- Department of Gastrointestinal Surgery, Clinical Medical College and The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
Background: Single-incision laparoscopy surgery (SILS) is a new laparoscopic technique that has emerged in the past decade. Whether it has advantages over conventionl laparoscopy surgery (CLS) is inconclusive. This article aimed to compare the short- and long-term outcomes of single-incision laparoscopic surgery and conventional laparoscopic surgery for colorectal cancer through high-quality literature text mining and meta-analysis.
Methods: Relevant articles were searched on the PubMed, Embase, and Cochrane Library databases from January 2012 to November 2021. All data was from randomized controlled trials (RCTs) in order to increase the confidence of the analytical results.The main outcomes were intraoperative and postoperative complications.
Results: A total of 10 RCTs were included, involving 1609 patients. The quality of the included studies was generally high. No significant difference was found between SILS and CLS in the postoperative complications, operation time, postoperative hospital stay, number of lymph nodes removed, readmission, reoperation, complication level I- II, complication level IIIa, complication level IIIb, prolonged Ileus, blood loss, infection, anastomotic leakage and operation time. The results showed that SILS group had a higher rate of intraoperative complications, but it had lower incision length and better cosmetic effects.
Conclusion: These results indicate that SILS did not have a comprehensive and obvious advantage over the CLS. On the contrary, SILS has higher intraoperative complications, which may be related to the more difficulty of SILS operation, but SILS still has better cosmetic effects, which is in line with the concept of surgical development. Therefore, the SILS needs to be selected in patients with higher cosmetic requirements and performed by more experienced surgeons.
Introduction
The incidence and mortality of colorectal cancer are disproportionately high, especially among men (1). In the past 60 years, general surgery has radically changed to minimally invasive surgery techniques to enhance the recovery rate, which became increasingly popular in the clinic (2). Conventional laparoscopy surgery (CLS) can decrease postoperative pain and accelerate patient recovery (3). Minimally invasive surgery has continued to play an important role as an alternative to traditional open surgery. Laparoscopic surgery demonstrated faster functional recovery rates, fewer postoperative complications, shorter length of the incision, and shorter hospital stay when compared with open surgery. Therefore, laparoscopic surgery has been recognized and recommended as a choice for colorectal cancer surgery without surgical contraindications (4). In order to pursue less trauma and better cosmetic effects, surgeons invented SILS in 2008. SILS not only strengthens the advantages of traditional laparoscopic surgery, but also has less surgical trauma, which represents the evolution of minimally invasive surgery towards scarless surgery (5, 6). However, Single-incision laparoscopy surgery (SILS) resented some new technical challenges compared with CLS (7, 8), for example, the limited number of working instruments which makes it difficult to achieve correct exposure and the necessary traction to tissues. Limited external working space, multiple instruments, and laparoscopies required for a procedure compete for the same space at the entry port, leading to external hand collisions and difficulty in internal manipulation of the instrument tip compared with CLS. Difficult to maintain pneumoperitoneum. The skills required for SILS differ from those required for CLS, SILS requires colorectal surgeons with superb laparoscopic skills, and surgeons need a learning curve cycle of 30-60 cases (9).
