- Department of Ultrasound, West China Hospital, Sichuan University, Chengdu, China
Melanoma is a malignant tumor that originates from melanocytes, most of which are of cutaneous origin. Most melanomas identified in the pancreas are metastatic, and primary pancreatic melanoma is extremely rare and has rarely been discussed. The correct preoperative diagnosis of pancreatic metastatic melanoma, especially primary melanoma, is challenging. Herein, we report a 43-year-old man who presented to our hospital due to unexplained left abdominal distension and pain. Abdominal ultrasound examination demonstrated multiple space-occupying lesions of the pancreas, and hypoechoic masses partially filled the splenic vein behind the pancreatic body. In the contrast-enhanced ultrasound (CEUS), all of these lesions showed iso-enhancement to slight hypo-enhancement in the arterial phase and hypo-enhancement in the venous phase. Masses in the splenic vein also showed hypo-enhancement. Imaging features suggested that the pancreatic lesions were malignant tumors. The tumor markers carcinoembryonic antigen, carbohydrate antigen 125 and carbohydrate antigen 19-9 were within normal limits. Based on clinical symptoms, imaging findings and incidence of pancreatic tumors, the patient’s clinical diagnosis was pancreatic carcinoma. Surgery was performed for the patient, while postoperative pathology confirmed malignant melanoma of the pancreas. Therefore, it is significant to identify the clinical and imaging manifestations of pancreatic melanoma in order to better manage the disease. Herein, we reported this case and reviewed the literature from 2000 to 2021 on the clinical and imaging features of 26 patients with pancreatic melanoma. It may improve clinicians’ awareness of the clinical and imaging performance of pancreatic melanoma, resulting in improved diagnosis, differential diagnosis, treatment, and outcomes.
Introduction
Malignant melanoma is a malignant tumor that originates from melanocytes and has a high mortality rate (1). Malignant melanoma of skin origin accounted for 91.2%, and other origin accounted for a small part (2). Most melanomas identified in the pancreas are metastatic, and primary pancreatic melanoma is extremely rare and has rarely been discussed. The clinical symptoms and imaging findings of pancreatic melanoma are not typical. Therefore, the correct preoperative diagnosis of pancreatic metastatic melanoma, especially primary melanoma, is challenging. Preoperative recognition of pancreatic melanoma is significant because of its different treatment and prognosis from other pancreatic malignant tumors. Although imaging features of pancreatic melanoma have been reported in a few cases, no features of pancreatic melanoma have been described through CEUS imaging before. To improve the diagnosis and differential diagnosis of pancreatic melanoma and related imaging findings, we report this unusual case and reviewed the related literature.
Case presentation
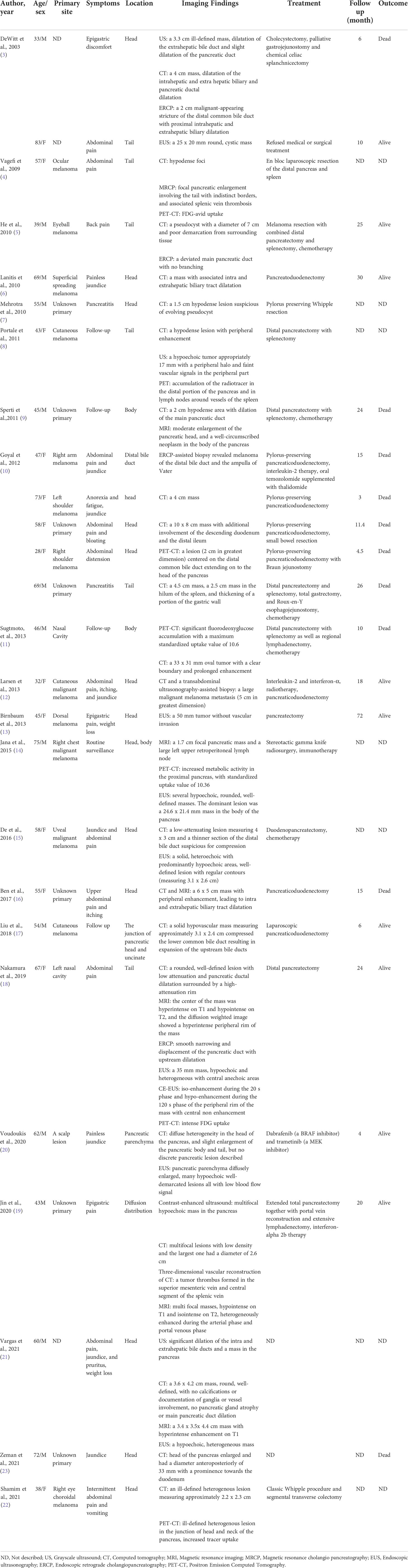
