- 1Gastrointestinal Department, Chongqing University Cancer Hospital, Chongqing, China
- 2Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing, China
Background: The advanced lung cancer inflammation index (ALI) has been identified as a scientific and clinical priority in multiple malignancies. The aim of this study is to investigate the value of the ALI before treatment in evaluating postoperative complications (POCs) and survival outcomes in patients with gastrointestinal (GI) cancer.
Methods: Electronic databases including PubMed, Embase and Web of Science were comprehensively reviewed up to June 2022. The endpoints were POCs and survival outcomes. Subgroup analyses and sensitivity analyses were also performed.
Results: Eleven studies including 4417 participants were included. A significant heterogeneity in the ALI cut-off value among studies was observed. Patients in the low ALI group showed increased incidence of POCs (OR=2.02; 95%CI:1.60-2.57; P<0.001; I2 = 0%). In addition, a low ALI was also significantly associated with worse overall survival (HR=1.96; 95%CI: 1.58-2.43; P<0.001; I2 = 64%), which remained consistent in all subgroups based on country, sample size, tumor site, tumor stage, selection method and Newcastle Ottawa Scale score. Moreover, patients in the low ALI group had an obviously decreased disease-free survival compared to these in the high ALI group (HR=1.47; 95%CI: 1.28-1.68; P<0.001; I2 = 0%).
Conclusion: Based on existing evidence, the ALI could act as a valuable predictor of POCs and long-term outcomes in patients with GI cancer. However, the heterogeneity in the ALI cut-off value among studies should be considered when interpreting these findings.
1 Introduction
Gastrointestinal (GI) cancers are one of the most common malignancies worldwide, accounting for about 25% of newly diagnosed cancer cases and more than 35% of cancer-related deaths (1). Although significant advances in surgery-based multimodal therapy for gastrointestinal cancers, the clinical efficacy of most of these patients is still poor (2, 3). Consequently, it is essential to develop a useful index to predict the short-term and long-term therapeutic outcomes in GI cancers.
Increasing evidence indicates that cancer-related inflammation and malnutrition status are prevalent in most patients with malignancy, which play an important role in postoperative recovery and prognosis (4, 5). Therefore, inflammation/nutrition-based biomarkers are expected to be valuable predictors of surgical and long-term outcomes. For example, preoperative neutrophil-to-lymphocyte ratio (NLR), based on two blood inflammatory factors, has been reported as a strong indicator for increased postoperative complications (POCs), prolongation of hospital stays and poor survival outcomes in breast cancer (6), lung cancer (7) and GI malignancies (8, 9). Meanwhile, reduced body mass index (BMI) and serum albumin (ALB), which reflect the nutritional status, have also been proven to be associated with adverse therapeutic outcomes in multiple cancers (10–12).
In recent years, a novel biomarker, the advanced lung cancer inflammation index (ALI), which integrates BMI, ALB and NLR (BMI *ALB/NLR), has been reported as a more promising predictor of surgical and long-term outcomes in cancers, because it incorporates multiple nutritional and inflammatory indicators (13, 14). A previous meta-analysis reported that low ALI before surgery indicates poor prognosis in Lung cancer patients (15). Another meta-analysis focusing on the relationship between the ALI and survival outcomes also found a similar conclusion in cancer patients (16). Nevertheless, the role of the ALI in POCs and survival outcomes of GI cancers remains inconclusive and no meta-analysis is available so far. In addition, a number of other studies on the ALI and therapeutic outcomes in GI cancers have been addressed in recent years.
Herein, we performed a systematic review and meta-analysis based on existing evidence to investigate the value of the ALI in POCs and long-term results in GI cancers.
2 Methods
The present study was conducted according to the requirements from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify studies that assess the association of pretreatment ALI with POCs and survival outcomes in GI cancer patients.
2.1 Search strategy
Relevant studies from PubMed, Embase and Web of Science were comprehensively examined up to June 30, 2022. Published language was not restricted during the search process. The following combination of key words in title/abstract were used to identify potential studies: [“advanced lung cancer inflammation index” OR “ALI”] AND [“cancer” OR “carcinoma” OR “neoplasm” OR “tumor”] AND [“survival” OR “prognostic” OR “prognosis” OR “mortality” OR “postoperative complications” OR “morbidity”]. In addition, the references of the included studies were scanned for potentially related reports. The search was independently performed by two investigators (HY-P and XF-C).
2.2 Inclusion and exclusion criteria
Studies that met the following criteria were included: (1) studies examined the relationship between the pretreatment ALI and POCs or long-term survival of patients with GI cancer; (2) Hazard ratio (HR) with 95% confidence interval (CI) was available or could be calculated based on survival curve; (3) The cutoff value of the ALI was clearly reported.
The exclusion criteria were studies (1) reported as case reports, reviews, letters and conferences; (2) with overlapping data.
2.3 Data extraction and quality assessment
Two reviewers (HY-P and XF-C) conducted the data extraction independently and cross-checked all the results. The extracted data included first author, publication year, study interval, country, study design and sample size, selection method, cut-off value, clinicopathological features like age, sex, tumor site and tumor stage, POCs and survival data.
The Newcastle Ottawa Scale (NOS) (17) was used to assess the quality of included studies and a study with NOS score >6 is regarded as of high quality.
2.4 Outcomes
In the present study, the primary outcomes were to investigate the relationship between the ALI and POCs or long-term results in patients with GI malignancy. POCs were defined as any morbidity classified as Clavien–Dindo (18) grade I or higher. Long-term outcomes included OS (from the date of surgery to the date of any cause of death) and DFS (from the date of surgery to the date of recurrence or the date of death from any cause).
Of note, since progression-free survival (PFS: from the date of surgery to the date of recurrence or any cause of death), recurrence-free survival (RFS: from the date of surgery to the date of recurrence), cancer-specific survival (CSS: from the date of surgery to the date of cancer-related death) and DFS share the similar endpoints, they were analyzed together as one outcome, DFS (19, 20).
2.5 Statistical analysis
The hazard ratios (HRs) and odds ratios (ORs) with their 95% confidence intervals (CIs) were used as the effect size for time-dependent outcomes and dichotomous variables, respectively. For studies that HR with 95%CI was not reported, the indirect data were calculated following the methods reported by Tierney et al. (21) Statistical heterogeneity among studies was assessed using I2 statistic. All pooled analyses were performed assuming the random-effects model, which accounts for variance across included studies. Subgroup analysis was used to explore the source of heterogeneity. Sensitivity analysis was conducted to evaluate the credibility of results. Publication bias was evaluated using Begg’s funnel plot. A two-tailed P value <0.05 was considered statistically significant. All of these statistical analyses were performed by Review Manager Software, version 5.3 (Cochrane, London, UK) and Stata, version 12.0 (Statacorp, College Station, TX).
