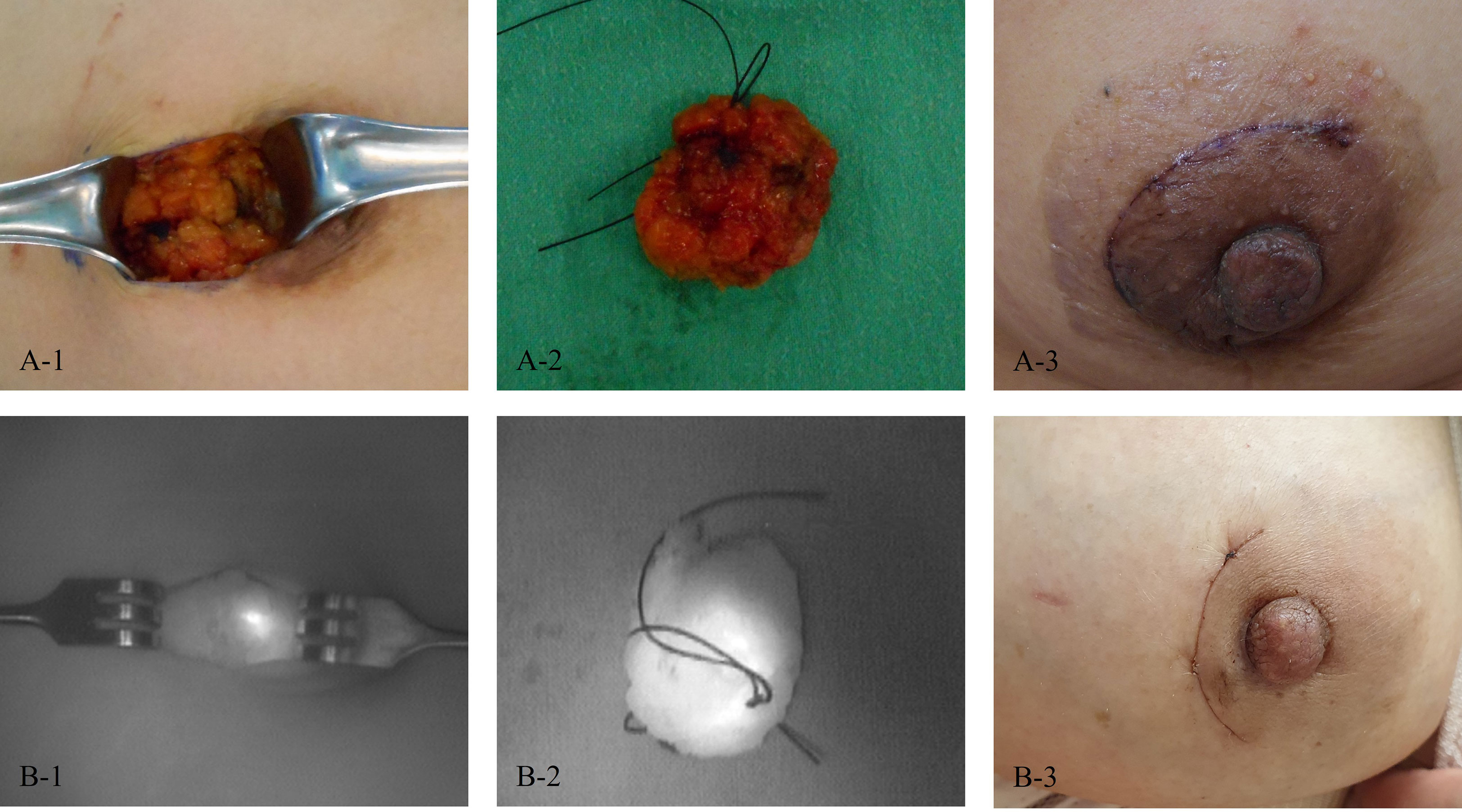
Figure 3 Photos of patients. (A) A patient in the control group (1, After skin incision; 2, After excision; and 3, On the last follow-up day). (B) A patient in the study group (1, After skin incision; 2, After excision; and 3, On the last follow-up day).
