
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 October 2022
Sec. Cardio-Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.988287
This article is part of the Research Topic HF2Cancer: Exploring bidirectional interaction between cardiovascular diseases and cancer View all 18 articles
Purpose: This study aimed to develop and validate a specific risk-stratification nomogram model for the prediction of venous thromboembolism(VTE) in hospitalized patients with lung cancer using readily obtainable demographic, clinical and therapeutic characteristics, thus guiding the individualized decision-making on thromboprophylaxis on the basis of VTE risk levels.
Methods: We performed a retrospective case–control study among newly diagnosed lung cancer patients hospitalized between January 2016 and December 2021. Included in the cohort were 234 patients who developed PTE and 936 non-VTE patients. The patients were randomly divided into the derivation group (70%, 165 VTE patients and 654 non-VTE patients) and the validation group (30%, 69 VTE patients and 282 non-VTE patients). Cut off values were established using a Youden´s Index. Univariate and multivariate regression analyses were used to determine independent risk factors associated with VTE. Variance Inflation Factor(VIF) was used for collinearity diagnosis of the covariates in the model. The model was validated by the consistency index (C-index), receiver operating characteristic curves(ROC) and the calibration plot with the Hosmer-Lemeshow goodness-of-fit test. The clinical utility of the model was assessed through decision curve analysis(DCA). Further, the comparison of nomogram model with current models(Khorana, Caprini, Padua and COMPASS-CAT) was performed by comparing ROC curves using the DeLong’s test.
Results: The predictive nomogram modle comprised eleven variables: overweight(24-28) defined by body mass index (BMI): [odds ratio (OR): 1.90, 95% confidence interval (CI): 1.19-3.07], adenocarcinoma(OR:3.00, 95% CI: 1.88-4.87), stageIII-IV(OR:2.75, 95%CI: 1.58-4.96), Central venous catheters(CVCs) (OR:4.64, 95%CI: 2.86-7.62), D-dimer levels≥2.06mg/L(OR:5.58, 95%CI:3.54-8.94), PT levels≥11.45sec(OR:2.15, 95% CI:1.32-3.54), Fbg levels≥3.33 g/L(OR:1.76, 95%CI:1.12-2.78), TG levels≥1.37mmol/L (OR:1.88, 95%CI:1.19-2.99), ROS1 rearrangement(OR:2.87, 95%CI:1.74-4.75), chemotherapy history(OR:1.66, 95%CI:1.01-2.70) and radiotherapy history(OR:1.96, 95%CI:1.17-3.29). Collinearity analysis with demonstrated no collinearity among the variables. The resulting model showed good predictive performance in the derivation group (AUC 0.865, 95% CI: 0.832-0.897) and in the validation group(AUC 0.904,95%CI:0.869-0.939). The calibration curve and DCA showed that the risk-stratification nomogram had good consistency and clinical utility. Futher, the area under the ROC curve for the specific VTE risk-stratification nomogram model (0.904; 95% CI:0.869-0.939) was significantly higher than those of the KRS, Caprini, Padua and COMPASS-CAT models(Z=12.087, 11.851, 9.442, 5.340, all P<0.001, respectively).
Conclusion: A high-performance nomogram model incorporated available clinical parameters, genetic and therapeutic factors was established, which can accurately predict the risk of VTE in hospitalized patients with lung cancer and to guide individualized decision-making on thromboprophylaxis. Notably, the novel nomogram model was significantly more effective than the existing well-accepted models in routine clinical practice in stratifying the risk of VTE in those patients. Future community-based prospective studies and studies from multiple clinical centers are required for external validation.
Venous thromboembolism (VTE), which manifests as deep vein thrombosis (DVT) and pulmonary thromboembolism (PE), is a major global burden of disease (1). DVT mostly affects the deep veins of the lower limbs. After the thrombi dislodge from clots in the deep veins of the lower limbs falls off, it can drift along with the blood flow and block the pulmonary arteries and its branches, resulting in PE. Hence, DVT and PE, collectively referred to as VTE, are the manifestations of the same disease at different stages. Of note, it has been established that there is a strong association between cancer and VTE events (2). On the one hand, patients with malignancy are at a high risk of VTE, account for approximately 20% of all patients complicated with vein thrombosis, and have a 4 to 7 fold increased risk of developing VTE compared to the general population (3). While on the other hand, cancer-associated thrombosis (CAT) is commonly associated with higher morbidity and mortality, increased hospital stay, reduced quality of life and higher medical costs (4, 5). Indeed, VTE is responsible for 9% of death in cancer patients, making it the second leading cause of death in cancer patients (6). As a result, VTE events continue to be common and potentially fatal complication in cancer inpatients.
However, the incidence of VTE might be underestimated due to the low rate of clinical detection, as well as the high rates of misdiagnosis and missed diagnosis (7). Several studies have reported that patients with lung cancer (LC) have a relatively higher of VTE development than patients with other solid tumors (8, 9). Furthermore, it has been recently recognized that VTE is surprisingly common in newly-diagnosed patients with LC and linked with poor prognosis (10). More importantly, considerable morbidity of long-term complications results from VTE, such as post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension, which not only affect the treatment of patients with primary diseases but also reduce the patients’ quality of life (11, 12). Given the diminishing evidence regarding the benefits of VTE thromboprophylaxis in low risk situations, overprophylaxis is clearly undesirable, and could result in an inherent risk of bleeding which may offset its clinical benefits (13). Consequently, early detection of high-risk factors for lung cancer combined with VTE should be paid for particular attention. There is an urgent need for useful clinical tools to accurately predict the risk of VTE in hospitalized patients with LC and to guide individualized decision-making on thromboprophylaxis.
Presently, nomogram-based prediction model has been widely used as a user-friendly screening tool for the diagnosis and prognosis of diseases (14). Nomogram is a visual display of complex mathematical formulas, which integrates multiple prediction variables and then uses the line with scale according to a certain proportion, so that the probability of occurrence of predicted events can be simply determined. Currently, comprehensive treatments such as surgery, chemotherapy, radiotherapy and more recently immunotherapy have been recognized as additional risk factors for VTE in patients with lung cancer (15–17). In particular, the current analysis demonstrates that nomogram has good risk-prediction ability for VTE in postoperative lung cancer patients (18). However, studies on the use of a nomogram model for predicting the risk of CAT are limited, especially for patients with LC receiving first-line systemic therapy (19–21). Meanwhile, there is less risk assessment models(RAMs) to evaluate the risk of VTE exclusively for hospitalized cancer patients. Therefore, more attention should be paid to the construction of specific VTE risk assessment model to guide prophylaxis decisions for hospitalized LC patients.
Considering the differences in the patient population characteristics and treatment modalities, it is necessary to develop an accurate, objective, and practical tool to predicting VTE in lung cancer patients using available clinical parameters, which would be helpful in guiding clinical decision-making on prophylaxis. Therefore, the aim of this study was to develop and validate a specific risk-stratification nomogram model for the prediction of VTE in lung cancer patients to provide a theoretical basis for the individualized treatment on the basis of VTE risk levels, thus guiding the implementation of clinical prevention and treatment.
This study was a matched case-control study. Data from a total of 10,053 newly diagnosed lung cancer patients admitted to The Affiliated Tumor Hospital of Xinjiang Medical University between January 2016 and December 2021 were collected and retrospectively analyzed. Patients enrolled in our study were all inpatients. The calculation of the sample size was based on demonstrating the probability of exposure among sampled control patients was 0.2 with 90% power and 5% statistical significance. Therefore, the obtained sample size of 234 VTE patients was adequate to address the study aims. To reduce potential selection bias between groups, a 1:4 ratio propensity score matching (PSM) method was performed with optimal full matching (22) with the covariates age, gender, and ethnicity. For each VTE patient, a matched sample of 4 non-VTE patients was also obtained. Thus, this sample of 936 non-VTE patients were selected out of the total 9819 control cases. Enrolled patients were further randomized into the derivation group (70%, 165 VTE patients and 654 non-VTE patients) and the validation group (30%, 69 VTE patients and 282 non-VTE patients) (Figure 1).
The study was conducted in accordance with the ethical standards revised in the 2013 Declaration of Helsinki. This study was approved by the Ethics Committee of The Affiliated Tumor Hospital of Xinjiang Medical University with the ethical approval number: 2019BC007. However, the requirement to obtain informed consent for any research utilizing patients’ medical information was waived owing to the retrospective design of the study.