Whether SILS has advantages over conventional laparoscopy (CLS) is inconclusive. However several high-quality RCTs comparing single-incision with conventional laparoscopic surgery for colorectal cancer were reported. This systematic review and meta-analysis aimed to compare the efficacy and safety of SILS and CLS for colorectal cancer. The study included only RCTs.
Methods
Search strategy and inclusion criteria
We conducted this study according to the PRISMA guidelines (preferred reporting program for systematic review and meta-analysis), and obtained relevant information on SILS and CLS from PubMed, Embase, and Cochrane databases (10, 11). The following terms were searched: single port, single incision, reduce port, laparoscopy, laparoscopic surgery. In order to extend the search, the related-articles function was adopted. Only the most recent or complete report was adopted if multiple repeated studies were found. The search was completed on December 24, 2021. Comparative studies were included only if they had at least one available primary or secondary outcome for evaluation. Articles such as reviews, letters, editorials, case reports, animal experimental studies, and meeting abstracts were excluded.
First, all the identified titles and abstracts were examined by two independent reviewers. Next, the same two reviewers independently examined the full text of potentially relevant articles. In the event of disagreement, a third reviewer was consulted and the relevant articles were discussed until a consensus was reached.
Data extraction and quality assessment
The following relevant information was extracted from all the included publications: reference, country/area, sample size, age, gender (M/F), BMI, tumor grade, study design. The main outcomes were intraoperative complications, postoperative complications. The secondary outcomes included operation time, postoperative hospital stay, number of lymph nodes removed, readmission, reoperation, complication level I-II, complication level IIIa, complication level IIIb, prolonged Ileus, blood loss, infection, anastomotic leakage, total incision length.
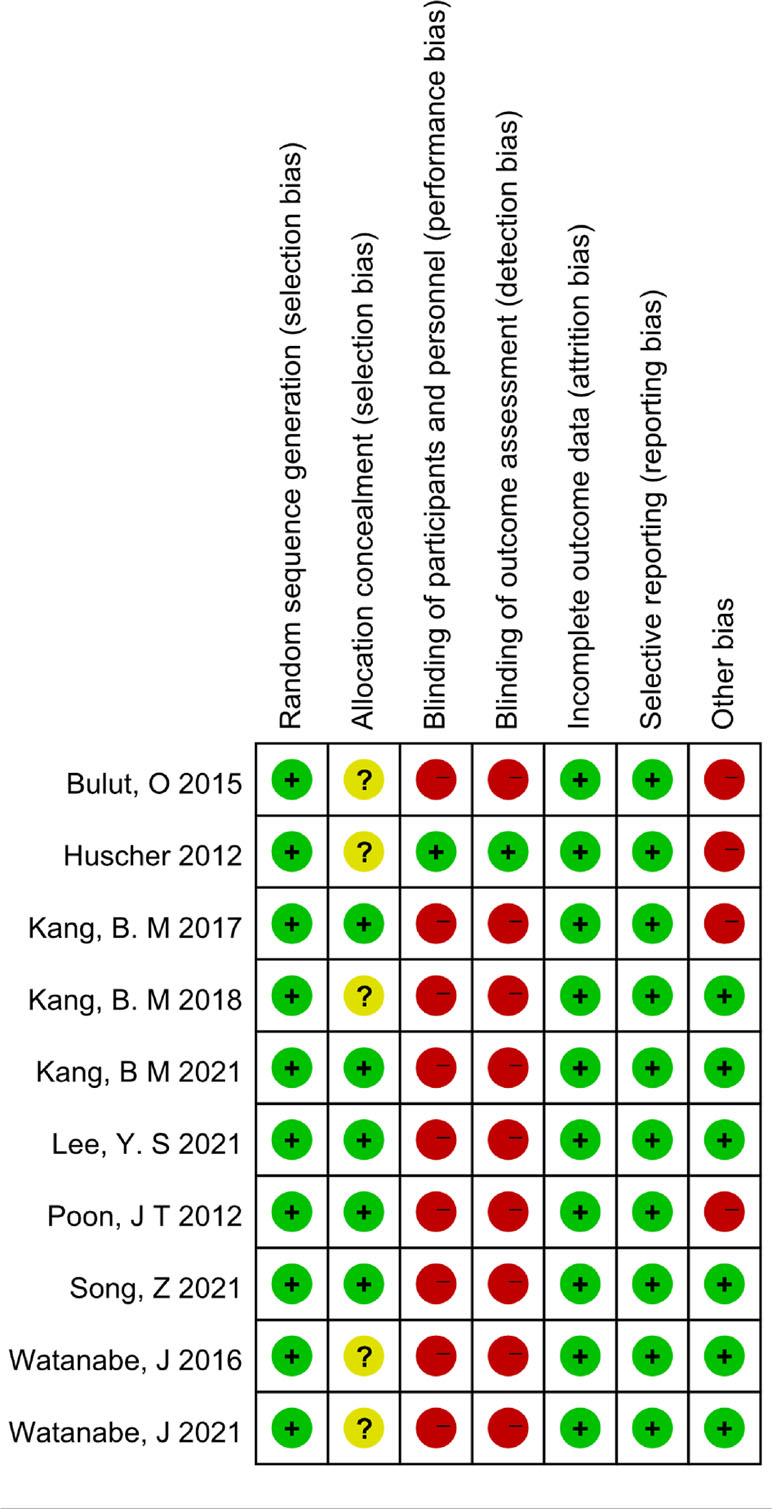
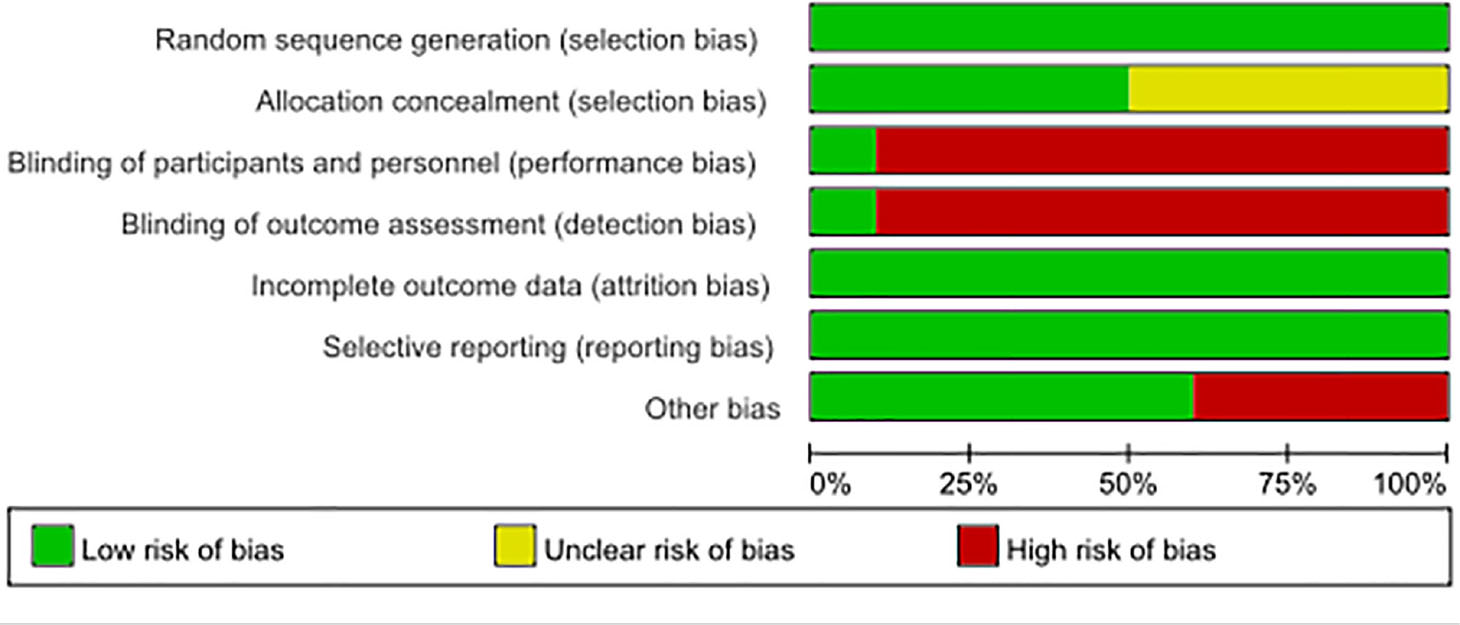
A 7-point Cochrane scale was used to assess the quality of the identified studies and includes seven assessment items: Random sequence generation (1 point), Allocation concealment (1 point), Blinding of participants and personnel (1 point), Blinding of outcome assessment (1 point), Incomplete outcome data (1 point), Selective reporting (1 point), Other bias (1 point), A score of 0 to 7 was assigned, and higher scores indicated higher quality. Any study scoring at least 4 was considered to have high methodology quality, and disagreements between the two researchers (Zeng DX and Li FH) were resolved by a third researcher (Tan L) who would make the final decision (Tables 1, 2)
Statistical analysis
Continuous variables and dichotomous variables were analyzed using weighted mean difference (WMD) and odds ratio (OR) respectively. The Chi2 and I2 statistics were used to assess the heterogeneity between studies, and the random-effects model was adopted if there was obvious heterogeneity between studies (p<0.05). Publication biases were determined using the funnel plot analyses. The analyses were performed with Review Manager (version 5.4.)
Results
Description of included and excluded studies
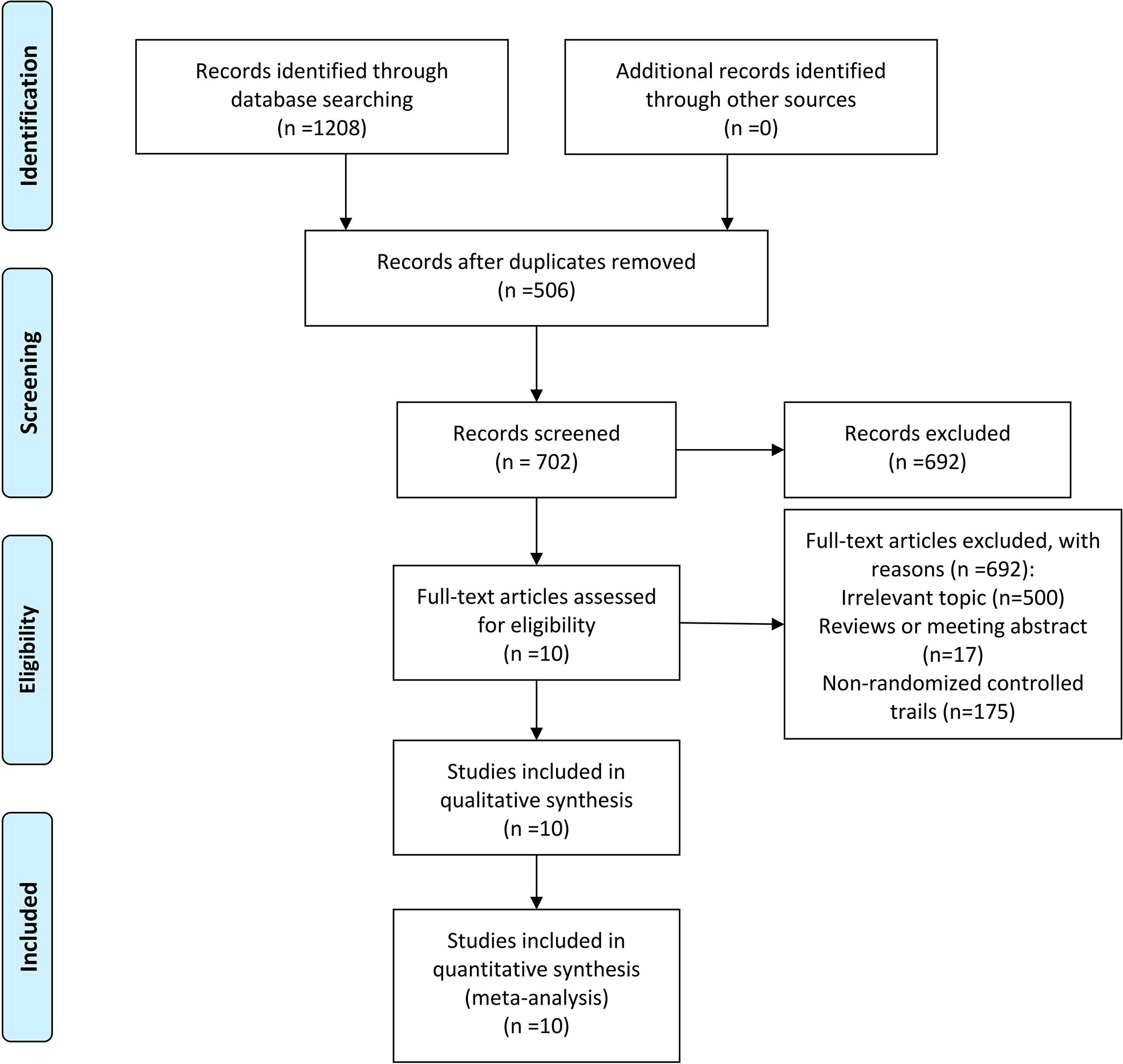
Through the search strategy as shown in Figure 1, a total of 10 studies (12–21), including 1609 patients, were identified fulfilling the inclusion criteria. A total of 506 duplications were excluded at the stage of title and abstract review. Full texts of the remaining 702 studies were screened. Of these studies, 692 were excluded. These included 500 irrelevant topics, 17 reviews or meeting abstracts, and 175 non-randomized controlled trials.
