
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 27 January 2023
Sec. Cancer Imaging and Image-directed Interventions
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1007464
This article is part of the Research Topic Cancer Imaging Techniques to Distinguish Benign and Malignant Tumors View all 13 articles
A correction has been applied to this article in:
Corrigendum: Association between diagnostic efficacy of acoustic radiation force impulse for benign and malignant thyroid nodules and the presence or absence of non-papillary thyroid cancer: A meta-analysis
 Jun Li1,2*
Jun Li1,2* Yu-Rui Zhang1
Yu-Rui Zhang1 Jia-Yu Ren3
Jia-Yu Ren3 Qiao-Li Li1
Qiao-Li Li1 Pei-Shan Zhu1
Pei-Shan Zhu1 Ting-Ting Du1
Ting-Ting Du1 Xiao-Yan Ge1
Xiao-Yan Ge1 Ming Chen1
Ming Chen1 Xin Wu Cui3*
Xin Wu Cui3*Purpose: The aim of this study was to investigate the diagnostic efficacy of Acoustic Radiation Force Impulse (ARFI) for benign and malignant thyroid nodules in the presence and absence of non-papillary thyroid cancer (NPTC) and to determine the cut-off values of Shear Wave Velocity (SWV) for the highest diagnostic efficacy of Virtual Touch Quantification (VTQ) and Virtual Touch Tissue Imaging and Quantification (VTIQ).
Methods: The diagnostic accuracy of ARFI for benign and malignant thyroid nodules was assessed by pooling sensitivity, specificity and area under the curve (AUC) in each group in the presence and absence of both non-papillary thyroid glands, using histology and cytology as the gold standard. All included studies were divided into two groups according to VTQ and VTIQ, and each group was ranked according to the magnitude of the SWV cutoff value to determine the SWV cutoff interval with the highest diagnostic efficacy for VTQ and VTIQ.
Results: A total of 57 studies were collected on the evaluation of ARFI for the diagnosis of benign and malignant thyroid nodules. The results showed that the presence of non-papillary thyroid carcinoma led to differences in the specificity of VTIQ for the identification of benign and malignant thyroid nodules, and the differences were statistically significant. In addition, the diagnostic efficacy of VTQ was best when the cutoff value of SWV was in the interval of 2.48-2.55 m/s, and the diagnostic efficacy of VTIQ was best when the cutoff value of SWV was in the interval of 3.01-3.15 m/s.
Conclusion: VTQ and VTIQ have a high diagnostic value for benign and malignant thyroid nodules; however, when the malignant nodules in the study contain non-papillary thyroid carcinoma occupying the thyroid gland, the findings should be viewed in a comprehensive manner.
Thyroid nodules are a very common thyroid disorder and the incidence of thyroid nodules has shown an increasing trend year by year over the last few decades (1). Thyroid cancer accounts for 5% of thyroid nodules (2). There are four main types of thyroid cancer pathology: papillary, follicular, medullary and interstitial. The most common of these pathological types is papillary thyroid cancer (PTC), which also has the best prognosis among thyroid cancers, while the others have a poor prognosis (3). Among them, interstitial thyroid cancer, although less common, is one of the most dangerous tumors and is an associated cause of death in nearly half of thyroid cancer patients (4). Therefore, the first prerequisite for clinical diagnosis is to identify the benign and malignant thyroid nodules and then to develop the most appropriate treatment plan based on this, in order to reduce unnecessary surgeries and surgical complications, and ultimately to improve the quality of life as well as the health status of patients.
Ultrasonography is the test of choice for thyroid disease. Preoperative ultrasound examination of thyroid nodules is the most commonly used clinical method. However, conventional ultrasonography, including color Doppler ultrasound, cannot accurately differentiate between benign and malignant thyroid nodules, even when combined with CT and MRI examinations (1).
Currently, fine-needle aspiration biopsy (FNAB) is one of the recommended adjuncts for the diagnosis of thyroid nodules, but studies have shown that the sensitivity and specificity of FNAB for the diagnosis of thyroid nodules are 65-98% and 72-100%, respectively, and 20%-30% of samples cannot be diagnosed pathologically, with a certain rate of underdiagnosis (5–10). Moreover, FNAB is an invasive procedure with potential complications that have a negative impact on the patient’s health.
In recent years, acoustic radiation force pulse elastography (ARFI) has been widely used in the examination of thyroid diseases, which can reflect the different hardness characteristics of benign and malignant lesions and is very useful for the identification of benign and malignant lesions (11). ARFI includes virtual touch tissue imaging and quantification (VTIQ) and virtual touch tissue quantification (VTQ) techniques, which are based on the principle of measuring the shear wave velocity (SWV) of the regions of interest (ROI) of the tissue. SWV is used to quantify the stiffness of the tissue. In a tissue lesion, the faster the shear wave velocity, the harder the lesion; the slower the shear wave velocity, the softer the lesion (2). Tissue stiffness is a characteristic that can reflect the nature of the nodule. The degree of fibrosis and the number of tumor cells vary among different histologic types of thyroid nodules, resulting in different stiffness in different histologic types of thyroid nodules. Compared to papillary carcinomas, other types of thyroid carcinomas, such as follicular, medullary, and undifferentiated carcinomas exhibit relatively soft structures (12).
In the past, a meta-analysis was performed to evaluate the diagnostic efficacy of ARFI in identifying benign and malignant thyroid nodules, and the results of the study showed that ARFI performed well in the differential diagnosis of benign and malignant thyroid nodules, and that ARFI may help guide the clinical choice of surgery for patients with thyroid nodules (13). However, this study only made a simple benign-malignant distinction between thyroid nodules and did not further delineate the pathological types of thyroid cancer.
The main objective of the present study, taken together with previous studies, was to assess whether the presence of nonpapillary thyroid cancer affects the diagnostic efficacy of ARFI for benign and malignant thyroid nodules and to determine the cut-off interval of SWV with optimal diagnostic efficacy for VTQ and VTIQ.
The search databases web of science, PubMed, and Embase were searched for relevant studies published up to May 1, 2022, with the search terms “(Acoustic Radiation Force Impulse or ARFI or VTIQ or VTQ or Virtual Touch tissue imaging and quantification or Virtual Touch tissue quantification) and (thyroid or thyroid nodules)”. The search language was English. In order to search as much relevant literature as possible, the search method of this paper was subject terms combined with free words, web search combined with manual search, and secondary search of the retrieved relevant literature was conducted.
Inclusion criteria: (i) the literature study must include the diagnostic analysis of thyroid nodules by ARFI; (ii) there is a gold standard for diagnosing the pathology of thyroid nodules in the literature, and the number of benign and malignant nodules must be given directly or indirectly; (iii) the number of patients must be ≥30; (iv) the literature should provide raw data and calculate the sensitivity, specificity, false positives and false negatives can be calculated directly or indirectly.
