- 1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Shanghai Clinical Research Center for Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 4Shanghai Key Laboratory of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 5Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: The predictive effects of liver metastases for immune-checkpoint inhibitors (ICIs) and the relationship between tumor mutational burden (TMB) and liver metastases (LM) remain unclear.
Methods: A systematic review and meta-analysis were conducted to explore the heterogeneity of ICIs efficacy between patients with or without LM. A pan-cancer cohort of 1,661 patients who received ICIs was downloaded and analyzed to assess the association between TMB and LM.
Results: Of 21053 studies identified in our search, eight single-arm studies and 24 randomized controlled trials were included. Overall, 17957 patients with advanced or metastatic cancers (4805 patients (26.8%) with LM and 13151 patients (73.2%) without LM) were enrolled. The pooled objective response rate (ORR) was 8.5% (95% CI 4%–13%) in the LM group versus 21% (95% CI 16%–21%) in the non-LM group. The pooled hazard ratio (HR) for death was 0.85 (95% CI 0.80–0.90) in the LM group treated with ICIs compared with the standard of care. In patients without LM who were treated with ICIs, the pooled HR for death was 0.78 (95% CI 0.73–0.82) compared with the standard of care. The difference in efficacy between patients with or without LM treated with ICIs was significant (p=0.04). Pan-cancer analysis revealed that the TMB-high rate was 10.8% in liver metastatic lesions versus 21.4% in other metastatic lesions (p=0.004). In addition, TMB was also significantly associated with OS as a binary cutoff (p=0.05) and was an independent prognostic variable (HR=0.98, P=0.047) as a continuous variable in patients with LM.
Conclusions: In patients with LM, the efficacy of immunotherapy was attenuated, but TMB-high could predict better survival outcomes.
Introduction
Cancer frequently metastasizes to the liver, and metastasizing of the liver contributed 30%-70% of cancer specific mortality (1). System therapies (including chemotherapy and target therapy) combined with local anatomic resections, radiation and interventional ablation were the standard therapeutics strategies for liver metastases. Given that patients with LM do not respond well to these conventional therapies and some were unable to continue due to liver dysfunction, these therapies offered a modest extension of survival.
ICIs targeting cytotoxic T-lymphocyte protein 4 (CTLA-4) and programmed death receptor-1 (PD-1) pathways have induced durable treatment responses in a wide variety of cancers. LM diminishes immunotherapy efficacy in preclinical models (2) and patients (2–4). Numerous trials showed that ICIs-based system therapy failed to obtain significant improvement in overall survival (OS) compared with the standard care in non–small cell lung cancer (NSCLC), small cell lung cancer (SCLC), and urothelial cancer (UCC) patients with LM (5, 6) (7, 8) (9). On the contrary, a favorable HR of death was shown in the renal-cell carcinoma (10), gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (11) patients with LM. Two meta-analyses done in advanced or metastatic cancers found no statistically significant association of LM with the efficacy of ICIs (12, 13). However, these meta-analyses included a limited number of trials and patients. Since the clinical trials showed contradictory results and the meta-analysis was not sufficiently powered, the clinical benefit of ICIs in patients with LM remains further investigated.
Higher TMB is associated with a higher number of tumor neoantigens that trigger a T cell response and clinically correlates with ICI outcomes (14, 15). A positive association of TMB and ICIs treatment efficacy was observed in advanced melanoma (16, 17), NSCLC (18, 19), gastroesophageal adenocarcinoma (20), and urothelial cancer (21). Samstein et al. reported that high TMB predicted superior survival across diverse types of human cancers (22), outside of these specific clinical trial populations. Whether high TMB predicts overall survival in patients with LM for ICIs treatment remains further investigated.
Here, we did a pan-cancer meta-analysis to assess the efficacy of ICIs both in patients with LM and patients without LM. We also analyzed a real-world cohort to explore the association between TMB and metastases sites and the predictive value of TMB on the efficacy of ICIs in the presence or absence of LM.
Methods
Search strategy and study selection
This analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (23).
We systemically searched PubMed, Embase, and Cochrane Library for published trials from January 01, 2011 to June 30, 2022, using the terms (“ipilimumab” OR “tremelimumab” OR “nivolumab” OR “pembrolizumab” OR “cemiplimab” OR “atezolizumab” OR “durvalumab” OR “avelumab” OR “tislelizumab” OR “sintilimab” OR “camrelizumab” OR “toripalimab”) AND (“trial” OR “clinical trial”) AND (“cancer” OR “carcinoma” OR “tumor” OR “tumour” OR “neoplasm”). Two authors (Ruiyan Wu and Kun Wang) independently conducted the searching process. The references of the included studies were manually searched for further eligible studies. Conference abstracts were excluded.
To be included, single arm studies had to assessed ORR for a single ICIs according to LM status; randomized trials had to meet the following criteria: (1) participants in the intervention group treated with a single ICIs or ICIs combinations or ICIs combined with standard care, and participants in the control group received standard care without ICIs; (2) have data available for the hazard ratio (HR) for death according to LM status. For studies with multiple reported data, we analyzed the most recent report and excluded the duplicates. All eligible studies were published in English. Any discrepancies were discussed and resolved by consensus.