3 Results
3.1 Study characteristics
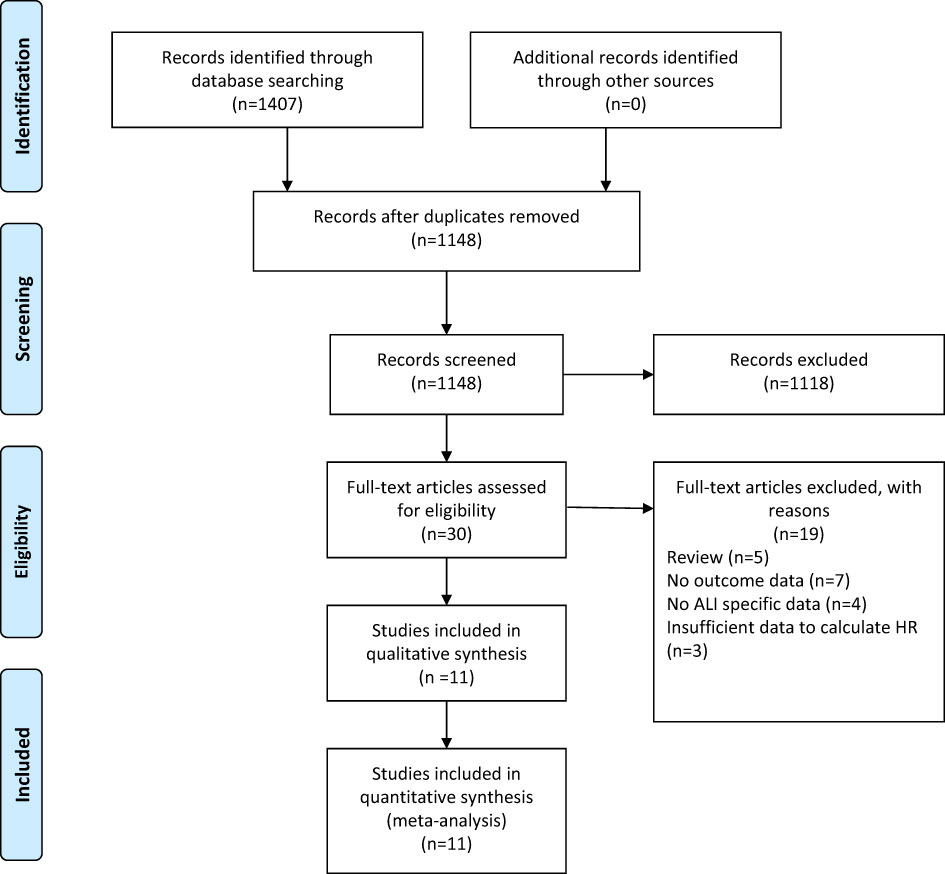
A flow chart of the selection process was shown in Figure 1. The search strategy yielded 1407 potential studies. After title, abstract assessment and full text assessment, 11 retrospective cohort studies (22–32) were finally included in the present study.
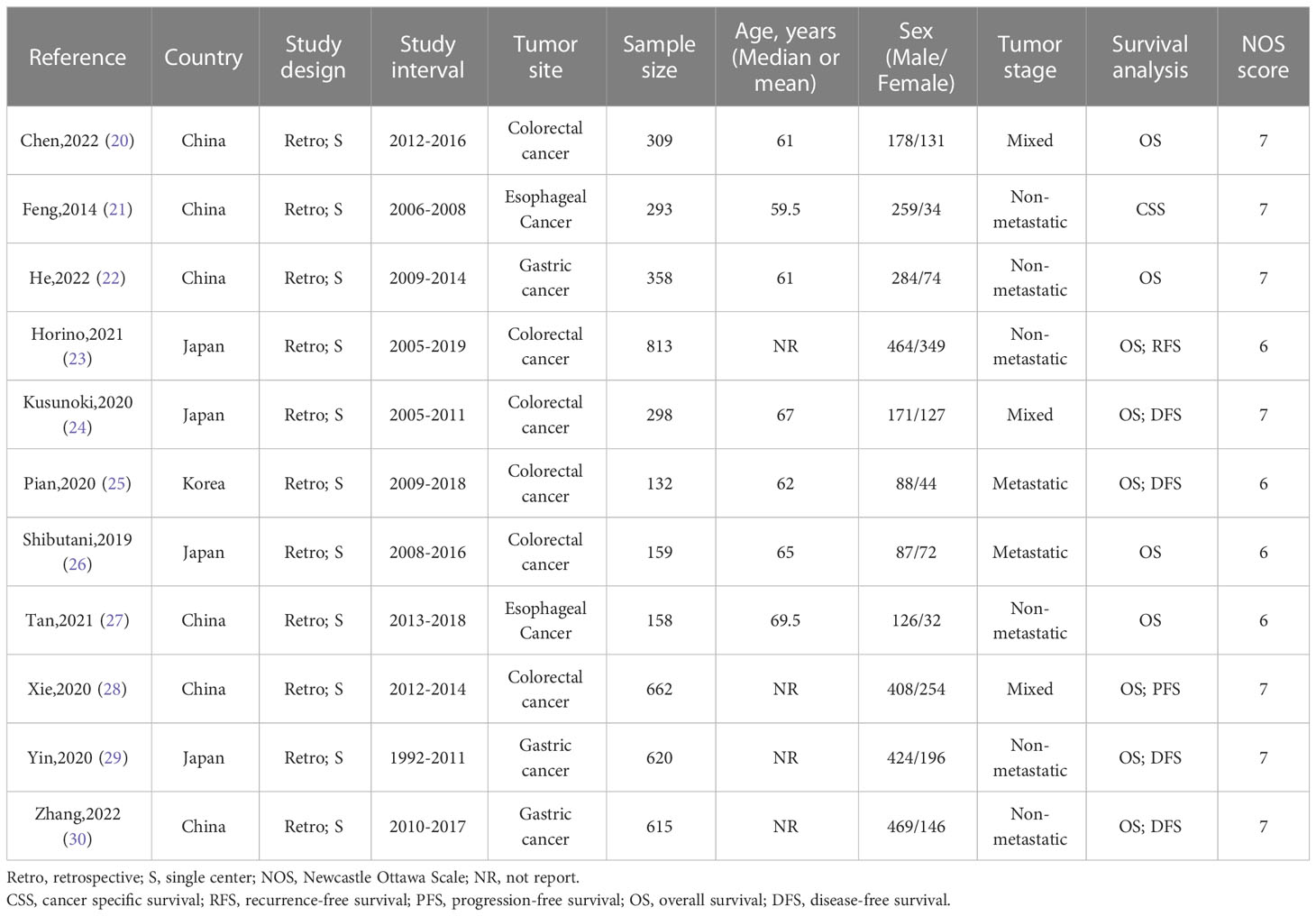
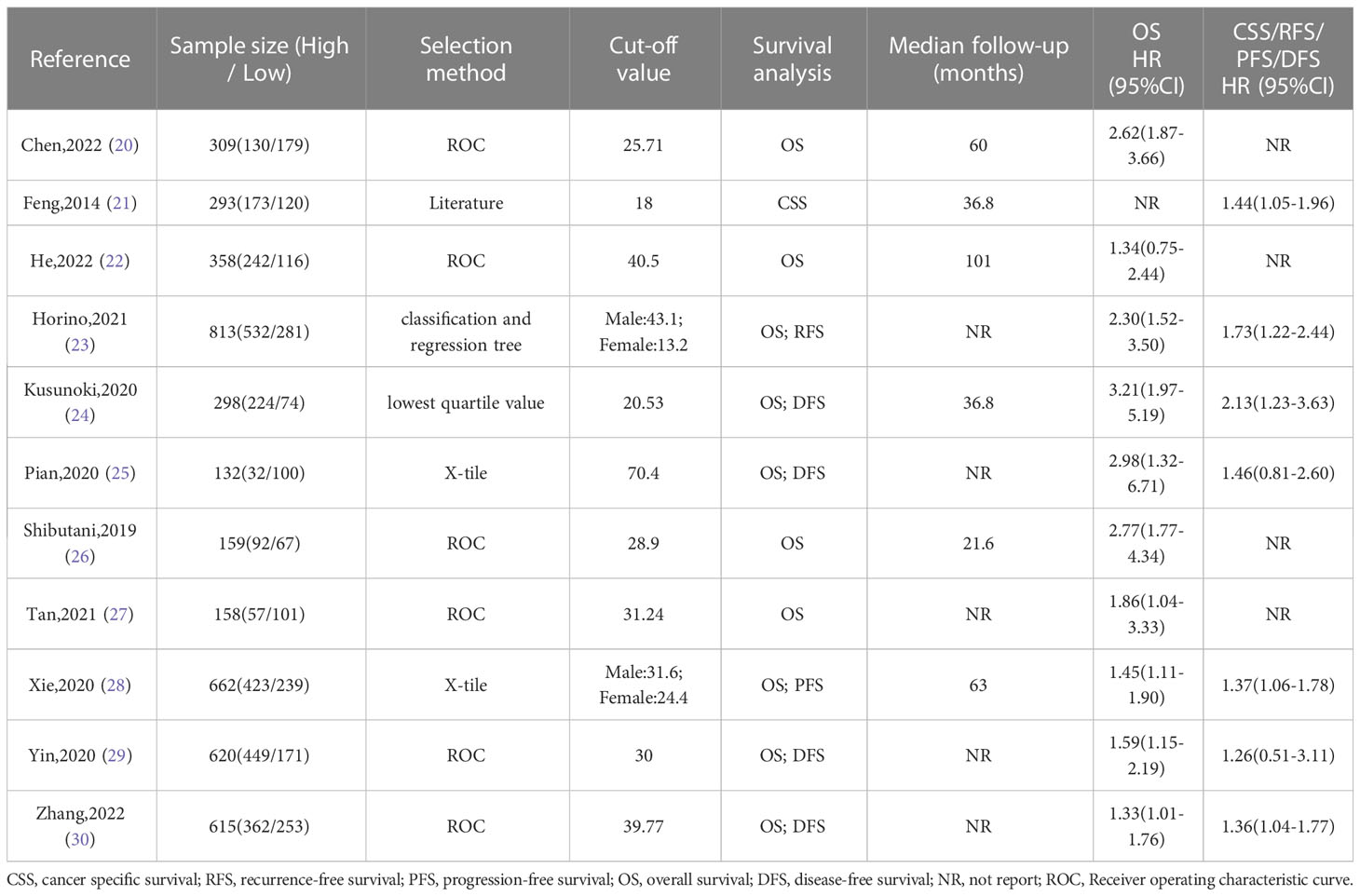
The baseline information of the included studies was shown in Tables 1, 2. A total of 4417 patients from China, Korea and Japan were included in this study. These studies were published from 2014 to 2022 with a sample size ranging from 132 to 813. Among these studies, two studies reported esophageal cancer, three studies reported gastric cancer, and six studies reported colorectal cancer. In addition, three studies reported overall POCs, ten studies reported