- 1Department of Gastroenterology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 3State Key Laboratory of Cancer Biology, Department of Gastrointestinal Surgery, Xijing Hospital of Digestive Diseases, Air Force Medical University, Xi’an, China
- 4Department of Hospital Management, Affiliated Hospital of Beihua University, Jilin, China
- 5Department of Gastrointestinal Surgery, Tianjin People’s Hospital, Tianjin, China
- 6Department of General Surgery, The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
Purpose: Recent studies have revealed the contrasting prognostic roles of body mass index (BMI) and tumor location in colorectal cancer (CRC). Given that right- and left-sided CRC may exhibit inverse effects on outcome and body weight, the present study aimed to examine whether the prognostic value of BMI and tumor location could be reciprocally stratified.
Methods: This prospective, observational study recruited 4,086 patients diagnosed with stage III CRC from five independent clinical centers in China. The association of patients’ outcomes with BMI and tumor location was evaluated hierarchically by Kaplan–Meier and Cox proportional-hazards models.
Results: Although BMI was not associated with overall outcome, the association was significantly modified by tumor location. Among left-sided tumors, obesity and overweight were significantly associated with adverse overall survival (OS) and disease-specific survival (DSS). In contrast, among right-sided tumors, overweight was significantly associated with more favorable OS and DSS compared with the normal-weight group. The association of survival with tumor location did not reach statistical significance. However, hierarchical analysis by BMI revealed that left-sided tumors were associated with more favorable outcomes in the normal-weight group, while there was no statistically significant difference in the overweight or obese group.
Conclusions: BMI and tumor location may have opposing effects on CRC prognosis, when stratified by each other, after adjusting for other known prognostic factors. These findings are the first to show the interactive prognostic impact of BMI and tumor location, which could be relevant to the stratification of patient management.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide (1–3). Previously, it occurred primarily in western countries whose populations frequently exhibit CRC risk factors, including obesity (2, 4–9). However, incidence rates of CRC have recently stabilized or decreased in most developed countries (3, 10–13). In contrast, the incidence rates of CRC are rapidly increasing in eastern Asia, including China, Japan, and South Korea (3, 14–16). In China, there has been a two- to four-fold increase in the incidence of CRC since the 1980s (17–19), which can be explained, at least in part, by changes in lifestyle factors (16, 19, 20).
Previous studies have found an association between high body mass index (BMI) and an elevated risk of developing CRC (11, 21–30). However, data on the potential effect of BMI on the prognosis of CRC are scarce, and the limited publications available on this topic have shown conflicting results (4, 31–37). In the National Cancer Institute (NCI)-sponsored Cancer and Leukemia Group B (CALGB) study, neither BMI nor weight change was found to be associated with outcome in patients with stage III colon cancer (31). In the National Surgical Adjuvant Breast and Bowel Project (NSABP), only severe obesity was found to be associated with colon cancer outcomes (32). However, another NCI-sponsored investigation found obesity to be an independent prognostic variable in colon cancer survivors (36). Two studies that focused on female patients obtained differing results regarding the prognostic value of BMI in CRC (33, 38).
Recently, the association between tumor location and the prognosis of human colorectal cancer has been investigated, and the studies to date have produced conflicting results. One study involving the Surveillance, Epidemiology, and End Results Program (SEER) database showed a significant increase in mortality for right-sided tumors compared with left-sided ones (39, 40). In contrast, another study using the SEER database found no overall difference between right- and left-sided colon cancers when stratified by tumor stage (41), and an investigation based on the NCIC CO.17 trial demonstrated that tumor location is not a prognostic factor in CRC (42). More recently, a study on metastatic CRC found that patients with left-sided tumors had superior overall and progression-free survival compared with right-sided ones (43). Considering that right- and left-sided tumors may exhibit different effects on body weight and even on outcomes, we hypothesized that there might be interactions between BMI and tumor location in the determination of prognosis (44, 45). Therefore, the intrinsic prognostic role of BMI and tumor location in CRC may be better understood after stratified analysis.
In the present study, we used stratified analysis to investigate the associations between BMI, tumor location, and outcomes in a large cohort of patients with resected stage III CRC.
Patients and methods
Study cohort
The study protocols of the present investigation were approved by the review boards of the participating institutions and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. This prospective cohort study was based on the Nutrition and Lifestyle Study Cohort of Colorectal Cancer in China (NCT02215642). The study population consisted of a series of participants consecutively diagnosed with stage III colorectal cancer in four separate clinical centers—namely, the Fourth Military Medical University, Tianjin Union Medical Center, Beihua University, and Chengdu Medical University—between January 2004 and December 2008. All included participants had pathologically confirmed CRC and received surgical resection with curative intent, as well as effective 5-fluorouracil-based adjuvant chemotherapy. Participants with any history of cancer (other than skin cancer), non-adenocarcinoma, inflammatory bowel disease, as well as those who received either ineffective chemotherapy or no treatment, had inaccurate follow-up, had missing BMI data, or who withdrew from the survey were excluded. The final parent study cohort comprised 4,086 patients who met all of the inclusion criteria. An independent data review was performed for quality assurance, and all of the entered data were checked for accuracy by administrative research staff.