A 43-year-old male patient was admitted to our hospital 1 month ago due to unexplained left abdominal distension and pain. Since the onset of the disease, the patient did not have nausea, vomiting, fever or jaundice. He experienced a weight loss of 7 kg over the course of the disease. There was no personal or family history of acute or chronic disease. No abnormal pigmentation of the skin or sclerae or enlarged superficial lymph nodes were observed. The patient showed no tenderness, rebound tenderness or muscle tension on abdominal palpation. The tumor markers carcinoembryonic antigen, carbohydrate antigen 125 and carbohydrate antigen 19-9 were within normal limits. Blood tests for liver and kidney function, electrolyte levels, and coagulation all demonstrated normal results. The patient underwent conventional ultrasound and CEUS examination by an ultrasound system (IU22, Philips Medical Solutions; Mountain View, CA, United States) equipped with a C5-1 abdominal convex transducer (frequency range of 1-5 MHz). Conventional ultrasound demonstrated an abnormal shape, large volume and uneven parenchymal echo of the pancreas. Meanwhile, three hypoechoic lesions were found at the head of the pancreas, the junction of the body and the tail of the pancreas, and the tail of the pancreas, with sizes of approximately 3.3x3.1 cm, 2.4x2.1 cm and 5.4x2.8 cm, respectively (Figures 1A, B). Hypoechoic masses partially filled in the splenic vein behind the pancreatic body (Figure 1B). All of these lesions had a slightly clear margins, and no obvious signal of blood flow was observed. Then, the patient underwent CEUS with the patient’s consent for further diagnosis. A 2.4-ml ultrasound contrast agent SonoVue (Bracco, Milan, Italy) suspension was injected through the left cubital vein followed by a flush with 5 ml saline. In the CEUS, all of these lesions showed iso-enhancement to slight hypo-enhancement in the arterial phase (Figure 1C) and hypo-enhancement in the venous phase (Figure 1D). Masses in the splenic vein also showed hypo-enhancement. Chest and abdominal CECT also showed hypo-enhancement in both the arterial and venous phases of these lesions (Figures 2A, B), with splenic vein emboli and no other lesions were found. CEUS and CECT imaging features both suggested that the pancreatic lesions were malignant tumors. Based on clinical symptoms, imaging findings and incidence of pancreatic tumors, the patient’s clinical diagnosis was pancreatic carcinoma. Total pancreatectomy and splenectomy and peripancreatic neurectomy with portal vein reconstruction were performed for the patient. Postoperative pathology confirmed a malignant tumor of the pancreas and some cytoplasm with deep brownish-black granules (Figure 3A), which invaded the fat, nerves and blood vessels outside the pancreas. Immunohistochemically, the tumor cells were positive for melanocytic marker S-100 (Figure 3B), Human Melanoma Black 45 (Figure 3C) and MART-1(Figure 3D). The above pathological findings revealed malignant melanoma. Four months postoperatively, the patient underwent whole-body CECT examination, which revealed multiple liver, lung metastases. The patient was then given gemcitabine combined with cisplatin chemotherapy and PD-1 immunotherapy with ominous details. The patient and his family gave up treatment because of persistent fever during chemotherapy and fever symptoms even after changing drugs. The patient died 11 months after surgery.
