
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 September 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.981226
This article is part of the Research Topic Prostate Cancer Genomics: Application at Different Stages of a Patient’s Journey View all 5 articles
Background: Epigenetic reprogramming through dysregulated histone lysine methylation (HLM) plays a crucial role in prostate cancer (PCa) progression. This study aimed to comprehensively evaluate HLM modification patterns in PCa microenvironment infiltration.
Materials and methods: Ninety-one HLM regulators in The Cancer Genome Atlas (TCGA) dataset were analyzed using bioinformatics. Differentially expressed genes (DEGs) and survival analyses were performed using TCGA-PRAD clinicopathologic and follow-up information. Consensus clustering analysis divided patients into subgroups. Gene ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed on the DEGs. Tumor mutation burden (TMB) and tumor microenvironment (TME) cell infiltration were evaluated in different HLM clusters. Quantitative real-time PCR (qPCR) analysis assessed HLM regulators in clinical PCa tissues.
Results: The tumor vs. normal (TN), Gleason score (GS) > 7 vs. GS < 7, pathological T stage (pT) = 2 vs. pT = 3, and TP53 mutation vs. wild-type comparisons using TCGA-PRAD dataset revealed 3 intersecting HLM regulators (EZH2, NSD2, and KMT5C) that were consistently upregulated in advanced PCa (GS > 7, pT3, HR > 1, and TP53 mutation) (P < 0.05) and verified in clinical PCa tissues. Consensus clustering analysis revealed three distinct HLM modification patterns (HLMclusters). However, no significant differences in recurrence-free survival (RFS) rates were found among the groups (P > 0.05). We screened 189 HLM phenotype-related genes that overlapped in the pairwise comparisons of HLMclusters and P < 0.01 in the Cox regression analysis. Three distinct subgroups (geneClusters) were revealed based on the 189 genes, in which cluster A involved the most advanced PCa (PSA > 10, T3-4, GS8-10, and biochemical recurrence) and the poorest RFS. The HLM score (HLMscore) was calculated by principal component analysis (PCA) of HLM phenotype-related genes that have positive predictive value for RFS (P < 0.001) and immune therapy responses (in the CTLA4-positive and -negative responses accompanied by a PD1-negative response).
Conclusion: We comprehensively evaluated HLM regulators in the PCa microenvironment using TCGA-PRAD, revealing a nonnegligible role of HLM patterns in PCa complexity and heterogeneity. Elucidating the effects of HLM regulators in PCa may enhance prognostics, aggressiveness assessments, and immunotherapy strategies.
Prostate cancer (PCa) is a common malignancy among men over the age of 50 (1). In 2022, there were 268,490 (27%; the highest incidence among men with all cancers) estimated new PCa cases and 34,500 (11%; the second most common cause of all cancer-related deaths among men) estimated PCa-related in the United States (1). PCa progresses to castration-resistant PCa (CRPC) after androgen-deprivation therapy (ADT); CRPC is more aggressive and can be resistant to subsequent chemotherapy, ultimately leading to cancer-related death. Therefore, identifying the molecular mechanisms associated with PCa progression is crucial for diagnosis, risk prediction, and treatment.
Nucleosome core particles can posttranslationally modify histones to maintain genomic integrity (2–4). Well-known histone modifications include methylation, acetylation, phosphorylation, and ubiquitylation, which alter the binding force of DNA to histones or recruit specific histone-binding proteins to epigenetically regulate genomic activity (4–6). Among these modifications, histone methylation is associated with heterochromatin formation and the regulation of target gene promoter activity (7, 8). Histone modifications occur on mainly the positively charged lysine (H3(K4, 9, 27, 36, and 79) and H4K20) and arginine (H3 (R2, 8, 17, and 26) and H4R3) residues in the N-terminal tails of wrapped DNA (9, 10). The histone lysine methylation (HLM) sites are highly conserved and precisely balanced by histone methyltransferases (HMT, “writers”), demethylases (HDM, “erasers), and methyl-lysine-recognizing proteins (MLRP, “readers”) (11–13).
Epigenetic reprogramming through inhibition of the enhancer of zeste homolog 2 (EZH2) has been found to enhance the effectiveness of enzalutamide (ENZ) treatment in CRPC patients (14, 15). EZH2 is a histone H3 lysine 27 (H3K27Me3) methyltransferase that silences the transcription of target genes. EZH2 has been found to be highly expressed in CRPC and neuroendocrine PCa (NEPC), and inhibition of EZH2 induces androgen receptor (AR) signaling reactivation in CRPC and further sensitivity to ADT (16–19). Studies of > 100 patients have indicated that EZH2 is a potential valuable, powerful prognostic parameter for PCa progression before or after treatment (20, 21). Authoritative studies have also elucidated that other HLM regulators, such as NSD2, SMYD3, LSD1, and DOT1L, play crucial roles in PCa progression by coordinating with transcription factors (such as AR and FOXA1) and performing the functions of histone methylation (22–27). Our previous study revealed that MLL5α (a smaller isoform of KMT2E) can prevent PCa progression by promoting AR/NDRG1 signaling via histone methylation (28). This finding implies a critical role for HLM regulators in PCa progression.
Nevertheless, few studies have focused on HLM modification patterns in different PCa risk stages and prognoses. Here, we performed a comprehensive transcriptome and genomic study of acknowledged HLM regulators (51 writers, 21 erasers, and 19 readers) in PCa by conducting bioinformatic analysis and using clinical PCa samples.
The data category of The Cancer Genome Atlas (TCGA) in transcriptome profiling, simple nucleotide variation, copy number variation (CNV), and clinical phenotype were downloaded from the Genomic Data Commons (GDC) Data Portal (https://portal.gdc.cancer.gov/) and University of California Santa Cruz Xena (UCSC) browser (https://xena.ucsc.edu/) as described in our previous study (29).
Transcripts per kilobase of exome per million (TPM) mapped reads were transformed from the transcriptome profiling data of HTSeq-FPKM. DEGs in variant phenotypes of PCa and normal prostate tissues were analyzed through the Wilcoxon or Kruskal−Wallis tests using the R packages “limma”, “reshape2”, and “ggpurb”.
The DEG-related functional and signaling pathways were evaluated through gene ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses utilizing the R packages “clusterProfiler” and “enrichplot”.
Recurrence-free survival (RFS) analyses were performed based on the clinical phenotype data of “days_to_first_biochemical_recurrence” and “days_to_last_follow_up.diagnoses”, which we previously described (29). The survival curves were generated via the Kaplan–Meier method, and statistical significance was evaluated through log-rank tests. Univariate Cox regression analysis was conducted based on the DEGs of HLM clusters (HLMclusters), and P < 0.01 was used for subsequent gene consensus clustering analysis. The survival analyses were implemented utilizing the R packages “survival” and “survminer”. Pearson or Spearman correlation analyses were performed, and a prognostic network map was drawn utilizing the R packages “igraph”, “psych”, “reshape2”, “RColorBrewer”, “corrplot”, and “ggpubr”.
To evaluate the characteristics of classifying patterns of HLM modification phenotypes in the clinicopathological features and prognosis of PCa patients, TCGA-PRAD patients were divided into subgroups by conducting consensus clustering analysis. This method identified distinct HLM modification patterns based on the expression of HLM regulators or HLM phenotype-related genes. Consensus clustering analysis was applied using the R package “ConsensusClusterPlus”. The clustering results were graphically displayed as a heatmap of the consensus matrices, consensus cumulative distribution function (CDF) plots, and delta area plots. The number of clusters and their stability were determined as previously described (29–31), including the criteria of relatively high consistency within the cluster, low variation coefficient, and no appreciable increase in the area under the CDF curve. PCA was conducted to confirm the fitness and correctness of the HLM modification patterns using the pcromp function of R software.
A set of scoring system (HLMscore) was constructed in PCa to quantify HLM modification patterns as previously described (31). The intersected HLM phenotype-related genes in the pairwise comparisons of three HLMclusters were extracted, and the significant prognostic genes were further screened via Cox regression models (P < 0.01). Then, PCA was conducted using screened genes to construct an HLM-relevant gene signature. Both principal component 1 (PC1) and component 2 (PC2) were regarded as signature scores. The HLMscore was calculated as follows:
where i = the expression of HLM phenotype-related genes.
GSVA was performed to assess the potential dysfunctional pathways in different clusters. GSVA comprehensively scored the DEGs and transformed them into KEGG pathways (32). The “KEGG gene sets as Gene Symbols” were downloaded from the website of Gene Set Enrichment Analysis (GSEA) (http://www.gsea-msigdb.org/gsea/), and the GSVA algorithm was implemented through the R packages “GSEABase” and “GSVA” to score each gene set.