The primary endpoints were accuracy of resection determined as the proportion of patients who had negative resection margins and histopathological accuracy. As assessed by the pathologist, a negative margin was defined as absence of the target lesion in any resected section of the breast specimen. Although additional resection was performed according to frozen biopsy results and a negative margin was finally obtained during breast cancer surgery, margin status was defined based on initial frozen biopsy results. Histopathological accuracy was defined as the longest length of the breast lesion compared to the longest length of the resected specimen for evaluating whether the target lesion was accurately excised without unnecessary wide resection. Secondary endpoints included the technical success rate, skin pigmentation rate, and adverse event rate. The technical success rate was defined as successful visualization after localization. It was evaluated for breast surface before surgery and excised specimen, respectively. Skin pigmentation was defined as discoloration due to the drug. It was evaluated by photographing the surgical site after surgery. The incidence of adverse events was defined as the proportion of patients with any confirmed adverse events after surgery. All events were collected including any harmful and unintended signs, symptoms, abnormalities in clinical laboratory test results, and diseases that occurred to the patients after injection of the test drug. In this clinical trial, postoperative pain, nausea, and vomiting were not collected because surgery was performed. The investigator evaluated whether the reported event was related to the test drug. Any events evaluated other than ‘definitely not related’ were defined as adverse events.
Primary efficacy analysis was based on Chan and Zhang’s 95% (two-sided) confidence interval (CI) for the difference in negative resection margin between the two groups. Non-inferiority of the study group compared to the control group was established if the difference in the negative resection margin rate was greater than the lower non-inferiority margin, i.e., if the lower boundary of the two-sided 95% CI was greater than or equal to -22.5%. All other statistical significance tests were performed as two-sided tests with a 5% significance level. Statistical significance was considered when p-values of< 0.05. Categorical data in this study were analyzed for differences using chi-squared test or Fisher’s exact test. In addition, McNemar’s test was used to analyze whether there was any change within the group. Continuous data in this study were analyzed for differences using paired t-test or Wilcoxon’s signed-rank test. All statistical analyses were performed using SAS software (SAS Institute Inc, version 9.4). Since results for the PP population were the same as those for the FA population, only PP data are presented in this paper. Safety population included all patients receiving at least one dose of the study drug.
This clinical trial was conducted in accordance with the protocol approved by the Ministry of Food and Drug Safety (MFDS) and the Institutional Review Board (IRB) for each institution. It complied with Korea’s Good Clinical Practice (KGCP) and Good Clinical Practice (GCP) set forth by the International Conference on Harmonisation (ICH).
We declare that this study has obtained a report of ethics board approval. Written informed consent was obtained from each participant. This study was approved by the Ethics Committee of Samsung Medical Center (IRB No. SMC 2019-12-117), Dongsan Medical Center (IRB No. DSMC 2020-01-047), National Cancer Center (IRB No. NCC2020-0070), and Myongji Hospital (IRB No. MJH2020-01-011).
A total of 104 patients were eligible for PP analysis (control group, n = 51; study group, n = 53). Table 2 shows patient characteristics. There were no significant differences in baseline characteristics between the two groups. Each dye was administered within 3 days of surgery. There was no difference in the mean exposure periods between the two groups (p = 0.86). The size of the lesion measured by preoperative US varied from 0.1 cm to 3.0 cm. However, there was no significant difference in mean size between the two groups (p = 0.35). According to histopathologic results, malignancy accounted for more than 70%. It showed no significant difference between the two group (p = 0.66). Benign lesions included fibroadenoma, phyllodes tumor, and intraductal papilloma.
