- 1Department of Dermatology, Yale School of Medicine, New Haven, CT, United States
- 2Department of Therapeutic Radiology, Yale School of Medicine, New Haven, CT, United States
- 3Department of Surgery, Yale School of Medicine, New Haven, CT, United States
- 4Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT, United States
Objective: The role of radiation therapy (RT) in melanoma has historically been limited to palliative care, with surgery as the primary treatment modality. However, adjuvant RT can be a powerful tool in certain cases and its application in melanoma has been increasingly explored in recent years. The aim of this study is to explore national patterns of care and associations surrounding the use of adjuvant RT for stage III melanoma.
Methods: The National Cancer Data Base (NCDB) was used to identify patients who were diagnosed with stage III melanoma between 2004 and 2014. Exclusion criteria included those with distant metastatic disease, in-situ histology, no confirmed positive nodes, palliative intent therapy, and dosing regimens inconsistent with National Comprehensive Cancer Network (NCCN) guidelines for adjuvant RT in melanoma. Patients treated with and without adjuvant RT were compared and factors associated with use of adjuvant RT were identified using multivariable logistic regression analyses.
Results: A total of 7,758 cases of stage III melanoma were analyzed, of which 11.7% received adjuvant RT. The mean age of the overall cohort was 58.5 years, and the majority of patients were male (64.7%), white (96.6%), on private insurance (51.3%), and presented to a non-high-volume facility (90.3%). Multivariable regression analyses revealed that patients who present to the hospital in 2009-2014 as compared to 2004-2008 (odds ratio [OR] 1.61, 95% confidence interval [CI] 1.36-1.92), had 4 or more positive nodes (OR 4.30, 95% CI 3.67-5.04), and had microscopic residual tumor (OR 2.11, 95% CI 1.46-3.04) were more likely to receive adjuvant RT. Factors that were negatively associated with receiving adjuvant RT included female gender (OR 0.72, 95% CI 0.61-0.85) and median income of at least $63,000 (OR 0.66, 95% CI 0.52-0.83).
Conclusions: This study demonstrates the rising use of RT for stage III melanoma in recent years and identifies demographic, social, clinical, and hospital-specific factors associated with patients receiving adjuvant RT. Further investigation is needed to explore disease benefits to improve guidance on the utilization of RT in melanoma.
1 Introduction
Melanoma has historically been thought to be radio-resistant, and postoperative radiation therapy (RT) has typically been sparingly used. Although the role of RT in melanoma remains controversial, RT has been established as a palliative treatment option for unresectable melanoma, and the ANZMTG 01.02/TROG 02.01 trial observed that RT following lymphadenectomy for selected patients with node-positive melanoma may reduce risk of locoregional recurrence (1).
Although surgery is the mainstay of treatment for melanoma, multiple factors affect the efficacy and eligibility for surgery. Depending on the site of malignancy, resection with completely negative margins may be difficult, if not impossible, to achieve. Comorbidities, unresectable disease, and patient preference are other possible reasons for consideration of other treatment modalities for melanoma. In these cases in which an operative approach is not recommended or not possible, RT as definitive therapy can be considered (2). Several investigators have additionally examined the impact of adjuvant RT on survival and locoregional control in melanoma (3–5). According to the National Comprehensive Cancer Network (NCCN) 2023 Guidelines, adjuvant RT can be considered for