Patient demographics
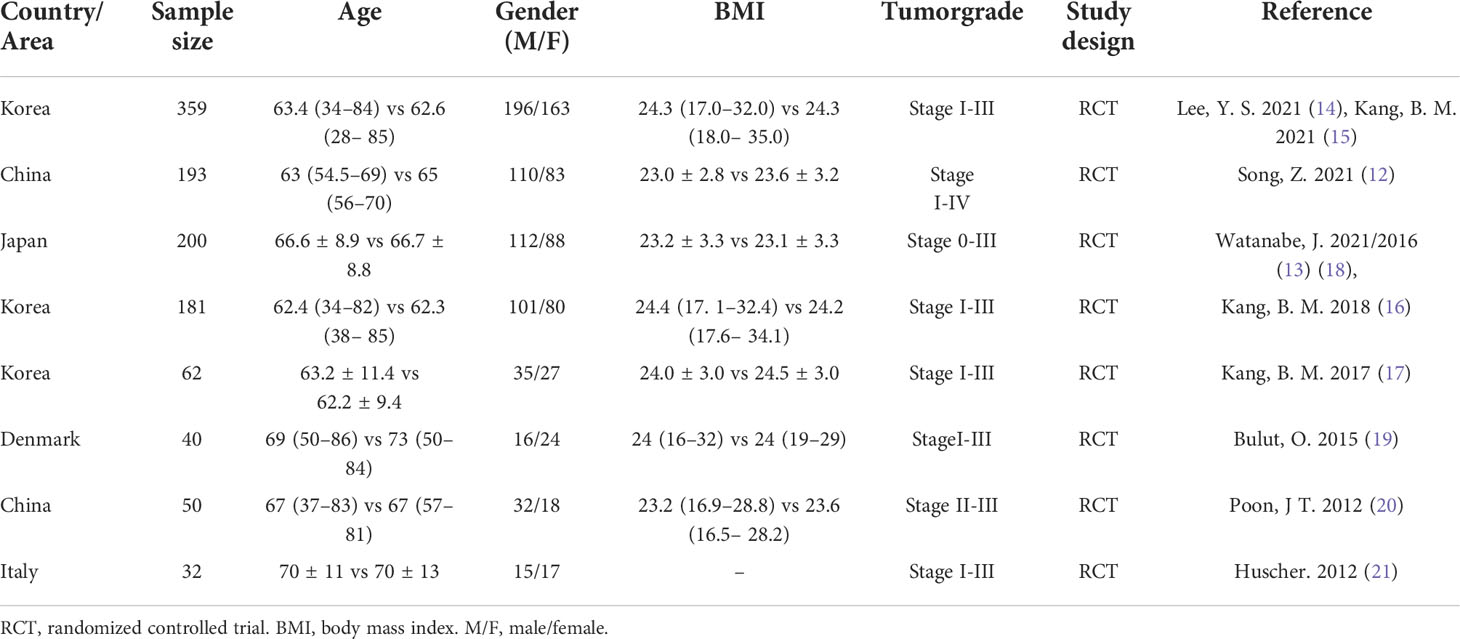
Main characteristics of the selected studies included reference, country/area, sample size, age, gender (M/F), BMI, tumor grade, study design are as follows (Table 3).
Meta-analysis results
Primary outcomes
1. Intraoperative Complications
Five studies participated the rate of intraoperative complications (12, 14, 16, 17, 21). Heterogeneity test: P = 0.86, I2 = 0%, showed no heterogeneity. The fixed-effect model was applied in the analyses. The results showed that the incidence of intraoperative complications in the SILS group was slightly higher than that in the CLS group. [OR = 1.98, 95% CI: 1.07 to 3.68, P = 0.03] (Figure 2).
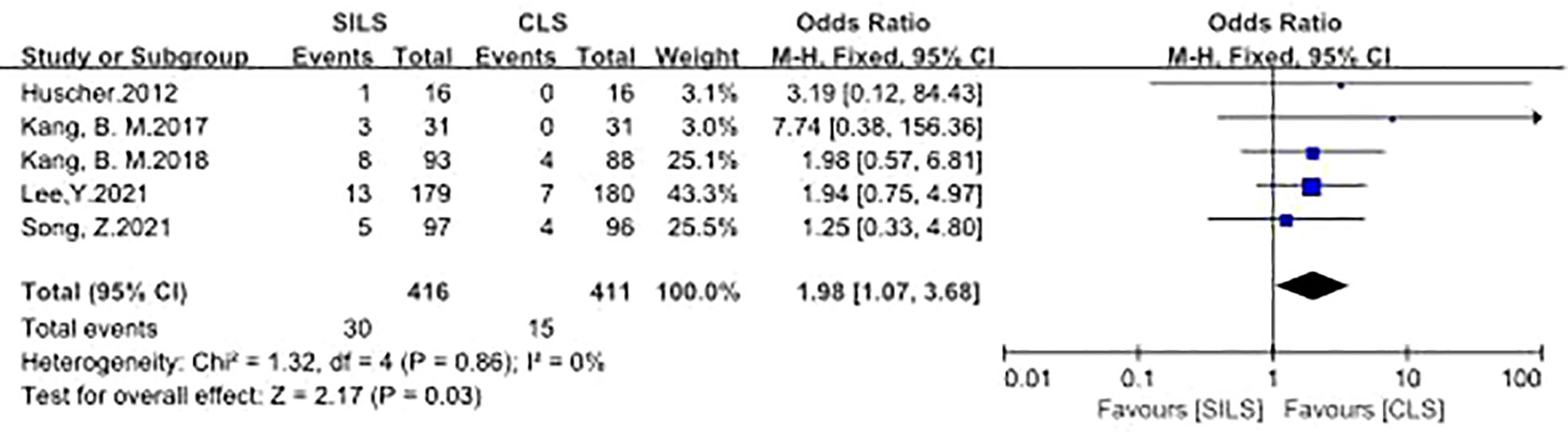
Figure 2 Meta-analysis of Intraoperative complications. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
2. Postoperative Complications
A total of eight studies involving 1125 patients participated the rate of postoperative complications (12, 14, 16–21). Heterogeneity test: P = 0.91, I2 = 0%, showed no heterogeneity. Fixed-effect model was applied in the analyses. The results of Meta-analysis showed that there was no significant difference in postoperative complications between the SILS group and the CLS group [OR = 0.75, 95% CI: 0.34 to 1.06, P = 0. 10] (Figure 3)
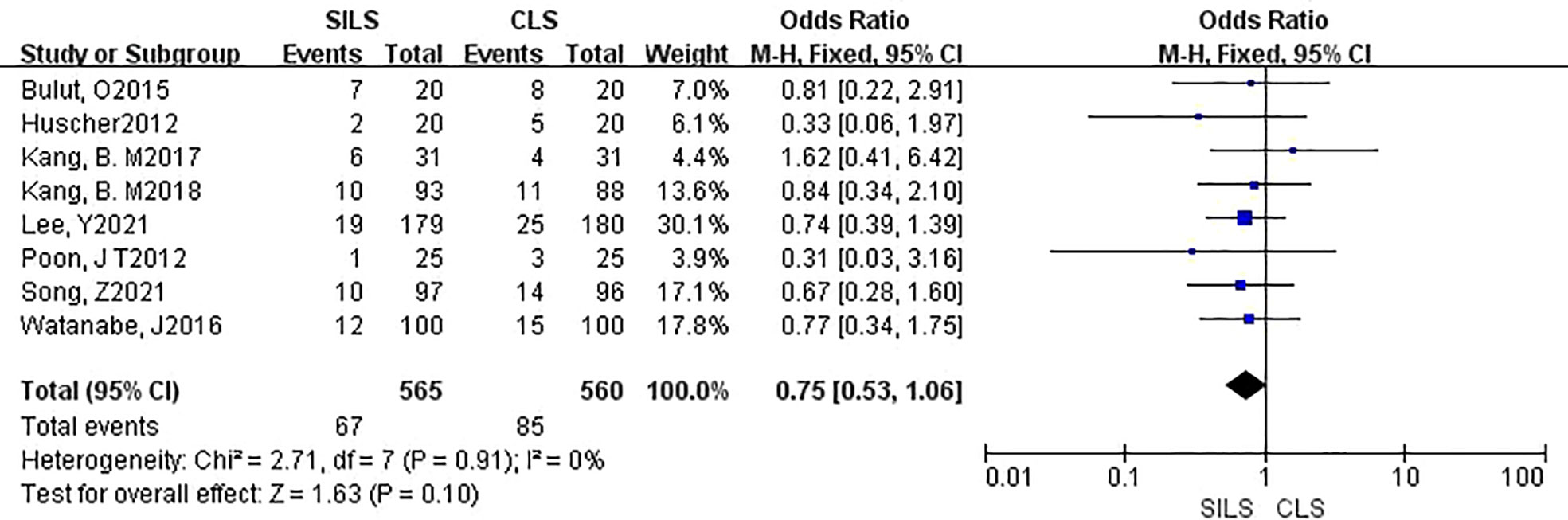
Figure 3 Meta-analysis of postoperative complications. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
Secondary outcomes
3. Total Incision Length
Two studies with a total of 262 patients participated the rate of total incision length (13, 17, 18). Heterogeneity test: P = 0.09, I2 = 64%, showed significant heterogeneity. The random model was applied in the analyses. Compared with the CLS group, the SILS group had a shorter total incision length and better cosmetic effects. [MD = -2.09, 95% CI: -3.41 to -0.78, P = 0.002] (Figure 4).
