- Thoracic Surgery Department, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objectives: This study aimed to evaluate the prognostic significance of the eighth edition TNM stage criteria in patients with combined small-cell lung cancer (C-SCLC) on a population level.
Methods: Using the Surveillance, Epidemiology, and End Results (SEER) database, patients diagnosed with C-SCLC (histology code 8245) between the years 2004 and 2015 were identified. We performed a Kaplan–Meier analysis and used the multivariable cox regression proportional hazards model to obtain prognostic overall survival estimates for each group of patients.
Results: A total of 477 patients diagnosed with C-SCLC were identified. The T, N, M, TNM, and combined TNM stage status of the eighth edition were all significant prognostic factors for patients’ overall survivals, with the best discrimination identified in the combined stages. Surgery was also found to be a prognostic factor (HR =1.95, 95%CI =1.49-2.56, p<0.01) for patients with C-SCLC.
Conclusions: The combined eighth edition of the TNM staging criteria shows reliable prognostic significance in patients with C-SCLC. Moreover, surgery might be significant for improving the patients’ prognosis.
Introduction
Lung cancer is one of the most prevalent malignancies and the leading cause of cancer-related mortality worldwide (1). Combined small-cell lung cancer (C-SCLC) is a relatively uncommon subset of lung neuroendocrine cancer (NEC), defined as small-cell lung cancer (SCLC; the most lethal subgroup) combined with additional components of non-small-cell lung cancer (NSCLC) (2). The NSCLC components could be different or mixed, without amount restrictions, unlike the minimum 10% of components for large-cell neuroendocrine carcinoma. The SCLC practice guidelines were used for the diagnosis and treatment of patients with C-SCLC, but aside from the NSCLC components, even the cell origins and overall survivals might be different (3).
The most recent eighth edition of the TNM cancer classification criteria for lung cancer was published in 2016, based on the International Association for the Study of Lung Cancer (IASLC) database (4). Compared to the seventh edition, there are significant refinements in the T and M components, decreased size boundaries, and more accurate metastases sites (5–7). Although these refinements were analyzed from the data of NSCLC patients, they were validated in SCLC in the IASLC database, National Cancer Data Base, and Chinese cohorts (8). However, to our knowledge, prognostic assessments of C-SCLC, another subset of NEC, have not yet been performed on a population level.
The purpose of our study was to evaluate the prognostic significance of the eighth edition TNM stage criteria in patients with C-SCLC by using the Surveillance, Epidemiology, and End Results (SEER) database on a population level (9). Moreover, we aimed to analyze the demographic and clinical treatments factors associated with survival outcomes. This might allow us to optimize the management procedures for these patients and finally improve the prognosis.
Methods
We searched for and collected data of patients from the Surveillance, Epidemiology, and End Results (SEER) database, which provides cancer treatment and survival information covering approximately 48.0 percent of the U.S. population (9). Based on the November 2020 submission, patients diagnosed with C-SCLC (histology code 8245) between the years 2004 and 2015 were identified. For the anonymized and public data, written informed consent and additional institutional review board approval were waived.
The patients included in this study were those who were reported from hospitals and clinics, were diagnosed with positive histology, had only one primary tumor, had a defined follow-up period, and who did not have missing staging information. Patients who died of other causes and represented unknown demographic and clinical variables were excluded. We determined the clinical TNM stages according to the eighth edition criteria. Because the numbers of T1a(n=11), IA1(n=4), IIA(n=7), and IIIC(n=8) stage tumors were small, we combined stages T1a and T1b (denoted as T1a&b), IIA, and IIB (denoted as II), as well as IIIB and IIIC (denoted as IIIB&C). Furthermore, stages IA1, IA2, and IA3 were grouped together as stage I for analysis.
We calculated the absolute numbers and percentages of each group of patients with C-SCLC. The median overall survivals were identified for each group. A Kaplan–Meier analysis was performed to obtain the prognostic overall survival estimates for each group of patients. The multivariable cox regression proportional hazards model was used to evaluate the prognostic significance of the eighth edition TNM stage criteria in C-SCLC. Statistical analysis was performed by the SPSS version 26.0(IBM) and PRISM version 9.3(GraphPad).
