- Department of Breast Disease, Peking Union Medical College Hospital, Peking Union Medical College, Beijing, China
Introduction: Although geriatric assessment (GA) has been used for a long time in the field of geriatrics and internal medicine, there are few studies on its application in the field of breast surgery. Therefore, the utility of specific GA domains for the assessment of older patients with breast cancer remains unclear. The aim of the present study was to evaluate the association between specific GA domains and the survival rate of older patients with breast cancer.
Methods: We used the database of Peking Union Medical College Hospital to identify older patients who were newly diagnosed with breast cancer between 2012 and 2018 and retrospectively analysed the data of 541 patients aged ≥65 years. Patients with metastatic cancer and those with missing vital status data were excluded. The primary outcomes were overall survival (OS) and breast cancer-specific survival. The GA domains used in this study included functional status, comorbidities, and psychological state. Multivariate regression analysis was used to estimate hazard ratios for these three domains.
Results: After a median follow-up of 72 months, we observed a significant relationship between functional impairment and mortality (adjusted HR: 3.06, 95% confidence interval [CI]: 1.83-5.10, P<0.001). Similarly, patients with severe comorbidities (adjusted HR: 2.35; 95% CI: 1.16-4.75, P=0.017) and an impaired psychological state (adjusted HR: 2.82, 95% CI: 1.45-5.50, P=0.002) showed worse OS rates. Accordingly, addition of the three GA domains to the basic model, which included age, tumour stage, lymph node stage, and intrinsic molecular subtype as baseline variables, yielded higher C‐statistics for mortality analysis (from 0.713 to 0.740).
Conclusion: To our knowledge, this is the first study to include specific GA domains in a prognostic model for older patients with breast cancer in China. Three domains, namely functional status, comorbidities, and psychological state, should be considered for survival analyses in this particular population. The full model including these three GA domains may be more accurate in predicting the survival of older patients with breast cancer.
1 Introduction
The number of older patients with cancer has steadily increased over the past10 years, with the progress of social aging (1). In addition, the prevalence of breast cancer in older adults (≥65 years) has been increasing of late (2). Cancer characteristics and treatment responsiveness tend to differ between older and younger patients with breast cancer (3). Therefore, the management of breast cancer in older patients is challenging because the disease is highly heterogeneous, and there is insufficient evidence specific to older adults. This can complicate the clinical decision-making process. The use of chronological age alone to determine treatment strategies increases the risk of overtreatment or undertreatment in older patients (4). Decision-making should involve use of the geriatric assessment (GA) and also consider patient preferences (5). Therefore, it is important to identify age‐related prognostic factors, such as comorbidities and functional status, that can support survival predictions and treatment decisions.
GA is believed to facilitate better estimations of life expectancy and assist treatment decisions in the field of geriatric oncology. GA typically comprises several domains, including physical performance, fall risk, functional status, multiple comorbidities, polypharmacy status, depressive symptoms, cognition, psychosocial distress, nutritional status, and socioeconomic support (6). The European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG) recommend that GA should be used for the management of all older patients with breast cancer (7).
There are several tools for GA, with each comprising different domains (6, 8, 9). Several GA domains have been reported as independent predictors of mortality in patients with cancer (10–13); among these, impaired functional status, comorbidities, and psychological distress are consistently identified as risk factors for mortality. Functional status measures in geriatric oncology commonly involve evaluations of activities of daily living (ADL) and instrumental ADL (IADL); the former encompasses basic self-care skills for independent home‐based living and is more representative and illustrative (14). Comorbidities refer to one or more disorders in addition to the specific cancer. These conditions become increasingly prevalent with advancing age and are associated with poorer outcomes in older patients with cancer (15). Emerging evidence also suggests that psychological distress is strongly correlated with cancer mortality in older patients (16). However, the mechanisms underlying psychological challenge-mediated tumour immune evasion have not been systematically explored.
Although there is increasing information on the impact of various GA domains on overall survival (OS) in patients with cancer, there is little relevant research involving older patients with breast cancer. Large-scale studies are warranted for more accurate identification of crucial GA domains that would assist survival predictions and treatment choices for this patient population. Accordingly, the aim of this retrospective study was to evaluate the associations of three GA domains, namely functional status, comorbidities, and psychological state, with survival in older patients with breast cancer.
2 Materials and methods
2.1 Study design and patient population
The Peking Union Medical College Hospital (PUMCH) database has been collecting the data of patients with breast cancer who have undergone treatment at PUMCH since 1975. These data includes patient age at cancer diagnosis, sex, vital status, surgical methods, tumour histology, treatment regimens, concomitant diseases, details regarding recurrence or metastasis (localised, regional, and distant), and survival information. For the present study, any hard copies of patient records were electronically filed by scanning. The necessary data were extracted and input into a new database to enable analysis of the associations between clinical information and mortality.
Eventually, data for 541 patients aged ≥65 years were collected. All patients underwent surgery and other treatments from 2012 to 2018 and underwent clinical follow-up at PUMCH. Written informed consent to undergo the procedure and follow-up assessments was obtained from all patients. The study was approved by the Institutional Review Board of Peking Union Medical College Hospital (ZS-2682) and performed in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Because the study was retrospective in nature and all patient data were anonymised, the need for informed consent for publication of this report was waived.
