- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 2Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
Background: Invisible cervical cancers on MRI can indicate less invasive surgery. Cervical cancers consist of squamous cell carcinoma (SCC) and non-SCC, each with different long-term outcomes. It is still unclear if surgical planning should be changed according to the histologic type of cervical cancer when it is not visible on MRI.
Purpose: The purpose of the study was to determine if surgical planning for cervical cancer that is not visible on MRI is influenced by the histologic type.
Materials and methods: Between January 2007 and December 2016, 155 women had Federation of Gynecology and Obstetrics (FIGO) stage 1B1 cervical cancer that was not visible on preoperative MRI. They underwent radical hysterectomies and pelvic lymph node dissections. Among them, 88 and 67 were histologically diagnosed with SCC and non-SCC, respectively. The size of the residual tumor, depth of stromal invasion, parametrial invasion, vaginal invasion, lymphovascular invasion, and lymph node metastasis were compared between these patients using the t-test, Mann–Whitney U test, Chi-squared test, or Fisher’s exact test. The recurrence-free and overall 10-year survival rates were compared between the groups by Kaplan–Meier analysis.
Results: The mean sizes of residual tumors were 8.4 ± 10.4 mm in the SCC group and 12.5 ± 11.9 mm in the non-SCC group (p = 0.024). The mean depth of stromal invasion in the SCC group was 12.4 ± 21.2% (0%–100%), whereas that in the non-SCC group was 22.4 ± 24.4 (0%–93%) (p = 0.016). However, there was no difference in parametrial or vaginal invasion, lymphovascular invasion, or lymph node metastasis (p = 0.504–1.000). The recurrence-free and overall 10-year survival rates were 98.9% (87/88) and 95.5% (64/67) (p = 0.246), and 96.6% (85/88) and 95.5% (64/67) (p = 0.872), respectively.
Conclusions: The non-SCC group tends to have larger residual tumors and a greater depth of stromal invasion than the SCC group, even though neither is visible on MRI. Therefore, meticulous care is necessary for performing parametrectomy in patients with non-SCC cervical cancer.
Introduction
Previously reported studies showed that postoperative outcomes were good when Federation of Gynecology and Obstetrics (FIGO) stage IB1 cervical cancer was not visible on preoperative magnetic resonance imaging (MRI) (1–3). This cancer has a much lower tumor burden than those visible on MRI. Accordingly, the former has a better prognosis than the latter. However, previous studies did not investigate whether postoperative outcomes differed according to histologic type. Patients with squamous cell carcinoma (SCC) frequently have better long-term outcomes than those without SCC.
Moreover, the tumor conspicuity of non-SCC is not as good as that of SCC, so it cannot be easily determined if non-SCC cervical cancer is visible on MRI (4–6). Minimizing parametrectomy is useful for avoiding postoperative complications (7–14). However, false-positive results for invisible tumors may lead to underestimating the extent of surgical resection needed. As a result, unnecessary additional treatments may follow a minimally invasive hysterectomy.
Thus, we hypothesized that the sizes of postoperative residual tumors differ according to the histologic types of FIGO stage IB1 cervical cancer, even though these are not visible on preoperative MRI. Rare studies have compared the postoperative outcomes of SCC and non-SCC patients. The purpose of this study was to determine if surgical planning for cervical cancer not visible on MRI is influenced by histologic type (SCC versus non-SCC).
Materials and methods
This study (File No.: 2022-04-030-001) was approved by the Institutional Review Board at Samsung Medical Center and the requirement for informed consent was waived due to the retrospective design.
Patients
Between January 2007 and December 2016, a total of 747 patients with FIGO IB1 cervical cancer underwent MRI prior to radical hysterectomy. Among them, 52 patients were excluded due to the poor image quality of the MRI examinations. Among the remaining 695 patients, 540 and 155 had visible cancer and invisible cancer, respectively, on preoperative MRI. Finally, 155 patients were included in the study population when they underwent 1.5 T or 3.0 T MRI. Of them, 88 patients were histologically confirmed to have squamous cell carcinoma (SCC) (SCC group). The remaining 67 patients were histologically confirmed to have other cervical cancers (non-SCC group). The medical records of the patients in the SCC group (48.5 ± 12.1 years; 20–81 years) and the non-SCC group (44.4 ± 8.5 years; 29–64 years) were reviewed. Colposcopic biopsy and conization were performed in 80.0% (124/155) and 60.0% (93/155), respectively.
Bimanual pelvic and rectovaginal examinations were done to determine the disease extent. Laboratory tests, chest radiography, cystoscopy, and sigmoidoscopy were routinely performed for clinical FIGO staging (15). The time interval between MRI and hysterectomy ranged from 1 to 47 days (median, 16 days) in the SCC group and from 0 to 39 days (median, 15 days) in the non-SCC group.
The MR images were preoperatively interpreted by one of two radiologists who had approximately five or more years of experience in gynecologic imaging. They were additionally reviewed by one radiologist who had approximately 19 years of experience in gynecologic imaging.