The inclusion criteria for the VTE group were as follows: (a) 18 years or older; (b) length of hospital stay >3 days;(c) all primary lung malignant tumors confirmed by histopathological examination; (d) with DVT and/or PE events confirmed by objective imaging methods and (f) complete case data. The primary diagnosis of VTE (DVT and/or PE) and comorbidities were abstracted from electronic medical records (EMR) according to the International Statistical Classification of Diseases, 10th Clinical Modification (ICD-10 CM).
The exclusion criteria were as follows: (a) inpatients hospitalized for<72h; (b) second malignancy other than lung cancer; (c) patients with acute coronary syndrome or history of implantation of intracardiac devices (pacemakers, prosthetic valves, or implantable cardioverter-defibrillators, etc.) or history of VTE prior to admission; (d) prophylactic anticoagulation before VTE occurring during antitumor therapies; (e) patients receiving long-term therapeutic anticoagulation (at least 3 months) before hospitalization; (f) previous hematological diseases, rheumatoid arthritis, limited liver and kidney function and/or damage.
Controls were selected by propensity score matching (PSM) method from adult lung cancer inpatients (length of hospital stay>3 days) admitted into the same departments during the same period as cases, without an ICD-10 code for VTE (DVT and/or PE) at discharge. The same exclusion criteria used for cases were also applied to controls. Controls were frequency-matched to cases at a ratio of 4:1.
Symptomatic or incidental VTE that occurred within the first 6 months of cancer diagnosis during the patients’hospitalization was the primary outcome of the study, including DVT and PE. The diagnosis of PE was made by computed tomography pulmonary angiography(CTPA) according to the consensus guidelines, with single/bilateral/multi-lobar pulmonary artery embolism and its branches being the main type. The diagnosis of DVT was made by computed tomography(CT) angiography or complete compression venous ultrasonography(CUS) according to standard ultrasonographic criteria (23), with venous blood stasis in the upper- and lower-extremities. All VTE events were was independently reviewed by two experienced experts in the field of angiology and radiology.
The abstracted data were extracted from the electronic EMR retrospectively: patients’ demographic and clinicopathological characteristics including age, gender, ethnicity, smoking history, blood type, body mass index (BMI) before initial treatment (baseline), Eastern Cooperative Oncology Group performance status (ECOG PS), central venous catheters(CVCs) indwelling history, tumor pathology(adenocarcinoma and non-adenocarcinoma) and clinical stage(early and advanced stage), PD-L1 expression(<50%, ≥50%), and diver genes status(EGFR and KRAS mutation, ALK and ROS1 rearrangement and wild type); Detailed information about historic treatment regimens including surgery, targeted therapy, chemotherapy, radiotherapy or immunotherapy during the follow-up period for case group and control group; Comorbid conditions including hypertension, diabetes and coronary heart disease. In order to avoid the effect of anticancer treatment on the value of the indicator, all laboratory examination data were obtained from the pre-treatment baseline assessment after admission. All D-dimer levels were assayed in plasma using the Innovance D-dimer immunoturbidimetric method (Siemens Healthcare, Eschborn, Germany). Laboratory examination data including routine blood indicators(haemoglobin(Hb), leucocyte platelet(Plt), neutrophil-to-lymphocyte ratio(NLR), platelet-to-lymphocyte ratio(PLR)); Coagulation function indexs(prothrombin time(PT), activated partial thromboplastin time(APTT), fibrinogen (Fbg), D-dimer (Ddi)); Biochemical routine (albumin, alanine transaminase(ALT), aspartate transaminase(AST), Lactate dehydrogenase (LDH), triglyceride(TG)); Pro-Brain natriuretic peptide (pro-BNP)) and tumor biomarkers(cytokeratin-19-fragment(CYFR121-1), carcinoembryonic antigen(CEA), carbohydrate antigen 125(CA125), Gastrin releasing peptide(Pro-GRP) and neuron-specific enolase(NSE)). Further, VTE risk was evaluated via Khorana Prediction Score, Padua Prediction Score, Caprini Risk Assessment model and COMPASS-cancerassociated thrombosis score (COMPASS-CAT), respectively. Predictor variables were identified from synthesis of the literatures about VTE risk (24–27). All patients were followed up by telephone or hospital visit until the occurrence of VTE, death or end of follow-up in March 2022.
The risk assessment model was developed in the derivation cohorts by binary multiple logistic regression analysis. Internal validation was performed in the internal validation cohorts. The Chi-square test for categorical variables were used to compare the baseline characteristics between the derivation and validation cohorts.
Logistic regression analysis for univariate and multivariate analyses and stepwise regression analysis were used to evaluate the independent factors influencing thrombosis in lung cancer patients. Variables with a P-value <0.05 in the univariate regression analysis were included in multivariate logistic regression analysis. Afterward, variables with clinical significance and those with P < 0.05 in the multivariate analysis were included in the backward stepwise logistic regression analysis. Backward stepwise selection was applied using the likelihood ratio test with Akaike’s information criterion (AIC) minimum method as the termination rule (28). The effect measure of each variable on VTE was presented as odds ratios (OR) and corresponding 95% confidence intervals (CI). Variance Inflation Factor (VIF) was used for collinearity diagnosis of the covariates in the model. Then the nomogram was constructed by using the RMS package in the R (r4.1.3) software to visually score individual risk probabilities of VTE in lung cancer patients.
The reliability of internal validation was assessed using the bootstrap method with 1000 replicates. The discrimination of the nomogram model was evaluated by the consistency index (C-index) and receiver operating characteristic curve (ROC) analysis. Further, the area under the ROC curve (AUC) was obtained to quantitatively evaluate the discriminative ability of the nomogram to predict VTE in patients with lung cancer. The possible value for an AUC ranges from 0.5 (no better discrimination than chance) to 1.0 (perfect discrimination). Moreover, calibration curve was plotted to assess the calibration of the nomogram with the Hosmer-Lemeshow goodness-of-fit test, and a p-value of the Hosmer-Lemeshow test > 0.05 indicates that a model has high goodness of fit. Finally, decision curve analysis (DCA) was performed to assess the clinical utility of the predictive nomogram model for guiding clinical decision making of thromboprophylaxis in lung cancer (29).
Risk of bias (ROB) and applicability was assessed using the Prediction Study Risk Of Bias Assessment Tool (PROBAST) (30). The assessment of ROB comprises four domains—participants, predictors, outcome, and analysis, questions are answered as “yes”, “probably yes”, “probably no”, “no”, or “no information”. The degree of ROB and applicability were judged as “low”, “high”, or “unclear” for each domain. Risk of bias and applicability assessment was performed by one reviewer and checked by a further reviewer. Any disagreements were mediated by a third reviewer.
The diagnostic performance of new prediction model and current models (Khorana, Caprini, Padua and COMPASS-CAT) were evaluated assessed by calculating the AUC. The diagnostic value of the nomogram was assessed by calculating the sensitivity, specificity, positive predictive values (PPV) and negative predictive values(NPV). Further, the comparison of new prediction model with current models was performed by comparing ROC curves using the DeLong’s test.
SPSS version 25.0 software (IBM, USA) and R version 4.1.3 software (https://www.r-project.org/) were performed for statistical analysis. PSM was performed with optimal full matching by the R package ‘Matchlt’. Multiple imputation with chained equations was used to replace missing data for BMI values. Considering the model’s extrapolation accuracy and clinical application, continuous variables were transformed into categorical variables by determining the optimal cut-off (OCF) value according to the maximum Youden index on the basis of the receiver operating characteristic(ROC) curves. The continuous BMI variable was categorized based on cut-off values routinely used in clinical practice for ease of interpretation. Categorical variables were presented as whole numbers and proportions. Comparisons between two groups were performed using the Chi-squared test for categorical variables. A two- sided p-value < 0.05 indicated statistical significance.