Data analysis
Basic characteristics concerning the first author, publication year, study design, study phase, primary tumor, line of therapy, study drugs, number of patients, age, sex, median follow-up time, HR for death in the overall population, ORR and HR according to patients LM status were collected. The primary endpoint was the difference of ORR and HR for death reported according to LM status. We derived the ORR and their 95% CIs from single-arm trials, HRs for death and their 95% CIs (separately in intervention group and control group) from randomized trials for patients with or without LM. Statistical heterogeneity was estimated using the Q tests and quantified the heterogeneity of the results using I2 statistic percentages. The pooled ORR and HR for death according to LM status were calculated using a fixed-effects model (Mantel-Haenszel method) if the heterogeneity test showed no statistical significance (I2 ≤ 50% or p ≥ 0.10). Otherwise, a random-effects model was adopted. The heterogeneity between two estimates HR was assessed by interaction test (24).
To ascertain the risk of bias, the Risk of bias (RoB2) tool was used to evaluate the quality of randomized studies. A controlled trial could be assessed as “Low risk of bias”, “Some concern” and “High risk of bias”.
Real-world cohort identification and analysis
A published cohort with clinical and genomic data of 1,661 advanced cancer patients treated with ICIs therapy from the cBioPortal online database (https://www.cbioportal.org) was downloaded (22). 930 patients were included in the analysis with genomic profiling conducted in metastasis tissue and 731 patients with primary tissue profiling were excluded. TMB was calculated by normalizing the total number of somatic non-synonymous mutations to the total number of megabases sequenced. We compared the TMB between liver metastasis tissues and other metastases tissues using wilcox.test (continuous variable) and chi-square test (binary cutoff). Kaplan–Meier (KM) survival analysis was used to assess the survival differences. Univariate and multivariate Cox regression analyses of clinicopathological variables were performed to select candidate predictors of survival. The statistically significant variables (P < 0.05) in univariate Cox regression analyses were incorporated into the multivariate Cox for predicting OS.
All reported p values are 2-sided, and p<0.05 was considered statistical significance. We did all analyses using R (version 4.1.0).
Results
A total of 21053 records (PubMed:6290; Embase:8792; Cochrane Library:5971) were identified. 17798 records were screened after duplicated (3255 records) removed, of which 239 were reviewed in full text. In total, 32 studies [8 single-arm studies (21, 25–31) and 24 RCTs (5–11, 32–48)] involving advanced and metastatic cancers patients were included for analysis (Figure 1). There were 18 trials with first-line therapy, 13 trials with second or additional lines of therapy, and 1 trial with maintenance therapy. The cancer types including in our analysis were non-small cell lung cancer (NSCLC, n=6), small cell lung cancer (SCLC, n=5), urothelial cancer (UC, n=4), oesophageal squamous cell carcinoma or gastric or gastro-oesophageal junction cancer (EC/GEJ/G, n=4), renal cell carcinoma (RCC, n=3), triple-negative breast cancer (TNBC, n=1) and prostate cancer (PC, n=1). Basic characteristics of the trials included in the systematic review and pooled analysis were summarized in Supplementary Table 1 and Supplementary Table 2.
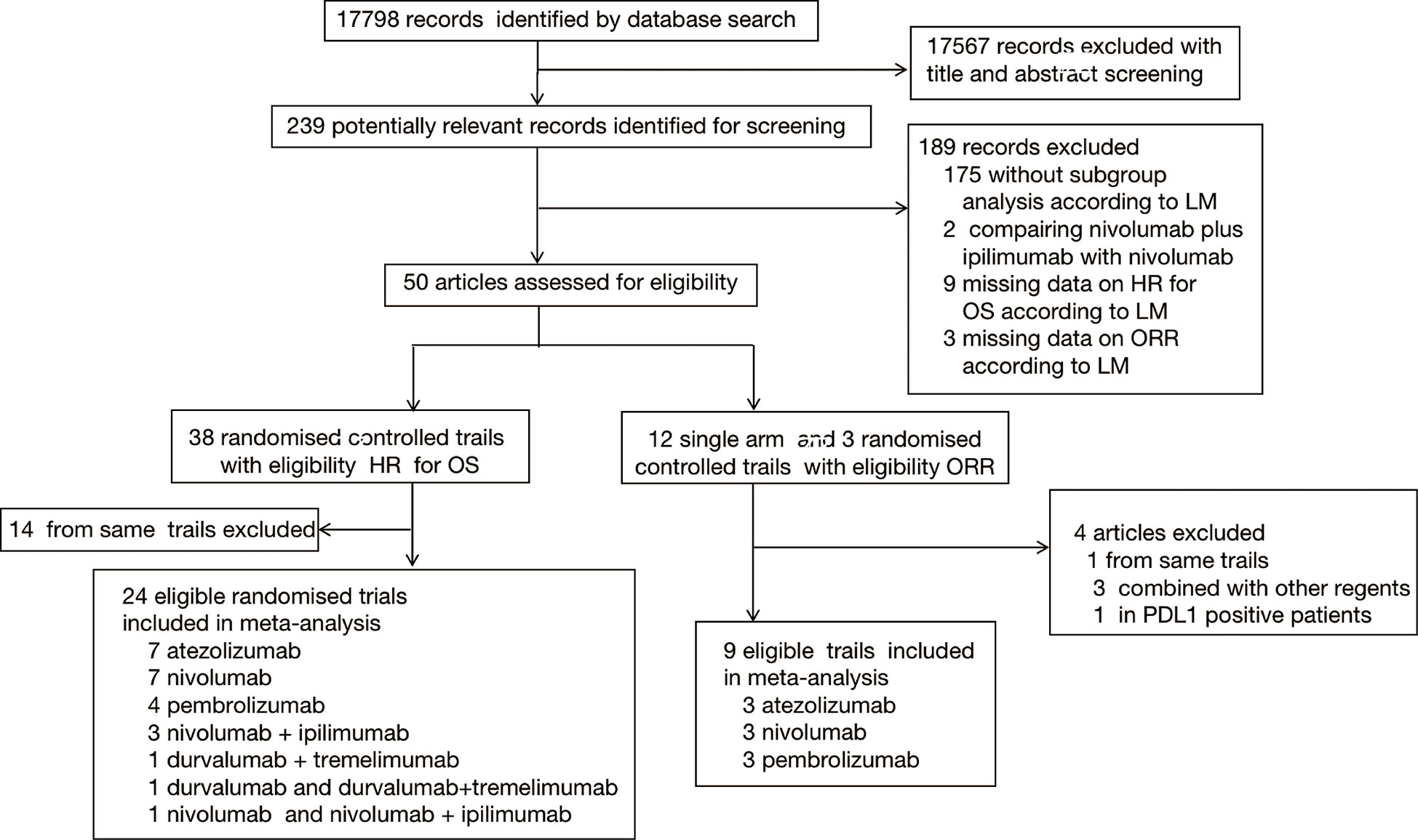
Figure 1 Study selection. Flowchart showing article identification, inclusion, and exclusion criteria.