Radical hysterectomy, vaginectomy, and lymph node (LN) dissection were performed on all patients. Additional surgical procedures depend on the clinical stage and the surgeon’s decision. When pelvic LNs were suspicious for metastasis at frozen sectioning, the para-aortic LNs were dissected.
Two pathologists examined the surgical specimens. They recorded the size of the residual tumor, histologic type, depth of stromal invasion, lymphovascular space (LVS) invasion, parametrial invasion, vaginal invasion, resection tumor margin, and LN metastasis.
After primary treatment, all patients received adequate follow-up procedures. During this period, patients underwent physical examinations, Pap smears, and tumor marker analysis every three months for the first two years and every six months for the next three years. Imaging studies, such as abdominopelvic computed tomography (CT) or pelvic MRI, were conducted every 6 – 12 months for the first two years and then annually for the next three years.
MR imaging
Pelvic scans were conducted with a 1.5 (n = 27) MRI scanner (Signa, GE Medical System, Milwaukee, USA) or 3 T (n = 128) MRI scanner (Intera Achiva 3T; Philips Medical System, Best, The Netherlands). The upper abdomen was scanned by MRI or CT. The 1.5 T MRI sequences of the pelvis included T2-weighted images (T2WI), T1-weighted images, and dynamic contrast-enhanced (DCE) images. Diffusion-weighted imaging (DWI) was added to the 3 T MRI examination. However, DWI could not be scanned at the 1.5 T MRI because the MR software did not have the capability. T2WI were obtained in the axial, sagittal, and coronal planes. The other sequences were obtained in the axial plane. The upper abdomen was scanned from the lower lung to the aortic bifurcation. The same MR parameters as those used by Park et al. were used (1).
Data analysis
Invisible cancer was defined when the cervical tumor was invisible on T2W and DCE 1.5T MR images and when it was invisible on T2WI, DWI, and DCE 3T MR images (Figures 1, 2). When post-biopsy inflammation was differentiated from cervical cancer on T2W because both were hypertense, DWI or DCE images were reviewed; the former had no diffusion restriction or showed iso- or higher enhancement compared to neighboring cervical tissue, unlike the latter.