Assessment of BMI and tumor location
To ensure the consistency of BMI values, weight (in kilograms) and height (in meters) were measured and recorded by trained staff at the point of preoperative assessment for each patient. BMI was categorized according to the World Health Organization (WHO) classification for Asian populations as normal weight (18.5 kg/m2 ≤ BMI < 23.0 kg/m2), overweight (23.0 kg/m2 ≤ BMI < 27.5 kg/m2), or obese (BMI ≥ 27.5 kg/m2). Participants with a BMI of <18.5 kg/m2 were defined as underweight and excluded. Self-reported weight was also recorded at the same time of BMI measurement, and it was found to have a strong correlation of 0.92 (p < 0.001) with that measured by clinical staff. Therefore, weight loss was calculated by subtracting the patient’s self-reported 12-month presurgical weight from the weight measured for BMI.
Tumor location was identified from medical records. Tumors proximal to the splenic flexure, such as those occurring in the cecum, ascending colon, or transverse colon, were classified as right-sided tumors, while those distal to the splenic flexure, such as tumors occurring in the descending colon, sigmoid colon, or rectum, were classified as left-sided tumors (43). Baseline performance status was recorded according to the Eastern Cooperative Oncology Group Performance Status Scale.
Follow-up and clinical endpoints assessment
All participants were followed up every 3 months by telephone conversation with the participant or their first-degree relatives. For participants who died during follow-up, notice of the death was ascertained by reports from the family, and the details were verified by reviewing death certificates. The cause of death, which was assigned by physicians blinded to information regarding lifestyle exposure, was obtained from a formal medical file or an official death certificate. For the primary purpose, we included overall survival (OS) and disease-specific survival (DSS) as the clinical endpoints. OS was defined as the time that elapsed from surgery to the date of death from any cause. DSS was defined as the time that from surgery to death related to CRC, censored at the date of death resulting from postoperative complications or other nonmalignant causes.
Statistical analysis