23 trials reported data on HR for death, and eight trials reported ORR according to LM status, one trial reported both HR for death and ORR, and the reported data was extracted for pooled HR for death and ORR analysis. 2 single-arm studies included cisplatin-ineligible patients. One trial enrolled IMDC intermediate- and poor-risk only UCC patients. We didn’t include studies reporting data on HR for death or ORR stratified by PD-L1 expression.
First, we explored whether LM could affect the response of ICIs. Of the total of 9 trials enrolling 2702 patients reported data on ORR, 740 patients with LM (27.4%) and 1962 patients without LM (72.6%). ICIs monotherapy had a significantly lower ORR patients with LM (pooled ORR, 0.08; 95% CI, 0.04-0.13) compared to patients without LM (ORR, 0.22; 95% CI, 0.17-0.27). There was substantial inter-study heterogeneity among single-arm studies estimates in both patients with LM (Q = 44.44, p<0 .001, I2 = 82.0%) patients without LM (Q = 64.15, p<0 .001, I2 = 87.5%) (Supplementary Figure 1).
Then, we compared the survival benefit of ICIs versus standard care in the presence or absence of LM. Of the total of 24 trials enrolling 17957 patients reported data on HR for death, 4805 with LM (26.8%) and 13151 without LM (73.2%). For patients without LM, immunotherapy achieved a prolonged OS compared with control therapy (pooled HR, 0.78; 95% CI, 0.73-0.82). An OS advantage of immunotherapy was also obtained for patients with LM but was smaller (HR, 0.85; 95% CI, 0.80-0.90). There was significant inter-study heterogeneity among single study estimates in patients without LM (Q=43.08, p=0.01, I2 = 43%), but not in patients with LM (Q=30.62, p=0.20, I2 = 18%). There was a significant difference in the efficacy of ICIs between different LM status, when compared with controls (pheterogeneity=0.04, Figure 2). The pooled interaction HR (the pooled estimate of the ratios of the HRs in patients with LM and patients without LM reported in each trial) was 1.12 (95% CI 1.03-1.21). The RoB of 23 RCTs was generally low to moderate (Supplementary Figure 2).
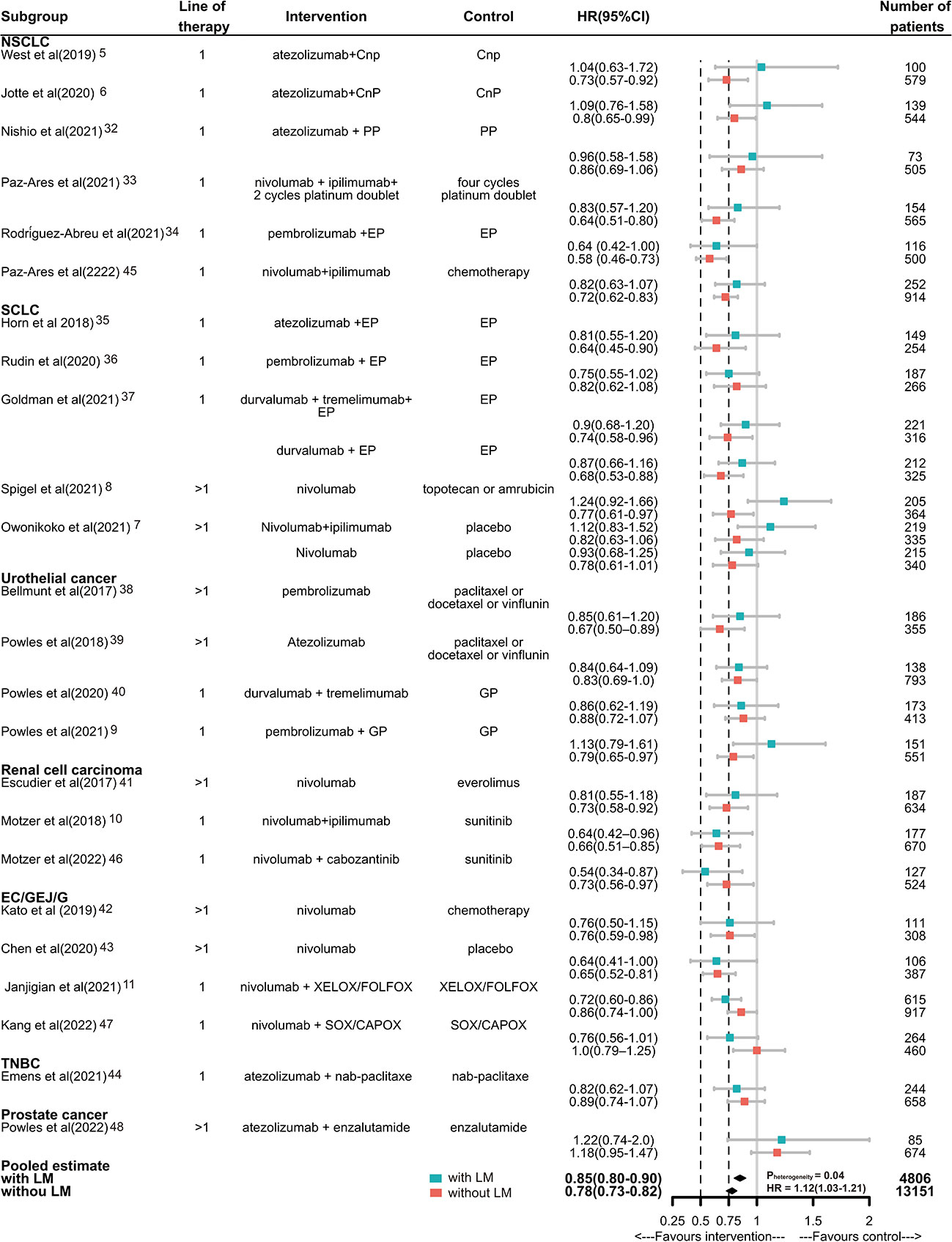
Figure 2 Hazard ratios of death for patients assigned to intervention treatment, compared with those assigned to control treatment, by LM status. Squares represent study-specific HRs. Horizontal lines indicate the 95% CIs. Diamonds indicate the meta-analytic pooled HRs, calculated separately in the presence or absence of LM, corresponding to 95% CIs. The p value for heterogeneity is from the meta-analysis of the interaction HRs and represents heterogeneity by LM status. Cnp100= carboplatin plus nab-paclitaxel. PP, pemetrexed plus cisplatin or carboplatin; EP/EC, etoposide plus cisplatin or carboplatin; GP, gemcitabine plus cisplatin or carboplatin; CAPOX/FOLFOX, capecitabine plus oxaliplatin or leucovorin, fluorouracil, plus oxaliplatin. HR, hazard ratio.