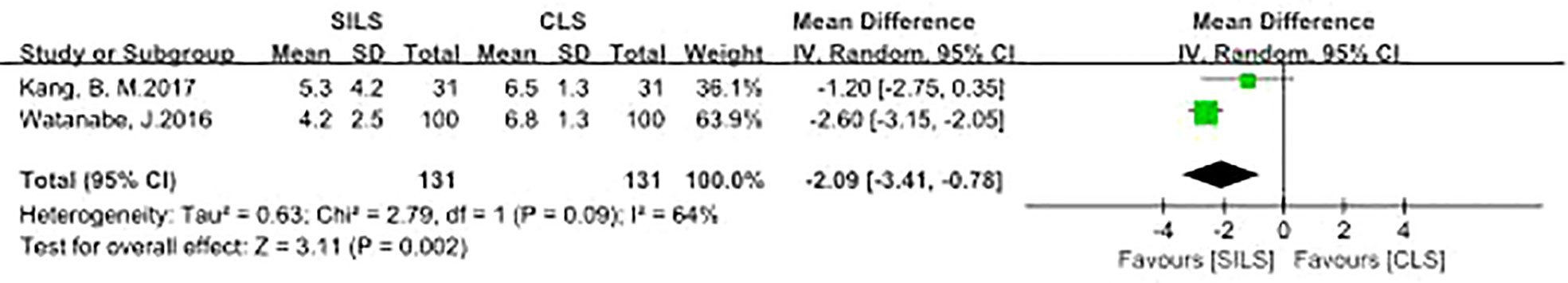
Figure 4 Meta-analysis of total incision length. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
4. Infection
A total of seven studies with 1055 patients reported the rate of infection (12, 14, 16, 18–21). The details contained wound infection (12, 14, 16, 18–21), central venous catheters infection (12), bronchopneumonia (21), Heterogeneity test: P = 0.74, I2 = 0%, showed no heterogeneity. Fixed-effect model was applied in the analyses. The results of Meta-analysis showed that there was no significant difference in postoperative complications between the SILS group and the CLS group [OR = 0.94, 95% CI: 0.49 to 1.79, P = 0.84] (Figure 5).
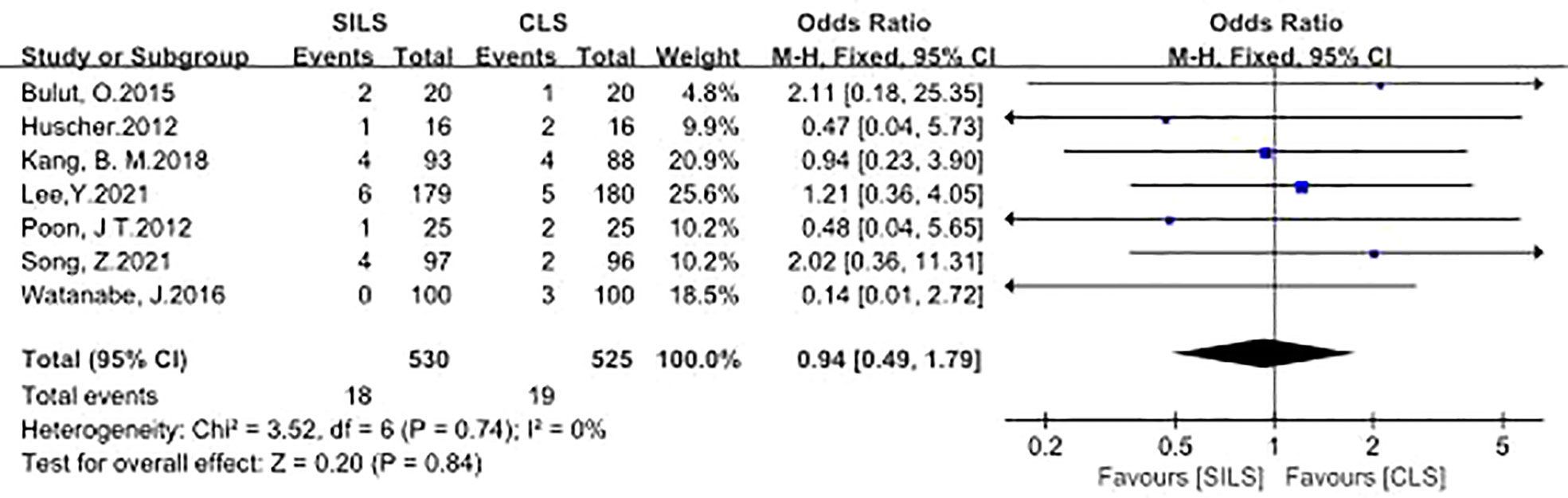
Figure 5 Meta-analysis of Infection. SILS, single-incision laparoscopic surgery, CLS; conventional laparoscopic surgery.
5. Anastomotic Leakage
A total of six studies with 1005 patients reported the rate of anastomotic leakage (12, 14, 16, 18, 19, 21). Heterogeneity test: P = 0.55, I2 = 0%, showed no heterogeneity. Fixed-effect model was applied in the analyses. Meta-analysis result showed that there was no significant difference between the two groups in the complications of anastomotic leakage [OR = 0.78, 95% CI: 0.35 to 1.71, P = 0.53] (Figure 6).
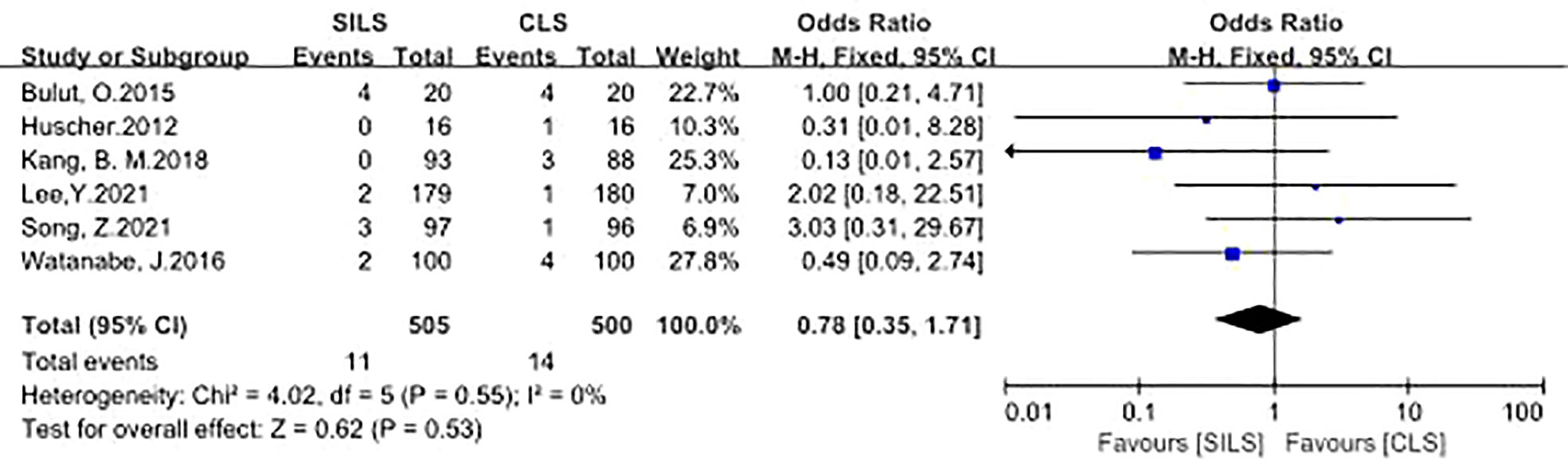
Figure 6 Meta-analysis of anastomotic leakage. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
6. Operation Time
A total of three studies with 294 patients reported the rate of operation time (17, 18, 21). Heterogeneity test: P = 0.37, I2 = 0%, showed no heterogeneity. A fixed-effect model was applied in the analyses. Meta-analysis result showed that there was no statistical difference in operation time between the two groups [MD = -2.86, 95% CI: -11.70 to 5.99, P = 0.53] (Figure 7).
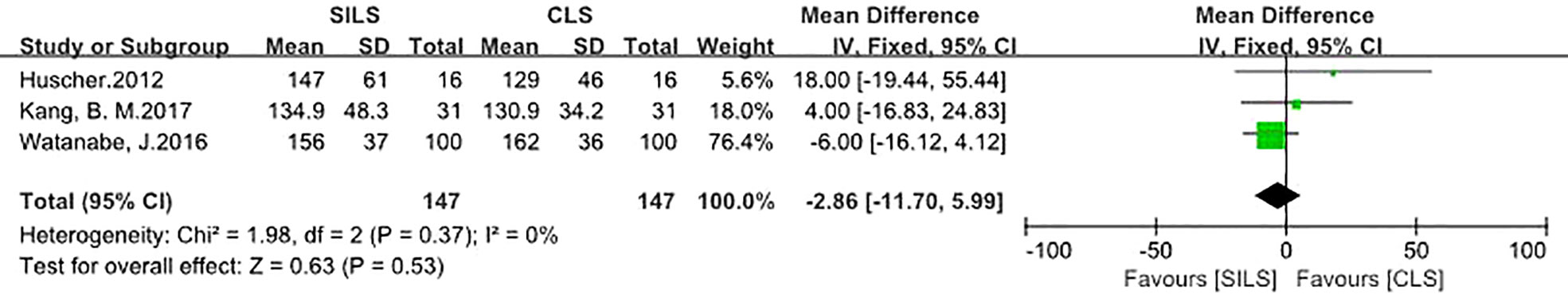
Figure 7 Meta-analysis of operation time. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
7. The following metrics included postoperative hospital stay, number of lymph nodes removed, readmission, reoperation, complication level (I-III), Prolonged Ileus showed no statistical difference (Figures 8–13).