patients with high-risk desmoplastic melanoma, those at high risk for regional recurrence after resection of the primary tumor, and in cases with brain metastases (2). Despite some evidence of benefit, there is a lack of clinical framework to guide the utilization of RT in stage III melanoma and optimal dosing regimens are not well-established. No study to our knowledge has examined the factors associated with the use of adjuvant RT in melanoma overall. To this end, our study seeks to examine the trends of adjuvant RT utilization over time, and to identify demographic, social, clinical, and hospital-specific factors associated with receipt of adjuvant RT.
The aim of this study is to explore the national patterns of care regarding utilization of adjuvant RT for stage III melanoma.
2 Methods
2.1 Data source
The National Cancer Data Base (NCDB) is a joint project by the American Cancer Society and the American College of Surgeons Commission on Cancer (CoC) that includes about 70% of the newly diagnosed cases of cancer in the United States from about 1,500 hospitals with CoC-accredited cancer programs. All of the data included in the NCDB is compliant with the privacy requirements of the Health Insurance Portability and Accountability Act (HIPAA). Institutional review board approval was not needed for this study since no patient, provider, or hospital identifiers were examined.
2.2 Study population
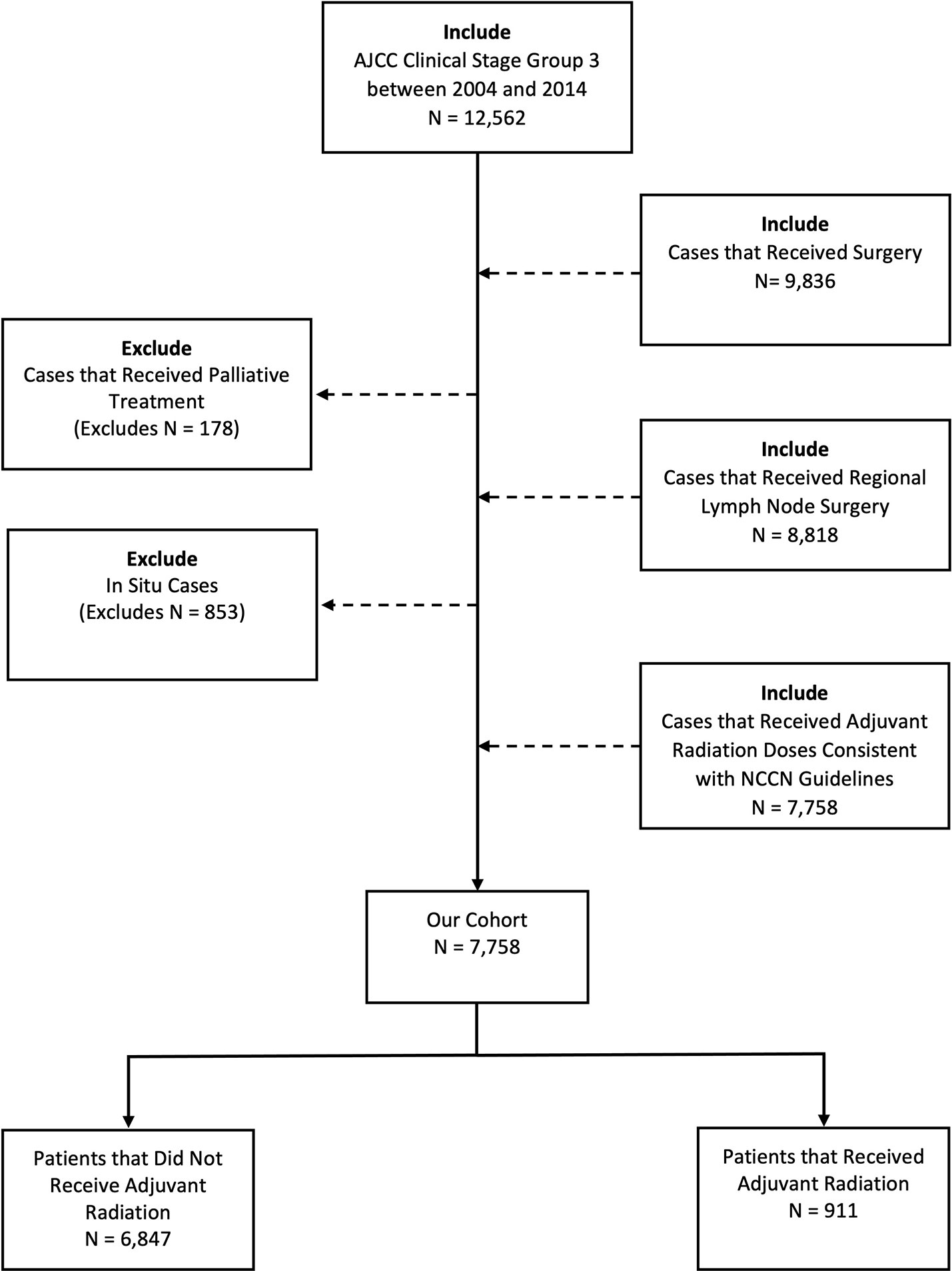
The NCDB was queried for patients diagnosed with stage III melanoma between 2004 and 2014. These years were chosen around the ANZMTG 01.02/TROG 02.01 trial and were further subcategorized into two groups, 2004-2008 and 2009-2014, to achieve an approximately even bifurcation. Our inclusion criteria included patient age older than 18 years and clinical stage III disease. Patients with distant metastatic disease, in-situ histology, no confirmed positive nodes, palliative intent therapy, and dosing regimens inconsistent with National Comprehensive Cancer Network (NCCN) guidelines for adjuvant RT in melanoma were excluded (Figure 1). Potential dosing regimens of adjuvant RT per NCCN guidelines include 30 Gy in 5 fractions over 2 weeks, 48 Gy in 20 fractions over 4 weeks, and 60-66 Gy in 30-33 fractions over 6-7 weeks (2). As such, cases with adjuvant radiation doses >66 Gy were excluded.
2.3 Statistical analysis
Baseline differences in demographic, socioeconomic, and hospital characteristics between patients treated with and without adjuvant radiation were assessed by chi-square and ANOVA testing. Statistical significance for these analyses was set at p<0.001, and hypothesis testing was two-sided. Multivariable logistic regression analyses were performed to identify factors associated with the use of adjuvant radiation. Demographic and clinical factors that were found to be significant on univariate analysis were included in the multivariable model. Age category, high facility volume status (>90th percentile volume), and Charlson-Deyo Score were also included in the multivariable model. These demographic, socioeconomic, clinical, and hospital-related factors were all included in the multivariable models. Stata Version 13.1 (StataCorp LP, College Station, TX) was used to perform data analyses.
3 Results
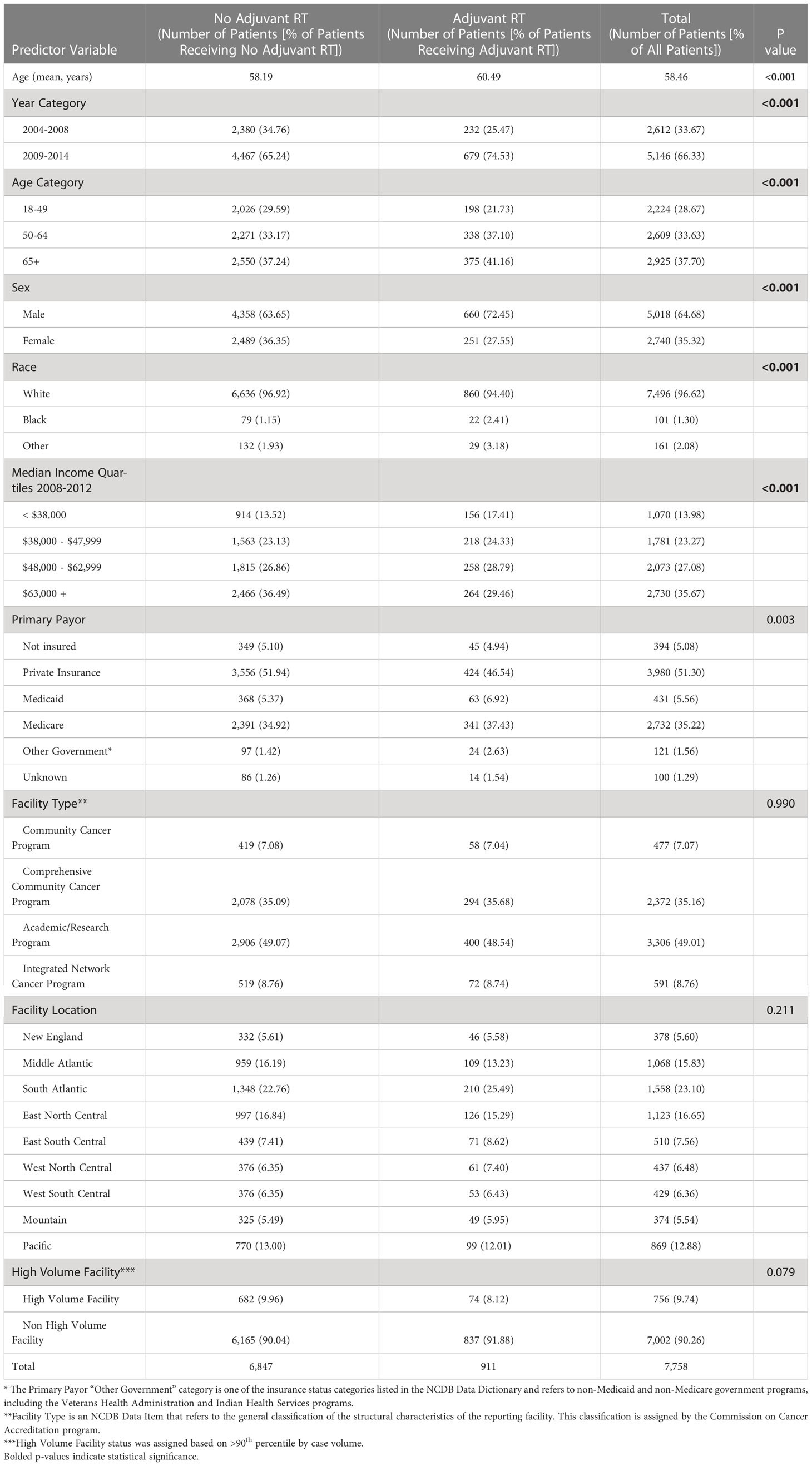
From 2004-2014, there were a total of 7,758 cases of stage III melanoma in the NCDB, of which 911 (11.7%) received adjuvant RT. Baseline characteristics of patients that did and did not receive adjuvant RT are outlined in Table 1. The mean age of the overall cohort was 58.5 years. The majority of patients presented in 2009-2014 (66.3%), were male (64.7%), white (96.6%), used private insurance (51.3%), and went to a non-high-volume facility (90.3%).