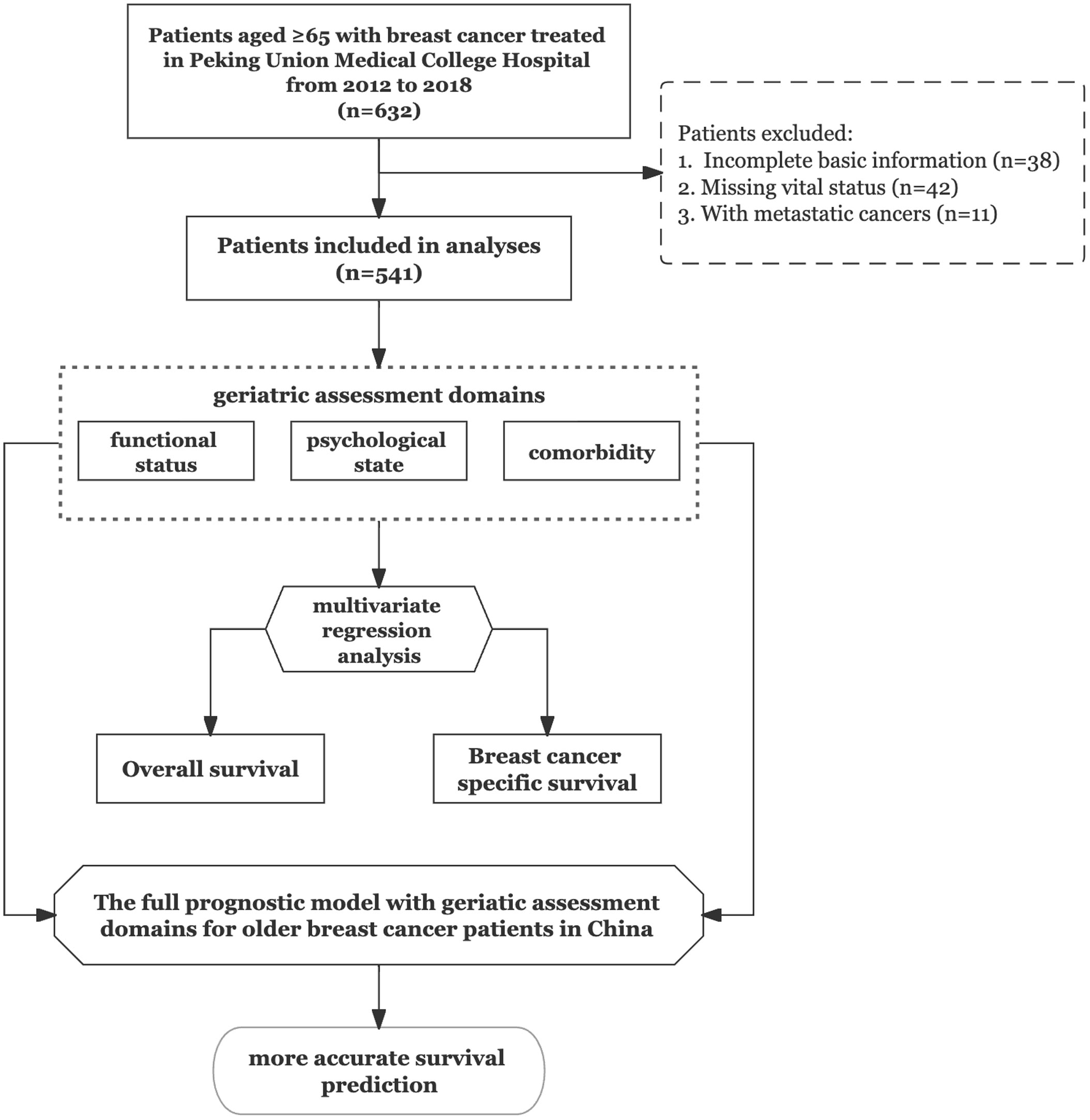
We first identified patients aged ≥65 years (n = 622) who were diagnosed with breast cancer between 1 January 2012 and December 2018. We used a cut-off age of 65 years because it is frequently designated as the age for GA implementation in geriatric oncology studies. At the same time, clinical trials related to breast cancer have often selected 65 years as the cut-off age to distinguish elderly patients. Patients with incomplete basic information (n = 38), missing vital status details (n = 42), or simultaneous metastatic cancer (n = 11) were excluded. All patients underwent monitoring to verify their vital status using the PUMCH follow-up system until December 2021.
2.2 Assessment of GA domains
Three specific GA domains, namely functional status, comorbidities, and psychological state, were used for assessments in this study. The patients admitted to our surgery ward are all ready for breast surgery; in other words, we tend to screen relatively ‘healthy’ older patients in our ward. Therefore, we first have to collect functional status and comorbidity data in the outpatient department and then collect psychological data; these data are supplied after admission of the patient. Data collection in the outpatient department, rather than administration of the entire GA questionnaire, facilitates a convenient and effective method for input.
Functional status was assessed using the Barthel Index score, which includes 10 items to measure ADL performance (17). These items include continence and independence in bathing, feeding, dressing, using the bathroom, getting up, and moving around the house. A total score ranging from 0 to 100 is calculated for each patient, with higher scores indicating higher levels of independence. We recorded the functional status as abnormal (0‐60) or normal (61‐100) based on the information provided by the patients. If the patient could not provide any detail precisely, we marked the status as unknown.
Comorbidities were assessed using the Charlson Comorbidity Index (CCI) score, which includes 17 comorbid conditions (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia or paraplegia, renal disease, any malignancy, and human immunodeficiency virus [HIV] infection) (18). From these, HIV infection was excluded because affected patients require treatment at designated hospitals. Furthermore, metastatic cancer was an exclusion criterion in the present study, whereas patients with paraplegia generally opt for drugs rather than surgery. Therefore, only 14 comorbid conditions were considered in the present study. There are four kinds of weights for each comorbid condition (1, 2, 3, and 6) based on the mortality risk associated with that condition. The following three categories were used for comorbidities in the present study: none (CCI score: 0–1), mild-to-moderate (CCI score: 2–3), and severe (CCI score: ≥4).
Psychological state was assessed using the updated Geriatric Depression Scale (GDS-15), which includes 15 items and is used to diagnose and evaluate depression in elderly individuals (19, 20). Psychological state was categorised as abnormal or depressive state (GDS ≥ 8), normal or non-depressive state (GDS < 8), and unknown.
2.3 Outcome variables
The primary outcomes were OS and breast cancer-specific survival (BCSS), estimated from the time of GA. We obtained detailed mortality data from our study database and retrieved the causes of death for all patients who died during the study period. Mortality was classified as BCSM and all-cause mortality.
2.4 Statistical analyses
Categorical variables are expressed as number and percentage. Pearson’s chi-square test was used to compare the results between the different groups. Continuous variables are presented as median with interquartile range.
