- 1Department of Breast Surgery, Shanghai Cancer Center, Fudan University, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Human Phenome Institute, Fudan University, Shanghai, China
Background: Neoadjuvant chemotherapy (NAC) has evolved significantly and has been widely accepted for downstaging disease in early-stage and locally advanced breast cancer patients. Since the optimal surgical intervention for patients receiving NAC remains controversial, we aim to investigate the survival outcome of patients treated with different surgical management.
Methods: A retrospective, nested case-control study was conducted in patients with invasive breast cancer that underwent NAC at Fudan University Shanghai Cancer Center from January 2010 to June 2019. Based on surgical intervention, patients were divided into mastectomy and breast conservation groups. Patients were matched on age at diagnosis, menopausal status, the year of the surgery, post neoadjuvant therapy pathological tumor (ypT) stage, post neoadjuvant therapy pathological node (ypN) stage, molecular subtypes, and axillary surgery by propensity score matching.
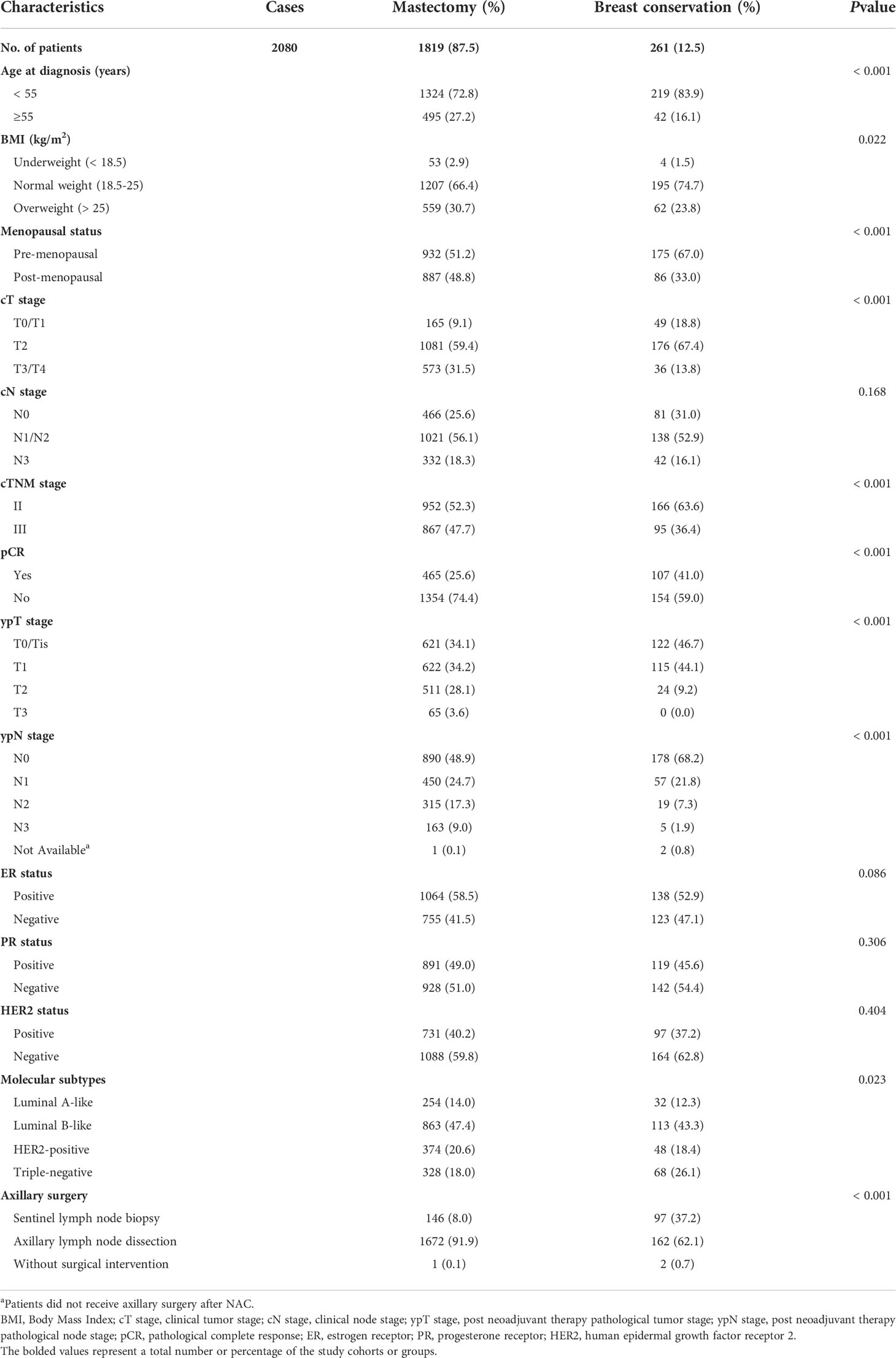
Results: A total of 2080 patients were enrolled in this study. Among them, 1819 (87.5%) patients were categorized as mastectomy group, and 261 (12.5%) patients were classed as breast conservation group. Over 9-years of research, the proportion of breast conservation steadily increased in patients after NAC. Data showed that younger (P<0.001) and pre-menopausal (P<0.001) patients with normal BMI (P=0.022) were more likely to receive breast conservation. Patients at advanced ypT stage (P<0.001), ypN stage (P<0.001), and clinical TNM stage (P<0.001) were more often to undergo mastectomy, while breast conservation rate was significantly higher in patients with triple-negative tumors (P=0.023). Compared with the mastectomy group, significant benefits in overall survival were observed in patients who received breast conservation (Hazard ratio 0.41, [95% confidence interval: 0.18-0.97]; p=0.049) in the matched cohort. There was no statistical difference between groups related to disease-free survival and locoregional recurrence.
Conclusions: Tumor biology can significantly impact the surgical decision in patients administrated with NAC. Breast conservation was a safe alternative for mastectomy in the NAC setting without compromising survival outcomes and locoregional control.
Introduction
Neoadjuvant chemotherapy (NAC) was initially applicated to locally advanced breast cancer in the 1970s (1). Since NAC could effectively downstage tumor burden in breast and axilla and allow an early evaluation of systematic therapy, it is increasingly used with extended indications for early-stage breast cancer (2, 3). With the evolvement modern of drugs and targeted therapy, dramatic improvement in rates of pathological complete response (pCR) was observed in patients receiving NAC, which was positively correlated with improved overall survival and disease-free survival (4, 5). Based on the National Cancer Database (NCDB) data, the overall proportion of NAC uses significantly increased from 15.7 to 26.0% from 2010 to 2015 for all subtypes, especially in triple-negative and HER2-positive tumors (6).
Although previous studies indicated that patients received NAC showed no difference in survival outcomes and locoregional recurrence rate compared with those who received adjuvant chemotherapy, it may contribute to a de-escalation of local treatment, which was repeatedly raised in the present therapeutic pattern (7). Thereby, NAC brought a shift of surgical management towards a less extensive paradigm in both axilla and breast surgery (8). According to the data from two early randomized studies, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-27 and the European Organization Research and Treatment of Cancer (EORTC) Trial 10902, the rate of breast-conserving surgery (BCS) was suggested increase up to 59.8% and 23% following NAC in patients initially recommended with mastectomy (9, 10). A meta-analysis enrolled in 14 prospective researches also suggested that NAC was correlated with a definite reduction in mastectomy rate by 16.6% (11).
Since the main purpose of breast-conserving surgery was to only remove tumors without resecting normal breast tissue, patients treated with BCS were proven to have favorable cosmetic outcomes, breast satisfaction, and quality of life. Moreover, BCS was identified with equivalent survival outcomes compared with mastectomy, even as the preferred treatment in early-stage breast cancer (12–14). Whereas, considering the difficulties in the clinical and radiologic assessment of residual tumors after NAC, accurate tumor size and localization were hard to accomplish. Based on previous studies, the NSABP B-18, B-27, and the EORTC 10902, BCS after NAC was correlated with a higher risk of locoregional disease (9, 10). However, a large retrospective study involving 6134 patients with operable or locally advanced breast cancer receiving NAC suggested that breast conservation could be a safe alternation of mastectomy in terms of local recurrence-free survival, even in patients with multifocal tumors (15). Therefore, whether those advantages of BCS could be maintained remains controversial in the NAC setting.
Additionally, regarding that oncoplastic surgical techniques were increasingly accepted in patients with breast cancer, the application of oncoplastic approaches in patients treated with NAC also gained a lot of attention. For patients who were not feasible for BCS, nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM) with immediate breast reconstruction (IBR) may be an alternative to conventional mastectomy, which identified with the improvement of aesthetic results and quality of life (16). From the perspective of breast conservation, oncoplastic breast-conserving surgery (OBCS) emerged as a surrogate option for conventional breast-conserving surgery with larger excised volume, lower margin involvement, and comparable cosmetic outcomes (17). Considerable amounts of studies focused on the feasibility of oncoplastic surgical interventions in patients without primary chemotherapy, whereas little was conducted to evaluate their oncological safety in the NAC settings.
Therefore, to address the optimal surgical intervention after NAC and influence factors for patient selection, we conducted this retrospective study to give an overview of the present therapeutic pattern of breast surgery, and compare the survival outcomes among different surgical groups in this matched case-control study.
