
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 September 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.994444
This article is part of the Research Topic Surgical Oncology in the Elderly: The State of the Art and Future Challenges View all 11 articles
 Quoc Riccardo Bao1*†‡
Quoc Riccardo Bao1*†‡ Filippo Crimì2†‡
Filippo Crimì2†‡ Giovanni Valotto1‡
Giovanni Valotto1‡ Valentina Chiminazzo3
Valentina Chiminazzo3 Francesca Bergamo4‡
Francesca Bergamo4‡ Alessandra Anna Prete4‡
Alessandra Anna Prete4‡ Sara Galuppo5‡
Sara Galuppo5‡ Badr El Khouzai5‡
Badr El Khouzai5‡ Emilio Quaia2‡
Emilio Quaia2‡ Salvatore Pucciarelli1‡
Salvatore Pucciarelli1‡ Emanuele Damiano Luca Urso1‡
Emanuele Damiano Luca Urso1‡Background: The aim of this study is to evaluate the correlation between body mass index (BMI) and body fat composition (measured with radiological fat parameters (RFP)) and pathological response after neoadjuvant chemoradiotherapy for locally advanced rectal cancer patients. The secondary aim of the study was to assess the role of BMI and RFP on major surgical complications, overall survival (OS), and disease-free survival (DFS).
Methods: All patients who underwent surgical resection following nCRT between 2005 and 2017 for mid-low rectal cancer were retrospectively collected. Visceral fat area (VFA), superficial fat area (SFA), visceral/superficial fat area ratio (V/S), perinephric fat thickness (PNF), and waist circumference (WC) were estimated by baseline CT scan. Predictors of pathologic response and postoperative complications were investigated using logistic regression analysis. The correlations between BMI and radiologic fat parameters and survival were investigated using the Kaplan–Meier method and log-rank test.
Results: Out of 144 patients included, a complete (TRG1) and major (TRG1+2) pathologic response was reported in 32 (22%) and 60 (45.5%) cases, respectively. A statistically significant correlation between BMI and all the RFP was found. At a median follow-up of 60 (35–103) months, no differences in terms of OS and DFS were found considering BMI and radiologic fat parameters. At univariable analysis, neither BMI nor radiologic fat parameters were predictors of complete or major pathologic response; nevertheless, VFA, V/S>1, and BMI were predictors of postoperative major complications.
Conclusions: We found no associations between BMI and body fat composition and pathological response to nCRT, although VFA, V/S, and BMI were predictors of major complications. BMI and RFP are not related to worse long-term OS and DFS.
Rectal cancer represents a major cause of morbidity and mortality worldwide, and the actual standard of care for locally advanced rectal cancer is total mesorectal excision (TME) following neoadjuvant chemoradiotherapy (nCRT) (1).
A high body mass index (BMI) was associated with an increased colorectal cancer risk (2, 3), and general and visceral obesity were reported as risk factors for the increased incidence of colorectal neoplasms (4). Furthermore, BMI has been linked to a worse outcome of colorectal cancer (5–8), probably due to the deregulation of IGFR-1 and other cytokines involved in metabolic syndrome, which are overexpressed in obese patients (9). IGFR-1 was correlated with a poor response after nCRT in rectal cancer (10), and visceral obesity was associated with worse outcomes in patients with stage II and III colorectal cancer in terms of surgical outcomes and recurrence (11–14).
The impact of obesity and visceral fat on response to neoadjuvant treatment was investigated in other neoplasms, such as breast cancer. Even with controversial results, the role of visceral fat in the mechanism of chemosensitivity was suggested (15, 16). In rectal cancer, obese patients were reported to have a lower rate of complete pathologic response (pCR) and a lower rate of sphincter-preserving procedures. However, no difference in terms of recurrence rate was described in obese and nonobese (6). Furthermore, Sun et al. confirmed that obese patients have a lower pCR rate, besides BMI was associated with an adverse effect on downstaging and tumor regression grade, and resulted as a strong predictor for recurrence (5).
Up to 20% of rectal cancer patients showed a pCR following nCRT, permitting also organ-sparing approaches. The identification of predictors of pathologic response, such as biological markers (i.e., carcinoembryonic antigen (CEA), microsatellite instability) (17, 18), is now essential to the best selection of patients in a rectum-sparing program. However, the impact of obesity and body fat composition on pathologic response has not been extensively assessed in the current literature.
The aim of this study is to evaluate the correlation between obesity (defined using BMI) and body fat composition (measured with radiological fat parameters (RFP)) and pathological response after nCRT for locally advanced rectal cancer patients. The secondary aim of the study was to assess the role of BMI and RFP on major surgical complications, overall survival (OS), and disease-free survival (DFS).
All patients who underwent surgical resection following nCRT for locally advanced rectal cancer between 2005 and 2017 were retrospectively collected from the prospectively maintained database of the Colorectal Surgery (General Surgery 3), University Hospital of Padova. The study was notified and approved by the local Ethical Committee. Inclusion criteria were histologically confirmed mid-low rectal adenocarcinoma up to 12 cm from the anal verge surgically treated following standard nCRT. For patients treated with upfront or emergency surgery, or with recurrent disease, short-course radiotherapy was excluded. The baseline work-up included clinical history, digital rectal examination, colonoscopy, CEA level, chest/abdomen computed tomography (CT) scan, and pelvic magnetic resonance imaging (MRI).