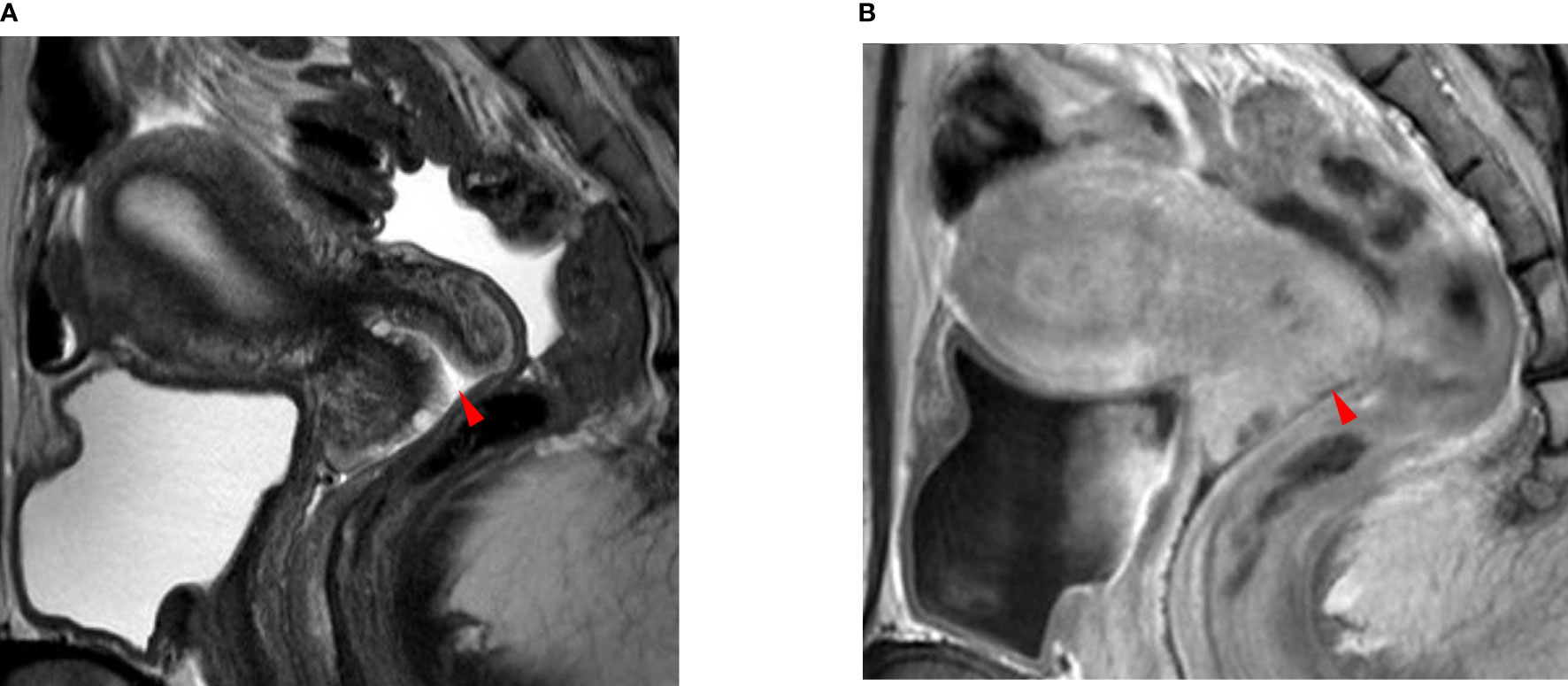
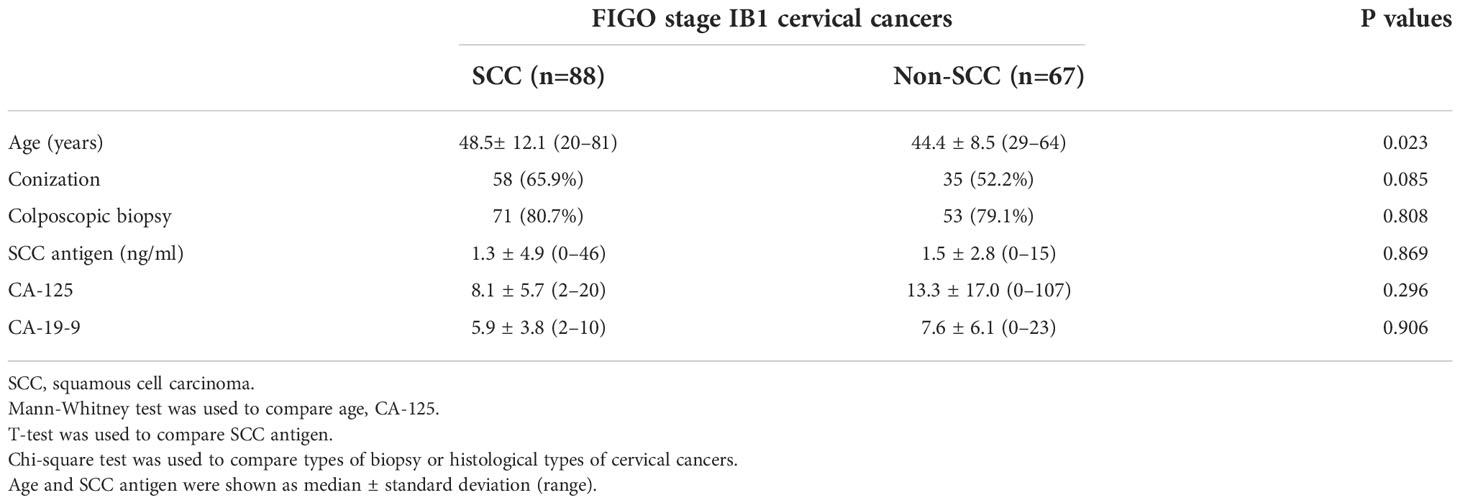
Figure 1 A 35-year-old woman with squamous cell carcinoma. (A) The T2-weighted sagittal MR image shows no focal lesion in the cervix. The red arrowhead indicates the external OS of the uterine cervix. (B) The delayed contrast-enhanced sagittal MR image shows no residual cancer in the cervix. The red arrowhead indicates the external OS of the uterine cervix. The pathologic report confirmed no residual cancer in the resected uterus. There was also no invasion of the lymphovascular space, vagina, parametrium, or lymph node metastasis.
Patient age, biopsy type, histologic type, and SCC antigens or other tumor markers were compared between the SCC and non-SCC groups. The size of the residual tumor, depth of stromal invasion, LVS invasion, parametrial invasion, vaginal invasion, and LN metastasis were also compared between the groups.
Recurrent tumors were assessed on follow-up CT or MR images. Recurrence-free and overall 10-year survival rates were calculated and compared between the SCC and non-SCC groups.
Statistical analysis
Patient age, the size of the residual tumor, and the depth of stromal invasion were compared by the Mann–Whitney test because these data did not show a Gaussian distribution. SCC antigens were compared between two groups using the t-test.
The proportions of biopsy type, cancer histology, LVS invasion, parametrial invasion, vaginal invasion, LN metastasis, and recurrence rate were compared using the chi-square or Fisher’s exact test.
Odds ratios (ORs) and 95% confidence intervals were calculated using the Woolf approximation. When the value was zero, 0.5 was added to each to make the calculation possible.
Recurrence-free and overall 10-year survival rates were compared using Kaplan–Meier survival curves.
Commercially available SPSS 24.0 software for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. A p-value of <0.05 was considered statistically significant.