In subgroup analyses, for NSCLC, SCLC, ICIs only without combination with chemotherapy or target therapy, anti-PD-1/PD-L1 plus anti-CTLA-4, the magnitude of efficacy of ICIs was greater for patients without LM than patients with LM, and the heterogeneity test for this LM-related interaction was significant. For UC, RCC, EC/GEJ/G, ICIs combination with chemotherapy therapy, anti-PD-1or anti-PD-L1, first or subsequent line of treatment, the efficacy of ICIs was not significant different. Notably, in TNBC and PC, the ICIs was not effective in reducing risk of death for patients with or without LM. But due to the limited trails available, we coudn’t draw specific conclusions(Figure 3).
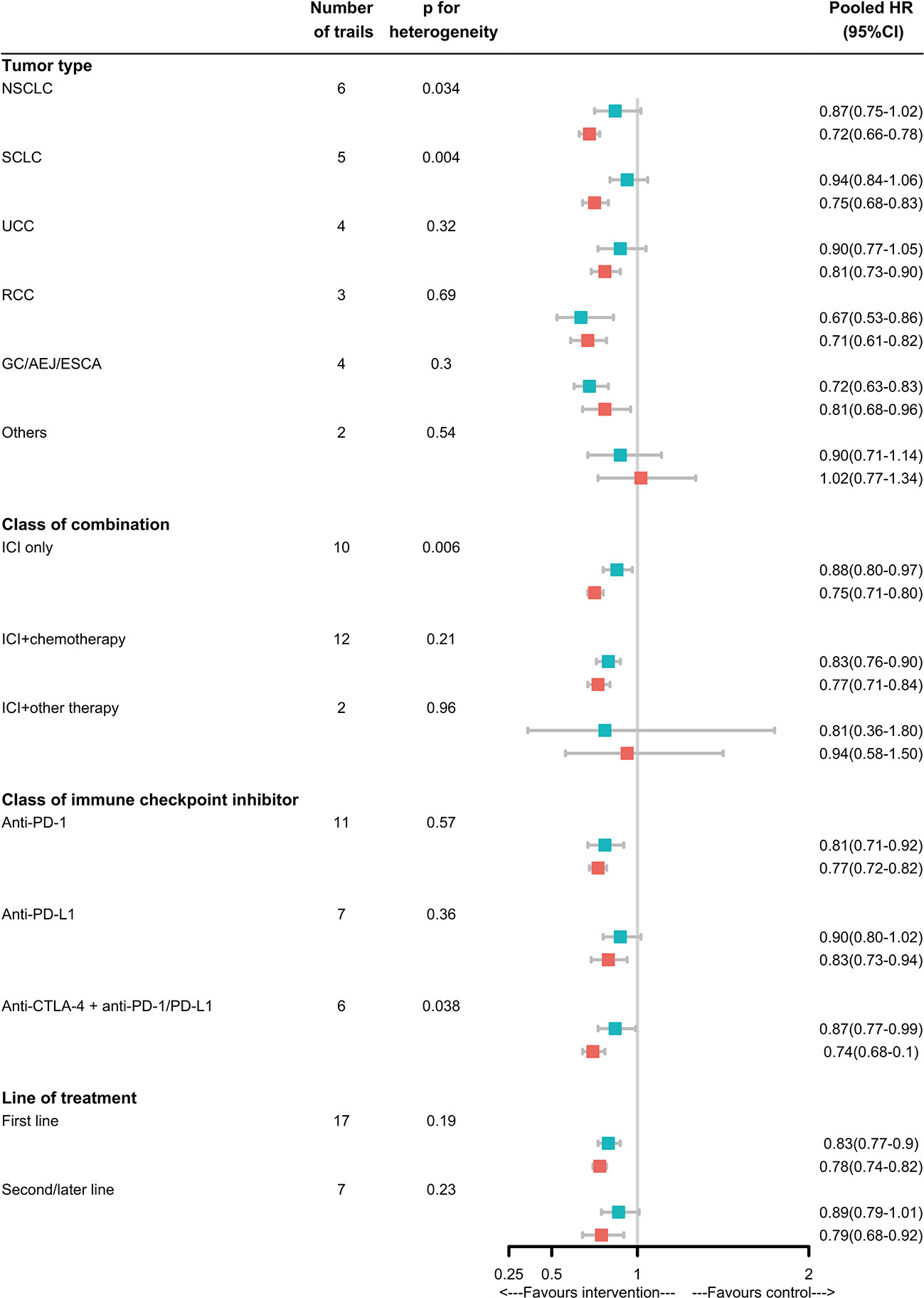
Figure 3 Analyses of LM-specific pooled hazard ratios, by subgroup. Squares represent subgroup-specific pooled hazard ratios (HRs). Horizontal lines indicate 95% CIs. The p-value for heterogeneity is from an interaction test that compares the estimated HRs across different LM statuses and represents heterogeneity within each subgroup.
Finally, we evaluated the prognostic impact of TMB in different metastases tissues since TMB is widely used to predict clinical outcomes of ICIs. We identified a cohort of 1,661 cancer patients with 11 cancer types. We included 930 patients with TMB score estimated in metastasis tissues (liver metastasis: 139, other site metastasis: 791). No significant differences between the clinicopathological characteristics (age, sex, and drug type of ICIs), except cancer type and TMB, were observed in the presence or absence of LM (Supplementary Table 3). TMB score was higher in patients with LM (p=0.048) (Figure 4A). We performed a stratified analysis by binarizing TMB level as TMB-high (top 20%) and TMB-other (bottom 80%) and found that TMB-high rate was half smaller in patients with LM patients without LM (p=0.004) (Figure 4B). TMB-high corresponded to a favor OS in patients with LM (p=0.05) and patients without LM (p<0.001) by Kaplan-Meier (KM) survival analysis (Figure 4C). Then, we further investigated whether TMB was an independent prognostic variable in patients with LM. Univariate and multivariate Cox regression analyses of clinicopathological variables were performed to select candidate predictors of survival. The statistically significant variables (P < 0.05) in univariate Cox regression analyses were incorporated into the multivariate Cox regression to test each factor’s independence for prediction OS. TMB was significantly associated with OS as a continuous variable (HR=0.98; 95% CI, 0.97-1.00, p=0.047), but the binary cutoff (top 20%, HR=0.42; 95% CI, 0.17-1.05, p=0.064) was marginal (Supplementary Table 4). Then, after adjusting for cancer type and drug type of ICIs by multivariable analysis, TMB as a continuous variable was still independency variable for prediction OS in patients with LM (adjusted HR=0.98; 95% CI, 0.96-1.00, p=0.049) (Supplementary Table 5).