A total of 1170 patients were enrolled and randomly assigned at a ratio of 7:3, resulting in 819 patients assigned to the derivation group and 351 assigned to the validation group (Figure 1). Based on the current sample size and effect size, our study has a statistical power of 91%, which exceeds the minimal statistical power of 80% required for the adequacy of sample sizes. The control’s lung cancer duration(time since cancer diagnosis) was ≥ the case’s duration to ensure that the control would have equal exposure to the risk of VTE induced by cancer. The demographic and clinical characristics of patients in the derivation and validation cohorts are illustrated in Table 1, indicate that most characterisics were similarly distirbuted between the two cohorts. Overall, 165(20.1%) patients in the development cohort and 69(19.7%) patients in the validation cohort developed VTE, and there was no significant difference in VTE morbidity between the two cohorts (c²=0.037, P = 0.848). Similarly, there were no significant differences of incidence in DVT alone(4.2% vs.3.1%), PE alone (14.9% vs.15.4%), and DVT&PE (1.1% vs.1.1%)(c²=0.407, 0.046, 0.004, P=0.688, 0.830, 0.951, respectively) (Table 1)
The results of univariate and multivariate logistic regression analyses based on the factors associated with VTE are presented in Table 2. In the validation group, univariate analysis showed that the following factors were statistically significant: BMI, histology, clinical stage, CVC history, hypertension, NLR, coagulation function indexs(D-dimer, ATTT, PT and Fbg levels), biochemical routine indexs(albumin, LDH and TG levels), Pro-BNP, tumor biomarkers(CEA, CA125 and Pro-GPR levels), molecular driver status(ROS1 rearrangement and PD-L1 high expression) and historic treatment regimens(chemotherapy, radiotherapy, and immunotherapy history)(P<0.05).
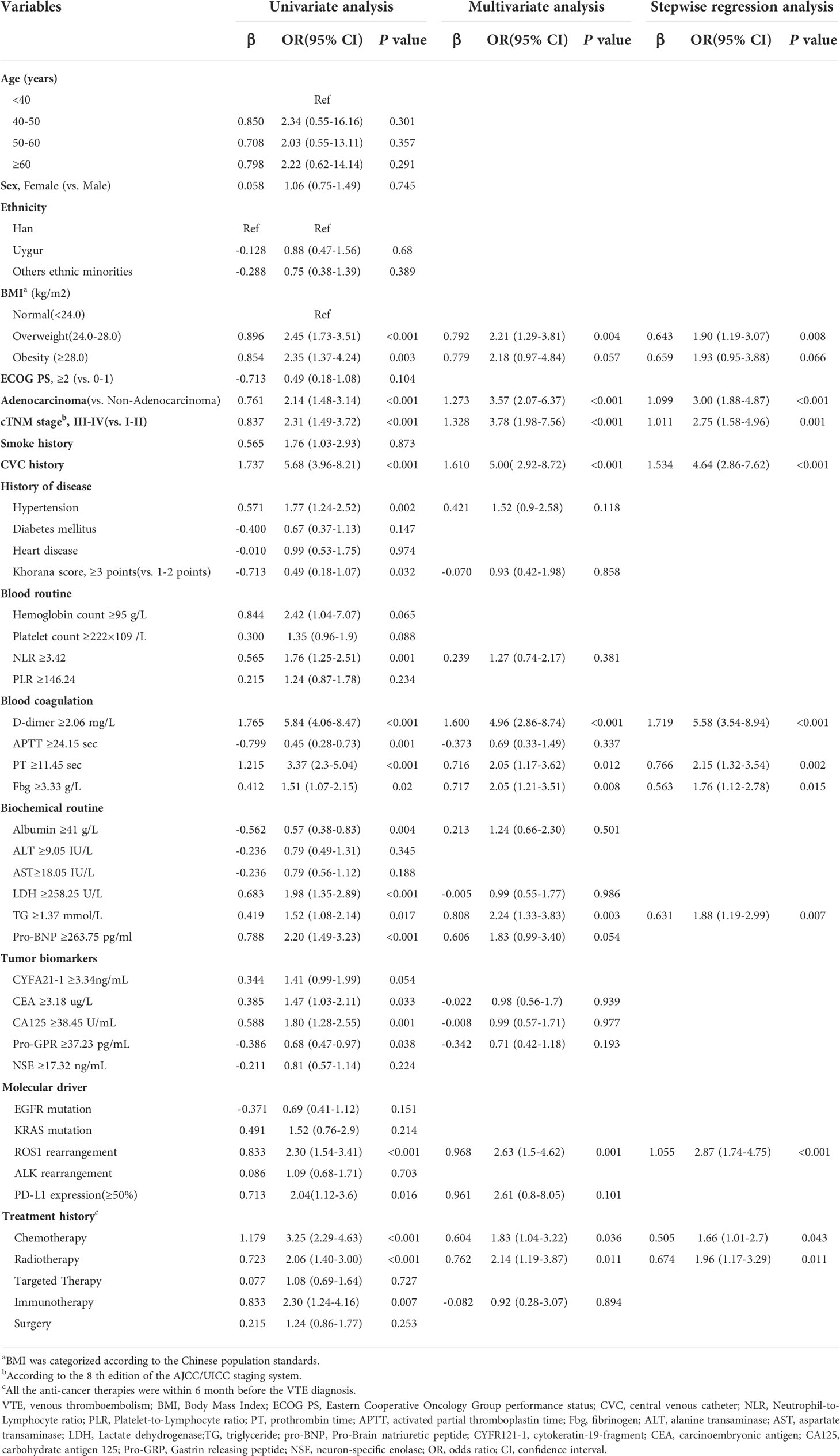
Table 2 Univariate and multivariate regression analysis of risk factors associated with VTE in lung cancer.
Afterward, according to the multivariate and backward stepwise logistic analysis, the results showed overweight(24-28) defined by BMI[1.90(1.19-3.07)], adenocarcinoma[3.00(1.88-4.87)], stageIII-IV[2.75(1.58-4.96)], CVC history[4.64(2.86-7.62)], D-dimer levels≥2.06 mg/L[5.58(3.54-8.94)], PT levels≥11.45sec[2.15(1.32-3.54)], Fbg levels≥3.33 g/L[1.76(1.12-2.78)], TGlevels≥1.37mmol/L[1.88(1.19-2.99)],ROS1rearrangement[2.87(1.74-4.75)],chemotherapy history[1.66(1.01-2.70)] and radiotherapy history[1.96(1.17-3.29)] ROS1rearrangementwere considered to be independent risk factors for VTE in lung cancer, and these factors were eventually incorporated into the final model (Table 2). Furthermore, the collinearity diagnostic analysis demonstrated that the VIFs of those risk factors were less than 4, indicating that there is no strong indication of multicollinearity among variables. Thus, there were eleven variables included in the final multivariable prediction model as predictors (Figure 2).
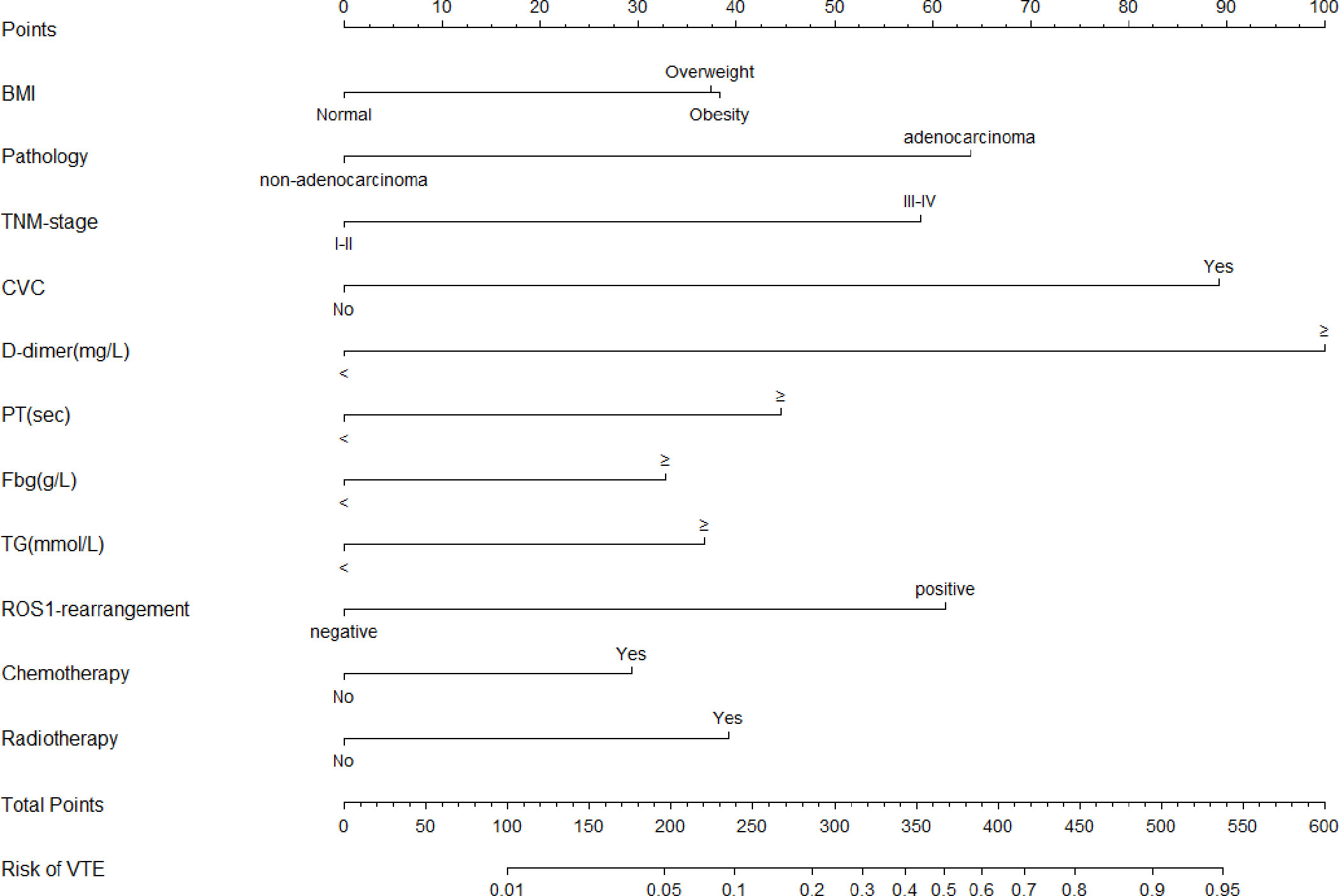
Figure 2 The Nomogram model for prediction of VTE in hospitalized patients with lung cancer. VTE, venous thromboembolism; BMI, Body Mass Index; CVC, central venous catheter; PT, prothrombin time; Fbg, fibrinogen; TG, triglyceride.