Clinical and pathological TNM staging were reported according to the American Joint Committee on Cancer (AJCC) Eighth Edition (19). Tumor regression grade (TRG) was assessed according to Mandard’s classification (20). pCR was defined as no viable tumor cell found in the surgical specimen (TRG1), while major pathologic responses as TRG1 and TRG2.
All the patients underwent standard nCRT with 5-FU/capecitabine and 50.4 Gy of fractioned radiotherapy. Patients who underwent short-course radiotherapy (5 × 5) were excluded to eliminate a possible confounding factor. Indication for nCRT was discussed during the multidisciplinary meeting, according to current guidelines (21, 22). Surgical resection was planned after a re-evaluation during the multidisciplinary meeting. If a complete or major clinical response was observed (23) and patients were eligible for a rectum-sparing approach in the context of other study protocols, currently running in our center (23–25), patients were treated with local excision [i.e., transanal excision, transanal endoscopic operation (TEO)]. On the contrary, if a partial/absent response was observed, or in the case of patients not eligible for rectum-sparing approach, a radical resection (i.e., TME), as low anterior resection (LAR), abdominoperineal resection (APR), or intersphinteric resection (ISR) with coloanal anastomosis, was planned. In patients treated by local excision, radicalization surgery (completion TME) was recommended when one of the high-risk features was present on the histopathological report as previously described (25). Patients treated with a rectum-sparing approach were followed up every 3 months according to study protocols (23–25). In patients treated with TME, adjuvant treatment was offered to patients with pTNM II stage with high-risk features or pTNM III stage according to national guidelines (22).
BMI was calculated at baseline assessment as the ratio between body weight (kg)/height (m2). For each patient, abdominal fat was calculated as previously described from the available preoperative baseline CT scan by an expert radiologist, who was blinded to clinical data (26) using reconstruction software (Fujifilm Synapse 5). The following RFP were estimated: superficial fat area (SFA), visceral fat area (VFA), total fat area (TFA), perinephric fat (PNF), waist circumference (WC), and visceral/superficial fat area ratio (V/S). The image attenuation range was set between −190 and −30 Hounsfield Unit (HU) (11). VFA and SFA were measured using a single slice at the level of the intervertebral space between L4 and L5 (Figure 1). The area of the psoas and sacrospinal muscles were excluded from the area since it may contain fatty tissue derived from age-related fatty degeneration (27). TFA was calculated by summing VFA and SFA. PNF was defined as the shortest distance (mm) between the kidney and the abdominal wall (28). WC was calculated at the level of the middle point between the last rib and the iliac crest (29). V/S was calculated as the ratio between VFA and SFA.
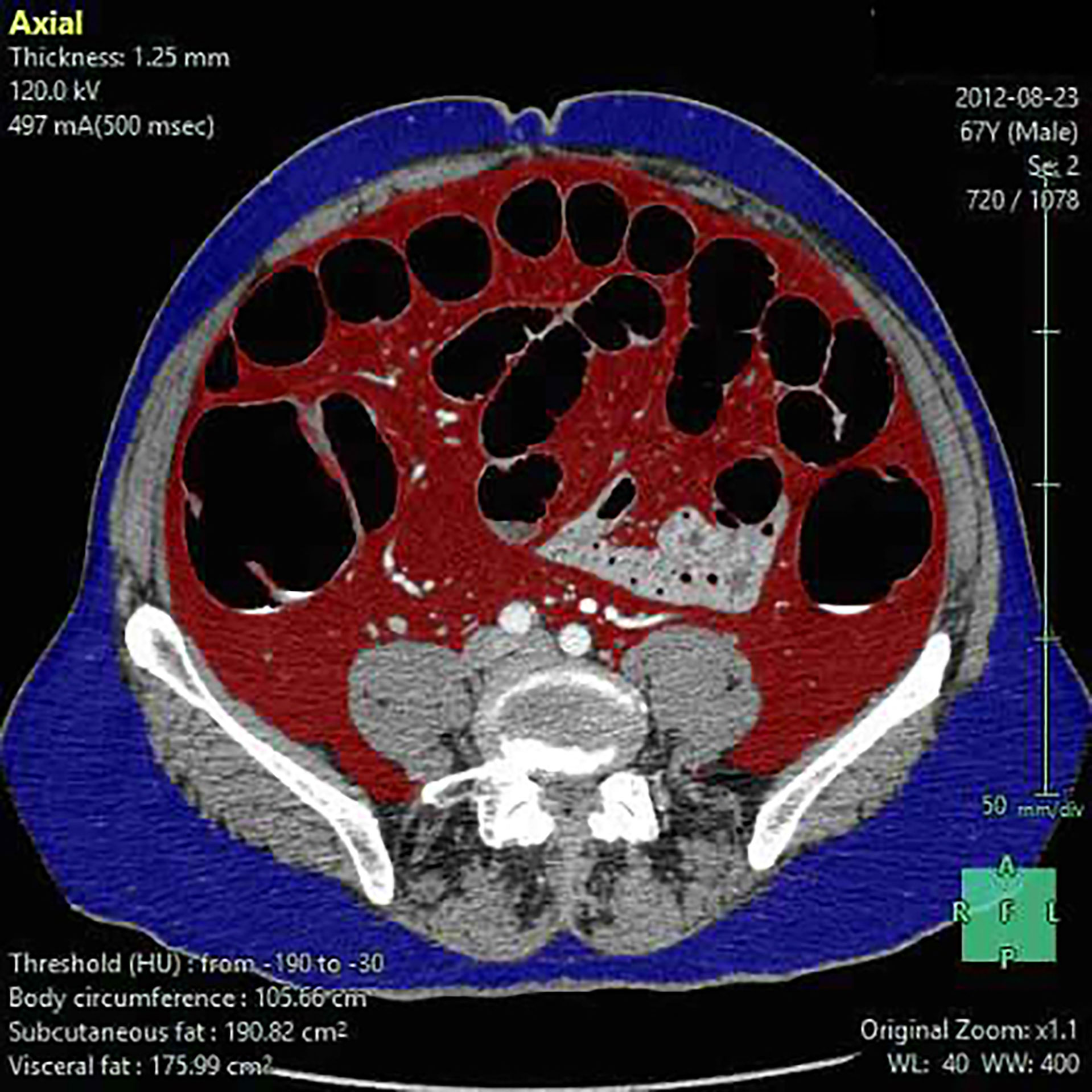
Figure 1 Visceral (VFA), superficial fat area (SFA), and waist circumference (WC) estimate. Superficial fat (blue), visceral fat (red), and waist circumference are estimated by using reconstruction software (Fujifilm Synapse 5).