Statistical analysis was conducted using SPSS statistical software (version 13.0). χ2 and Wilcoxon rank-sum tests were used to test for an association between the categorical variables of the different groups. The survival curves were determined using the Kaplan–Meier method, and differences in the survival distributions were evaluated using the log-rank test. The factors potentially related to survival were analyzed via Cox’s proportional hazards modeling to determine which of them might have had a significant influence on survival. To determine whether the tumor location and BMI modified outcomes, the categorical BMI, tumor location, and data on weight loss were included in the Cox model. Differences with a p-value of 0.05 or less were considered to be significant, and all of the p-values were determined using two-sided tests.
Results
Study cohort characteristics
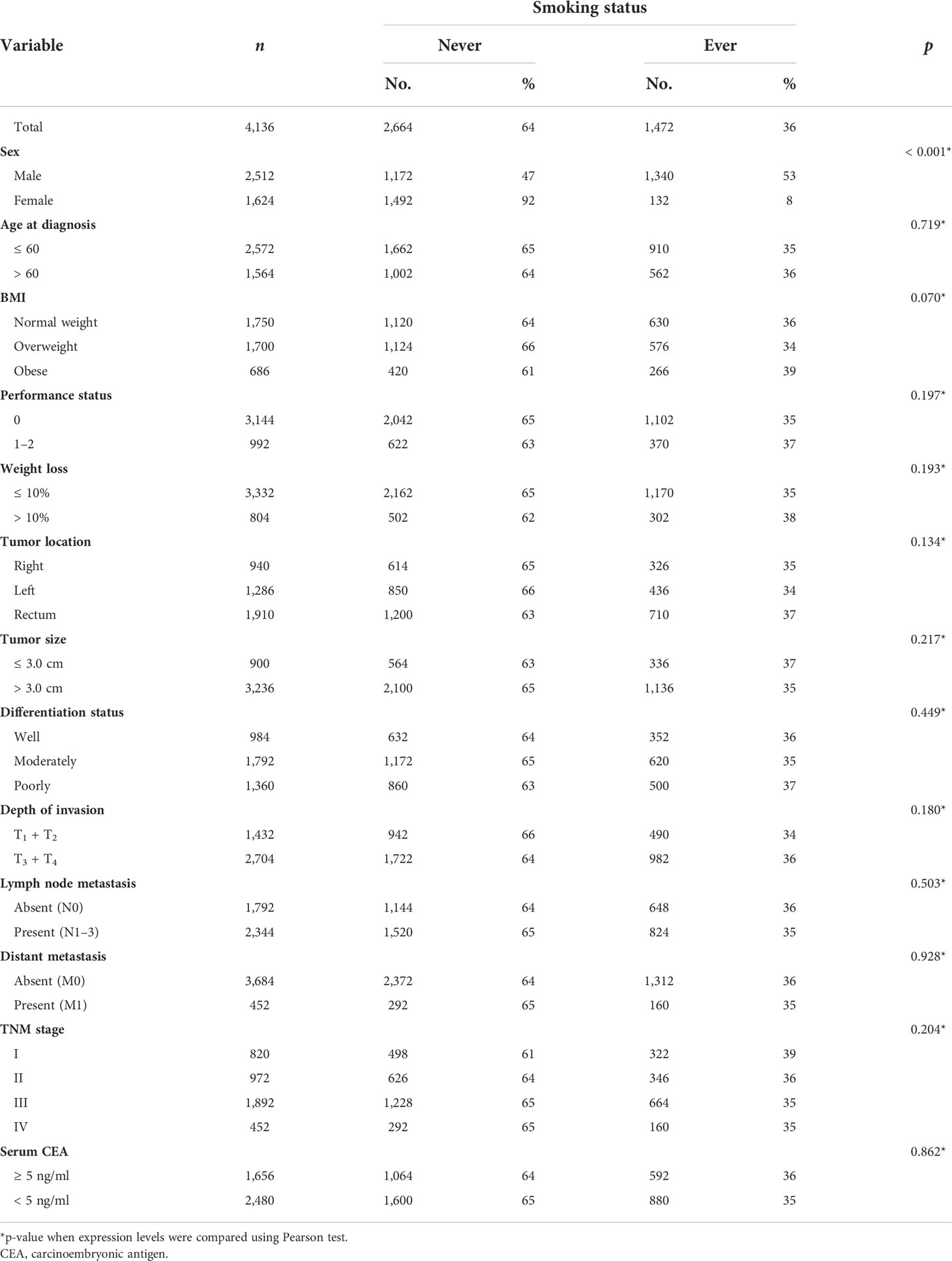
The baseline clinicopathological data for the study cohort is shown in Table 1. According to our predetermined BMI categories, 672 patients (16%) were obese, 1,691 patients (41%) were overweight, and 1,723 patients (42%) were of normal weight. Analysis of the baseline characteristics showed that a higher percentage of patients with right-sided tumors were female compared with those with left-sided tumors (42% vs. 38%; p = 0.004). Patients with right-sided tumors were significantly older than those with left-sided tumors (39% vs. 36% > 60 years old; p = 0.042). Patients with right-sided tumors were also less likely to be obese or overweight than those with left-sided tumors (15% vs. 17% and 40% vs. 42%, respectively; p = 0.007). In addition, right-sided tumors were also less likely to be well-differentiated than left-sided tumors (11% vs. 14%; p = 0.004). Although no significant prognostic difference was found among the obese, overweight, and normal weight groups (Figure 1A), statistical analysis revealed that the multiplicative interaction of BMI and tumor location was significant for OS (p = 0.001), indicating that the prognostic value of BMI differed according to tumor location. We therefore further investigated the association between BMI and OS/DSS within left-sided and right-sided tumors.
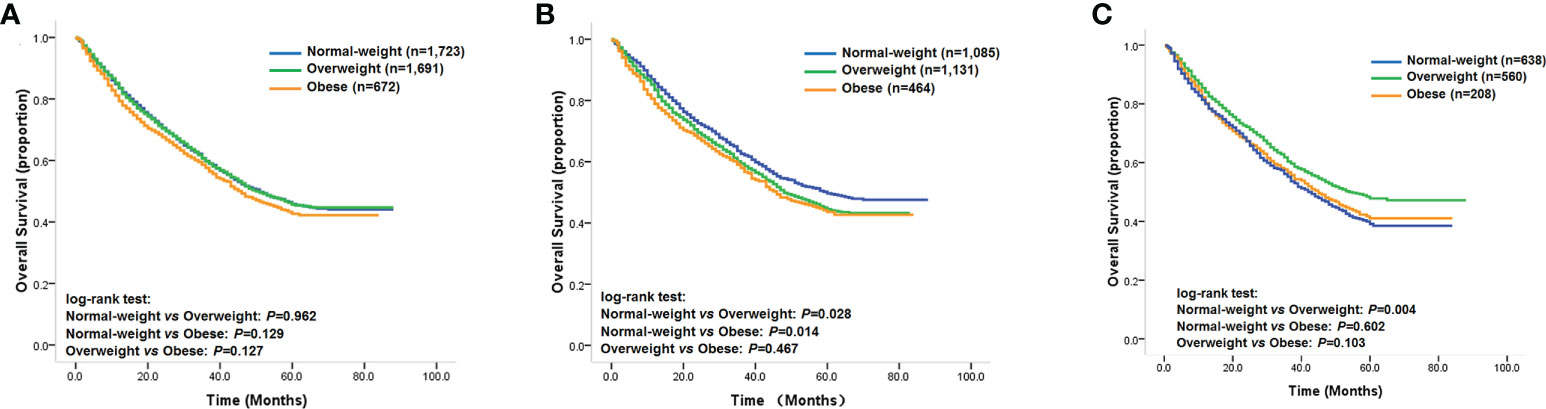
Figure 1 (A) Kaplan–Meier survival curves of the overall study cohort; (B) Kaplan–Meier survival curves of patients with left-sided tumors; (C) Kaplan–Meier survival curves of patients with right-sided tumors.