A Cox proportional hazards model was constructed to estimate the independent effects of the three GA domains on the survival rate during a 6-year follow-up period, after adjustment for age, tumour stage, lymph node (LN) stage, and intrinsic molecular subtype. The primary outcome was OS time, which was defined as the time from the date of cancer diagnosis to the date of death from any cause or the date on which the patient was last known to be alive. The estimated effects of the three GA domains on OS were calculated as hazard ratios (HRs) and 95% confidence intervals (CIs). Adjusted survival curves stratified by the categories of the three GA domains were also generated. To examine the effects of the three GA domains on OS and BCSS, we constructed a Cox proportional hazards model after adjusting for age, tumour stage, LN stage, and intrinsic molecular subtype.
To assess the incremental prognostic value of each GA domain, we estimated Harrell’s concordance statistic (C‐statistic) for different models for OS and BCSS. The C‐statistic is equivalent to the area under the receiver operating characteristic curve, with a value of 0.5 indicating random predictions and a value of 1.0 indicating perfect discrimination between survivors and non-survivors. The first model was a ‘basic’ model that controlled for age, tumour stage, LN stage, and intrinsic molecular subtype. The GA domains were then individually added to the basic model. The final model was a ‘full’ model that included all three GA domains in addition to the covariates in the basic model.
All analyses were conducted using R software version 4.1.3. A two-sided P-value of <0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
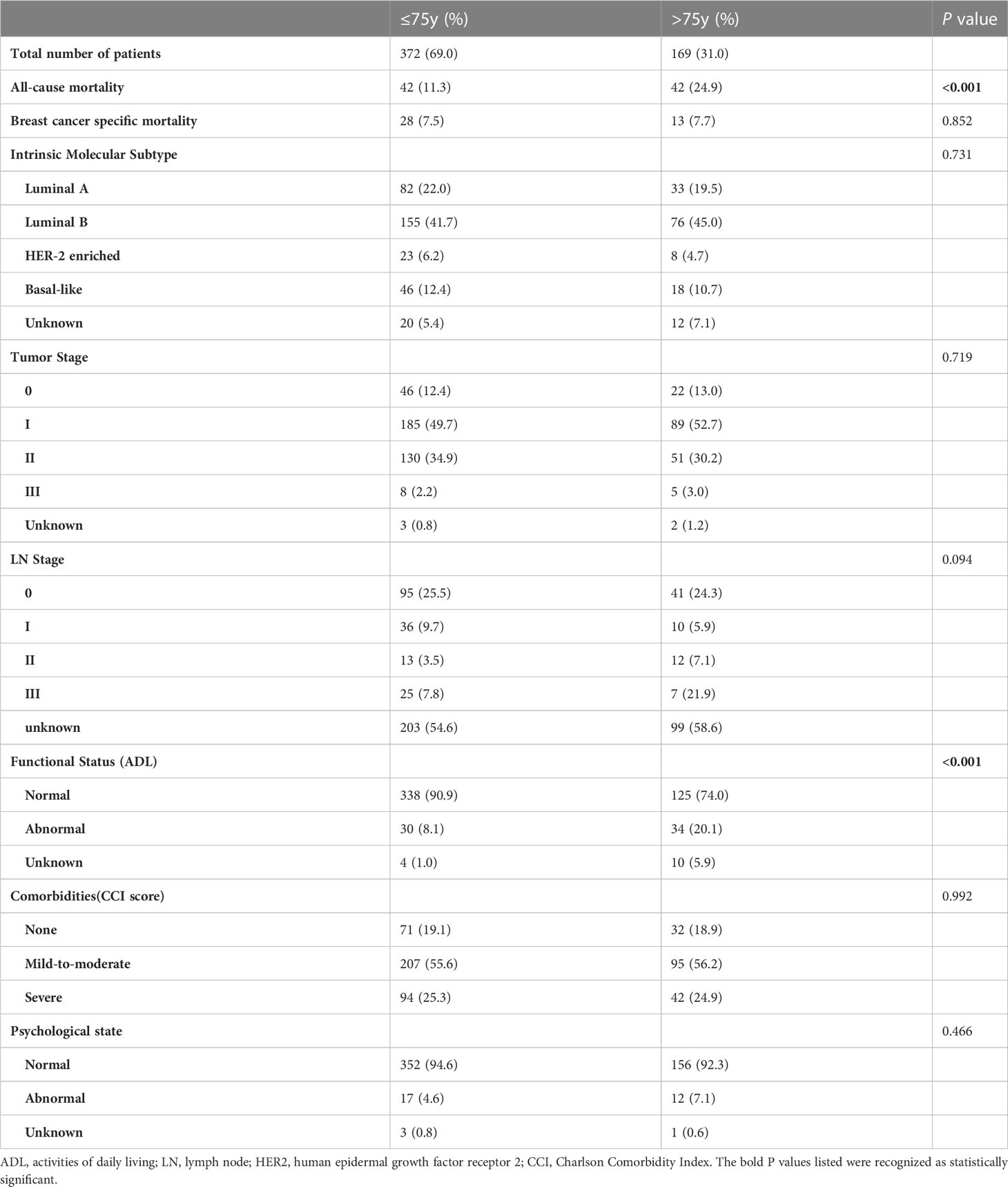
The study flowchart is shown in Figure 1. We recruited older patients (≥65 years) who underwent breast surgery at our cancer centre. From the 632 patients considered eligible, we identified 541 patients as study subjects. Patient characteristics are listed in Table 1. Of the 541 patients, 169 (31%) were aged ≥75 years at the time of cancer diagnosis. The most common cancer subtype was luminal B, and the most common tumour stage was stage II. During the 6‐year follow‐up period, the all-cause mortality rates for the <75-year and ≥75-year groups were 11.3% and 24.9%, respectively, with a significant difference between groups. There was no significant difference in the BCSM rate between the two groups. We also observed significant difference in functional status between the two groups, with no significant differences in the distributions of intrinsic molecular subtype, tumour stage, LN stage, comorbidities, and psychological state.