Exclusion criteria: (i) diagnostic criteria were not described; (ii) data could not be extracted; (iii) duplicate literature; (iv) pathological histology was not used as the gold standard; (v) cutoff values for SWV were not indicated; (vi) editorials, letters, case reports, review articles, commentaries, case-control studies, and conference articles.
Two authors independently searched and read the titles, abstracts, and keywords of the detected literature to initially identify eligible literature that could be selected, and then carefully read the full text of the literature to finalize the eligible literature that could be included.
If the 2 authors disagreed on whether the literature should be included, a third author helped to suggest a solution.
Relevant database literature was screened by 2 independent authors using a blinded method and in strict accordance with the inclusion and exclusion criteria of the literature; those that met the requirements were included and those that did not were excluded. The extracted literature included the authors’ names, the location of the study, the time of publication, the number of included lesions, the number of benign and malignant lesions, the pathology of malignant nodules, and the sensitivity, specificity, and accuracy of the test to be evaluated.
Both stata 16.0 software and RevMan 5.3 software were used for statistical analysis in this study.
The statistical software was used to produce summary receiver operating characteristic (SROC) curves, publication bias funnel plots, and to calculate the sensitivity, specificity, and area under curve (AUC) of the diagnosis, respectively.
All included literature was evaluated for quality using RevMan 5.3, a revised tool for quality assessment of diagnostic accuracy studies, including patient selection, index tests, reference standards, processes, and timelines.
In this meta-analysis, 1405 original articles were retrieved based on the search terms “(Acoustic Radiation Force Impulse or ARFI or VTIQ or VTQ or Virtual Touch tissue imaging and quantification or Virtual Touch tissue quantification) and (thyroid or thyroid nodules)”. By carefully reading the titles and abstracts of the articles, 134 papers were initially included, and then the papers were strictly screened and excluded according to the inclusion and exclusion criteria, and finally 57 papers met the conditions of meta-analysis. The specific inclusion process of the literature is shown in Figure 1.
A total of 8802 thyroid nodules were included in the pooled 57 studies. The nature of all thyroid nodules in all included studies was histologically confirmed. Benign thyroid nodules included nodular goiter, eosinophilia, Hashimoto’s thyroiditis, subacute thyroiditis, and thyroid adenoma, while malignant thyroid nodules included papillary, follicular, undifferentiated, metastatic, and medullary carcinomas.
The pooled 57 papers were divided into two groups according to the different pathological characteristics of malignant nodes, with group A being studies in which all included malignant nodes were papillary carcinomas, 21 in total, and group B being studies in which included malignant nodes included medullary carcinomas, follicular carcinomas, undifferentiated carcinomas, and other metastatic carcinomas in addition to papillary carcinomas, 36 in total (see Tables 1 and 2 for the specific data of the two groups, respectively), and then classified according to VTQ and VTIQ two techniques were classified again, and groups A and B were divided into AVTQ group, AVTIQ group,BVTQ group and BVTIQ group, respectively. Regression analysis was done for each of the four data groups, and the data showed that the sensitivity, specificity, and AUC of the AVTQ group were 0.82 (95CI%, 0.76-0.87), 0.84 (95CI%, 0.78-0.89), and 0.90 (95CI%, 0.87-0.92) (Figure 2), respectively; the sensitivity, specificity, and AUC of the AVTIQ group were 0.75 (95CI%, 0.69-0.80), 0.83 (95CI%, 0.75-0.89) and 0.79 (95CI%, 0.75-0.82), respectively (Figure 3); the sensitivity, specificity and AUC of BVTQ group were 0.82 (95CI%, 0.77-0.85), (95CI%, 0.82-0.90) and 0.90 (95CI%, 0.87-0.93) (Figure 4); the sensitivity, specificity, and AUC of the BVTIQ group were 0.81 (95CI%, 0.76-0.86), 0.85 (95CI%, 0.74-0.91), and 0.89 (95CI%, 0.85-0.91), respectively (Figure 5). The data from the AVTQ group were compared with the BVTQ group and the AVTIQ group with the BVTIQ group. The results of data analysis showed that the difference in sensitivity and specificity between the AVTQ and BVTQ groups was small and not statistically significant (p < 0.01 for both sensitivity and specificity), and there was no difference in sensitivity but a difference in specificity between the AVTIQ and BVTIQ groups and the difference was statistically significant (p = 0.08 > 0.05).
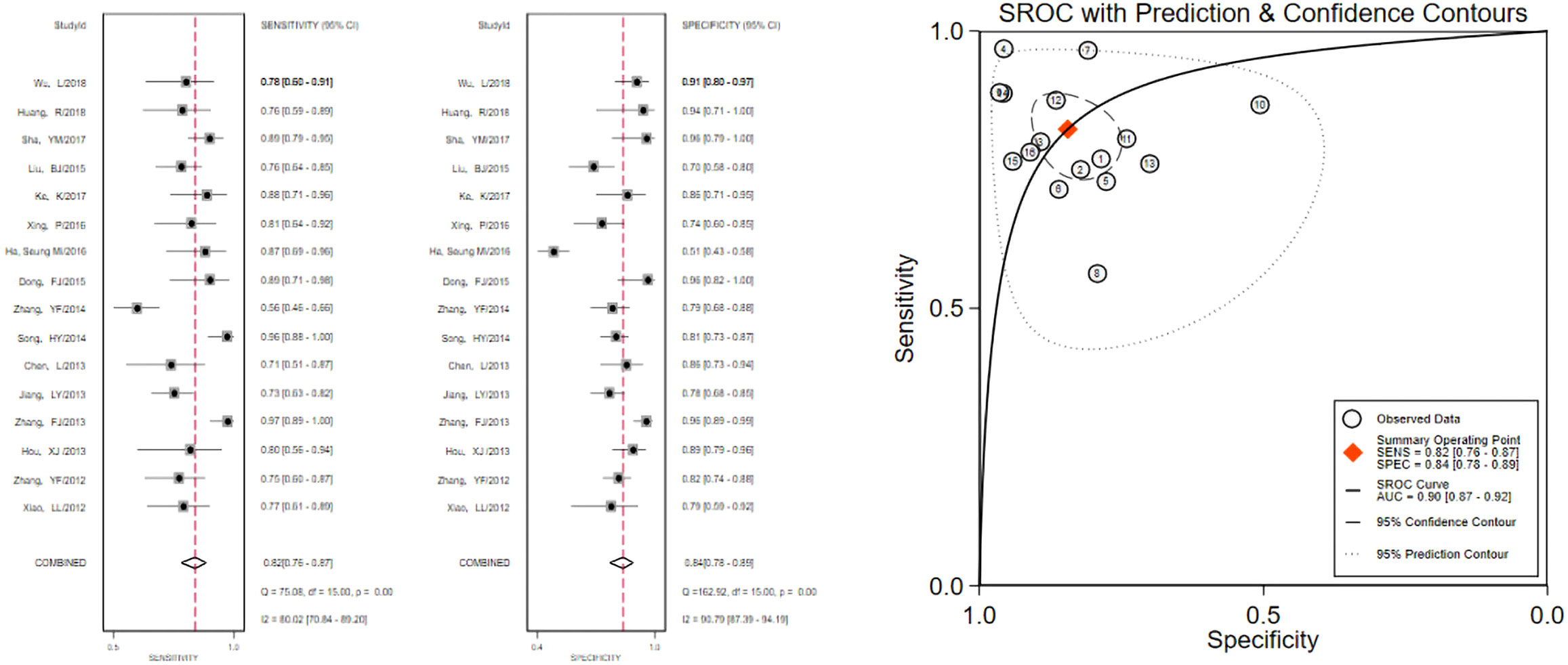
Figure 2 The sensitivity and specificity of the AVTQ group in the diagnosis of thyroid nodules and the summary ROC (Summary ROC) curve of the AVTQ group were analyzed. the AUC indicates the area under the curve.