Based on the regression coefficient of these risk factors, the risk score model of VTE[Logit (P)] was constructed as Logit(P) = -6.306+0.643×BMI+1.099×Pathology+1.011×TNM-stage+ 1.543×CVC history+1.719×D-dimer+0.766×PT+0.563×Fbg+0.631×TG+1.055×ROS1-rearrangement+0.505×chemotherapy history+0.674×radiotherapy history. For visualization and convenient clinical use of the predictive model, the mathematical risk prediction model was visualized as a nomogram to predict the likelihood of VTE in hospitalized patients with lung cancer (Figure 2). The probability of developing VTE can be determined by assigning points for each variable by drawing a line upward to the Points axis, summing all the points from the variables plotted on the total points axis and then drawing a vertical line from the total points axis straight down to the risk of VTE axis. For example, the application of this model to a 53-year-old patient with lung cancer would show the following results: BMI of 28 kg/m2, histology of adenocarcinoma, IV stage, with CVC history, D-dimer of 5.97 mg/L, PT of 11.8 sec, Fbg of 2.48 sec, TG of 1.2g/L, ROS1 rearrangement(+), without chemotherapy and radiotherapy history. The total score of the above predictors was 38 + 64+59+89+100+44+0+0+61+0+0 = 455 and the corresponding risk probability of VTE was 0.81 (81%)
The AUCs in the development and validation group were 0.865 (95% CI: 0.832-0.897) and 0.904 (95%CI: 0.869-0.939), respectively, which indicated the good prediction performance of the model (Figure 3). The proposed nomogram was validated internally using the bootstrap method with 1000-bootstrap repetitions in the development cohort, with a C-index of 0.904, which indicated that the novel proposed model achieved high prediction accuracy. Furthermore, the calibration curve of the nomogram for the prediction of the risk of VTE in patients with lung cancer demonstrated good agreement between prediction and observation in the development (Figure 4A) and validation (Figure 4B) cohorts. The findings of the Hosmer–Lemeshow goodness-of-fit test also was not significant in the development and validation sets(c²=14.848, 4.276, P = 0.062, 0.831, respectively).
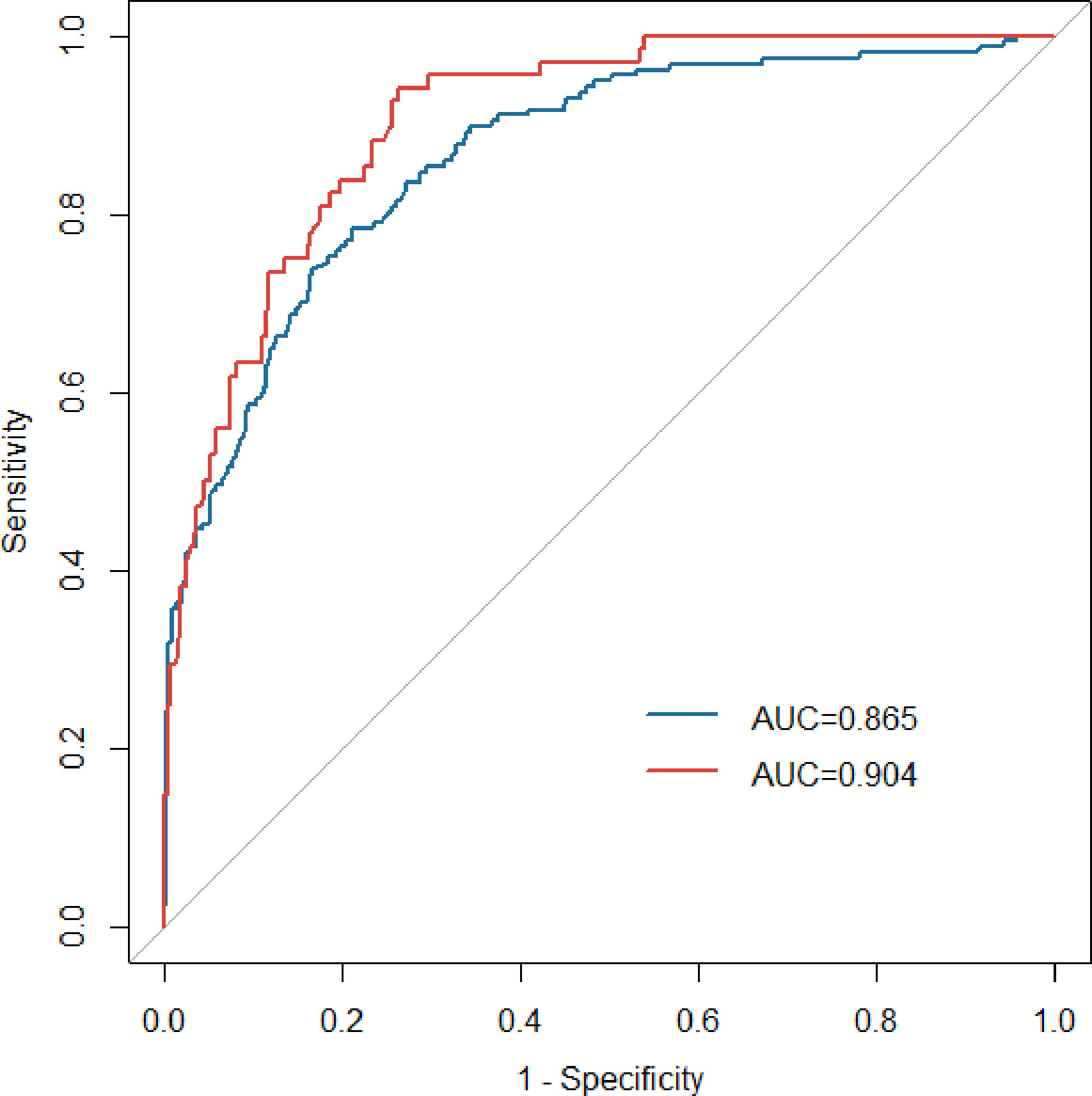
Figure 3 ROC curves of the nomogram in the development (blue line) and validation (red line) groups. ROC, receiver operating characteristic; AUC, area under the curve.
The DCA curves for the predictive nomogram are presented in Figure 5. The clinical utility of nomogram model was estimated using DCA by quantifying the net benefits at different threshold probabilities. The DCA displayed that the nomogram provided superior net benefit of thromboprophylaxis in patients at VTE risk than strategies of treating all and treating none, with a probability threshold interval of 2%–82% (Figure 5).