Results
A cohort of 477 patients diagnosed with C-SCLC between the years 2004 and 2015 were included in this study, including 296 (62.05%) patients aged between 40 and 70 years old, 247 (51.78%) male patients, 250 (52.41%) married patients, and 397 (83.23%) white patients. Regarding the primary sites of the tumors, 6.08%, 62.05%, 4.19%, and 23.69% were located in the main bronchus and the upper, middle, and lower lobe, respectively, while 3.98% were not located in any specific lung sites. Most patients (213, 44.65%) were in stage IV, 36.89% in stage IVA, and 7.76% in stage IVB. Meanwhile, 11.95%, 5.66%, 9.01%, 11.74%, and 16.98% of the patients were in stages IA, IB, II, IIIA, and IIIB&C, respectively. In total, 350 (73.38%) cases received chemotherapy, while 245 (51.36%) and 146 (30.61%) patients received radiation and surgery, respectively. For combination treatments, 138 (28.93%) patients underwent both surgery and chemotherapy, while 103 (21.59%) patients underwent both surgery and radiation (Table 1).
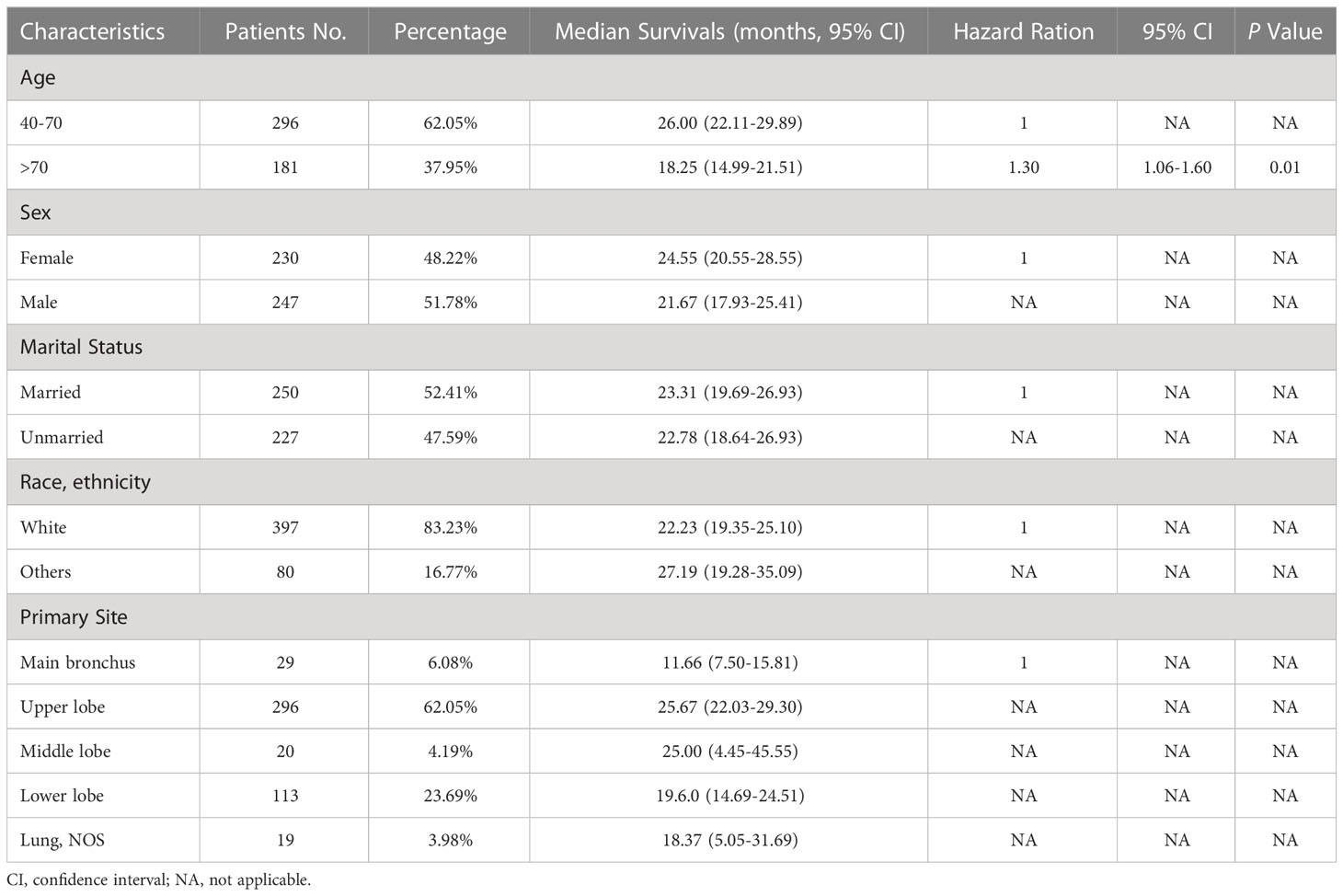
Table 1 Demographic variables, median survivals, and multivariable cox regression for patients with C-SCLC.