Results
The median age of the patients in the SCC group was higher than that in the non-SCC group (p = 0.023) (Table 1). In the SCC group, 65.9% (58/88) underwent conization and 80.7% (71/88) had colposcopic biopsies, whereas in the non-SCC group, 52.2% (35/67) underwent conization and 79.1% (53/67) had colposcopic biopsies (p = 0.085 and p =0.808, respectively). The histologic diagnoses in the non-SCC group included adenocarcinoma in 89.6% (60/67) and adenosquamous carcinoma in 10.4% (7/67). There was no difference in tumor markers (p = 0.296–0.906) between the groups.
The median size of the residual tumor was 8.4 ± 10.4 mm (0–36 mm) in the SCC group and 12.5 ± 11.9 mm (0–55 mm) in the non-SCC group (p = 0.024) (Table 2) (Figures 1, 2). The median depth of stromal invasion was 12.4 ± 21.2% (0–100%) in the SCC group and 22.4 ± 24.4% (0–93%) in the non-SCC group (p = 0.016) (Figures 1, 2). Residual tumors in these groups were detected in 52.1% (50/88) and 68.7% (46/67) (p = 0.133), respectively. SCC group (n = 88) underwent conization in 58 (65.9%) who had residual cancer in 27 (46.6%). Non-SCC group (n = 67) underwent conization in 35 (52.2%) who had residual cancer in 16 (45.7%). There was no difference between SCC and non-SCC groups regarding the incidence of residual cancer following conization (p = 1.000).
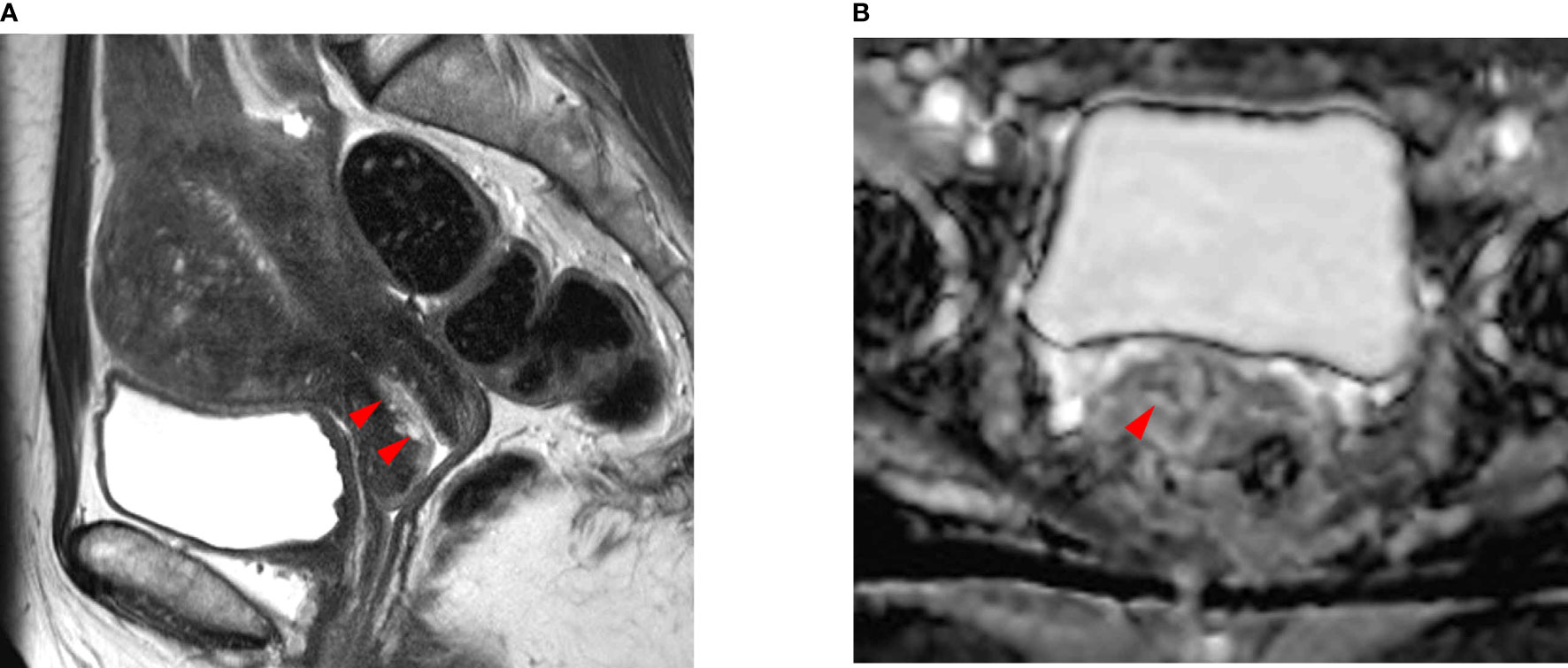
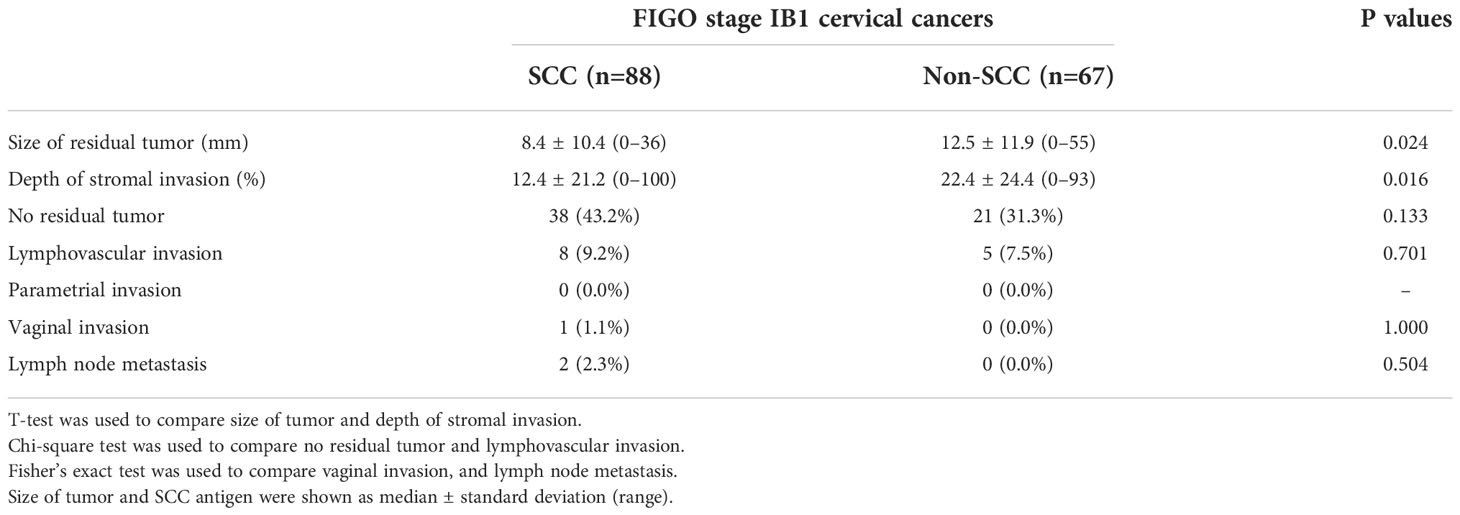
Figure 2 A 48-year-old woman with endocervical adenocarcinoma. (A) The T2-weighted sagittal MR image shows no tumor in the uterine cervix. The red arrows indicate a poorly demarcated cystic mass, which was preoperatively interpreted as normal endocervical glands. (B) The apparent diffusion coefficient (ADC) axial image shows no focal lesion with low ADC values in the cervical canal (red arrowhead). However, the pathologic report confirmed that there was a residual tumor in the endocervical canal. The tumor size was measured as 2.0 × 1.5 cm and the depth of stromal invasion was 0.4 cm in a 1.3-cm cervical wall. It was well-correlated with the endocervical lesion in (A). Tumor invasion to the lymphovascular space, vagina, and parametrium and lymph node metastasis were all negative.