TMB contributes to immune recognition of cancer and was calculated by the total number of somatic mutations per million bases (33). TCGA-PRAD patients were classified into low-TMB and high-TMB groups based on the TMB value of optimum threshold segmentation (lowest log-rank P value in the Kaplan‒Meier analysis) in the RFS analysis. The correlation between the HLM score (shown as HLMscore below) and TMB value was then analyzed.
Sigle-sample gene set enrichment analysis (ssGSEA) was performed to quantify TME cell infiltration by calculating enrichment scores (32). The gene set related to immune cell type in each TME cell infiltration was obtained as previously reported (34, 35). Then, we quantitatively analyzed the immune cell type infiltration in different HLMclusters.
As reported previously, IPS determines immunogenicity and is calculated according to the expression levels of the genes in representative cell types (36). The IPSs of TCGA-PRAD were obtained from The Cancer Immunome Atlas (TCIA) website (https://tcia.at/home) and then statistically analyzed in different HLMscore groups.
Forty-two PCa tissues (14 with Gleason score (GS) < 7, 14 with GS = 7, and 14 with GS > 7) and 14 adjacent normal tissues were collected from PCa patients in accordance with Ethics Committee guidelines. All patients underwent prostatectomy between 2016 and 2021 at Beijing Tongren Hospital and Beijing Chaoyang Hospital. Table 1 summarizes the clinicopathological characteristics of the patients.
Total RNA extraction and complementary DNA (cDNA) reverse transcription were performed using TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA) and One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. qPCR was performed with Top Green qPCR SuperMix (TransGen Biotech) on an SDS 7500 FAST Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The endogenous reference genes 18S ribosomal RNA and GAPDH were used as controls. Supplementary Table 1 (Table S1) shows the relevant primer sequences.
R software (4.0.3), SPSS software version 23 (IBM, Armonk, New York, USA), Microsoft Excel 2019 software (Microsoft Corp., Redmond, WA, USA), and GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA) were used to conduct the statistical analyses. Continuous data were analyzed using Wilcoxon or Kruskal–Wallis tests. Pearson and Spearman correlation analyses were used to determine the association between two variables. Kaplan–Meier survival analyses with a log-rank test and univariate Cox regression models with a hazard ratio (HR) were utilized for the survival analyses.
This study was conducted using CNV and TPM mapped reads in the TCGA-PRAD dataset. The flow chart of this study is shown in Figure 1. The DEGs of HLM regulators in TCGA-PRAD data stratified by CNV loss or gain (CNV), tumor vs. normal (TN), GS > 7 vs. GS < 7 (GS), pT3 vs. pT2 (pT), TP53 mutation vs. wild type (TP53), and RFS high vs. low expression were analyzed, and the intersecting genes were validated using clinical PCa tissues. Then, the TCGA-PRAD patients were divided into three groups (HLMcluster) based on 91 HLM regulators via consensus clustering analysis. The HLM phenotype-related genes overlapped in the pairwise comparisons of HLMclusters were screened via univariate Cox regression analysis. TCGA-PRAD patients were further divided into three groups (geneCluster) according to the expression of screened genes, and the HLMscore was calculated through PCA of the HLM phenotype-related genes. The clusters (HLMclusters and geneClusters), screened genes, and HLMscores were evaluated using RFS, TMB, and TME analyses.
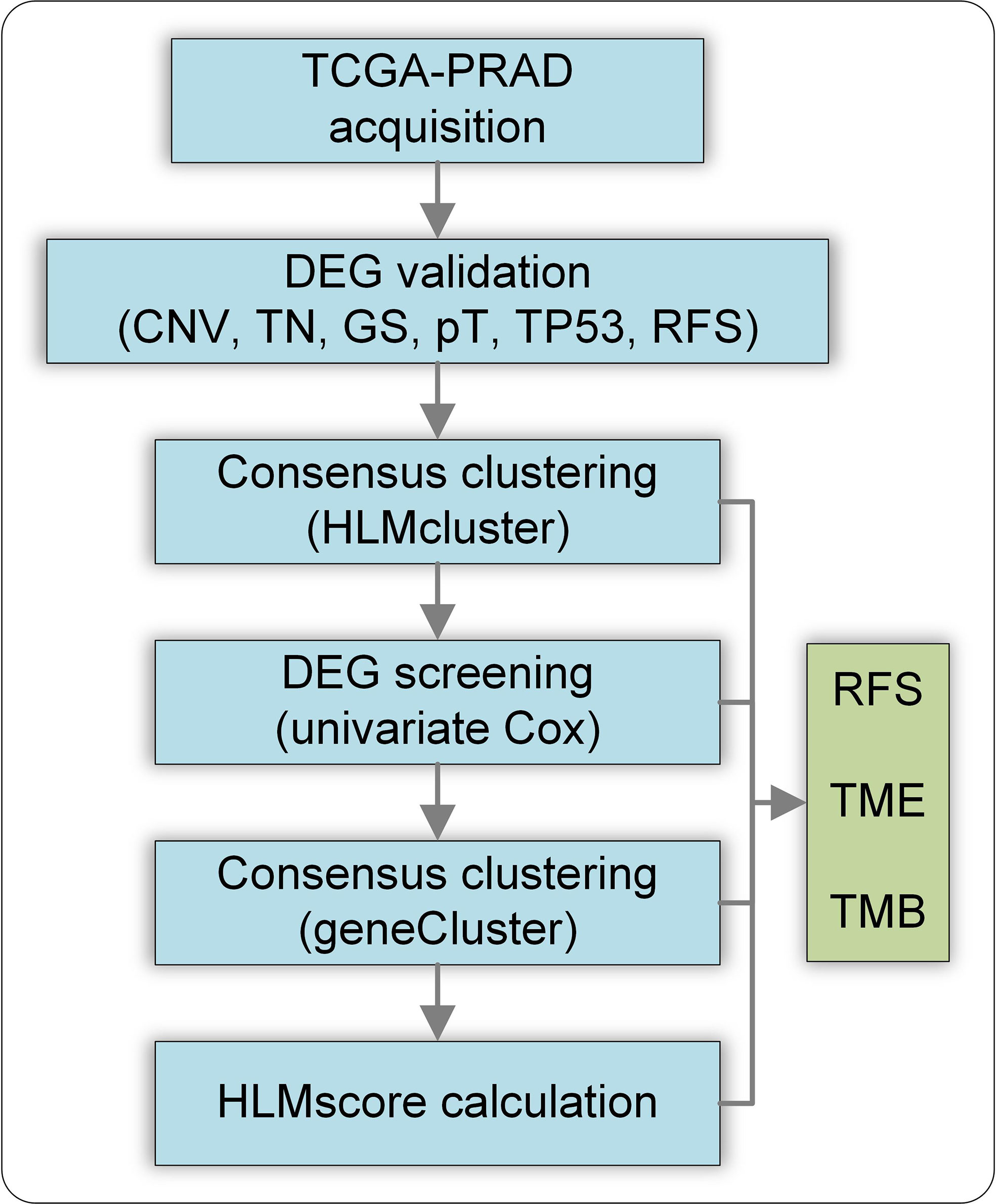
Figure 1 Flow chart of this study. CNV, CNV loss or gain; TN, tumor vs. normal; GS, GS >7 vs. GS< 7; pT, pT3 vs. pT2 (pT); TP53, TP53 mutation vs. wild type; RFS, RFS high vs. low expression of genes.
The average estimated CNVs were previously considered a method to determine PCa malignancy (37). We analyzed the CNV alteration frequency and DEGs in HLM regulators in TCGA-PRAD and normal tissues. Regarding CNV events, approximately 7.87% (7 of 89) of HLM regulators had widespread CNV deletion levels (> 10%), among which SETDB2 (25.50%), PRDM13 (21.91%), and PRDM1 (21.31%) had the three highest degrees of copy number loss. All HLM regulators had a low proportion of CNV amplification (< 5%), with MECOM (4.58%) and SETDB1 (4.58%) having the highest CNV gain (Figure 2A). The chromosomal location and CNV alterations in HLM regulators are shown in Figure S1A. DEGs in HLM regulators in PCa and normal prostate tissues were statistically analyzed using the TPM dataset of TCGA-PRAD. Twenty-six HLM regulator genes with significantly higher expression levels (including EZH2, PRDM12, and KMT5C) and 25 with lower expression levels (including MECOM, PRDM11, CBX7, and so on) were found in PCa tissues compared with normal prostate tissues (P < 0.05) (Figure 2B and Table S2). Unlike the findings in a previous study of gastric cancer (31), no significant correlation between CNV alteration and DEG expression was found in TCGA-PRAD. In the mutation frequency analysis, we found that only 58 gene symbols of HLM regulators transformed from the “ensemble ID” were matched with the gene symbol in the TCGA-PRAD data category of “simple nucleotide variation” and data type of “Masked Somatic Mutation”. Among the 58 identified HLM regulators, mutations were found in 112 of 484 samples (23.14%), among which KMT2D (24 of 484), KMT2C (20 of 484), and KDM6A (11 of 484) were the most frequently mutated genes in TCGA-PRAD (Figure S1B). Nevertheless, most of the genes exhibited low mutation rates (≤ 1%), implying highly conserved and stable expression levels of HLM regulators in PCa.