After the determination of the eighth edition TNM stages, it was found that the T stage, N stage, M stage, TNM stage, and combined TNM stage statuses were all significant prognostic factors for the patients’ overall survivals (Figures 1A-E). With the increasing stage status, decreasing overall survivals were found in nearly all different stage status groups. In the groups of the T1a&b, T1c, T2a, T2b, T3, and T4 stages, the median overall survivals were 38.52, 24.93, 27.79, 22.80, 16.63, and 14.12 months, respectively. Only one exception was found in the overall survivals between the N2 stage (13.20 months) and N3 stage (19.83 months), increasing overall survivals with the stages. The N0 stage group conferred a median overall survival of 36.81 months, compared with those for the N1 stage (20.15 months). The M0 stage group had a median overall survival of 32.78 months and declined to 17.94 months in the M1a stage group, 10.61 months in the M1b stage, and 6.19 months in the M1c stage groups. For the TNM stage status groups, the IA and IB stage groups had almost the same median overall survivals of 52.67 and 52.59 months, respectively, which then declined steeply to 30.14 months in the II stage group. Similarly, the median overall survivals of the IIIA and IIIB&C stage groups were almost the same, at 21.64 and 21.28 months, respectively. In the IVA and IVB stage groups, the median overall survivals were the shortest, at 12.02 and 6.19 months, respectively. Moreover, the median overall survivals of the combined TNM stage status groups were 52.64, 30.14, 21.43, and 11.01 months from stages I to IV, respectively. Analysis of other clinical treatment different status groups gave the maximum difference in surgery status groups, and it was found that the overall survival of patients who received surgery was 41.58 months, compared with those without surgery (Table 2).
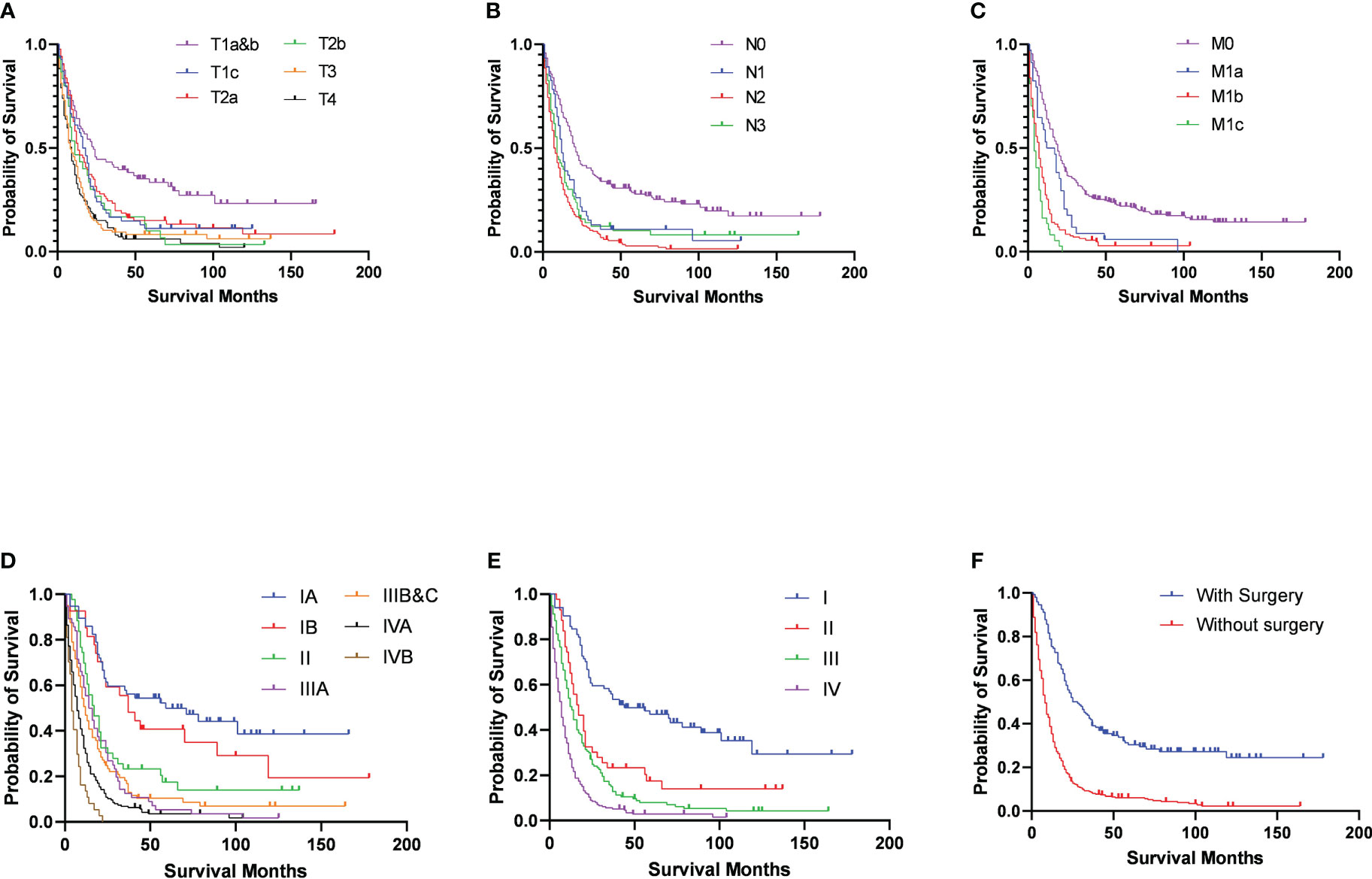
Figure 1 Survival outcomes in patients with C-SCLC stratified by eighth edition TNM stage criteria (A) T-stage; (B) N-stage; (C) M-stage; (D) TNM-stage; (E) combined TNM-stage) and surgery (F).
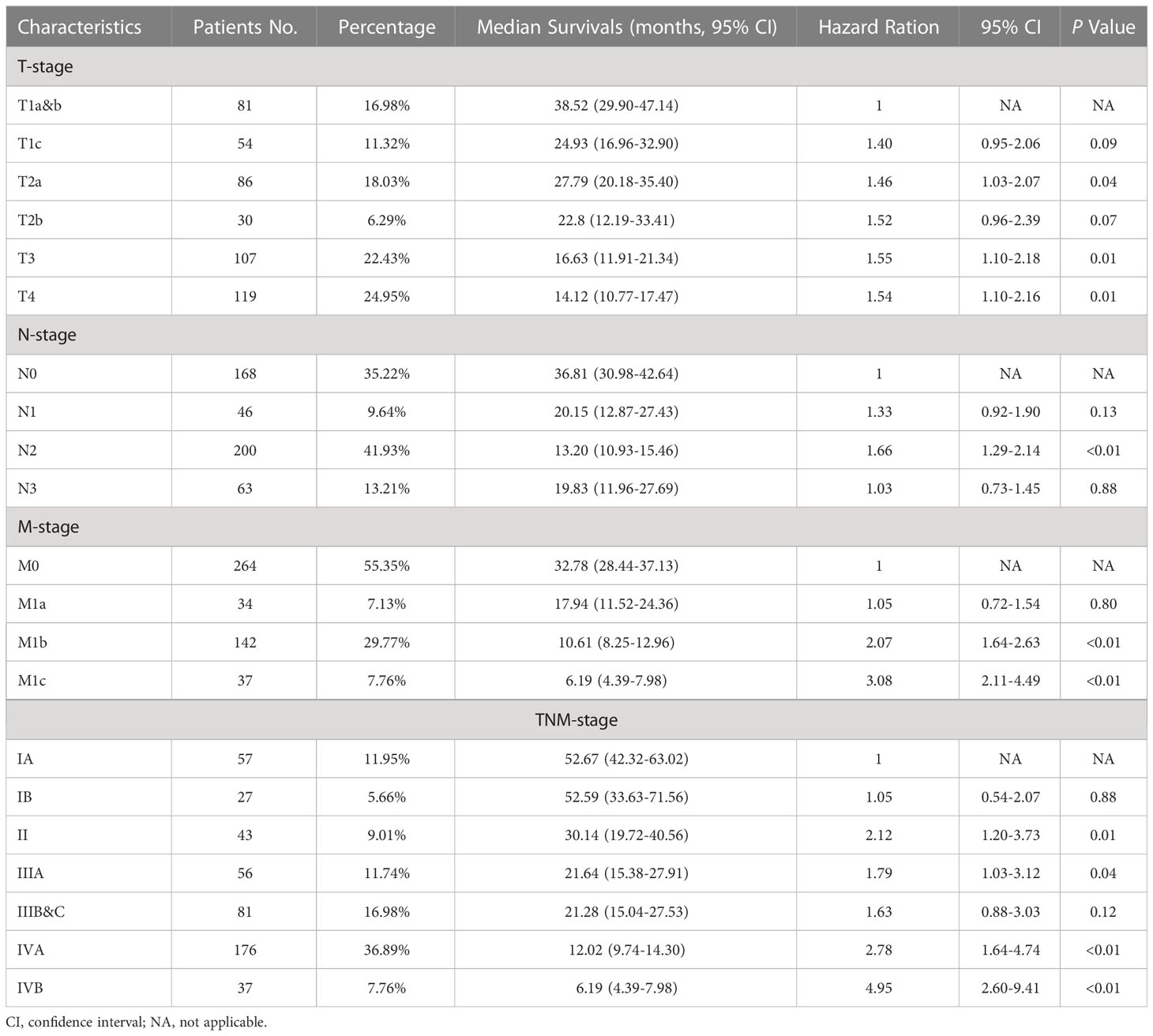
Table 2 TNM stage characteristics, median overall survivals, and multivariable cox regression for patients with C-SCLC.