Parametrial invasion in the SCC and non-SCC groups was detected at 0% (0/88) and 0% (0/67), respectively. LVS invasion was 9.1% (8/88) in the SCC group and 7.5% (5/67) in the non-SCC group, respectively (p = 0.701). LN metastasis was detected in 2.3% (2/88) and 0% (0/67) of the SCC and non-SCC groups, respectively (p = 0.504). Vaginal invasion was detected in 1.1% (1/88) and 0% (0/67) of the SCC and non-SCC groups, respectively (p = 1.000).
The tumor recurrence rate was 1.1% (1/88) in the SCC group and 4.5% (3/67) in the non-SCC group on follow-up CT or MR images (p = 0.316). The recurrence-free 10-year survival rate in the SCC and non-SCC groups was 98.9% (87/88) and 94.5% (64/67) (p = 0.246), respectively. The overall 10-year survival rate was 96.6% (85/88) and 95.5% (64/67) in the SCC and non-SCC groups, respectively (p = 0.872).
Recurrent tumors had the highest OR, at 4.078 in the SCC group versus the non-SCC group. The other ORs ranged from 0 to 1.665 for residual tumor, LN metastasis, LVS invasion, and vaginal invasion.
Discussion
Our results showed that the residual tumor size in the SCC group was smaller than that in the non-SCC group, even though none of these tumors were visible on MRI. The depth of stromal invasion in the SCC group was also smaller than that in the non-SCC group.
Currently, MRI is more available for women with cervical cancer because it is more precise for measuring tumor size than a physical examination (16–18). These MR images can be scanned in the axial, sagittal, and coronal planes. Therefore, the greatest tumor diameter and tumor volume are measured more accurately by palpation. Gynecologists inspect the outer tumor surface alone, but not the inner margin, which is well-depicted on MRI. This imaging modality provides precise tumor staging, and thus, it is more sensitive to detecting parametrial invasion or endocervical cancer than visual assessment (16–18). MRI also has the potential to avoid intravenous urography, cystoscopy, and sigmoidoscopy if cervical cancer is in the early stages (19–22). Moreover, current FIGO staging requires metastatic work-up in iliac or paraaortic LNs, which are not palpable (22). T2WI is useful for detecting morphologic changes, such as increased size, round shape, and the obliterated fatty hilum of metastatic LNs (23, 24). DWI is sensitive to changes in the tissue cellularity of metastatic LNs (25, 26). These MRI findings are currently used to determine if there is LN metastasis.