OS, and seven studies reported DFS. Moreover, the cut-off value of the ALI varies a lot among these studies. The details of quality assessment of the included studies were shown in the supplementary file (Table S1), and the scores of these studies ranged from 6 to 7 after careful assessment with NOS.
3.2 Overall survival
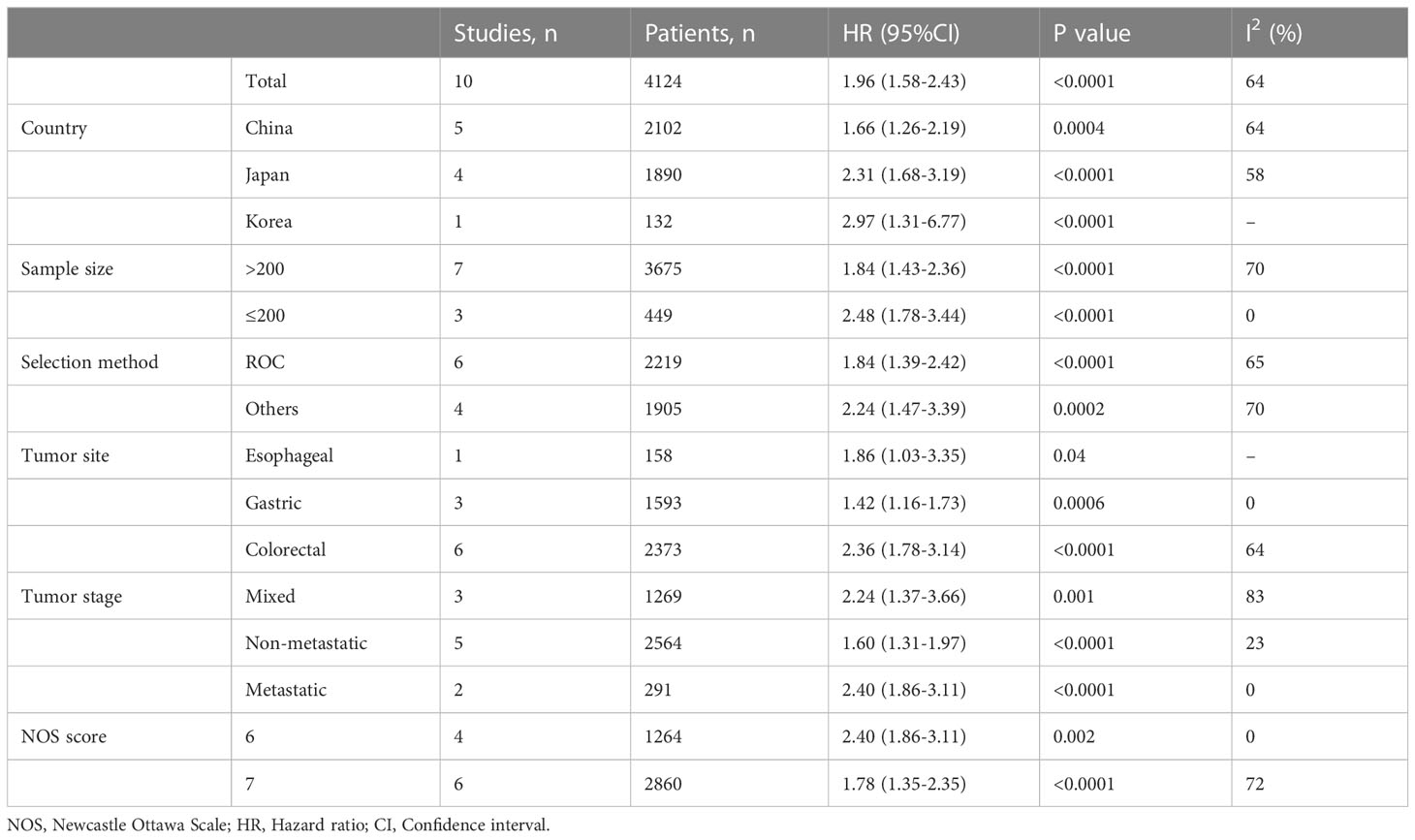
Ten studies involving 4124 patients (2543 in the high ALI group and 1581 in the low ALI group) reported the OS outcome. The pooled HR was 1.96 (95%CI: 1.58-2.43; P<0.001), which indicated that a low ALI was associated with decreased OS in patients with GI cancer (Figure 2A, Table 3). Given the presence of moderate heterogeneity (I2 = 64%; P=0.003), subgroup analyses based on country, sample size, tumor site, tumor stage, selection method and NOS score were performed. As shown in Table 3 and Figure S1, the