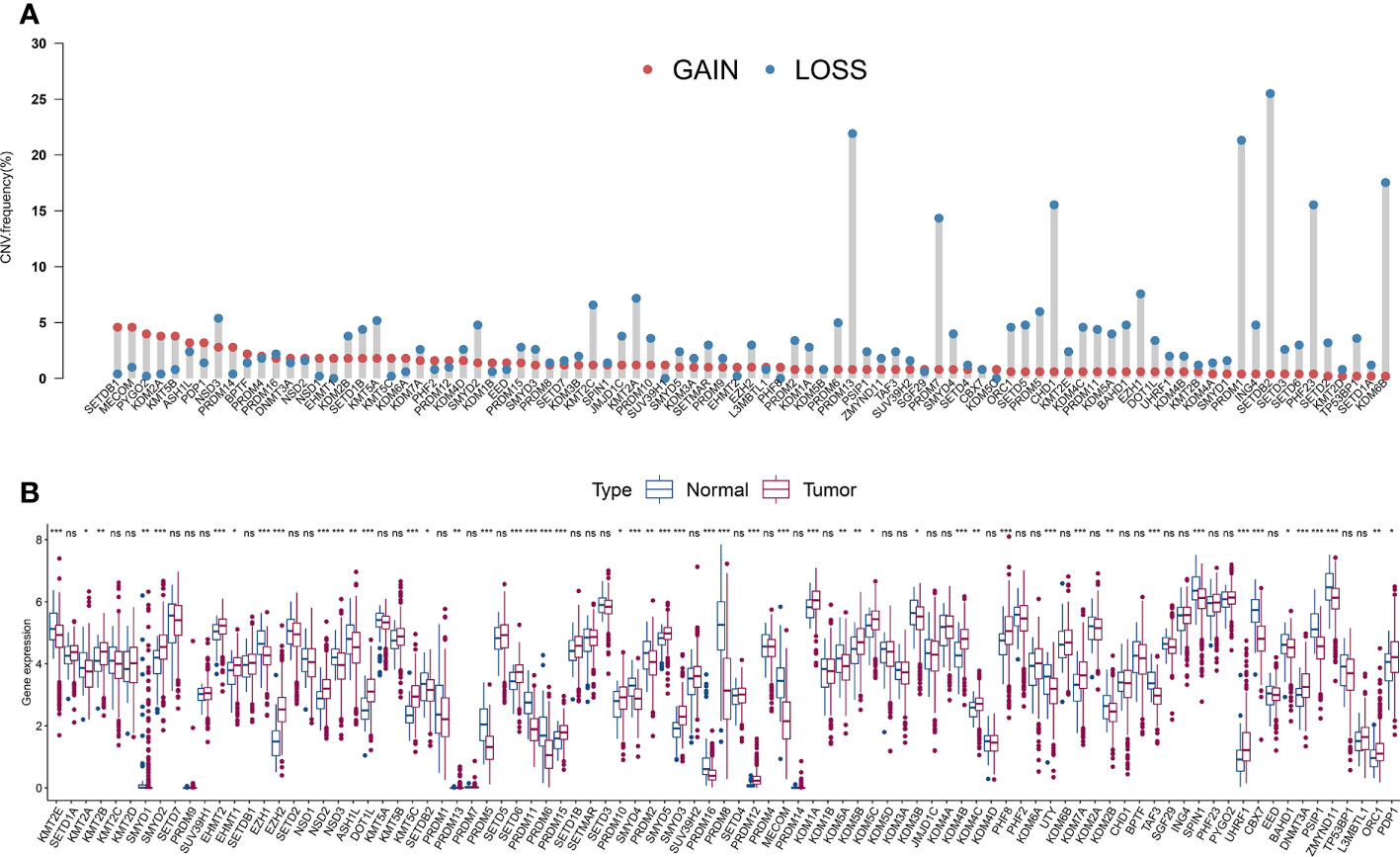
Figure 2 The CNV variation frequency and the expression levels of HLM regulators in PCa. (A) The CNV variation frequency of HLM regulators in TCGA-PRAD. Red dot, amplification frequency; Blue dot, deletion frequency. (B) The expression levels of the 91 HLM regulators in PCa and normal prostate tissues are shown as boxplot. The values of median ± interquartile ranges are shown in the graph. ns P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
PCa patients with GS ≥ 7 were previously reported to have more aggressive disease and a worse prognosis than those with GS < 7 (38, 39). Hence, we divided TCGA-PRAD into three groups: 45 with GS < 7; 246 with GS = 7; and 204 with GS > 7. Next, we estimated the expression levels of HLM regulators using TCGA-PARD TPM data. Compared with those in the GS < 7 group, the expression levels of EZH2, ORC1, UHRF1, NSD2, KMT5C, PRDM12, EED, DNMT3A, KDM2A, and KDM2B were significantly elevated in the GS > 7 group (P < 0.001) (Figure 3A and Table S3). The DEGs in HLM regulators in different pathological T (pT) stages (187 with T2; 291 with T3; and 10 with T4) were also analyzed, and UHRF1, EZH2, ORC1, NSD2, EED, DNMT3A, KMT5C, and PRDM12 were significantly elevated in T3 stage PCa compared with T2 stage PCa (P < 0.001) (Figure 3B and Table S4).
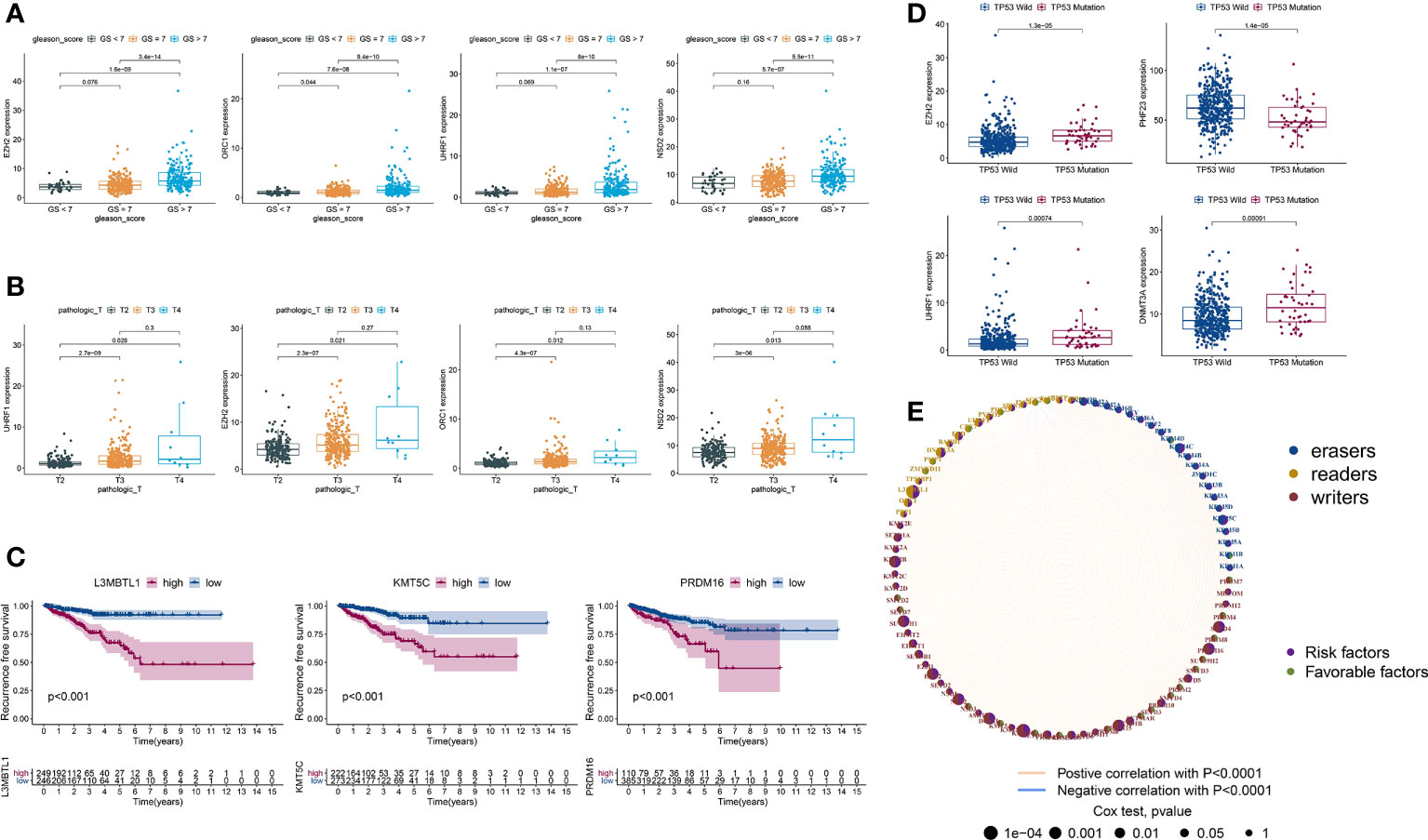
Figure 3 DEGs of HLM regulators in relation to different PCa clinicopathological characteristics and prognoses. (A–D) Distribution of the 3–4 lowest P values of HLM regulators in TCGA-PRAD data stratified by GS (GS > 7 vs. GS < 7) (A), pT (T3 vs. T2) (B), RFS (lowest log-rank P value in the Kaplan‒Meier analysis) (C), and TP53 (mutation vs. wild type) (D). The boxplots show the median ± interquartile range values, and P values are presented above each pair of comparisons. (E) Prognostic network of interactions among HLM regulators in PCa. Different circle sizes represent the P values of each HLM regulator with respect to the prognosis. Right hemisphere of purple, risk factors for RFS; green, favorable factors for RFS. Left hemisphere of blue, erasers; orange, readers; red, writers. Lines of pink, positive correlations of HLM regulators; blue, negative correlations.
