
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 23 August 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.984932
This article is part of the Research TopicLocal Ablative Therapies for The Management of Lung CancerView all 16 articles
Background: Thermal ablation (TA) is considered a safe alternative to surgical resection for the treatment of non-small cell lung cancer (NSCLC). While previous studies have shown that TA is beneficial for stage I NSCLC patients, however, few have reported on TA efficacy in patients with stage II-III NSCLC. The current study investigated the impact of TA on the overall survival (OS) and cancer-specific survival (CSS) of patients with stage II-III NSCLC.
Methods: Data on patients with stage II-III NSCLC who did not undergo surgical resection between 2004 and 2015 were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Propensity score matching (PSM), Kaplan-Meier survival curves, and Cox regression were used for statistical analyses.
Results: A total of 57,959 stage II-III NSCLC patients who did not undergo surgical resection were included in this study, 261 of whom received TA. Overall, TA was associated with a longer OS (p = 0.035) and CSS (p = 0.005) than non-ablation. After 1:3 PSM, 252 patients receiving TA and 732 patients not receiving ablation were enrolled in the matched cohort. The OS (p = 0.047) and CSS (p = 0.029) remained higher in the TA group than in the non-ablation group after PSM. Cox regression analysis showed that age, sex, primary tumor site, pathological type, tumor size, radiotherapy, chemotherapy, and thermal ablation were independently associated with OS and CSS (p <0.05). Subgroup analysis found that the advantages of TA were more pronounced among individuals ≥70 years of age, with tumor size ≤3.0 cm, or who did not receive radiotherapy.
Conclusion: TA could be an effective alternative treatment for stage II-III NSCLC patients unsuitable for surgical resection, particularly those ≥70 years of age, with tumor size ≤3.0 cm, or who have not received radiotherapy.
Lung cancer, one of the most prevalent and lethal malignancies worldwide, is a significant public health concern (1). In the United States, it is estimated that 235,760 new cases of lung cancer will be diagnosed in 2021, and 131,800 patients will die from the disease (2). Surgical resection is the preferred treatment for early-stage non-small cell lung cancer (NSCLC) (3, 4). However, a proportion of NSCLC patients are unsuitable for surgical resection for several reasons including advanced age, severe underlying disease, and refusal to undergo surgical intervention (5, 6). Thus, non-surgical treatment for NSCLC, including thermal ablation (TA) and stereotactic body radiotherapy (SBRT), has attracted increasing attention (7–11).
TA includes radiofrequency ablation (RFA), microwave ablation (MWA), and ultrasound ablation using various energy sources (12, 13). As a minimally invasive treatment technique, TA utilizes thermal energy to cause injuries to the targeted tissue (14). Of the ablation technologies in clinical use, RFA is the most widely used for the treatment of multiple conditions, including cardiac arrhythmias and liver, lung, kidney, and other tumors (12). MWA devices use higher frequency electromagnetic waves than RFA, allowing larger tissue volumes to be heated (13). Thermal injuries caused by TA may result in protein denaturation, enzyme inactivation, vascular injury, and ischemia-reperfusion, leading to irreversible cellular damage (15, 16). Previous studies indicate that TA is beneficial for stage I NSCLC patients (7, 17–19). Current guidelines from multiple societies specify that the best candidates for TA are stage I NSCLC patients who are not eligible for or amenable to surgical resection and SBRT (20, 21). However, few studies have reported on TA efficacy in patients with stage II-III NSCLC. Thus, it remains unknown whether inoperable stage II-III NSCLC patients may benefit from this technique.
According to the American Society of Clinical Oncology and Chinese Medical Association guidelines, inoperable stage III NSCLC patients should be offered concurrent chemoradiotherapy as a standard treatment (22, 23). Those who are not candidates for concurrent chemoradiation but are candidates for chemotherapy should be offered both sequential chemotherapy and radiation therapy. For inoperable stage II-III NSCLC patients who are unsuitable for chemoradiation, radiotherapy alone is the standard treatment with a survival benefit (23–25). The survival benefit of TA is comparable to SBRT in stage I NSCLC patients (26, 27). The current study performed a retrospective analysis to investigate whether inoperable stage II-III NSCLC patients can benefit from TA using patient data from the Surveillance, Epidemiology, and End Results (SEER) database.
Data were retrieved from the SEER 18 Registries Database (2000–2018). SEER is a national population-based registry program that collects the tumor-related clinical data and basic demographics of cancer patients (28). Since this database is publicly available and all records are de-identified, no ethics committee approval or informed consent is required.
Data from patients diagnosed with NSCLC during 2004–2015 were extracted from SEER. The third edition of the International Classification of Diseases for Oncology (ICD-O-3) was used to identify NSCLC. Patients were included in the study if they had a pathological diagnosis of NSCLC and their age at diagnosis was >18 years. Patients were excluded if (1) they received other surgical procedures beyond thermal ablation, or surgery information was unknown (2), their tumors were identified as TNM stage I or IV (3), they were identified in autopsy or death certificates (4), their survival data was unknown, or (5) information regarding age, sex, race, pathological type, primary tumor site, laterality, lymph node staging, marital status, or tumor size was missing.
The following information was extracted: year of diagnosis, age at diagnosis, sex, race, histological grade, pathological type, primary tumor site, laterality, lymph node staging, tumor size, radiotherapy, chemotherapy, surgery, marital status, cancer-specific survival (CSS), overall survival (OS), and survival months. TNM staging was reclassified according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging manual. Patients were divided into TA and non-ablation groups based on the treatment they received. The primary endpoints in this study were OS and CSS. CSS was defined as the time from diagnosis to death attributed to NSCLC.
In retrospective cohort studies, treatment-related selection bias resulting from an imbalance in the baseline characteristics is inevitable (29). Propensity score matching (PSM) can reduce the selection bias, offset differing clinical features among groups, and bolster the evidence of a retrospective cohort study (30). The current study created a logistic regression model with propensity scores to balance the baseline characteristics between the TA and non-ablation groups. TA was defined as the dependent variable, while other baseline characteristics were included as the covariables. The PSM was performed in a 1:3 ratio using nearest neighbor matching with a caliper of 0.001. Chi-square or Fisher’s exact tests were used to compare baseline characteristics between groups.
Analyses were performed using R version 4.0.0 and SPSS version 26.0. Kaplan-Meier methods and log-rank tests were used for survival analysis. Univariate and multivariable analyses were performed using Cox proportional hazards regression. Variables with p <0.10 from the univariable analysis were considered for multivariable analysis. Differences with p <0.05 were considered significant.
Data on 57,959 non-surgical patients who were diagnosed with stage II-III NSCLC during 2004–2015 were extracted from the SEER database. A total of 261 patients (0.45%) received TA and 57,698 patients (99.55%) did not. The baseline characteristics of the patients are shown in Table 1. There were significant differences in age (p = 0.003), race (p = 0.013), tumor site (p <0.001), pathological type (p <0.001), tumor size (p = 0.002), lymph node staging (p = 0.022) and chemotherapy (p = 0.003) between the TA and non-ablation groups in the unmatched cohort. After 1:3 PSM, 252 patients receiving TA and 732 patients without ablation were enrolled in the matched cohort. The baseline characteristics were well-balanced between the TA and non-ablation groups in the matched cohorts.