pooled results of all subgroup analyses revealed that patients in the high ALI group had a significantly better OS when compared with these in the low ALI group. Additionally, sensitivity analysis by deleting one study at a time showed that the pooled outcome did not substantially change (Figure 2B).
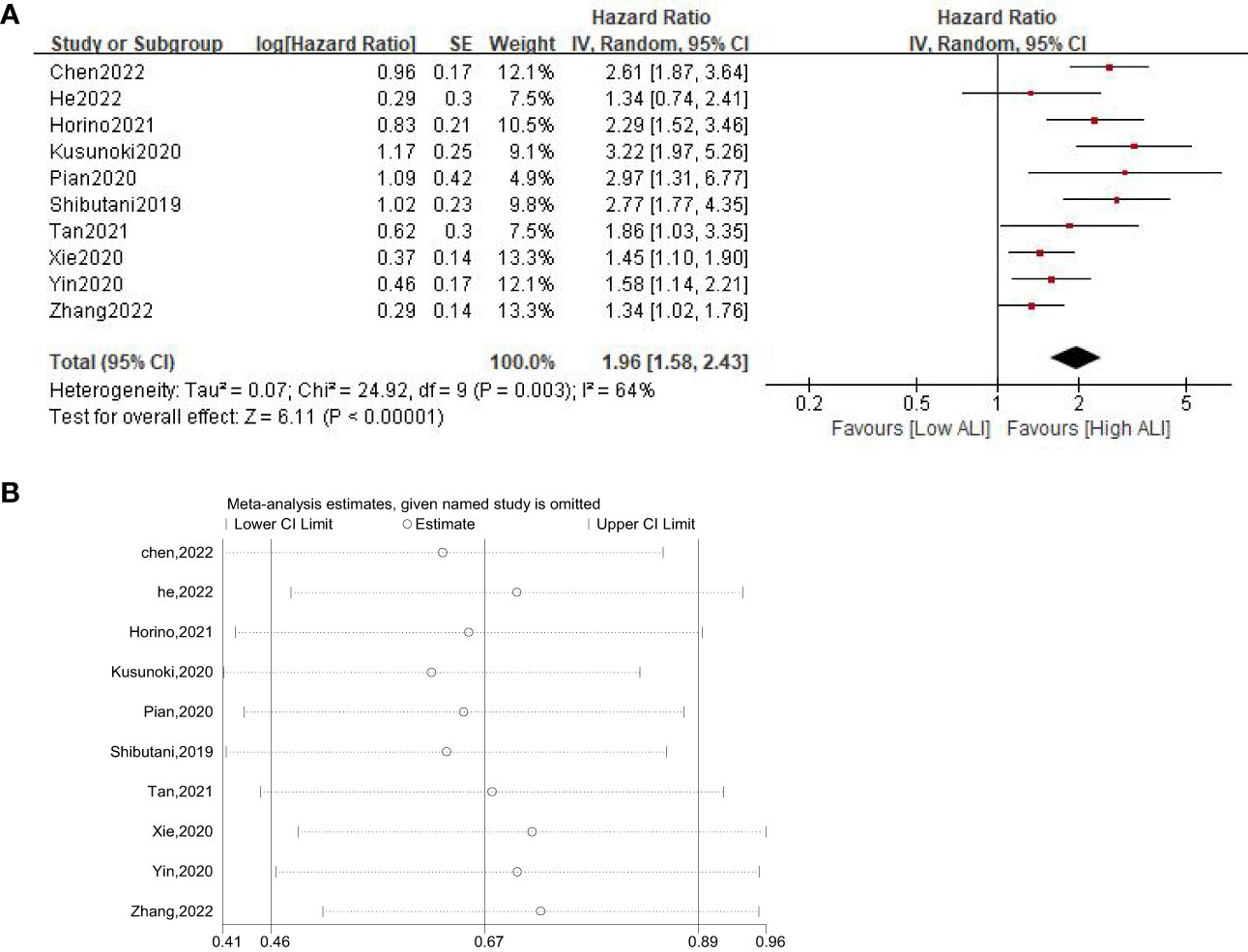
Figure 2 Forest plot (A) and sensitivity analysis (B) assessing the relationship between ALI and OS.
3.3 Disease-free survival
A total of seven studies involving 3433 patients (2195 in the high ALI group and 1238 in the low ALI group) reported on DFS. The pooled HR was 1.47 (95%CI: 1.28-1.68; P<0.001; I2 = 0%), which suggested that patients in the low ALI group had a worse DFS compared with patients in the high ALI group (Figure 3A). Sensitivity analysis showed that the combined effect was not significantly changed (Figure 3B).