Materials and methods
Inclusion and exclusion criteria of patients
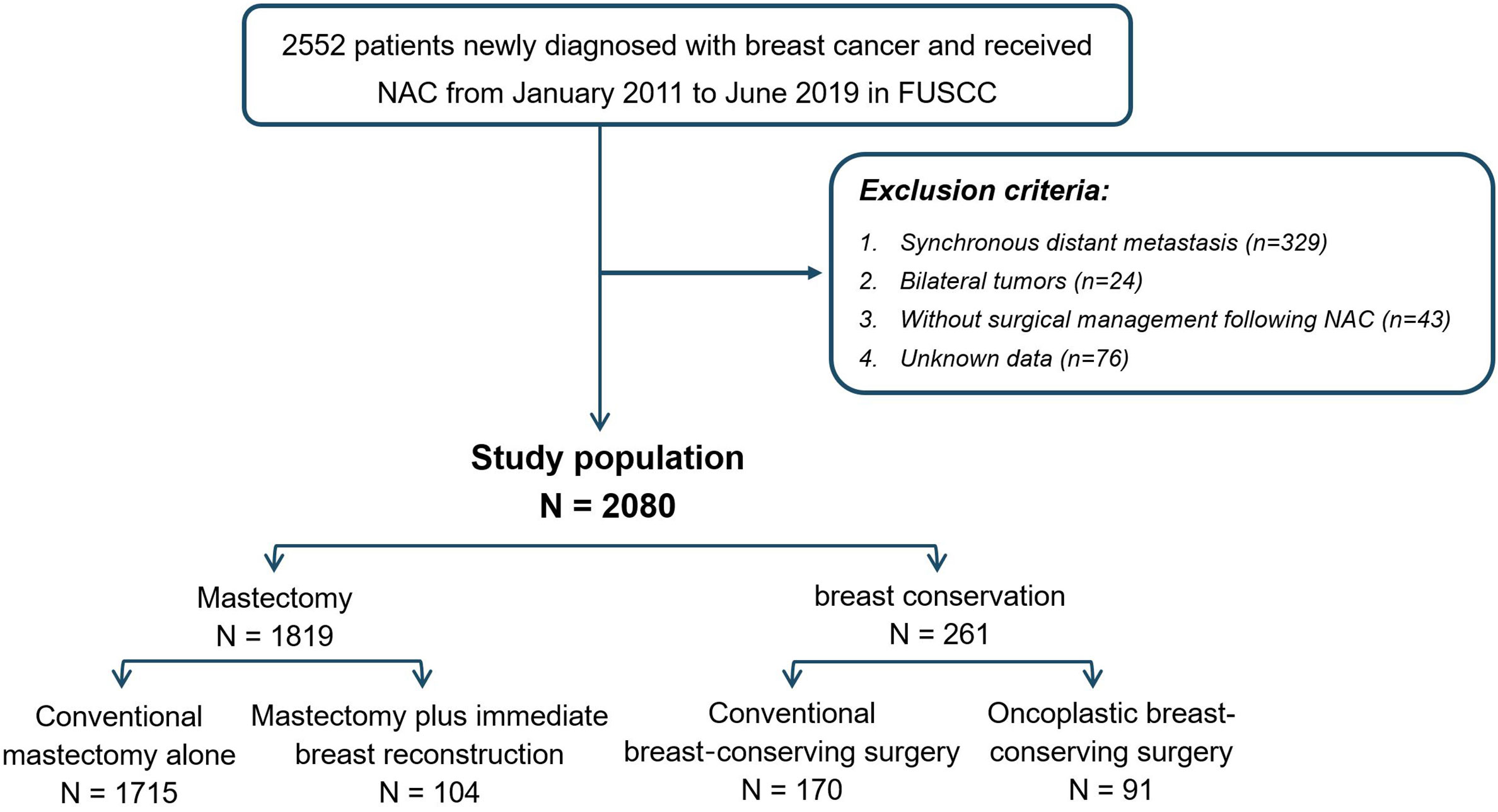
A total of 2552 female patients diagnosed with primary invasive breast cancer receiving neoadjuvant chemotherapy between January 2010 and June 2019 at Fudan University Shanghai Cancer Center (FUSCC) were enrolled. After excluding patients with distant metastasis (n=329), bilateral tumor (n=24), missing assessment of tumor immunohistology (n=76), and without surgical intervention following neoadjuvant chemotherapy (n=43), 2080 patients met the criteria and served as the study population. Based on surgical intervention performed after NAC, patients were divided into mastectomy (n=1819) and breast conservation groups (n=261). To be specific, the mastectomy group involved patients who received conventional mastectomy (CM) or mastectomy plus immediate breast reconstruction (M+IBR), while the breast conservation group evolved patients who underwent conventional breast-conserving surgery (CBCS) and oncoplastic breast-conserving surgery (OBCS). Surgical interventions were performed by highly qualified breast surgeons who had a long period of professional training in oncoplastic surgery. All the oncoplastic breast-conserving surgeries involved in this study were accomplished by volume displacement approach, which consisted of extent lumpectomy and using the remaining breast tissue to remedy the deformation resulting from tumor resection. In addition, M+IBR surgeries were accomplished by nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM) combined with immediate breast reconstruction (IBR). NSM was conducted under the condition that patients without nipple involvement clinically pre-operation, and the retro-areolar frozen-section specimens were confirmed to be tumor-free intra-operation.
Data extraction
Demographic, clinical, pathological, and treatment information were retrospectively collected by medical record review. All the patients were followed up through outpatient interviews or telephone calls. The survival status of patients was extracted from the Department of Clinical Statistics of FUSCC based on the medical records or telephone follow-up records. Overall survival (OS) was measured from the date of starting NAC to the date of the last follow-up records or death from any causes. Disease-free survival (DFS) was calculated from the date of starting NAC to the date of first locoregional recurrence, metastatic relapse, or death. Local regional recurrence (LRR) was defined from the date of starting NAC to the date of first locoregional recurrence (relapse at ipsilateral breast or chest wall, in ipsilateral axillary, supra- and/or infra-clavicular, and/or internal mammary lymph nodes).
Clinicopathological characteristics
All the patients receiving NAC were pathological proven primary invasive breast cancer by core-needle biopsy, supplemented by mammography, breast and axillary ultrasound, and magnetic resonance imaging (MRI). The selection criteria for NAC treatment was patients diagnosed with stage II-III breast cancer, while some patients subsequently identified with synchronous distant metastasis were excluded in the current study. The NAC regimens were administrated based on National Comprehensive Cancer Network Guidelines (NCCN) by the same team of treating oncologists, which generally included, taxanes or anthracycline combined with cyclophosphamide, taxanes combined with anthracycline and cyclophosphamide, and taxanes combined with platinum. Patients diagnosed with HER2-positive received HER2-targeted therapy in addition to chemotherapy. Since the radiological methods for residual tumor assessment were not unified earlier time in the study, response assessment was accomplished by pathological diagnosis after surgery. The radiotherapy following surgery depended on the surgical choice and pathological diagnosis after surgery. Endocrine therapy was offered to patients with estrogen receptor-positive or progesterone receptor-positive tumors. All of the pathological variables were determined according to the same guidelines at any time point. The staging of the tumor and axilla were categorized by the eighth edition of the American Joint Committee on Cancer (AJCC) Tumor Node Metastasis (TNM) stage criterion. Clinical T (cT) and N (cN) stages were evaluated by physical examination or imaging techniques (ultrasound, mammography, and magnetic resonance imaging) supplemented by pathological measurements. The clinical prognostic (cTNM) stage was applied to patients staging before NAC treatment. Hormone receptor (HR) status, including estrogen receptor (ER) and progesterone receptor (PR), were identified using immunohistochemistry (IHC). Human epidermal growth factor receptor-2 (HER2) positivity was defined as IHC 3+ or amplification by fluorescence in situ hybridization (FISH). Accordingly, tumors were divided into four molecular subtypes: (I) the luminal A-like subtype (HR-positive, HER2-negative, and Ki-67 ≤ 20%); (II) the luminal B-like subtype (HR-positive and HER2-positive; or HR-positive, HER2-negative, and Ki-67>20%); (III) the HER2-positive subtype (HER2-positive and HR-negative); (IV) the triple-negative subtype (negative ER, PR, and HER2). Pathologic complete response (pCR) is defined as the presence of in situ cancer after treatment in the absence of residual invasive disease in the breast, while in absence of tumor cells (invasive or in situ) in the axilla. Negative margins for breast-conserving surgery were defined as no ink on the tumor.
Statistical analysis
The chi-square test and Fisher’s exact test were used to compare the demographic characteristics and clinicopathological data among groups. To minimize the bias caused by differences in clinical features and variations in treatments throughout time, we employed 1:1 propensity score matching (PSM) without replacement to balance the significant variables between groups. Patients were chosen and matched based on the age at diagnosis, menopausal status, the year of the surgery (Patients were divided into five groups based on the year of surgery, from 2010 to 2011, from 2012 to 2013, from 2014 to 2015, from 2016 to 2017, and from 2018 to 2019), post-neoadjuvant therapy pathological tumor (ypT) stage, post-neoadjuvant therapy pathological node (ypN) stage, molecular subtypes, and axillary surgery. Overall survival (OS), disease-free survival (DFS), and locoregional recurrence (LRR) were analyzed by Kaplan-Meier analysis and compared with the log-rank test. 5-year OS, 5-year DFS, and 5-year LRR were measured according to the description of Greenwood’s variance estimated by Anderson et al. (18). The hazard ratio (HR) and 95% confidence interval (95%CI) were estimated using the Cox proportional hazards regression model. A p-value <0.05 was considered statistically significant. All of the statistical analysis was carried out with IBM SPSS, version 27.0.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research has been approved by the Medical Ethics Committee of Fudan University Shanghai Cancer Center (1905202-7). Informed consent has been waived by the ethics committee as this retrospective research poses no risk to patients.