In the survival analysis of Kaplan–Meier curves and univariate Cox regression analysis, TCGA-PRAD patients were divided into two groups according to the optimum threshold segmentation of the expression levels of HLM regulators, which had the lowest log-rank P value in the Kaplan−Meier analysis. High expression levels of KMT5C, L3MBTL1, PRDM16, NSD2, KMT2B, SUV39H1, SETD4, DOT1L, EZH2, and PRDM15 were associated with poor RFS rates (HR > 1 and P value of Cox regression analysis < 0.001) (Figure 3C). PCa with TP53 mutation was previously reported to have poor radiographic progression-free survival (rPFS) rates and a shorter time to CRPC progression (40). We divided TCGA-PRAD into two groups according to TP53 mutation status (44 with TP53 mutation and 429 with TP53 wild-type) and found that the expression levels of EZH2, PHF23, UHRF1, DNMT3A, and SMYD2 were differentially expressed between the TP53 mutation and wild-type groups (P < 0.001) (Figure 3D and Table S5). The comprehensive landscape of HLM regulator interactions and prognosis based on RFS outcomes is depicted in the network (Figure 3E).
We then analyzed the intersecting differentially expressed HLM regulators by comparing TN (tumor versus (vs.) normal), GS (GS > 7 vs. GS < 7), pT (T3 vs. T2), RFS (high vs. low in the univariate Cox regression analysis), and TP53 (mutation vs. wild type) (P < 0.05). Six intersecting DEGs of EZH2, NSD2, KMT5C, UHRF1, ORC1, and DNMT3A were found in the 5 comparisons, and all were consistently highly expressed in tumor and other advanced-stage parameters (in the GS > 7, pT3, HR > 1, and TP53 mutation groups) (Figure 4A). Forty-two clinical PCa tissues were used to validate these oncogenes, including 14 adjacent normal prostate tissues, 14 with GS < 7, 14 with GS = 7, and 14 with GS > 7 (Table 1). The expression levels of 6 genes were assessed via qPCR analysis. Compared with adjacent normal tissues, the relative expression levels of EZH2, NSD2, KMT5C, and UHRF1 were higher in PCa tissues (P < 0.05), but no significant differences were found in the expression levels of ORC1 and DNMT3A (P > 0.05) (Figure 4B). The expression levels of EZH2, NSD2, and KMT5C were significantly higher in the GS > 7 group than in the GS < 7 group (P < 0.05) (Figure 4C). These data confirmed the roles of EZH2, NSD2, and KMT5C oncogenes in the occurrence and progression of PCa.
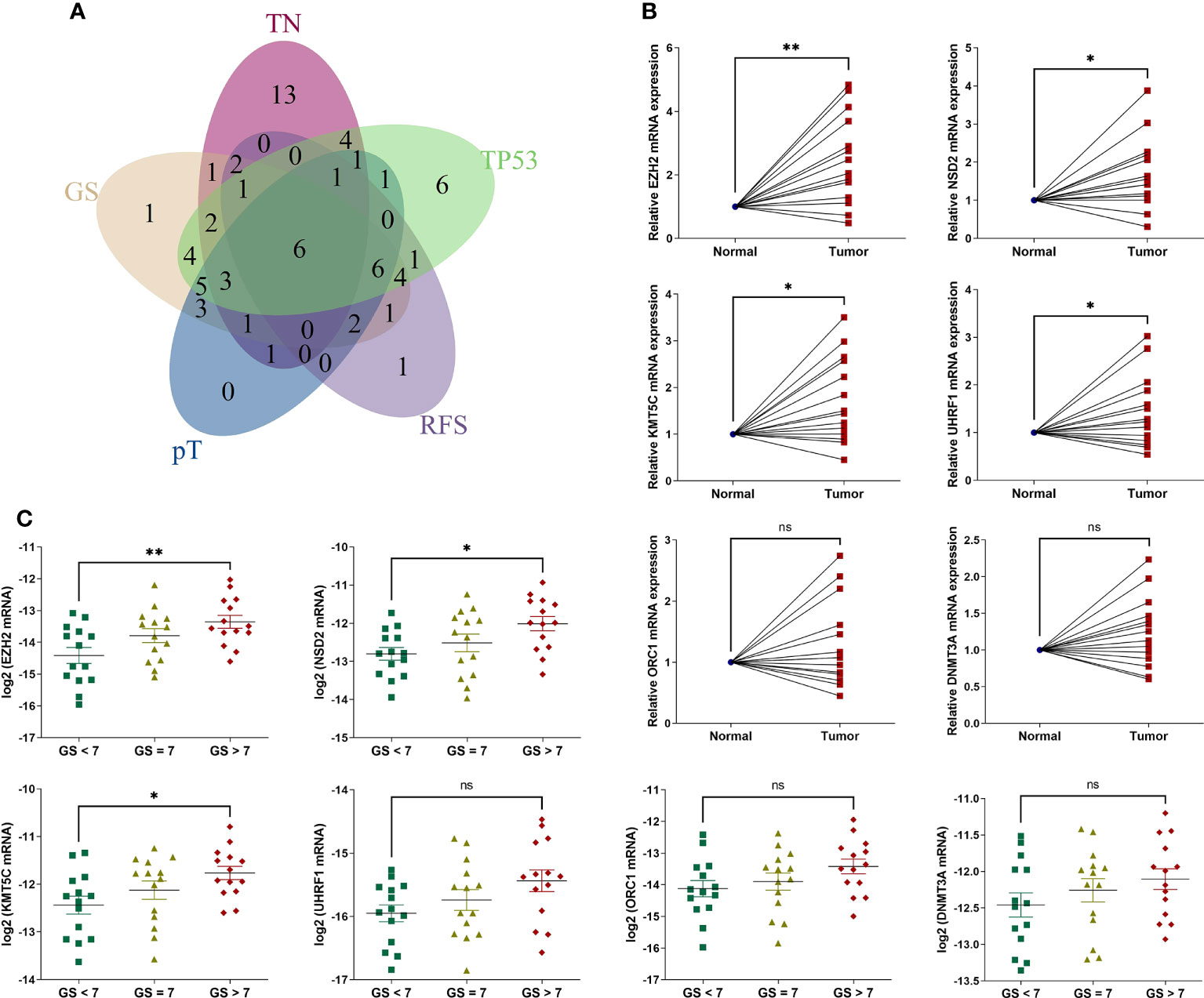
Figure 4 The expression levels of 6 intersecting differentially expressed HLM regulators in clinical PCa tissues. (A) The intersecting DEGs of 91 HLM regulators in relation to the comparisons of TN (tumor vs. normal PCa tissues), GS (GS > 7 vs. GS < 7), pT (T3 vs. T2), RFS (P value of univariate Cox regression analysis < 0.05), and TP53 (mutation vs. wild type) are shown as a Venn diagram. (B, C) The expression levels of 6 intersecting HLM regulators (EZH2, NSD2, KMT5C, UHRF1, ORC1, and DNMT3A) were compared in PCa vs. adjacent normal prostate tissues (B) and GS > 7 vs. GS = 7 vs. GS< 7 (C). The results were normalized to adjacent normal tissues or those of endogenous reference genes. The means ± SEMs are shown in the graphs. ns P > 0.05; *P < 0.05; **P < 0.01.