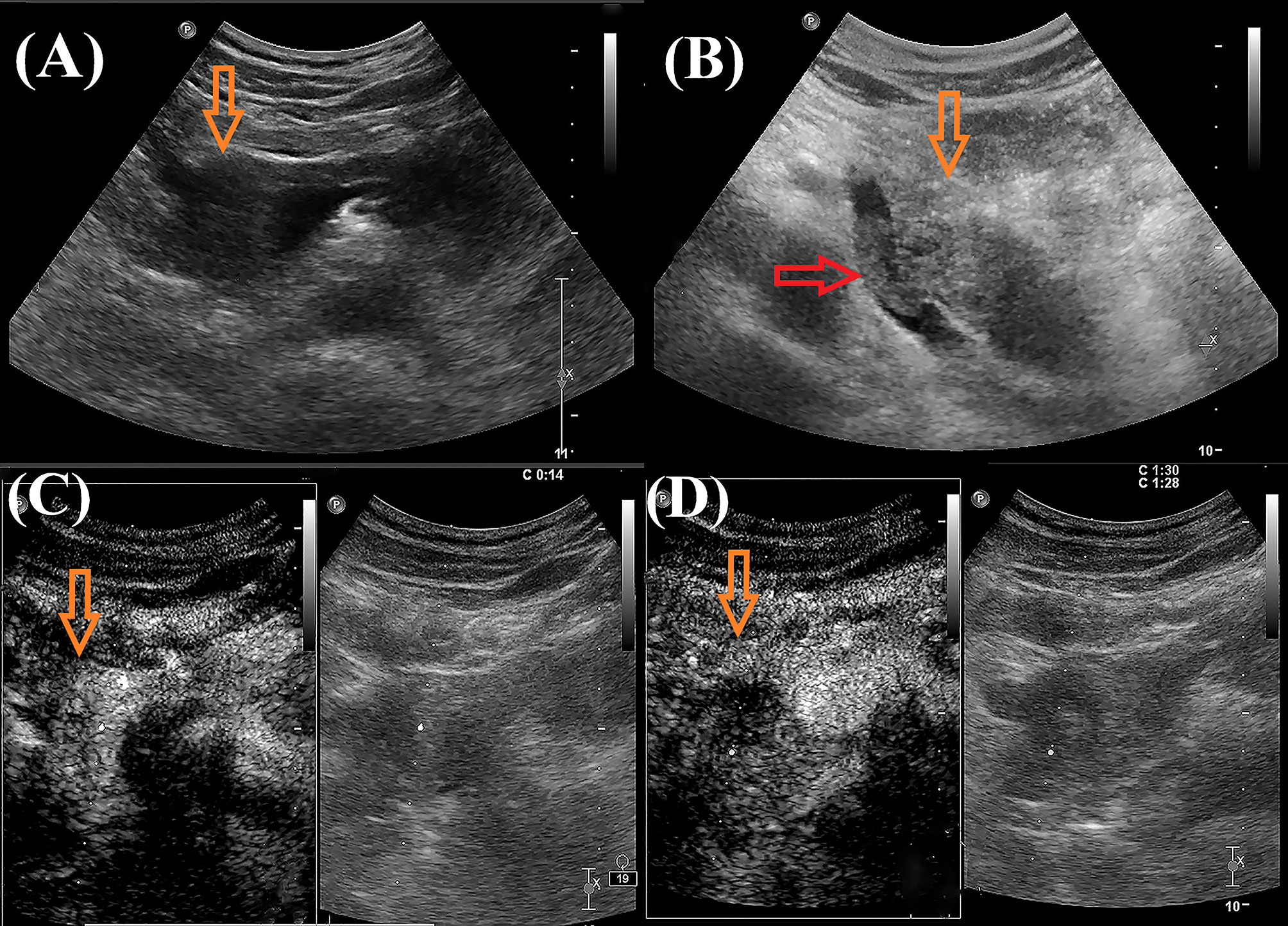
Figure 1 Ultrasound images of the patient. (A, B): Grayscale ultrasound showed hypoechoic mass in the pancreatic parenchyma (orange arrow) and hypoechoic masses partially filled in the splenic vein (red arrow).In the arterial phase, contrast-enhanced US (CEUS) showed iso-enhancement to slight hypo-enhancement [(C), orange arrow)]; In the venous phase, CEUS showed hypo-enhancement [(D), orange arrow].