For the multivariable cox regression analysis in patients with C-SCLC, T-stage (T2a, hazard ratio [HR] =1.46, 95%CI =1.03-2.07, p=0.04; T3, HR =1.55, 95%CI =1.10-2.18, p=0.01; T4, HR =1.54, 95%CI =1.10-2.16, p=0.01; referred to T1a&b), N-stage (N2, HR =1.66, 95%CI =1.29-2.14, p<0.01; referred to N0), M-stage (M1b, HR =2.07, 95%CI =1.64-2.63, p<0.01; M1c, HR =3.08, 95%CI =2.11-4.49, p<0.01 referred to M0), TNM-stage (II, HR =2.12, 95%CI =1.20-3.73, p=0.01; IIIA, HR=1.79, 95%CI =1.03-3.12, p=0.04; IVA, HR =2.78, 95%CI =1.64-4.74, p<0.01; IVB, HR=4.95, 95%CI =2.60-9.41, p<0.01; referred to IA), and the combined TNM stage (II, HR =2.07, 95%CI =1.26-3.40, p<0.01; IV, HR=2.84, 95%CI =1.84-4.39, p<0.01; referred to IA) were significantly associated with bigger HRs of patients with C-SCLC. Moreover, ages older than 70 years (HR =1.30, 95%CI =1.06-1.60, p=0.01) compared to ages between 40 and 70 years, and cases without surgery (HR =1.95, 95%CI =1.49-2.56, p<0.01) compared to those with surgery, were significantly associated with bigger HRs of patients with C-SCLC (Figure 1F). There were bigger HRs, but without statistical significance in the other stage status groups. Likely, T1c (HR =1.40, 95%CI =0.95-2.06, p=0.09) and T2b (HR =1.53, 95%CI =0.96-2.39, p=0.07) were bigger HRs, but not significantly associated with those referred to T1a&b (Table 3).