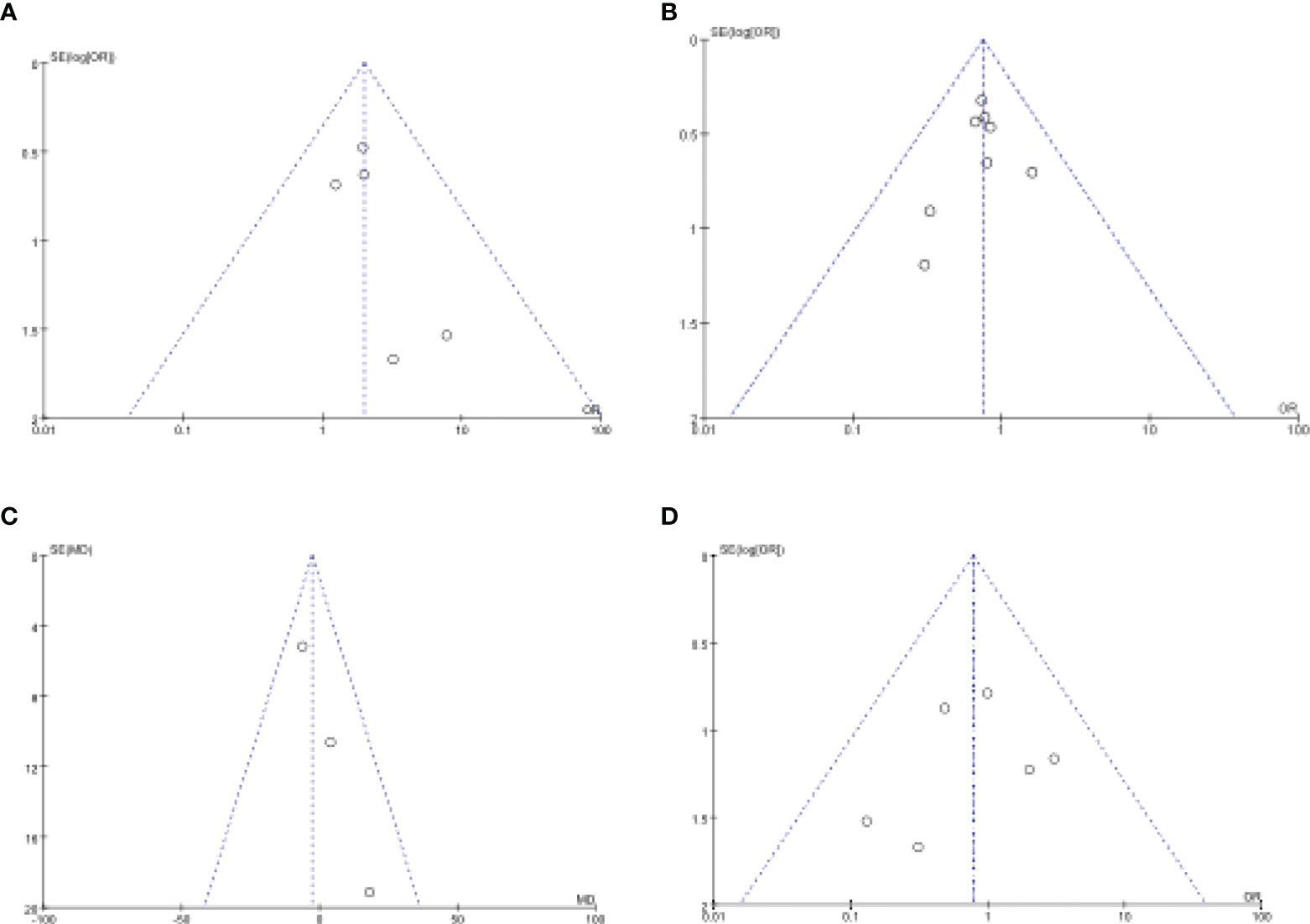
Figure 8 Funnel plot of publication bias in the meta-analysis. (A) Intraoperative Complications. (B) Postoperative Complications. (C) Operation Time. (D) Anastomotic Leakage.
Figure 9 Meta-analysis of number of lymph nodes removed. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.

Figure 10 Meta-analysis of Readmission. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
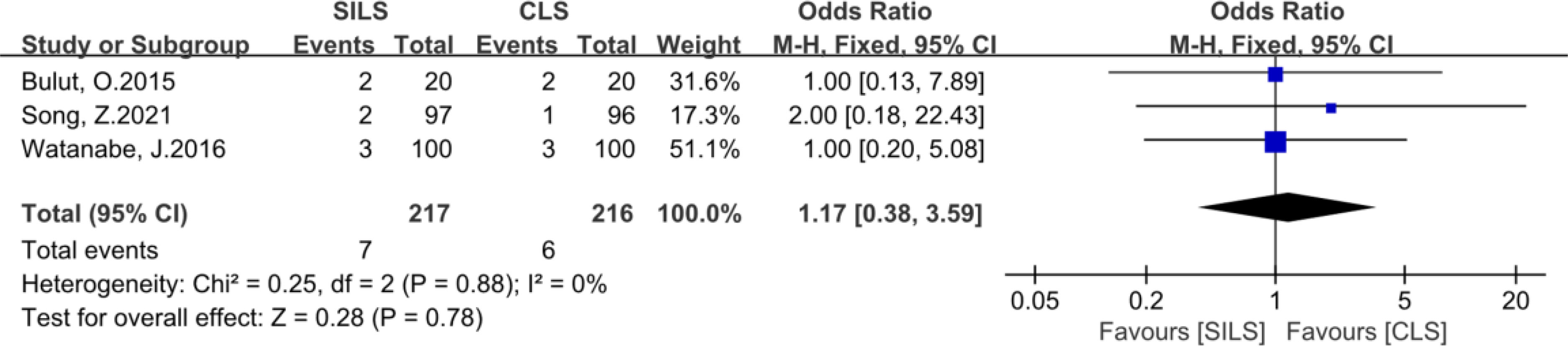
Figure 11 Meta-analysis of reoperation. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
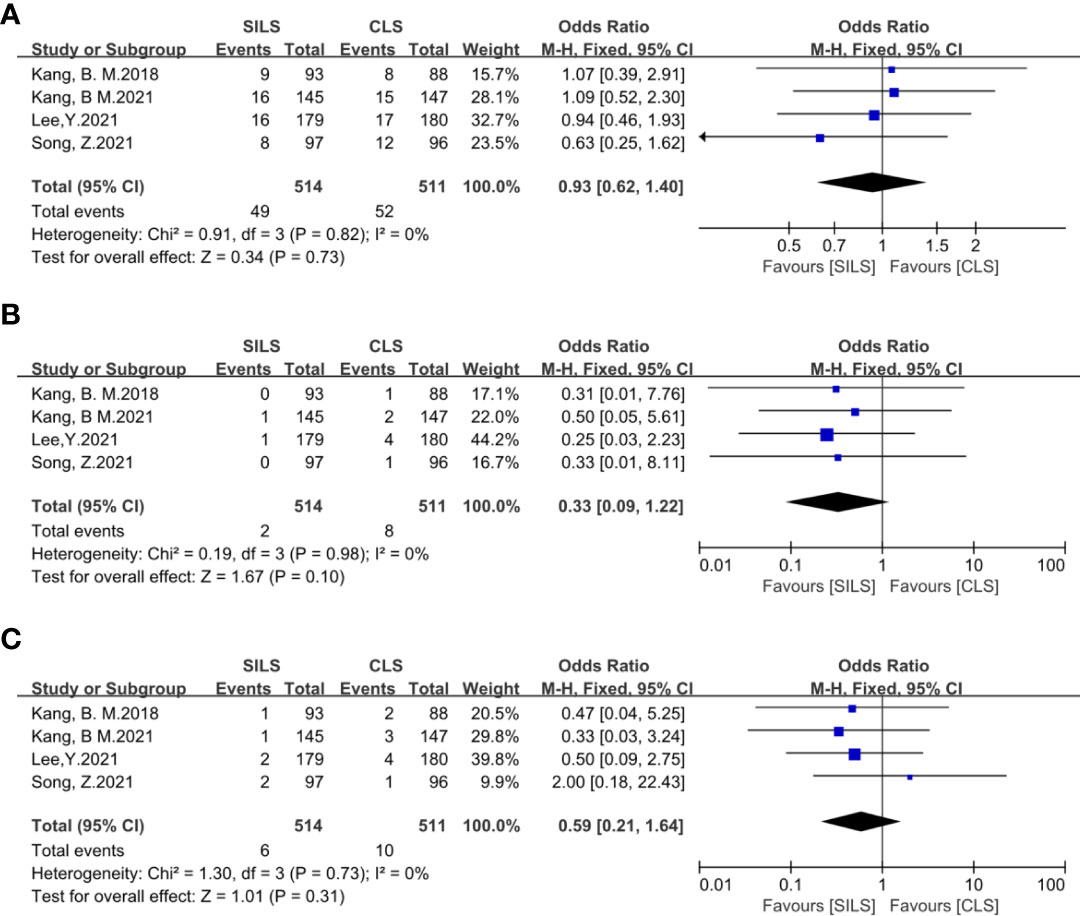
Figure 12 Meta-analysis of complication level I-III (A: I-II, B: IIIa, C: IIIb). SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
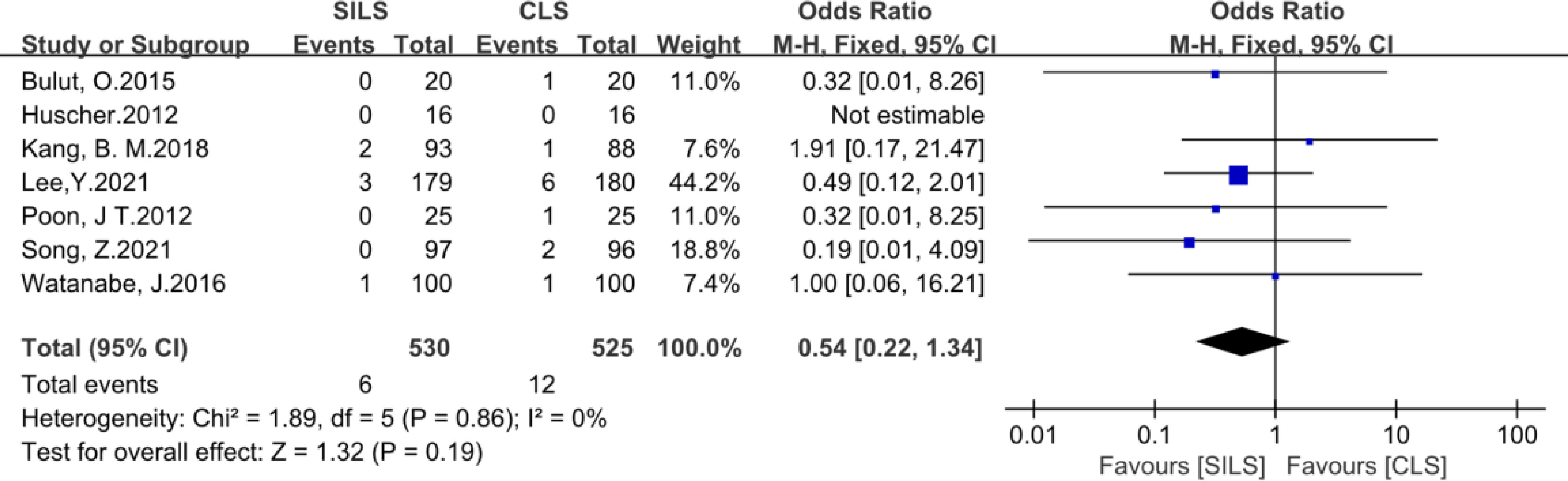
Figure 13 Meta-analysis of prolonged Ileus. SILS, single-incision laparoscopic surgery; CLS, conventional laparoscopic surgery.
Sensitivity analysis
Sensitivity analysis showed that there was no heterogeneity in remaining results except the total incision length (Heterogeneity test: P = 0.09, I2 = 64%). In our included articles, only two of them contained effective continuous variables, heterogeneity could not be analyzed by excluding any article.
Publication bias
We evaluated publication bias by looking at the symmetry of funnel plots. Our results showed no publication bias (Figure 8).
Discussion
Single-incision laparoscopic surgery (SILS) is an emerging minimally invasive technique (22). Patients and surgeons pay a lot of attention to it, because of its potential advantages such as smaller incision length, lower rate of postoperative complications, and so on (23, 24). However, SILS also has the same weaknesses as laparoscopic surgery, such as less tactile sense and limited instrument movement (25). These weaknesses are even enhanced during single incision laparoscopic surgery. The poor ergonomics and resulting technical difficulty with SILS contribute to the most important reason that this technique has not been rapidly adopted. When we talk about CLS, we are likely to consider that, comparing with SILS, it probably leads to a longer operative time, more intraoperative blood loss and higher intraoperative or postoperative complication rates. To determine whether SILS has advantages over CLS and whether its safety and efficacy are not inferior to CLS, we performed this meta-analysis of RCTs only.
In this study, we found that SILS had higher rates of intraoperative complications (vascular injury and adjacent organ injury). Besides the potential selection bias, on the one hand, this may be attributed to inadequate exposure of the surgical field and more difficult operation for surgeons. SILS combines multiple puncture holes of laparoscopy into a single hole, which violates the triangular operation control principle of laparoscopic technology. Single-incision operation requires colorectal surgeons to have superb laparoscopic skills. Usually, surgeons need a learning curve cycle of 30 to 60 cases to be proficient in application. Therefore, compared with CLS, SILS is more likely to lead to intraoperative complications, especially in some obese or difficult exposure cases. As for the obese patients especially BMI>30kg/m2, many studies agree that it’s a challenge for minimally invasive surgery, because abdominal exposure is poor compared with non-obese patients. However, the BMI of our cohort was relatively low, the patients with BMI>30kg/m2 was not enough to carry out statistical analysis.