Kaplan‐Meier analysis revealed a significant difference in the OS of patients receiving TA and those who did not (p = 0.035) (Figure 1A). The median OS was 15 months (95% confidence interval (CI): 11.275–18.775) and 12 months (95% CI: 11.847–12.153) for the TA and non-ablation groups, respectively. The 3- and 5-year OS of the TA and non-ablation groups was 24.14% versus 18.82% and 14.39% versus 10.84%, respectively. Patients who received TA also had longer CSS than non-ablation patients (p = 0.005) (Figure 1B). After 1:3 matching, patients who received TA also had longer OS than non-ablation patients (p = 0.047) (Figure 1C). The median OS was 15 months (95% CI: 11.111–18.889) and 11 months (95% CI: 9.811–12.189) for the TA and non-ablation groups, respectively. The 3- and 5-year OS of the TA and non-ablation groups was 24.21% versus 19.51% and 14.98% versus 10.71%, respectively. CSS was also longer for patients in the TA group than in the non-ablation group (p = 0.029) (Figure 1D).
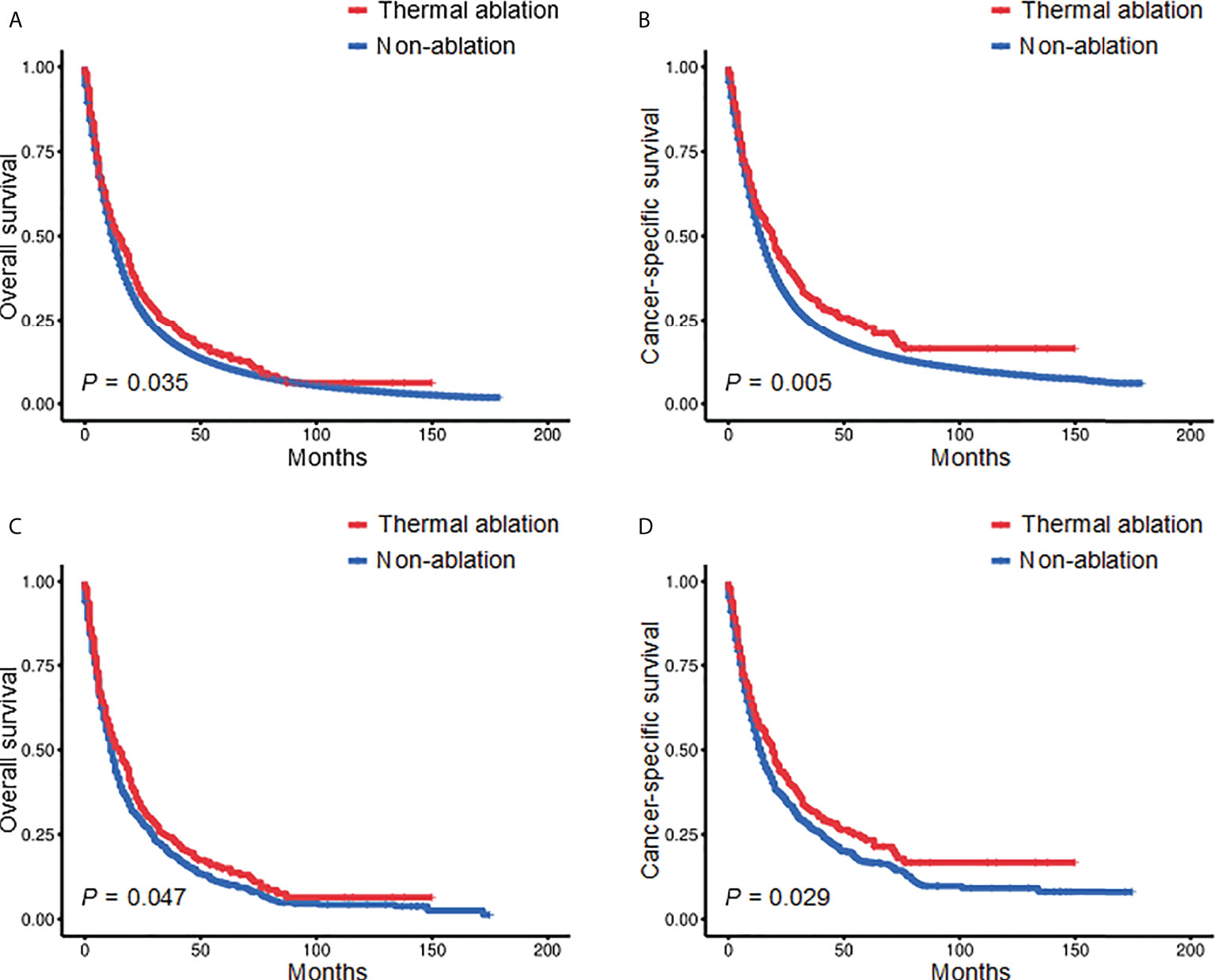
Figure 1 Kaplan-Meier survival curves of stage II-III NSCLC patients before (A, B) and after (C, D) PSM. NSCLC, non-small cell lung cancer; PSM, propensity score matching.
Univariate survival analysis showed that age, sex, race, primary tumor site, histological grade, pathological type, tumor size, radiotherapy, and chemotherapy were significantly associated with OS (p < 0.05) (Table 2). Variables with p <0.10 from the univariable analysis were considered for multivariable analysis. The multivariable analysis showed that age, sex, primary tumor site, pathological type, tumor size, radiotherapy, chemotherapy, and thermal ablation were independently associated with OS (p <0.05). Regarding the pathological type, squamous cell carcinoma served as a reference, and adenocarcinoma (hazard ratio (HR) = 0.81, 95% CI: 0.69–0.95, p = 0.009) was shown to be a favorable prognostic factor for OS. However, large cell lung cancer (HR = 1.10, 95% CI: 0.68–1.77, p = 0.703) and the other pathological types (HR = 1.00, 95% CI: 0.64–1.56, p = 0.997) were not statistically related to OS. Compared with tumor size ≤3.0 cm, tumor sizes of 5.1–7.0 cm (HR = 1.31, 95% CI: 1.07–1.60, p = 0.009) and >7.0 cm (HR = 1.91, 95% CI: 1.52–2.40, p <0.001) were adverse prognostic factors for OS, and tumor sizes of 3.1–5.0 cm (HR = 1.12, 95% CI: 0.94–1.33, p = 0.203) did not significantly impact OS. The multivariable analysis of CSS showed that age, sex, primary tumor site, pathological type, tumor size, lymph node staging, radiotherapy, chemotherapy, and TA were independently associated with CSS (p <0.05) (Table 3). Overall, TA was associated with longer OS (p = 0.005) and CSS (p = 0.020).