Cervical cancer that is not visible on MRI strongly suggests a lower tumor volume compared to those that are visible on MRI (1–3). Therefore, tumor invasion of the parametrium or vagina is extremely rare in invisible cervical cancer. The likelihood of cervical stromal or lymphovascular space invasion is much lower in cervical cancer that is not visible on MRI. LN, or hematogenous metastasis, is also rare. As a result, the long-term survival of patients with invisible cancer is better than that of patients with cancer visible on MRI. Moreover, additional post-operative treatments, such as radiation therapy or chemotherapy, are rarely necessary for women with invisible cervical cancer. Tumor invisibility on MRI can be a strong indicator of minimally invasive surgery.
Huang et al. reported that DCEI improved the depiction of cervical cancer that was not visible on T2WI and DWI (27). Quantitative analysis of DCEI parameters helps enhance the residual tumor after conization. Unfortunately, our study analyzed DCEI by visual assessment alone. Therefore, DCE-MRI quantitative parameters should be added to exclude the likelihood of residual cancer after conization. Hu et al. reported that radiomics had the potential to additionally detect cervical cancer that is not visible on conventional MRI (28). They demonstrated that analyzing radiomics improved diagnostic performance for detecting residual cancer after biopsy or conization. Xia et al. studied radiomics based on a nomogram to predict pelvic LN metastasis in women with early cervical cancer. They achieved high diagnostic accuracy for detecting preoperative pelvic LN metastasis (29).
Park et al. showed that many residual cancers were detected postoperatively even if the tumors were not visible on conventional MR images (1). They tried to identify useful MRI features to allow for minimally invasive surgery because radical hysterectomy with LN dissection results in serious postoperative morbidities. As such, invisible tumors on conventional MR images alone help gynecologists minimize parametrectomy procedures and reduce the extent of LN dissection.
We also agree with their point of view about the clinical significance of cancer-invisible MRI findings. In their research, almost half of the cases had a residual tumor, whose median size was 5 mm. Their 10-year recurrence-free survival rate was almost 100%. As a result, if small residual tumors are detected with new MRI techniques, the patients may undergo unnecessary radical surgery, which seems to be an excessive treatment. When cervical cancer is invisible on T2WI, DWI, and DCEI with visual assessment alone, these MRI findings can provide a clue for indicating minimally invasive surgery.
SCC cervical cancer tends to manifest as a solid tumor on MRI, and thus, the tumor size is easily measured (30). It is well correlated with the tumor size on the hysterectomy specimen. In contrast, the tumor margin of non-SCC cervical cancer is not easily demarcated on preoperative MRI because a cystic component is frequent (4–6). Therefore, if non-SCC is composed mainly of cysts, it is frequently difficult to differentiate from nabothian cysts. Besides, if a few cancer cells are just lining the surface of cysts, current MRI techniques make it difficult to determine if there is a residual tumor following a biopsy. For these reasons, the size of the residual tumor and the depth of stromal invasion in the non-SCC group could not be easily identified on preoperative MRI. These findings in the non-SCC group tend to be more frequent than in the SCC group, although neither are visible on MRI.
Radical hysterectomy is the standard treatment for FIGO stage IB1 cervical cancer and, subsequently, improves the long-term survival rate. This surgical technique consists of parametrectomy and LN dissection. Accordingly, patients have a higher risk of postoperative complications, such as voiding difficulty (7–9), anorectal dysfunction (10, 11), sexual dissatisfaction (10, 11), and lymphedema (12–14), if parametrectomy or LN dissection becomes aggressive. Therefore, greater attention is being paid to minimally invasive surgery to minimize these postoperative complications. The patients in our cases had a relatively younger median age (less than 50 years) and a higher overall survival rate. Because of the radical hysterectomy procedure, they have a high likelihood of postoperative morbidities for a long period. From this point of view, excessive surgical resection can be avoided in women who have cervical cancer that is not visible on MRI because local invasion or metastasis is histologically negative in almost all cases.
This study had several limitations. First, it was conducted retrospectively. Therefore, the likelihood of selection bias cannot be excluded. Second, the number of 1.5 T MRI examinations was relatively large. Unfortunately, our 1.5 T scanner could not provide DWI sequences because it was an old version. However, a 1.5 T scanner has a lower signal-to-noise ratio than a 3 T scanner. Third, the number of SCC cases was relatively small, and the proportion of SCC cases was relatively less than that of non-SCC. There was no difference in long-term survival rates, even though the recurrence rate of SCC was not the same as that of non-SCC.