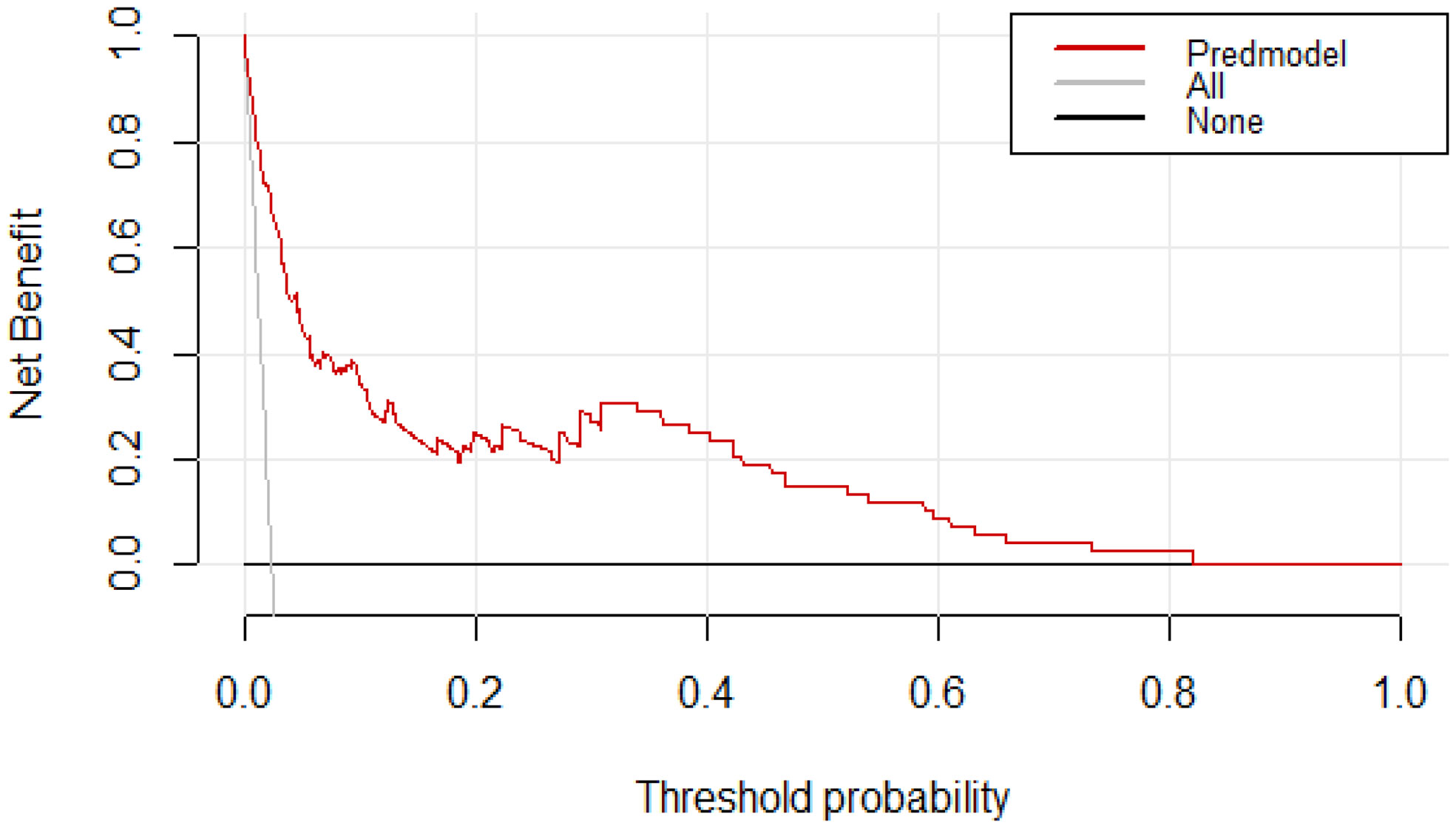
Figure 5 Decision curve analysis (DCA) for assessment of the clinical utility for thromboprophylaxis. The threshold probability represents the predicted risk of VTE for recommending primary thromboprophylaxis.The net benefit balances the risk of VTE with the potential harms of unnecessary thromboprophylaxis and is equal to the true-positive rate minus the weighted false-positive rate.
An overview of the risk of bias(ROB) and the applicability for this prediction model is provided in Supplementary Table 1. The model was rated as high risk of bias in two domains: Predictors and analysis. The risk model had a high ROB and good applicability. The high risk of bias was judged according to some specific issues in the study design and statistical analysis (see the rationale of rating in Supplementary Table 1)
The ROC curves of different VTE risk assessment models are shown in Figure 6. The area under the ROC curve for the specific VTE risk-stratification nomogram model (0.904; 95% CI:0.869-0.939) was significantly higher than those of the KRS, Caprini, Padua and COMPASS-CAT models(Z=12.087, 11.851, 9.442, 5.340, all P<0.001, respectively). Additionally, the risk score of 300.6 was determined as the optimal cutoff value with the maximum Youden index(OR 14.17, 95% CI 9.45-21.64, P < 0.001) and was divided into a low-risk group (562 patients with risk score ≤ 300.6) and high-risk group (220 patients with risk score>300.6), respectively. The nomogram model presentedwith a sensitivity (Se) of 73.9%, specificity (Sp) of 83.4%, positive predictive value (PPV) of 52.7% and negative predictive value (NPV) of 92.7%.
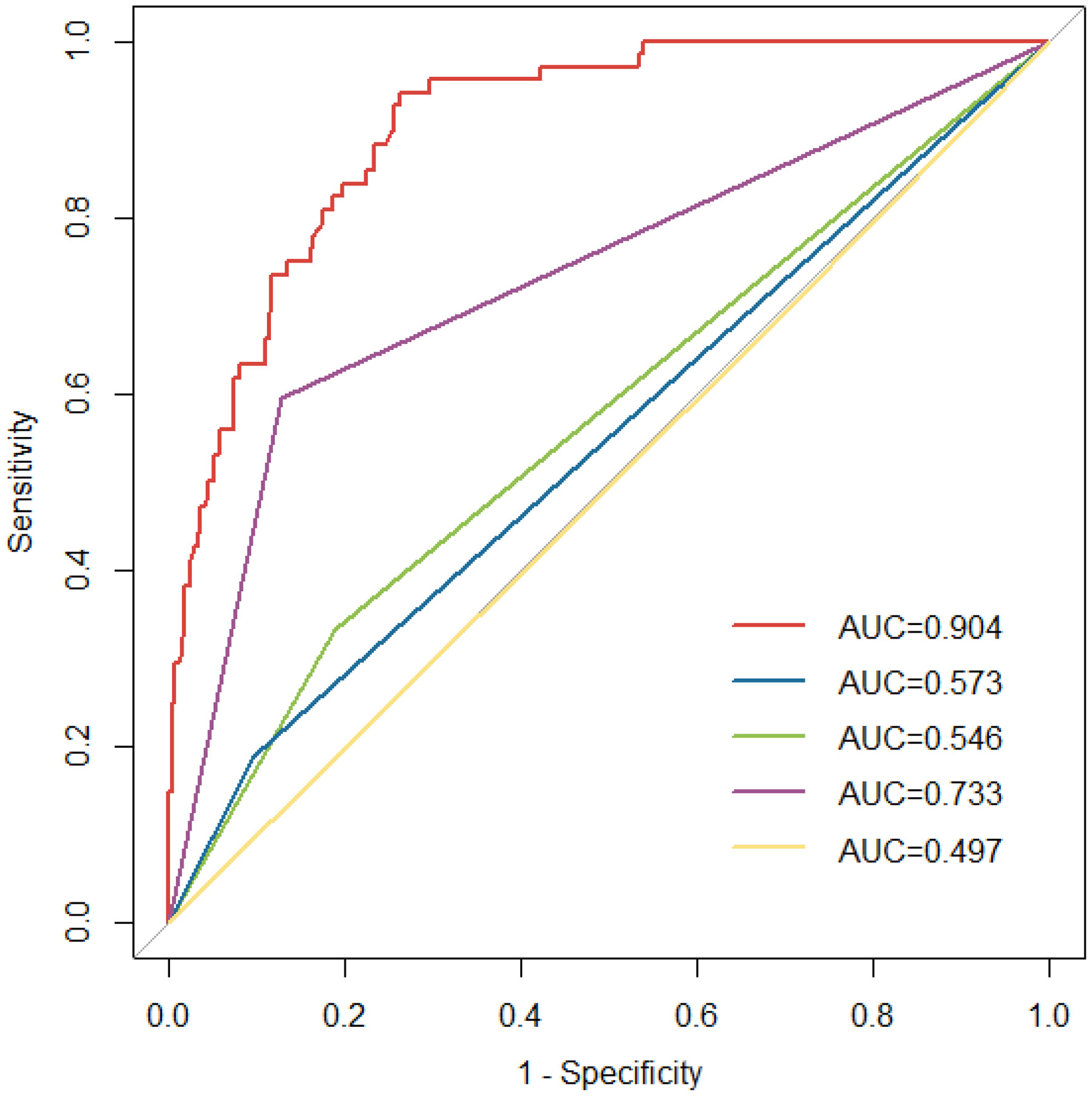
Figure 6 ROC curves for the existing risk assessment models [Khorana score(green line), Caprini risk assessment model(yellow line), Padua score(blue line) and COMPASS-CAT model(purple line)] and nomogram model (red line). ROC, receiver operating characteristic; AUC, area under the curve.