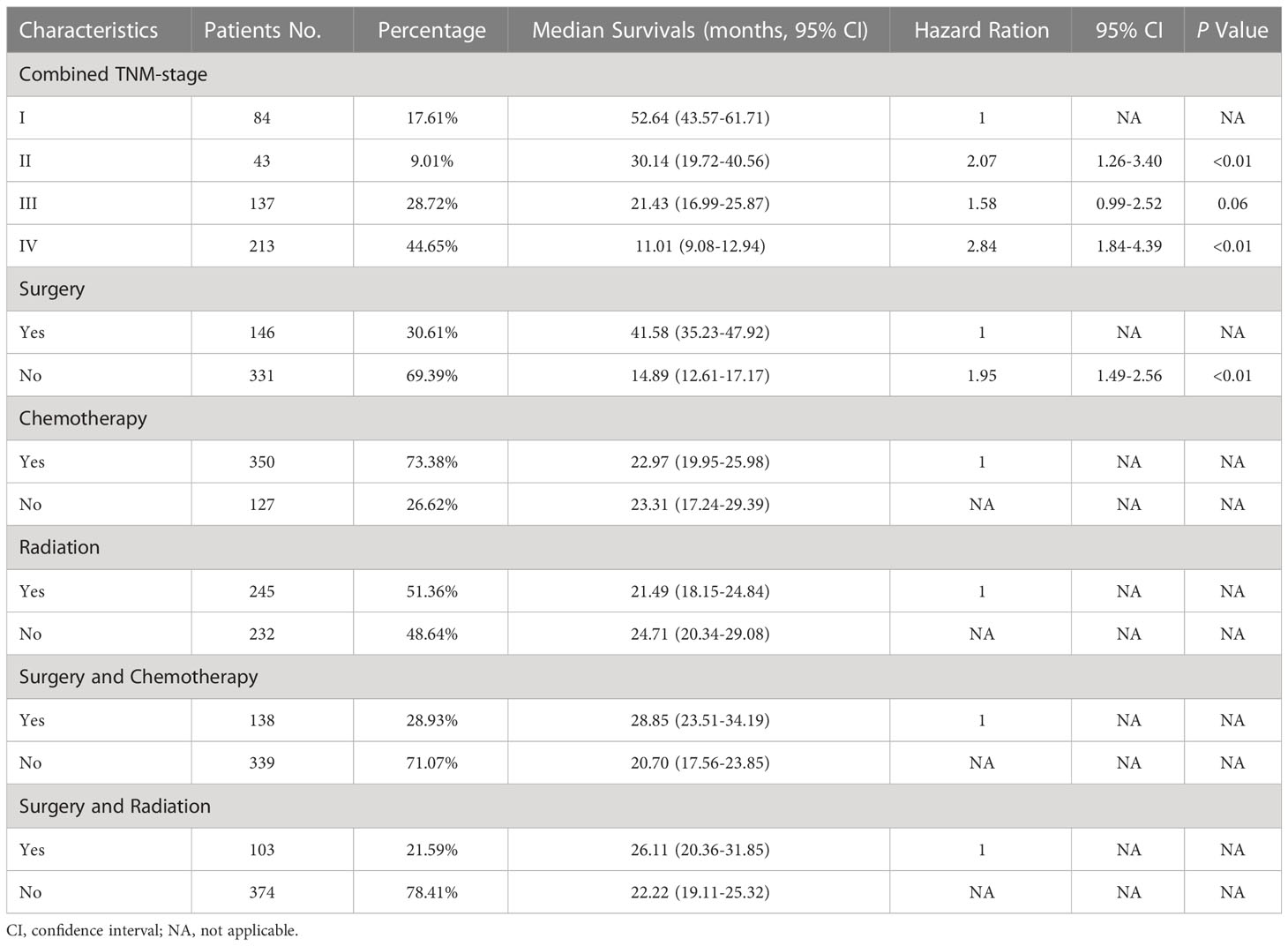
Table 3 Combined TNM stage and treatment characteristics, median overall survivals, and multivariable cox regression for patients with C-SCLC.
Discussion
In this study, we described and validated the prognostic significance of the eighth edition TNM stage criteria in C-SCLC using population-based data from the SEER plus database. Approximately half of the population of the USA was encompassed in the SEER database; the prognostic significance of the eighth edition TNM stage criteria in C-SCLC might be generalizable and better in representing the population experience. Moreover, this huge and relatively uniform number of patients with C-SCLC and the adequate follow-up information enhance the power of our study and improve the ability to characterize reliable key factors associated with the prognosis.