Continuous variables were reported as median (I–III quartiles), while qualitative variables were reported as absolute numbers and percentages. Descriptive statistical analysis was performed by dividing patients with pCR and non-pCR, and complete/major vs partial/absent pathological response (TRG3–5). Significant differences between the two groups were tested by Pearson’s Chi-square for categorical variables and the Mann–Whitney U test for continuous variables. PNF and BMI were evaluated as both continuous and categorical variables, using as cutoff the median value (14.7 mm) for PNF and generally accepted cutoffs defined for overweight (BMI>25) and obesity (BMI>30). For V/S, two cutoffs of 0.4 and 1 were used according to the cutoffs used in the previous literature (11, 28). The correlation between BMI and RFP was evaluated with Spearman’s correlation coefficient and graphically presented using a correlation plot. Predictors of pathologic response were investigated using a univariable logistic regression approach, and a multivariable model was planned to investigate the independent predictors. The Kaplan–Meier method was used to estimate the OS, DFS, local-recurrence free survival (LRFS), and distant-recurrence free survival (DRFS). The survival curves were compared using the log-rank test. Local recurrence (LR) was defined as any recurrence in the pelvis, while distant recurrence (DR) was defined as any other recurrence. The association between RFP and OS and DFS was evaluated with univariable Cox proportional hazard models. All statistical analyses were performed using R software (version 4.0.3) (30), using the RMS package (31).
Patients, tumor, and treatment characteristics are summarized in Table 1. Overall, 144 patients were included for analysis, 97 (67.4%) were men and 47 (32.6%) were women. The median age was 66 (58–74) years, the median distance of the tumor from the anal verge was 6.0 (4.0–9.0) cm, and the median preoperative CEA was 2.1 (1.2–4.2) ng/ml.
The median time from the completion of nCRT and surgery was 8.6 (7.0–11.4) weeks. After nCRT, 91 (63.2%) patients underwent LAR, 24 (16.7%) APR, 20 (13.9%) local excision, and nine (6.3%) ISR with coloanal anastomosis. Among TME procedures, 106 (73.6%) patients had an open traditional approach and 18 (12.5%) had a laparoscopic approach. Among the 20 patients treated with local excision, only three (15%) had negative histopathological features and required a completion TME according to the study protocol. BMI, RFP, and postoperative complications according to Clavien–Dindo are described in Supplementary Table S2. Postoperative complications occurred in 55 (38.2%) patients, and in 11 (7.6%) patients requiring re-operation (Clavien–Dindo >3a). The histopathological analysis reported a pCR in 32 (22.2%) patients, whereas a major pathologic response in 28 (21.2%), respectively.
Median BMI was 25.0 (22.7–27.0), median SFA, VFA, and TFA were 175.5 (124.8–227.6), 140.8 (99.9–205.1), and 318 (249–430) cm2, respectively. Median PNF was 14.7 (7.4–22.6) mm, and median WC was 95.7 (88.4–103.8) cm. Median V/S ratio was 0.827 (0.620–1.141). The Spearman’s correlation coefficient showed a statistically significant correlation between SFA (p = 0.63, p < 0.001), VFA (p = 0.76, p < 0.001), V/S (p = 0.17, p = 0.04), TFA (p = 0.78, p < 0.001) PNF (p = 0.55, p < 0.001), and BMI (Figure 2).