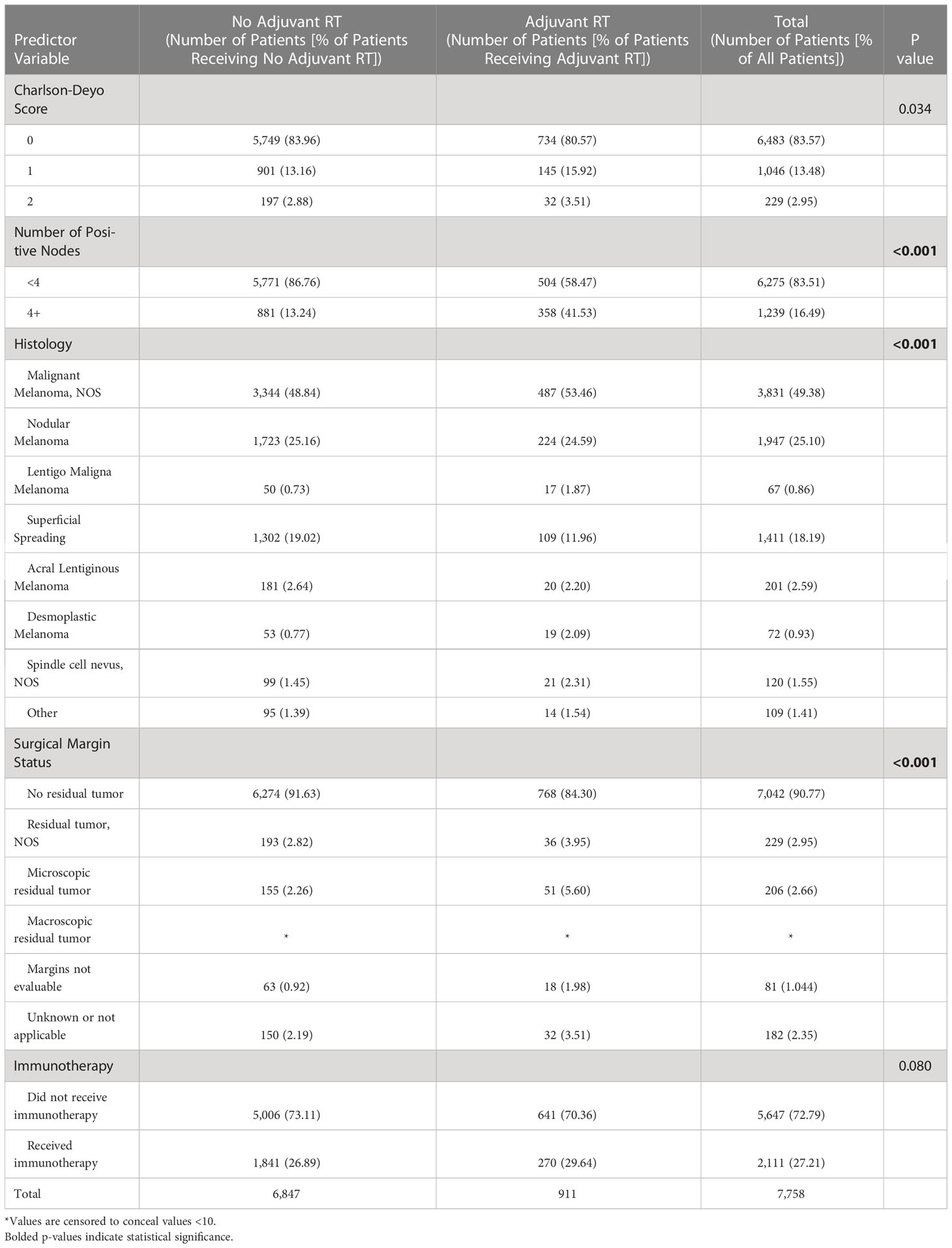
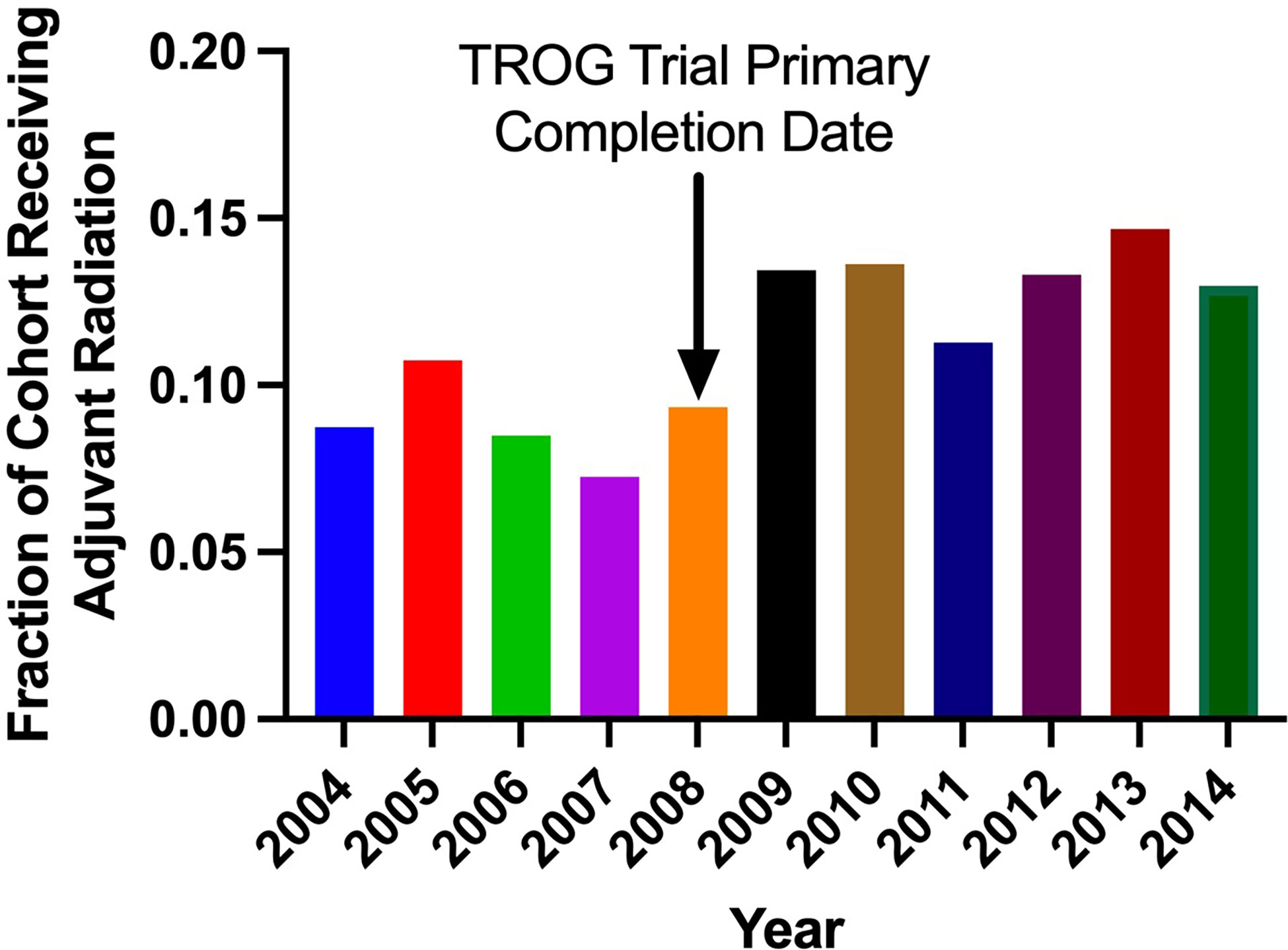
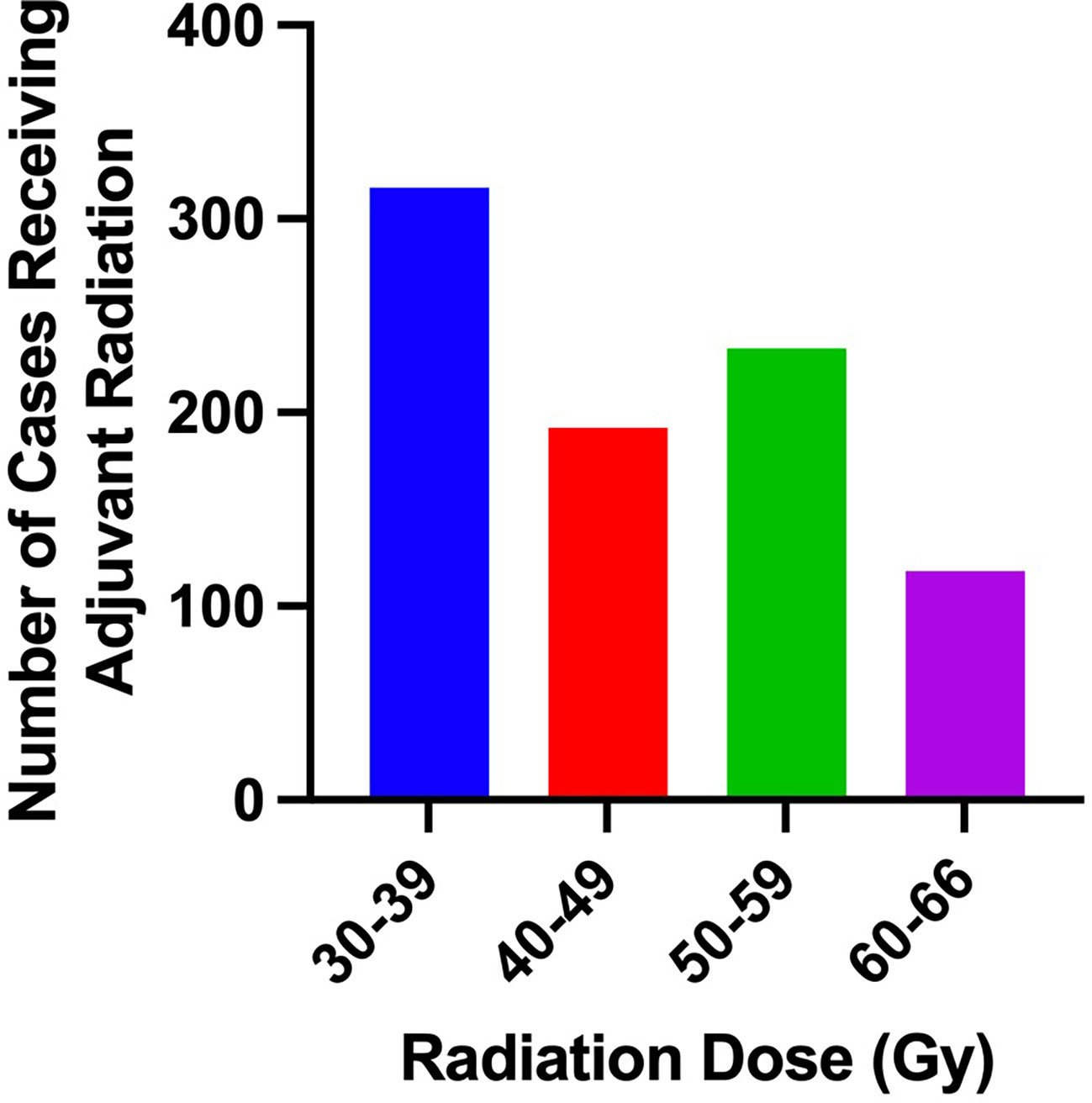
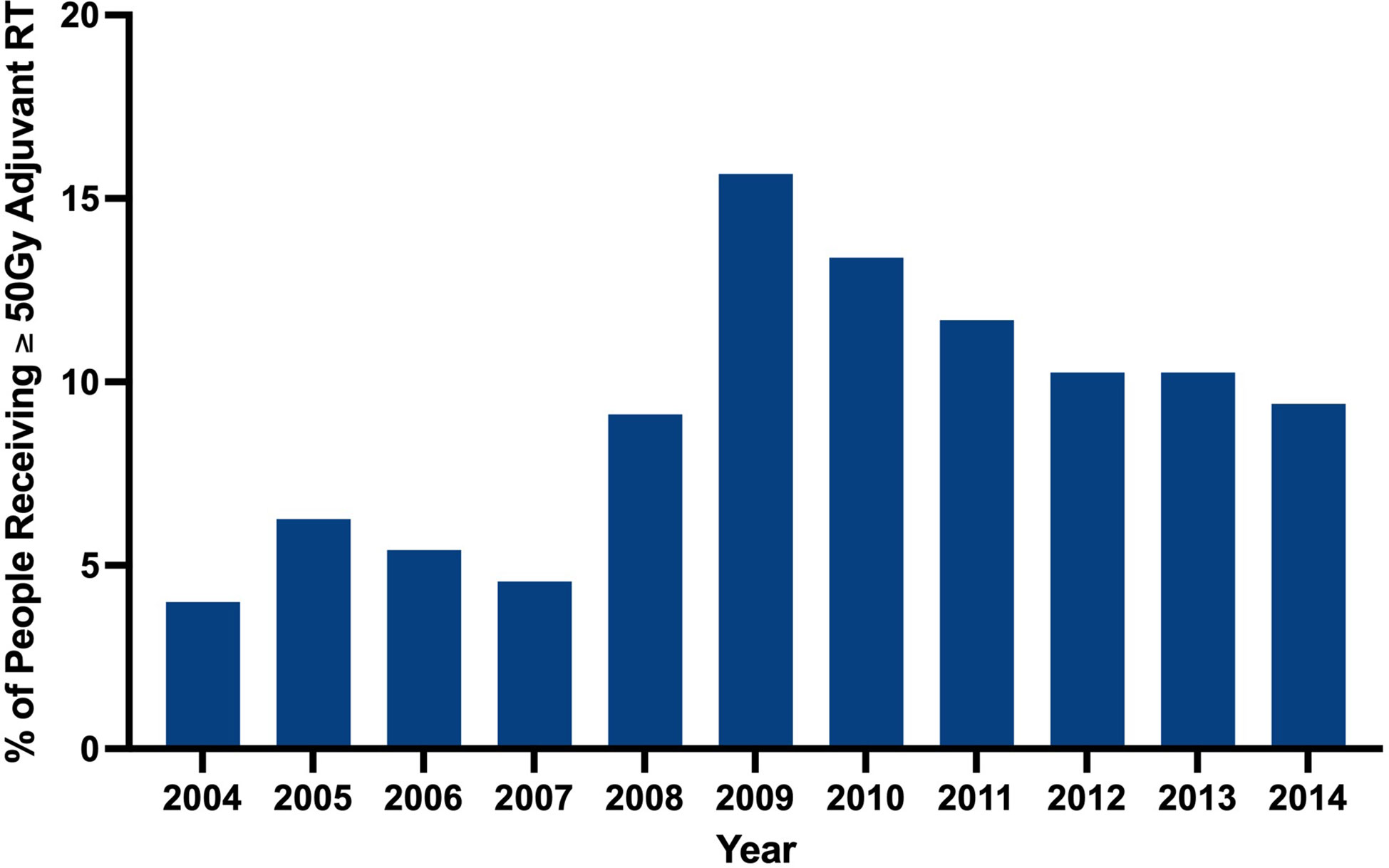
Clinical characteristics of patients that did and did not receive adjuvant RT are outlined in Table 2. The proportion of patients receiving adjuvant RT in each year are displayed in Figure 2 and the dosages received by the patients are displayed in Figure 3. The proportion of patients receiving at least 50 Gy of radiation are displayed in Figure 4. The majority of patients had a Charlson-Deyo score of 0 (83.6%), less than 4 positive nodes (83.5%), and no residual tumor (90.8%).
On multivariable regression, multiple patient and hospital-related factors were associated with use of adjuvant RT. Patients who presented to the hospital in 2009-2014 as compared to 2004-2008 (odds ratio [OR] 1.61; 95% confidence interval [CI] 1.36-1.92), had 4 or more positive nodes (OR 4.30, 95% CI 3.67-5.04), had desmoplastic melanoma (OR 3.15, 95% CI 1.77-5.59), and had microscopic residual tumor (OR 2.11, 95% CI 1.46-3.04) were more likely to receive adjuvant RT. Female gender (OR 0.72, 95% CI 0.61-0.85), median income of at least $63,000 (OR 0.66, 95% CI 0.52-0.83), and a diagnosis of superficial spreading melanoma (OR 0.62, 95% CI 0.49-0.78) were negatively associated with receiving adjuvant RT (Table 3).