Kaplan-Meier was used to conduct subgroup analyses stratified by age and tumor size. When age was <70 years, there was no significant difference in the OS and CSS of the TA and non-ablation groups. The 3- and 5-year OS of the TA and non-ablation groups was 22.48% versus 25.95% and 17.40% versus 16.09%, respectively (p = 0.718, Figure 2A). The 3- and 5-year CSS of the TA and non-ablation groups was 29.07% versus 32.10% and 22.60% versus 22.51%, respectively (p = 0.760, Figure 2B). When age was ≥70 years, the TA group had longer OS and CSS than the non-ablation group. The 3- and 5-year OS of the TA and non-ablation groups was 26.02% versus 13.30% and 12.29% versus 5.61%, respectively (p = 0.001, Figure 2C). The 3- and 5-year CSS of the TA and non-ablation groups was 34.59% versus 20.82% and 23.84% versus 10.79%, respectively (p <0.001, Figure 2D).
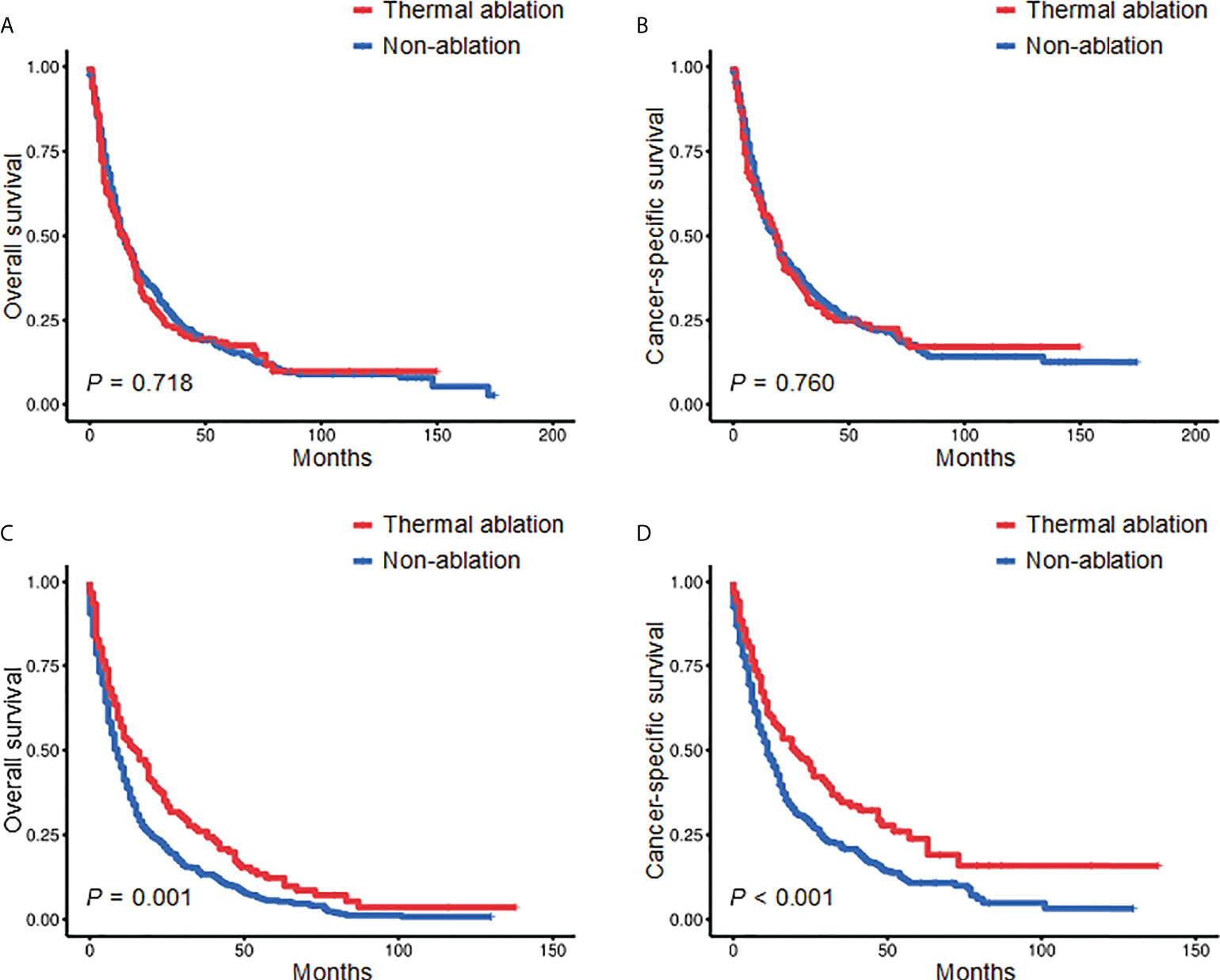
Figure 2 Kaplan-Meier survival curves comparing the TA and non-ablation groups when age was <70 years (A, B) or ≥70 years (C, D) after PSM. TA, thermal ablation; PSM, propensity score matching.
In patients with tumor size ≤3.0 cm, the 3- and 5-year OS of the TA and non-ablation groups was 34.44% versus 17.87% and 18.86% versus 8.89%, respectively (p = 0.001, Figure 3A). The 3- and 5-year CSS of the TA and non-ablation groups was 46.55% versus 29.85% and 32.35% versus 17.06%, respectively (p = 0.002, Figure 3B). However, no significant difference was observed in the OS and CSS of the TA and non-ablation groups for tumor sizes of 3.1–5.0 cm (3-year OS: 22.97% versus 24.77%, p = 0.803, Figure 3C; 3-year CSS: 29.57% versus 30.65%, p = 0.716, Figure 3D), 5.1–7.0 cm (3-year OS: 18.75% versus 18.99%, p = 0.672, Figure 3E; 3-year CSS: 22.12% versus 23.76%, p = 0.721, Figure 3F), and >7.0 cm (3-year OS: 10.00% versus 13.64%, p = 0.920, Figure 3G; 3-year CSS: 15.12% versus 15.98%, p = 0.884, Figure 3H).