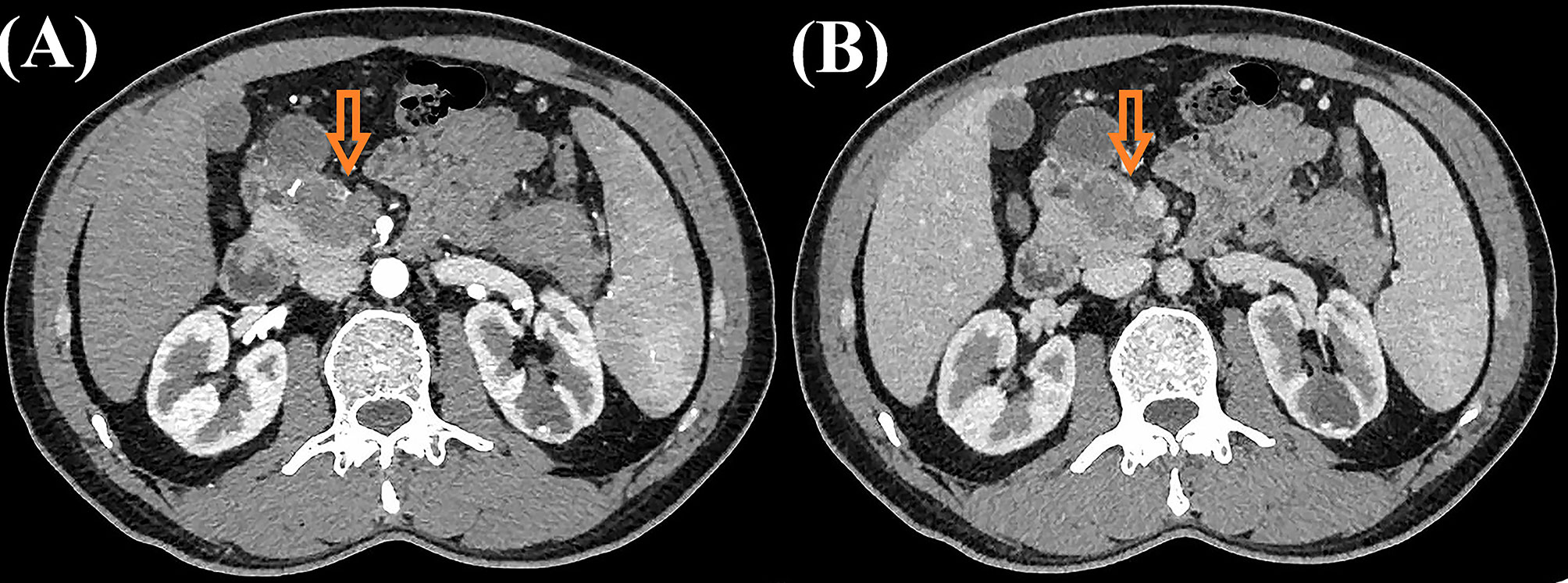
Figure 2 Contrast-enhanced computed tomography images of the patient. Contrast-enhanced computed tomography showed both hypo-enhancement masses in the arterial phase [(A), orange arrow)] and venous phase [(B), orange arrow)].
Figure 3 Postoperative histopathological images of the patient. (A) Hematoxylin and eosin staining showed some cytoplasm with deep brownish-black granules (magnification, × 400). Immunohistochemical staining displayed S100(+) (B), Human Melanoma Black 45(+) (C) and MART-1(+) (magnification, × 400) (D).
Discussion
Melanoma is characterized by uncontrolled proliferation of melanocytes, and cutaneous melanoma accounts for 91.2% of all melanomas (2). Non-cutaneous forms of primary melanoma include ocular and mucosal lesions and represent 5.2% and 1.3% of all melanomas, respectively (2). Most melanomas identified in the pancreas are metastatic, and primary pancreatic melanoma is extremely rare and has rarely been discussed. Combined with medical history, physical examination, and imaging, we did not identify the primary site of our patient, so we thought this might be a case of primary pancreatic melanoma.
We reviewed the literature from 2000 to 2021 and found 21 publications regarding the imaging features of pancreatic melanomas (3–23). The clinical findings and imaging features of these 21 reported cases are summarized in Table 1. Finally, 26 patients were included for further analysis. The age of the patients ranged from 32 to 75 years, with equal number of men and women. Most of the patients had a clear history of primary melanoma, while the primary lesion search was not performed or not found in some patients. The major symptoms found in these patients were abdominal pain, jaundice, and weight loss, while some patients had no apparent symptoms. Pancreatic melanoma is mainly distributed in the head and tail of the pancreas, with a few diffusely distributed in the pancreatic parenchyma. The tumor marker CA-199 was described in six cases, of which five were normal and one was slightly elevated. Most patients were treated with pancreatectomy, while a few were treated with surgery combined with chemotherapy and immunotherapy. Due to the difference of primary site, time in pancreatic lesion detection and treatment, the survival of patients is different to some extent.