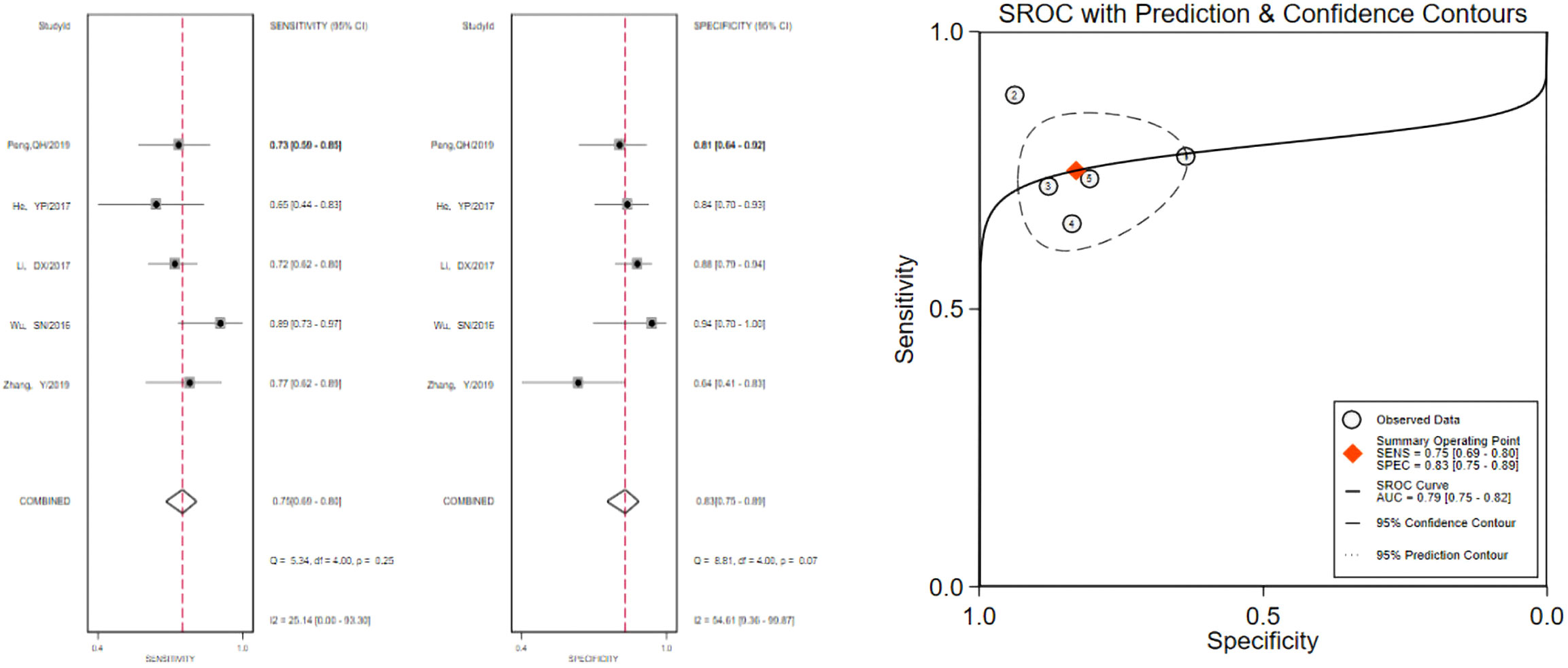
Figure 3 The sensitivity and specificity of the AVTIQ group in the diagnosis of thyroid nodules and the summary ROC (Summary ROC) curve of the AVTIQ group were analyzed. the AUC indicates the area under the curve.
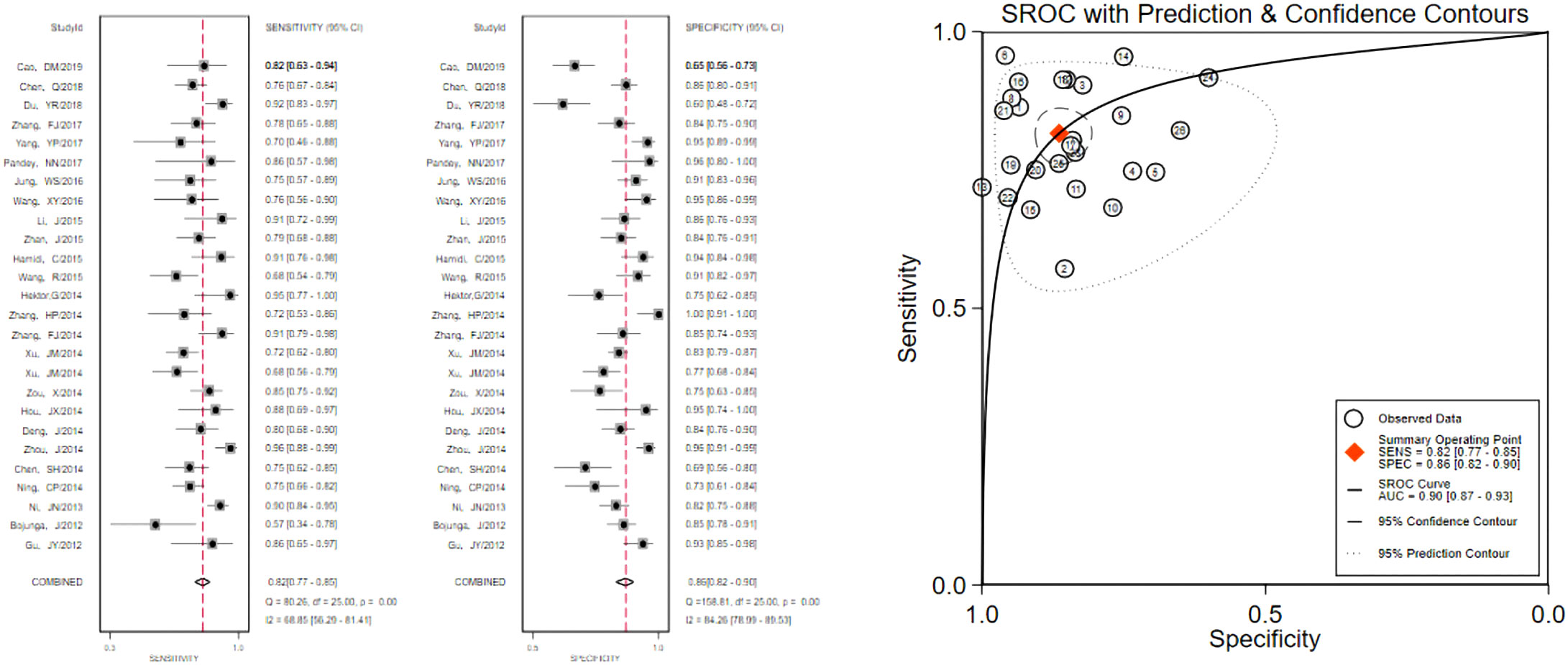
Figure 4 The sensitivity and specificity of the BVTQ group in the diagnosis of thyroid nodules and the summary ROC (Summary ROC) curve of the BVTQ group were analyzed. the AUC indicates the area under the curve.