We divided TCGA-PRAD patients into subgroups (HLMcluster) through consensus clustering analysis using HLM regulators to explore the influence of HLM modification patterns on PCa prognosis and immune cell infiltration. The cluster number, k = 3, was determined to be the optimal category number considering the appreciable delta area under the CDF curves (Figure 5A). The transcriptome profiles of HLM regulators between different clusters (named “clusters 1–3 or A-C”) were significantly distinguished by PCA (Figure 5B). The levels of HLM regulators were integrally highly expressed in cluster A and weakly expressed in cluster B (Figure 5C). Among the three clusters, the number of biochemical recurrences, pathological N1 (pN1), and PSA grade 3 (grade 1: > 0 and < 1, grade 2: 1–10, grade 3: > 10) stages were visually lowest in cluster B, implying relatively low malignancy of PCa in cluster B (Figure 5C). The different KEGG pathways were analyzed via GSVA in every pairwise comparison among the three clusters (Figure S2A-C). The KEGG lysine degradation, adherens junction, and neurotrophin signaling pathways were dysregulated in the comparison of clusters A and B (Figure S2A). However, the RFS Kaplan–Meier curves for the HLMclusters revealed no significant differences among these subgroups (P = 0.194) (Figure 5D). Thus, HLM clusters may not be suitable for prognostic risk prediction. Immune cells can infiltrate more oncogenic mutated tumors, and these tumors are more sensitive to immunotherapy (41, 42). We analyzed immune cell infiltrations in three HLMclusters and found that 13 of 23 subpopulations of immune cells were significantly differentially infiltrated among these clusters (Figure 5E and Table S6).
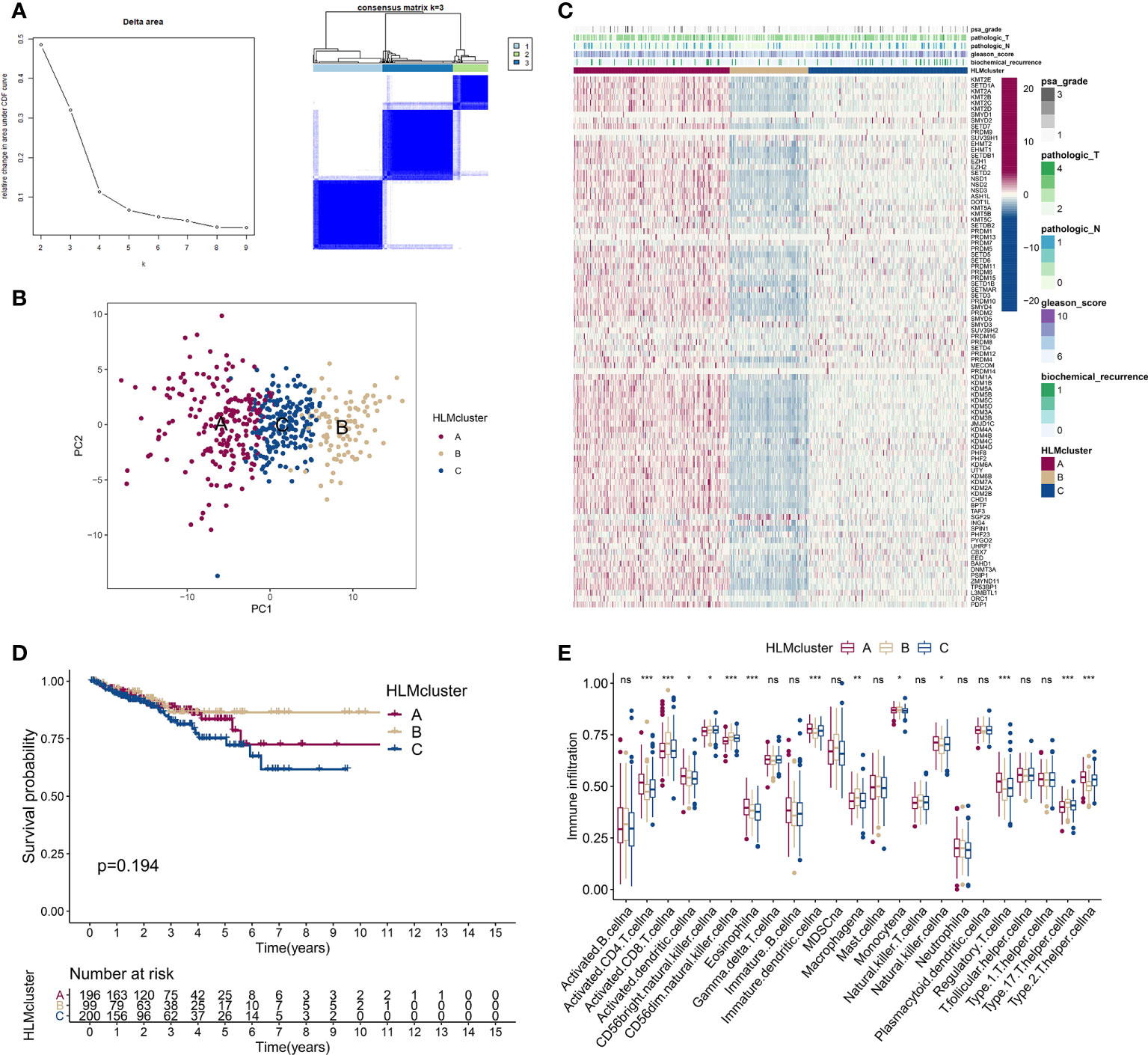
Figure 5 Consensus clustering analysis based on HLM regulators. (A) (left) Relative changes in the area under the CDF curve from k = 2 to 9. (right) Color-coded heatmap of the consensus matrix for k = 3 obtained from applying consensus clustering. Color gradients represent values from 0–1: white: 0; dark blue: 1. (B) PCA of the transcriptome profiles of HLM regulators in three HLMcluster patterns. Red, orange, and blue dots in the scatter diagram represent HLMclusters (A–C) respectively. (C) The expression levels of HLM regulators in the unsupervised three HLMclusters were estimated, and the distribution of the various clinicopathological characteristics of TCGA-PRAD are shown as a heatmap. PSA grade, pT, pN, GS, and biochemical recurrence were used for patient annotation. Red in the heatmap, high expression; blue, low expression. (D) RFS analysis among the three HLMclusters using Kaplan–Meier curves. Log-rank P values are shown in the graph, and the numbers at risk are shown at the bottom. (E) The abundance of infiltration of each immune cell type among the three HLMclusters is shown in the boxplot. The values of the median ± interquartile range are shown in the graph. ns P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
We screened DEGs (P value of Bayes test < 0.00001) in every pairwise comparison among the three HLMclusters to investigate the effect of downstream genes of HLM regulators and found 3,297 intersecting genes via a Venn diagram (Figure 6A). In the GO enrichment analysis, epigenetic reprogramming, such as covalent chromatin modification, histone modification, RNA splicing, nuclear speck, and transcription coregulator activities, was significantly enriched according to the DEGs (Figure S3A, B). KEGG pathway signaling enrichment analysis of the DEGs revealed that nucleocytoplasmic transport, protein processing in the endoplasmic reticulum, ubiquitin-mediated proteolysis, and mRNA surveillance pathways were dysregulated (Figure S3C, D).