Unfortunately, no specific imaging features for pancreatic melanomas have been found at present. Although some imaging modalities, such as ultrasonography, computed tomography, nuclear magnetic resonance imaging, endoscopic ultrasound, and positron emission tomography, are valuable to some extent, it remains a challenge to diagnose pancreatic melanomas preoperatively if there is no clear history of primary melanoma. Conventional ultrasound was performed in 4 of the 26 patients, and it usually presented as a hypoechoic mass with or without dilation of the bile duct and pancreatic duct. CT was performed in 21 of the 26 patients, and most of the findings were hypoechoic solid masses with or without dilatation of the bile duct and pancreatic duct, and only two of them presented pseudocysts. CECT findings were described in three cases, two with peripheral enhancement and one with delayed enhancement. Endoscopic ultrasound was performed in six cases, most of which presented as well-defined hypoechoic solid masses, and only one presented as cystic mass. There have been a few reports of other imaging techniques for diagnosing pancreatic melanomas, such as magnetic resonance imaging, magnetic resonance cholangiopancreatography, endoscopic retrograde cholangiopancreatography and positron emission computed tomography. None of these imaging methods revealed specific features for pancreatic melanomas.
In this patient, the symptoms of abdominal distension and pain and weight loss were similar to those in the literature. CEUS and CECT indicated the pancreatic lesions were malignant tumors. In addition, pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic carcinoma, which accounts for the majority (90%) of pancreatic neoplasms (24–26). Therefore, based on the clinical symptoms, imaging findings and incidence of pancreas neoplasms, the surgeons misdiagnosed it as pancreatic carcinoma. It is crucial for us to accurately distinguish PDAC from pancreatic melanomas, which determines the patients’ treatment options and the doctors’ management of patients. Carbohydrate antigen 19-9 levels are elevated in 80% of pancreatic cancer patients (27). However, in this patient, the tumor markers carcinoembryonic antigen, carbohydrate antigen 125, and carbohydrate antigen 19-9 were within normal limits. We can also see some differences between pancreatic melanomas and PDAC in ultrasound images. PDACs are derived from epithelial cells that line the pancreatic duct (28), and most PDACs are localized in the pancreatic head (29). Consequently, the typical imaging features of PDAC in conventional ultrasound are hypoechoic mass, dilatation of the pancreatic duct, and dilatation of the bile duct (30). And PDAC is generally a solitary poorly defined lesion (31). Nonetheless, in this case, the large size of the pancreatic head lesion did not cause dilation of the main pancreatic duct. And the margins of these lesions were more clearly relative to PDAC. In the CEUS, PDAC is typically significantly hypo-enhancing in the arterial phase, because of the desmoplastic reaction with low vascular density that is present in 90% of cases (32–38). However, the lesions of this patients showed iso-enhancement to slightly hypo- enhancement in the arterial phase. Another common solid pancreatic neoplasm pancreatic neuroendocrine tumors typically present as hyper-enhancing lesions in the arterial phase of CEUS examinations (39–41). So when we encounter a solid lesion of the pancreas like this patient with a different sonographic appearance than typical PDAC and pancreatic neuroendocrine tumor, the tumor markers are also not significantly elevated. We should consider other rare tumor possibilities, there is no doubt that pancreatic melanoma is one of the possibilities. It is important for clinicians to consider a broad differential diagnosis when faced with inconclusive imaging studies of pancreatic tumors. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) plays an important role in providing cytological confirmation for diagnosis (14). In the literature we reviewed, 9 of 26 patients underwent EUS-FNA and all were confirmed melanoma, which demonstrated the important role of EUS-FNA in the differential diagnosis of pancreatic tumors. In addition, EUS-FNA is now the gold standard method for sampling the pancreas (15). If conditions permit, patients can be clearly diagnosed by EUS-FNA.