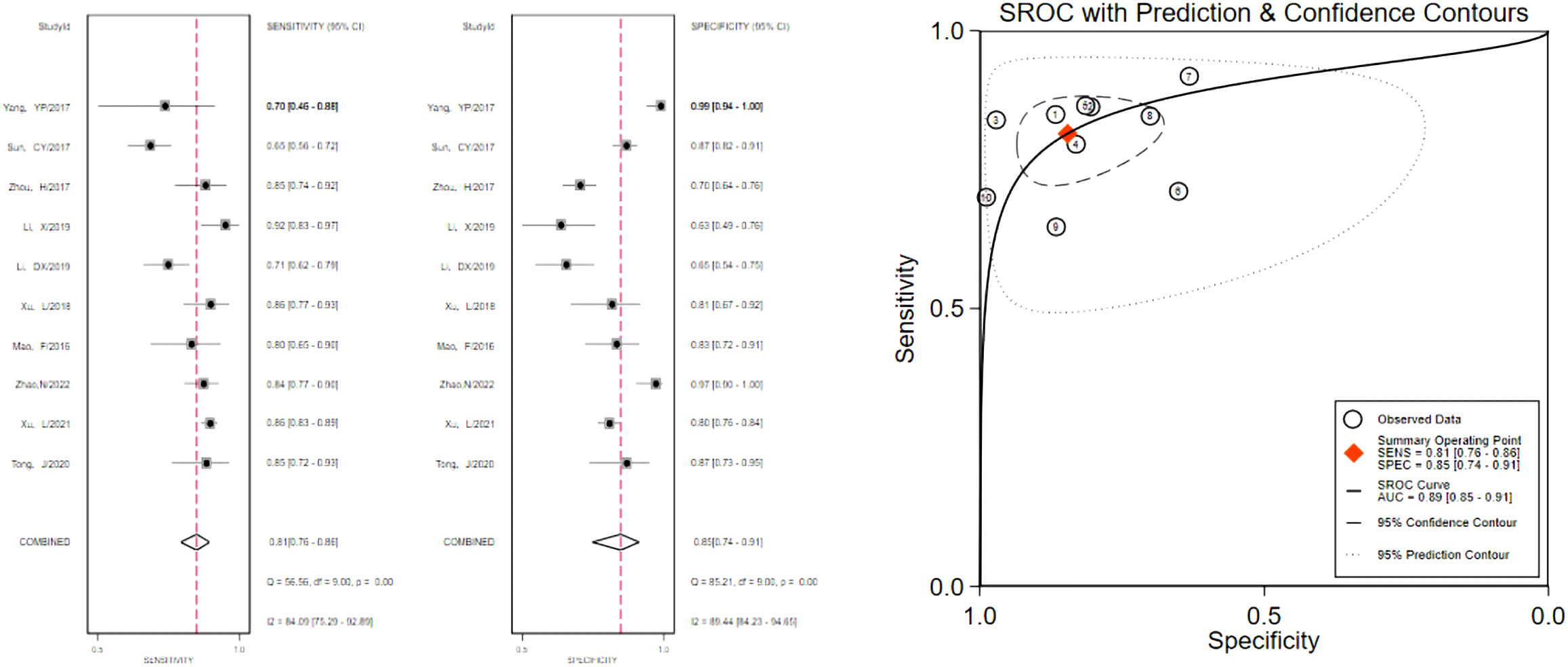
Figure 5 The sensitivity and specificity of the BVTIQ group in the diagnosis of thyroid nodules and the summary ROC (Summary ROC) curve of the BVTIQ group were analyzed. the AUC indicates the area under the curve.
Then, the 58 papers were divided into two groups according to the two techniques of VTIQ and VTQ, and each group was sorted according to the size of the cut-off value from smallest to largest, and then the sensitivity, specificity and AUC of each group were calculated in every three groups. The sensitivity, specificity, and AUC of VTQ were 0.91 (95CI%, 0.80-0.97), 0.88 (95CI%, 0.73-0.95), and 0.96 (95CI%, 0.93-0.97), respectively; when the cut-off value was in the interval of 3.01-3.15m/s, the diagnostic efficacy of VTIQ was the best. The best diagnostic performance of VTIQ was achieved when the cut-off value was in the interval of 3.01-3.15m/s, with sensitivity, specificity and AUC of 0.74 (95CI%, 0.59-0.58), 0.92 (95CI%, 0.75-0.98) and 0.88 (95CI%, 0.84-0.90), respectively.
When using meta-analysis in diagnostic trials, Deeks funnel plots are usually chosen to assess publication bias, and the results of Deeks funnel plots are shown in Figure 6. p > 0.05, suggesting no publication bias in this study.
All included literature was evaluated for quality using RevMan 5.3, and the results of the literature quality evaluation are shown in Figures 7 and 8.
In this study, the included literature was divided into four groups according to whether all malignant nodules were papillary thyroid carcinomas and the difference between VTQ and VTIQ. From the results, it is clear that there was a statistically significant difference in specificity between group A and group B only when VTIQ was used to identify benign and malignant thyroid nodules, and the specificity of diagnosis was better when non-papillary thyroid carcinomas were included in malignant thyroid nodules.
From past studies, it is known that non-thyroidal papillary carcinomas such as follicular and medullary carcinomas are pathologically different from papillary thyroid carcinomas, with follicular and medullary carcinomas having less fibrous content and more cellular components compared to papillary carcinomas. Papillary carcinomas are often accompanied by sand-like calcification formation, so the pathological specimens of papillary carcinomas are harder, while follicular and medullary carcinomas are softer in texture (11). However, the results of this study showed that there was no difference in the diagnostic efficacy of VTQ for malignant nodules regardless of whether they contained non-papillary thyroid carcinoma, whereas the specificity of VTIQ was superior for the group of malignant nodules containing non-papillary thyroid carcinoma.