Prognostic value of BMI in left-sided tumors
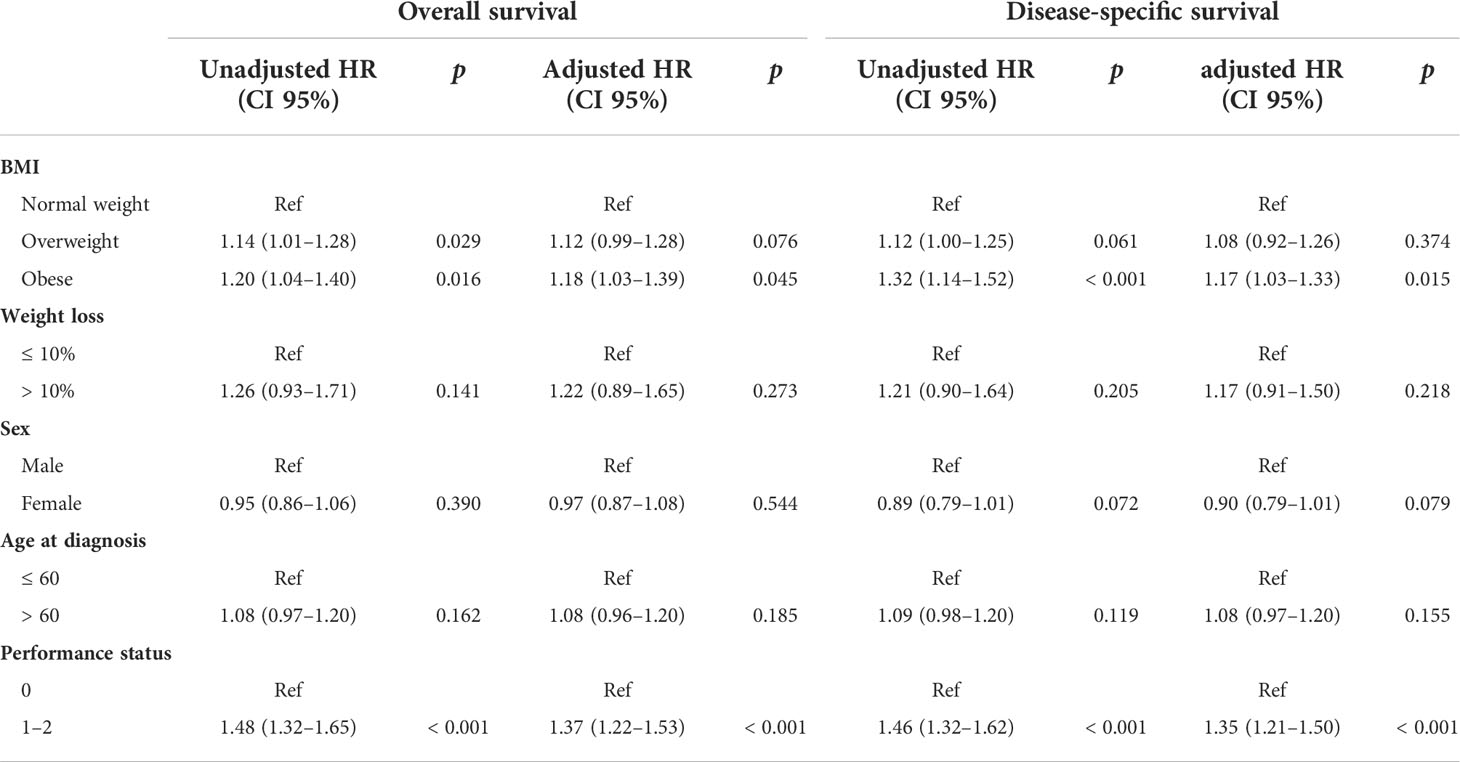
Among patients with left-sided tumors, Kaplan–Meier analysis showed that obese and overweight patients had worse OS compared with normal-weight patients, while no significant prognostic differentiation was found between obese and overweight patients (Figure 1B). The unadjusted hazard ratios (HR) for the overweight and obese patients with left-sided tumors were 1.14 (95% CI, 1.01–1.28; p=0.029) and 1.20 (95% CI, 1.04–1.40; p=0.016), respectively, compared to normal-weight patients. Next, we evaluated the effect of BMI on DSS status. The univariate analysis showed a similar trend as the OS analysis in that categorical BMI was significantly associated with DSS; i.e., patients with normal weight had favorable DSS compared with those who were overweight or obese (Table 2). The unadjusted HRs of DSS for overweight and obese patients relative to normal-weight patients were 1.12 (95% CI, 1.00–1.25; p=0.061) and 1.32 (95% CI, 1.14–1.52; p<0.001), respectively. Among other clinicopathological characteristics, performance status, differentiation status, depth of tumor invasion, node metastasis, and distant metastasis were found to be associated outcomes of left-sided tumors (Table 2, p<0.05).
To control for confounding, we used a Cox proportional hazards model adjusted for sex, age at diagnosis, performance status, differentiation status, and stage according to TNM classification. We found that only obesity was independently associated with worse OS and DSS compared with those of normal-weight patients. No statistically significant difference was detected between overweight and normal-weight patients. In addition, performance status was also found to be an independent prognostic factor (Table 2).
Prognostic value of BMI in right-sided tumors
We next investigated the prognostic value of BMI among patients with right-sided tumors. Interestingly, the Kaplan–Meier analysis revealed that overweight patients with right-sided tumors had better OS compared with that of normal-weight patients (Figure 1C), with an unadjusted HR of 0.80 (95% CI, 0.68–0.93; p = 0.004), while the univariate analysis did not find significant differences in OS for obese patients compared with normal-weight or overweight patients (Figure 1C). In contrast to the results for left-sided tumors, sex, weight loss, performance status, differentiation status, depth of invasion, node metastasis, and distant metastasis were found to be associated with clinical outcome (Table 3, p < 0.05). Further evaluation of the effect of BMI on DSS status in right-sided tumors found a similar survival pattern as that in the OS analysis in that overweight patients had better DSS compared with that of normal-weight patients (log-rank test: p = 0.001). Beyond the significant association between BMI and DSS, female patients were also found to have lower risk of DSS.
In the multivariate analysis, we utilized the Cox proportional-hazards regression models described in the analysis of left-sided tumors. The results showed that overweight status was significantly associated with prolonged OS and DSS compared with normal-weight status, indicating that overweight status might be a protective factor for patients with right-sided tumors (Table 3). However, the difference in OS and DSS between obese and normal-weight patients was not statistically significant (Table 3). In contrast to the results obtained for left-sided tumors, female sex and a lesser degree of weight loss were associated with better OS and DSS among right-sided tumors, and these associations reached statistical significance (Table 3).