Figure 4 Association between TMB and metastases. (A)Analyses of TMB score between different metastases sites, the p value is from wilcox test. (B)Analyses of TMB-high and TMB-other distribution between different metastases sites, TMB-high was defined as the top 20% of patients and the bottom 80% patients were defined as TMB-other. The p value is from Chi-square test. (C) Kaplan-Meier graph of overall survival of 914 patients with overall survival longer than one month. Two-sided log-rank p value indicated the Top20% groups compared to the bottom80% group for patients with or without LM.
Discussion
This study showed that ICI-based systemic therapy could improve overall survival for patients with or without LM. However, patients with LM had a smaller treatment benefit from these drugs versus control treatments than patients without LM. To our knowledge, this was the first study to clearly show significant heterogeneity in the efficacy of immune checkpoint inhibitors according to the patient’s liver metastases status. The benefit difference of our findings was strengthened by single-arm studies of ICIs monotherapy. The pooled ORR was smaller than half the size for patients with LM than for patients without LM. This result was clinical reverence to the previous report that patients with LM had significantly shorter overall survival (OS) than those without LM (10 vs. 20 months; P < 0.0001) (4).
The liver is naturally programmed as an immune privilege organ with hypo-reactivity to food-derived antigens and bacterial products through the portal vein (49–51). The classic hypothesis was that a variety of hepatic cell types can induce activated T-cell accumulation and apoptosis in the liver, including liver-resident antigen-presenting cells (APCs) (52), Kupffer cells (53), liver sinusoidal endothelial cells (54), hepatocytes (55), NKT cells (56) and stellate cells (57). Liver metastases further prompted hepatic immunotolerance by eliminating systemic tumor-specific CD8+ T cells through macrophages induced, Fas-FasL pathway dependent apoptosis (2). In fact, the presence of liver metastases was associated with fewer infiltrating CD8+ T cells at the invasive margin in distant tumors (3). Besides, Lee et al. reported that liver could lead to the systemic suppression of antitumor immunity and response to anti-PD-1 immunotherapy through activation of regulatory T cells (Tregs) and modulation of intratumoral CD11b+ monocytes (58). This general state of tolerance enabled the expansion of local metastases and had systemic consequences that manifested poor response to immunotherapy in patients with LM. Subgroup analysis from a total of 11 trials of NSCLC and SCLC showed that the efficacy of ICIs was evidently decreased and marginal for patients with LM. The fundamental inhibition mechanisms and magnitude of decreased efficacy, suggested that more assessment should be paid for patients with LM in the routine clinical practice of ICIs treatment, especially in NSCLC and SCLC.