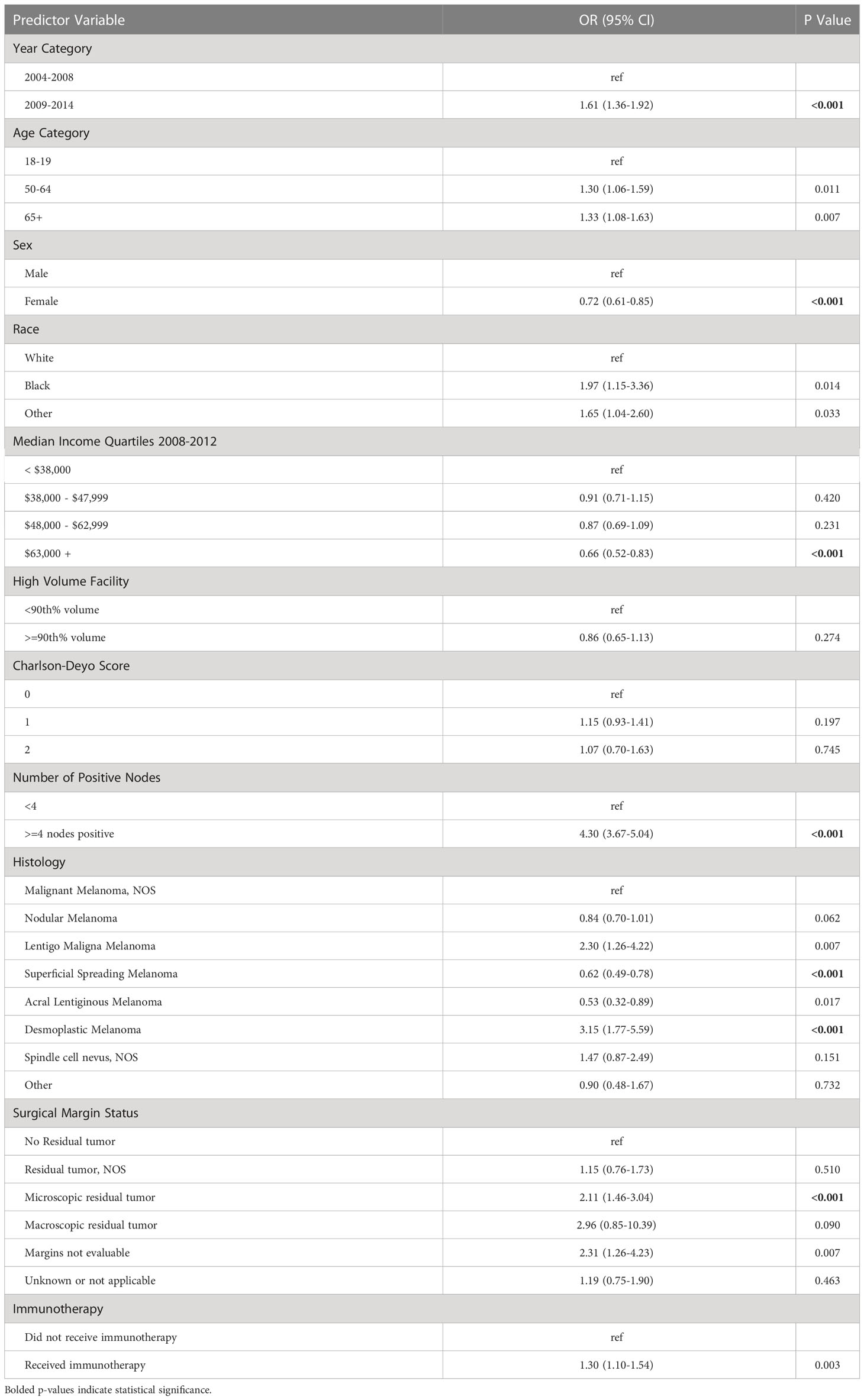
Table 3 Multivariable model for demographic, hospital-related factors, and disease characteristics associated with adjuvant radiation use.
4 Discussion
While surgery remains the mainstay of treatment for melanoma, for unresectable cases, high-risk surgical candidates, and those at high risk of toxicity to systemic adjuvant therapy, RT can be a powerful treatment modality. Although melanoma has been widely considered radio-resistant, with the past role of RT limited to palliative care, the applications for adjuvant RT in melanoma has been explored extensively in recent years. A phase II study TROG 96.06 found that the use of adjuvant RT in patients with melanoma involving lymph nodes was associated with a high rate of locoregional control (91%) (6). This study was followed by randomized study ANZMTG 01.02/TROG 02.01, which demonstrated that RT following lymphadenectomy for selected patients with node-positive melanoma reduces risk of locoregional recurrence (1). In this trial, the patients who derived the greatest benefit from treatment with adjuvant RT were those with pathological evidence of extracapsular extension (ECE) of nodal disease (1). Notably, we were unable to identify and thus analyze this subgroup of patients in our study due to database limitations. Despite evidence of benefit, there is no established consensus or clinical framework to guide the utilization of RT in melanoma. To this end, our study seeks to identify demographic, social, clinical, and hospital-specific factors associated with receipt of adjuvant RT and to examine the trends of adjuvant RT utilization over time.
4.1 Trend of adjuvant RT usage
Our analysis found that RT was utilized significantly more for stage III melanoma in more recent years (2004-2008 vs. 2009-2014; p<0.001). The wealth of recent studies in the literature that explore the use of RT in melanoma corroborate this observed trend. In a retrospective analysis of 1,675 patients with extracranial metastatic melanoma by Gabani et al., it was suggested that RT plays an increasing role in the management of metastatic melanoma in the era of immunotherapy (7). The rising use of RT in melanoma within the past decade may reflect the updated reappraisal of the value of adjuvant RT in melanoma. Our results suggest that higher RT dosages have been employed in more recent years despite lack of well established guidelines to dictate adjuvant RT dosing.
4.2 Demographic factors associated with adjuvant RT usage
Our analysis found that patients with stage III melanoma of higher income were significantly less likely to receive adjuvant RT (p<0.001). The discrepancy in disease severity upon presentation among different socioeconomic classes may serve as a possible explanation. In a retrospective review of 49,772 patients with cutaneous melanoma, Cormier et al. found that overall 5-year survival was significantly less in minorities than in their white counterparts, even after adjusting for age, sex, and region (8). Notably, minorities were significantly more likely to present with stage IV melanoma than were whites. These racial and socioeconomic disparities in severity of melanoma at presentation is corroborated by many other studies in the literature (9–11). Patients of lower income quartiles may be more likely to receive adjuvant RT possibly due to increased severity of disease that is not amenable to surgery upon presentation (12). Although our study did not find a statistically significant difference in receipt of RT with respect to insurance status (p=0.003), disparities in outcomes with respect to insurance status have been reported previously in the literature. In an epidemiological analysis of 26,958 melanoma patients by Kooistra et al., Medicaid patients were more likely to present with a late stage (9). Another retrospective study examining the association of health insurance with outcomes in 61,650 melanoma patients by Amini et al. found that patients with Medicaid or no insurance were more likely to present with increased tumor thickness and ulceration (12). A nationwide review of 15,941 metastatic melanoma patients conducted by Hague et al. revealed that African American race, Medicaid, and lower income status were associated with significantly decreased receipt of immunotherapy. In light of the social disparities in receipt of immunotherapy for melanoma (13), it is conceivable that disparities also exist in the receipt of RT.
4.3 Clinical factors associated with adjuvant RT usage
Increased disease burden, unresectable disease, and patients with high risk of locoregional spread are likely strong indicators for use of adjuvant RT. Consistent with this hypothesis, our study found that patients with at least 4 positive lymph nodes were more likely to receive adjuvant RT (p<0.001). The ANZMTG 01.02/TROG 02.01 trial stratified patients into categories of 4 or more involved lymph nodes and less than 4 involved lymph nodes, suggesting an important clinical distinction in the number of involved nodes (1). The trial additionally stratified patients based on ECE, finding that patients with ECE of nodal disease achieved the greatest benefit from treatment with adjuvant RT (1). Multiple studies in the literature have examined the impact of adjuvant RT on locoregional control in melanoma. In a retrospective review of 160 patients with cervical lymph node metastases from melanoma, Ballo et al. found that adjuvant RT use resulted in a 10-year regional control rate of 94% (3). In a study conducted by Owens et al. that examined the role of postoperative RT in mucosal melanoma of the head and neck, adjuvant RT versus surgery alone demonstrated a nonsignificant trend toward a lower rate of locoregional recurrence (p=0.13) (14).