Negative resection rate, the primary efficacy endpoint, was 98.04% (50/51) in the study group and 90.57% (48/53) in the control group. As a result of a non-inferiority test, the lower limit of Chan and Zhang’s 95% two-sided accurate confidence interval for the difference between the two groups was -0.0231, which exceeded the non-inferiority threshold of -0.225. Thus, the accuracy of resection in the study group was not inferior to that of the control group (90.57% vs 98.04%, 95% CI: -2.31 – 18.91, p = 0.21). As a result of pathology, there was no significant difference in mean length of breast lesion or mean length of excised specimen between the two groups. However, there was a statistically significant difference in histopathological accuracy (0.26 ± 0.13 vs 0.33 ± 0.17, p = 0.01) (Table 3). The technical success rate was 98.0% in the study group and 88.24% in the control group for the breast. It was 100% in the study group and 92.54% in the control group for the excised specimen. Technical success rates show no statistically significant difference between the two groups (marking on breast, p = 0.11, marking on the excised specimen, p = 0.12). In the control group, skin pigmentation was observed in 16 patients, but there was no skin pigmentation case in the study group (0.00% vs. 30.77%, p< 0.01) (Table 4). Adverse events were not reported in either group.
Due to an increase in screening examinations and the development of imaging methods, the detection of small breast lesions is increasing. Thus, accurate localization is required for proper diagnosis and treatment (20). Among several localization techniques, needle localization has been widely used. However, it has several disadvantages, including risk of wire migration or withdrawal, patient’s pain, and interference with surgical approaches (2, 6, 7). Localization using dyes for visualizing non-palpable breast lesions is rapid and easy to perform. Among various bioavailable dyes, charcoal has been widely used as a material without the risk of fast dye dispersion. Thus, surgery can be planned several days after localization. However, it has disadvantages of skin pigmentation and foreign body reaction (9).
Because the use of ICG-HA for breast localization was not reported yet, we previously conducted a phase 2 clinical trial of ICG-HA. However, the sample size was small (n = 44). In addition, only breast benign diseases were included (18). In this study, we evaluated the efficacy and safety of ICG-HA for localizing non-palpable breast lesions including breast cancer with a larger sample size (n = 108). The accuracy of resection in the study group was not inferior to that of the control group (90.57% vs. 98.04%, 95% CI: -2.31 – 18.91, p = 0.21) (Table 3). There were significant intergroup differences in histopathological accuracy (0.26 ± 0.13 vs. 0.33 ± 0.17, p = 0.01) and skin pigmentation rates (0% vs. 30.8%, p< 0.01) (Table 4). However, there was no significant differences in technical success rate or adverse drug reaction.
Histopathological accuracy defined as the longest length of the breast lesion compared to the longest length of the resected specimen was evaluated to determine whether the target lesion was accurately excised without unnecessary wide resection. Similarly, in the phase 2 clinical trial, the accuracy of resection defined as the maximum diameter of the resected specimen divided by the maximum diameter of the preoperative lesion detected in the US was evaluated and the control group was found to have a higher value than the study group. The reason for the wider resection in the control group was because the injection amount of activated charcoal was higher than that of the ICG-HA (18). On the contrary, histopathological accuracy was higher in the control group than that in the study group, although the injection amount of activated charcoal was not reduced in this phase 3 clinical study. This inconsistency between the two studies might be because radical excision was intended to excise a wider area for a malignant tumor than for a benign lesion.
Skin pigmentation was observed in 30.8% of patients in the control group but in none of patients in the study group (p< 0.01). Skin pigmentation caused by charcoal localization can be removed by excising the overlying skin (21). However, excessive skin excision might cause poor postoperative breast shape. Most charcoal are removed during the surgery, although small amounts might remain in the breast around the surgical area. In most cases, residual charcoal does not appear on follow-up imaging. However, in some cases, residual charcoal can develop into foreign body granulomas (22). We observed only skin pigmentation as a disadvantage of charcoal in this study. Long-term follow-up is required to determine the development of foreign body granulomas that can cause unnecessary biopsy or surgery.
The technical success rate evaluated by two aspects showed a higher success rate in the study group, although the differences between the two groups was not statistically significant. In some cases of the control group, charcoal spread around the lesion with target lesion not localized. The possible cause of failure in these cases might have been the difficulty of injection into a very dense breast or hard mass. Because activated charcoal is in particulate form and insoluble in water, it usually does not disperse into the surrounding tissues, allowing surgery to be planned over several days. However, a thick needle is required for injection. Blockage of the needle tip could occur. Thus, so accurate localization is not possible because it is not gently injected into a hard tissue. On the other hand, ICG-HA has the advantages of being gently injected into hard tissues even with a thinner needle, leading to less pain for the patient.