Prognostic value of tumor location stratified by BMI
Our Kaplan–Meier analysis of the association between tumor location and OS showed that the prognostic difference between right- and left-sided tumors did not reach statistical significance (log-rank test: p=0.067, Figure 2A). However, as our statistical analysis revealed that the multiplicative interaction of BMI and tumor location was significant for OS, we further analyzed the impact of tumor location on outcomes according to patients’ BMI status in order to reveal how BMI could stratify the prognostic value of tumor location. Our results demonstrated that patients with right-sided tumors achieved statistically significantly unfavorable OS compared with patients with left-sided tumors (log-rank test: p < 0.001, Figure 2B), with an unadjusted HR of 1.31 (95% CI, 1.15–1.50; p < 0.001). Multivariable analysis also showed that primary tumor location was an independent prognostic factor for OS in normal-weight patients, with an adjusted HR for right-sided tumors of 1.28 (95% CI, 1.13–1.46; p < 0.001), while differences in DSS remained statistically significant in normal-weight patients, with unadjusted HR and adjusted HRs for right-sided tumors of 1.34 (95% CI, 1.19–1.58; p < 0.001) and 1.31 (95% CI, 1.16–1.51; p < 0.001), respectively, compared with left-sided tumors. However, a significantly prognostic difference between left- and right-sided tumors was detected in overweight and obese patients (Figures 2C, D). These results indicated that tumor location might have opposing effects on patients’ outcomes depending on BMI category.
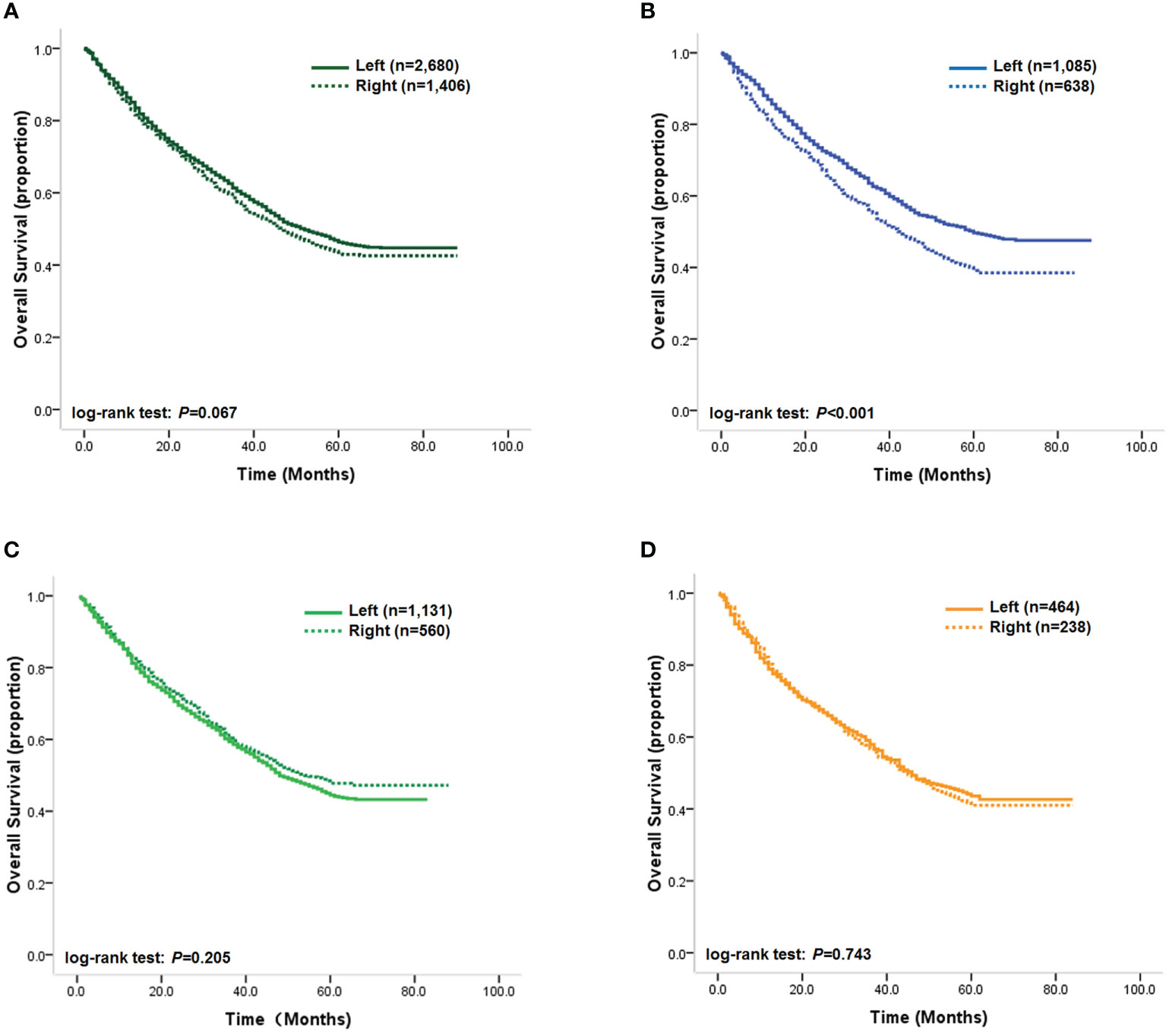
Figure 2 (A) Kaplan–Meier survival curves of the overall study cohort; (B) Kaplan–Meier survival curves of normal-weight patients; (C) Kaplan–Meier survival curves of overweight patients; (D) Kaplan–Meier survival curves of obese patients.