The clinicopathological characteristics of patients with a pCR and a complete/major pathological response are summarized in Table 2 and Supplementary Table S1. A statistically significant difference was found between pCR and non-pCR patients regarding preoperative CEA (p = 0.04), distance from the anal verge (p = 0.002), and baseline cT stage (p = 0.03). No difference between the group pCR or a major pathological response regarding BMI, SFA, VFA, TFA, PNF, WC, or V/S.
Following a median follow-up of 59 (20–104) months, 20 (14.1%) patients died and 36 (25.0%) experienced recurrence. Five (3.4%) of these patients had LR, 27 (18.8%) had DR, and four (2.8%) had both LR and DR. The median OS of the whole cohort was 60.0 (34–104) months, and the median DFS was 32.0 (12.2–66.0) months. In patients with LR, the median LRFS was 18 (17–20) months, whereas the median DRFS was 15 (9–25) months. Out of the 20 patients treated with local excision, the median follow-up was 65.5 (48.8–110) months. Of these, one patient suffered LR requiring salvage TME, one patient had DR, and one patient had both LR and DR.
No differences in terms of OS, DFS, LRFS, and DRFS were found considering PNF (log-rank p = 0.89, p = 0.63, p = 0.38, and p = 0.72, respectively) (Supplementary Figure S1), BMI>25 (log-rank p = 0.66, p = 0.46, p = 0.48, and p = 0.51, respectively), BMI>30 (log-rank p = 0.55, 0.82, p = 0.93, and p = 0.99, respectively) (Supplementary Figure S2), V/S using a cutoff of 0.4 (log-rank p = 0.82, p = 0.23, p = 0.85, and p = 0.24, respectively), and a cutoff of 1 (log-rank p = 0.58, p = 0.14, p = 0.30, and p = 0.19, respectively) (Figure 3).
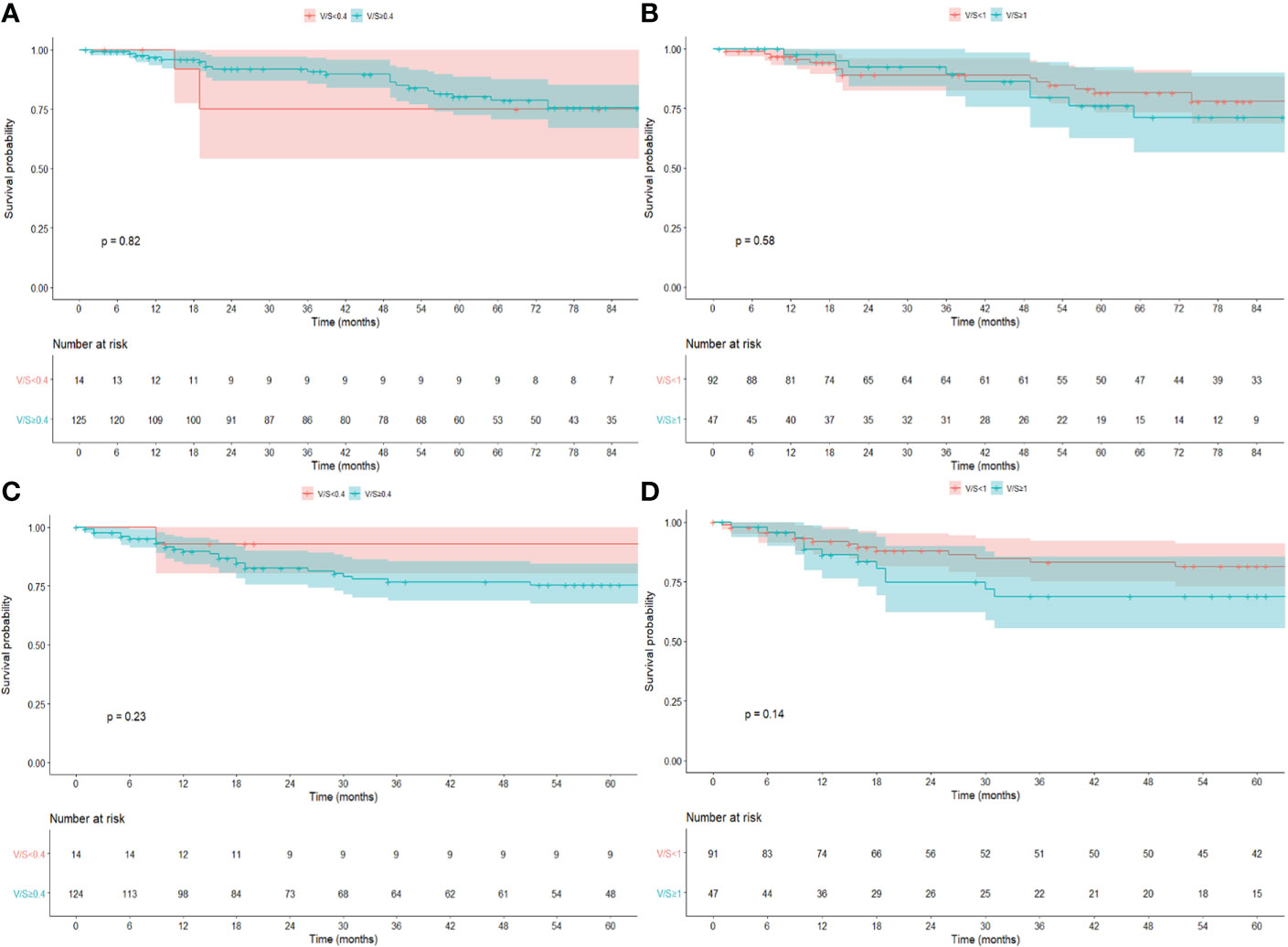
Figure 3 Kaplan–Meier survival estimate for OS and DFS for V/S using a cutoff of 0.4 (A, C) and 1.0 (B, D).