Results
Trends of surgical intervention in patients who underwent NAC
Among 2552 patients diagnosed with primary invasive breast cancer and received neoadjuvant chemotherapy (NAC), 2080 patients were involved in this study according to the inclusion and exclusion criteria. Among them, 1819 (87.5%) patients were categorized as mastectomy group, and 261 (12.5%) patients were classed as breast conservation group. In the mastectomy group, patients who underwent conventional mastectomy (CM) and M+IBR accounted for 82.5% (1715/2080) and 5% (104/2080) patients respectively. As for breast conservation, 170 (8.2%) and 91 (4.4%) patients were treated with CBCS and OBCS after NAC (Figure 1). Data showed that the proportion of breast conservation gradually increased in patients treated with NAC over the 9-years of research. Notably, the research cohort also suggested a growing prevalence of M+IBR from 1.26% to 8.70%, while fewer patients opted for CM-alone as surgical intervention after NAC. Moreover, the percentage of patients undergoing OBCS increased from 0.42% to 7.60% throughout the 9 years (Figure 2).
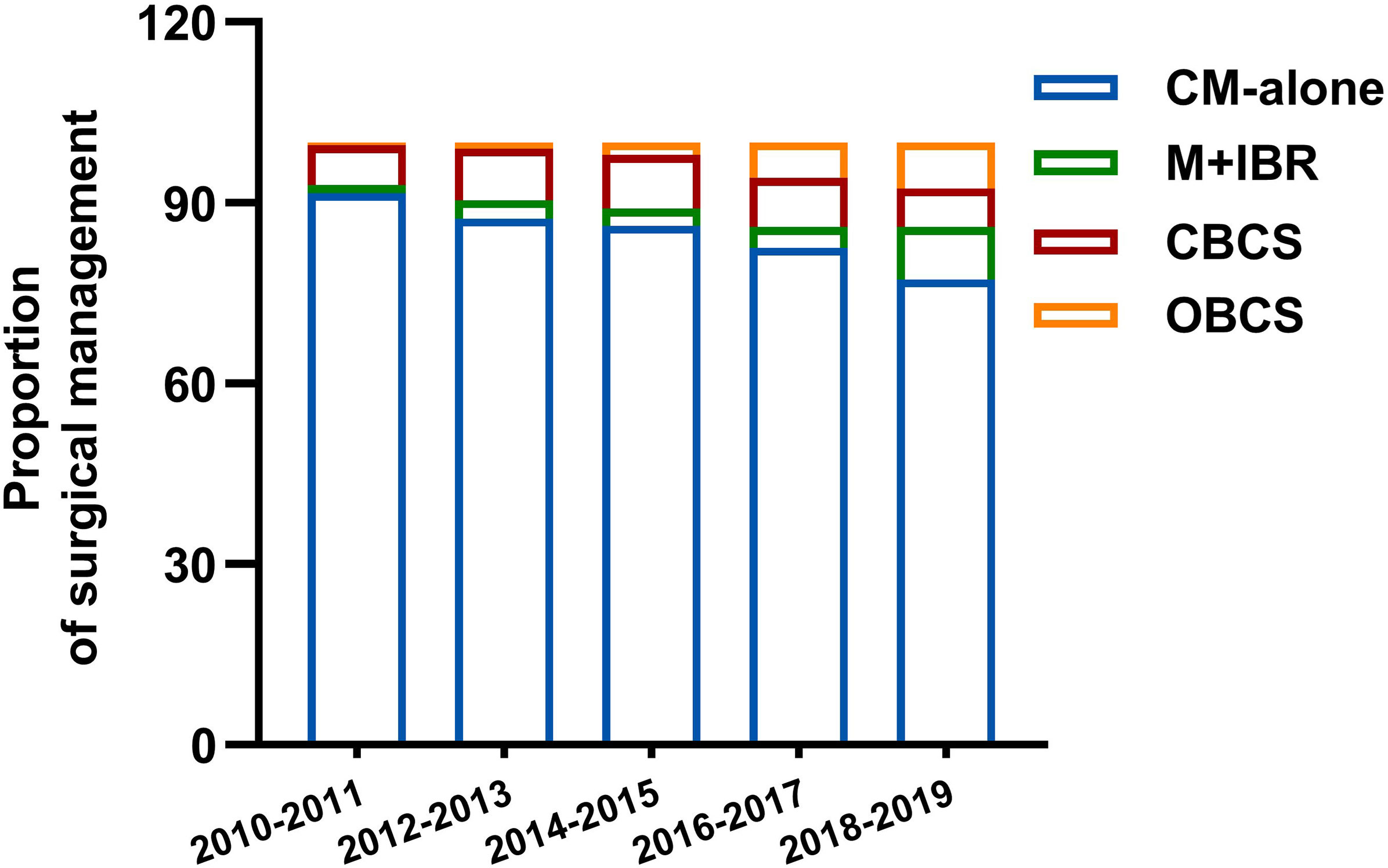
Figure 2 Distribution of surgical management in different period. CM-alone, conventional mastectomy alone; M+IBR, mastectomy plus immediate breast reconstruction; CBCS, conventional breast-conserving surgery; OBCS, oncoplastic breast-conserving surgery.
Demographics and clinicopathological characteristics of patients.
Data indicated that demographics and clinicopathological characteristics are various among the surgical groups (Table 1). Patients in the mastectomy group were older (p<0.001), pre-menopausal (p<0.001), and had normal BMI (p=0.022). In addition, a larger proportion of advanced clinical T stage (p<0.001), clinical TNM stage (p<0.001), ypT stage (p<0.001), ypN stage (p<0.001), as well as non-pCR rate (p<0.001) were also observed in patients receiving mastectomy. While the rate of breast conservation was higher in patients with triple-negative tumors (p=0.023). Over 90% (1672/1819) of patients in the mastectomy group received axillary lymph node dissection (ALND), while 62.1% (162/261) of patients were conducted with sentinel lymph node biopsy (SLNB) in the breast conservation group. No significant differences were identified in the cN stage, ER status, PR status, and HER2 status among the surgical groups.
Among the patients in the mastectomy group, data indicated that a larger proportion of younger (p<0.001) and premenopausal (p<0.001) patients with low or normal BMI (p<0.001) received M+IBR. The rate of M+IBR was significantly higher in patients with ER-positive (p=0.013), and PR-positive (p=0.042) tumors. There was no statistically significant difference in tumor and nodal stage at presentation or after NAC, HER2 status, and molecular subtypes of the tumor, along with the type of axillary surgery between patients who received CM-alone and M+IBR. In addition, the baseline of patients and tumor biologic characteristics did not significantly differ across patients underwent CBCS and OBCS (Table 2).
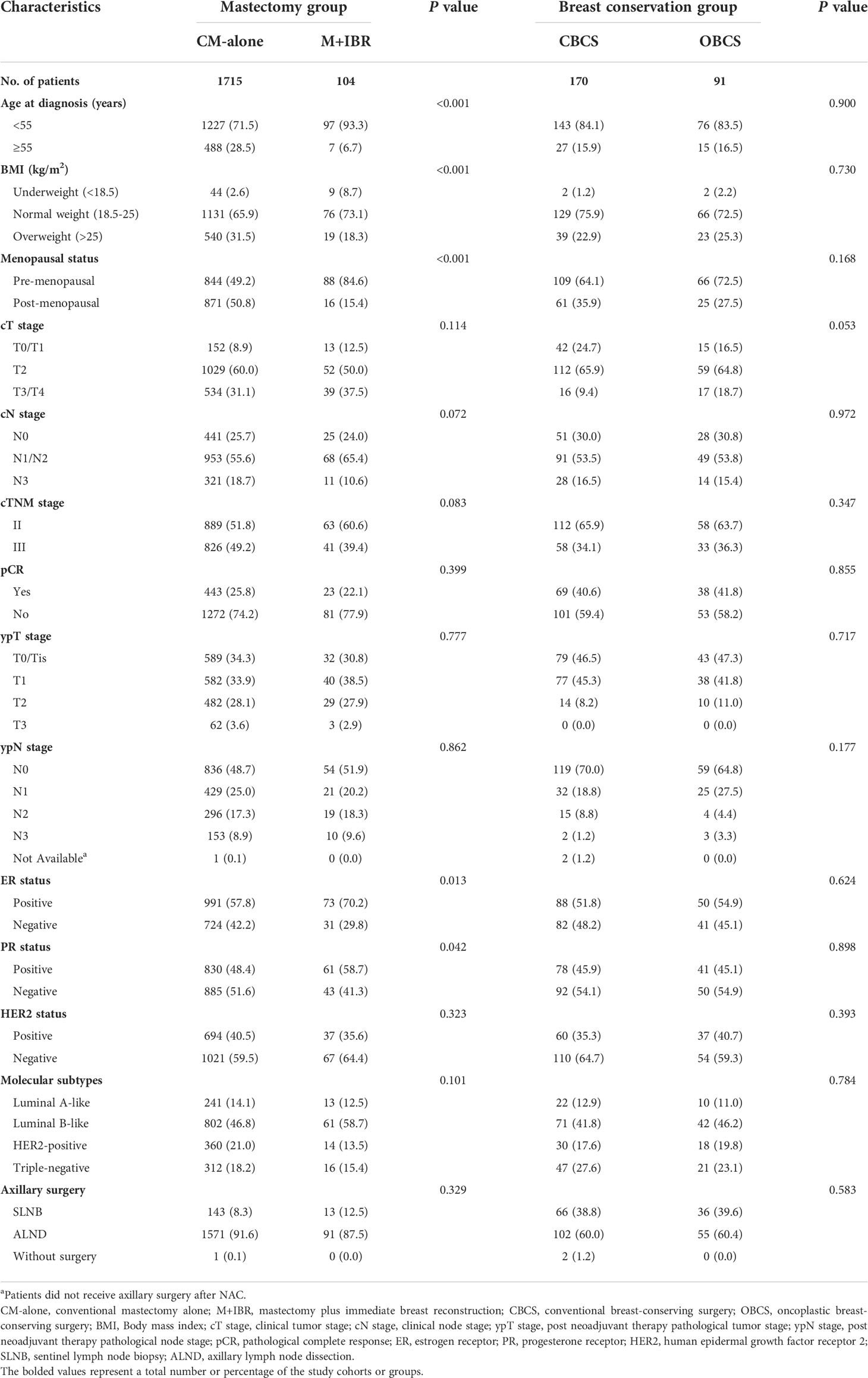
Table 2 Comparison of characteristics of patients receiving different surgical interventions in mastectomy and breast conservation groups.