On the other hand, complete mesocolic excision with central vascular ligation is essential for oncologic resection of right colon cancer (26), which needs to carefully clean the Henle superior mesenteric vein and artery and gastrocolic trunk. Anatomic variations of the blood vessels increases the challenges of operation as well. In addition, some previous studies reported that SILS is lack of triangular dissection, restricted degrees of freedom of movement the number of ports that can be used is restricted, the proximity of the instruments to each other during the operation-crossing fighting (16, 17, 21, 27), above factors may be more likely to lead to vascular injury in the SILS group. Single-port right hemicolectomy may be particularly challenging for SILS. There are certain technical differences between single-port right hemicolectomy and left hemicolectomy. Technical difficulty of the right hemicolon is more difficult to master, and the learning curve is slightly longer. As for the subgroup analysis of SILS, Lee,Y et al. reported that right hemicolectomy was associated with more operative complications than anterior resection with SILS (14). Because Complete mesocolic excision with central vascular ligation is vital of importance, which carefully dissection around the superior mesenteric vein and artery and gastrocolic trunk of Henle is required. In addition, The above vessels are prone to anatomical variation. These undoubtedly increase the difficulty of laparoscopic surgery, especially magnified in SILS because of lack of triangular dissection and difficulty achieving traction and countertraction in the SILS group. Therefore, to address a potential technical bias regarding learning curve, all procedures were performed by expert senior surgeons with good experience of both SPL and MPL techniques (28). However, Say-June Kim et al. showed lower rates of intraoperative complications, they agreed that a significant disadvantage of conventional laparoscopic surgery is the separation of the operator’s hands and eyes. Conventional laparoscopic surgery provides a surgical area controlled by a human assistant. This easily leads to unsatisfactory interactions between the operation and the assistant surgeon, which can compromise optimal visual field (29). On the contrary, it’s beneficial to SILS especially for skilled surgeons. Of course, this also requires surgeons to spend more time training to master the application of this technology. Khayat, A et al. were cautious about the two groups of similar results of their own experiments, because of selection bias, the patients operated with the SILS approach might be easier cases (30). This may compensate for deficiencies of SILS in other areas, resulting in equal complications in both groups.
This meta-analysis showed that SILS had less total length of skin incision than CLS [MD = -2.09cm, P = 0.002]. The umbilical cord is a natural scar, keeping the incision in the umbilical ring could improve the cosmetic effect. SILS not only strengthens the advantages of traditional laparoscopic surgery, but also has less surgical trauma, representing the evolution of minimally invasive surgery towards scarless surgery. This is also the advantage and value of this technology. However, Bush et al’s study (31) investigating women’s satisfaction for minimally invasive surgery (conventional vs. single-incision vs. robotic surgery) showed that the proportion of the CLS was the highest (56.4%) and the second was SILS (41. 1%). The comparative assessment of long-term complications (SILS-related incisional hernia) was not addressed in our study, but a recent RCT study suggested no significant difference in the incidence of incisional hernia after SILS arm versus SILS arm versus CLS arm with a long-term follow-up (13). Whether SILS has advantages in the following aspects needs further high-quality evidence: such as Less post operative pain, Less wound related complications, Faster recovery, Early return to work. The continuous data in the most of the introduced studies are in the form of median and extreme values which can’t be imported into the forest map, thus the conclusion is one-sided to some extent. Further research and better evaluation methods are needed to confirm the results.