In a univariable logistic regression (Table 3) analysis, the baseline cT stage (OR, 4.86 (95% CI, 1.36–17.29); p = 0.04) and distance from the anal verge (OR, 0.22 (95% CI, 0.16–0.56); p = 0.00) were found to be predictors of pCR. Sex (OR, 0.42 (95% CI, 0.20–0.85); p = 0.02) and preoperative CEA (OR, 0.275 (95% CI, 1.07–1.98); p = 0.01) were linked to a significant pathological response. The general obesity index (BMI, WC) and abdominal obesity (SFA, VFA, TFA, and V/S ratio) were not predictive of pCR or major pathological response (TRG1–2).
At logistic regression analysis, VFA (OR, 2.14 (95% CI, 1.05–4.38)), V/S>1 (OR, 0.04 (95% CI, 0.04)), and BMI (OR, 1.71 (95% CI, 1.04–2.82)) were predictors of postoperative major complications.
The present study failed to demonstrate the correlation of BMI and RFP to pathologic response after nCRT and to the long-term outcomes in locally advanced rectal cancer patients. In our findings, general obesity and visceral fat did not correlate with pathologic response, so these parameters are not to be considered contraindications for the organ-sparing approach. Moreover, obesity and visceral fat were confirmed to be predictors of postoperative major complications. Obesity is known to be associated with increased intra- and postoperative complications, and some studies reported a worse survival after surgery in rectal cancer patients after nCRT (32–34). The role of obesity and abdominal fat as prognostic factors and their impact on oncological short- and long-term outcomes were studied with controversial results. We found no significant association between obesity and radiological abdominal fat parameters considered (BMI, SFA, VFA, TFA, PNF, WC, and V/S) and the pathologic response to nCRT. In the previous literature, a few authors investigated the correlation between obesity or abdominal fat and oncological outcomes in rectal cancer after nCRT (5, 6, 28, 35), whereas others investigated the role of obesity indexes in colon and rectal cancer patients altogether (14, 33). Park et al. and Sun et al. reported on two large series of rectal cancer patients and correlated only BMI to oncological outcomes (5, 6). Park et al. reported a lower rate of pCR and a lower rate of sphincter-saving procedures in obese patients (patients with a BMI>30) (6). Similarly, Sun et al. reported that obese patients had a lower pCR rate and adverse effects on downstaging and TRG. In this study, BMI>30 was found to be a strong predictor of recurrence, with an increased 5-year LR rate in severely obese patients (5). On the other hand, in both studies, OS was not affected by BMI. However, these authors used only BMI as an obesity index, instead of a more specific radiological index such as VFA and V/S.
Han et al. (36) described the association between obesity (defined as a BMI >25) and visceral obesity (defined as a VFA ≥100) and pCR in 536 rectal cancer patients after nCRT without finding any statistical correlation between those parameters. Similarly to our study, Lee et al. investigated the role of RFP in 125 rectal cancer patients. They found that only V/S>1 was related to a higher recurrence rate, and a worse DFS and OS. However, this study did not include patients treated with nCRT (11). AV/S cutoff of 0.4 was used by Clark et al., which found that higher VFA, V/S, and BMI were related to a minor tumor downstaging, a decreased DFS, and an increased recurrence rate. Furthermore, PNF was associated with a worse OS (28). Interestingly, patients with a V/S>0.4 were statistically older and affected by other comorbidities (hypertension, hypercholesterolemia). These conditions could explain a trend toward a worse OS. Moon et al. demonstrated that a higher V/S was related to a lower DFS, without difference in terms of OS (14). Finally, Goulart et al., including colon and rectal cancer patients, reported no difference in OS and DFS by dividing VFA into quartiles (33).
In our study, BMI and VFA were confirmed to be associated with postoperative complications. It is widely assumed that surgery in obese patients is affected by increased postoperative comorbidity due to the more difficult surgery and all the comorbidity associated with metabolic syndrome. Similarly to our finding, Zhou et al. reported VFA as a strong independent predictor of postoperative complications in rectal cancer (32). However, in this study, all the patients treated with nCRT were excluded. Heus et al. reported VFA and TFA in rectal cancer patients undergoing long-course nCRT. Using a cutoff of 100 cm2 for VFA, an increase in operative blood loss and postoperative complications were reported (35). Even if the role of postoperative complications on the long-term oncological outcome is still debated (37), postoperative complications, as far as worse general performance status, may result in a delay in adjuvant therapy and in an increased rate of LR and a decreased survival (38, 39).