Survival analysis between patients receiving mastectomy and breast conservation
The median follow-up time for patients in the mastectomy group was 55.0 months, and 55.2 months for the patients who underwent breast conservation. During the period of follow-up, the overall survival was 88.1% and 93.9% for patients who underwent mastectomy and breast conservation. In the mastectomy group, 81.9% (n=1490) of patients live without recurrence, with rates of recurrence were 3.2% (n=58) for locoregional recurrence, 1.0% (n=18) for local recurrence. In the breast conservation group, 88.1% (n=230) of patients live without recurrence, with rates of recurrence were 5.0% (n=13) for locoregional recurrence, 2.0% (n=5) for local recurrence. A total of 133 (6.4%) patients were lost to follow-up.
The overall survival (HR 1.92, [95%CI: 1.30-2.83]; p=0.011) and disease-free survival (HR 1.57, [95%CI: 1.16-2.14]; p=0.015) were higher in patients underwent breast conservation, whereas no statistically significant difference was observed in locoregional recurrence (HR 0.62, [95%CI: 0.30-1.26); p=0.115) (Figure 3). However, characteristics of demographics and tumor biology were considerably variable in patients who underwent different surgical management, which may lead to selection bias in the types of surgery and act as confounding factors in the comparisons of oncologic outcomes. To reduce the impact of confounding variables in comparison to survival outcomes, we developed matched cohort of patients based on age at diagnosis, menopausal status, ypT stage, ypN stage, molecular subtypes, year of NAC, and axillary surgery. Consequently, 388 patients were matched successfully (Table 3).
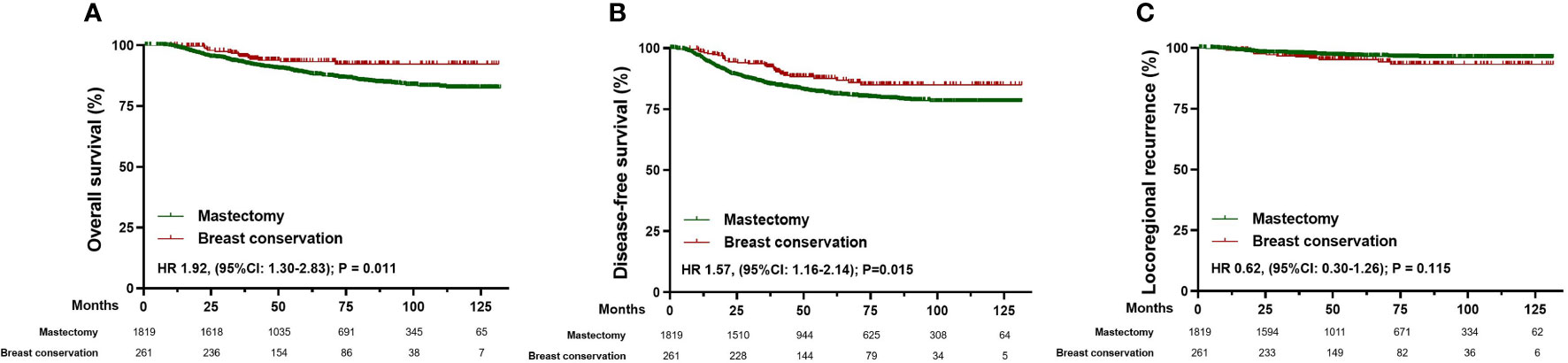
Figure 3 Survival analysis in the total cohort of patients receiving mastectomy (n=1819) and breast conservation (n=261). (A) Kaplan-Meier curves for overall survival analysis in patients of different surgical groups. (B) Kaplan-Meier curves for disease-free survival analysis in patients of different surgical groups. (C) Kaplan-Meier curves for locoregional recurrence analysis in patients of different surgical groups. HR, Hazard ratio; 95% CI, 95% confidence interval.
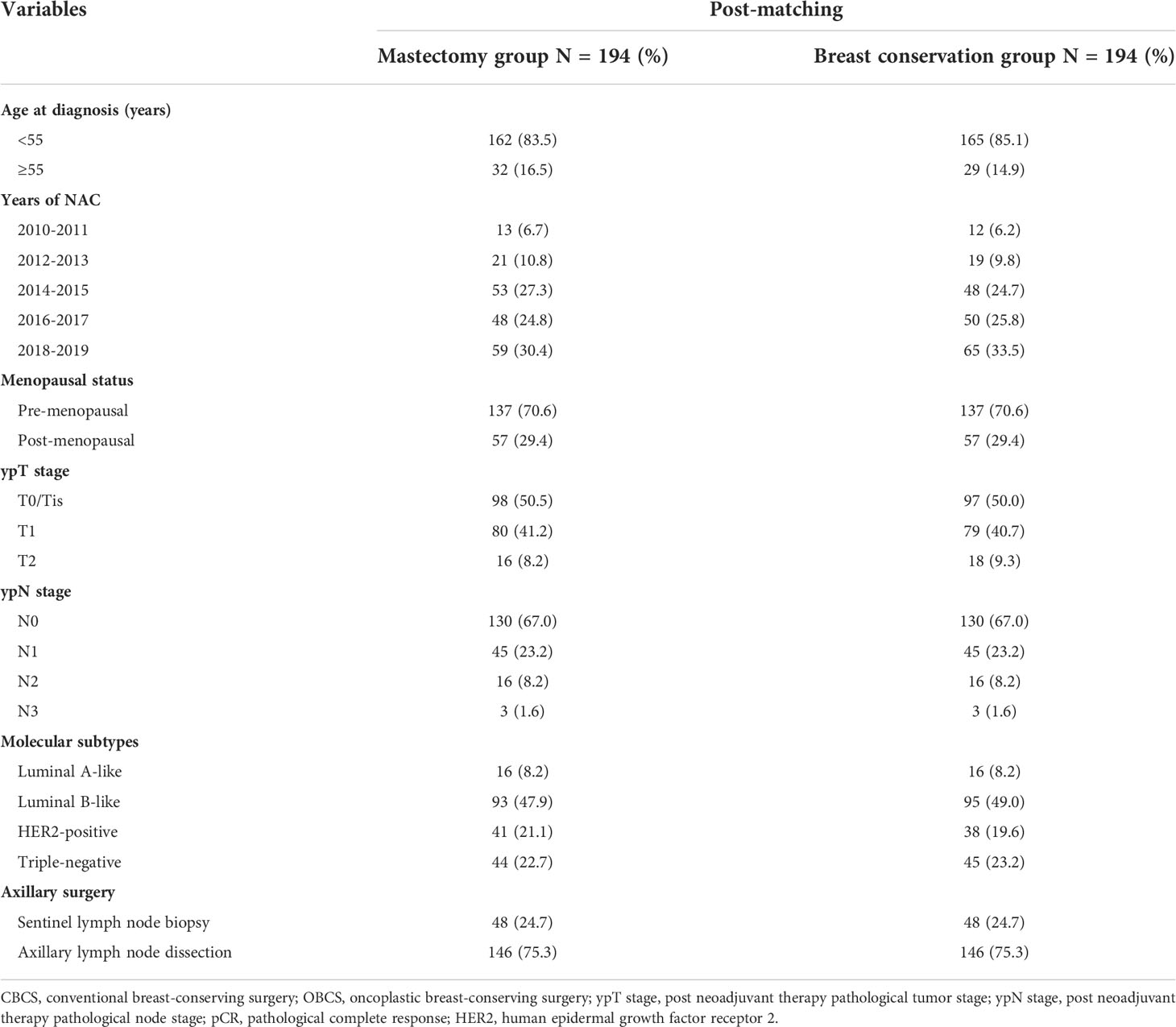
Table 3 Balanced statistics of patients receiving neoadjuvant chemotherapy in different surgical groups after propensity score matching.