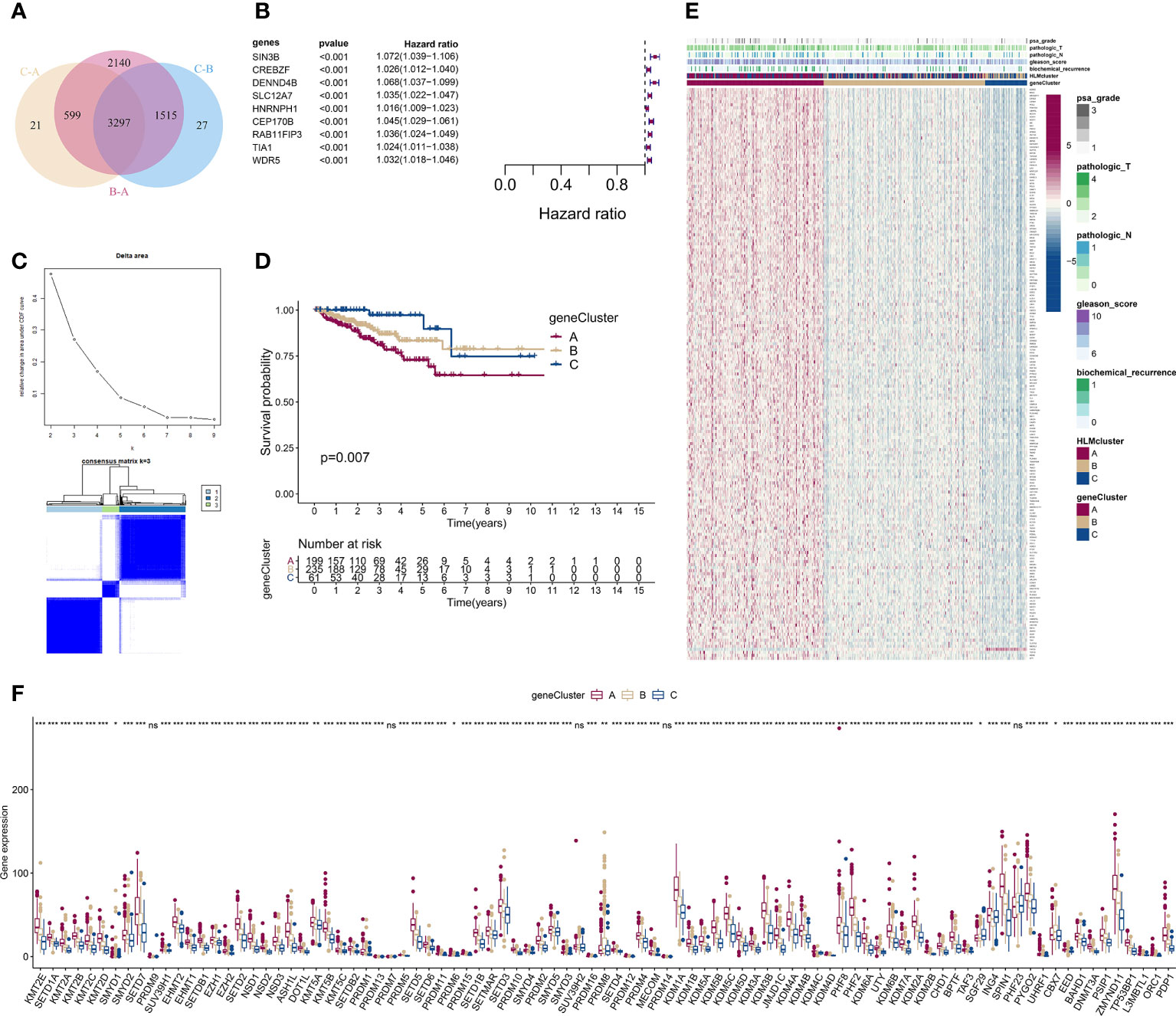
Figure 6 Consensus clustering analysis based on 189 HLM phenotype-related genes. (A) Intersecting DEGs identified in every pairwise comparison of HLMclusters are shown as a Venn diagram. (B) Univariate Cox regression analysis for RFS was performed in relation to 3,297 intersecting genes. The statistical significance (P in the Kaplan–Meier survival and Cox regression analysis < 0.001) of 9 genes (SIN3B, CREBZF, DENND4B, SLC12A7, HNRNPH1, CEP170B, RAB11FIP3, TIA1, and WDR5), P value, and HR value (with 95% confidence interval (CI)) are shown in the plot. (C) Consensus clustering analysis based on the 189 HLM phenotype-related genes (overlap in the pairwise comparisons of HLMclusters and P < 0.01 in the Cox regression analysis) was performed. (up) Relative change in area under the CDF curve from k = 2 to 9. (down) Color-coded heatmap of the consensus matrix for k = 3. Color gradients represent values from 0–1: white: 0; dark blue: 1. (D) RFS analysis among the three geneClusters using Kaplan–Meier curves. Log-rank P values are shown in the graph, and the numbers of at-risk patients are shown at the bottom. (E) The expression levels of 189 HLM phenotype-related genes in the unsupervised three geneClusters were estimated, and the distribution of the various clinicopathological characteristics of TCGA-PRAD are shown as a heatmap. PSA grade, pT, pN, GS, biochemical recurrence, and HLMclusters were used for patient annotation. Red in the heatmap, high expression; blue, low expression. (F) Boxplot of the expression levels of HLM regulators among the three geneClusters. The values of the median ± interquartile range are shown in the graph. ns P > 0.05; * P < 0.05; ** P < 0.01; ***P < 0.001.
We then performed a univariate Cox analysis using these DEGs and found that 189 genes (HLM phenotype-related genes) had significant differences in the context of RFS outcomes (P value of Cox regression< 0.01). Nine genes (SIN3B, CREBZF, DENND4B, SLC12A7, HNRNPH1, CEP170B, RAB11FIP3, TIA1, and WDR5) among these had a P value < 0.001 in the Kaplan–Meier survival and Cox regression analysis (Figure 6B).
We next evaluated the characteristics of classify patterns of HLM phenotype-related genes. Three consensus clusters (geneCluster 1–3 or A–C) of TCGA-PRAD patients were identified by analyzing 189 HLM phenotype-related genes (Figure 6C). In the survival analysis, significant differences in the RFS outcomes were found among geneClusters, and cluster A had the poorest RFS rate (P value of Kaplan–Meier survival analysis = 0.007) (Figure 6D). The distribution of clinicopathological characteristics in the geneClusters showed that the number of biochemical recurrences, GS, pN, pT, and PSA grade 3 were visually highest in geneCluster A (Figure 6E). The TME of immune cell infiltration characteristics in the three geneClusters were evaluated, and 7 of 23 subpopulations of immune cells were differentially infiltrated among these clusters (Figure S4A and Table S7). The expression levels of HLM regulators in the geneClusters revealed that 86 of 91 HLM regulators were significantly dysregulated, most of which were highly expressed in cluster A (Figure 6F and Table S8).
Considering the complexity of HLM regulators and the individual heterogeneity of PCa patients, the patterns of HLM modification in individual patients were quantitatively assessed (HLMscore) by constructing a set of scoring system by PCA of the 189 HLM phenotype-related genes (Figure 7A). The HLMscores in geneClusters/HLMclusters were analyzed and were significantly different in both clusters; geneCluster C and HLMcluster B had the highest values (Figure 7B). In the survival analysis, TCGA-PRAD patients were divided into two groups according to the optimum threshold segmentation of the HLMscore with the lowest log-rank P value in the Kaplan−Meier analysis (202 with low-HLMscore group and 293 with high-HLMscore group). The results showed that the low-HLMscore group was associated with poor RFS outcomes (P < 0.001) (Figure 7C). A low HLMscore was also associated with a higher rate of biological recurrence (fustat of 1) (19%), and the recurrence status was matched with lower HLMscores (Figure 7D). We then analyzed the RFS outcomes of the HLMscore groups stratified by clinicopathological features of GS and pT (Figure 7E and Figure S4B, C). The low HLMscore group also exhibited poor RFS outcomes in the GS 8-10 (P = 0.004) and pT3 (P < 0.001) stages (Figure 7E). A summary of the attribute changes in HLMcluster, geneCluster, HLMscore, and biological recurrence status is shown in the alluvial diagram (Figure 7F). Consistent with the findings above, most patients in geneCluster A were classified into the low-HLMscore group, which is relevant to the higher rate of biological recurrence.