To our knowledge, this is the retrospective cohort study with the largest sample size that focuses on the prognostic significance of the new edition of the TNM stage criteria in C-SCLC. We confirmed the survival outcomes separation and the progressive decline of overall survival by advancing the stage groups of the new edition. In the new edition, the size boundaries between the groups were decreased, such as stage T2, which includes tumor diameters of 3 to 5 cm instead of 3 to 7 cm in the seventh edition criteria, resulting in better distinguishing performance in the T-stage subcategories (10). Furthermore, the metastases subcategory was refined, while the previous staging categorized any metastatic case to stage IV (11, 12). Our results showed that M1c had a significantly worse prognosis than M1b, consolidating better distinguishing performance in the metastases subcategory refinement by the eighth edition. However, the categories of the N stage saw nearly no update from the previous criteria, while the N2 and N3 groups showed overlap and no significant differences in overall survivals. This finding may present a limitation on the application of the new TNM staging criteria, particularly in the N stages, which should be tailored in the upcoming staging system for patients with C-SCLC.
It was thought that surgery therapy is mostly suitable for the patients with early-stage C-SCLC (13). Our study showed the all-different-staged patients without surgery were significantly associated with a bigger HR and were referred to surgery. The median overall survival time in the surgery group was much longer than that in the non-surgery group. For the chemotherapy and radiation therapy status groups, the median overall survival time became longer when the therapy combined with surgery. This was consistent with the findings of previous studies, which showed that the 5-year overall survival rate in the C-SCLC surgery group was 48.9%, which is much higher than the 36.6% recorded in the non-surgery group (14). For all C-SCLC patients, even those with extended disease, it was considered to be potentially helpful to receive surgery for better prognosis. This was consistent with the finding in the NSCLC patients, as well as in some late-stage malignancies such as breast cancer and colorectal cancer (15–18). Therefore, surgery might be significant for improving the prognosis, and the surgical indication should be broader in patients with C-SCLC. Radiation was proved to improve the prognosis only in patients with stage III C-SCLC, and chemotherapy was significantly associated with better prognosis in both stage III and IV patients. This appears reasonable, and it is almost the same with NSCLC patients. It might be necessary for future studies to investigate patients who would be suitable for surgery and what kind of surgery would be suitable for them.
Despite the sufficient evidence and reasonable arguments, there are still some limitations that should be considered in our study. The first limitation is that our study was a retrospective cohort analysis, with retrospective research limitations such as selection bias. In addition, the lack of the component information of C-SCLC—whether small-cell lung cancer combined with adenocarcinoma or the others—might influence the outcomes of the patients and thus produce bias. Finally, the number of patients was limited in some of the subgroups, which were poorly represented. This might limit the overall survival estimation in these groups. A larger cohort of patients and detailed information are needed to validate the observed survival difference.
Conclusion
In conclusion, our study shows that the combined eighth edition of the TNM staging criteria has reliable prognostic significance in patients with C-SCLC, and surgery might be significant for improving the prognosis. While the new TNM stages subcategories do not have good applicability and discrimination from their adjacent groups, further study is needed for the reclassification of the upcoming TNM staging and in patients with C-SCLC.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: www.seer.cancer.gov.
Author contributions
Conception and design: ZZ, SG, and JH; collection and assembly of data: ZZ and YG; data analysis and interpretation: all authors; manuscript writing: ZZ, FT, and QX; final approval of manuscript: all authors; accountability for all aspects of the work: all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (2020AAA0109500), National Natural Science Foundation of China (82122053), the Beijing Municipal Science & Technology Commission (Z191100006619118), R&D Program of Beijing Municipal Education Commission (KJZD20191002302), CAMS Initiative for Innovative Medicine (2021-1-I2M-012, 2021-1-I2M-015), Key-Area Research and Development Program of Guangdong Province (2021B0101420005), Shenzhen High-level Hospital Construction Fund, and Sanming Project of Medicine in Shenzhen.