Currently, early detection of high-risk factors for cancer patients combined with VTE should be paid for particular attention. Previous studies mainly focused on the analysis of risk factors of lung cancer-associated VTE to establish the risk score system (19–21). However, few studies have been performed for the development of novel nomograms for the prediction of VTE in lung cancer patients, particularly given other factors influencing cancer-associated VTE such as genetic and therapeutic factors (31).Therefore, with a specific focus on both genetic and therapeutic factors, we developed and validated a simple yet highly discriminating, well-calibrated, and parsimonious nomogram prediction model for the occurrence of VTE in hospitalized lung cancer patients in this study, which can provide a theoretical basis for clinical decision-making on thromboprophylaxis on the basis of VTE risk levels. To our knowledge, this is the first to integrate readily obtainable clinical parameters, genetic and therapeutic factors into a modeling of the nomogram for the prediction of VTE in lung cancer. Furthermore, the nomogram prediction model proposed in this study was superior to other established scoring models in risk stratification of VTE patients.
According to the results of the logistic regression analyses, we established a simplified eleven-variables nomogram model, which contains four clinical variables(BMI, histology, clinical stage and CVC history), four biomarkers(D-dimer, PT, Fbg and TG), oncogenic abnormalities(ROS-1 rearrangement) and history of previous radiotherapy and chemotherapy treatment. Many of the risk factors of cancer-associated VTE identified in this analysis, particularly in lung cancer, were mostly consistent with those of the previous literatures (18–21). Among the numerous risk factors, it is widely accepted that higher BMI were patient-related risk factors related to VTE. Several studies have revealed that being obesity or overweight was associated with higher risk of VTE (8, 18, 32), while being underweight was associated with a lower risk of VTE (33). This study confirmed the previous data with a calculated OR of 1.90(95%CI: 1.19-3.07) for overweight. Furthermore, an OR value of 1.93(95%CI: 0.95-3.88) for obesity was observed but was not significant. Considering the body structure, dietary habits and ethnic differences exist between Chinese and Caucasian populations, the Chinese criteria for BMI were used instead of the World Health Organization(WHO) standard for classification in this study. Moreover most studies noted that the modified version of Khorana scale setting the cut-off points of BMI at 24kg/m2 for Chinese population could improve the risk stratification and identification of patients with VTE (34). Therefore, we have adjusted the cut-off points of BMI according to the Chinese population standards in our models, for a better evaluation of association between BMI and VTE risk in a Chinese population.
With respect to cancer-related factors, adenocarcinoma was shown to be one of the most powerful predictors for VTE development in our nomogram model. Based on our analysis, the results showed that patients with adenocarcinoma have an approximately 3-fold higher risk of developing VTE than that of non-adenocarcinom apopulation. This result is consistent with the findings of previous studies (19, 21, 35). There is also clear evidence that patients with adenocarcinoma was associated with a markedly higher risk of VTE compared with other pathological types (8, 35). Another recent studies by Tsubata et al. (19) and Li et al. (21) has demonstrated that adenocarcinoma was one of the most powerful predictors for VTE development in the predictive scoring system. These studies led to widespread belief that adenocarcinoma plays a critical role in activating pro-coagulants factors by secreting mucin that may result in thrombus formation. Additionally, we found that patients with advanced stage have a risk of VTE that is 2- to 3-fold higher than those from patients with early stages, which is in accordance with the findings of more recent studies (8, 19, 21, 36, 37). These results suggest that patients with advanced and metastatic cancer has been linked to an increased risk of VTE as compared to tumors that are localized. It is now well acknowledged that high prevalence of VTE occurrence in advanced stage patients may likely be related to the synchronicity of tumor progression and increased coagulation activity (38).
Moreover, most previous studies noted that the use of long-term central venous catheters(CVCs) especially peripheral insertion central catheter (PICC) was strongly associated with increased risk of upper-extremity DVT (39, 40). This study shows that a prior history of CVCs was an independent risk factor for VTE in patients with lung cancer, with an OR of 4.64. This is in agreement with previous studies, which showed CVCs indwelling might be the main cause of upper extremity DVT among cancer patients (8). Furthermore, it should be noted that the incidence of catheter‐related thrombosis (CRT) is closely related to the type of CVC, the thickness of the Catheter and the position of the CVC tip (39–41). This phenomenon might be correlated with vascular endothelial injury and slow blood flow caused by the deep venous indwelling catheter, or compression of the cervical lymph nodes metastasis.
D-dimer is the specific degradation products of crosslinked fibrin and is used as sensitive marker of hypercoagulability status and of endogenous fibrinolysis. It has been established by several studies that D-dimer levels was an independent risk predictor of VTE in various types of cancer (21, 42–44). There is even some evidence suggesting that incorporating D-dimer into the VTE risk score significantly improves VTE risk discrimination and reclassification (45). These results are highly consistent with those from our study, which suggested that D-dimer≥2.06 mg/L can be considered independent risk factors of VTE and can be useful in stratifying risk for VTE in lung cancer. However, it should be noted that the exact D-dimer threshold for the diagnosis of VTE in the models is still controversial. We applied a highly sensitive cut-off of 2.06 mg/L that adapted from the cut-off given by the Youden index, which is similar or higher than these previous studies (20, 42, 43). This may be due to the fact that the tumors of the enrolled patients were mostly at advanced stage, which might resulted in higher baseline D-dimer levels.
Moreover, we have incorporated other noninvasive and obtained easily in clinic indicators into our analyses. The present study found that PT levels≥11.45sec, Fbg levels≥3.33 g/L and TG levels≥1.37mmol/L were an independent risk factor for VTE in patients with lung cancer, with an OR of 2.15, 1.76 and 1.88, respectively. Currently, the association of inflammatory parameters(PLR and NLR) and the risk of thromboembolism has been attracted increasing interest in recent years (46, 47). Based on the logistic regression analysis, our results showed that NLR at baseline were statistically significant in the univariate analysis, whereas elevated NLR were not associated with an increased risk of VTE in the multivariate analysis(P=0.381), which is partly compatible with the findings of more recent studies (46, 48). The reason might be related to the effects of neutrophil extracellular traps(NETs) released by tumor-activated neutrophils.
Currently, there is a growing interest in exploring the potential correlation between driver genes and VTE risk in lung cancer (49–51). Clearly, there is a critical need to incorporating positive driver genes into a risk assessment model for lung cancer to improve performance. One of the strengths of our study is the inclusion of molecular drivers in the construction of this model. Interestingly, our results showed that the risk of VTE in ROS1 rearrangements(ROS1+) patients is 2.63-fold greater than that in ROS1- patients, and the odds of VTE in ROS1+ lung cancer were higher than ALK+, EGFR+ and KRAS+ cohorts in the univariate analysis. Similar to our findings, more recent studies by Zhu et al. (50) and Ng et al. (51) have also found that the risk of VTE is significantly increased in patients with ROS+ NSCLC compared to EGFR+ and KRAS+ cases. Although the mechanism is not clear yet, The mucus produced in ROS1 fusions lung adenocarcinoma probably contributes to further platelet recruitment and, consequently, to the thrombus development.
Considering the the treatment-related factors suggested in the literatures including history of chemo-, radiation- and immuno-therapies, several studies have confirmed that chemotherapy, widely used in more than half of the patients, has been reported to be associated with a 2- to 6-fold increased VTE risk (8, 15). By multivariate logistic regression analysis our study showed that having previously undergone radiation or chemotherapy within 6 months before VTE diagnosis was independently associated with an increased risk of VTE for patients with lung cancer, which is in accordance with the findings of Li et al. (21). Plausible explanations for this could be attributed to vascular endothelium damage, reduced endogenous anticoagulant factors(antithrombin, protein C, protein S) and platelet activation (15). Nonetheless, there is less evidence available on the influence of RT on outcome in cancer patients with VTE. Still, a few studies have displayed that there remained a significant correlation between RT and VTE in patients with cancer (16, 52). This may be in part due to the endothelial damage to veins caused by radiation exposure.