3.2 Survival data for the GA domains
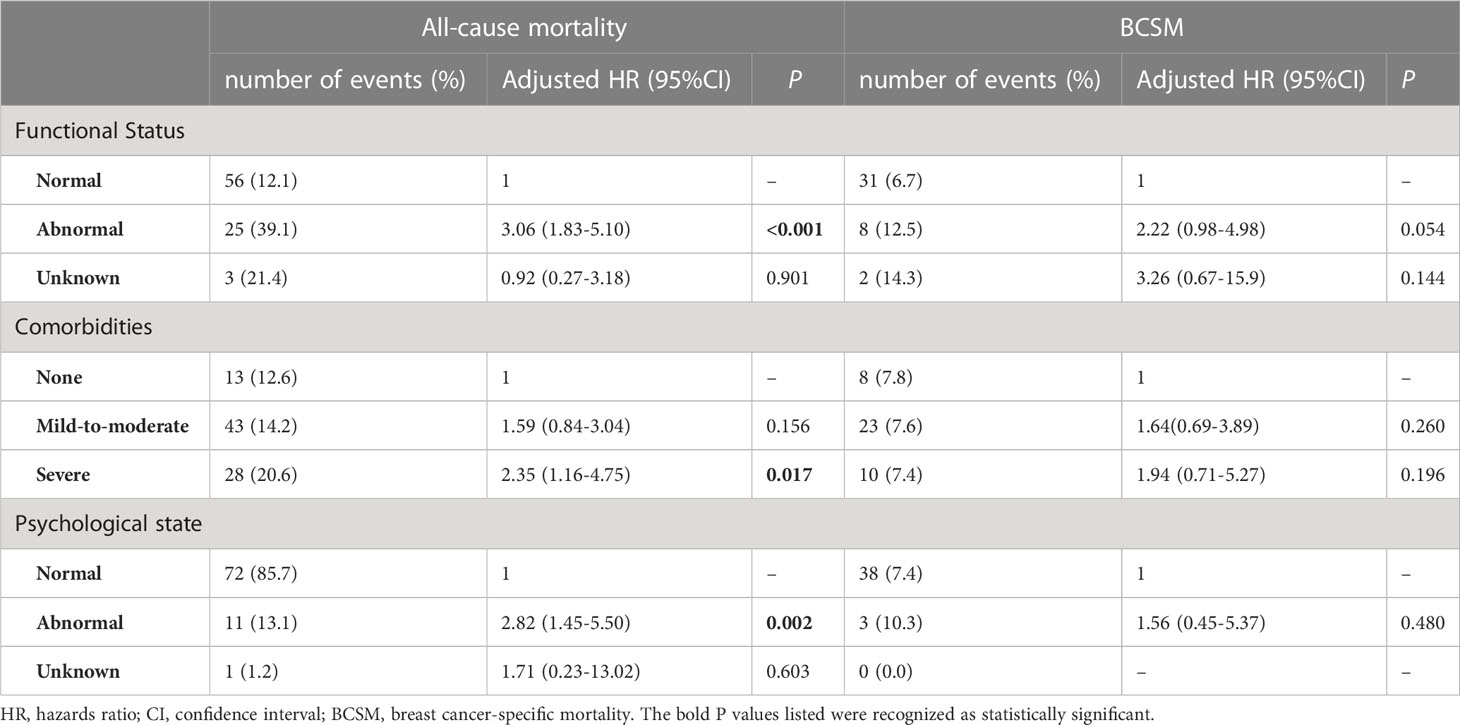
Multivariate Cox proportional hazards models were used to assess all-cause mortality and BCSM according to the three GA domains. HRs were calculated using Cox proportional hazards models adjusted for age, tumour stage, LN stage, and intrinsic molecular subtype. Table 2 presents the results.
In the all‐cause mortality analysis, we observed a significant relationship between ADL impairment and mortality. Breast cancer patients with abnormal ADL had a significantly higher risk of all-cause mortality (adjusted HR: 3.06, 95% CI: 1.83-5.10, P<0.001) than did functionally independent patients. There was no significant difference in BCSM between patients with ADL impairment and those without ADL impairment (adjusted HR: 2.22, 95% CI: 0.98-4.98, P=0.054).
Meanwhile, the models for comorbidities and psychological state yielded similar results for geriatric impairment and mortality. Patients with severe comorbidities had a significantly higher hazard of all-cause mortality (adjusted HR: 2.35, 95% CI: 1.16-4.75, P=0.017) than did those without comorbidities. With regard to BCSM, there was no significant difference between patients with severe comorbidities and those with no comorbidity after adjustment (adjusted HR: 1.94, 95% CI:0.71-5.27, P=0.196). This was also observed in the analysis of psychological state. Relative to normal patients, patients with psychological abnormalities had an adjusted HR of 2.82 (95% CI: 1.45-5.50, P=0.002) for all-cause mortality. For BCSM, there was no significant difference between normal patients and patients with psychological abnormalities (adjusted HR: 1.56, 95% CI: 0.45-5.37, P=0.480).
Figure 2 shows the OS and BCSS curves for each GA domain after adjusting for age, tumour stage, LN stage, and intrinsic molecular subtype. OS was significantly higher for patients with an inferior geriatric status. In BCSS analysis, there was no significant relationship with the GA domains.