Acknowledgments
We thank all individuals who took part in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Zhao X, McCutcheon JN, Kallakury B, Chahine JJ, Pratt D, Raffeld M, et al. Combined small cell carcinoma of the lung: Is it a single entity? J Thorac Oncol (2018) 13(2):237–45. doi: 10.1016/j.jtho.2017.10.010
3. Lei Y, Feng H, Qiang H, Shang Z, Chang Q, Qian J, et al. Clinical characteristics and prognostic factors of surgically resected combined small cell lung cancer: A retrospective study. Lung Cancer (2020) 146:244–51. doi: 10.1016/j.lungcan.2020.06.021
4. Yoon JY, Sigel K, Martin J, Jordan R, Beasley MB, Smith C, et al. Evaluation of the prognostic significance of TNM staging guidelines in lung carcinoid tumors. J Thorac Oncol (2019) 14(2):184–92. doi: 10.1016/j.jtho.2018.10.166
5. Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, et al. The international association for the study of lung cancer lung cancer staging project: Proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11(3):300–11. doi: 10.1016/j.jtho.2015.10.008
6. Chiang CL, Guo Q, Ng WT, Lin S, Ma TSW, Xu Z, et al. Prognostic factors for overall survival in nasopharyngeal cancer and implication for TNM staging by UICC: A systematic review of the literature. Front Oncol (2021) 11:703995. doi: 10.3389/fonc.2021.703995
7. Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallieres E, Groome P, et al. The IASLC lung cancer staging project: External validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol (2017) 12(7):1109–21. doi: 10.1016/j.jtho.2017.04.011
8. Tan F, Bi N, Zhang H, Li R, Wang Z, Duan J, et al. External validation of the eighth edition of the TNM classification for lung cancer in small cell lung cancer. Lung Cancer (2022) 170:98–104. doi: 10.1016/j.lungcan.2022.03.011
9. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: Incidence - SEER research data, 8 registries, Nov 2021 Sub (1975-2019) . National Cancer Institute, DCCPS, Surveillance Research Program. Available at: http://www.seer.cancer.gov (Accessed May 5, 2022).
10. Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemanski L, et al. The IASLC lung cancer staging project: Methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (Eighth) edition of the TNM classification of lung cancer. J Thorac Oncol (2016) 11(9):1433–46. doi: 10.1016/j.jtho.2016.06.028
11. Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A 3rd, et al. The IASLC lung cancer staging project: Proposals for the revision of the m descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol (2015) 10(11):1515–22. doi: 10.1097/JTO.0000000000000673
12. Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, et al. The IASLC lung cancer staging project: Proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol (2015) 10(7):990–1003. doi: 10.1097/JTO.0000000000000559
13. Simbolo M, Centonze G, Ali G, Garzone G, Taormina S, Sabella G, et al. Integrative molecular analysis of combined small-cell lung carcinomas identifies major subtypes with different therapeutic opportunities. ESMO Open (2022) 7(1):100308. doi: 10.1016/j.esmoop.2021.100308
14. Yang L, Zhou Y, Wang G, Liu D, Chen B, Pu D, et al. Clinical features and prognostic factors of combined small cell lung cancer: Development and validation of a nomogram based on the SEER database. Transl Lung Cancer Res (2021) 10(11):4250–65. doi: 10.21037/tlcr-21-804
15. Thomas A, Khan SA, Chrischilles EA, Schroeder MC. Initial surgery and survival in stage IV breast cancer in the united states, 1988-2011. JAMA Surg (2016) 151(5):424–31. doi: 10.1001/jamasurg.2015.4539
16. Tarantino I, Warschkow R, Worni M, Cerny T, Ulrich A, Schmied BM, et al. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: A population-based, propensity score-adjusted trend analysis. Ann Surg (2015) 262(1):112–20. doi: 10.1097/SLA.0000000000000860
17. Wang K, Shi Y, Li ZY, Xiao YL, Li J, Zhang X, et al. Metastatic pattern discriminates survival benefit of primary surgery for de novo stage IV breast cancer: A real-world observational study. Eur J Surg Oncol (2019) 45(8):1364–72. doi: 10.1016/j.ejso.2019.02.013
Keywords: combined small cell lung cancer (c-SCLC), 8th TNM stage criteria, prognostic significance, SEER database, surgery
Citation: Zhao Z, Gao Y, Tan F, Xue Q, Gao S and He J (2023) Prognostic significance of eighth edition TNM stage criteria in combined small-cell lung cancer. Front. Oncol. 13:1018288. doi: 10.3389/fonc.2023.1018288
Received: 13 August 2022; Accepted: 13 February 2023;
Published: 08 March 2023.
Edited by:
Luigi Tornillo, University of Basel, SwitzerlandReviewed by:
Ming Li, Fudan University, ChinaAntonio Mazzella, European Institute of Oncology (IEO), Italy
Copyright © 2023 Zhao, Gao, Tan, Xue, Gao and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie He, cHJvZl9oZWppZUAxMjYuY29t
 Ziran Zhao
Ziran Zhao Yibo Gao
Yibo Gao