This result was unexpected, for which several speculations were made: one, it may be because some malignant nodules such as follicular carcinoma and medullary carcinoma have more distinct ultrasound features due to their poor differentiation. According to the latest European Thyroid Association guidelines, when a lesion has one of the above features of irregular shape, irregular border, microcalcifications and deep hypoechogenicity, the nodule may be malignant up to 26-87%. The more malignant features a tumor has, the highest its risk of malignancy. In a study by Zhao,J 2020, it was shown that some medullary carcinomas have more obvious malignant ultrasound features, specifically the irregular morphology of the tumor, poor demarcation with surrounding tissues, solid hypoechoic or very hypoechoic, and intra-nodular calcification (69); secondly, it is also possible that there are many microscopic thyroid papillary carcinomas among the papillary thyroid carcinomas, and The ROI range of ARFI is 6mm×5mm, which is not suitable for the diagnosis of smaller nodules, and this may also be the reason for this result (70). For example, in the included study by Chen, SH in 2014, they included a total of 275 nodules and 23 microscopic papillary thyroid carcinomas out of 60 papillary thyroid carcinomas. The sensitivity of VTQ for thyroid nodules in that article was 75% and the specificity was 70% (44); in addition, all nodules included in Zhu,J’s article in 2015 were microscopic nodules, and the sensitivity of VTQ for diagnosing benign and malignant thyroid nodules in that study was 76.4% and the specificity was 75.8% (70).
In addition, the measurement range of SWV is 0.5-8.4 m/s, a characteristic that makes ARFI unable to achieve satisfactory measurement results for extremely hard or soft tissues, and the quality of imaging is difficult to guarantee (2). Therefore, it will have an unavoidable impact on the quality of the article. In addition to this, there are some studies that did not exclude nodules with a background of diffuse thyroid lesions when they were included. Pathologically, diffuse thyroid lesions are caused by infiltration of thyroid follicular cells by diffuse lymphocytes, destruction of follicles by atrophy and fibrosis, and these factors can make the texture of the thyroid gland harder (71). It is also possible that although the malignant nodules in group B included nonpapillary carcinomas, the proportion of nonpapillary carcinomas in thyroid cancer was so low that the results in this section were biased, but because of the low incidence of nonpapillary thyroid carcinomas and the paucity of data, there is not a large body of data to support the exclusion of this speculation.
The sensitivity, specificity and AUC of each group were calculated by comparing the data of each group and found that the best diagnostic efficacy was achieved when the cut-off values were in the range of 2.48-2.55m/s. The sensitivity, specificity and AUC of VTQ were 0.91 (95CI%, 0.80-0.97), 0.88 (95CI%, 0.73-0.95) and 0.96 (95CI%, 0.93-0.97), respectively, and the diagnostic efficacy of VTIQ was best when the cut-off value was in the range of 3.01-3.15m/s. The sensitivity, specificity and AUC of VTQ were best when the cut-off value was in the range of 3.01-3.15m/s. The diagnostic efficacy of VTIQ was best when the cut-off value was in the range of 3.01-3.15m/s, with sensitivity, specificity and AUC of 0.74 (95CI%, 0.59-0.58), 0.92 (95CI%, 0.75-0.98) and 0.88 (95CI%, 0.84-0.90), respectively.
This meta-analysis has several limitations. We searched only three databases, PubMed, Web of science, and Embase, suggesting that there may be relevant studies that were missed. Also, this meta-analysis included only English-language literature, so there may be language bias.
In summary, ARFI imaging is a highly effective imaging tool to identify benign and malignant thyroid nodules. There is no difference in the diagnostic effectiveness of VTQ for malignant nodules with or without non-papillary thyroid cancer, while VTIQ has a better specificity for the diagnosis of malignant nodules with non-papillary thyroid cancer. Therefore, ARFI imaging of benign and malignant thyroid nodules must take into account various clinical information of the patient and be analyzed critically to make a more accurate diagnosis.
JL, Y-RZ and X-WC contributed to the conception and design of the study. Y-RZ and P-SZ searched and reviewed studies, extracted and analyzed the data, and wrote the first draft of the manuscript. Q-LL, MC and J-YR reviewed and edited the manuscript. X-YG and T-TD directed the project and contributed to discussion as well as reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
(No. 2020-PT330-003):Supported by Open Research Fund of NHC Key Laboratory of Prevention and Treatment of Central Asia High Incidence Diseases. Supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences. (No. 2019DB012): The Corps Science and Technology Key Project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhuo J, Ma Z, Fu WJ, Liu SP. Differentiation of benign from malignant thyroid nodules with acoustic radiation force impulse technique. Br J Radiol (2014) 87(1035):20130263. doi: 10.1259/bjr.20130263
2. Liu BJ, Li DD, Xu HX, Guo LH, Zhang YF, Xu JM, et al. Quantitative shear wave velocity measurement on acoustic radiation force impulse elastography for differential diagnosis between benign and malignant thyroid nodules: A meta-analysis. Ultrasound Med Biol (2015) 41(12):3035–43. doi: 10.1016/j.ultrasmedbio.2015.08.003
3. Xu J, Xu X, Xu H, Zhang Y, Guo L, Liu L, et al. Prediction of cervical lymph node metastasis in patients with papillary thyroid cancer using combined conventional ultrasound, strain elastography, and acoustic radiation force impulse (Arfi) elastography. Eur Radiol (2016) 26(8):2611–22. doi: 10.1007/s00330-015-4088-2