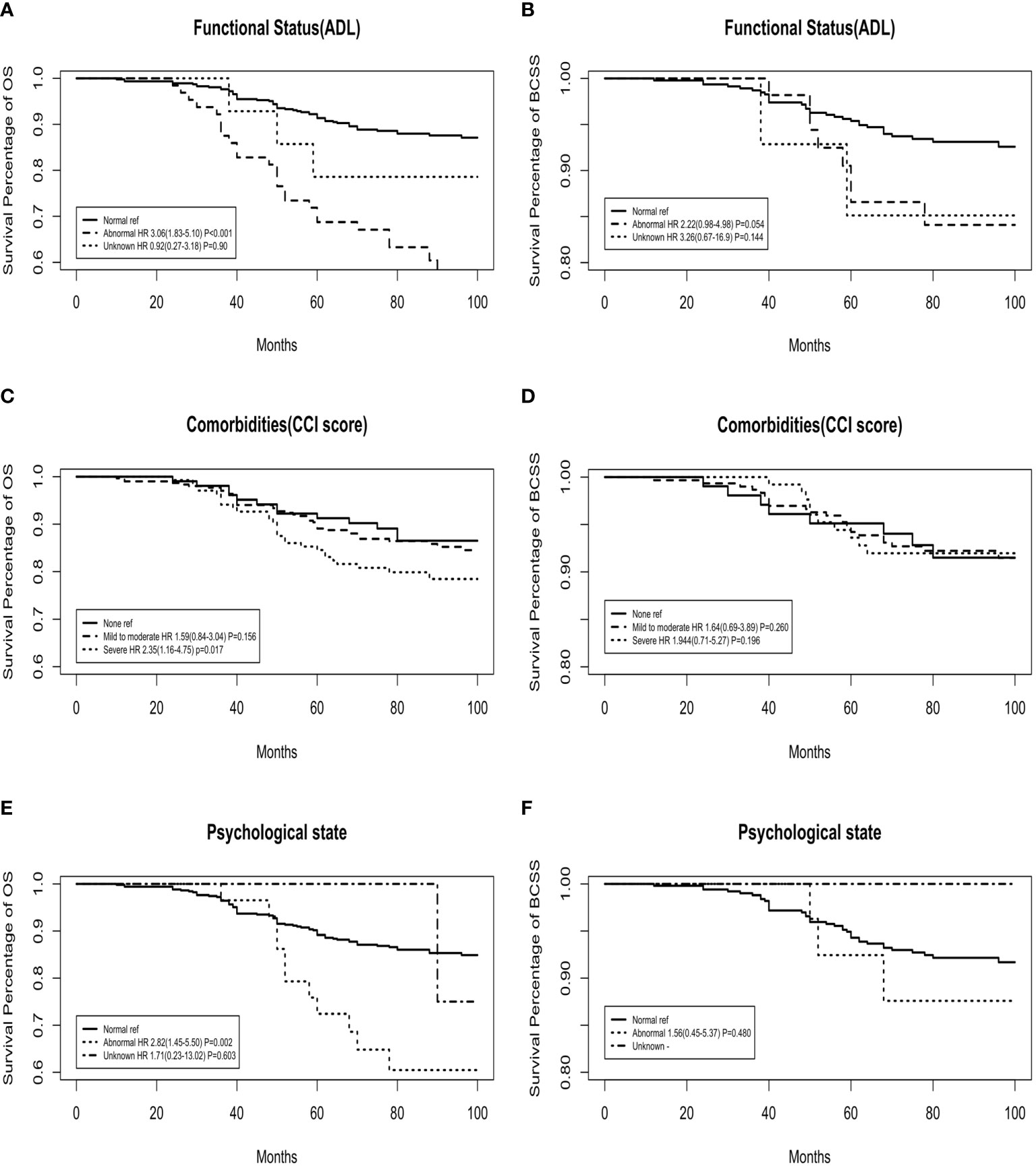
Figure 2 Adjusted overall survival (OS) and breast cancer-specific survival (BCSS) curves for functional status, comorbidities, and psychological state domains of the geriatric assessment. (A, C, E) OS curves; (B, D, F) BCSS curves.
The OS rate was significantly lower in the abnormal function group than in the normal function group (adjusted HR: 3.06, 95% CI: 1.83‐5.10, P<0.001), while the BCSS analysis showed no significant interaction between these two groups. The comorbidity model yielded results that were similar to those of the functional model. The OS rate for patients with none‐to‐moderate comorbidities was higher than that for patients with severe comorbidities (for severe comorbidities, adjusted HR: 2.35, 95% CI: 1.16‐4.75, P=0.017). Similarly, there was a significant difference in HR according to the psychological state. All-cause mortality was significantly higher in patients with an abnormal psychological state (adjusted HR: 2.82, 95% CI: 1.45‐5.50, P=0.002). However, BCSS analysis showed no significant interaction between these two groups (adjusted HR: 1.56, 95% CI: 0.45‐5.37, P=0.480).
3.3 Survival data for tumour domains
The results of mortality analyses for patients stratified by tumour stage, LN stage, and intrinsic molecular subtype are shown in Table 3.
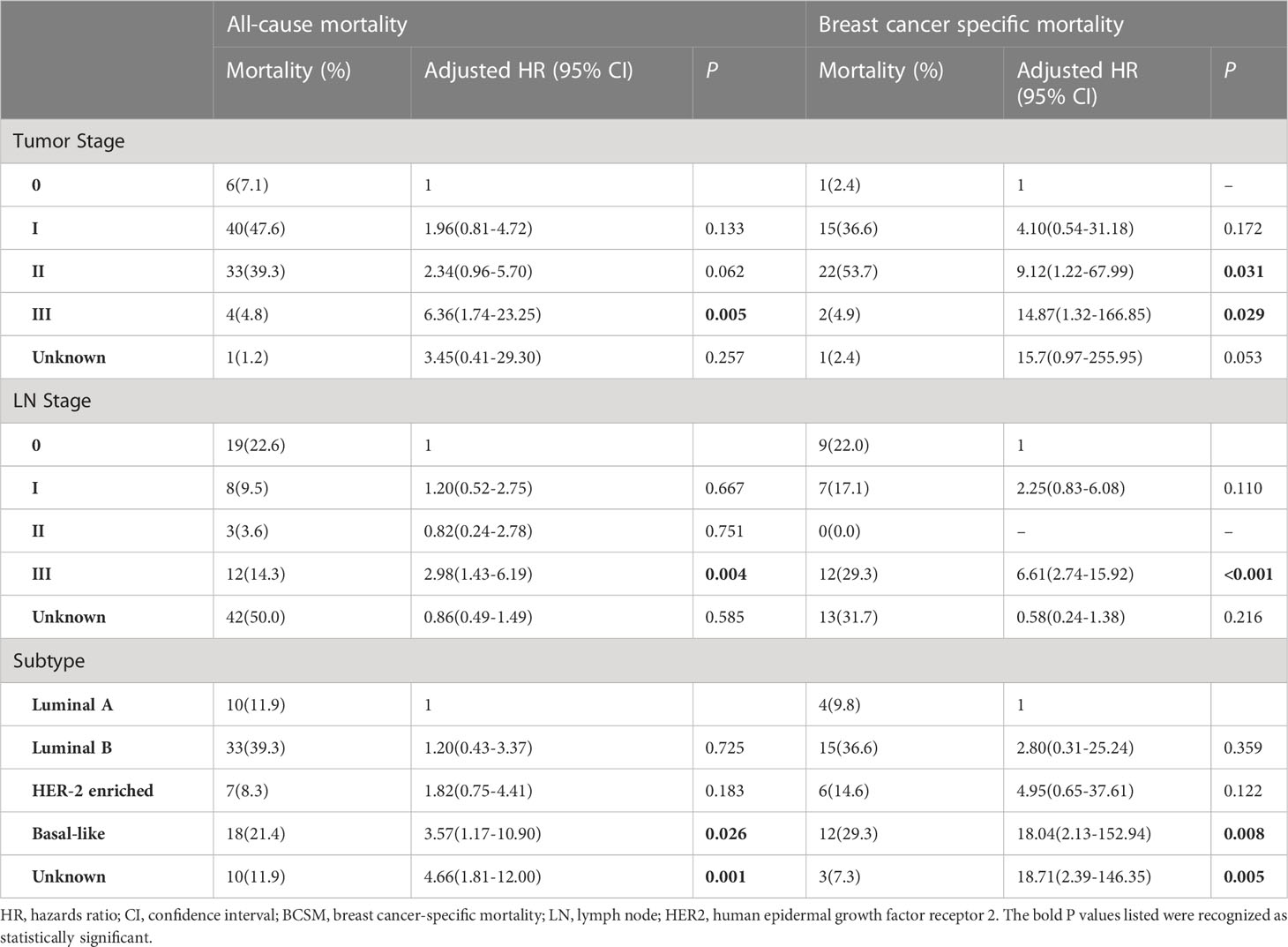
Table 3 Adjusted HRs for all-cause mortality and BCSM according to tumour stage, LN stage, and subtype.