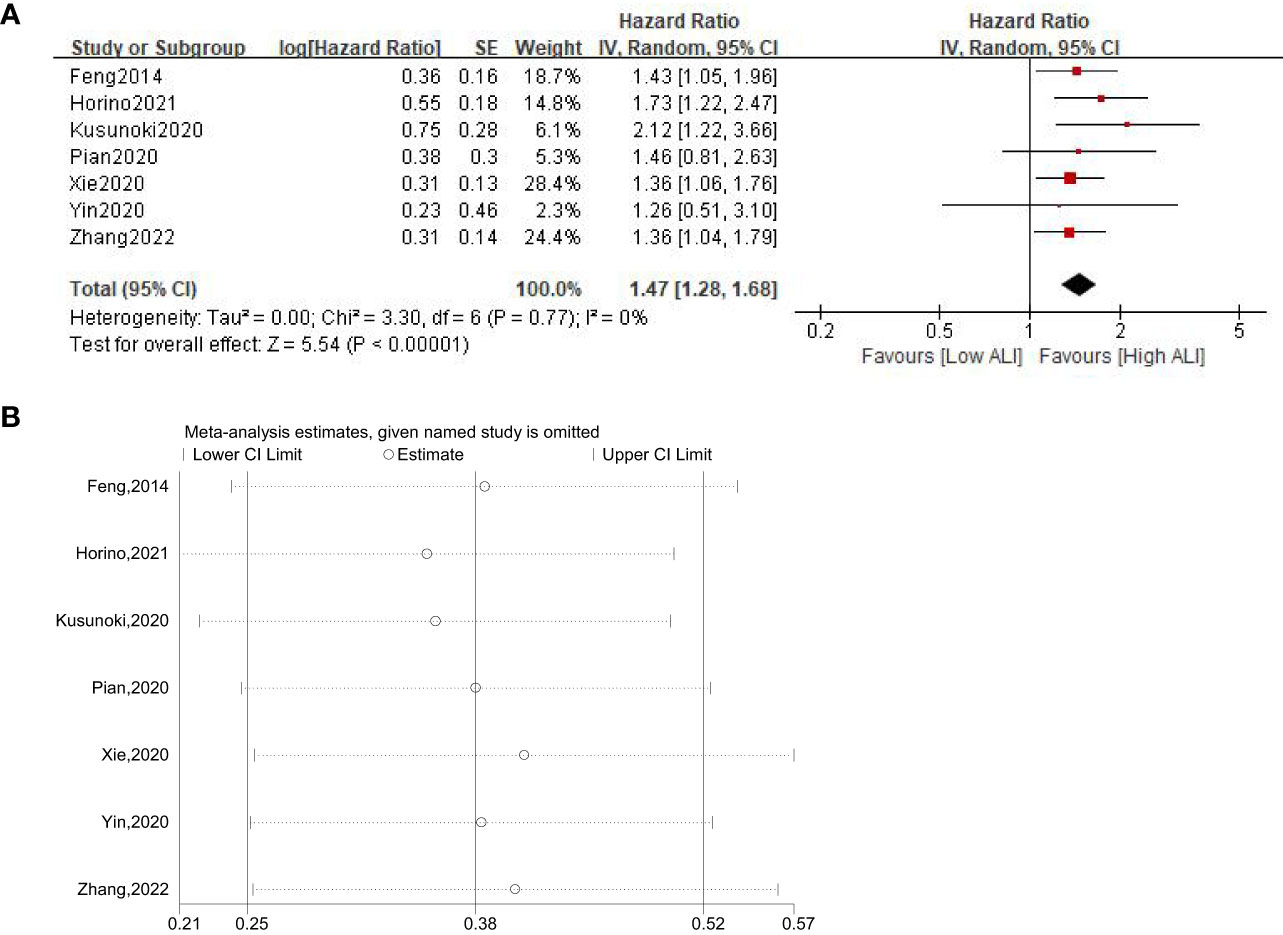
Figure 3 Forest plot (A) and sensitivity analysis (B) assessing the relationship between ALI and DFS.
3.4 Postoperative complications
Three studies involving 1607 patients (987 in the high ALI group and 620 in the low ALI group) reported this outcome. The pooled OR was 2.02 (95%CI: 1.60-2.57; P<0.001; I2 = 0%), which suggested that patients with a low ALI had a higher risk of POCs than those with a high ALI (Figure 4A). Similarly, sensitivity analysis did not show significant change for the pooled outcome (Figure 4B).
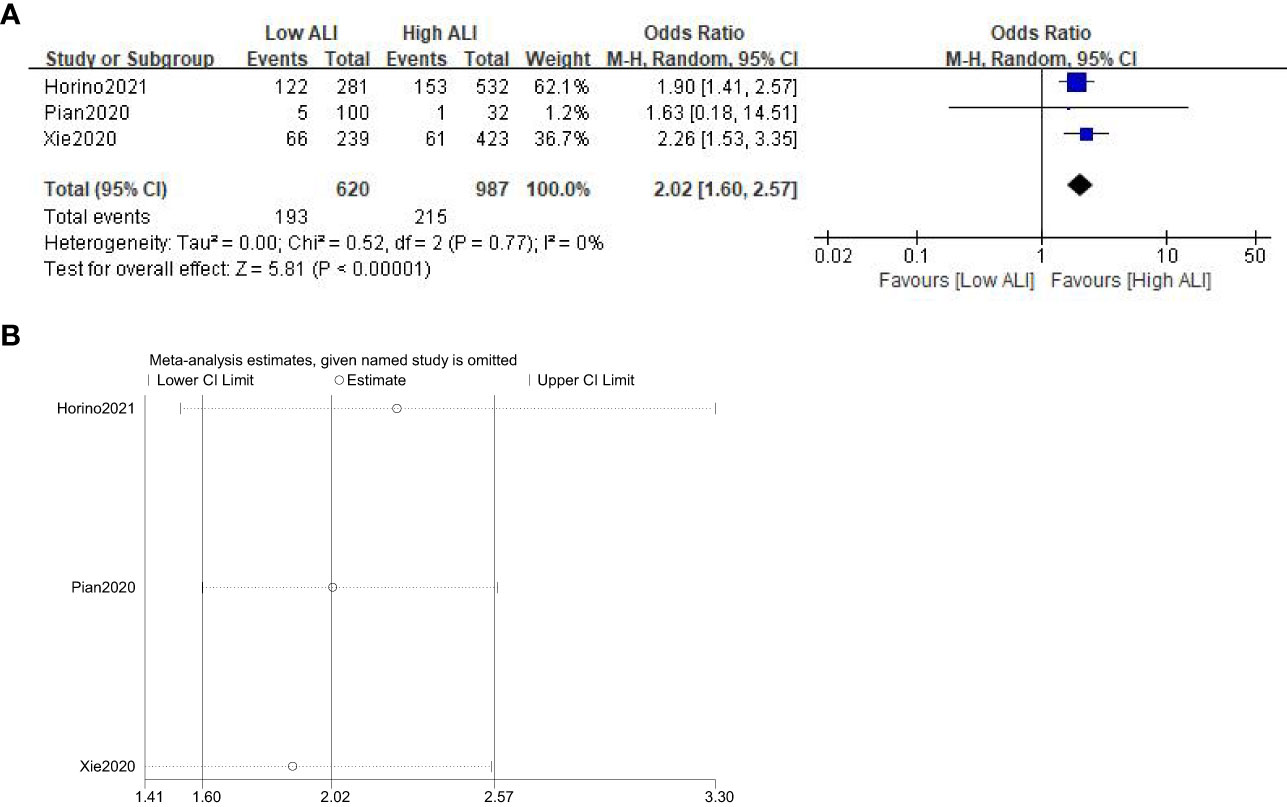
Figure 4 Forest plot (A) and sensitivity analysis (B) assessing the relationship between ALI and POCs.