In summary, it is difficult to correctly diagnose pancreatic melanoma before surgery, especially if there is no history of primary lesions. The sonographic features of pancreatic melanoma in our patient are different from common solid lesions PDAC and pancreatic neuroendocrine tumors, but more cases are needed to summarize and validate these findings. Preoperative EUS-FNA could be considered for further confirmation, and preoperative biopsy combined with comprehensive imaging examination will contribute to identifying adequate surgical candidate (42, 43). Hence, awareness of pancreatic melanoma from imaging features and tumor markers may aid in the management of patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZY performed the literature review and wrote the manuscript. HY and WL supported the data collection and manuscript revision. YL supervised the writing and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Natural Science Foundation of China, No. 82071940.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Peng Q, Wang J. Non-coding rnas in melanoma: Biological functions and potential clinical applications. Mol Ther Oncolytics (2021) 22:219–31. doi: 10.1016/j.omto.2021.05.012
2. Chang AE, Karnell LH, Menck HR. The national cancer data base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. the American college of surgeons commission on cancer and the American cancer society. Cancer (1998) 83(8):1664–78. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
3. DeWitt JM, Chappo J, Sherman S. Endoscopic ultrasound-guided fine-needle aspiration of melanoma metastatic to the pancreas: Report of two cases and review. Endoscopy (2003) 35(3):219–22. doi: 10.1055/s-2003-37258
4. Vagefi PA, Stangenberg L, Krings G, Forcione DG, Wargo JA. Ocular melanoma metastatic to the pancreas after a 28-year disease-free interval. Surgery (2010) 148(1):151–4. doi: 10.1016/j.surg.2009.06.013
5. He MX, Song B, Jiang H, Hu XG, Zhang YJ, Zheng JM. Complete resection of isolated pancreatic metastatic melanoma: A case report and review of the literature. World J Gastroenterol (2010) 16(36):4621–4. doi: 10.3748/wjg.v16.i36.4621
6. Lanitis S, Papaioannou N, Sgourakis G, Seitz A, Zacharakis E, Karaliotas C. Prolonged survival after the surgical management of a solitary malignant melanoma lesion within the pancreas: A case report of curative resection. J Gastrointest Liver Dis (2010) 19(4):453–5.
7. Mehrotra R, Heer M, Wright T, Chen S. Malignant melanoma of the pancreas. ANZ J Surg (2010) 80(11):858–9. doi: 10.1111/j.1445-2197.2010.05517.x
8. Portale TR, Di Benedetto V, Mosca F, Trovato MA, Scuderi MG, Puleo S. Isolated pancreatic metastasis from melanoma. Case Rep Il Giornale di chirurgia (2011) 32(3):135–7.
9. Sperti C, Polizzi ML, Beltrame V, Moro M, Pedrazzoli S. Pancreatic resection for metastatic melanoma. case report and review of the literature. J Gastrointest Cancer (2011) 42(4):302–6. doi: 10.1007/s12029-010-9169-5
10. Goyal J, Lipson EJ, Rezaee N, Edil BH, Schulick R, Wolfgang CL, et al. Surgical resection of malignant melanoma metastatic to the pancreas: Case series and review of literature. J Gastrointest Cancer (2012) 43(3):431–6. doi: 10.1007/s12029-011-9320-y
11. Sugimoto M, Gotohda N, Kato Y, Takahashi S, Kinoshita T, Shibasaki H, et al. Pancreatic resection for metastatic melanoma originating from the nasal cavity: A case report and literature review. Anticancer Res (2013) 33(2):567–73.
12. Larsen AK, Krag C, Geertsen P, Jakobsen LP. Isolated malignant melanoma metastasis to the pancreas. Plast Reconstr Surg Glob Open (2013) 1(8):e74. doi: 10.1097/gox.0000000000000018
13. Birnbaum DJ, Moutardier V, Turrini O, Gonçalves A, Delpero JR. Isolated pancreatic metastasis from malignant melanoma: Is pancreatectomy worthwile? J Surg Technique Case Rep (2013) 5(2):82–4. doi: 10.4103/2006-8808.128733
14. Jana T, Caraway NP, Irisawa A, Bhutani MS. Multiple pancreatic metastases from malignant melanoma: Conclusive diagnosis with endoscopic ultrasound-guided fine needle aspiration. Endosc Ultrasound (2015) 4(2):145–8. doi: 10.4103/2303-9027.156746
15. De Moura DT, Chacon DA, Tanigawa R, Coronel M, Cheng S, Artifon ÉL, et al. Pancreatic metastases from ocular malignant melanoma: The use of endoscopic ultrasound-guided fine-needle aspiration to establish a definitive cytologic diagnosis: A case report. J Med Case Rep (2016) 10(1):332. doi: 10.1186/s13256-016-1121-2
16. Ben Slama S, Bacha D, Bayar R, Gharbi L, Lahmar A. Pancreatic resection for isolated metastasis from melanoma of unknown primary. Acta Gastro-enterologica Belgica (2017) 80(2):323–4.