After adjustment for ADL, CCI, and the psychological state, stratifications of these three tumour domains markedly affected the results for both all-cause mortality and BCSM.
Specifically, compared with other tumour stages, stage III was significantly associated with higher all-cause mortality (adjusted HR: 6.36, 95% CI: 1.74‐23.25) and BCSM (adjusted HR: 1.51, 95% CI: 1.34‐1.71). Likewise, patients with stage 3 LNs showed a higher risk of all-cause mortality (adjusted HR: 2.98, 95% CI: 1.43‐6.19) and BCSM (adjusted HR: 6.61, 95% CI: 2.74‐15.92). With regard to the intrinsic molecular subtype, the basal-like subtype was significantly associated with higher all-cause mortality (adjusted HR: 3.57, 95% CI: 1.17‐10.90) and BCSM (adjusted HR: 18.04, 95% CI: 2.13‐152.94). However, the unknown group was associated with lower mortality.
3.4 Estimates for the prognostic model
The incremental prognostic value of the three GA domains in our statistical models was analysed by comparing the C-statistics of the different models for all-cause mortality and BCSM (Table 4). The basic model included the baseline variables of age at diagnosis, tumour stage, LN stage, and intrinsic molecular subtype. The full model included functional status, comorbidities, and psychological status in addition to the baseline variables.
Compared with the basic model, models that added any of the three GA domains showed higher C‐statistics for all-cause mortality and BCSM. Among the three GA domains, functional status facilitated the largest increase in model performance. The full model with all three GA domains yielded the highest C‐statistics for both all-cause mortality and BCSM.
4 Discussion
For older patients with breast cancer, oncologists have to determine the eligibility of patients to receive treatment according to the general guidelines; furthermore, they must identify patients who require surveillance because of poor tolerance. Previous studies have demonstrated that age alone cannot determine treatment strategies for older patients with cancer (21), and the implementation of comprehensive GA involving numerous domains has been recommended by EUSOMA and SIOG (7). However, comprehensive GA is time-consuming, and not all domains have been validated in terms of breast cancer treatment (22). To our knowledge, this is the first study in China to focus on the prognostic value of functional status, comorbidities, and psychological state for survival analyses of older patients with breast cancer. Our findings support the utility of these GA domains for achieving improved prognostic accuracy and making informed treatment decisions for older patients with breast cancer.
In the present study, functional status, comorbidities, and psychological state were independently associated with the OS rate after adjustment for clinical variables commonly used by oncologists in the risk assessment of BCSM (age, tumour stage, LN stage, and intrinsic molecular subtype).
The Eastern Cooperative Oncology Group Performance Status (ECOG PS) is the most widely used functional assessment tool in oncology. However, several studies have demonstrated that ECOG PS may underestimate the degree of functional impairment, particularly in older patients with cancer (23). Therefore, ADL evaluation tools such as the Barthel Index are recommended as a better alternative by EUSOMA and SIOG (24). In the present study, we found that ADL impairment at the time of cancer diagnosis was associated with poorer OS; this was consistent with the findings of previous clinical trials (22, 25). According to our findings, there was no significant association between the ADL score and BCSS. In addition, we determined an association between ADL impairment and poorer OS, which can be explained by the increased risk of mortality associated with their general health status, lower treatment feasibility, and increased treatment-related adverse reactions.
In addition, the prognostic impact of comorbidities was observed in this study. While the presence of comorbidities was associated with a poorer OS rate, it was not associated with the BCSS rate, probably because of the increased risk of mortality from concurrent diseases, which can be considered to have a direct effect on OS. The coexistence of breast cancer with another disease affects the treatment of both conditions; this is also an important reason for the lower OS rate (26). Our findings support the belief that comorbidities are an essential component of GA, as observed in other relevant studies (27–29).
Psychological state is another important factor in terms of the prognosis and treatment decision for breast cancer, particularly that in elderly patients (30–32). Our finding of a significant association between an impaired psychological state at cancer diagnosis and poorer OS is concordant with the findings in previous studies (31–33). As observed with the other two GA domains, this association was observed only with OS, not BCSS. A possible explanation for this finding is that the psychological state affects overall immune function in an individual; therefore, it is significantly related to OS, which is associated with all concurrent diseases (34, 35).
In addition to focusing on the above GA domains, the present study also demonstrated the prognostic impact of tumour-related domains in older patients with breast cancer. Several clinical studies have confirmed that tumour-related factors such as tumour stage, LN stage, and intrinsic molecular subtype are significant independent factors for the prognosis of elderly patients with breast cancer (24, 36, 37). The present study yielded consistent results for both OS and BCSS.