In the present study, it showed a similar operation time for the SILS group to the CLS group [MD: -6min] which did not differ significantly (p=0.53), Some previous studies are similar to ours (17, 18, 32), However, some research required a longer time (33–35) while others required a shorter time than the CLS group (18, 36), besides the potential selection bias, The reasons for the rich results may be as follows, On the one hand, surgeons who focus on SILS and manage more surgical cases will reach a higher level of expertise, and the accumulating experience may considerably decrease operative time (37, 38), on the contrary, inexperienced surgeons may need more time. Yimei Jiang et al. reported that their surgeon was skilled in 3-port laparoscopic colorectal surgery before performing SILS for colorectal cancer, which is helpful for overcoming the learning curve of SILS. On the other hand, because of the reduced length and number of apertures of the skin incision, it takes less time for both making and closing the skin incision in SILS. All in all, though SILS for rectal cancer is much more difficult than CLS, surgeons accumulated more surgeries, overcame the learning curve of single-incision laparoscopy, a further reduction in operating time is only a matter of time.
This meta-analysis did not confirmed that SILS reduced the rate of postoperative complications. However, some studies showed different results, Bulut et al. pointed out opposite results. We analyzed the factors leading to this result from many aspects. First, there is no uniform standard for patient inclusion. Postoperative complications such as bleeding, incision infection and anastomotic fistula were related to individual inclusion criteria such as age, nutritional status, underlying diseases and tumor scope. Under the same operation, the postoperative complications of good individual condition were significantly less than those of poor individual condition, so the occurrence of postoperative complications was difficult to be measured by the difference of surgical methods (9). Second, although SILS has a shorter incision length and fewer incisions than CLS (39), the improvement of equipment in recent years has led to more and more changes in the skills of surgeons. Shorten the operation time, shorten the length of the incision, reduce postoperative pain, promote early activity to enhance recovery. All these can reduce the incidence of postoperative complications. Third, patients with different postoperative nursing specifications, postoperative hospital and family nursing support will affect the occurrence of postoperative complications. Kang,B,M et al. have published two RCTs with different conclusions on postoperative complications, so we believe that the occurrence of postoperative complications is not related to the effect of operation type.
In this meta-analysis, only 2 studies provided data on length of hospital stay. Because the included studies did not use the same discharge criteria and had a low sample size, differences in length of stay were of low reference value (9). In condition, different surgical instrument specifications and inconsistent surgical methods for colorectal cancer (such as low rectal cancer anterior resection, radical abdominal perineotomy combined with rectal cancer) may also affect the operative time and incidence of intraoperative complications. All of these indirectly increase the length of hospital stay. Therefore, it is difficult to accurately evaluate the advantage of SILS in hospital stay.
Due to insufficient data from included randomized controlled studies, the long-term outcomes of SILS was not evaluated. Some previous studies showed that the long-term outcomes of patients undergoing SILS were comparable to those of patients undergoing CLS (40–42). Hirano, Y. et al. reported the median follow-up interval was 60 months. The 5-year relapse free survival for stage I, stage II and stage III disease were 90.5%, 88.1% and 79.6%, respectively. The 5-year overall survival for stage I, stage II, stage III and stage IV disease were 97.6%, 92.9%, 88.6% and 40.9%. The factors of long-term outcomes are comprehensive. Besides the operation factors, postoperative care and personalized chemo-radiotherapy are essential as well. More randomized controlled trials are needed to demonstrate the advantages and disadvantages of long-term outcomes in patients with SILS.
Compared with other previous meta-analysis (43–45) including retrospective studies or clinical controlled trials (CCTs), this meta-analysis only included and analyzed all relevant RCTs in the present to ensure that the results were more reliable. However, this study has some limitations. First, our searched database is limited, some open data is not included, and included articles had few data samples. Second, each surgeon had a different technical proficiency which might be likely to cause the data of the article to be offset. Thirdly, only one study blinded the participants, while the others were open-labeled RCTs, which can lead to substantial implementation bias. What’s more, the included literature lacks long-term follow-up results, including the rate of local tumor recurrence or distant metastasis, and survival rate.manipulation of the instrument tip compared with CLS.
Conclusion
SILS showed shorter incision length and higher rates of intraoperative complications compared with CLS, it means that SILS has good cosmetic effects with relatively high surgical risk. Therefore, SILS needs to be selected in patients with higher cosmetic requirements and performed by more experienced surgeons.What’s more, systematic review and meta-analysis did not prove that the SILS had a comprehensive and obvious advantage over the CLS. Some extra metrics such as postoperative patient recovery, postoperative hospital stay, postoperative pain may have potential benefits which needs to be further explored by high-quality RCTs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
Author contributions: F-hL and D-xZ performed data acquisition, analysis, and interpretation and drafted the manuscript; LC contributed to data interpretation and revised the manuscript; XC-f Provided suggestions for modification; TL and ZP aimed to revise the article; J-wX contributed to the study conception and design and critical revision of the manuscript; all authors approved the final version of the submitted manuscript.
Funding
National Natural Science Foundation of China, NO. 81070378 and NO. 81270561; Special Research Fund for the First Affiliated Hospital of Chengdu Medical College, NO. CYFY2019YB08; and High-level Talents Introduction Fund for the First Affiliated Hospital of Chengdu Medical College, NO. CYFY2018GQ17.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Abu GM, Wexner SD. Re-appraisal and consideration of minimally invasive surgery in colorectal cancer. Gastroenterol Rep (Oxf) (2017) 5(1):1–10. doi: 10.1093/gastro/gox001
3. Brockhaus AC, Sauerland and S. Saad S. Single-incision versus standard multi-incision laparoscopic colectomy in patients with malignant or benign colonic disease: a systematic review, meta-analysis and assessment of the evidence. BMC Surg (2016) 16(1):71. doi: 10.1186/s12893-016-0187-5
4. Sato T, Watanabe M. The present status and developments of laparoscopic surgery for colorectal cancer. J Anus Rectum Colon (2017) 1(1):1–6. doi: 10.23922/jarc.2016-010
5. Keller D, Haas E. Single-incision laparoscopic colon and rectal surgery. Clinics Colon Rectal Surg (2015) 28(03):135–9. doi: 10.1055/s-0035-1555004
6. Wormser C, Runge JJ. Advances in laparoscopic surgery. Vet Clinics North America: Small Anim Pract (2016) 46(1):63–84. doi: 10.1016/j.cvsm.2015.08.001
7. Islam A, Castellvi AO, Tesfay ST, Castellvi AD, Wright AS, Scott DJ, et al. Early surgeon impressions and technical difficulty associated with laparoendoscopic single-site surgery: a society of American gastrointestinal and endoscopic surgeons learning center study. Surg Endosc (2011) 25(8):2597–603. doi: 10.1007/s00464-011-1594-4
8. Parker JM, Feldmann TF, Cologne KG. Advances in laparoscopic colorectal surgery. Surg Clinics North America (2017) 97(3):547–60. doi: 10.1016/j.suc.2017.01.005
9. Yuan Y, Jian J, Jing H, Yan R, You F, Fu X, et al. Single-incision vs. conventional laparoscopic surgery for colorectal cancer: An update of a systematic review and meta-analysis. Front Surg (2021) 8:704986. doi: 10.3389/fsurg.2021.704986
10. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
11. Cumpston M, Li T, Page MJ, Chandler J, Higgins JPt, Welch VA, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
12. Song Z, Liu K, Li Y, Shi Y, Jiang Y, Wang C, et al. Short-term outcomes of single-incision laparoscopic surgery for colorectal cancer: A single-center, open-label, non-inferiority, randomized clinical trial. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.762147