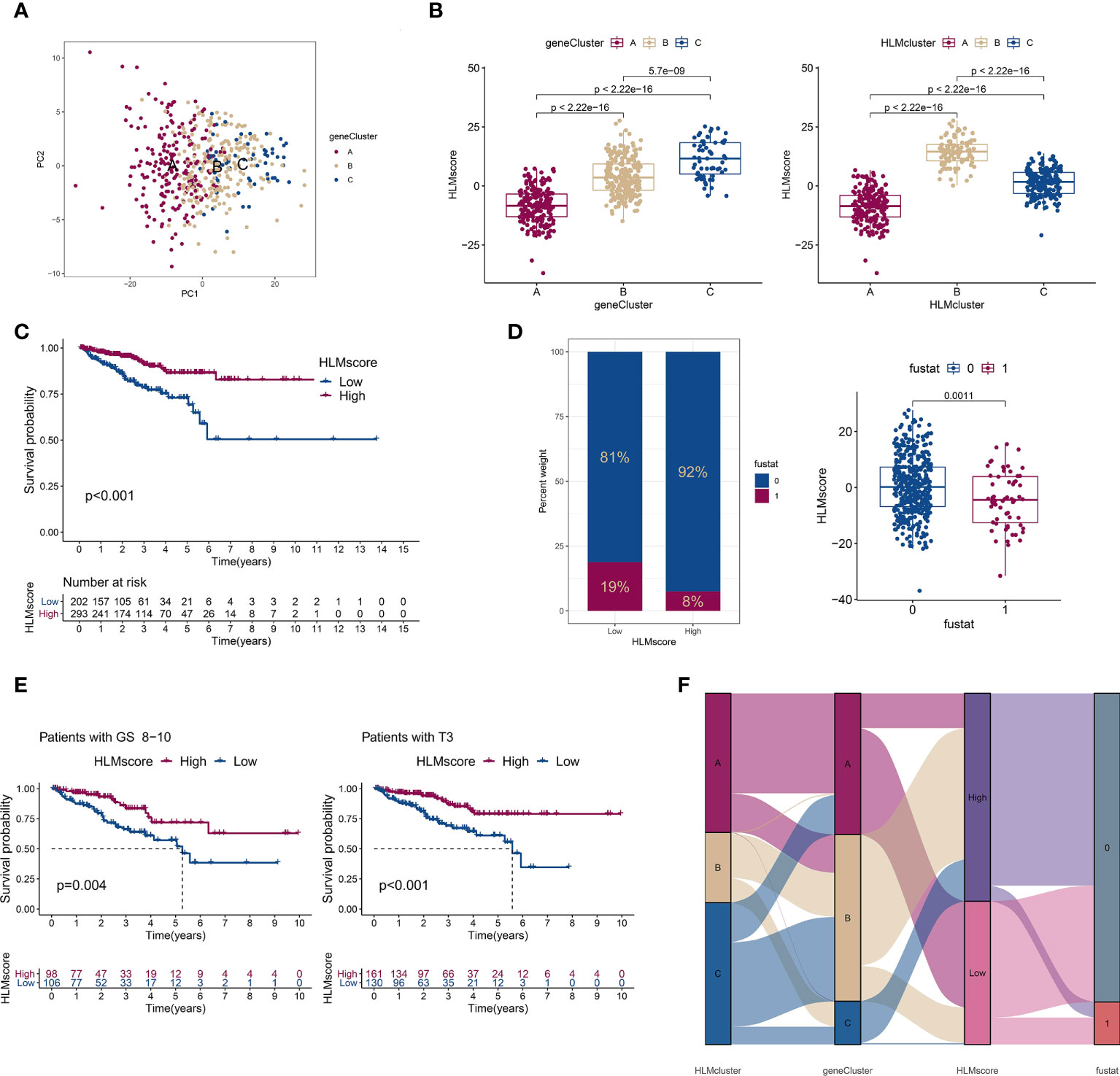
Figure 7 The low-HLMscore group based on the 189 HLM phenotype-related genes was associated with poor prognosis in terms of RFS. (A) PCA of the transcriptome profiles of 189 HLM phenotype-related genes in three geneGluster patterns. Red, orange, and blue dots in the scatter diagram represent geneClusters A to C, respectively. (B) The HLMscore was calculated via PCA based on 189 gene levels in TCGA-PARD. Then, we compared the levels of the HLMscore among geneClusters (left) and HLMclusters (right). The values of median ± interquartile range and P are shown in the boxplots. (C) TCGA-PRAD patients were divided into two groups (202 with low-HLMscore group and 293 with high-HLMscore group) according to the optimum threshold segmentation of the HLMscore in relation to the lowest log-rank P value in the Kaplan‒Meier analysis. RFS analysis of the two HLMscore groups is performed using Kaplan–Meier curves. (D) (left) The proportion among TCGA-PRAD patients of biochemical recurrence status in low- and high-HLMscore groups (fustat of 0: no recurrence; 1: recurrence). (right) HLMscores in the different statuses of biochemical recurrence. The P value of the Wilcoxon test is shown in the boxplot. (E) RFS analysis of the two HLMscore groups among TCGA-PRAD patients with GS 8–10 (left) and pT3 (right). (F) The attribute changes of TCGA-PRAD in HLMclusters, geneClusters, HLMscore, and recurrence status are shown as an alluvial diagram.
TMB is positively associated with oncogenic mutations and the immunotherapeutic response (41, 42). The correlation between the HLMscore and TMB was analyzed to estimate PCa malignancy. However, the results showed that there was no difference in the level of TMB between the low- and high-HLMscore groups (P = 0.4), and TMB also showed no correlation with the HLMscore in the Spearman correlation analysis (R = -0.058 and P = 0.21) (Figure 8A). The patients were first divided into two groups (the high- and low-TMB groups) according to the TMB value via an RFS analysis as previously reported (29). Then, the patients were further divided into four groups by combining the HLMscore and TMB groups. As shown in Figure 8B, the high-TMB with low-HLMscore group had the poorest RFS among the four groups. The distribution of somatic mutations in the low- and high-HLMscore groups indicated a more extensive mutation frequency of TP53 (13% vs. 7%) and a reduced frequency of SPOP (5% vs. 14%) mutation in the low-HLMscore group vs. the high-HLMscore group (Figure 8C).
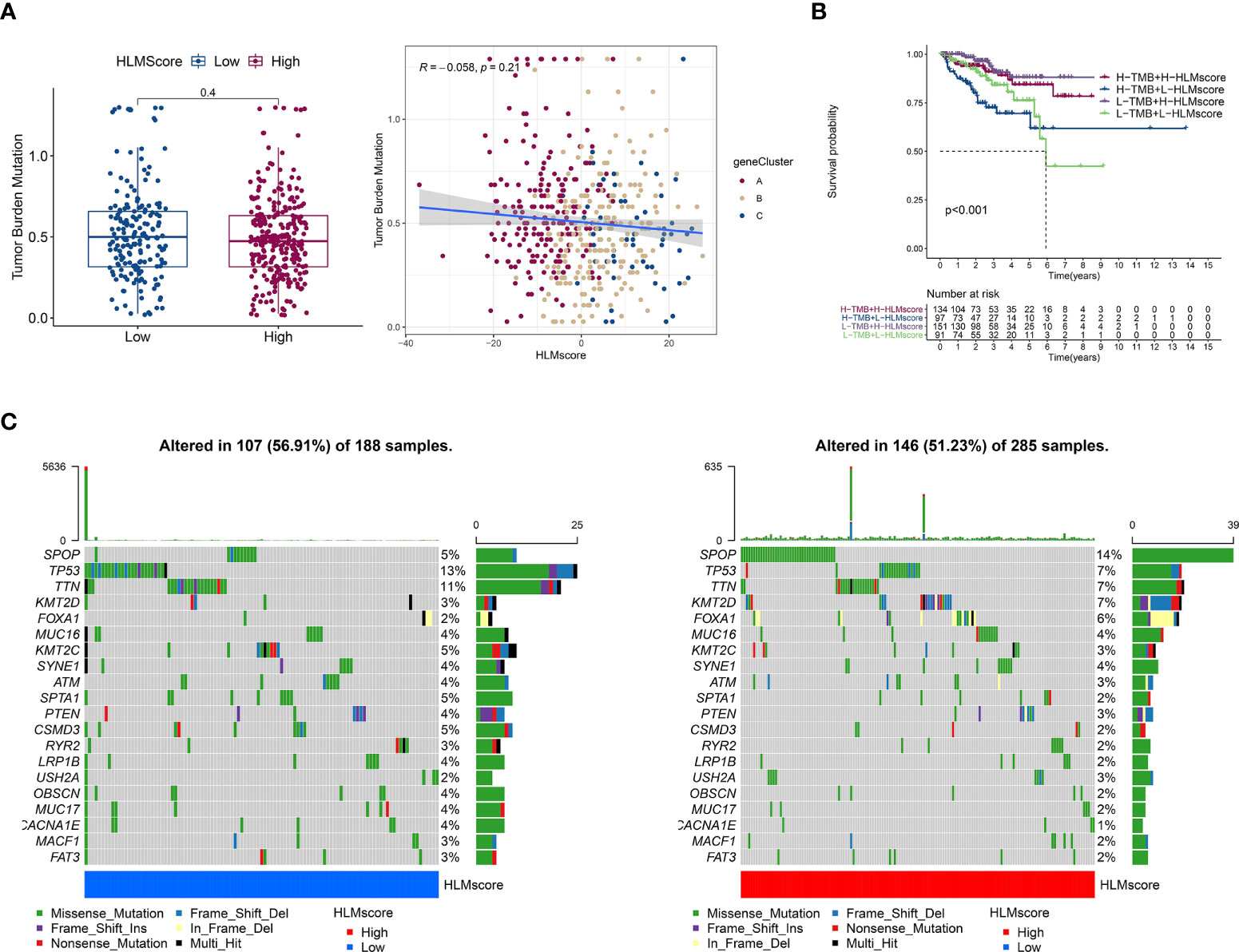
Figure 8 Characteristics associated with TCGA-PRAD tumor mutations based on HLMscore status. (A) (left) TMB values of the two HLMscore groups. The P value of the Wilcoxon test is shown in the boxplot. (right) Spearman correlation analysis between the HLMscores and TMB values in TCGA-PRAD was performed and is shown as a scatter diagram. Red, orange, and blue dots represent geneClusters A to C, respectively. (B) TCGA-PRAD patients were divided into two statuses (low and high TMB) according to the optimum threshold segmentation of TMB values in relation to the RFS analysis. Then, the TCGA-PRAD patients were further split into four groups based on TMB and HLMscore status (high (H)-TMB + H-HLMscore; H-TMB + low (L)-HLMscore; L-TMB + H-HLMscore; and L-TMB + L-HLMscore), and survival analysis for RFS was performed. (C) The somatic mutations of TCGA-PRAD patients with low-HLMscore (left) and high-HLMscore (right) groups are shown as waterfall plots.