In the matched cohorts, the median follow-up time was 63.1 months for the mastectomy group and 55.0 months for the breast conservation group. Kaplan-Meier analysis showed that patients receiving breast conservation had better OS (HR 0.41, [95% CI 0.18-0.97]; p=0.049) compared with those who received mastectomy after NAC (Figure 4A). The 5-year OS for patients in the mastectomy group was 92.3% (95% CI 89.7%-93.8%) versus 96.4% (95% CI 94.5%-97.3%) in the breast conservation group. No statistical differences between the mastectomy and breast conservation group in 5-year DFS (86.9% versus 91.4%; HR 0.76, [95%CI: 0.43-1.37]; p=0.372) and LRR (95.6% versus 96.4%; HR 1.062, [95%CI: 0.40-2.83]; p=0.905) were observed (Figures 4B, C).

Figure 4 Survival analysis in the matched cohort of patients receiving mastectomy (n=194) and breast conservation (n=194). (A) Kaplan-Meier curves for overall survival analysis in patients of different surgical groups. (B) Kaplan-Meier curves for disease-free survival analysis in patients of different surgical groups. (C) Kaplan-Meier curves for locoregional recurrence analysis in patients of different surgical groups. HR, Hazard ratio; 95% CI, 95% confidence interval.
Survival analysis between patients with and without application of oncoplastic techniques in surgery following NAC
We further compared the survival outcomes of patients with and without the application of oncoplastic techniques in the surgical process. Given the evolvement of surgical management, and substantial differences in the baseline of patients and tumor biologic characteristics between patients receiving CM-alone and M+IBR, or CBCS and OBCS, well-balanced groups were generated to reduce the selection bias for survival analysis.
After matching, 91 patients receiving CM-alone and 91 patients receiving M+IBR were matched successfully (Table 4). The median follow-up was 48.1 months for the patients underwent CM-alone and 45.1 months for the patients underwent M+IBR. Data indicated no significant differences between the CM-alone and M+IBR groups in 5-year OS (85.3% versus 94.2%; HR 0.44, [95%CI: 0.16-1.17]; p=0.116), DFS (78.3% versus 85.6%; HR 0.72, [95%CI: 0.35-1.48]; p=0.378), and LRR (98.9% versus 95.5%; HR 2.83, [95%CI: 0.40-20.17]; p=0.300) (Figure 5). Regarding the breast conservation group, due to the relatively recent application of oncoplastic breast-conserving surgery in the NAC setting, matched patients were limited between CBCS (n=31) and OBCS (n=31) groups (Table 4). The median follow-up for patients who underwent CBCS and OBCS was 50.1 months and 50.1 months respectively. Moreover, no significant differences were identified in OS (96.4% versus 96.4%; HR 0.91, [95%CI: 0.06-14.54]; p=0.945), DFS (92.4% versus 92.2%; HR 1.33, [95%CI: 0.23-7.67]; p=0.757), LRR (92.4% versus 96.4%; HR 0.90, [95%CI: 0.17-6.40]; p = 0.914) between patients in those two groups (Figure 6).
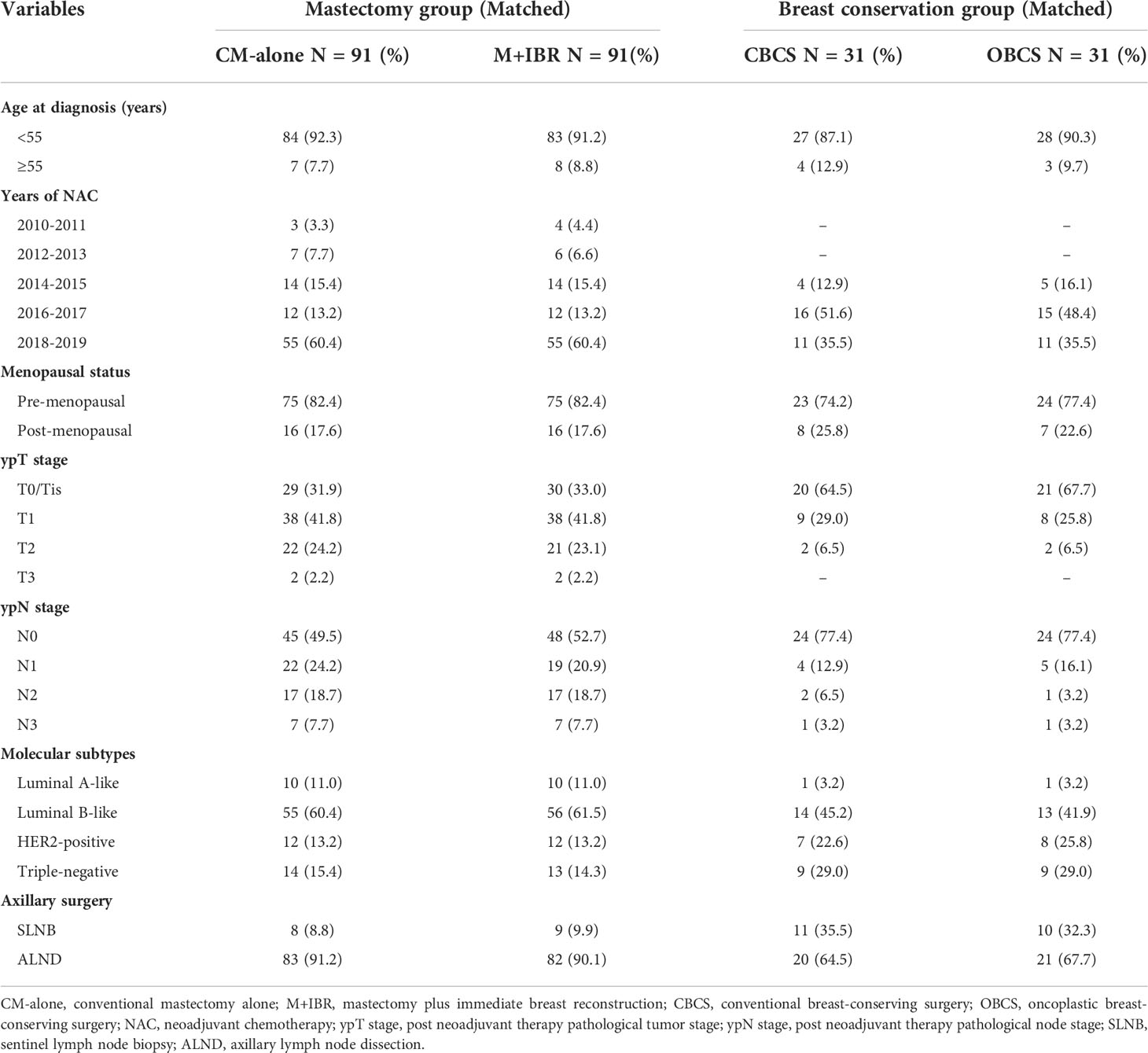
Table 4 Matched patients receiving different surgical intervention in mastectomy group and breast conservation group after propensity score matching.
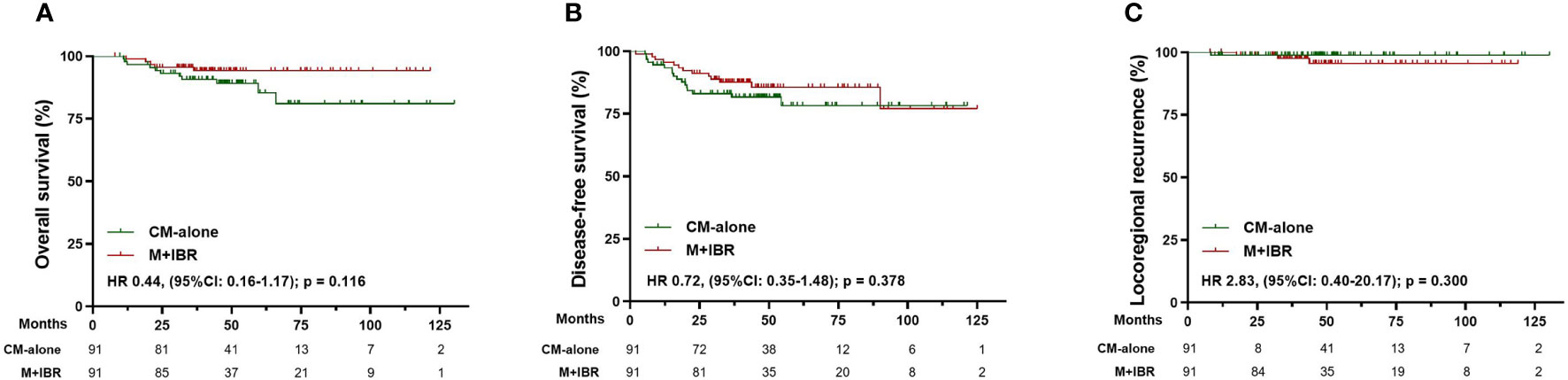
Figure 5 Survival analysis in the matched cohort of patients receiving conventional mastectomy alone (n=91) and mastectomy plus immediate breast reconstruction (n=91). (A) Kaplan-Meier curves for overall survival analysis in patients of different surgical groups. (B) Kaplan-Meier curves for disease-free survival analysis in patients of different surgical groups. (C) Kaplan-Meier curves for locoregional recurrence analysis in patients of different surgical groups. CM-alone, conventional mastectomy alone; M+IBR, mastectomy plus immediate breast reconstruction; HR, Hazard ratio; 95% CI, 95% confidence interval.