Furthermore, we evaluated the prognostic value of a novel competing risk approach including the three GA domain and tumour-related variables and showed the incremental prognostic value of functional status, comorbidities, and psychological status by adding them to a basic statistical model including age, tumour stage, LN stage, and intrinsic molecular subtype as baseline variables. The full model with all three GA domains significantly increased the prediction ability of the basic statistical model; this indicates that comprehensive consideration of tumour factors and GA domains is valuable for predicting the prognosis and deciding the treatment of elderly patients with breast cancer.
4.1 Limitations
This study has several limitations. First, we extracted clinical information from a data source at a single cancer centre, which was unable to provide extensive and universal clinical information. Therefore, our study population may not be representative of the general population. Second, the psychological state at cancer diagnosis may be an inaccurate indicator because it is a subjective domain, particularly for elderly patients with cancer. However, this is a common problem in clinical psychological evaluation. Third, because of the lack of previous research on the utility of GA for elderly patients with breast cancer, we selected the three GA domains based on data for other cancer types. Accordingly, selection bias could not be avoided because we were unable to examine other GA domains. Fourth, we simplified the grades for ADL and CCI to adapt to the clinical application of surgery; thus, the indicators may not be sufficiently precise. However, implementation of the original form of GA would be too cumbersome and not conducive to clinical implementation. Future studies are warranted for the development of an algorithm with improved accuracy for convenient clinical applications. Fifth, the present study only focused on the relationship between GA domains and prognosis, and the applicability and effectiveness of GA domains in terms of treatment options remain unclear and will be the scope of our future research.
5 Conclusion
In the present study, we validated the association between three specific GA domains and OS in older patients with breast cancer and found that addition of functional status, comorbidities, and psychological state to a basic model including tumour-relevant variables was valuable and useful for comprehensive assessments to predict long-term survival in older patients with breast cancer. Although further studies are needed to verify the contribution of these GA domains to the treatment decision-making process, this predictive model should be considered when discussing the risks and benefits of clinical intervention for older patients with breast cancer.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YL and YX: conceptualisation, methodology, formal analysis, writing, editing, and review. YS: conceptualisation, methodology, investigation, data curation, and writing. YLX: methodology, investigation, and resources. CJW: investigation, resources, and editing. XZ: methodology and review. XH: methodology and review. QS: conceptualisation, methodology, review, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2021-I2M-1-014).
Acknowledgments
We would like to thank Eidtage (Editage.com) for the language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pilleron S, Alqurini N, Ferlay J, Haase KR, Hannan M, Janssen-Heijnen M, et al. International trends in cancer incidence in middle-aged and older adults in 44 countries. J Geriatr Oncol (2022) 13(3):346–55. doi: 10.1016/j.jgo.2021.11.011
2. DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin (2014) 64(1):52–62. doi: 10.3322/caac.21203
3. Abdel-Razeq H, Iweir S, Abdel-Razeq R, Rahman FA, Almasri H, Bater R, et al. Differences in clinicopathological characteristics, treatment, and survival outcomes between older and younger breast cancer patients. Sci Rep (2021) 11(1):14340. doi: 10.1200/JCO.2021.39.15_SUPPL.E18604
4. Nightingale G, Schwartz R, Kachur E, Dixon BN, Cote C, Barlow A, et al. Clinical pharmacology of oncology agents in older adults: A comprehensive review of how chronologic and functional age can influence treatment-related effects. J Geriatr Oncol (2019) 10(1):4–30. doi: 10.1016/j.jgo.2018.06.008
5. Giri S, Al-Obaidi M, Weaver A, Kenzik KM, McDonald A, Clark D, et al. Association between chronologic age and geriatric assessment-identified impairments: Findings from the CARE registry. J Natl Compr Canc Netw (2021) 19(8):922–7. doi: 10.6004/jnccn.2020.7679
6. Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol (2014) 32(24):2595–603. doi: 10.1200/JCO.2013.54.8347
7. Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, et al. Management of elderly patients with breast cancer: updated recommendations of the international society of geriatric oncology (SIOG) and European society of breast cancer specialists (EUSOMA). Lancet Oncol (2012) 13(4):e148–60. doi: 10.1016/S1470-2045(11)70383-7
8. Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol (2018) 36(22):2326–47. doi: 10.1200/JCO.2018.78.8687
9. Soto-Perez-de-Celis E, Li D, Yuan Y, Lau YM, Hurria A. Functional versus chronological age: Geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol (2018) 19(6):e305–16. doi: 10.1016/S1470-2045(18)30348-6
10. Caillet P, Laurent M, Bastuji-Garin S, Liuu E, Culine S, Lagrange JL, et al. Optimal management of elderly cancer patients: usefulness of the comprehensive geriatric assessment. Clin Interv Aging (2014) 9:1645–60. doi: 10.2147/CIA.S57849
11. Corre R, Greillier L, Le Caer H, Audigier-Valette C, Baize N, Berard H, et al. Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non-Small-Cell lung cancer: The phase III randomized ESOGIA-GFPC-GECP 08-02 study. J Clin Oncol (2016) 34(13):1476–83. doi: 10.1200/JCO.2015.63.5839
12. Magnuson A, Sattar S, Nightingale G, Saracino R, Skonecki E, Trevino KM. A practical guide to geriatric syndromes in older adults with cancer: A focus on falls, cognition, polypharmacy, and depression. Am Soc Clin Oncol Educ Book (2019) 39:e96–e109. doi: 10.1200/EDBK_237641