3.5 Publication bias
The Begg’s funnel plot was performed to assess the potential publication bias. As shown in Figure 5, the funnel plots of OS, DFS and POCs were virtually symmetric, and the P values of Begg’s test were 0.239, 0.230, and 1.000, respectively, indicating that these pooled outcomes had a low risk of publication bias.
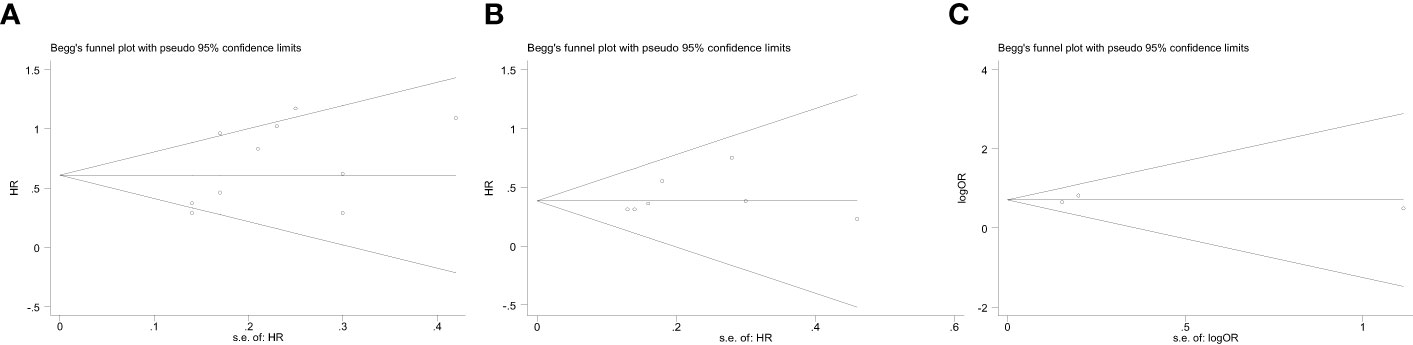
Figure 5 Begg’s funnel plot assessing publication bias between ALI and therapeutic outcomes, including (A) OS, (B) DFS, (C) POCs. The Begg’s P values were 0.239, 0.230, and 1.000, respectively.
4 Discussion
In 2013, Jafri et al. (13) first established the ALI based on commonly used clinical parameters as a systemic inflammation-related prognostic score tool for metastatic non-small cell lung cancer. Since then, the ALI has been widely used as a readily available and reliable biomarker to evaluate the prognosis of coronary artery disease (33), lung cancer (34) and pancreatic cancer (35). As shown in Table 1, most of included studies were published after 2019, indicating that the ALI is still a very new index in the field of GI cancer.
In this study, we included eleven studies with 4417 patients with GI cancer and found that a low ALI was significantly associated with decreased OS (HR=1.96; 95%CI: 1.58-2.43; P<0.001) and DFS (HR=1.47; 95%CI: 1.28-1.68; P<0.001). Meanwhile, we have further identified that the ALI could also act as a predictor for POCs (OR=2.02; 95%CI:1.60-2.57; P<0.001) in patients with GI cancer. Therefore, the ALI may have a good discriminatory value and remains an effective inflammatory/nutrition factor for predicting therapeutic outcomes in GI cancer.
Systemic inflammatory reflection (SIR) is recognized as the 7th hallmark of cancer which is closely associated with the occurrence and progression of malignancies (36). Consistent with this evidence, the SIR as a potential prognostic marker is also demonstrated for various malignancies, and the NLR is one of the well-established SIR markers (37, 38). Increased neutrophils in the tumor microenvironment have been reported to prompt the tumor growth and metastasis by releasing chemokines and cytokines (39). Besides, neutrophils can also inhibit the activation of T lymphocytes, thereby inhibiting the anti-tumor immunity of the host (39, 40). While lymphocytes, especially CD4+ T lymphocytes and CD8+ T lymphocytes, as the most important immune cells, play an anti-tumor role by inducing the lysis and apoptosis of tumor cells (41). Lymphopenia has been demonstrated to be associated with poor prognosis in cancer patients (42, 43). On the other hand, nutritional status is also reported as an important predictive factor of therapeutic outcomes in several types of malignancies (44, 45). Studies have proven that malnutrition leads to an inadequate anti-tumor immune response and reduces wound healing ability, thereby reducing treatment efficacy and leading to severe POCs (46, 47). As an objective and common measurement reflecting patients’ nutritional status, baseline BMI and ALB have been indicated to be positively associated with the short-term and long-term outcomes of cancer patients and have been used to triage patients in clinic care (48). Reasonably, the ALI, combined with these factors, is a useful comprehensive indicator of nutritional and inflammatory status, may enable better understanding of the functional state of patients and predict the therapeutic results of patients with GI cancer.
In our pooled analysis involving 4124 participants, we found that the ALI is an independent factor influencing the OS of patients with GI cancer. Even though significant heterogeneity existed, our subgroup analyses based on country, sample size, tumor site, selection method, tumor stage and NOS score showed our results were reliable and robust. Meanwhile, the sensitivity analysis showed that there was no change of the evident correlation between low ALI and poor OS. In addition, we have further investigated the relationship between the ALI and DFS. The combined result including 3433 patients showed that a low ALI was significantly associated with decreased DFS in GI cancer patients without obvious heterogeneity. Likewise, the sensitivity analysis supported the consistence and reliability of the result. We have also explored the correlation between ALI and POCs. The integrated result demonstrated that low ALI could act as a predictor of the incidence of POCs without heterogeneity. Furthermore, sensitivity analysis suggested that the result of meta-analysis for POCs was reliable. Based on these results, the ALI may be regarded as an effective clinical indicator predicting the short-term and long-term results of GI cancer patients.