4. Luo H, Xia X, Kim G, Xu H. Characterizing dedifferentiation of thyroid cancer by integrated analysis. Sci Adv (2021) 7(31):eabf3657. doi: 10.1126/sciadv.abf3657
5. La Rosa GL, Belfiore A, Giuffrida D, Sicurella C, Ippolito O, Russo G, et al. Evaluation of the fine needle aspiration biopsy in the preoperative selection of cold thyroid nodules. Cancer (1991) 67(8):2137–41. doi: 10.1002/1097-0142(19910415)67:8<2137::aid-cncr2820670822>3.0.co;2-y
6. Bojunga J, Herrmann E, Meyer G, Weber S, Zeuzem S, Friedrich-Rust M. Real-time elastography for the differentiation of benign and malignant thyroid nodules: A meta-analysis. Thyroid (2010) 20(10):1145–50. doi: 10.1089/thy.2010.0079
7. Ogilvie JB, Piatigorsky EJ, Clark OH. Current status of fine needle aspiration for thyroid nodules. Adv Surg (2006) 40:223–38. doi: 10.1016/j.yasu.2006.06.003
8. Al-azawi D, Mann GB, Judson RT, Miller JA. Endocrine surgeon-performed us guided thyroid fnac is accurate and efficient. World J Surg (2012) 36(8):1947–52. doi: 10.1007/s00268-012-1592-2
9. Bohacek L, Milas M, Mitchell J, Siperstein A, Berber E. Diagnostic accuracy of surgeon-performed ultrasound-guided fine-needle aspiration of thyroid nodules. Ann Surg Oncol (2012) 19(1):45–51. doi: 10.1245/s10434-011-1807-z
10. Tee YY, Lowe AJ, Brand CA, Judson RT. Fine-needle aspiration may miss a third of all malignancy in palpable thyroid nodules: A comprehensive literature review. Ann Surg (2007) 246(5):714–20. doi: 10.1097/SLA.0b013e3180f61adc
11. Shuzhen C. Comparison analysis between conventional ultrasonography and ultrasound elastography of thyroid nodules. Eur J Radiol (2012) 81(8):1806–11. doi: 10.1016/j.ejrad.2011.02.070
12. Chang N, Zhang X, Wan W, Zhang C, Zhang X. The preciseness in diagnosing thyroid malignant nodules using shear-wave elastography. Med Sci Monit (2018) 24:671–7. doi: 10.12659/msm.904703
13. Zhan J, Jin JM, Diao XH, Chen Y. Acoustic radiation force impulse imaging (Arfi) for differentiation of benign and malignant thyroid nodules–a meta-analysis. Eur J Radiol (2015) 84(11):2181–6. doi: 10.1016/j.ejrad.2015.07.015
14. Xiao L, Zhao Y, Gao L. Value of virtual touch tissue quantification technique in the diagnosis of small solid thyroid nodules. Chin J Ultrasonogr (2012) 21(9):771–4. doi: 10.3760/cma.j.issn.1004-4477.2012.09.012
15. Hou XJ, Sun AX, Zhou XL, Ji Q, Wang HB, Wei H, et al. The application of virtual touch tissue quantification (Vtq) in diagnosis of thyroid lesions: A preliminary study. Eur J Radiol (2013) 82(5):797–801. doi: 10.1016/j.ejrad.2012.12.023
16. Dong FJ, Xu JF, Liu HY. Virtual touch tissue quantification in differential diagnosis of benign and malignant thyroid nodules. Chin J Med Imaging Technol (2015) 31(3):347–50. doi: 10.13929/j.1003-3289.2015.03.007
17. Liu BJ, Zhao CK, Xu HX, Zhang YF, Xu JM, Li DD, et al. Quality measurement on shear wave speed imaging: Diagnostic value in differentiation of thyroid malignancy and the associated factors. ONCOTARGET (2017) 8(3):4948–59. doi: 10.18632/oncotarget.13996
18. Wu L, Li SY, Ni ZL. Virtual touch tissue quantification imaging in differential diagnosis of thyroid imaging report and data system 4 thyroid nodules. Chin J Interv Imaging Ther (2018) 15(12):732–5. doi: 10.13929/j.1672-8475.201807005
19. Chen L, Chen Y, Chen L, Zhan J. Comparison of ultrasound elastography imaging area ratio with acoustic radiation force impulse in differential diagnosis of thyroid nodules. Chin J Ultrasound (2013) 29(9):772–4. doi: 10.3969/j.issn.1002-0101.2013.09.002
20. Zhang YF, Liu C, Xu HX, Xu JM, Zhang J, Guo LH, et al. Acoustic radiation force impulse imaging: A new tool for the diagnosis of papillary thyroid microcarcinoma. BioMed Res Int (2014) 2014:416969. doi: 10.1155/2014/416969
21. Huang R, Wang XT, Wang R, Sha YM. Shear wave elastography and acoustic radiation force impulse imaging in differential diagnosis of benign and malignant thyroid nodules. Chin J Interv Imaging Ther (2018) 15(5):277–81. doi: 10.13929/j.1672-8475.201710050
22. Zhang FJ, Han RL. The value of acoustic radiation force impulse (Arfi) in the differential diagnosis of thyroid nodules. Eur J Radiol (2013) 82(11):e686–90. doi: 10.1016/j.ejrad.2013.06.027
23. Jiang LY, Cai YY, He YQ, Gu JY, Du LF, Yang YR. Grey-scale sonography and arfi-vtq in diagnosis of papillary thyroid microcarcinoma smaller than 1 Cm. TUMOR (2016) 36:202–7. doi: 10.3781/j.issn.1000-7431.2016.33.988
24. Sha YM, Wang XT, Wang R. Acoustic radiation force impulse imaging in differential diagnosis of small solid thyroid nodules coexisting with hashimoto’s thyroiditis. Chin J Interv Imaging Ther (2017) 14(8):504–8. doi: 10.13929/j.1672-8475.201703044
25. Song HY, Huang DZ, Yang JZ. Virtual touch tissue quantification in differential diagnosis of thyroid benign and malignant nodules and its infouence factors. Chin J Ultrasonogr (2014) 23(3):227–30. doi: 10.3760/cma.j.issn.1004-4477.2014.03.017
26. Xing P, Chen Q, Yang ZW, Liu CB, Wu CJ. Combination of conventional ultrasound and tissue quantification using acoustic radiation force impulse technology for differential diagnosis of small thyroid nodules. Int J Of Clin And Exp Med (2016) 9(5):8288–95. doi: 10.1055/s-0042-1757423
27. Ha SM, Cho SW. Virtual touch tissue quantification in the differential diagnosis of benign and malignant thyroid nodules. J Korean Soc Radiol (2016) 74(6):365. doi: 10.3348/jksr.2016.74.6.365
28. Ke K, Zhang QX, Wang ZG. Thyroid imaging reporting and data system, virtual tough tissues quantification technique and ceus in differential diagnosis of benign and malignant thyroid nodules. Chin J Interv Imaging Ther (2017) 14(5):287–91. doi: 10.13929/J.1672-8475.201610034
29. Zhang YF, Xu HX, He Y, Liu C, Guo LH, Liu LN, et al. Virtual touch tissue quantification of acoustic radiation force impulse: A new ultrasound elastic imaging in the diagnosis of thyroid nodules. PloS One (2012) 7(11):e49094. doi: 10.1371/journal.pone.0049094