Our analysis also found that patients with desmoplastic melanoma were also more likely to receive adjuvant RT than patients with other subtypes of melanoma (p<0.001). In line with this finding, in a retrospective study of 130 patients with nonmetastatic desmoplastic melanoma, Guadagnolo et al. found that adjuvant RT provided superior local control compared to surgery alone (15). This finding is reflected in the NCCN guideline to consider adjuvant RT in cases of high-risk desmoplastic melanoma (2).
Although a clear relationship between Charlson-Deyo score and receipt of RT was not identified in our study, the influence of increased comorbidities on treatment strategies in melanoma merits further investigation. It is possible that patients with increased disease burden and comorbidities may be deemed at higher risk for surgical complications, and thus, RT may be considered a more viable treatment option for such patients.
4.4 Potential disease benefits of adjuvant RT
Several studies have sought to examine the benefits of adjuvant RT for the treatment of melanoma. In a randomized phase III trial of 215 patients who underwent local treatment of one to three melanoma brain metastases assigned to whole brain radiation therapy or observation cohorts, no clinical benefit was observed (16). In a retrospective study of 56 patients with high-risk melanoma, Chang et al. found that adjuvant RT provided excellent locoregional control (4). Furthermore, they found that hypofractionation was equally effective as conventional fractionation. In a retrospective analysis of 200 melanoma patients with axillary metastases, Beadle et al. found that adjuvant RT to the axilla produced equivalent locoregional control when compared to adjuvant RT to both the axilla and supraclavicular fossa (5). The ANZMTG 01.02/TROG 02.01 trial was a randomized control trial that examined the use of adjuvant RT in melanoma patients who had undergone lymphadenectomy and were at high risk of recurrence (1). The trial found that adjuvant RT did not improve overall survival or relapse-free survival in the patient population analyzed, but did improve locoregional control.
The benefit of using RT in melanoma as adjuvant treatment has become particularly controversial in recent years given the promising results associated with the use of modern systemic therapies such as immune checkpoint and BRAF/MEK inhibitors, several of which have been approved for resected stage IIB, IIC, and III melanoma (17). Currently, systemic therapies approved as adjuvant therapy for melanoma include pembrolizumab, ipilimumab, and nivolumab as well as dabrafenib plus trametinib for patients with a BRAF V600 mutation (18). Our study revealed that patients who received immunotherapy were more likely to have received adjuvant RT, although this finding was not significant (p=0.003). Of note, since NCDB does not provide drug-specific data, we were unable to analyze the association between the specific type of immunotherapy and adjuvant RT.
Several studies have examined the combination of systemic therapy and RT in treating melanoma. In a retrospective cohort study examining 98 patients with melanoma lymph node metastases who underwent lymphadenectomy, patients receiving combined systemic and radiation therapies exhibited a lower 3-year cumulative incidence of lymph node basin recurrence relative to patients receiving only systemic therapy; however, the difference was not statistically significant (19). Additionally, no significant difference was noted in terms of in-transit/distant recurrences, disease-free survival, or melanoma-specific survival. In a retrospective study of 23 patients with mucosal melanoma, target lesion control rate at 1-year follow up was significantly higher in the cohort that received RT and pembrolizumab as compared to either treatment used alone (20). This suggests that immune checkpoint therapy may have a potential radiosensitizing effect on tumors. Several clinical studies support the existence of an interaction between immunotherapy and RT (21). In a phase I clinical trial, among seven patients with metastatic melanoma who were treated with stereotactic body radiation therapy followed by interleukin-2 (IL-2), 71% achieved a complete or partial response compared to 16% that was previously reported in association with IL-2 monotherapy (22, 23). Another retrospective study of melanoma patients with brain metastases revealed that 40% of those treated with ipilimumab prior to RT exhibited a partial response to RT whereas only 9% achieved a partial response among those who were not treated with ipilimumab (24). These results support the possibility that immunotherapy and RT act synergistically in the treatment of melanoma and can be used in combination as adjuvant therapy for stage III disease.
With the advent and increasing number of promising adjuvant systemic therapies for melanoma over the past decade, the role of adjuvant RT has potentially been superseded for most patients. However, adjuvant RT can be a powerful treatment modality particularly for patients who are not candidates for adjuvant systemic options. Patient characteristics that may favor RT over systemic therapies for adjuvant treatment include lack of a targetable BRAF mutation, high risk of toxicity to systemic therapies including those with a history of autoimmune disease, and/or patient preference. In these cases, consideration of adjuvant RT may be beneficial. Patients with significant ECE on pathology should also be considered for adjuvant RT with or without concurrent systemic therapy.
4.5 Limitations
The limitations of this study are inherent to retrospective database studies. The NCDB may contain coding and reporting biases and misclassified or incomplete data. Furthermore, relapse patterns could not be identified due to the lack of coding for local or distant recurrence, and we were unable to access detailed pathology information including the presence or absence of ECE. Nevertheless, this study elucidates trends of adjuvant RT utilization over time, and identifies demographic, social, clinical, and hospital-specific factors associated with receipt of adjuvant RT. Further investigation into the disease benefits of RT for the treatment of melanoma is warranted.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
AK, BY, and FM contributed to writing the manuscript. CZ, BY, and VL conducted the statistical analyses. DY, TT, JL, and YA provided oversight and guidance for this study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Yale Skin Cancer SPORE Career Enhancement Program Grant (TT). This publication was made possible in part by the Yale Clinical and Translational Science Award #TL1 TR001864 from the National Center for Advancing Translational Sciences of the National Institutes of Health and the Yale Center for Clinical Investigation (FM).
Conflict of interest
JL serves on the La Roche Posay Advisory Board.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Henderson MA, Burmeister BH, Ainslie J, Fisher R, Di Iulio J, Smithers BM, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol (2015) 16(9):1049–60. doi: 10.1016/S1470-2045(15)00187-4
2. Swetter SM, Thompson JA, Johnson D, Albertini MR, Barker CA, Baumgartner J, et al. Melanoma: Cutaneous (Version 1.2023). NCCN Clinical Practice Guidelines in Oncology. (2022).