There are some limitations in our study. First, all of the involved studies were retrospective studies performed at a single center, which may increase the risk of bias, and more prospective studies are required to further investigate this issue. Second, all included studies are Asian cohorts and studies from western countries are lacking, which may lead to potential publication bias and limit the generalization of the results. Third, due to the limited number of included studies, the value of the ALI in POCs, especially in specific POCs, needs to be further clarified. Finally, the cut-off value of the ALI among studies varied a lot, which might affect the validity and clinical utility of these findings; and further studies that use ALI as a continuous variable are warranted to verify this issue.
5 Conclusions
The findings of the meta-analysis suggested that the ALI prior to initial treatment is of great significance in predicting POCs and long-term survival results in patients with GI cancer. However, high-quality prospective studies with large sample size are still required to further validate the value of ALI in GI cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
H-YP wrote the manuscript. H-YP, X-FC and M-HY performed the data search and data analysis. H-YP, M-HY and L-HC prepared figures. All authors reviewed the manuscript. HS approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Chongqing Technology innovation and Application Development Special general project (cstc2019jscx-msxmX0194).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1021672/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Matsuoka T, Yashiro M. Precision medicine for gastrointestinal cancer: recent progress and future perspective. World J Gastrointest Oncol (2020) 12(1):1–20. doi: 10.4251/wjgo.v12.i1.1
3. Gusakov EA, Topchu IA, Mazitova AM, Dorogan IV, Bulatov ER, Serebriiskii IG, et al. Design, synthesis and biological evaluation of 2-quinolyl-1,3-tropolone derivatives as new anti-cancer agents. RSC advances (2021) 11(8):4555–71. doi: 10.1039/D0RA10610K
4. McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J cancer (2009) 125(6):1298–305. doi: 10.1002/ijc.24409
5. Yamada T, Morita T, Furukawa Y, Tamaki S, Kawasaki M, Kikuchi A, et al. A new systemic inflammation-nutrition index provides the additional long-term prognostic information to the get with the guidelines-heart failure risk score in acute decompensated heart failure. Circulation (2019) 140.
6. Connor DO, Griffin ML, O’Sullivan JS, Millar S, O'Keeffe J, Bird BR, et al. (2015). Pre-surgical neutrophil-to-lymphocyte ratio (NLR) is a prognostic indicator of recurrence free and overall survival in breast cancer patients undergoing primary surgery, in: Cancer Research Conference: 37th Annual CTRC AACR San Antonio Breast Cancer Symposium San Antonio, TX United States Conference Publication. 75(9 SUPPL. 1).
7. Mandaliya H, Jones M, Nordman I, Oldmeadow C. Prognostic importance of neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR) and advanced lung cancer inflammation index (ALI) at diagnosis and post treatment in elderly with stage IV NSCLC. Ann Oncol (2017) 28(Supplement 10):x142. doi: 10.1093/annonc/mdx671.051
8. Tanaka H, Tamura T, Toyokawa T, Muguruma K, Miki Y, Kubo N, et al. Clinical relevance of postoperative neutrophil-lymphocyte ratio (NLR) to recurrence after adjuvant chemotherapy of s-1 for gastric cancer. Anticancer Res (2018) 38(6):3745–51. doi: 10.21873/anticanres.12655
9. Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J gastroenterol (2015) 21(43):12410–20. doi: 10.3748/wjg.v21.i43.12410
10. Wada T, Kunisaki C, Ono HA, Makino H, Akiyama H, Endo I. Implications of BMI for the prognosis of gastric cancer among the Japanese population. Digestive Surgery (2015) 32(6):480–6. doi: 10.1159/000440654
11. Fan X, Wang D, Zhang W, Liu J, Liu C, Li Q, et al. Inflammatory markers predict survival in patients with advanced gastric and colorectal cancers receiving anti-PD-1 therapy. Front Cell Dev Biol (2021) 9:638312. doi: 10.3389/fcell.2021.638312
12. Tan GH, Novo CA, Dayal S, Chandrakumaran K, Mohamed F, Cecil T, et al. The modified Glasgow prognosis score predicts for overall and disease-free survival following cytoreductive surgery and HIPEC in patients with pseudomyxoma peritonei of appendiceal origin. Eur J Surg oncology: J Eur Soc Surg Oncol Br Assoc Surg Oncol (2017) 43(2):388–94. doi: 10.1016/j.ejso.2016.10.009
13. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC cancer (2013) 13:158. doi: 10.1186/1471-2407-13-158
14. Park YH, Lim JH, Yi HG, Lee MH, Kim CS. Prognostic value of pretreatment advanced lung cancer inflammation index (ALI) in diffuse large b-cell lymphoma patients treated with r-CHOP chemotherapy. Haematologica (2016) 101(Supplement 1):687. doi: 10.1159/000452991
15. Zhang Y, Chen B. Prognostic value of the advanced lung cancer inflammation index in patients with lung cancer: a meta-analysis. Dis Markers (2019) 2019:2513026. doi: 10.1155/2019/2513026
16. Hua X, Chen J, Wu Y, Sha J, Han S, Zhu X. Prognostic role of the advanced lung cancer inflammation index in cancer patients: a meta-analysis. World J Surg Oncol (2019) 17(1):177. doi: 10.1186/s12957-019-1725-2
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
18. Clavien PA, Strasberg SM. Severity grading of surgical complications. Ann Surgery (2009) 250(2):197–8. doi: 10.1097/SLA.0b013e3181b6dcab
19. Pang HY, Yan MH, Chen LH, Chen XF, Chen ZX, Zhang SR, et al. Detection of asymptomatic recurrence following curative surgery improves survival in patients with gastric cancer: a systematic review and meta-analysis. Front Oncol (2022) 12:1011683. doi: 10.3389/fonc.2022.1011683
20. Papakonstantinou A, Gonzalez NS, Pimentel I, Suñol A, Zamora E, Ortiz C, et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: a systematic review and meta-analysis. Cancer Treat Rev (2022) 104:102362. doi: 10.1016/j.ctrv.2022.102362
21. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
22. Chen C, Mao S, Li M, Xu W, Zhu ZQ. Correlation analysis of preoperative advanced lung cancer inflammation index and prognosis of colorectal cancer. [Chinese] Chin J Cancer Prev Treat (2022) 29(4):285–9. doi: 10.16073/j,cnki,cjcpt.2022.04.09
23. Feng JF, Chen QX, Huang Y. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. OncoTargets Ther (2014) 7:1811–5. doi: 10.2147/OTT.S68084