Since specific adverse events and the significant economic cost of ICIs, efforts are an urgent need for identifying predictive biomarkers to select patients who would derive the maximum potential benefit from immunotherapies with liver metastases. We found that TMB, as a continuous variable, was an independent prognostic factor (HR=0.98, P=0.047) adjustment for cancer type and drug class of ICIs by multivariate Cox regression analyses for patients with LM. As a binary cutoff, TMB was also significantly associated with OS by Kaplan-Meier (KM) survival analysis (p=0.05) but the HR was marginal. We supposed that the contradiction result might due to limited patients number (n=15) in TMB-high group.
We also found that both TMB score and TMB-high rate was smaller in patients with LM, which might partly contribute to the relatively poor response. A previous pan-cancer analysis based on the same cohort of 1,661 cancer patients showed that TMB level was comparable between patients with and without LM (22). In our analysis, we included 930 patients with TMB estimated in metastasis tissue, the patients with TMB estimated in primary tissue were excluded for analysis. Besides, we compared the high TMB (defined as the highest 20%) rate instead of TMB level since the former is widely used in clinical practice. There were studies reported that the TMB status differed between the liver metastatic lesions, primary lesions and lung metastatic lesions. Hoshion et al. found that TMB was found to be high (10 or more per 1 Mb) in 8 out of 24 patients with primary lesions and in 5 of 24 patients with liver metastatic lesions (59). Wang et al. found the lung metastases from colorectal cancer (CRC) demonstrated notably higher TMB levels than those of liver metastases from CRC (6.022 vs. 2.02 SNVs/Mb, P=0.044) and a TMB >10 SNVs/Mb was observed more frequently in samples from the lung metastases than liver metastases cohort (P=0.004) (60).
Data from clinical trials (IMpower150 (61)) indicated that chemotherapy could restore systemic efficacy of ICIs for patients with LM. Our result showed that for ICIs combined with chemotherapy, comparable benefit (pheterogeneity=0.56) was yielded regardless of LM status, while for ICIs only without combination, the benefit was marginal and evidently decreased (Pheterogeneity=0.006) in patients with LM. These data were in line with the hypothesis that tumor cell destruction by chemotherapy may provide a broad neoantigen to facilitate immune recognition and anti-tumor immune response (62). Radiotherapy could reshape the liver immune microenvironment via increasing hepatic T cell infiltration, diminishing liver myeloid cell number and diminishing the ratio of CD11b+F4/80+ myeloid cells to CD8+ T cells, thus abolished immunotherapy resistance induced by liver metastasis in MC38 mouse modle (2). With appropriate biomarker and manipulation of the liver metastases microenvironment, a positive response of ICIs for patients with LM could be elicit.
Subgroup analysis showed that, anti-PD-1/PD-L1 combined with CTLA-4 can significantly improve overall survival in both patients with or without LM, but the magnitude of this benefit is largely dependent on liver metastasis status. For patients with LM, the efficacy of ICIs was valid for patients in anti-PD-1 and first-line settings but was marginal in anti-PD-L1 and second-line settings. The biological difference between anti-PD-1 and anti-PD-L1 attributed to disrupting different aspects of the same ligand-receptor interaction: blockade of additional ligand-receptor interactions such as PD-L2-PD-1 with anti-PD-1, but not with anti-PD-L1 (63). Human adult liver expressed PD-L2 but not PD-L1 (63), and these might relate to the benefit difference between anti-PD-1 and anti-PD-L1 in patients with LM. In addition, chemotherapy resistance increased the level of terminally differentiated and immunosuppressive cells (i.e., SPP1+ and MRC1+ CCL18+ macrophages) while decreased the cytotoxic immune cells (i.e., FGFBP2+ GZMB+ CD8+ T cells) in liver metastases patients (64), providing evidence to support first-line use of ICIs for patients with LM. For these reasons, anti-PD1 or combination with an-CTLA-4, the first-line setting was more favorable for patients with LM.
This study had several limitations. Firstly, our meta-analysis relied on published results rather than on individual patients’ data and we could not exclude the variables that might influence our results. Apart from TMB, factors associated with ICIs efficacy, such as age, gender, the expression of PD-L1 or the EGFR or ALK mutational status, were not so far known to be distributed differentially associated with patients’ liver metastases status. Secondly, a relatively small number of eligible trials for RCC, EC/GEJ/G, TNBC and PC were included in the analysis and we cannot make a solid conclusion on the true predictive or prognostic significance of LM in the tumor type mentioned above. Thirdly, no eligible trial for melanoma patients was included in our analysis due to no RCT reported HR for death according to liver metastases status.