Discussion
As an area experiencing a rapid increase in CRC incidence, Asia has seen only a few published studies to date on the prognostic role of BMI on CRC, and these studies’ results have been confusingly discordant with investigations conducted in Western countries (46–49). Recently, tumor location in CRC has been associated with outcomes, although the limited investigations so far have shown conflicting results. This suggests that tumor location might be a powerful confounding factor that could modify both body weight and the risk of death. In addition, the potential impact of BMI on the survival of CRC patients could also be a confounding factor for the prognostic role of tumor location. Therefore, stratifying the analysis according to the tumor location and BMI would be an effective method of addressing the potential bias effect. However, previous work on CRC has omitted the prognostic effect of BMI might be stratified by tumor location, which would lack validity.
We therefore conducted this study to test the hypothesis that there might be interactions between BMI and tumor location in of the context of determining prognosis. We used a study cohort consisting of 4,086 patients from different areas of China. Our results showed that, in addition to an association between tumor location and sex, patients with right-sided tumors were more likely to be of normal weight, which is probably because right-sided tumors cause weight loss. Moreover, tumor location was also found to be associated with tumor differentiation status, in that right-sided tumors were more likely to be poorly differentiated. Although the overall prognosis did not differ according to BMI, our analysis showed a substantial stratifying effect of tumor location on the association of BMI with outcome. Specifically, obese patients with left-sided tumors had significantly unfavorable OS and DSS compared with those of normal-weight patients in both univariate and multivariate analysis, while overweight status was found to only be associated univariately with unfavorable OS in left-sided tumors. Interestingly, among patients with right-sided tumors, overweight patients were found to have more favorable outcomes in both univariate and multivariate analyses compared with normal-weight patients, while the outcome of obese patients with right-sided tumors was not significantly different from that of normal-weight patients. In addition, it was found that the significant association of outcome with sex and weight loss was limited to right-sided tumors. The results of our overall analysis for the total participant cohort are consistent with the CALGB study, which found no association of stage III CRC outcome with BMI status; however, we found statistically significant prognostic differences among BMI groups after we stratified the analysis by tumor location (31). Although the prognostic role of weight change is also different, the weight change in our study is presurgical, while that in the CALGB study took place after adjuvant chemotherapy. Considering the conflicting prognostic role of BMI represented in other studies, which did not stratify by tumor location, our results demonstrate that BMI could adversely impact tumor outcome in CRC patients according to the tumor location.
The specific mechanism by which tumor location stratifies the prognostic value of BMI in CRC might be quite complex. It is most likely related to the host–tumor interaction, which appears to modify tumor cell behavior and corresponding outcome. Increased BMI is associated with a chronic inflammatory state that may elevate circulating factors such as insulin, estrogen, insulin-like growth factor, steroid hormones, and leptin (50–52). These factors may promote a favorable microenvironment for tumor cell survival, proliferation, invasion, and metastasis, thereby heightening risks of tumor recurrence and death. In light of this reasoning, overweight and obesity can be understood to facilitate tumor progression and, hence, poorer outcomes. Although the impact of tumor location on CRC is still controversial, it can cause varying effects on weight loss.
Left- and right-sided tumors differ in their development, clinical presentation, environmental epidemiology, and sex distribution. In addition, recent investigations have found that left-sided tumors are frequently infiltrating, constricting lesions, with a phenotype that involves chromosomal instability, aneuploidy, KRAS, and p53 mutation (53–55). In contrast, right-sided tumors are more likely to be diploid and to be characterized by mucinous histology, high microsatellite instability, CpG island methylation, and BRAF mutations (56, 57). These differences between left- and right-sided tumors could harbor the prognostic value of BMI. In the present study, our results demonstrated that patients with right-sided tumors were more likely to be in a relatively low BMI category, further supporting this notion. In terms of the confounding impact of BMI on the prognostic value of tumor location, we also found that tumor location in patients with different BMI statuses might have opposing prognostic effects.
Our study has several strengths. Our cohort is the largest in Asia to date in the context of investigating prognostic role of BMI, and it is representative of the Chinese patient population. All patients received curative surgical resection and standard adjuvant chemotherapy, and the bias caused by adjuvant chemotherapy was excluded (58). BMI was measured at a uniform time point relative to surgery. In addition, the hospital-based cohort provided detailed data that allowed tumor recurrence and/or the details of the clinical variables to be investigated.
However, the present study had several limitations: we did not consider post-surgical weight loss; we only assessed obesity by BMI without considering other measures of body habitus, such as waist–hip ratio or waist circumference; and in the survival analysis, information related to BMI, such as lifestyle factors, was not accessed or adjusted.