13. Watanabe J, Ishibe A, Suwa H, Ota M, Fujii S, Kubota K. Long-term outcomes of a randomized controlled trial of single-incision versus multi-port laparoscopic colectomy for colon cancer. Ann Surg (2021) 273(6):1060–5. doi: 10.1097/SLA.0000000000004252
14. Lee YS, Kim JH, Kim HJ, Lee SC, Kang BM, Kim CW. Short-term outcomes of single-port versus multiport laparoscopic surgery for colon cancer. Ann Surg (2021) 273(2):217–23. doi: 10.1097/SLA.0000000000003882
15. Kang BM, Lee YS, Kim JH, Kim HJ, Lee SC, Kim CW. Quality of life and patient satisfaction after single- and multiport laparoscopic surgery in colon cancer: a multicentre randomised controlled trial (SIMPLE trial). Surg Endosc (2021) 35(11):6278–90. doi: 10.1007/s00464-020-08128-9
16. Kang BM, Kim HJ, Kye B-H, Lee SC, Lee KY, Park SJ. Multicenter, randomized single-port versus multiport laparoscopic surgery (SIMPLE) trial in colon cancer: an interim analysis. Surg Endosc (2018) 32(3):1540–9. doi: 10.1007/s00464-017-5842-0
17. Kang BM, Park SJ, Lee KY, Lee S-H. Single-port laparoscopic surgery can be performed safely and appropriately for colon cancer: Short-term results of a pilot randomized controlled trial. J Laparoendosc Adv Surg Tech (2017) 27(5):501–9. doi: 10.1089/lap.2016.0467
18. Watanabe J, Ota M, Fujii S, Suwa H, Ishibe A, Endo I. Randomized clinical trial of single-incision versus multiport laparoscopic colectomy. Br J Surg (2016) 103(10):1276–81. doi: 10.1002/bjs.10212
19. Bulut O, Aslak KK, Levic K, Nielsen CB, Rømer E, Sørensen S, Christensen IJ, Nielsen HJ, et al. A randomized pilot study on single-port versus conventional laparoscopic rectal surgery: effects on postoperative pain and the stress response to surgery. Tech Coloproctol (2015) 19(1):11–22. doi: 10.1007/s10151-014-1237-6
20. Poon JTC, Cheung C-W, Fan JKM, Lo OSH, Law W-L. Single-incision versus conventional laparoscopic colectomy for colonic neoplasm: a randomized, controlled trial. Surg Endosc (2012) 26(10):2729–34. doi: 10.1007/s00464-012-2262-z
21. Huscher CG, Mingoli A, Sgarzini G, Mereu A, Binda B, Brachini G, Trombetta S, et al. Standard laparoscopic versus single-incision laparoscopic colectomy for cancer: early results of a randomized prospective study. Am J Surg (2012) 204(1):115–20. doi: 10.1016/j.amjsurg.2011.09.005
22. Saeed Far S, Miraj S. Single-incision laparoscopy surgery: a systematic review. Electr phys (2016) 8(10):3088–95. doi: 10.19082/3088
23. Podda M, Saba A, Porru F, Pisanu A. Systematic review with meta-analysis of studies comparing single-incision laparoscopic colectomy and multiport laparoscopic colectomy. Surg Endosc (2016) 30(11):4697–720. doi: 10.1007/s00464-016-4812-2
24. Dong B, Luo Z, Lu J, Yang Y, Song Y, Cao J, Li W, et al. Single-incision laparoscopic versus conventional laparoscopic right colectomy: A systematic review and meta-analysis. Int J Surg (2018) 55:31–8. doi: 10.1016/j.ijsu.2018.05.013
25. Takahashi H, Takemasa I, Haraguchi N, Nishimura J, Hata T, Yamamoto H, et al. The single-center experience with the standardization of single-site laparoscopic colectomy for right-sided colon cancer. Surg Today (2017) 47(8):966–72. doi: 10.1007/s00595-016-1457-7
26. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation - technical notes and outcome. Colorectal Dis (2009) 11(4):354–64. doi: 10.1111/j.1463-1318.2008.01735.x
27. Tei M, Wakasugi M, Akamatsu H. Comparison of perioperative and short-term oncological outcomes after single- or multiport surgery for colorectal cancer. Colorectal Dis (2015) 17(7):O141–7. doi: 10.1111/codi.12986
28. Maggiori L, Tuech JJ, Cotte E, Lelong B, Denost Q, Karoui M. Single-incision laparoscopy versus multiport laparoscopy for colonic surgery: A multicenter, double-blinded, randomized controlled trial. Ann Surg (2018) 268(5):740–6. doi: 10.1097/SLA.0000000000002836
29. Kim SJ, Choi B-J, Jeong W, Lee SC. The feasibility of single-port laparoscopic appendectomy using a solo approach: a comparative study. Ann Surg Treat Res (2016) 90(3):164. doi: 10.4174/astr.2016.90.3.164
30. Khayat A, Maggiori L, Vicaut E, Ferron M, Panis Y. Does single port improve results of laparoscopic colorectal surgery? a propensity score adjustment analysis. Surg Endosc (2015) 29(11):3216–23. doi: 10.1007/s00464-015-4063-7
31. Bush AJ, Morris SN, Millham FH, Isaacson KB. Women’s preferences for minimally invasive incisions. J Minimally Invasive Gynecol (2011) 18(5):640–3. doi: 10.1016/j.jmig.2011.06.009
32. Waters JA, Guzman MJ, Fajardo AD, Selzer DJ, Wiebke EA, Robb BW. Single-port laparoscopic right hemicolectomy: A safe alternative to conventional laparoscopy. Dis Colon Rectum (2010) 53(11):1467–72. doi: 10.1007/DCR.0b013e3181f23ca0
33. Kim S, Ryu G-O, Choi B-J, Kim J-G, Lee K-J, Lee SC. The short-term outcomes of conventional and single-port laparoscopic surgery for colorectal cancer. Ann Surg (2011) 254(6):933–40. doi: 10.1097/SLA.0b013e318237826b
34. Lim SW, Kim HR, Kim YJ. Single incision laparoscopic colectomy for colorectal cancer: comparison with conventional laparoscopic colectomy. Ann Surg Treat Res (2014) 87(3):131. doi: 10.4174/astr.2014.87.3.131
35. Song Z, Li Y, Liu K, Jiang Y, Shi Y, Ji X. Clinical and oncologic outcomes of single-incision laparoscopic surgery for right colon cancer: a propensity score matching analysis. Surg Endosc (2019) 33(4):1117–23. doi: 10.1007/s00464-018-6370-2
36. Jiang Y, Song Z, Cheng X, Liu K, Shi Y, Wang C. Clinical and oncological outcomes of single-incision vs. convent laparosc Surg rectal cancer Surg Endosc (2020) 34(12):5294–303. doi: 10.1007/s00464-019-07317-5
37. van den Boezem PB, Sietses C. Single-incision laparoscopic colorectal surgery, experience with 50 consecutive cases. J Gastrointest Surg (2011) 15(11):1989–94. doi: 10.1007/s11605-011-1626-3
38. Lim SW, Kim HJ, Kim CH, Huh JW, Kim YJ, Kim HR. Umbilical incision laparoscopic colectomy with one additional port for colorectal cancer. Tech Coloproctol (2013) 17(2):193–9. doi: 10.1007/s10151-012-0900-z
39. Arezzo A, Passera R, Forcignanò E, Rapetti L, Cirocchi R, Morino M. Single-incision laparoscopic cholecystectomy is responsible for increased adverse events: results of a meta-analysis of randomized controlled trials. Surg Endosc (2018) 32(9):3739–53. doi: 10.1007/s00464-018-6143-y
40. Hirano Y, Hattori M, Hiranuma C, Douden K, Hashizume Y, Taniguchi K. Long-term oncologic outcomes of single-incision laparoscopic surgery for colorectal cancer. Surg Endosc Other Intervent Tech (2017) 31(2):S123. doi: 10.1007/s00464-017-5541-x
41. Ishii Y, Yahagi M, Ochiai H, Sako H, Amemiya R, Maeda H. Short-term and midterm outcomes of single-incision laparoscopic surgery for right-sided colon cancer. Asian J Endosc Surg (2019) 12(3):275–80. doi: 10.1111/ases.12654
42. Hirano Y, Hiranuma C, Hattori M, Douden K, Yamaguchi S. Long-term oncological outcomes of single-port laparoscopic surgery for colon cancer. ANZ J Surg (2019) 89(4):408–11. doi: 10.1111/ans.15076
43. Lv C, Wu S, Wu Y, Shi J, Su Y, Fan Y. Single-incision laparoscopic versus traditional multiport laparoscopic colorectal surgery–a cumulative meta-analysis and systematic review. Int J Colorectal Dis (2013) 28(5):611–21. doi: 10.1007/s00384-013-1653-5
44. Hoyuela C, Juvany M, Carvajal F. Single-incision laparoscopy versus standard laparoscopy for colorectal surgery: A systematic review and meta-analysis. Am J Surg (2017) 214(1):127–40. doi: 10.1016/j.amjsurg.2017.03.002
Keywords: single-incision laparoscopic surgery (SILS), conventional laparoscopic surgery (CLS), colorectal cancer, randomized controlled trials, meta-analysis, complication
Citation: Li F-h, Zeng D-x, Chen L, Xu C-f, Tan L, Zhang P and Xiao J-w (2022) Comparison of clinical efficacy of single-incision and traditional laparoscopic surgery for colorectal cancer: A meta-analysis of randomized controlled trials and propensity-score matched studies. Front. Oncol. 12:997894. doi: 10.3389/fonc.2022.997894
Received: 23 July 2022; Accepted: 22 September 2022;
Published: 13 October 2022.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
Jacopo Andreuccetti, Civil Hospital of Brescia, ItalyPietro Anoldo, Federico II University Hospital, Italy
Stefano Bona, Humanitas Research Hospital, Italy
Copyright © 2022 Li, Zeng, Chen, Xu, Tan, Zhang and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang-wei Xiao, eGlhb2ppYW5nd2VpMjAxOEAxNjMuY29t
†These authors share first authorship
‡ORCID: Fang-han Li, orcid.org/0000-0002-5514-9334
De-xin Zeng, orcid.org/0000-0002-8965-0725
Li Chen, orcid.org/0000-0003-1232-2659
Jiang-wei Xiao, orcid.org/0000-0002-4288-7581
Cheng-fei Xu, orcid.org/0000-0001-9031-537X
Lin Tan, orcid.org/0000-0001-5442-5184
Pan Zhang, orcid.org/0000-0002-6333-9349
 Fang-han Li
Fang-han Li De-xin Zeng
De-xin Zeng Li Chen‡
Li Chen‡ Jiang-wei Xiao
Jiang-wei Xiao