Conclusion
The non-SCC group tends to have a larger size of residual tumor and a deeper depth of stromal invasion than the SCC group. Despite these histologic results, non-SCC cervical cancer is frequently invisible on preoperative MRI. Therefore, the extent of parametrectomy for non-SCC cervical cancer should be different from that for SCC cervical cancer, even though these tumors are not visible on preoperative MRI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Samsung Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization, JJ, BP, and J-WL. Methodology, JJ, BP, and J-WL. Software, BP. Validation, BP and J-WL. Formal analysis, JJ and BP. Investigation, JJ and BP. Resources, BP. Data curation, JJ and BP. Writing-original draft preparation, JJ and BP. Writing-review and editing, all authors. Visualization, BP. Supervision, BP and J-WL. Project administration, BP. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Park JY, Lee JW, Park BK, Lee YY, Choi CH, Kim TJ, et al. Postoperative outcomes of MR-invisible stage IB1 cervical cancer. Am J Obstet Gynecol (2014) 211(2):168.e1–7. doi: 10.1016/j.ajog.2014.02.032
2. Park BK, Kim TJ. Useful MRI findings for minimally invasive surgery for early cervical cancer. Cancers (Basel) (2021) 13(16). doi: 10.3390/cancers13164078
3. Jeong SY, Park BK, Choi CH, Lee YY, Kim TJ, Lee JW, et al. Utility of 3T MRI in women with IB1 cervical cancer in determining the necessity of less invasive surgery. Cancers (Basel) (2022) 14(1). doi: 10.3390/cancers14010224
4. Kido A, Mikami Y, Koyama T, Kataoka M, Shitano F, Konishi I, et al. Magnetic resonance appearance of gastric-type adenocarcinoma of the uterine cervix in comparison with that of usual-type endocervical adenocarcinoma: a pitfall of newly described unusual subtype of endocervical adenocarcinoma. Int J Gynecol Cancer (2014) 24(8):1474–9. doi: 10.1097/igc.0000000000000229
5. Ando H, Miyamoto T, Kashima H, Takatsu A, Ishii K, Fujinaga Y, et al. Usefulness of a management protocol for patients with cervical multicystic lesions: A retrospective analysis of 94 cases and the significance of GNAS mutation. J Obstet Gynaecol Res (2016) 42(11):1588–98. doi: 10.1111/jog.13083
6. Saida T, Sakata A, Tanaka YO, Ochi H, Ishiguro T, Sakai M, et al. Clinical and MRI characteristics of uterine cervical adenocarcinoma: Its variants and mimics. kjr (2019) 20(3):364–77. doi: 10.3348/kjr.2018.0458
7. Panici PB, Angioli R, Palaia I, Muzii L, Zullo MA, Manci N, et al. Tailoring the parametrectomy in stages IA2–IB1 cervical carcinoma: is it feasible and safe? Gynecol Oncol (2005) 96(3):792–8. doi: 10.1016/j.ygyno.2004.11.018
8. Artman LE, Hoskins WJ, Bibro MC, Heller PB, Weiser EB, Barnhill DR, et al. Radical hysterectomy and pelvic lymphadenectomy for stage IB carcinoma of the cervix: 21 years experience. Gynecol Oncol (1987) 28(1):8–13. doi: 10.1016/S0090-8258(87)80002-1
9. Landoni F, Maneo A, Cormio G, Perego P, Milani R, Caruso O, et al. Class II versus class III radical hysterectomy in stage IB–IIA cervical cancer: A prospective randomized study. Gynecol Oncol (2001) 80(1):3–12. doi: 10.1006/gyno.2000.6010
10. Rob L, Halaska M, Robova H. Nerve-sparing and individually tailored surgery for cervical cancer. Lancet Oncol (2010) 11(3):292–301. doi: 10.1016/S1470-2045(09)70191-3
11. Bonneau C, Cortez A, Lis R, Mirshahi M, Fauconnier A, Ballester M, et al. Lymphatic and nerve distribution throughout the parametrium. Gynecol Oncol (2013) 131(3):708–13. doi: 10.1016/j.ygyno.2013.10.006
12. Rebegea LF, Stoleriu G, Manolache N, Serban C, Craescu M, Lupu MN, et al. Associated risk factors of lower limb lymphedema after treatment of cervical and endometrial cancer. Exp Ther Med (2020) 20(6):181. doi: 10.3892/etm.2020.9311
13. Togami S, Kawamura T, Fukuda M, Yanazume S, Kamio M, Kobayashi H. Risk factors for lymphatic complications following lymphadenectomy in patients with cervical cancer. Japanese J Clin Oncol (2018) 48(12):1036–40. doi: 10.1093/jjco/hyy151