30. Zhang YL, Yu K, Yang M, Zhang Y, Li JL, Tang J. Comparison of elasticity imaging and virtual touchtm tissue imaging and quantification in the diagnosis of thyroid nodules. Zhongguo yi xue ke xue yuan xue bao Acta Academiae Medicinae Sinicae (2019) 41(1):383–7. doi: 10.3881/j.issn.1000-503X.10772
31. Wu SN, He JD, Jiang TA, Zhong LY, Zhang XF Virtual touch tissue imaging quantification in differential diagnosis of benign and malignant Ti-rads 4 thyroid nodules. Chin J Ultrasonography (2016) 25(7):573–8. doi: 10.3760/cma.j.issn.1004-4477.2016.07.006
32. Li DX, Liu QQ, Guo BB, Tian J, Liu Q, Wu CJ. Study on the diagnostic values of virtual touch tissue imaging quantification and virtual touch tissue quantification techniques in differentiating thyroid benign and malignant nodules. Chin J Med Ultrasound(Electronic Edition) (2017) 14(10):749–54. doi: 10.3877/cma.j.issn.1672-6448.2017.10.007
33. He Y-P, Xu H-X, Li X-L, Li D-D, Bo X-W, Zhao C-K, et al. Comparison of virtual touch tissue imaging & quantification (Vtiq) and Toshiba shear wave elastography (T-swe) in diagnosis of thyroid nodules: Initial experience. Clin Hemorheology Microcirculation (2017) 66(1):15–26. doi: 10.3233/ch-16217
34. Peng Q, Niu C, Zhang M, Peng Q, Chen S. Sonographic characteristics of papillary thyroid carcinoma with coexistent hashimoto’s thyroiditis: Conventional ultrasound, acoustic radiation force impulse imaging and contrast-enhanced ultrasound. Ultrasound Med Biol (2019) 45(2):471–80. doi: 10.1016/j.ultrasmedbio.2018.10.020
35. Cao DM, Lin MF, Lin DM. Diagnosis of benign and malignant thyroid nodules by acoutic pulsed radiation elastography combined with conventional ultrasound. Chin J Ultrasound (2019) 35(7):577–80. doi: CNKI:SUN:ZGCY.0.2019-07-001
36. Deng J, Zhou P, Tian SM, Zhang L, Li JL, Qian Y. Comparison of diagnostic efficacy of contrast-enhanced ultrasound, acoustic radiation force impulse imaging, and their combined use in differentiating focal solid thyroid nodules. PloS One (2014) 9(3):e90674. doi: 10.1371/journal.pone.0090674
37. Wang R, Wang XT, Hou XJv. Virtual touch tissue quantification in differential diagnosis of solid thyroid nodules. Chin J Med Imaging Technol (2015) 31(10):1506–9. doi: 10.13929/j.1003-3289.2015.10.014
38. Ning CP, Xu LH, Fang SB, Sun YM, Wang JH. Clinical value and impact factors of virtual touch tissue quantification techniques in evaluating benign and malignant thyroid nodules. Chin J Ultrasonogr (2014) 23(7):594–7. doi: 10.3760/cma.j.issn.1004-4477.2014.07.013
39. Gu J, Du L, Bai M, Chen H, Jia X, Zhao J, et al. Preliminary study on the diagnostic value of acoustic radiation force impulse technology for differentiating between benign and malignant thyroid nodules. J Of Ultrasound In Med (2012) 31(5):763–71. doi: 10.7863/jum.2012.31.5.763
40. Wang XY, Chen XX, Ling B, Huang XL, He D, Zhang B. Value of contrast-enhanced ultrasound and acoustic radiation force impulse-imaging in differential diagnosis of benign and malignant thyroid nodules. Chin J Ultrasound Med (2016) 32(8):673–6. doi: 10.3969/j.issn.1002-0101.2016.08.001
41. Hamidi C, Goya C, Hattapoglu S, Uslukaya O, Teke M, Durmaz MS, et al. Acoustic radiation force impulse (Arfi) imaging for the distinction between benign and malignant thyroid nodules. Radiol Med (2015) 120(6):579–83. doi: 10.1007/s11547-014-0495-8
42. Bojunga J, Dauth N, Berner C, Meyer G, Holzer K, Voelkl L, et al. Acoustic radiation force impulse imaging for differentiation of thyroid nodules. PloS One (2012) 7(8):e42735. doi: 10.1371/journal.pone.0042735
43. Zhan J, Diao XH, Wan M, Wang HM, Chen Y. The re-evaluation of united stiffness score system for thyroid nodules. Chin J Ultrasound Med (2015) 31(11):964–7. doi: CNKI:SUN:ZGCY.0.2015-11-002
44. Chen S, Li Q, Li DD. Acoustic radiation force impulse technology in identification of benign and mlignant solid thyroid nodules. Chin J Med Imaging Technol (2014) 30(5):711–4. doi: 10.13929/j.1003-3289.2014.05.020
45. Hou JX, Wang XT, Wang R, et al. Applicative value of acoustic radiation force impluse imaging in differential diagnosis of small hypoechoic thyroid nodules. Chin J Ultrasonogr (2014) 23(1):27–30. doi: 10.3760/cma.j.issn.1004-4477.2014.01.008
46. Zou X, Li QS, Li QS, Chen SH, Luo CR, Xiong HH. Value of acoustic radiation force impulse imaging vtq in the differential diagnosis between benign and malignant thyroid nodules. Chin J Ultrasound Med (2014) 30(7):588–91. doi: CNKI:SUN:ZGCY.0.2014-07-005
47. Xu J, Xu X, Xu H, Zhang Y, Zhang J, Guo LH, et al. Conventional us, us elasticity imaging, and acoustic radiation force impulse imaging for prediction of malignancy in thyroid nodules. Radiology (2014) 272(2):577–86. doi: 10.1148/radiol.14132438
48. Chen Q, Xing P, Liu Q, Wu C-J. Malignancy risk assessment of category 4 nodules by applying acoustic radiation force impulse in thyroid imaging reporting and data system. Int J Of Clin And Exp Med (2018) 11(4):3473–83. doi: 10.2174/1573405619666221115135842
49. Yang Y-P, Xu X-H, Bo X-W, Liu B-J, Guo L-H, Xu J-M, et al. Comparison of virtual touch tissue imaging & quantification (Vtiq) and virtual touch tissue quantification (Vtq) for diagnosis of thyroid nodules. Clin Hemorheology Microcirculation (2017) 65(2):137–49. doi: 10.3233/ch-16142
50. Zhang FJ, Zhao XM, Han RL, Du M, et al. Comparison of acoustic radiation force impulse imaging and strain elastography in differentiating malignant from benign thyroid nodules. J Ultrasound Med (2017) 36(12):2533–43. doi: 10.1002/jum.14302
51. Li J, Xu SZ, Du TT, et al. Conventional ultrasound, ultrasonic elastography and acoustic radiation force impulse elastography in the diagnostic tests of benign and malignant thyroid nodules. Chin Gen Pract (2015) 18(6):720–3. doi: 10.3969/j.issn.1007-9572.2015.06.028
52. Xu JM, Xu HX, Xu XH, Liu C, Zhang YF, Guo LH, et al. Solid hypo-echoic thyroid nodules on ultrasound: The diagnostic value of acoustic radiation force impulse elastography. Ultrasound Med Biol (2014) 40(9):2020–30. doi: 10.1016/j.ultrasmedbio.2014.04.012