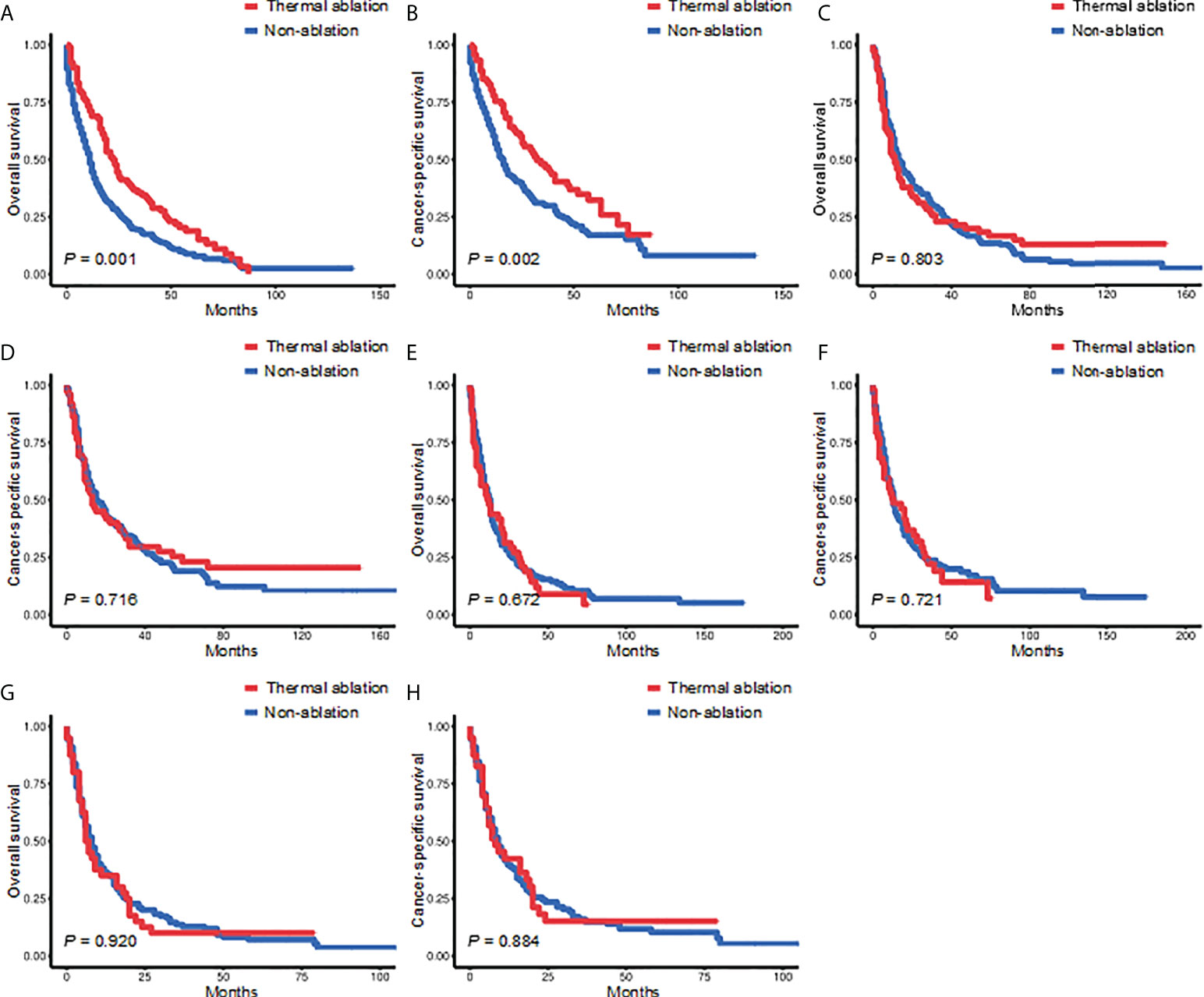
Figure 3 Kaplan-Meier survival curves for the TA and non-ablation groups when tumor size was ≤3.0 cm (A, B), 3.1-5.0 cm (C, D), 5.1-7.0 cm (E, F), or >7.0 cm (G, H) after PSM. TA, thermal ablation; PSM, propensity score matching.
Cox proportional hazard analyses were performed to explore the survival benefit of TA for patients in different subgroups. TA improved the OS of patients who were ≥70 years of age (HR = 0.693, 95% CI: 0.560–0.857, p <0.001) or white (HR = 0.841, 95% CI: 0.715–0.989, p = 0.037) and had right laterality (HR = 0.813, 95% CI: 0.670–0.987, p = 0.037), lung lobes (HR = 0.845, 95% CI: 0.716–0.998, p = 0.047), adenocarcinoma (HR = 0.743, 95% CI: 0.555–0.994, p = 0.046), tumor size ≤3.0 cm (HR = 0.647, 95% CI: 0.501–0.834, p = 0.001), N0 staging (HR = 0.708, 95% CI: 0.547–0.916, p = 0.009), non-radiotherapy (HR = 0.642, 95% CI: 0.508–0.812, p <0.001) and non-chemotherapy (HR = 0.704, 95% CI: 0.573–0.864, p = 0.001) (Figure 4). For CSS, subgroup analysis stratified by age, sex, race, histological grade, tumor site, laterality, lymph node staging, tumor size, radiotherapy, chemotherapy, and marital status gave similar results (Figure 5). A statistically improved OS (p = 0.046) but not CSS (p = 0.060) was observed in patients with TA when the pathology type was adenocarcinoma.
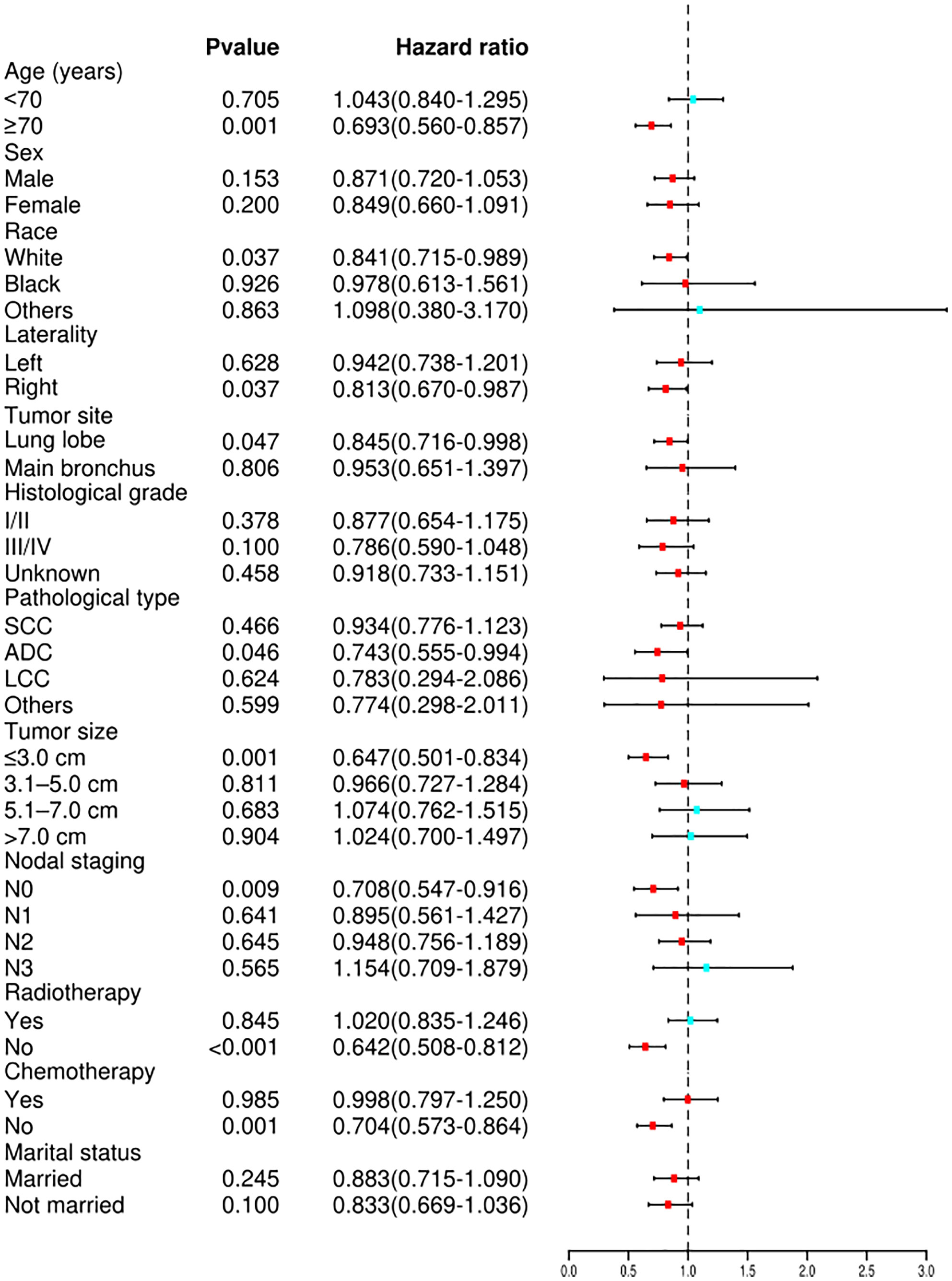
Figure 4 Forest plot depicting subgroup analysis of the OS between the TA and non-ablation groups after PSM. SCC, squamous cell carcinoma; ADC, adenocarcinoma; LCC, large cell carcinoma; OS, overall survival; TA, thermal ablation; PSM, propensity score matching.