17. Liu X, Feng F, Wang T, Qin J, Yin X, Meng G, et al. Laparoscopic pancreaticoduodenectomy for metastatic pancreatic melanoma: A case report. Med (Baltimore) (2018) 97(44):e12940. doi: 10.1097/md.0000000000012940
18. Nakamura Y, Yamada R, Kaneko M, Naota H, Fujimura Y, Tabata M, et al. Isolated pancreatic metastasis from malignant melanoma: A case report and literature review. Clin J Gastroenterol (2019) 12(6):626–36. doi: 10.1007/s12328-019-00996-6
19. Jin Y, Ran C, Li F, Cheng N. Melanoma of unknown primary in the pancreas: Should it be considered primary? BMC Surg (2020) 20(1):76. doi: 10.1186/s12893-020-00731-w
20. Voudoukis E, Mpitouli A, Giannakopoulou K, Velegraki M, Datseri G, Bachlitzanaki M, et al. Disseminated metastatic cutaneous melanoma to pancreas and upper gastrointestinal tract diagnosed by endoscopic ultrasound: An unusual case. Clin J Gastroenterol (2020) 13(1):134–8. doi: 10.1007/s12328-019-01004-7
21. Vargas-Jiménez J, Vargas-Madrigal J, Arias-Mora R, Ulate-Ovares D, Solis-Ugalde B. Pancreatic metastasis from malignant melanoma: Not all that glitters is gold. Case Rep Gastroenterol (2021) 15(1):131–6. doi: 10.1159/000511864
22. Shamim SA, Tripathy S, Rastogi S, Barwad A, Prakash S. Isolated pancreatic metastasis from choroidal melanoma after 10 years of enucleation mimicking neuroendocine tumor on 68ga-dotanoc Pet/Ct. Clin Nucl Med (2021) 46(1):e21–e2. doi: 10.1097/rlu.0000000000003112
23. Zeman J, Olivová L, Hrudka J, Hajer J, Rychlík I. Obstructive jaundice secondary to pancreatic head metastasis of malignant amelanotic melanoma as the first clinical manifestation. Prague Med Rep (2021) 122(1):45–51. doi: 10.14712/23362936.2021.6
24. de la Santa LG, Retortillo JA, Miguel AC, Klein LM. Radiology of pancreatic neoplasms: An update. World J Gastrointest Oncol (2014) 6(9):330–43. doi: 10.4251/wjgo.v6.i9.330
25. Park W, Chawla A, O'Reilly EM. Pancreatic cancer: A review. JAMA (2021) 326(9):851–62. doi: 10.1001/jama.2021.13027
26. Hussain SP. Pancreatic cancer: Current progress and future challenges. Int J Biol Sci (2016) 12(3):270–2. doi: 10.7150/ijbs.14950
27. Teich N, Kleeff J, Lochs H, Mössner J, Keim V, Friess H, et al. The presence of the proteolysis-inducing factor in urine does not predict the malignancy of a pancreatic tumour. BMC Gastroenterol (2005) 5:20. doi: 10.1186/1471-230x-5-20
28. Grant TJ, Hua K, Singh A. Molecular pathogenesis of pancreatic cancer. Prog Mol Biol Trans Sci (2016) 144:241–75. doi: 10.1016/bs.pmbts.2016.09.008
29. Schlitter AM, Häberle L, Richter C, Huss R, Esposito I. [Standardized diagnosis of pancreatic head carcinoma]. Der Pathologe (2021) 42(5):453–63. doi: 10.1007/s00292-021-00971-4
30. Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: A state-of-the-Art review. World J Gastroenterol (2014) 20(24):7864–77. doi: 10.3748/wjg.v20.i24.7864
31. Schawkat K, Manning MA, Glickman JN, Mortele KJ. Pancreatic ductal adenocarcinoma and its variants: Pearls and perils. Radiographics (2020) 40(5):1219–39. doi: 10.1148/rg.2020190184
32. Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut (2004) 53(6):854–9. doi: 10.1136/gut.2003.029934