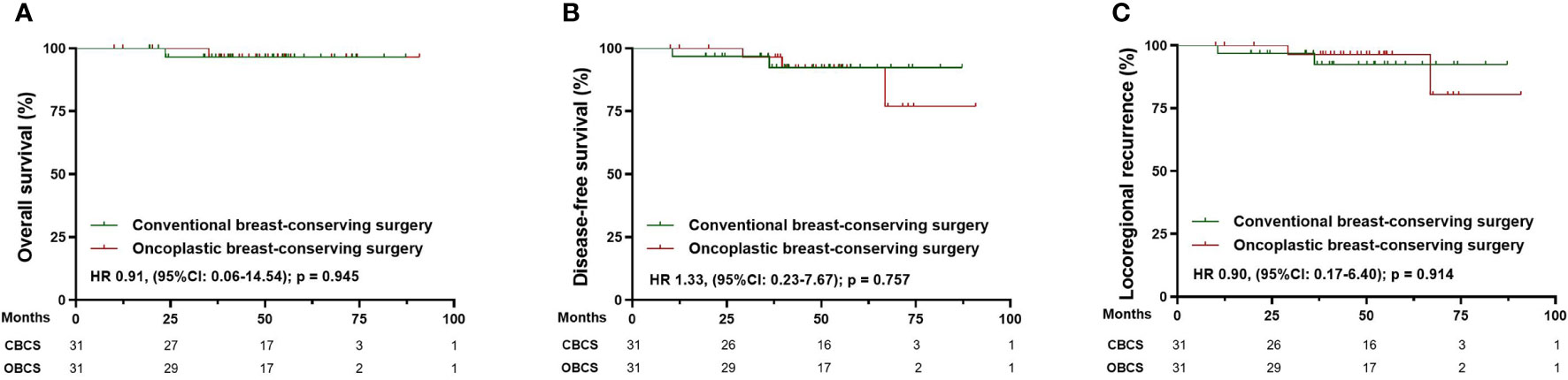
Figure 6 Survival analysis in the matched cohort of patients receiving conventional breast-conserving surgery (n=31) and oncoplastic breast-conserving surgery (n=31). (A) Kaplan-Meier curves for overall survival analysis in patients of different surgical groups. (B) Kaplan-Meier curves for disease-free survival analysis in patients of different surgical groups. (C) Kaplan-Meier curves for locoregional recurrence analysis in patients of different surgical groups. CBCS, conventional breast-conserving surgery; OBCS, oncoplastic breast-conserving surgery; HR, Hazard ratio; 95% CI, 95% confidence interval.
Discussion
In the current study, patients who received breast conservation accounted for a small proportion of surgical management in the NAC setting and were identified with distinct features of demographic and clinicopathological variables compared with patients who underwent mastectomy. Moreover, we demonstrated comparable survival outcomes between patients receiving mastectomy and breast conservation, and further supported the application of oncoplastic techniques in the surgical intervention following NAC.
Neoadjuvant chemotherapy is increasingly used with extended indications, which brought consequential advantages and implications for de-escalation of local therapeutic decisions. Breast conservation is considered a reasonable option for selected patients receiving NAC in several guidelines (19, 20). Nevertheless, our results revealed an excessive inclination to mastectomy in the current clinical practice in China, which was accordant with another study conducted in northwest China (21). Moreover, the rate of breast-conserving surgery following NAC in the current study was much lower than research in Europe and the United States (7, 22). According to a recent retrospective study from Memorial Sloan-Kettering Cancer Center, the rate for patients successfully conducted with BCS after NAC was 44.96% (23). Likewise, Golshan et al. reported that 47.5% of patients with stage II-III triple-negative breast cancer underwent BCS after NAC in a prospective study, with a BCS conversion rate of 53.2% from ineligible to eligible owing to administration of NAC. Golshan et al. suggested that Asian and European patients showed significantly lower BCS rates, compared with patients in North America (24). However, our data revealed a steadily increasing tendency toward breast conservation, suggesting that our current surgical patterns may experience a similar shift from total mastectomy to breast conservation as earlier in those developed countries (10, 25).
Furthermore, with the comprehensive application of oncoplastic techniques in breast surgery, we revealed a considerable growing proportion of oncoplastic surgery in the NAC setting, including M+IBR and OBCS surgeries. As a combination of gland removal with breast skin preservation and immediate breast reconstruction, M+IBR was often called conservative mastectomy (26). Studies showed that M+IBR often correlated with improved patient satisfaction and aesthetic outcomes, which made it gain popularity in the selected patients (16, 27). We also reported a marked trend toward higher proportions of patients receiving M+IBR in the NAC setting. Regarding breast conservation, compared with CBCS, the addition of the oncoplastic technique in surgery allows a larger resection volume with rearrangement of resident breast tissue to achieve better cosmetic outcomes for the breast. Consequently, OBCS was increasingly utilized in patients eligible for breast conservation to further ensure complete tumor removal and correct breast deformities (28–30), and a similar trend was observed in patients undergoing NAC in our study. All these above indicated a rising need for improved cosmetic outcomes in patients receiving NAC. However, there were limited studies of matched groups on the efficacy and oncologic safety of oncoplastic surgery compared to conventional surgery in the NAC setting (31–33).
As for axilla management, ALND was more prevalent in the mastectomy groups, which implies an overall aggressive surgical paradigm in the NAC setting. Whereas, the proportion of SLNB increased appreciably among the patients who opted for breast conservation. In patients initially treated with surgery, SLNB offered good effectiveness in pathologic nodal staging and cancer control, along with a significant decline in arm morbidity, which led to a shift of axillary surgery from ALND to SLNB (34–36). Moreover, studies indicated that SLNB following NAC could achieve the acceptable false-negative rate (FNR) by sophisticated identification procedures, such as increasing the number of removed SLNs and applying of combined detection technique (37, 38). A growing number of studies further suggested that SLNB showed comparable oncologic safety in patients with clinically positive nodes in the NAC setting (39–41). Based on these clinical trials, SLNB was feasible and applicable after NAC for evaluation of residual disease in the axilla and local control. Consequently, considering the improvement of SLNB on arm function and quality of life, SLNB could be a reliable diagnostic alternative option for ALND for selected patients after NAC.
To determine the reasons that may contribute to the current surgical patterns, we compared characteristics between surgical groups. Consistent with recent research, we found that age and menopausal status could significantly influence surgical management after NAC (21). One possible explanation might be that elderly patients were often at weakened functional status with increasing comorbidities, resulting in extensive tension and anxiety about the disease and less concern about cosmetic appearance. Therefore, older patients were more likely to receive mastectomy to minimize the risk of recurrence and avoid the radiotherapy following BCS (42–44). Moreover, our data suggested that tumor biological features at presentation and after NAC were also variant among surgical options, including tumor size, disease stage, and molecular subtypes. Consistently, a growing number of evidence indicated that clinical tumor size was a significant predictor of pCR rate, which interferes with the preference of surgical options in patients administrated with NAC (23, 45–47). In addition, previous studies also demonstrated that patients diagnosed with triple-negative or HER2-positive breast cancer were less likely to be offered mastectomy (48). Petruolo O et al. and Golshan M et al. reported that HER2-positive and triple-negative were positively associated with successful downstaging of disease after NAC, which facilitated breast conservation (23, 49, 50).
Even though the mastectomy group had a higher proportion of older, post-menopausal patients with advanced tumor and disease stages before and after NAC, there was still a substantial number of patients who were eligible for breast conservation. In the current study, the pCR rate of patients with luminal A-like tumors was 5.2%, 20.4% for luminal B-like tumors, 51.7% for HER2-positive tumors, and 35.6% with triple-negative tumors, which were similar to the pCR rate in the ACOSOG Z1071 (Alliance) study and a German pooled analysis (22, 51). However, we found that more than 80% of patients who achieved pCR opted for mastectomy following NAC. Therefore, our findings suggested that disease-specific features were not the primary reason for the low rate of breast conservation. One of the possible explanations might be the lack of accurate and executable methods for the assessment of tumor regression in standard breast cancer management pathways (52). Additionally, according to the earlier studies, conservative attitude toward breast conservation in patients may also be an important factor that may lead to inclination toward mastectomy in the current surgical pattern, which implied insufficiency in evidence-based patient counseling (53).
To figure out whether the inclination toward mastectomy is necessary for patients treated with NAC, survival analysis was performed in matched cohorts of patients who underwent mastectomy or breast conservation. The survival outcomes of patients receiving NAC in the present study are in keeping with those in previous western studies (7, 54–56). Recently, Simons, J. M. et al. reported DFS of 90.9% for BCS and 82.9% for mastectomy, while OS of 95.3% for BCS and 85.9% for mastectomy (57). Moreover, our data showed a survival benefit on OS in patients who received breast conservation, while no significant difference was observed in DFS and LRR between the two groups, which were corroborated with the data of earlier studies with matched control groups (14, 56). Therefore, breast conservation should be considered as an alternative to mastectomy owing to better survival outcomes and equivalent locoregional control in the NAC setting.