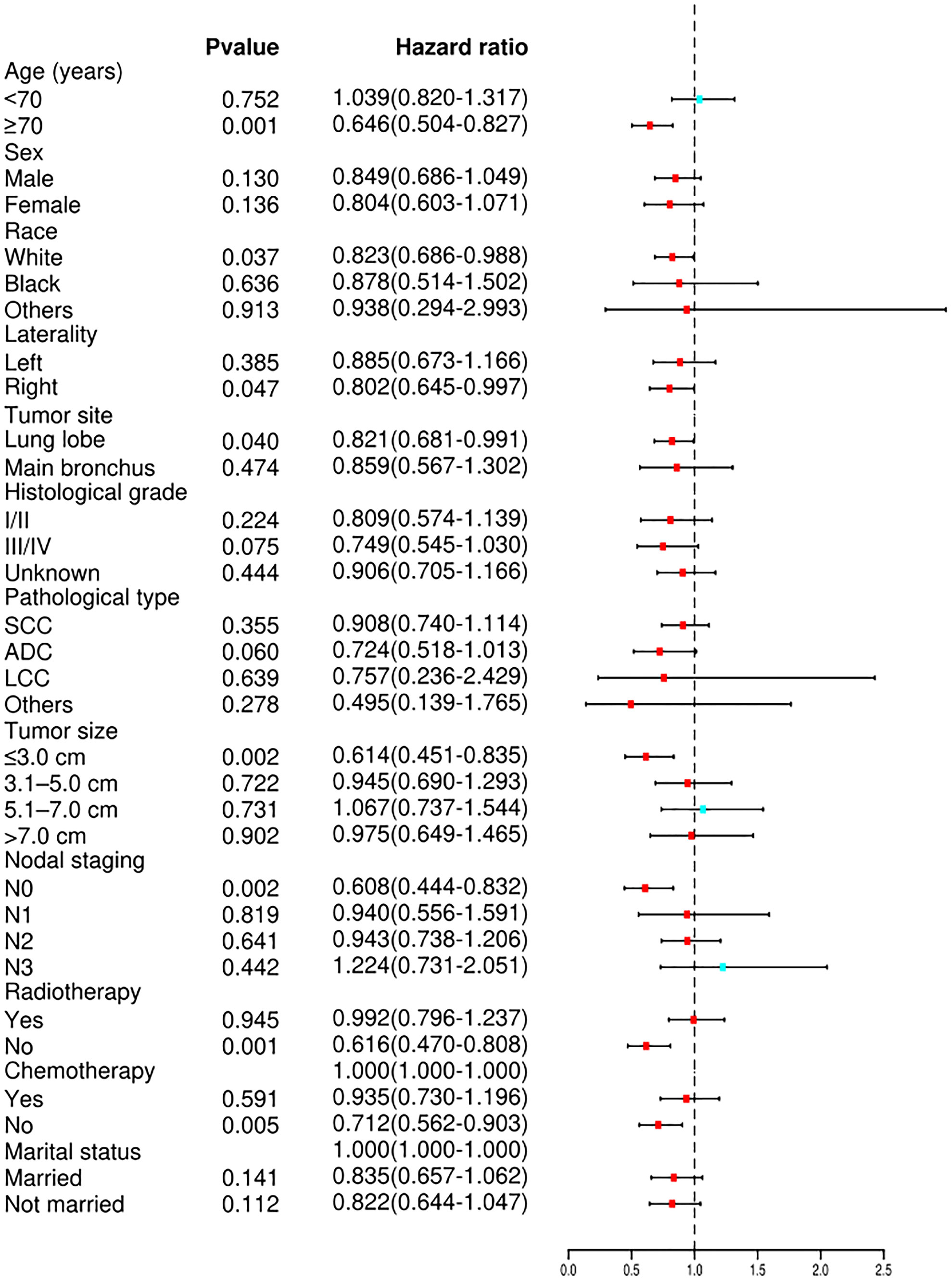
Figure 5 Forest plot depicting subgroup analysis of the CSS between the TA and non-ablation groups after PSM. SCC, squamous cell carcinoma; ADC, adenocarcinoma; LCC, large cell carcinoma; CSS, cancer-specific survival; TA, thermal ablation; PSM, propensity score matching.
Data on 57,959 stage II-III NSCLC patients who were unsuitable for surgical resection were extracted from the SEER database. The findings revealed that TA improved the OS and CSS of stage II-III NSCLC patients who did not undergo surgical resection, particularly for those ≥70 years of age, with tumor size ≤3.0 cm, or who did not receive radiotherapy.