The available literature on rectal cancer patients, obesity parameters, pCR, and other oncological outcomes has given conflicting results, mainly because in different studies, there are different inclusion criteria, methods, and main objectives and endpoints. With these limitations, there is no agreement on the role of obesity and its related radiological parameters on oncological outcomes in locally advanced rectal cancer patients treated with nCRT and surgery. Conflicting data exist on the role of obesity index on pathological response to nCRT, and only BMI was considered by Sun et al. and Park et al. (the studies with the largest number of patients included), while RFPs were not investigated (5, 6). Unlike oncological outcomes, we can find agreement in the literature on finding an association between most obesity index and perioperative complications. Based on the data available in the present study, obesity cannot basically influence oncological decision-making, but it is a predictor of a higher rate of surgical complications.
Our study does have some limitations. First is the small number of enrolled patients when compared with a few similar previous publications, even if only the study of Clark et al. has the same inclusion criteria and the same methods we used (28). Clark et al. analyzed the same obesity parameters we considered in a group of 99 rectal cancer patients treated with nCRT, finding that elevated V/S or PNF was associated with shorter DFS and OS. The relationships between BMI, RFPs, pathologic response, and postoperative complications were not investigated. From this point of view, our study investigated the largest group of locally advanced rectal cancer patients, all surgically treated after a neoadjuvant approach, considering BMI and all the most known RFPs, in association with pCR, OS, DFS, and perioperative complications. Despite these considerations, the relatively small number of patients enrolled could cover the predictive prognostic potential of some of the parameters studied in many of the analyses presented. For this reason, also, we did not use a multivariable model to investigate the independent predictors of complete/major pathologic response since none of the parameters considered resulted as a predictor of pathologic response.
Second, its retrospective design, even if the clinical data were prospectively maintained in our database, whereas RFP was retrospectively collected from CT scans, and the number of enrolled patients is smaller than in other studies. Third, we arbitrarily used different cutoffs for the RFP considered since there is no strong evidence, nor agreement, in the current literature about this topic. Further studies are needed to establish the proper cutoff of these indexes. Furthermore, we considered altogether different surgical procedures such as TME and local excision. Considering that the primary aim of the study is to assess a correlation between body fat and tumor regression, we think that patients enrolled in an organ-preservation prospective clinical study (ReSARCH trial) (23) with an accurate and standardized histopathological analysis and a long-term follow-up are eligible for the analysis, even if no pathological data are available on their mesorectal status. Last, we are analyzing the effect of nCRT on an Italian cohort, where the median BMI ranges between 24 and 26, and the rate of obesity and overweight is 10% and 35%, respectively (40). To note, this is the first study to analyzed the relationship between obesity and oncological outcomes in a group of Italian rectal cancer patients, whereas most of the cited studies are from the USA or China, where the estimated rate of obesity is greater than 36% and 16%, respectively (41, 42).
We found no associations between BMI and RFP and pathological response to nCRT, although VFA, V/S, and BMI were predictors of major surgical complications. BMI and RFP are not related to worse long-term OS and DFS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Comitato Etico per la Sperimentazione Clinica della Provincia di Padova. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Study concepts: QB, FC, EQ, SP, EU. Study design: QB, FC, EU. Data acquisition: QB, FC, GV, FB, AP, SG, BK. Data analysis and interpretation: QB, FC, GV, FB, AP, SG, BK. Statistical analysis: FC, VC. Manuscript preparation: QB, FC, GV. Manuscript editing: VC, FB, AP, SG, BK. Manuscript review: QB, EQ, SP, EU. All the authors revised and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.994444/full#supplementary-material
1. Ma B, Gao P, Wang H, Xu Q, Song Y, Huang X, et al. What has preoperative radio(chemo)therapy brought to localized rectal cancer patients in terms of perioperative and long-term outcomes over the past decades? A systematic review and meta-analysis based on 41,121 patients. Int J Cancer (2017) 141(5):1052–65. doi: 10.1002/ijc.30805
2. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut (2013) 62(6):933–47. doi: 10.1136/gutjnl-2013-304701
3. Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: Findings from cancer and leukemia group b 89803. J Clin Oncol (2008) 26(25):4109–15. doi: 10.1200/JCO.2007.15.6687
4. Murphy N, Carreras-Torres R, Song M, Chan AT, Martin RM, Papadimitriou N, et al. Circulating levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 associate with risk of colorectal cancer based on serologic and mendelian randomization analyses. Gastroenterology (2020) 158(5):1300–12:e20. doi: 10.1053/j.gastro.2019.12.020
5. Sun Y, Xu Z, Lin H, Lu X, Huang Y, Huang S, et al. Impact of body mass index on treatment outcome of neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur J Surg Oncol (2017) 43(10):1828–34. doi: 10.1016/j.ejso.2017.07.022
6. Park IJ, You YN, Skibber JM, Rodriguez-Bigas MA, Das P, Eng C, et al. Oncologic and functional hazards of obesity among patients with locally advanced rectal cancer following neoadjuvant chemoradiation therapy. Am J Clin Oncol (2017) 40(3):277–82. doi: 10.1097/COC.0000000000000150
7. Choi Y, Lee YH, Park SK, Cho H, Ahn KJ. Association between obesity and local control of advanced rectal cancer after combined surgery and radiotherapy. Radiat Oncol J (2016) 34(2):113–20. doi: 10.3857/roj.2016.01725
8. Scarpa M, Ruffolo C, Erroi F, Fiorot A, Basato S, Pozza A, et al. Obesity is a risk factor for multifocal disease and recurrence after colorectal cancer surgery: A case-control study. Anticancer Res (2014) 34(10):5735–41.