14. Togami S, Kubo R, Kawamura T, Yanazume S, Kamio M, Kobayashi H. Comparison of lymphatic complications between sentinel node navigation surgery and pelvic lymphadenectomy in patients with cervical cancer. Japanese J Clin Oncol (2020) 50(5):543–7. doi: 10.1093/jjco/hyaa001
15. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet (2009) 105(2):103–4. doi: 10.1016/j.ijgo.2009.02.012
16. Steed H, Capstick V, Schepansky A, Honore L, Hiltz M, Faught W. Early cervical cancer and parametrial involvement: Is it significant? Gynecol Oncol (2006) 103(1):53–7. doi: 10.1016/j.ygyno.2006.01.027
17. Yamazaki H, Todo Y, Okamoto K, Yamashiro K, Kato H. Pretreatment risk factors for parametrial involvement in FIGO stage IB1 cervical cancer. J Gynecol Oncol (2015) 26(4):255–61. doi: 10.3802/jgo.2015.26.4.255
18. Kamimori T, Sakamoto K, Fujiwara K, Umayahara K, Sugiyama Y, Utsugi K, et al. Parametrial involvement in FIGO stage IB1 cervical carcinoma: Diagnostic impact of tumor diameter in preoperative magnetic resonance imaging. Int J Gynecol Cancer (2011) 21(2):349. doi: 10.1097/IGC.0b013e3182072eea
19. Rockall AG, Ghosh S, Alexander-Sefre F, Babar S, Younis MT, Naz S, et al. Can MRI rule out bladder and rectal invasion in cervical cancer to help select patients for limited EUA? Gynecol Oncol (2006) 101(2):244–9. doi: 10.1016/j.ygyno.2005.10.012
20. Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet (2009) 105(2):107–8. doi: 10.1016/j.ijgo.2009.02.009
21. Jeong BK, Huh SJ, Choi DH, Park W, Oh D, Kim T, et al. Indications for endoscopy according to the revised FIGO staging for cervical cancer after MRI and CT scanning. J Gynecol Oncol (2012) 23(2):80–5. doi: 10.3802/jgo.2012.23.2.80
22. Merz J, Bossart M, Bamberg F, Eisenblaetter M. Revised FIGO staging for cervical cancer - a new role for MRI. Rofo (2020) 192(10):937–44. doi: 10.1055/a-1198-5729
23. Kim SH, Kim SC, Choi BI, Han MC. Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology (1994) 190(3):807–11. doi: 10.1148/radiology.190.3.8115631
24. Bipat S, Glas AS, van der Velden J, Zwinderman AH, Bossuyt PM, Stoker J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: a systematic review. Gynecol Oncol (2003) 91(1):59–66. doi: 10.1016/s0090-8258(03)00409-8
25. Shen G, Zhou H, Jia Z, Deng H. Diagnostic performance of diffusion-weighted MRI for detection of pelvic metastatic lymph nodes in patients with cervical cancer: a systematic review and meta-analysis. Br J Radiol (2015) 88(1052):20150063. doi: 10.1259/bjr.20150063
26. He XQ, Wei LN. Diagnostic value of lymph node metastasis by diffusion-weighted magnetic resonance imaging in cervical cancer. J Cancer Res Ther (2016) 12(1):77–83. doi: 10.4103/0973-1482.148726
27. Huang JW, Song JC, Chen T, Yang M, Ma ZL. Making the invisible visible: improving detectability of MRI-invisible residual cervical cancer after conisation by DCE-MRI. Clin Radiol (2019) 74(2):166.e15–.e21. doi: 10.1016/j.crad.2018.10.013
28. Hu Q, Shi J, Zhang A, Duan S, Song J, Chen T. Added value of radiomics analysis in MRI invisible early-stage cervical cancers. Br J Radiol (2022) 95(1133):20210986. doi: 10.1259/bjr.20210986
29. Xia X, Li D, Du W, Wang Y, Nie S, Tan Q, et al. Radiomics based on nomogram predict pelvic lymphnode metastasis in early-stage cervical cancer. Diagnostics (2022) 12(10):2446. doi: 10.3390/diagnostics12102446
Keywords: uterus, cervical cancer, histology, MRI, FIGO staging
Citation: Jeon J, Park BK, Lee J-W, Choi CH, Lee Y-Y, Kim T-J and Kim B-G (2022) Invisible cervical cancers on MRI: Can the type of histology (SCC versus non-SCC) influence surgical planning? Front. Oncol. 12:996516. doi: 10.3389/fonc.2022.996516
Received: 17 July 2022; Accepted: 21 November 2022;
Published: 08 December 2022.
Edited by:
Alessio G. Morganti, University of Bologna, ItalyReviewed by:
Komsun Suwannarurk, Thammasat University, ThailandMilly Buwenge, University of Bologna, Italy
Copyright © 2022 Jeon, Park, Lee, Choi, Lee, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Byung Kwan Park, cmFwYXJrQHNra3UuZWR1; Jeong-Won Lee, Z2FyZGVuLmxlZUBzYW1zdW5nLmNvbQ==
 Jungeun Jeon1
Jungeun Jeon1 Byung Kwan Park
Byung Kwan Park Chel Hun Choi
Chel Hun Choi Yoo-Young Lee
Yoo-Young Lee