TA is considered a safe treatment for primary lung cancer (7, 20, 21). As an alternative therapy to surgical resection, TA causes irreversible damage to tumor cells using thermal energy (14, 31, 32). While previous studies have shown that TA is beneficial for stage I NSCLC patients (7, 18, 33), however, there is minimal data on the use of TA in stage II-III NSCLC patients. The current study found that TA could significantly improve the OS and CSS of stage II-III NSCLC patients without surgical resection. However, the effect of differences in clinicopathological features, such as age, race, tumor site, pathological type, tumor size, lymph node staging, and chemotherapy, cannot be overlooked. After PSM, baseline characteristics were similar between the TA and non-ablation groups in the adjusted cohorts and patients with TA still had longer OS and CSS than those without. This finding is consistent with results from a study by Heon et al. (34) which retrospectively evaluated the efficacy of CT-guided RFA in 77 NSCLC patients. Among stage I-II NSCLC patients, the median OS for those receiving RFA alone was 28.2 months, which was similar to patients receiving surgery alone (p >0.05). For stage III-IV NSCLC patients who received chemotherapy, the median OS of those receiving RFA was longer than the OS of those with no ablation (42 months versus 29 months, p = 0.03).
Multivariate Cox regression analysis showed that age, sex, tumor site, pathological type, tumor size, radiotherapy, chemotherapy, and thermal ablation were independently associated with the OS and CSS of stage II-III NSCLC patients without surgical resection. Subgroup analysis stratified by age showed that TA improved the OS and CSS of individuals ≥70 years of age with stage II-III NSCLC. When age was <70 years, no significant difference in OS or CSS was observed between the TA and non-ablation groups. These results were similar to a study on stage I NSCLC patients (35). Zeng et al. compared the efficacy of TA and wedge resection and found that the OS and CSS of stage I NSCLC patients who received TA and wedge resection were comparable for individuals >75 years of age (p >0.05). However, the OS and CSS were significantly shorter for patients who received TA than those who received wedge resection among individuals ≤75 years of age (p <0.05). This may be because older patients have more medical comorbidities and poorer performance status. As a minimally invasive treatment technique, TA benefits elderly patients by significantly decreasing the likelihood of complications (36, 37).
There was a statistically significant difference in the OS and CSS of patients receiving TA and those without when tumor size was ≤3.0 cm. However, when tumor size was >3.0 cm, no significant differences in OS and CSS were observed between the groups. Xu et al. retrospectively evaluated the efficacy of MWA in 234 NSCLC patients (38). The median OS was 35 months for patients with tumor size <3.0 cm and only 16 months for patients with tumor size ≥3.0 cm (p <0.001). This may be because of the limited amount of tumor necrosis caused by TA. The occurrence of incomplete ablation for patients with large tumors may increase the risk of leaving tumor remnants and disease recurrence.
Cox proportional hazard analyses were performed to distinguish between several groups that benefitted more from TA. TA improved the OS and CSS of patients who were white or had right laterality, lung lobes, N0 staging, non-radiotherapy, and non-chemotherapy (p <0.05). Steber et al. reported the long-term outcomes from a prospective single-arm, phase 2 study of 13 inoperable NSCLC patients receiving combined RFA and external beam radiotherapy (EBRT) (39). The median progression-free survival of patients with combined RFA and EBRT was 37.8 months, similar to the survival of patients who received EBRT alone. Thus, RFA combined with EBRT was not recommended for patients with NSCLC.
No statistically significant differences were observed in the OS and CSS of patients receiving TA or no ablation among those receiving chemotherapy. The advantage of TA was more pronounced in patients without chemotherapy (p <0.05). Wei et al. (40) reported that the median OS of Stage III-IV lung adenocarcinoma patients was not significantly different between groups receiving MWA combined with chemotherapy or chemotherapy alone (23.9 months versus 17.3 months, p = 0.140). A study of 49 NSCLC patients by Li et al. (41) also showed no statistical difference in 3‐year OS between those receiving MWA and chemotherapy or chemotherapy alone (21.057 months versus 17.843 months, p >0.05). In contrast, a study of 256 NSCLC patients by Xu et al. showed that the median OS was longer for patients with CT-guided RFA in combination with systemic chemotherapy than for those receiving chemotherapy alone (17.5 months versus 13.4 months, p <0.05) (42). Another retrospective study of 66 NSCLC patients reported by Feng et al. showed that patients who received MWA in combination with systemic chemotherapy had a longer median OS than those who received systemic chemotherapy alone (289.0 days versus 190.0 days, p = 0.018) (43). Overall, the effects of TA in combination with chemotherapy on OS and CSS remain unclear. Additional prospective multicenter randomized controlled trials are needed to explore the efficacy of TA in combination with chemotherapy in NSCLC patients.
Several factors may contribute to the survival benefits of TA among stage II-III NSCLC patients. First, TA can cause direct thermal injuries to targeted tissues. Temperatures exceeding 60°C can cause cellular membrane lysis, protein denaturation, and enzyme inactivation, which lead to rapid coagulative necrosis (15, 44). Second, in the tumor periphery beyond the border of immediate tissue coagulation, TA can cause indirect thermal injuries, including vascular injury, ischemia-reperfusion, release of lysosomal contents (16, 45, 46). Finally, TA may result in the release of abundant immunogenic intracellular substrates, which can initiate and upregulate steps in the cancer immunity cycle required to elicit an anti-cancer immune response (47, 48).
The current study has several limitations. First, there was a lack of detail in the SEER database on targeted therapy, immunotherapy, and the results of genetic testing. Targeted therapies can inhibit the protein products of aberrant genes. Currently, oncogenic aberrations in eight genes (EGFR, ALK, ROS1, BRAF, KRAS, NTRK, MET, and RET) are approved therapies for NSCLC (49). Immunotherapies have also proven efficacious in NSCLC patients (50). However, the status of targeted therapy or immunotherapy and the results of genetic testing were not evaluated as variables in this study so the effects of these factors on prognosis could not be explored. Second, the type of TA modality may impact the prognosis. However, the SEER database was unable to differentiate between TA modalities (e.g., RFA, MWA, and ultrasound ablation), so this information could not be included. Third, a large proportion of cases in the SEER database had no detailed information on tumor extension, such as the description of various organ invasions, so factors regarding the sith TNM stage could not be accurately transformed into the eighth TNM stage (subgroup, IIa, IIb, IIIa, IIIb, and IIIc) in stage II-III patients. Thus, stage II-III patients were not further stratified by stage, which may further bias the results. Finally, this study was retrospective so the strength of the results is weaker than it is for randomized controlled trials or prospective studies. Selection bias may be present because only patients with complete information were included.
In summary, TA could be an effective alternative treatment for stage II-III NSCLC patients unsuitable for surgical resection, particularly those ≥70 years of age, with tumor size ≤3.0 cm, or who did not receive radiotherapy.
Publicly available data were analyzed in this study. The data can be found here: https://seer.cancer.gov/.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
W-YY and FY designed the study. W-YY and YH collected the data. W-YY, QH, and MP analyzed and interpreted the data. W-YY, ZZ, and SX drafted the manuscript. MP and FY proofread the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant number 81972195), the Scientific Research Program of Hunan Provincial Health Commission (grant number 20201047), the Clinical Medical Technology Innovation Guide Project of Hunan Province (grant number 2020SK53408), and the National Clinical Key Specialty Construction Project.