3. Ballo MT, Bonnen MD, Garden AS, Myers JN, Gershenwald JE, Zagars GK, et al. Adjuvant irradiation for cervical lymph node metastases from melanoma. Cancer (2003) 97(7):1789–96. doi: 10.1002/cncr.11243
4. Chang DT, Amdur RJ, Morris CG, Mendenhall WM. Adjuvant radiotherapy for cutaneous melanoma: Comparing hypofractionation to conventional fractionation. Int J Radiat oncol biol phys (2006) 66(4):1051–5. doi: 10.1016/j.ijrobp.2006.05.056
5. Beadle BM, Guadagnolo BA, Ballo MT, Lee JE, Gershenwald JE, Cormier JN, et al. Radiation therapy field extent for adjuvant treatment of axillary metastases from malignant melanoma. Int J Radiat oncol biol phys (2009) 73(5):1376–82. doi: 10.1016/j.ijrobp.2008.06.1910
6. Burmeister BH, Mark Smithers B, Burmeister E, Baumann K, Davis S, Krawitz H, et al. A prospective phase II study of adjuvant postoperative radiation therapy following nodal surgery in malignant melanoma-trans Tasman radiation oncology group (TROG) study 96.06. Radiother Oncol J Eur Soc Ther Radiol Oncol (2006) 81(2):136–42. doi: 10.1016/j.radonc.2006.10.001
7. Gabani P, Robinson CG, Ansstas G, Johanns TM, Huang J. Use of extracranial radiation therapy in metastatic melanoma patients receiving immunotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol (2018) 127(2):310–7. doi: 10.1016/j.radonc.2018.02.022
8. Cormier JN, Xing Y, Ding M, Lee JE, Mansfield PF, Gershenwald JE, et al. Ethnic differences among patients with cutaneous melanoma. Arch Internal Med (2006) 166(17):1907–14. doi: 10.1001/archinte.166.17.1907
9. Kooistra L, Chiang K, Dawes S, Gittleman H, Barnholtz-Sloan J, Bordeaux J. Racial disparities and insurance status: An epidemiological analysis of Ohio melanoma patients. J Am Acad Dermatol (2018) 78(5):998–1000. doi: 10.1016/j.jaad.2017.11.019
10. Harvey VM, Patel H, Sandhu S, Wallington SF, Hinds G. Social determinants of racial and ethnic disparities in cutaneous melanoma outcomes. Cancer control J Moffitt Cancer Center (2014) 21(4):343–9. doi: 10.1177/107327481402100411
11. Dawes SM, Tsai S, Gittleman H, Barnholtz-Sloan JS, Bordeaux JS. Racial disparities in melanoma survival. J Am Acad Dermatol (2016) 75(5):983–91. doi: 10.1016/j.jaad.2016.06.006
12. Amini A, Rusthoven CG, Waxweiler TV, Jones BL, Fisher CM, Karam SD, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the united states. J Am Acad Dermatol (2016) 74(2):309–16. doi: 10.1016/j.jaad.2015.09.054
13. Haque W, Verma V, Butler EB, Teh BS. Racial and socioeconomic disparities in the delivery of immunotherapy for metastatic melanoma in the united states. J immunother (Hagerstown Md 1997). (2019) 42(6):228–35. doi: 10.1097/CJI.0000000000000264
14. Owens JM, Roberts DB, Myers JN. The role of postoperative adjuvant radiation therapy in the treatment of mucosal melanomas of the head and neck region. Arch otolaryngol–head Neck surg (2003) 129(8):864–8. doi: 10.1001/archotol.129.8.864
15. Guadagnolo BA, Prieto V, Weber R, Ross MI, Zagars GK. The role of adjuvant radiotherapy in the local management of desmoplastic melanoma. Cancer (2014) 120(9):1361–8. doi: 10.1002/cncr.28415
16. Hong AM, Fogarty GB, Dolven-Jacobsen K, Burmeister BH, Lo SN, Haydu LE, et al. djuvant Whole-Brain Radiation Therapy Compared With Observation After Local Treatment of Melanoma Brain Metastases: A Multicenter, Randomized Phase III Trial. Journal of clinical oncology. Journal of clinical oncology (2019) 37(33):3132–41. doi: 10.1200/JCO.19.01414
17. Dimitriou F, Long GV, Menzies AM. Novel adjuvant options for cutaneous melanoma. Ann Oncol (2021) 32(7):854–65. doi: 10.1016/j.annonc.2021.03.198
18. Seth R, Messersmith H, Kaur V, Kirkwood JM, Kudchadkar R, McQuade JL, et al. Systemic therapy for melanoma: ASCO guideline. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(33):3947–70. doi: 10.1200/JCO.20.00198
19. Straker RJ 3rd, Song Y, Sun J, Shannon AB, Cohen LS, Muradova E, et al. Adjuvant radiation therapy for clinical stage III melanoma in the modern therapeutic era. Ann Surg Oncol (2021) 28(7):3512–21. doi: 10.1245/s10434-020-09384-8
20. Kim HJ, Chang JS, Roh MR, Oh BH, Chung K-Y, Shin SJ, et al. Effect of radiotherapy combined with pembrolizumab on local tumor control in mucosal melanoma patients. Front Oncol (2019) 9:835. doi: 10.3389/fonc.2019.00835
21. Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: A review of clinical outcomes. Int J Radiat oncol biol phys (2014) 88(5):986–97. doi: 10.1016/j.ijrobp.2013.08.035
22. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol Off J Am Soc Clin Oncol (1999) 17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105
23. Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2–tumor and immunological responses. Sci Transl Med (2012) 4(137):137ra74. doi: 10.1126/scitranslmed.3003649
Keywords: adjuvant radiation therapy, melanoma, radiation oncology, skin neoplasm, trends, NCDB
Citation: King ALO, Lee V, Yu B, Mirza FN, Zogg CK, Yang DX, Tran T, Leventhal J and An Y (2023) Factors associated with the use of adjuvant radiation therapy in stage III melanoma. Front. Oncol. 13:1005930. doi: 10.3389/fonc.2023.1005930
Received: 28 July 2022; Accepted: 18 January 2023;
Published: 01 February 2023.
Edited by:
Silvia Cammelli, University of Bologna, ItalyReviewed by:
Bryan Burmeister, GenesisCare, AustraliaWen Jiang, University of Texas MD Anderson Cancer Center, United States
Copyright © 2023 King, Lee, Yu, Mirza, Zogg, Yang, Tran, Leventhal and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi An, eWkuYW5AeWFsZS5lZHU=
 Amber L. O. King
Amber L. O. King Victor Lee
Victor Lee Beverly Yu
Beverly Yu Fatima N. Mirza
Fatima N. Mirza Cheryl K. Zogg3
Cheryl K. Zogg3 Daniel X. Yang
Daniel X. Yang Yi An
Yi An