Our findings extend the collective knowledge on the effects of BMI and tumor location beyond their known associations with CRC outcomes by showing that their prognostic role in CRC survivors is stratified by both BMI and tumor location. Although further study is needed to determine the exact mechanism of this stratifying effect, the present study is relevant to efforts to stratify prognostic predictions for CRC patients, with the goals of better management and improved outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was reviewed and approved by the Ethics Committee of Xi’an Jiaotong University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DC designed the experimental method and evaluated its feasibility. ZZ, XY, YL, and XG carried out the experiments. WW, BS, YZ, and QH collected the clinical and prognostic information. ZZ wrote the manuscript. DC revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 81201927, 81672460, 82002957, and 82173337) and the Shaanxi Innovative Talents Promotion Plan (grant numbers 2022TD-58 and 2020JM-391).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin (2013) 63:11–30. doi: 10.3322/caac.21166
2. Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin (2009) 59:366–78. doi: 10.3322/caac.20038
3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin (2011) 61:69–90. doi: 10.3322/caac.20107
4. Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev (2011) 20:1410–20. doi: 10.1158/1055-9965.EPI-11-0079
5. Eberth JM, Vernon SW, White A, Abotchie PN, Coan SP. Accuracy of self-reported reason for colorectal cancer testing. Cancer Epidemiol Biomarkers Prev (2010) 19:196–200. doi: 10.1158/1055-9965.EPI-09-0335
6. Cheskin LJ, Prosser BJ. Obesity and the risk of colon polyps. J Clin Gastroenterol (2007) 41:229–30. doi: 10.1097/01.mcg.0000248020.54256.b0
7. Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual report to the nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer (2012) 118:2338–66. doi: 10.1002/cncr.27514
8. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (2008) 371:569–78. doi: 10.1016/S0140-6736(08)60269-X
9. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer (1993) 54:594–606. doi: 10.1002/ijc.2910540413
10. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA (2012) 307:491–7. doi: 10.1001/jama.2012.39
11. Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer (2009) 125:171–80. doi: 10.1002/ijc.24343
12. Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr (2010) 92:471–90. doi: 10.3945/ajcn.2009.29005
13. Jacobs ET, Ahnen DJ, Ashbeck EL, Baron JA, Greenberg ER, Lance P, et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: a pooling study. Am J Epidemiol (2009) 169:657–66. doi: 10.1093/aje/kwn401
14. Matsuo K, Mizoue T, Tanaka K, Tsuji I, Sugawara Y, Sasazuki S, et al. Association between body mass index and the colorectal cancer risk in Japan: pooled analysis of population-based cohort studies in Japan. Ann Oncol (2012) 23:479–90. doi: 10.1093/annonc/mdr143
15. Sung JJ, Lau JY, Goh KL, Leung WK, Asia Pacific Working Group on Colorectal C. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol (2005) 6:871–6. doi: 10.1016/S1470-2045(05)70422-8
16. Sung JJ, Lau JY, Young GP, Sano Y, Chiu HM, Byeon JS, et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut (2008) 57:1166–76. doi: 10.1136/gut.2007.146316
17. Deng SX, Gao J, An W, Yin J, Cai QC, Yang H, et al. Colorectal cancer screening behavior and willingness: an outpatient survey in China. World J Gastroenterol (2011) 17:3133–9. doi: 10.3748/wjg.v17.i26.3133
18. Zhang S, Cui Y, Weng Z, Gong X, Chen M, Zhong B, et al. Changes on the disease pattern of primary colorectal cancers in southern China: a retrospective study of 20 years. Int J Colorectal Dis (2009) 24:943–9. doi: 10.1007/s00384-009-0726-y
19. Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol (2005) 11:4685–8. doi: 10.3748/wjg.v11.i30.4685
20. Meng W, Cai SR, Zhou L, Dong Q, Zheng S, Zhang SZ, et al. Performance value of high risk factors in colorectal cancer screening in China. World J Gastroenterol (2009) 15:6111–6. doi: 10.3748/wjg.15.6111
21. Odegaard AO, Koh WP, Yu MC, Yuan JM. Body mass index and risk of colorectal cancer in Chinese singaporeans: the Singapore Chinese health study. Cancer (2011) 117:3841–9. doi: 10.1002/cncr.25936
22. Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer (2010) 126:692–702. doi: 10.1002/ijc.24803
23. Oxentenko AS, Bardia A, Vierkant RA, Wang AH, Anderson KE, Campbell PT, et al. Body size and incident colorectal cancer: a prospective study of older women. Cancer Prev Res (Phila) (2010) 3:1608–20. doi: 10.1158/1940-6207.CAPR-10-0116
24. Hughes LA, Simons CC, van den Brandt PA, Goldbohm RA, van Engeland M, Weijenberg MP. Body size and colorectal cancer risk after 16.3 years of follow-up: an analysis from the Netherlands cohort study. Am J Epidemiol (2011) 174:1127–39. doi: 10.1093/aje/kwr247
25. Russo A, Franceschi S, La Vecchia C, Dal Maso L, Montella M, Conti E, et al. Body size and colorectal-cancer risk. Int J Cancer (1998) 78:161–5. doi: 10.1002/(SICI)1097-0215(19981005)78:2<161::AID-IJC7>3.0.CO;2-X
26. Adams KF, Leitzmann MF, Albanes D, Kipnis V, Mouw T, Hollenbeck A, et al. Body mass and colorectal cancer risk in the NIH-AARP cohort. Am J Epidemiol (2007) 166:36–45. doi: 10.1093/aje/kwm049
27. Campbell PT, Cotterchio M, Dicks E, Parfrey P, Gallinger S, McLaughlin JR. Excess body weight and colorectal cancer risk in Canada: associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev (2007) 16:1735–44. doi: 10.1158/1055-9965.EPI-06-1059