13. Morris EP, Zaheed AB, Sharifian N, Sol K, Kraal AZ. Subjective age, depressive symptoms, and cognitive functioning across five domains. J Clin Exp Neuropsychol (2021) 43(3):310–23. doi: 10.1080/13803395.2021.1926436
14. Wang DXM, Yao J, Zirek Y, Reijnierse EM, Maier AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle (2020) 11(1):3–25. doi: 10.1002/jcsm.12502
15. Williams GR, Deal AM, Lund JL, Chang Y, Muss HB, Pergolotti M, et al. Patient-reported comorbidity and survival in older adults with cancer. Oncologist (2018) 23(4):433–9. doi: 10.1634/theoncologist.2017-0404
16. Adeyemi OJ, Gill TL, Paul R, Huber LB. Evaluating the association of self-reported psychological distress and self-rated health on survival times among women with breast cancer in the U.S. PloS One (2021) 16(12):e0260481. doi: 10.1371/journal.pone.0260481
17. Pashmdarfard M, Azad A. Assessment tools to evaluate activities of daily living (ADL) and instrumental activities of daily living (IADL) in older adults: A systematic review. Med J Islam Repub Iran (2020) 34:33. doi: 10.47176/mjiri.34.33
18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis (1987) 40(5):373–83. doi: 10.1016/0021-9681(87)90171-8
19. Dias F, Teixeira AL, Guimaraes HC, Barbosa MT, Resende EPF, Beato RG, et al. Accuracy of the 15-item geriatric depression scale (GDS-15) in a community-dwelling oldest-old sample: the pieta study. Trends Psychiatry Psychother (2017) 39(4):276–9. doi: 10.1590/2237-6089-2017-0046
20. Mitchell AJ, Bird V, Rizzo M, Meader N. Diagnostic validity and added value of the geriatric depression scale for depression in primary care: A meta-analysis of GDS30 and GDS15. J Affect Disord (2010) 125(1-3):10–7. doi: 10.1016/j.jad.2009.08.019
21. Barthelemy P, Heitz D, Mathelin C, Polesi H, Asmane I, Litique V, et al. Adjuvant chemotherapy in elderly patients with early breast cancer. impact of age and comprehensive geriatric assessment on tumor board proposals. Crit Rev Oncol Hematol (2011) 79(2):196–204. doi: 10.1016/j.critrevonc.2010.06.005
22. Sourdet S, Brechemier D, Steinmeyer Z, Gerard S, Balardy L. Impact of the comprehensive geriatric assessment on treatment decision in geriatric oncology. BMC Cancer (2020) 20(1):384. doi: 10.1186/s12885-020-06878-2
23. Liu H, Gao M, Mei D, Han HX, Li JT, Bai JF, et al. A comparative study of comprehensive geriatric assessment in elder patients with non-hodgkin's lymphoma. Zhonghua Nei Ke Za Zhi (2018) 57(5):330–4. doi: 10.3760/cma.j.issn.0578-1426.2018.05.005
24. Biganzoli L, Battisti NML, Wildiers H, McCartney A, Colloca G, Kunkler IH, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European society of breast cancer specialists (EUSOMA) and the international society of geriatric oncology (SIOG). Lancet Oncol (2021) 22(7):e327–40. doi: 10.1016/S1470-2045(20)30741-5
25. Clough-Gorr KM, Thwin SS, Stuck AE, Silliman RA. Examining five- and ten-year survival in older women with breast cancer using cancer-specific geriatric assessment. Eur J Cancer (2012) 48(6):805–12. doi: 10.1016/j.ejca.2011.06.016
26. de Glas NA, Kiderlen M, Bastiaannet E, de Craen AJ, van de Water W, van de Velde CJ, et al. Postoperative complications and survival of elderly breast cancer patients: A FOCUS study analysis. Breast Cancer Res Treat (2013) 138(2):561–9. doi: 10.1007/s10549-013-2462-9
27. Okonji DO, Sinha R, Phillips I, Fatz D, Ring A. Comprehensive geriatric assessment in 326 older women with early breast cancer. Br J Cancer (2017) 117(7):925–31. doi: 10.1038/bjc.2017.257
28. Munir A, Huws A, Khan S, Sharaiha Y, Holt S, Khawaja S. Geriatric assessment tool application in treatment recommendations for older women with breast cancer. Breast (2022) 63:101–7. doi: 10.1016/j.breast.2022.03.012
29. Jorgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer (2012) 106(7):1353–60. doi: 10.1038/bjc.2012.46
30. Stanton AL, Bower JE. Psychological adjustment in breast cancer survivors. Adv Exp Med Biol (2015) 862:231–42. doi: 10.1007/978-3-319-16366-6_15
31. Pai T, Cornell L, Seneviratne D, Niazi S, Mussallem D, Vallow L. Pre-diagnosis major life stressors and breast cancer outcomes. Breast Cancer Res Treat (2021) 188(2):459–64. doi: 10.1007/s10549-021-06218-3
32. Buscariollo DL, Cronin AM, Borstelmann NA, Punglia RS. Impact of pre-diagnosis depressive symptoms and health-related quality of life on treatment choice for ductal carcinoma in situ and stage I breast cancer in older women. Breast Cancer Res Treat (2019) 173(3):709–17. doi: 10.1007/s10549-018-5006-5
33. Nilsen M, Stalsberg R, Sand K, Haugan G, Reidunsdatter RJ. Meaning making for psychological adjustment and quality of life in older long-term breast cancer survivors. Front Psychol (2021) 12:734198. doi: 10.3389/fpsyg.2021.734198
34. Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol (2004) 5(10):617–25. doi: 10.1016/S1470-2045(04)01597-9
35. Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer (2019) 125(9):1417–31. doi: 10.1002/cncr.31943
36. Alberts AS, Falkson G, van der Merwe R. Factors influencing prognosis in elderly patients with primary breast cancer. S Afr J Surg (1991) 29(1):8–10.
Keywords: breast cancer, older patients, geriatric analysis, survival rate, survival model
Citation: Lin Y, Xu Y, Wang C, Song Y, Xu Y, Zhang X, Huang X and Sun Q (2023) Geriatric assessment for older patients with breast cancer: A single-institution study. Front. Oncol. 13:1031682. doi: 10.3389/fonc.2023.1031682
Received: 30 August 2022; Accepted: 14 February 2023;
Published: 23 February 2023.
Edited by:
Atif Ali Hashmi, Liaquat National Medical College, PakistanReviewed by:
Xiaojiong Jia, Harvard Medical School, United StatesDavide Soldato, University of Genoa, Italy
Copyright © 2023 Lin, Xu, Wang, Song, Xu, Zhang, Huang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Sun, cWlhbmdzdW5wdW1jaEAxMjYuY29t
†These authors have contributed equally to this work
 Yan Lin†
Yan Lin† Changjun Wang
Changjun Wang Yu Song
Yu Song Yali Xu
Yali Xu Xiaohui Zhang
Xiaohui Zhang Qiang Sun
Qiang Sun