This study had several limitations. First, although ICG is safe and the most widely used fluorophore in various clinical fields, long-term follow-up of ICG-HA has not been performed. Additional study should be conducted regarding the safety issue in the future. Second, our patient group was heterogeneous as both malignant and benign lesions were included. Therefore, there was a difference in setting an appropriate resection area for the targeted lesion. There may have been intentions of surgeons to remove wider region during surgery to obtain safer negative resection margin for cancer patients. In addition, skin pigmentation might have been underestimated because some malignant lesions required skin resection, although skin resection was not required in most benign cases. Third, since it was a multicenter study, errors might have occurred by each institution and by each researcher. Even with these limitations, this study was meaningful because it was prospectively designed and conducted on breast cancer. No studies on ICG-HA for breast localization have been reported yet.
In conclusion, this multicenter phase 3 clinical trial evaluated the efficacy and safety of an ICG-HA mixture for localization of non-palpable breast lesions relative to those of activated charcoal. ICG-HA injection is a new method for localizing non-palpable breast lesions. It is useful method for obtaining accurate resections and cosmetic benefits in breast cancer. To avoid skin pigmentation, localization with ICG-HA could be considered.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the ethics committee of Samsung Medical Center. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
SK, M-HL, SYL, and HS contributed to conception and design of the study. YB organized the database. YB and HC performed the statistical analysis. YB wrote the first draft of the manuscript. YB and HC wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
This study was sponsored by Hanlim Pharm. Co., Ltd., a Korean pharmaceutical company dedicated to developing new medicine. The sponsor covered the cost of collecting data. The funder had no role in data analysis, data interpretation, or writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Altomare V, Guerriero G, Giacomelli L, Battista C, Carino R, Montesano M, et al. Management of nonpalpable breast lesions in a modern functional breast unit. Breast Cancer Res Treat (2005) 93(1):85–9. doi: 10.1007/s10549-005-3952-1
2. Homer MJ, Smith TJ, Safaii H. Prebiopsy needle localization. methods, problems, and expected results. Radiol Clin North Am (1992) 30(1):139–53. doi: 10.1016/S0033-8389(22)02491-5
3. Rosenberg AL, Schwartz GF, Feig SA, Patchefsky AS. Clinically occult breast lesions: Localization and significance. Radiology (1987) 162(1 Pt 1):167–70. doi: 10.1148/radiology.162.1.3024209
4. Svane G. A stereotaxic technique for preoperative marking of non-palpable breast lesions. Acta Radiol Diagn (Stockh) (1983) 24(2):145–51. doi: 10.1177/028418518302400207
5. Bristol JB, Jones PA. Transgression of localizing wire into the pleural cavity prior to mammography. Br J Radiol (1981) 54(638):139–40. doi: 10.1259/0007-1285-54-638-139
6. Mayo RC 3rd, Kalambo MJ, Parikh JR. Preoperative localization of breast lesions: Current techniques. Clin Imaging (2019) 56:1–8. doi: 10.1016/j.clinimag.2019.01.013
7. Helvie MA, Ikeda DM, Adler DD. Localization and needle aspiration of breast lesions: Complications in 370 cases. AJR Am J Roentgenol (1991) 157(4):711–4. doi: 10.2214/ajr.157.4.1892023
8. Acosta JA, Greenlee JA, Gubler KD, Goepfert CJ, Ragland JJ. Surgical margins after needle-localization breast biopsy. Am J Surg (1995) 170(6):643–5. doi: 10.1016/s0002-9610(99)80033-6