33. D'Onofrio M, Zamboni G, Tognolini A, Malago R, Faccioli N, Frulloni L, et al. Mass-forming pancreatitis: Value of contrast-enhanced ultrasonography. World J Gastroenterol (2006) 12(26):4181–4. doi: 10.3748/wjg.v12.i26.4181
34. Kersting S, Konopke R, Kersting F, Volk A, Distler M, Bergert H, et al. Quantitative perfusion analysis of transabdominal contrast-enhanced ultrasonography of pancreatic masses and carcinomas. Gastroenterology (2009) 137(6):1903–11. doi: 10.1053/j.gastro.2009.08.049
35. Numata K, Ozawa Y, Kobayashi N, Kubota T, Shimada H, Nozawa A, et al. Contrast-enhanced sonography of pancreatic carcinoma: Correlations with pathological findings. J Gastroenterol (2005) 40(6):631–40. doi: 10.1007/s00535-005-1598-8
36. D'Onofrio M, Barbi E, Dietrich CF, Kitano M, Numata K, Sofuni A, et al. Pancreatic multicenter ultrasound study (Pamus). Eur J Radiol (2012) 81(4):630–8. doi: 10.1016/j.ejrad.2011.01.053
37. Serra C, Felicani C, Mazzotta E, Piscitelli L, Cipollini ML, Tomassetti P, et al. Contrast-enhanced ultrasound in the differential diagnosis of exocrine versus neuroendocrine pancreatic tumors. Pancreas (2013) 42(5):871–7. doi: 10.1097/MPA.0b013e31827a7b01
38. Wang Y, Yan K, Fan Z, Sun L, Wu W, Yang W. Contrast-enhanced ultrasonography of pancreatic carcinoma: Correlation with pathologic findings. Ultrasound Med Biol (2016) 42(4):891–8. doi: 10.1016/j.ultrasmedbio.2015.12.008
39. Dietrich CF, Braden B, Hocke M, Ott M, Ignee A. Improved characterisation of solitary solid pancreatic tumours using contrast enhanced transabdominal ultrasound. J Cancer Res Clin Oncol (2008) 134(6):635–43. doi: 10.1007/s00432-007-0326-6
40. D'Onofrio M, Mansueto G, Falconi M, Procacci C. Neuroendocrine pancreatic tumor: Value of contrast enhanced ultrasonography. Abdominal Imaging (2004) 29(2):246–58. doi: 10.1007/s00261-003-0097-8
41. Al-Hawary MM, Francis IR, Anderson MA. Pancreatic solid and cystic neoplasms: Diagnostic evaluation and intervention. Radiologic Clinics North America (2015) 53(5):1037–48. doi: 10.1016/j.rcl.2015.05.005
42. Pang JC, Roh MH. Metastases to the pancreas encountered on endoscopic ultrasound-guided, fine-needle aspiration. Arch Pathol Lab Med (2015) 139(10):1248–52. doi: 10.5858/arpa.2015-0200-RA
Keywords: pancreatic melanoma, pancreatic ductal adenocarcinoma, pancreatic carcinoma, contrast-enhanced ultrasound, enhancement, case report
Citation: Yuan Z, Yan H, Ling W and Luo Y (2022) Contrast-enhanced ultrasound of pancreatic melanoma: A case report and literature review. Front. Oncol. 12:989638. doi: 10.3389/fonc.2022.989638
Received: 08 July 2022; Accepted: 10 August 2022;
Published: 06 September 2022.
Edited by:
Xin-Wu Cui, Huazhong University of Science and Technology, ChinaReviewed by:
J. Wang, Anhui Medical University, ChinaXinyi Wang, First Affiliated Hospital of Anhui Medical University, China
Copyright © 2022 Yuan, Yan, Ling and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Luo, eWFubHVvQHNjdS5lZHUuY24=
 Zhiqiang Yuan
Zhiqiang Yuan Hualin Yan
Hualin Yan Wenwu Ling
Wenwu Ling Yan Luo
Yan Luo