9. Berryman DE, Glad CA, List EO, Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol (2013) 9(6):346–56. doi: 10.1038/nrendo.2013.64
10. Wu XY, Wu ZF, Cao QH, Chen C, Chen ZW, Xu Z, et al. Insulin-like growth factor receptor-1 overexpression is associated with poor response of rectal cancers to radiotherapy. World J Gastroenterol (2014) 20(43):16268–74. doi: 10.3748/wjg.v20.i43.16268
11. Lee KH, Kang BK, Ahn BK. Higher visceral fat area/subcutaneous fat area ratio measured by computed tomography is associated with recurrence and poor survival in patients with mid and low rectal cancers. Int J Colorectal Dis (2018) 33(9):1303–7. doi: 10.1007/s00384-018-3065-z
12. Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle (2018) 9(1):53–9. doi: 10.1002/jcsm.12234
13. Rickles AS, Iannuzzi JC, Mironov O, Deeb AP, Sharma A, Fleming FJ, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointest Surg (2013) 17(1):133–43. doi: 10.1007/s11605-012-2045-9
14. Moon HG, Ju YT, Jeong CY, Jung EJ, Lee YJ, Hong SC, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol (2008) 15(7):1918–22. doi: 10.1245/s10434-008-9891-4
15. Omarini C, Palumbo P, Pecchi A, Draisci S, Balduzzi S, Nasso C, et al. Predictive role of body composition parameters in operable breast cancer patients treated with neoadjuvant chemotherapy. Cancer Manag Res (2019) 11:9563–9. doi: 10.2147/CMAR.S216034
16. Farr A, Stolz M, Baumann L, Bago-Horvath Z, Oppolzer E, Pfeiler G, et al. The effect of obesity on pathological complete response and survival in breast cancer patients receiving uncapped doses of neoadjuvant anthracycline-taxane-based chemotherapy. Breast (2017) 33:153–8. doi: 10.1016/j.breast.2017.04.001
17. Wallin U, Rothenberger D, Lowry A, Luepker R, Mellgren A. CEA - a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum (2013) 56(7):859–68. doi: 10.1097/DCR.0b013e31828e5a72
18. Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, et al. Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: A national cancer database (NCDB) analysis. Ann Surg (2020) 271(4):716–23. doi: 10.1097/SLA.0000000000003051
19. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: A Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
20. Mandard A-M, Dalibard F, Mandard J-C, Marnay J, Henry-Amar M, Petiot J-F, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathol Correlations Cancer (1994) 73(11):2680–6. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c
21. National comprehensive cancer network. rectal cancer (Version 1.2022) (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
22. AIOM. Linee guida NEOPLASIE DEL RETTO e ANO (2021). Available at: https://snlg.iss.it/wp-content/uploads/2021/11/LG-279-Retto-e-Ano_agg2021.pdf.
23. Barina A, De Paoli A, Delrio P, Guerrieri M, Muratore A, Bianco F, et al. Rectal sparing approach after preoperative radio- and/or chemotherapy (RESARCH) in patients with rectal cancer: a multicentre observational study. Tech Coloproctol (2017) 21(8):633–40. doi: 10.1007/s10151-017-1665-1
24. Valentini V, Gambacorta MA, Cellini F, Aristei C, Coco C, Barbaro B, et al. The INTERACT trial: Long-term results of a randomised trial on preoperative capecitabine-based radiochemotherapy intensified by concomitant boost or oxaliplatin, for cT2 (distal)-cT3 rectal cancer. Radiother Oncol (2019) 134:110–8. doi: 10.1016/j.radonc.2018.11.023
25. D'Alimonte L, Bao QR, Spolverato G, Capelli G, Del Bianco P, Albertoni L, et al. Long-term outcomes of local excision following neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Ann Surg Oncol (2021) 28(5):2801–8. doi: 10.1245/s10434-020-09243-6
26. Gradmark AM, Rydh A, Renström F, De Lucia-Rolfe E, Sleigh A, Nordström P, et al. Computed tomography-based validation of abdominal adiposity measurements from ultrasonography, dual-energy X-ray absorptiometry and anthropometry. Br J Nutr (2010) 104(4):582–8. doi: 10.1017/S0007114510000796
27. Yang KL, Yang SH, Liang WY, Kuo YJ, Lin JK, Lin TC, et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat Oncol (2013) 8:43. doi: 10.1186/1748-717X-8-43
28. Clark W, Siegel EM, Chen YA, Zhao X, Parsons CM, Hernandez JM, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg (2013) 216(6):1070–81. doi: 10.1016/j.jamcollsurg.2013.01.007
29. Ma WY, Yang CY, Shih SR, Hsieh HJ, Hung CS, Chiu FC, et al. Measurement of waist circumference: midabdominal or iliac crest? Diabetes Care (2013) 36(6):1660–6. doi: 10.2337/dc12-1452