53. Zhang FJ, Han RL, Zhao XM. The value of virtual touch tissue image (Vti) and virtual touch tissue quantification (Vtq) in the differential diagnosis of thyroid nodules. Eur J Radiol (2014) 83(11):2033–40. doi: 10.1016/j.ejrad.2014.08.011
54. Du Y-R, Ji C-L, Wu Y, Gu X-G. Combination of ultrasound elastography with Ti-rads in the diagnosis of small thyroid nodules (≤10 mm): A new method to increase the diagnostic performance. Eur J Radiol (2018) 109:33–40. doi: 10.1016/j.ejrad.2018.10.024
55. Zhang HP, Shi QS, Gu JY, Jiang LY, Bai M, Liu L, et al. Combined value of virtual touch tissue quantification and conventional sonographic features for differentiating benign and malignant thyroid nodules smaller than 10 mm. J Ultrasound Med (2014) 33(2):257–64. doi: 10.7863/ultra.33.2.257
56. Ni JN, Huang PT, Zhang HW, Su N, et al. Diagnostic value of virtual touch tissue quantification in diacriminating thyroid benign and malignant nodule. Chin J Ultrasonogr (2013) 22(2):137–40. doi: 10.3760/cma.j.issn.1004-4477.2013.02.015
57. Jung WS, An YY, Ihn YK, Park YH. The diagnostic performance of acoustic radiation force impulse elasticity imaging to differentiate malignant from benign thyroid nodules: Comparison with conventional b-mode sonographic findings. J Korean Soc Radiol (2016) 74(2):96. doi: 10.3348/jksr.2016.74.2.96
58. Pandey NN, Pradhan GS, Manchanda A, Garg A. Diagnostic value of acoustic radiation force impulse quantification in the differentiation of benign and malignant thyroid nodules. Ultrason Imaging (2017) 39(5):326–36. doi: 10.1177/0161734617706170
59. Grazhdani H, Cantisani V, Lodise P, Di Rocco G, Proietto MC, Fioravanti E, et al. Prospective evaluation of acoustic radiation force impulse technology in the differentiation of thyroid nodules: Accuracy and interobserver variability assessment. J Ultrasound (2014) 17(1):13–20. doi: 10.1007/s40477-013-0062-5
60. Tong J, Huang L, Li J, Cao CL, Du TT Virtual touch tissue imaging quantification combined with ceus in differential diagnosis of benign and malignant Ti-rads 4 thyroid nodules. Chin J Med Imaging Technol (2020) 36(6):828–33. doi: 10.13929/j.issn.1003-3289.2020.06.006
61. Xu L, Zhou Y, Li Y, Lu B, Liu T Reducing unnecessary biopsy of American college of radiology thyroid imaging reporting and data system category 4 nodules. J Ultrasound Med (2020) 40(2):227–36. doi: 10.1002/jum.15391
62. Zhao N, Chen J, Yao M, Han R. The diagnostic value of the different acoustic radiation force impulse technique for solid thyroid nodules with different diameters: A case–control study. Asian J Surg (2022) 45(11):2246–52. doi: 10.1016/j.asjsur.2021.12.056
63. Mao F, Xu HX, Zhang SM, Zhang YF, Bo XY, Li XL, et al Study on the application of virtual touch tissue imaging quantification in combination with the 2015 guidelines of American thyroid association for differentiating benign thyroid nodules from malignant thyroid nodules. Chin Gen Pract (2016) 19(21):2585–90. doi: 10.3969/j.issn.1007-9572.2016.21.019
64. Xu L, Zhou YB, Xu C, Tian G, Jiang TA The value of virtual touch tissue imaging quantification and real-time elastography techniques in the differentiation of thyroid imaging reporting and data syatem 4 nodules of thyroid. Chin J Med Ultrasound(Electronic Edition) (2018) 15(1):53–8. doi: 10.3877/cma.j.issn.1672-6448.2018.01.010
65. Li DX, Chen Q, Liu QQ, Tian J, Wu CJ Study on the diagnostic vaiue of acoustic radiation for impulse imaging combined with acr-tirads in differentiating thyroid benign and malignant noduies Co-exists with hashimoto’s thyroiditis. Chin J Ultrasonic Med (2019) 35(11):961–4. doi: CNKI:SUN:ZGCY.0.2019-11-034
66. Li X, Hou X-J, Du L-Y, Wu J-Q, Wang L, Wang H, et al. Virtual touch tissue imaging and quantification (Vtiq) combined with the American college of radiology thyroid imaging reporting and data system (Acr Ti-rads) for malignancy risk stratification of thyroid nodules. Clin Hemorheology Microcirculation (2019) 72(3):279–91. doi: 10.3233/ch-180477
67. Zhou H, Zhou X-L, Xu H-X, Li D-D, Liu B-J, Zhang Y-F, et al. Virtual touch tissue imaging and quantification in the evaluation of thyroid nodules. J Ultrasound Med (2017) 36(2):251–60. doi: 10.7863/ultra.15.12070
68. Sun C-Y, Lei K-R, Liu B-J, Bo X-W, Li X-L, He Y-P, et al. Virtual touch tissue imaging and quantification (Vtiq) in the evaluation of thyroid nodules: The associated factors leading to misdiagnosis. Sci Rep (2017) 7(1):41958. doi: 10.1038/srep41958
69. Zhao J, Yang F, Wei X, Mao Y, Mu J, Zhao L, et al. Ultrasound features value in the diagnosis and prognosis of medullary thyroid carcinoma. Endocrine (2020) 72(3):727–34. doi: 10.1007/s12020-020-02510-2
70. Zhu J, Zhan J, Diao XH, Wan M. The value of combined stiffness score system for the diagnosis of thyroid micronodules. Chin J Med Ultrasound(Electronic Edition) (2015) 12(10):768–72. doi: 10.3877/cma.j.issn.1672-6448.2015.10.006
Keywords: non-papillary thyroid cancer (NPTC), virtual touch quantification (VTQ), virtual touch tissue imaging and quantification (VTIQ), shear wave velocity (SWV), meta-analysis
Citation: Li J, Zhang Y-R, Ren J-Y, Li Q-L, Zhu P-S, Du T-T, Ge X-Y, Chen M and Cui XW (2023) Association between diagnostic efficacy of acoustic radiation force impulse for benign and malignant thyroid nodules and the presence or absence of non-papillary thyroid cancer: A meta-analysis. Front. Oncol. 13:1007464. doi: 10.3389/fonc.2023.1007464
Received: 30 July 2022; Accepted: 09 January 2023;
Published: 27 January 2023.
Edited by:
Rosaria Maddalena Ruggeri, University of Messina, ItalyCopyright © 2023 Li, Zhang, Ren, Li, Zhu, Du, Ge, Chen and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, MTI4NzQyNDc5OEBxcS5jb20=; Xin Wu Cui, Y3VpeGlud3VAbGl2ZS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.