In the present study, we further revealed comparable outcomes in well-matched cohorts of patients who underwent CM-alone and M+IBR. Previous studies also demonstrated the feasibility of M+IBR in terms of OS, DFS, and local recurrence. Zhen-Yu Wu, et al. compared oncologic outcomes between patients who underwent IBR with NSM/SSM and CM following NAC by a retrospective, propensity score-matched case-control study, which indicated comparable 5-year OS, DFS, LRR, and distant metastasis-free survival (32). And these findings were further confirmed in subgroups analysis of breast cancer patients with young age or locally advanced breast cancer (58, 59). In addition, our results further supported the oncologic safety of OBCS in the NAC setting. Compared with patients who underwent CBCS groups, we observed no differences in OS and DFS, along with comparable locoregional control in patients who received OBCS. A matched-cohort analysis even reported that OBCS could be a valuable option for tumors that regressed sub-optimally after NAC without compromising oncologic safety (33). Regarding locoregional control, a meta-analysis of two NSABP neoadjuvant trials indicated that LRR was not related to surgical options (54). Moreover, Chen et al. demonstrated an equivalent rate of negative margins in patients with large tumor size between OBCS and CBCS groups (60). Besides, wider resection margins in locally advanced tumors were also reported in OBCS (61). Hence, expanding the indication of oncoplastic techniques in conventional surgical intervention following NAC were feasible for patient administrated with NAC.
There were several limitations in the current study that should be taken into consideration while deducing the results. The most important limitation was caused by the retrospective study design. Since patients were retrospectively involved and divided into different surgical groups, the selection bias of surgical management was inevitable. Accordingly, we reported substantial heterogeneity in clinical features between patients undergoing mastectomy and breast conservation following NAC. In addition, considerate progression in surgical intervention and NAC regimens for breast cancer patients have occurred in recent decades, which lead to substantial differences in terms of the available treatment for patients. As a single cancer center in Shanghai, we had more than 2,000 breast cancer patients diagnosed and hospitalized every year since 2010, and started to perform oncoplastic surgery in 1999 (62). Therefore, oncoplastic breast surgery was performed by highly qualified breast surgeons who had undergone a long period of professional training in our institute. However, the application of oncoplastic techniques in surgical management after NAC started relatively late. Consequently, M+IBR and OBCS were new surgical options for patients with breast cancer receiving NAC, and they have lately gained popularity. Under this situation, it is critical to ensure that patients involved in the survival analysis were offered comparable therapies. Altogether, to minimize the inherent bias on the patient baseline, tumor biology, and treatment characteristics, nested case-control studies were required, and PSM was performed to balance such variations and generate matched cohorts for survival analysis. Also, the regression rate of tumor after NAC and original cup size were not included in the present study, which were also presumed to be potential impact factors for the surgical options. Moreover, the patient cohort of the current study was from a single institution. Therefore, although this cohort represents a real-world practice, multi-institutional investigations were required to further validate our results.
Altogether, although a growing proportion of patients opted for breast preservation after NAC, a considerate inclination toward mastectomy was observed in the current clinical practice in China. Moreover, we revealed a substantial evolvement in the application of the oncoplastic technique in surgical management following NAC. Our results strengthen the evidence that breast conservation was a feasible and safe alternative for mastectomy, which provided evidence to support surgical de-escalation in the NAC setting. Therefore, patient-centered clinical trials to guide treatment decisions, qualified pre-surgical assessment of tumor regression, thorough consultation of challenges and benefits of surgical de-escalation, along with a collaborative decision-making process between surgeons and patients, were necessary to improve the rate of breast conservation and quality of life for breast cancer patients receiving NAC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Conception and design: YS, BY, SH, XH, GL, ZS, and JW; administrative support: JC, BY, GL, ZS, and JW; provision of study materials or patients: YS, WC, and BY; collection and assembly of data: YS, WC, and XZ; data analysis and interpretation: YS and JW; manuscript writing: All authors. All authors contributed to the article and approved the submitted version.
Funding
This study was supported in part by grants from the National Key R&D Program of China (2017YFC1311004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Swain SM, Sorace RA, Bagley CS, Danforth DN Jr, Bader J, Wesley MN, et al. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res (1987) 47:3889–94.
2. King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol (2015) 12:335–43. doi: 10.1038/nrclinonc.2015.63
3. Montagna G, Mamtani A, Knezevic A, Brogi E, Barrio AV, Morrow M. Selecting node-positive patients for axillary downstaging with neoadjuvant chemotherapy. Ann Surg Oncol (2020) 27:4515–22. doi: 10.1245/s10434-020-08650-z
4. Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol (2012) 13:25–32. doi: 10.1016/S1470-2045(11)70336-9
5. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National surgical adjuvant breast and bowel project protocol b-27. J Clin Oncol (2006) 24:2019–27. doi: 10.1200/JCO.2005.04.1665
6. Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: Triple-negative and HER2+ subtypes. Ann Surg Oncol (2018) 25:2241–8. doi: 10.1245/s10434-018-6531-5
7. Early Breast Cancer Trialists' Collaborative G. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol (2018) 19:27–39. doi: 10.1016/S1470-2045(17)30777-5
8. Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol (2008) 26:814–9. doi: 10.1200/JCO.2007.15.3510
9. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols b-18 and b-27. J Clin Oncol (2008) 26:778–85. doi: 10.1200/JCO.2007.15.0235
10. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European organization for research and treatment of cancer trial 10902. J Clin Oncol (2001) 19:4224–37. doi: 10.1200/JCO.2001.19.22.4224
11. Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg (2007) 94:1189–200. doi: 10.1002/bjs.5894
12. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med (2002) 347:1233–41. doi: 10.1056/NEJMoa022152
13. Jacobson JA, Danforth DN, Cowan KH, d'Angelo T, Steinberg SM, Pierce L, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med (1995) 332:907–11. doi: 10.1056/NEJM199504063321402
14. Wrubel E, Natwick R, Wright GP. Breast-conserving therapy is associated with improved survival compared with mastectomy for early-stage breast cancer: A propensity score matched comparison using the national cancer database. Ann Surg Oncol (2021) 28:914–9. doi: 10.1245/s10434-020-08829-4
15. Ataseven B, Lederer B, Blohmer JU, Denkert C, Gerber B, Heil J, et al. Impact of multifocal or multicentric disease on surgery and locoregional, distant and overall survival of 6,134 breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol (2015) 22:1118–27. doi: 10.1245/s10434-014-4122-7
16. Galimberti V, Vicini E, Corso G, Morigi C, Fontana S, Sacchini V, et al. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast (2017) 34 Suppl 1:S82–4. doi: 10.1016/j.breast.2017.06.034
17. Kosasih S, Tayeh S, Mokbel K, Kasem A. Is oncoplastic breast conserving surgery oncologically safe? A meta-analysis 18,103 patients. Am J Surg (2020) 220:385–92. doi: 10.1016/j.amjsurg.2019.12.019
18. Anderson JR, Bernstein L, Pike MC. Approximate confidence intervals for probabilities of survival and quantiles in life-table analysis. Biometrics (1982) 38:407–16. doi: 10.2307/2530454
19. National Comprehensive Cancer Network. (NCCN) clinical practice guidelines in oncology (NCCN guideline®). breast cancer, version 3. (2022) Available at: https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf.
20. C.o.B.C.S. Chinese Anti-Cancer Association. Guideline and standard for diagnosis and treatment of breast cancer (2021). China Oncol (2021) 31:954–1040.