30. R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria (2022). Available at: http://www.r-project.org/index.html
31. Harrell FE Jr. Rms: Regression modeling strategies (2020). Available at: https://CRAN.R-project.org/package=rms.
32. Zhou CJ, Cheng YF, Xie LZ, Hu WL, Chen B, Xu L, et al. Metabolic syndrome, as defined based on parameters including visceral fat area, predicts complications after surgery for rectal cancer. Obes Surg (2020) 30(1):319–26. doi: 10.1007/s11695-019-04163-1
33. Goulart A, Malheiro N, Rios H, Sousa N, Leão P. Influence of visceral fat in the outcomes of colorectal cancer. Dig Surg (2019) 36(1):33–40. doi: 10.1159/000486143
34. Balentine CJ, Wilks J, Robinson C, Marshall C, Anaya D, Albo D, et al. Obesity increases wound complications in rectal cancer surgery. J Surg Res (2010) 163(1):35–9. doi: 10.1016/j.jss.2010.03.012
35. Heus C, Cakir H, Lak A, Doodeman HJ, Houdijk AP. Visceral obesity, muscle mass and outcome in rectal cancer surgery after neo-adjuvant chemo-radiation. Int J Surg (2016) 29:159–64. doi: 10.1016/j.ijsu.2016.03.066
36. Han JS, Ryu H, Park IJ, Kim KW, Shin Y, Kim SO, et al. Association of body composition with long-term survival in non-metastatic rectal cancer patients. Cancer Res Treat (2020) 52(2):563–72. doi: 10.4143/crt.2019.249
37. Bao QR, Pellino G, Spolverato G, Restivo A, Deidda S, Capelli G, et al. The impact of anastomotic leak on long-term oncological outcomes after low anterior resection for mid-low rectal cancer: extended follow-up of a randomised controlled trial. Int J Colorectal Dis (2022) 37(7):1689–98. doi: 10.1007/s00384-022-04204-9
38. Koedam TWA, Bootsma BT, Deijen CL, van de Brug T, Kazemier G, Cuesta MA, et al. Oncological outcomes after anastomotic leakage after surgery for colon or rectal cancer: Increased risk of local recurrence. Ann Surg (2022) 275(2):e420–7. doi: 10.1097/SLA.0000000000003889
39. Kulu Y, Tarantio I, Warschkow R, Kny S, Schneider M, Schmied BM, et al. Anastomotic leakage is associated with impaired overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol (2015) 22(6):2059–67. doi: 10.1245/s10434-014-4187-3
40. Istituto Superiore di Sanità. Dati epidemiologici . Available at: https://www.epicentro.iss.it/obesita/epidemiologia-italia.
41. Obesity rate by state (2021). Available at: https://worldpopulationreview.com/state-rankings/obesity-rate-by-state.
Keywords: radiologic fat parameters and rectal cancer outcomes rectal cancer, neoadjuvant chemoradiotherapy, pathological response, obesity, visceral fat, BMI
Citation: Bao QR, Crimì F, Valotto G, Chiminazzo V, Bergamo F, Prete AA, Galuppo S, El Khouzai B, Quaia E, Pucciarelli S and Urso EDL (2022) Obesity may not be related to pathologic response in locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Front. Oncol. 12:994444. doi: 10.3389/fonc.2022.994444
Received: 14 July 2022; Accepted: 13 September 2022;
Published: 29 September 2022.
Edited by:
Felix Berlth, Johannes Gutenberg University Mainz, GermanyReviewed by:
Shenghui Huang, Fujian Medical University Union Hospital, ChinaCopyright © 2022 Bao, Crimì, Valotto, Chiminazzo, Bergamo, Prete, Galuppo, El Khouzai, Quaia, Pucciarelli and Urso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quoc Riccardo Bao, UXVvY3JpY2NhcmRvLmJhb0B1bmlwZC5pdA==
†These authors share first authorship
‡ORCID: Quoc Riccardo Bao, https://orcid.org/0000-0003-3463-6659
Filippo Crimì, https://orcid.org/0000-0001-6822-1430
Giovanni Valotto, https://orcid.org/0000-0002-6412-6150
Francesca Bergamo, https://orcid.org/0000-0002-8795-4653
Alessandra Anna Prete, https://orcid.org/0000-0002-6263-9278
Sara Galuppo, https://orcid.org/0000-0002-2290-9157
Badr El Khouzai, https://orcid.org/0000-0002-7124-4331
Emilio Quaia, https://orcid.org/0000-0003-2020-9365
Salvatore Pucciarelli, https://orcid.org/0000-0001-5289-9925
Emanuele Damiano Luca Urso, https://orcid.org/0000-0002-2789-2470
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.