To date, many randomized controlled trials(RCTs) have developed several risk assessment models suitable for cancer patients, such as KRS, Caprini, Padua and COMPASS-CAT score. Concerning the comparison of the current model with other existing models, our current data suggest that the area under the ROC curve for the specific VTE risk-stratification nomogram model(0.904; 95% CI:0.869-0.939) was significantly higher than those of the KRS, Caprini, Padua and COMPASS-CAT models. The reason for this differences may have been due to the scale discrimination in the applicable population. KS scale was originally designed for cancer outpatients, which did not include some therapeutic factors (e.g. chemotherapy) occurred during hospitalization. Therefore, the proportion of patients who were at a high risk of VTE may be underestimated. Additionally, Caprini scale has a good predictive value in cancer inpatients, particularly among patients undergoing surgery, which can easily lead to pharmacologic overprophylaxis, and thus result in an inherent risk of bleeding (53). Consequently, it is necessary to establish a meticulous benefit and risk assessment model for VTE in patients with malignancies to balance of the risk of bleeding against the risk of thromboembolism. Based on our results, DCA revealed that the nomogram provided superior net benefit of thromboprophylaxis in patients with high VTE risk for threshold probabilities between 2% and 82%. Further large randomised‐controlled trials(RCTs) are needed to evaluate the benefits of thromboprophylaxis in patients at high risk of VTE.
Some limitations of the current study must be considered. First, selection bias could not be avoided due to the single-center retrospective study design. Thus, this model needs to be further validated with larger sample sizes, and/or performed in other centers and other geographic regions to determine its generalizability and efficiency. Second, it is noted that we did not performroutine VTE screening for enrolled patients. This study only identified patients with symptomatic or incidental VTE, which may result in a bias underestimating of the prevalence of VTE in enrolled patients. Moreover, local compression of vascular structures via mass lesion and presence of genetic mutations associated with increased thrombosis are risk factors for developing VTEs that cannot be ignored.As a result, limitations of the absence of the above datas must be considered in the current study. Finally, further multi-center prospective clinical trials and community-based prospective studies are needed to validate and refine the model.
In conclusion, this study systematically developed and validated a novel VTE risk prediction nomogram model for patients with newly diagnosed lung cancer, which incorporated available clinical parameters, genetic and therapeutic factors into the assessment system. Notably, the novel nomogram model was significantly more effective than the existing well-accepted models in routine clinical practice in stratifying the risk of VTE in those patients. We have provided evidence to support that high-performance nomogram model can be reliably used to identify hospitalized patients with lung cancer at a high risk of VTE and to guide individualized decision-making on thromboprophylaxis. However, the model needs external validation in other clinical centers, and should be extended to other care settings (e.g. community-based ambulatory patients).
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HL contributed to the study design, statistical analyses, and manuscript writing. YT, HN, LH, GC, CZ and KK performed data collection. HL and YT analyzed and interpreted the data. QL designed and supervised the research and revised the manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by National Natural Science Foundation of China (grant number 81760014).
The authors are thankful to Mrs. Tiantian Sun and Jiali Lu, biostatistician, for their help and guidance in carrying out the statistical analysis for the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.988287/full#supplementary-material
1. Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet (2016) 388(10063):3060–73. doi: 10.1016/S0140-6736(16)30514-1
2. Grilz E, Posch F, Nopp S, Königsbrügge O, Lang IM, Klimek P, et al. Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer-a nationwide analysis. Eur Heart J (2021) 42(23):2299–307. doi: 10.1093/eurheartj/ehab171
3. Mulder FI, Horváth-Puhó E, van Es N, van Laarhoven HWM, Pedersen L, Moik F, et al. Venous thromboembolism in cancer patients: a population-based cohort study. Blood (2021) 137(14):1959–69. doi: 10.1182/blood.2020007338
4. Lyman GH, Culakova E, Poniewierski MS, Kuderer NM. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb Res (2018) 164 Suppl 1:S112–8. doi: 10.1016/j.thromres.2018.01.028
5. Lloyd AJ, Dewilde S, Noble S, Reimer E, Lee AYY. What impact does venous thromboembolism and bleeding have on cancer patients' quality of life? Value Health (2018) 21(4):449–55. doi: 10.1016/j.jval.2017.09.015
6. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. (2007) 5(3):632–4. doi: 10.1111/j.1538-7836.2007.02374.x
7. Khalil J, Bensaid B, Elkacemi H, Afif M, Bensaid Y, Kebdani T, et al. Venous thromboembolism in cancer patients: an underestimated major health problem. World J Surg Oncol (2015) 13:204. doi: 10.1186/s12957-015-0592-8
8. Peng M, Yang S, Li G, Zhang T, Qin X, Shi C, et al. Solid tumor complicated with venous thromboembolism: A 10-year retrospective cross-sectional study. Clin Appl Thromb Hemost. (2021) 27:1076029620975484. doi: 10.1177/1076029620975484
9. Mahajan A, Brunson A, White R, Wun T. The epidemiology of cancer-associated venous thromboembolism: An update. Semin Thromb Hemost. (2019) 45(4):321–5. doi: 10.1055/s-0039-1688494
10. Shen Q, Dong X, Tang X, Zhou J. Risk factors and prognosis value of venous thromboembolism in patients with advanced non-small cell lung cancer: a case-control study. J Thorac Dis (2017) 9(12):5068–74. doi: 10.21037/jtd.2017.11.116
11. Galanaud JP, Monreal M, Kahn SR. Epidemiology of the post-thrombotic syndrome. Thromb Res (2018) 164:100–9. doi: 10.1016/j.thromres.2017.07.026
12. Gandhi V, Hewston M, Yadam S, Ma K, Singh AC, Cheema T. Consequences of venous thromboembolism, including chronic thromboembolic pulmonary hypertension. Crit Care Nurs Q (2017) 40(3):260–75. doi: 10.1097/CNQ.0000000000000164
13. Chaudhary R, Damluji A, Batukbhai B, Sanchez M, Feng E, Chandra Serharan M, et al. Venous thromboembolism prophylaxis: Inadequate and overprophylaxis when comparing perceived versus calculated risk. Mayo Clin Proc Innov Qual Outcomes. (2017) 1(3):242–7. doi: 10.1016/j.mayocpiqo.2017.10.003
14. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol (2015) 16(4):e173–80. doi: 10.1016/S1470-2045(14)71116-7
15. Debbie Jiang MD, Alfred Ian Lee MD. Thrombotic risk from chemotherapy and other cancer therapies. Cancer Treat Res (2019) 179:87–101. doi: 10.1007/978-3-030-20315-3_6
16. Temraz S, Moukalled N, Gerotziafas GT, Elalamy I, Jara-Palomares L, Charafeddine M, et al. Association between radiotherapy and risk of cancer associated venous thromboembolism: A Sub-analysis of the COMPASS-CAT study. Cancers (Basel). (2021) 13(5):1033. doi: 10.3390/cancers13051033
17. Roopkumar J, Swaidani S, Kim AS, Thapa B, Gervaso L, Hobbs BP, et al. Increased incidence of venous thromboembolism with cancer immunotherapy. Med (2021) 2(4):423–34. doi: 10.1016/j.medj.2021.02.002
18. Li Y, Shen L, Ding J, Xie D, Yang J, Zhao Y, et al. Derivation and validation of a nomogram model for pulmonary thromboembolism in patients undergoing lung cancer surgery. Transl Lung Cancer Res (2021) 10(4):1829–40. doi: 10.21037/tlcr-21-109
19. Tsubata Y, Hotta T, Hamai K, Furuya N, Yokoyama T, Saito R, et al. A new risk-assessment tool for venous thromboembolism in advanced lung cancer: a prospective, observational study. J Hematol Oncol (2022) 15(1):40. doi: 10.1186/s13045-022-01259-7
20. Li J, Yi J, Hua L, Su Y, Huo M, Dou F, et al. Development and validation of a predictive score for venous thromboembolism in newly diagnosed non-small cell lung cancer. Thromb Res (2021) 208:45–51. doi: 10.1016/j.thromres.2021.10.013
21. Li Z, Zhang G, Zhang M, Mei J, Weng H, Peng Z. Development and validation of a risk score for prediction of venous thromboembolism in patients with lung cancer. Clin Appl Thromb Hemost. (2020) 26:1076029620910793. doi: 10.1177/1076029620910793
22. Hansen BB, Klopfer SO. Optimal full matching and related designs via network flows. J Comput Graphical Stat (2006) 15(3):609–27. doi: 10.1198/106186006X137047
23. Schellong SM, Schwarz T, Halbritter K, Beyer J, Siegert G, Oettler W, et al. Complete compression ultrasonography of the leg veins as a single test for the diagnosis of deep vein thrombosis. Thromb Haemost. (2003) 89(2):228–34. doi: 10.1055/s-0037-1613436
24. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood (2008) 111(10):4902–7. doi: 10.1182/blood-2007-10-116327
25. Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. (1991) 17 Suppl 3:304–12.
26. Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the padua prediction score. J Thromb Haemost. (2010) 8(11):2450–7. doi: 10.1111/j.1538-7836.2010.04044.x
27. Gerotziafas GT, Taher A, Abdel-Razeq H, AboElnazar E, Spyropoulos AC, El Shemmari S, et al. A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: The prospective COMPASS-Cancer-Associated thrombosis study. Oncologist (2017) 22(10):1222–31. doi: 10.1634/theoncologist.2016-0414
28. Zhang Z. Variable selection with stepwise and best subset approaches. Ann Transl Med (2016) 4(7):136. doi: 10.21037/atm.2016.03.35
29. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. (2006) 26(6):565–74. doi: 10.1177/0272989X06295361
30. Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med (2019) 170(1):51–8. doi: 10.7326/M18-1376
31. Di W, Xu H, Xue T, Ling C. Advances in the prediction and risk assessment of lung cancer-associated venous thromboembolism. Cancer Manag Res (2021) 13:8317–27. doi: 10.2147/CMAR.S328918
32. Weze KO, Obisesan OH, Dardari ZA, Cainzos-Achirica M, Dzaye O, Graham G, et al. The interplay of Race/Ethnicity and obesity on the incidence of venous thromboembolism. Am J Prev Med (2022) S0749-3797(22):00064–2. doi: 10.1016/j.amepre.2021.12.023
33. Delluc A, Mottier D, Le Gal G, Oger E, Lacut K. Underweight is associated with a reduced risk of venous thromboembolism. results from the EDITH case-control study. J Thromb Haemost. (2009) 7(4):728–9. doi: 10.1111/j.1538-7836.2009.03280.x
34. Wang MM, Qin XJ, He XX, Qiu MJ, Peng G, Yang SL. Comparison and screening of different risk assessment models for deep vein thrombosis in patients with solid tumors. J Thromb Thrombolysis. (2019) 48(2):292–8. doi: 10.1007/s11239-019-01840-x
35. Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost. (2004) 2(10):1760–5. doi: 10.1111/j.1538-7836.2004.00928.x
36. Yang J, Zhang Y, Yang P, Zhang X, Li M, Zou L. A novel nomogram based on prognostic factors for predicting venous thrombosis risk in lymphoma patients. Leuk Lymphoma. (2021) 62(10):2383–91. doi: 10.1080/10428194.2021.1913149
37. Gade IL, Braekkan SK, Naess IA, Hansen JB, Cannegieter SC, Overvad K, et al. The impact of initial cancer stage on the incidence of venous thromboembolism: the Scandinavian thrombosis and cancer (STAC) cohort. J Thromb Haemost. (2017) 15(8):1567–75. doi: 10.1111/jth.1375223
38. Ahlbrecht J, Dickmann B, Ay C, Dunkler D, Thaler J, Schmidinger M, et al. Tumor grade is associated with venous thromboembolism in patients with cancer: results from the Vienna cancer and thrombosis study. J Clin Oncol (2012) 30(31):3870–5. doi: 10.1200/JCO.2011.40.1810
39. Winters JP, Callas PW, Cushman M, Repp AB, Zakai NA. Central venous catheters and upper extremity deep vein thrombosis in medical inpatients: the medical inpatients and thrombosis (MITH) study. J Thromb Haemost. (2015) 13(12):2155–60. doi: 10.1111/jth.13131
40. Al-Asadi O, Almusarhed M, Eldeeb H. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single centre cohort study. Thromb J (2019) 17:2. doi: 10.1186/s12959-019-0191-y
41. Gaddh M, Antun A, Yamada K, Gupta P, Tran H, El Rassi F, et al. Venous access catheter-related thrombosis in patients with cancer. Leuk Lymphoma. (2014) 55(3):501–8. doi: 10.3109/10428194.2013.813503
42. Jiang XX, Li XY, Zhang J, Wang XX, Lin CQ. A nomogram model can predict the risk of venous thromboembolism in postoperative patients with gynecological malignancies. Int J Gynaecol Obstet (2021) 158(3):689–99. doi: 10.1002/ijgo.14061
43. Yuan HL, Zhang X, Li Y, Guan Q, Chu WW, Yu HP, et al. A nomogram for predicting risk of thromboembolism in gastric cancer patients receiving chemotherapy. Front Oncol (2021) 11:598116. doi: 10.3389/fonc.2021.598116
44. Wang P, Zhao H, Zhao Q, Ren F, Shi R, Liu X, et al. Risk factors and clinical significance of d-dimer in the development of postoperative venous thrombosis in patients with lung tumor. Cancer Manag Res (2020) 12:5169–79. doi: 10.2147/CMAR.S256484
45. Gibson CM, Spyropoulos AC, Cohen AT, Hull RD, Goldhaber SZ, Yusen RD, et al. The IMPROVEDD VTE risk score: Incorporation of d-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open (2017) 1(1):e56–65. doi: 10.1055/s-0037-1603929
46. Grilz E, Posch F, Königsbrügge O, Schwarzinger I, Lang IM, Marosi C, et al. Association of platelet-to-Lymphocyte ratio and neutrophil-to-Lymphocyte ratio with the risk of thromboembolism and mortality in patients with cancer. Thromb Haemost. (2018) 118(11):1875–84. doi: 10.1055/s-0038-1673401
47. Otasevic V, Mihaljevic B, Milic N, Stanisavljevic D, Vukovic V, Tomic K, et al. Immune activation and inflammatory biomarkers as predictors of venous thromboembolism in lymphoma patients. Thromb J (2022) 20(1):20. doi: 10.1186/s12959-022-00381-3
48. Fuentes HE, Paz LH, Wang Y, Oramas DM, Simons CR, Tafur AJ. Performance of current thromboembolism risk assessment tools in patients with gastric cancer and validity after first treatment. Clin Appl Thromb Hemost. (2018) 24(5):790–6. doi: 10.1177/1076029617726599
49. Qian X, Fu M, Zheng J, Zhou J, Zhou J. Driver genes associated with the incidence of venous thromboembolism in patients with non-Small-Cell lung cancer: A systematic review and meta-analysis. Front Oncol (2021) 11:680191. doi: 10.3389/fonc.2021.680191
50. Zhu VW, Zhao JJ, Gao Y, Syn NL, Zhang SS, Ou SI, et al. Thromboembolism in ALK+ and ROS1+ NSCLC patients: A systematic review and meta-analysis. Lung Cancer. (2021) 157:147–55. doi: 10.1016/j.lungcan.2021.05.019
51. Ng TL, Smith DE, Mushtaq R, Patil T, Dimou A, Yang S, et al. ROS1 gene rearrangements are associated with an elevated risk of peridiagnosis thromboembolic events. J Thorac Oncol (2019) 14(4):596–605. doi: 10.1016/j.jtho.2018.12.001
52. Guy JB, Bertoletti L, Magné N, Rancoule C, Mahé I, Font C, et al. Venous thromboembolism in radiation therapy cancer patients: Findings from the RIETE registry. Crit Rev Oncol Hematol (2017) 113:83–9. doi: 10.1016/j.critrevonc.2017.03.006
Keywords: lung cancer, venous thromboembolism, risk assessment, nomogram model, thromboprophylaxis
Citation: Li H, Tian Y, Niu H, He L, Cao G, Zhang C, Kaiweisierkezi K and Luo Q (2022) Derivation, validation and assessment of a novel nomogram-based risk assessment model for venous thromboembolism in hospitalized patients with lung cancer: A retrospective case control study. Front. Oncol. 12:988287. doi: 10.3389/fonc.2022.988287
Received: 07 July 2022; Accepted: 27 September 2022;
Published: 10 October 2022.
Edited by:
Canan G. Nebigil, INSERM U1260 Nanomedicine régénératrice (RNM), FranceReviewed by:
Yusuf Ziya Şener, Hacettepe University, TurkeyCopyright © 2022 Li, Tian, Niu, He, Cao, Zhang, Kaiweisierkezi and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Luo, bHVvcWluMDcwNTIwMjJAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.