21. Li X, Yan C, Xiao J, Xu X, Li Y, Wen X, et al. Factors associated with surgical modality following neoadjuvant chemotherapy in patients with breast cancer. Clin Breast Cancer (2021) 21:e611-e617. doi: 10.1016/j.clbc.2021.03.011
22. Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg (2014) 260:608–14. doi: 10.1097/SLA.0000000000000924
23. Petruolo O, Sevilimedu V, Montagna G, Le T, Morrow M, Barrio AV. How often does modern neoadjuvant chemotherapy downstage patients to breast-conserving surgery? Ann Surg Oncol (2021) 28:287–94. doi: 10.1245/s10434-020-08593-5
24. Golshan M, Loibl S, Wong SM, Houber JB, O'Shaughnessy J, Rugo HS, et al. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: Surgical results from the BrighTNess randomized clinical trial. JAMA Surg (2020) 155:e195410. doi: 10.1001/jamasurg.2019.5410
25. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from national surgical adjuvant breast and bowel project b-18. J Clin Oncol (1997) 15:2483–93. doi: 10.1200/JCO.1997.15.7.2483
26. Veronesi U, Stafyla V, Luini A, Veronesi P. Breast cancer: from "maximum tolerable" to "minimum effective" treatment. Front Oncol (2012) 2:125. doi: 10.3389/fonc.2012.00125
27. Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg (2015) 150:9–16. doi: 10.1001/jamasurg.2014.2895
28. Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg (2014) 72:145–9. doi: 10.1097/SAP.0b013e3182605598
29. Piper ML, Esserman LJ, Sbitany H, Peled AW. Outcomes following oncoplastic reduction mammoplasty: A systematic review. Ann Plast Surg (2016) 76 Suppl 3:S222–6. doi: 10.1097/SAP.0000000000000720
30. Kelemen P, Pukancsik D, Ujhelyi M, Savolt A, Kovacs E, Ivady G, et al. Comparison of clinicopathologic, cosmetic and quality of life outcomes in 700 oncoplastic and conventional breast-conserving surgery cases: A single-centre retrospective study. Eur J Surg Oncol (2019) 45:118–24. doi: 10.1016/j.ejso.2018.09.006
31. Ryu JM, Park S, Paik HJ, Nam SJ, Kim SW, Lee SK, et al. Oncologic safety of immediate breast reconstruction in breast cancer patients who underwent neoadjuvant chemotherapy: Short-term outcomes of a matched case-control study. Clin Breast Cancer (2017) 17:204–10. doi: 10.1016/j.clbc.2016.10.009
32. Wu ZY, Kim HJ, Lee JW, Chung IY, Kim JS, Lee SB, et al. Long-term oncologic outcomes of immediate breast reconstruction vs conventional mastectomy alone for breast cancer in the setting of neoadjuvant chemotherapy. JAMA Surg (2020) 155:1142–50. doi: 10.1001/jamasurg.2020.4132
33. van la Parra RFD, Clough KB, Thygesen HH, Levy E, Poulet B, Sarfati I, et al. Oncological safety of oncoplastic level II mammoplasties after neoadjuvant chemotherapy for Large breast cancers: A matched-cohort analysis. Ann Surg Oncol (2021) 28:5920–8. doi: 10.1245/s10434-021-09829-8
34. Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: Long-term follow-up from the American college of surgeons oncology group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg (2016) 264:413–20. doi: 10.1097/SLA.0000000000001863
35. Donker M, Tienhoven Gv, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol (2014) 15:1303–10. doi: 10.1016/S1470-2045(14)70460-7
36. Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer (2000) 88:608–14. doi: 10.1002/(SICI)1097-0142(20000201)88:3<608::AID-CNCR17>3.0.CO;2-K
37. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol (2013) 14:609–18. doi: 10.1016/S1470-2045(13)70166-9
38. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA (2013) 310:1455–61. doi: 10.1001/jama.2013.278932
39. Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: Results from ACOSOG Z1071 (Alliance). Ann Surg (2016) 263:802–7. doi: 10.1097/SLA.0000000000001375
40. Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: Results from the American college of surgeons oncology group Z1071 trial (Alliance). J Clin Oncol (2015) 33:3386–93. doi: 10.1200/JCO.2014.57.8401
41. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J Clin Oncol (2016) 34:1072–8. doi: 10.1200/JCO.2015.64.0094
42. Field TS, Bosco JL, Prout MN, Gold HT, Cutrona S, Pawloski PA, et al. Age, comorbidity, and breast cancer severity: impact on receipt of definitive local therapy and rate of recurrence among older women with early-stage breast cancer. J Am Coll Surg (2011) 213:757–65. doi: 10.1016/j.jamcollsurg.2011.09.010
43. Fisher CS, Martin-Dunlap T, Ruppel MB, Gao F, Atkins J, Margenthaler JA. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol (2012) 19:3246–50. doi: 10.1245/s10434-012-2525-x
44. de Glas NA, Kiderlen M, Bastiaannet E, de Craen AJ, van de Water W, van de Velde CJ, et al. Postoperative complications and survival of elderly breast cancer patients: A FOCUS study analysis. Breast Cancer Res Treat (2013) 138:561–9. doi: 10.1007/s10549-013-2462-9
45. Wang Y, Li L, Liu X, Wang Y, Tang Z, Wu Y, et al. Treatment response correlation between primary tumor and axillary lymph nodes after neoadjuvant therapy in breast cancer: A retrospective study based on real-world data. Gland Surg (2021) 10:656–69. doi: 10.21037/gs-20-686
46. Livingston-Rosanoff D, Schumacher J, Vande Walle K, Stankowski-Drengler T, Greenberg CC, Neuman H, et al. Does tumor size predict response to neoadjuvant chemotherapy in the modern era of biologically driven treatment? A Nationwide Study US Breast Cancer Patients. Clin Breast Cancer (2019) 19:e741-7. doi: 10.1016/j.clbc.2019.05.014
47. Goorts B, van Nijnatten TJ, de Munck L, Moossdorff M, Heuts EM, de Boer M, et al. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat (2017) 163:83–91. doi: 10.1007/s10549-017-4155-2
48. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res (2007) 13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109
49. Golshan M, Cirrincione CT, Sikov WM, Berry DA, Jasinski S, Weisberg TF, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: Surgical results from CALGB 40603 (Alliance). Ann Surg (2015) 262:434–9. doi: 10.1097/SLA.0000000000001417
50. Golshan M, Cirrincione CT, Sikov WM, Carey LA, Berry DA, Overmoyer B, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II-III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). Breast Cancer Res Treat (2016) 160:297–304. doi: 10.1007/s10549-016-4006-6
51. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol (2012) 30:1796–804. doi: 10.1200/JCO.2011.38.8595
52. Tasoulis MK, Lee HB, Yang W, Pope R, Krishnamurthy S, Kim SY, et al. Accuracy of post-neoadjuvant chemotherapy image-guided breast biopsy to predict residual cancer. JAMA Surg (2020) 155:e204103. doi: 10.1001/jamasurg.2020.4103
53. Yang B, Ren G, Song E, Pan D, Zhang J, Wang Y, et al. Current status and factors influencing surgical options for breast cancer in China: A nationwide cross-sectional survey of 110 hospitals. Oncologist (2020) 25:e1473-e1480. doi: 10.1634/theoncologist.2020-0001
54. Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE Jr., et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project b-18 and b-27. J Clin Oncol (2012) 30:3960–6. doi: 10.1200/JCO.2011.40.8369
55. Caudle AS, Yu TK, Tucker SL, Bedrosian I, Litton JK, Gonzalez-Angulo AM, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res (2012) 14:R83. doi: 10.1186/bcr3198
56. Arlow RL, Paddock LE, Niu X, Kirstein L, Haffty BG, Goyal S, et al. Breast-conservation therapy after neoadjuvant chemotherapy does not compromise 10-year breast cancer-specific mortality. Am J Clin Oncol (2018) 41:1246–51. doi: 10.1097/COC.0000000000000456
57. Simons JM, Jacobs JG, Roijers JP, Beek MA, Boonman-de Winter LJM, Rijken AM, et al. Disease-free and overall survival after neoadjuvant chemotherapy in breast cancer: breast-conserving surgery compared to mastectomy in a large single-centre cohort study. Breast Cancer Res Treat (2021) 185:441–51. doi: 10.1007/s10549-020-05966-y
58. Wu ZY, Kim HJ, Lee J, Chung IY, Kim J, Lee SB, et al. Oncologic outcomes of immediate breast reconstruction in young women with breast cancer receiving neoadjuvant chemotherapy. Breast Cancer Res Treat (2022) 191:345–54. doi: 10.1007/s10549-021-06428-9
59. Wu ZY, Han HH, Kim HJ, Chung IY, Kim J, Lee SB, et al. A propensity score-matched analysis of long-term oncologic outcomes after nipple-sparing versus conventional mastectomy for locally advanced breast cancer. Ann Surg (2020) 276(2022):386–90. doi: 10.1097/SLA.0000000000004416
60. Chen Y, Hao S, Chen J, Huang X, Cao A, Hu Z, et al. A retrospective cohort study comparing traditional breast conservation with oncoplastic surgery in breast cancer patients after neoadjuvant chemotherapy. Ann Plast Surg (2022) 88:144–51. doi: 10.1097/SAP.0000000000002971
61. Youssef MMG, Namour A, Youssef OZ, Morsi A. Oncologic and cosmetic outcomes of oncoplastic breast surgery in locally advanced breast cancer after neoadjuvant chemotherapy, experience from a developing country. Indian J Surg Oncol (2018) 9:300–6. doi: 10.1007/s13193-017-0689-3
Keywords: breast cancer, neoadjuvant chemotherapy, surgery, mastectomy, breast conservation, oncoplastic surgery
Citation: Sang Y, Zhou X, Chi W, Chen J, Yang B, Hao S, Huang X, Liu G, Shao Z and Wu J (2022) Surgical options of the breast and clinical outcomes of breast cancer patients after neoadjuvant chemotherapy: A single-center retrospective study. Front. Oncol. 12:984587. doi: 10.3389/fonc.2022.984587
Received: 02 July 2022; Accepted: 10 October 2022;
Published: 27 October 2022.
Edited by:
Xiaowei Qi, Army Medical University, ChinaReviewed by:
Min-Ying Lydia Su, University of California, Irvine, United StatesRené Aloisio Da Costa Vieira, Barretos Cancer Hospital, Brazil
Sanjit Agrawal, Tata Medical Centre, India
Copyright © 2022 Sang, Zhou, Chi, Chen, Yang, Hao, Huang, Liu, Shao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiong Wu, d3VqaW9uZzExMjJAdmlwLnNpbmEuY29t
 Yuting Sang
Yuting Sang Xujie Zhou1,2,3
Xujie Zhou1,2,3 Benlong Yang
Benlong Yang Jiong Wu
Jiong Wu