28. Giacosa A, Franceschi S, La Vecchia C, Favero A, Andreatta R. Energy intake, overweight, physical exercise and colorectal cancer risk. Eur J Cancer Prev (1999) 8 Suppl 1:S53–60. doi: 10.1097/00008469-199912001-00009
29. Nock NL, Thompson CL, Tucker TC, Berger NA, Li L. Associations between obesity and changes in adult BMI over time and colon cancer risk. Obes (Silver Spring) (2008) 16:1099–104. doi: 10.1038/oby.2008.42
30. Fanipakdel A HS, Javadinia SA, Afkhami Jeddi F, Vasei M. The prognostic role of body mass index in survival of non-metastatic postoperative patients with colorectal cancer. Int J Cancer Manag (2021) 14(3):e110257. doi: 10.5812/ijcm.110257
31. Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from cancer and leukemia group b 89803. J Clin Oncol (2008) 26:4109–15. doi: 10.1200/JCO.2007.15.6687
32. Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O'Connell MJ, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst (2006) 98:1647–54. doi: 10.1093/jnci/djj442
33. Prizment AE, Flood A, Anderson KE, Folsom AR. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa women's health study. Cancer Epidemiol Biomarkers Prev (2010) 19:2229–37. doi: 10.1158/1055-9965.EPI-10-0522
34. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
35. Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol (2000) 152:847–54. doi: 10.1093/aje/152.9.847
36. Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res (2010) 16:1884–93. doi: 10.1158/1078-0432.CCR-09-2636
37. Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst (2013) 105:1344–54. doi: 10.1093/jnci/djt223
38. Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control (2012) 23:1939–48. doi: 10.1007/s10552-012-0071-2
39. Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol (2008) 15:2388–94. doi: 10.1245/s10434-008-0015-y
40. Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum (2010) 53:57–64. doi: 10.1007/DCR.0b013e3181c703a4
41. Weiss JM, Pfau PR, O'Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results–Medicare data. J Clin Oncol (2011) 29:4401–9. doi: 10.1200/JCO.2011.36.4414
42. Brule SY, Jonker DJ, Karapetis CS, O'Callaghan CJ, Moore MJ, Wong R, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO. 17. Eur J Cancer (2015) 51:1405–14. doi: 10.1016/j.ejca.2015.03.015
43. Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst (2015) 107(3):dju427. doi: 10.1093/jnci/dju427
44. Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol (2015) 41:300–8. doi: 10.1016/j.ejso.2014.11.001
45. Nawa T, Kato J, Kawamoto H, Okada H, Yamamoto H, Kohno H, et al. Differences between right- and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenterol Hepatol (2008) 23:418–23. doi: 10.1111/j.1440-1746.2007.04923.x
46. Min YW, Kim SA, Lee JH, Kim JY, Chang DK, Rhee PL, et al. Overweight is associated with a favorable survival in patients with colorectal cancer: a prospective cohort study in an Asian population. Ann Surg Oncol (2012) 19:3460–4. doi: 10.1245/s10434-012-2436-x
47. Moon HG, Ju YT, Jeong CY, Jung EJ, Lee YJ, Hong SC, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol (2008) 15:1918–22. doi: 10.1245/s10434-008-9891-4
48. Yamamoto N, Fujii S, Sato T, Oshima T, Rino Y, Kunisaki C, et al. Impact of body mass index and visceral adiposity on outcomes in colorectal cancer. Asia Pac J Clin Oncol (2012) 8:337–45. doi: 10.1111/j.1743-7563.2011.01512.x
49. Liu D, Li Q, Yang Z, Hu X, Qian W, Du Y, et al. Association of body mass index and smoking on outcome of Chinese patients with colorectal cancer. World J Surg Oncol (2013) 11:271. doi: 10.1186/1477-7819-11-271
50. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr (2004) 92:347–55. doi: 10.1079/BJN20041213
51. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol (2005) 115:911–9. quiz 920. doi: 10.1016/j.jaci.2005.02.023
52. Conde J, Scotece M, Gomez R, Lopez V, Gomez-Reino JJ, Lago F, et al. Adipokines: biofactors from white adipose tissue. a complex hub among inflammation, metabolism, and immunity. Biofactors (2011) 37:413–20. doi: 10.1002/biof.185
53. Breivik J, Lothe RA, Meling GI, Rognum TO, Borresen-Dale AL, Gaudernack G. Different genetic pathways to proximal and distal colorectal cancer influenced by sex-related factors. Int J Cancer (1997) 74:664–9. doi: 10.1002/(SICI)1097-0215(19971219)74:6<664::AID-IJC18>3.0.CO;2-5
54. Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med (1990) 113:779–88. doi: 10.7326/0003-4819-113-10-779
55. Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. Colorectal cancer: a tale of two sides or a continuum? Gut (2012) 61:794–7. doi: 10.1136/gutjnl-2012-302014
56. Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer (2002) 101:403–8. doi: 10.1002/ijc.10635
57. Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol (2011) 29:1261–70. doi: 10.1200/JCO.2010.30.1366
Keywords: colorectal cancer, body mass index (BMI), tumor location, overall survival, disease specific survival (DSS)
Citation: Zhang Z, Yan X, Lu Y, Guo X, Jiao M, Wang W, Sun B, Zhou Y, Hu Q and Chu D (2022) The prognostic impact of BMI on colorectal cancer is stratified by tumor location. Front. Oncol. 12:987518. doi: 10.3389/fonc.2022.987518
Received: 06 July 2022; Accepted: 10 October 2022;
Published: 07 November 2022.
Edited by:
Haiqing Ma, Guangdong Provincial People’s Hospital, ChinaReviewed by:
Seyed Alireza Javadinia, Sabzevar University of Medical Sciences, IranMohamad Amin Pourhoseingholi, Shahid Beheshti University of Medical Sciences, Iran
Copyright © 2022 Zhang, Yan, Lu, Guo, Jiao, Wang, Sun, Zhou, Hu and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dake Chu, Y2h1ZGFrZUB4anR1LmVkdS5jbg==
 Zixi Zhang1
Zixi Zhang1 Dake Chu
Dake Chu