The authors thank all the staff and participants of the SEER program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Dai C, Shen J, Ren Y, Zhong S, Zheng H, He J, et al. Choice of surgical procedure for patients with non-Small-Cell lung cancer ≤ 1 Cm or > 1 to 2 Cm among lobectomy, segmentectomy, and wedge resection: A population-based study. J Clin Oncol (2016) 34(26):3175–82. doi: 10.1200/jco.2015.64.6729
4. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-Small-Cell lung cancer (Jcog0802/Wjog4607l): A multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet (London England) (2022) 399(10335):1607–17. doi: 10.1016/s0140-6736(21)02333-3
5. Okami J, Higashiyama M, Asamura H, Goya T, Koshiishi Y, Sohara Y, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: Prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol (2009) 4(10):1247–53. doi: 10.1097/JTO.0b013e3181ae285d
6. Qiang G, Liang C, Guo Y, Shi B, Tian Y, Song Z, et al. Video-assisted thoracoscopic lobectomy for elderly nonsmall cell lung cancer: Short-term and long-term outcomes. J Cancer Res Ther (2015) 11(4):793–7. doi: 10.4103/0973-1482.140930
7. Uhlig J, Ludwig JM, Goldberg SB, Chiang A, Blasberg JD, Kim HS. Survival rates after thermal ablation versus stereotactic radiation therapy for stage 1 non-small cell lung cancer: A national cancer database study. Radiology (2018) 289(3):862–70. doi: 10.1148/radiol.2018180979
8. Ni Y, Huang G, Yang X, Ye X, Li X, Feng Q, et al. Microwave ablation treatment for medically inoperable stage I non-small cell lung cancers: Long-term results. Eur Radiol (2022) 32(8):5616–22. doi: 10.1007/s00330-022-08615-8
9. Iezzi R, Cioni R, Basile D, Tosoratti N, Posa A, Busso M, et al. Standardizing percutaneous microwave ablation in the treatment of lung tumors: A prospective multicenter trial (Malt study). Eur Radiol (2021) 31(4):2173–82. doi: 10.1007/s00330-020-07299-2
10. Lindberg K, Grozman V, Karlsson K, Lindberg S, Lax I, Wersäll P, et al. The hilus-trial-a prospective Nordic multicenter phase 2 study of ultracentral lung tumors treated with stereotactic body radiotherapy. J Thorac Oncol (2021) 16(7):1200–10. doi: 10.1016/j.jtho.2021.03.019
11. Detillon D, Driessen EJM, Aarts MJ, Janssen-Heijnen MLG, van Eijck CHJ, Veen EJ. Changes in treatment patterns and survival in elderly patients with stage I non-Small-Cell lung cancer with the introduction of stereotactic body radiotherapy and video-assisted thoracic surgery. Eur J Cancer (Oxford Engl 1990) (2018) 101:30–7. doi: 10.1016/j.ejca.2018.06.016
12. Olive G, Yung R, Marshall H, Fong KM. Alternative methods for local ablation-interventional pulmonology: A narrative review. Trans Lung Cancer Res (2021) 10(7):3432–45. doi: 10.21037/tlcr-20-1185
13. Fallahi H, Prakash P. Antenna designs for microwave tissue ablation. Crit Rev Biomed Eng (2018) 46(6):495–521. doi: 10.1615/CritRevBiomedEng.2018028554
14. Maxwell AWP, Park WKC, Baird GL, Martin DW, Lombardo KA, Dupuy DE. Effects of a thermal accelerant gel on microwave ablation zone volumes in lung: A porcine study. Radiology (2019) 291(2):504–10. doi: 10.1148/radiol.2019181652
15. Huang L, Yang S, Bai M, Lin Y, Chen X, Li G, et al. Thermal shielding performance of self-healing hydrogel in tumor thermal ablation. Colloids surfaces B Biointerfaces (2022) 213:112382. doi: 10.1016/j.colsurfb.2022.112382
16. Bianchi L, Cavarzan F, Ciampitti L, Cremonesi M, Grilli F, Saccomandi P. Thermophysical and mechanical properties of biological tissues as a function of temperature: A systematic literature review. Int J hyperthermia (2022) 39(1):297–340. doi: 10.1080/02656736.2022.2028908
17. Chan MV, Huo YR, Cao C, Ridley L. Survival outcomes for surgical resection versus ct-guided percutaneous ablation for stage I non-small cell lung cancer (Nsclc): A systematic review and meta-analysis. Eur Radiol (2021) 31(7):5421–33. doi: 10.1007/s00330-020-07634-7
18. Ambrogi MC, Fanucchi O, Dini P, Melfi F, Davini F, Lucchi M, et al. Wedge resection and radiofrequency ablation for stage I nonsmall cell lung cancer. Eur Respir J (2015) 45(4):1089–97. doi: 10.1183/09031936.00188014
19. Hiraki T, Gobara H, Mimura H, Matsui Y, Toyooka S, Kanazawa S. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg (2011) 142(1):24–30. doi: 10.1016/j.jtcvs.2011.02.036
20. Venturini M, Cariati M, Marra P, Masala S, Pereira PL, Carrafiello G. Cirse standards of practice on thermal ablation of primary and secondary lung tumours. Cardiovasc interventional Radiol (2020) 43(5):667–83. doi: 10.1007/s00270-020-02432-6
21. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-Small-Cell lung cancer (Nsclc): Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28(suppl_4):iv1–iv21. doi: 10.1093/annonc/mdx222
22. Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J, et al. Management of stage iii non-Small-Cell lung cancer: Asco guideline. J Clin Oncol (2022) 40(12):1356–84. doi: 10.1200/jco.21.02528
23. Oncology Society of Chinese Medical AssociationChinese Medical Association Publishing House (2022). Chinese medical association guideline for clinical diagnosis and treatment of lung cancer (2022 Eds). Zhonghua Zhong Liu Za Zhi [Chinese J Oncol] 44(6):457–90. doi: 10.3760/cma.j.cn112152-20220413-00255
24. Dawe DE, Christiansen D, Swaminath A, Ellis PM, Rothney J, Rabbani R, et al. Chemoradiotherapy versus radiotherapy alone in elderly patients with stage iii non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer (Amsterdam Netherlands) (2016) 99:180–5. doi: 10.1016/j.lungcan.2016.07.016
25. Sigel K, Lurslurchachai L, Bonomi M, Mhango G, Bergamo C, Kale M, et al. Effectiveness of radiation therapy alone for elderly patients with unresected stage iii non-small cell lung cancer. Lung Cancer (Amsterdam Netherlands) (2013) 82(2):266–70. doi: 10.1016/j.lungcan.2013.06.011
26. Li M, Xu X, Qin Y, Zhang P, Shen C, Xia Q, et al. Radiofrequency ablation vs. stereotactic body radiotherapy for stage ia non-small cell lung cancer in nonsurgical patients. J Cancer (2021) 12(10):3057–66. doi: 10.7150/jca.51413