Two main conclusions can be drawn from this study. First, LM was an important prognostic variable and should be considered in the assessment of risk versus benefit for ICI-based systemic therapy. ICI-based systemic therapy could significantly improve overall survival in both patients with or without LM but the magnitude of this benefit is largely LM status dependent. Future research should focus on improving outcomes for patients with LM. Second, high TMB was distributed differentially according to LM status and was also a favor biomarker for ICI-based systemic therapy regardless of LM status. However, due to the relatively small number of eligible patients into the final analysis, the prognostic value of high TMB in the presence of LM remained further investigated.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
Study design: ZZ, J-FW and R-YW. Data extraction: R-YW, B-CW, KW, FX, Z-YZ. Data analysis: R-YW, B-CW, KW, FX, Z-YZ. Manuscript writing: R-YW, B-CW, KW. Manuscript edition: ZZ, J-FW. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82002905, 82003229), the Shanghai sailing plan (20YF1408300) and Key Clinical Specialty Project of Shanghai.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.994276/full#supplementary-material
Abbreviations
ICIs, Immune checkpoint inhibitors; CTLA-4, Cytotoxic T lymphocyte antigen 4; PD-1, Programmed death 1; PD-L1, Programmed cell death 1 ligand 1; TMB, Tumor mutational burden; LM, Liver metastases; OS, Overall survival; ORR, Objective response; HR, Hazard ratio; CI, Confidence interval; Cnp, Carboplatin plus nab-paclitaxel; PP, Pemetrexed plus cisplatin or carboplatin; EP/EC, Etoposide plus cisplatin or carboplatin; GP, Gemcitabine plus cisplatin or carboplatin; CAPOX, Capecitabine plus oxaliplatin; FOLFOX, Leucovorin, fluorouracil, plus oxaliplatin.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med (2021) 27(1):152–64. doi: 10.1038/s41591-020-1131-x
3. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res (2017) 5(5):417–24. doi: 10.1158/2326-6066.CIR-16-0325
4. Chen XJ, Ren A, Zheng L, Zheng ED, Jiang T. Pan-cancer analysis identifies liver metastases as negative predictive factor for immune checkpoint inhibitors treatment outcome. Front Immunol (2021) 12:651086. doi: 10.3389/fimmu.2021.651086
5. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6
6. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
7. Owonikoko TK, Park K, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J Clin Oncol (2021) 39(12):1349–59. doi: 10.1200/JCO.20.02212
8. Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331(). Ann Oncol (2021) 32(5):631–41. doi: 10.1016/j.annonc.2021.01.071
9. Powles T, Csoszi T, Ozguroglu M, Matsubara N, Geczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(7):931–45. doi: 10.1016/S1470-2045(21)00152-2
10. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
11. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
12. Li S, Sun S, Xiang H, Yang J, Peng M, Gao Q. Liver metastases and the efficacy of the PD-1 or PD-L1 inhibitors in cancer: a meta-analysis of randomized controlled trials. Oncoimmunology (2020) 9(1):1746113. doi: 10.1080/2162402X.2020.1746113
13. Wei Y, Li Y, Du Q, Peng X, Jin J, Guo H, et al. Effects of clinicopathological characteristics on the survival of patients treated with PD-1/PD-L1 inhibitor monotherapy or combination therapy for advanced cancer: A systemic review and meta-analysis. J Immunol Res (2020) 2020:5269787. doi: 10.1155/2020/5269787
14. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (2015) 348(6230):69–74. doi: 10.1126/science.aaa4971
15. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science (2018) 362(6411):eaar3593. doi: 10.1126/science.aar3593
16. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med (2014) 371(23):2189–99. doi: 10.1056/NEJMoa1406498
17. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science (2015) 350(6257):207–11. doi: 10.1126/science.aad0095
18. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (2015) 348(6230):124–8. doi: 10.1126/science.aaa1348
19. Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic features of response to combination immunotherapy in patients with advanced non-Small-Cell lung cancer. Cancer Cell (2018) 33(5):843–852 e4. doi: 10.1016/j.ccell.2018.03.018
20. Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Molecular determinants of clinical outcomes with pembrolizumab versus paclitaxel in a randomized, open-label, phase III trial in patients with gastroesophageal adenocarcinoma. Ann Oncol (2021) 32(9):1127–36. doi: 10.1016/j.annonc.2021.05.803
21. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (2016) 387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4
22. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet (2019) 51(2):202–6. doi: 10.1038/s41588-018-0312-8
23. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA (2015) 313(16):1657–65. doi: 10.1001/jama.2015.3656
24. Collins GG, Youdim MB. The binding of (14C)phenethylhydrazine to rat liver monoamine oxidase. Biochem Pharmacol (1975) 24(6):703–6. doi: 10.1016/0006-2952(75)90246-4
25. Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol (2016) 17(11):1590–8. doi: 10.1016/S1470-2045(16)30496-X
26. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (2017) 389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2
27. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol (2017) 18(3):312–22. doi: 10.1016/S1470-2045(17)30065-7
28. Vuky J, Balar AV, Castellano D, O'Donnell PH, Grivas P, Bellmunt J, et al. Long-term outcomes in KEYNOTE-052: Phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol (2020) 38(23):2658–66. doi: 10.1200/JCO.19.01213
29. Apolo AB, Ellerton JA, Infante JR, Agrawal M, Gordon MS, Aljumaily R, et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase ib JAVELIN solid tumor study: 2-year updated efficacy and safety analysis. J Immunother Cancer (2020) 8(2):e001246. doi: 10.1136/jitc-2020-001246
30. Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort a of the phase II KEYNOTE-086 study. Ann Oncol (2019) 30(3):397–404. doi: 10.1093/annonc/mdy517
31. Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncol (2019) 5(1):74–82. doi: 10.1001/jamaoncol.2018.4224
32. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: Results from the randomized phase 3 IMpower132 trial. J Thorac Oncol (2021) 16(4):653–64. doi: 10.1016/j.jtho.2020.11.025
33. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
34. Rodriguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol (2021) 32(7):881–95. doi: 10.1016/j.annonc.2021.04.008
35. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
36. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol (2020) 38(21):2369–79. doi: 10.1200/JCO.20.00793
37. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2021) 22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8
38. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
39. Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (2018) 391(10122):748–57. doi: 10.1016/S0140-6736(17)33297-X
40. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol (2020) 21(12):1574–88. doi: 10.1016/S1470-2045(20)30541-6
41. Escudier B, Sharma P, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate 025 randomized phase 3 study: Outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol (2017) 72(6):962–71. doi: 10.1016/j.eururo.2017.02.010
42. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
43. Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer (2020) 23(3):510–9. doi: 10.1007/s10120-019-01034-7
44. Emens LA, Adams S, Barrios CH, Dieras V, Iwata H, Loi S, Rugo HS, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol (2021) 32(8):983–93. doi: 10.1016/j.annonc.2021.05.355
45. Paz-Ares LG, Ramalingam SS, Ciuleanu TE, Lee JS, Urban L, Caro RB, Park K, et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 part 1 trial. J Thorac Oncol (2022) 17(2):289–308. doi: 10.1016/j.jtho.2021.09.010
46. Motzer RJ, Powles T, Burotto M, Escudier B, Bourlon MT, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(7):888–98. doi: 10.1016/S1470-2045(22)00290-X
47. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(2):234–47. doi: 10.1016/S1470-2045(21)00692-6
48. Powles T, Yuen KC, Gillessen S, Kadel EE 3rd, Rathkopf D, Matsubara N, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med (2022) 28(1):144–53. doi: 10.1038/s41591-021-01600-6
49. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol (2013) 14(10):996–1006. doi: 10.1038/ni.2691
50. Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer (2021) 21(9):541–57. doi: 10.1038/s41568-021-00383-9
51. Zheng M, Tian Z. Liver-mediated adaptive immune tolerance. Front Immunol (2019) 10:2525. doi: 10.3389/fimmu.2019.02525
52. Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol (2013) 10(4):292–302. doi: 10.1038/cmi.2013.7
53. Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis b virus-associated hepatocellular carcinoma. Hepatology (2012) 56(4):1342–51. doi: 10.1002/hep.25777
54. Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med (2000) 6(12):1348–54. doi: 10.1038/82161
55. Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T Lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology (2006) 44(5):1182–90. doi: 10.1002/hep.21378
56. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science (2018) 360(6391):eaan5931. doi: 10.1126/science.aan5931
57. Horst AK, Neumann K, Diehl L, Tiegs G. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol (2016) 13(3):277–92. doi: 10.1038/cmi.2015.112
58. Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol (2020) 5(52):eaba0759. doi: 10.1126/sciimmunol.aba0759
59. Hoshino I, Yokota H, Iwatate Y, Mori Y, Kuwayama N, Ishige F, et al. Prediction of the differences in tumor mutation burden between primary and metastatic lesions by radiogenomics. Cancer Sci (2022) 113(1):229–39. doi: 10.1111/cas.15173
60. Wang Z, Zheng X, Wang X, Chen Y, Li Z, Yu J, et al. Genetic differences between lung metastases and liver metastases from left-sided microsatellite stable colorectal cancer: next generation sequencing and clinical implications. Ann Transl Med (2021) 9(12):967. doi: 10.21037/atm-21-2221
61. Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol (2021) 16(11):1909–24. doi: 10.1016/j.jtho.2021.07.009
62. Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, et al. Epitope landscape in breast and colorectal cancer. Cancer Res (2008) 68(3):889–92. doi: 10.1158/0008-5472.CAN-07-3095
63. Niglio SA, Jia R, Ji J, Ruder S, Patel VG, Martini A, et al. Programmed death-1 or programmed death ligand-1 blockade in patients with platinum-resistant metastatic urothelial cancer: A systematic review and meta-analysis. Eur Urol (2019) 76(6):782–9. doi: 10.1016/j.eururo.2019.05.037
Keywords: immune-checkpoint inhibitors, liver metastases, tumor mutational burden (TMB), immunotherapy, meta - analysis
Citation: Wu R-Y, Wang B-C, Wang K, Xia F, Zhang Z-Y, Wan J-F and Zhang Z (2023) Immunotherapy and tumor mutational burden in cancer patients with liver metastases: A meta and real word cohort analysis. Front. Oncol. 12:994276. doi: 10.3389/fonc.2022.994276
Received: 14 July 2022; Accepted: 20 December 2022;
Published: 19 January 2023.
Edited by:
Chunxia Su, Shanghai Pulmonary Hospital, ChinaReviewed by:
Yizhuo Wang, University of Texas MD Anderson Cancer Center, United StatesEswar Shankar, The Ohio State University, United States
Copyright © 2023 Wu, Wang, Wang, Xia, Zhang, Wan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Zhang, emhlbl96aGFuZ0BmdWRhbi5lZHUuY24=; Jue-Feng Wan, d2pmNjIzMTMxNzJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Rui-Yan Wu
Rui-Yan Wu Bi-Cheng Wang
Bi-Cheng Wang Kun Wang1,2,3,4†
Kun Wang1,2,3,4† Fan Xia
Fan Xia Zhi-Yuan Zhang
Zhi-Yuan Zhang Zhen Zhang
Zhen Zhang