9. Rose A, Collins JP, Neerhut P, Bishop CV, Mann GB. Carbon localisation of impalpable breast lesions. Breast (2003) 12(4):264–9. doi: 10.1016/s0960-9776(03)00105-x
10. Sound S, Okoh A, Yigitbas H, Yazici P, Berber E. Utility of indocyanine green fluorescence imaging for intraoperative localization in reoperative parathyroid surgery. Surg Innov (2019) 26(6):774–9. doi: 10.1177/1553350615613450
11. Alander JT, Kaartinen I, Laakso A, Patila T, Spillmann T, Tuchin VV, et al. A review of indocyanine green fluorescent imaging in surgery. Int J BioMed Imaging (2012) 2012:940585. doi: 10.1155/2012/940585
12. Anayama T, Qiu J, Chan H, Nakajima T, Weersink R, Daly M, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg (2015) 99(1):224–30. doi: 10.1016/j.athoracsur.2014.07.050
13. Nagaya T, Nakamura YA, Choyke PL, Kobayashi H. Fluorescence-guided surgery. Front Oncol (2017) 7:314. doi: 10.3389/fonc.2017.00314
14. Motomura K, Inaji H, Komoike Y, Kasugai T, Noguchi S, Koyama H. Sentinel node biopsy guided by indocyanine green dye in breast cancer patients. Jpn J Clin Oncol (1999) 29(12):604–7. doi: 10.1093/jjco/29.12.604
15. Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer (2009) 115(11):2491–504. doi: 10.1002/cncr.24291
16. Holt D, Okusanya O, Judy R, Venegas O, Jiang J, DeJesus E, et al. Intraoperative near-infrared imaging can distinguish cancer from normal tissue but not inflammation. PloS One (2014) 9(7):e103342. doi: 10.1371/journal.pone.0103342
17. Wapnir I, Dua M, Kieryn A, Paro J, Morrison D, Kahn D, et al. Intraoperative imaging of nipple perfusion patterns and ischemic complications in nipple-sparing mastectomies. Ann Surg Oncol (2014) 21(1):100–6. doi: 10.1245/s10434-013-3214-0
18. Kim I, Choi HJ, Ryu JM, Lee SK, Yu JH, Lee JE, et al. The efficacy and safety of indocyanine green-hyaluronic Acid mixture (LuminoMark) for localization in patients with non-palpable breast lesions: A multi-center open-label parallel phase-2 clinical trial. BMC Surg (2021) 21(1):134. doi: 10.1186/s12893-021-01129-y
19. Duarte C, Bastidas F, de los Reyes A, Martinez MC, Hurtado G, Gomez MC, et al. Randomized controlled clinical trial comparing radioguided occult lesion localization with wire-guided lesion localization to evaluate their efficacy and accuracy in the localization of nonpalpable breast lesions. Surgery (2016) 159(4):1140–5. doi: 10.1016/j.surg.2015.09.023
20. Haakinson DJ, Stucky CC, Dueck AC, Gray RJ, Wasif N, Apsey HA, et al. A significant number of women present with palpable breast cancer even with a normal mammogram within 1 year. Am J Surg (2010) 200(6):712–7. doi: 10.1016/j.amjsurg.2010.08.005
21. Canavese G, Catturich A, Vecchio C, Tomei D, Estienne M, Moresco L, et al. Pre-operative localization of non-palpable lesions in breast cancer by charcoal suspension. Eur J Surg Oncol (1995) 21(1):47–9. doi: 10.1016/s0748-7983(05)80067-8
Keywords: localization, non-palpable, excision, indocyanine green, indocyanine green (ICG)
Citation: Bang YJ, Choi HJ, Kim I, Lee M-H, Lee S, Shin HJ, Nam SJ, Lee JE, Chae B-J, Lee SK, Ryu JM and Kim SW (2023) The efficacy and safety of an indocyanine green−hyaluronic acid mixture (LuminoMark™) for localization in patients with non-palpable breast lesions: A multicenter, randomized, open-label, parallel phase 3 clinical trial. Front. Oncol. 13:1039670. doi: 10.3389/fonc.2023.1039670
Received: 08 September 2022; Accepted: 13 March 2023;
Published: 24 March 2023.
Edited by:
Luigi Cavanna, Ospedaliera di Piacenza, ItalyReviewed by:
Yunmei Wang, Shaanxi Provincial Cancer Hospital, ChinaCopyright © 2023 Bang, Choi, Kim, Lee, Lee, Shin, Nam, Lee, Chae, Lee, Ryu and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seok Won Kim, c2Vva3dvbjEua2ltQHNhbXN1bmcuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.