27. Uhlig J, Mehta S, Case MD, Dhanasopon A, Blasberg J, Homer RJ, et al. Effectiveness of thermal ablation and stereotactic radiotherapy based on stage I lung cancer histology. J Vasc interventional Radiol (2021) 32(7):1022–8.e4. doi: 10.1016/j.jvir.2021.02.025
28. Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G, et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin (2021) 71(5):381–406. doi: 10.3322/caac.21693
29. Lim YJ, Kim Y, Kong M. Comparative survival analysis of preoperative and postoperative radiotherapy in stage ii-iii rectal cancer on the basis of long-term population data. Sci Rep (2018) 8(1):17153. doi: 10.1038/s41598-018-35493-2
30. Li X, An B, Ma J, He B, Qi J, Wang W, et al. Prognostic value of the tumor size in resectable colorectal cancer with different primary locations: A retrospective study with the propensity score matching. J Cancer (2019) 10(2):313–22. doi: 10.7150/jca.26882
31. Kyrylenko S, Gogotsi O, Baginskiy I, Balitskyi V, Zahorodna V, Husak Y, et al. Mxene-assisted ablation of cells with a pulsed near-infrared laser. ACS Appl Materials Interfaces (2022) 14(25):28683–96. doi: 10.1021/acsami.2c08678
32. Chen Y, Huang H, Li Y, Xiao W, Liu Y, Chen R, et al. Tigit blockade exerts synergistic effects on microwave ablation against cancer. Front Immunol (2022) 13:832230. doi: 10.3389/fimmu.2022.832230
33. Ambrogi MC, Fanucchi O, Cioni R, Dini P, De Liperi A, Cappelli C, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: A prospective intention-to-Treat study. J Thorac Oncol (2011) 6(12):2044–51. doi: 10.1097/JTO.0b013e31822d538d
34. Lee H, Jin GY, Han YM, Chung GH, Lee YC, Kwon KS, et al. Comparison of survival rate in primary non-Small-Cell lung cancer among elderly patients treated with radiofrequency ablation, surgery, or chemotherapy. Cardiovasc interventional Radiol (2012) 35(2):343–50. doi: 10.1007/s00270-011-0194-y
35. Zeng C, Lu J, Tian Y, Fu X. Thermal ablation versus wedge resection for stage I non-small cell lung cancer based on the eighth edition of the tnm classification: A population study of the us seer database. Front Oncol (2020) 10:571684. doi: 10.3389/fonc.2020.571684
36. Gironés R, Torregrosa D, Gómez-Codina J, Maestu I, Tenias JM, Rosell R. Prognostic impact of comorbidity in elderly lung cancer patients: Use and comparison of two scores. Lung Cancer (Amsterdam Netherlands) (2011) 72(1):108–13. doi: 10.1016/j.lungcan.2010.07.001
37. Yang H, Li M, Mei T. Survival benefit of thermal ablation combined with chemotherapy for the treatment of stage iv nonsmall cell lung cancer: A propensity-matched analysis. Int J hyperthermia (2022) 39(1):348–57. doi: 10.1080/02656736.2022.2038281
38. Xu S, Qi J, Li B, Li XG. Survival prediction for non-small cell lung cancer patients treated with ct-guided microwave ablation: Development of a prognostic nomogram. Int J hyperthermia (2021) 38(1):640–9. doi: 10.1080/02656736.2021.1914353
39. Steber CR, Hughes RT, Urbanic J, Clark H, Petty WJ, Blackstock AW, et al. Long-term outcomes from a phase 2 trial of radiofrequency ablation combined with external beam radiation therapy for patients with inoperable non-small cell lung cancer. Int J Radiat oncology biology Phys (2021) 111(1):152–6. doi: 10.1016/j.ijrobp.2021.04.020
40. Wei Z, Ye X, Yang X, Huang G, Li W, Wang J, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol (Northwood London England) (2015) 32(2):464. doi: 10.1007/s12032-014-0464-z
41. Li C, Wang J, Shao JB, Zhu LM, Sun ZG, Zhang N. Microwave ablation combined with chemotherapy improved progression free survival of iv stage lung adenocarcinoma patients compared with chemotherapy alone. Thorac Cancer (2019) 10(7):1628–35. doi: 10.1111/1759-7714.13129
42. Xu F, Cai Z, Xu B, Song J, Liu H, Li X, et al. Clinical research on the combined use of systemic chemotherapy and ct-guided radiofrequency ablation in treatment of lung cancer. Lasers Med Sci (2022) 37(1):233–9. doi: 10.1007/s10103-020-03222-9
43. Feng K, Lu Y. Clinical analysis of systemic chemotherapy combined with microwave ablation in the treatment of lung cancer. Asian J Surg (2022) 45(5):1107–12. doi: 10.1016/j.asjsur.2021.08.013
44. Geoghegan R, Ter Haar G, Nightingale K, Marks L, Natarajan S. Methods of monitoring thermal ablation of soft tissue tumors - a comprehensive review. Med Phys (2022) 49(2):769–91. doi: 10.1002/mp.15439
45. Huang XW, Nie F, Wa ZC, Hu HT, Huang QX, Guo HL, et al. Thermal field distributions of ablative experiments using cyst-mimicking phantoms: Comparison of microwave and radiofrequency ablation. Acad Radiol (2018) 25(5):636–42. doi: 10.1016/j.acra.2017.11.010
46. Kok HP, Cressman ENK, Ceelen W, Brace CL, Ivkov R, Grüll H, et al. Heating technology for malignant tumors: A review. Int J hyperthermia (2020) 37(1):711–41. doi: 10.1080/02656736.2020.1779357
47. Rangamuwa K, Leong T, Weeden C, Asselin-Labat ML, Bozinovski S, Christie M, et al. Thermal ablation in non-small cell lung cancer: A review of treatment modalities and the evidence for combination with immune checkpoint inhibitors. Trans Lung Cancer Res (2021) 10(6):2842–57. doi: 10.21037/tlcr-20-1075
48. Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology (2009) 251(1):58–66. doi: 10.1148/radiol.2511072175
49. Rivera-Concepcion J, Uprety D, Adjei AA. Challenges in the use of targeted therapies in non-small cell lung cancer. Cancer Res Treat (2022) 54(2):315–29. doi: 10.4143/crt.2022.078
Keywords: non-small cell lung carcinoma, thermal ablation, survival, stage II-III, SEER
Citation: Yang WY, He Y, Hu Q, Peng M, Zhang Z, Xie S and Yu F (2022) Survival benefit of thermal ablation therapy for patients with stage II-III non-small cell lung cancer: A propensity-matched analysis. Front. Oncol. 12:984932. doi: 10.3389/fonc.2022.984932
Received: 02 July 2022; Accepted: 03 August 2022;
Published: 23 August 2022.
Edited by:
Xin Ye, Qianfoshan Hospital, Shandong University, ChinaReviewed by:
Zhonglun Mai, Southern Medical University Cancer Center, ChinaCopyright © 2022 Yang, He, Hu, Peng, Zhang, Xie and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenglei Yu, eXVmZW5nbGVpQGNzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.