24. He K, Si L, Pan X, Sun L, Wang Y, Lu J, et al. Preoperative systemic immune-inflammation index (SII) as a superior predictor of long-term survival outcome in patients with stage I-II gastric cancer after radical surgery. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.829689
25. Horino T, Tokunaga R, Miyamoto Y, Hiyoshi Y, Akiyama T, Daitoku N, et al. The advanced lung cancer inflammation index is a novel independent prognosticator in colorectal cancer patients after curative resection. Ann Gastroenterological Surgery (2022) 6(1):83–91. doi: 10.1002/ags3.12499
26. Kusunoki K, Toiyama Y, Okugawa Y, Yamamoto A, Omura Y, Ohi M, et al. Advanced lung cancer inflammation index predicts outcomes of patients with colorectal cancer after surgical resection. Dis Colon Rectum (2020) 63(9):1242–50. doi: 10.1097/DCR.0000000000001658
27. Pian G, Hong SY, Oh SY. Prognostic value of advanced lung cancer inflammation index in patients with colorectal cancer liver metastases undergoing surgery. Tumori J (2022) 108(1):56–62. doi: 10.1177/0300891620983465
28. Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Kimura K, et al. The prognostic significance of the advanced lung cancer inflammation index in patients with unresectable metastatic colorectal cancer: a retrospective study. BMC Cancer (2019) 19(1):241. doi: 10.1186/s12885-019-5468-9
29. Tan X, Peng H, Gu P, Chen M, Wang Y. Prognostic significance of the L3 skeletal muscle index and advanced lung cancer inflammation index in elderly patients with esophageal cancer. Cancer Manage Res (2021) 13:3133–43. doi: 10.2147/CMAR.S304996
30. Xie H, Huang S, Yuan G, Kuang J, Yan L, Wei L, et al. The advanced lung cancer inflammation index predicts short and long-term outcomes in patients with colorectal cancer following surgical resection: a retrospective study. PeerJ (2020) 8:e10100. doi: 10.7717/peerj.10100
31. Yin C, Toiyama Y, Okugawa Y, Omura Y, Kusunoki Y, Kusunoki K, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: a propensity score matching analysis. Clin Nutr (2021) 40(3):1130–6. doi: 10.1016/j.clnu.2020.07.018
32. Zhang X, Wang D, Sun T, Li W, Dang C. Advanced lung cancer inflammation index (ALI) predicts prognosis of patients with gastric cancer after surgical resection. BMC Cancer (2022) 22(1):684. doi: 10.1186/s12885-022-09774-z
33. Fan W, Zhang Y, Liu Y, Ding Z, Si Y, Shi F, et al. Nomograms based on the advanced lung cancer inflammation index for the prediction of coronary artery disease and calcification. Clin Appl Thrombosis-Hemostasis (2021) 27:10760296211060455. doi: 10.1177/10760296211060455
34. Bacha S, Sghaier A, Habibech S, Cheikhrouhou S, Racil H, Chaouch N, et al. Advanced lung cancer inflammation index: a prognostic score in patients with metastatic non-small cell lung cancer. La Tunisie Medicale (2017) 95(11):976–81.
35. Barth DA, Brenner C, Riedl JM, Prinz F, Klocker EV, Schlick K, et al. External validation of the prognostic relevance of the advanced lung cancer inflammation index (ALI) in pancreatic cancer patients. Cancer Med (2020) 9(15):5473–9. doi: 10.1002/cam4.3233
36. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis (2009) 30(7):1073–81. doi: 10.1093/carcin/bgp127
37. Li Q-Q, Lu Z-H, Yang L, Lu M, Zhang X-T, Li J, et al. Neutrophil count and the inflammation-based Glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pacific J Cancer Prev (2014) 15(2):945–50. doi: 10.7314/APJCP.2014.15.2.945
38. Rossana B, Rinaldi S, Santoni M, Newsom-Davis T, Tiberi M, Morgese F, et al. Systemic immune-inflammation index (SII) in patients with advanced non-small cell lung cancer (nsclc). Eur J Cancer (2015) 51:S630–0. doi: 10.1016/S0959-8049(16)31733-6
39. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer (2013) 13(11):759–71. doi: 10.1038/nrc3611
40. Li J, Zhang Y, Xu Q, Wang G, Jiang L, Wei Q, et al. Systemic inflammatory markers of resectable colorectal cancer patients with different mismatch repair gene status. Cancer Manag Res (2021) 13:2925–35. doi: 10.2147/CMAR.S298885
41. Rabinowich H, Cohen R, Bruderman I, Steiner Z, Klajman A. Functional analysis of mononuclear cells infiltrating into tumors: lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res (1987) 47(1):173–7.
42. Shi DD, Youssef GC, Nassar AH, Lim-Fat MJ, Ligon KL, Wen PY, et al. Improved survival among females and association with lymphopenia in patients with newly diagnosed glioblastoma. Neuro-Oncology (2022) 24(11):2005–7. doi: 10.1093/neuonc/noac190
43. Zhao Q, Bi Y, Xue J, Liu Y, Zhu J, Qin S. Prognostic value of absolute lymphocyte count in patients with advanced esophageal cancer treated with immunotherapy: a retrospective analysis. Ann Transl Med (2022) 10(13):744. doi: 10.21037/atm-22-2669
44. Vashi PG, Gupta D, Lammersfeld CA, Braun DP, Popiel B, Misra S, et al. The relationship between baseline nutritional status with subsequent parenteral nutrition and clinical outcomes in cancer patients undergoing hyperthermic intraperitoneal chemotherapy. Nutr J (2013) 12:118. doi: 10.1186/1475-2891-12-118
45. Yildirim M, Yildiz M, Duman E, Goktas S, Kaya V. Prognostic importance of the nutritional status and systemic inflammatory response in non-small cell lung cancer. J Buon (2013) 18(3):728–32
46. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr (Edinburgh Scotland) (2007) 26(6):698–709. doi: 10.1016/j.clnu.2007.06.009
47. Chen F, Chi J, Liu Y, Fan L, Hu K. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: a meta-analysis of cohort studies. Arch Gerontol Geriatrics (2022) 98:104534. doi: 10.1016/j.archger.2021.104534
Keywords: gastrointestinal cancer, advanced lung cancer inflammation index, postoperative complications, prognosis, meta-analysis
Citation: Pang H-Y, Chen X-F, Yan M-H, Chen L-H, Chen Z-X, Zhang S-R and Sun H (2023) Clinical significance of the advanced lung cancer inflammation index in gastrointestinal cancer patients: a systematic review and meta-analysis. Front. Oncol. 13:1021672. doi: 10.3389/fonc.2023.1021672
Received: 01 September 2022; Accepted: 02 June 2023;
Published: 19 June 2023.
Edited by:
Kanjoormana Aryan Manu, Amala Cancer Research Centre, IndiaReviewed by:
Ming Yang, Capital Medical University, ChinaEmil Bulatov, Kazan Federal University, Russia
Copyright © 2023 Pang, Chen, Yan, Chen, Chen, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Sun, c3VuaGFvNjhAc2luYS5jb20=
†These authors share first authorship
 Hua-Yang Pang
Hua-Yang Pang Xiu-Feng Chen
Xiu-Feng Chen Meng-Hua Yan
Meng-Hua Yan Li-Hui Chen1
Li-Hui Chen1 Hao Sun
Hao Sun