The correlation between the HLMscore and genes related to immune-related cell infiltration was analyzed to better illustrate the HLMscore characteristics based on immune cell infiltration. Previous research suggested that low immune-cell infiltration was markedly related to prolonged overall survival times (30). Our results revealed that 8 of 23 immune cell infiltrates were negatively correlated with the HLMscore, and 4 were positively correlated (Figure 9A). Thus, it is unlikely that the HLMscore and immune response patterns interact with each other to influence RFS prognosis (Figure 7C).
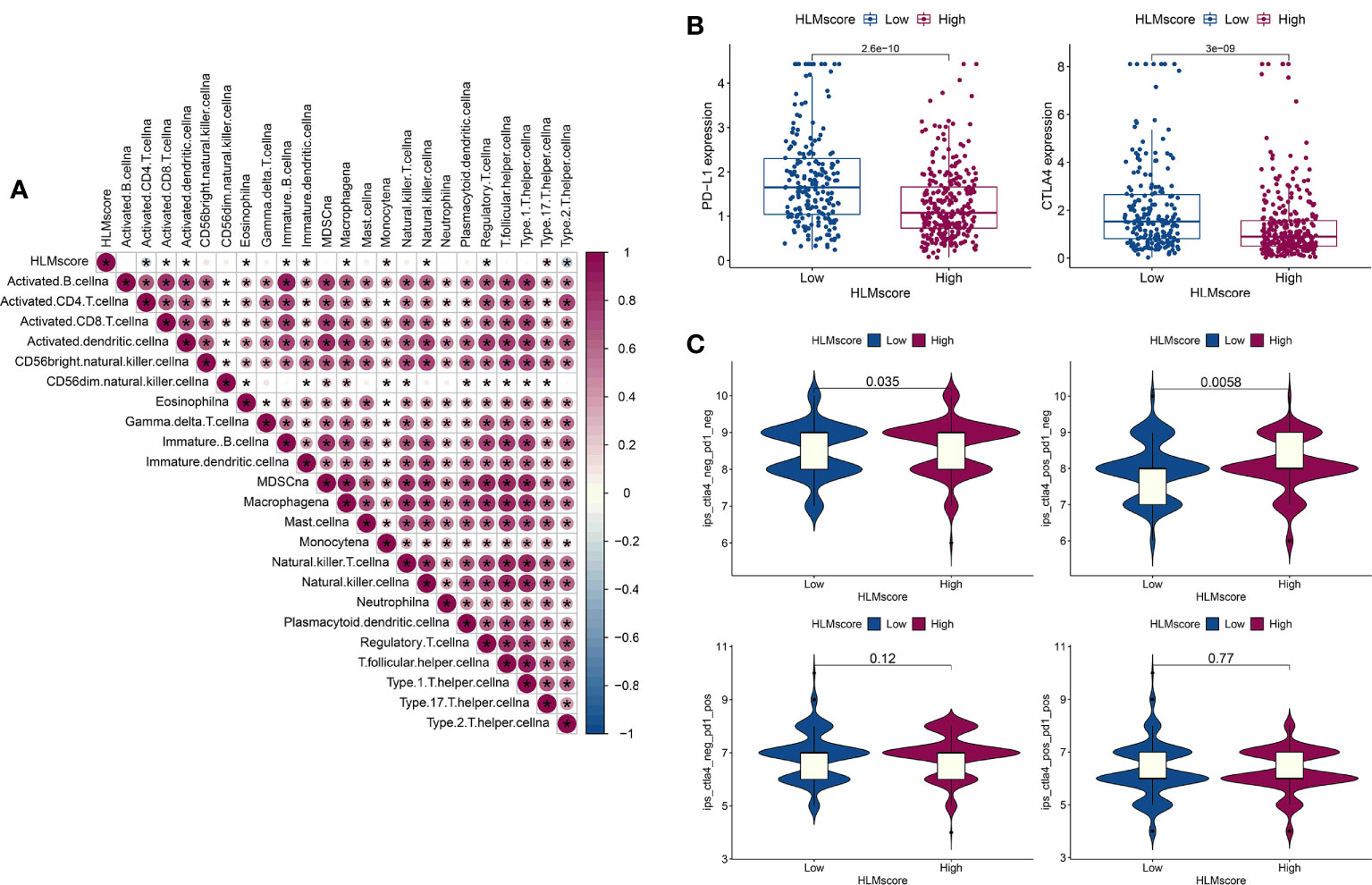
Figure 9 Characteristics of the HLMscore status in PCa with an immune-related microenvironment. (A) Correlation analysis between the HLMscore and infiltrating immune cell type in TCGA-PRAD. Red, positive correlation, blue, negative correlation.*P < 0.05. (B) The expression levels of PD-L1 and CTLA4 in the two HLMscore groups are shown as boxplots. (C) The IPS scores in ips_ctla4_neg_pd1_neg (CTLA4 and PD1 negative response), ips_ctla4_pos_pd1_neg (CTLA4 positive and PD1 negative response), ips_ctla4_neg_pd1_pos, and ips_ctla4_pos_pd1_pos in relation to the respective HLMscore groups are shown as violin plots. The P values of the Wilcoxon test are shown in the violin plots.
Immunotherapy as represented by immunological checkpoint (PD-1/L1 and CTLA-4) blockade (ICB) was confirmed to have pronounced efficacy in the clinical treatment of durable response patients (43). However, unfortunately, the majority of tumor patients are insensitive and experience minimal or no clinical benefit, which is far from ideal (43). Predicting sensitivity to ICB is key for improving ICB therapeutic success and exploring novel immunotherapeutic strategies (42, 44). PD-L1 and CTLA4 expression in the HLMscore groups was evaluated to determine the potential sensitivity to immunotherapy. The results showed that both PD-L1 and CTLA4 were highly expressed in the low-HLMscore group (Figure 9B). We then performed value prediction of the risk score for ICB. TCGA-PRAD patients were classified into the following four groups: ips_ctla4_neg_pd1_neg (CTLA4 and PD1 negative response); ips_ctla4_pos_pd1_neg (CTLA4 positive and PD1 negative response); ips_ctla4_neg_pd1_pos; and ips_ctla4_pos_pd1_pos (Figure 9C). The results showed that CTLA4-positive and -negative responses accompanied by PD1-negative regions (ips_ctla4_pos/neg_pd1_neg) had significantly different IPSs in the two HLMscore groups (P < 0.05) but not in the PD1-positive regions (ips_ctla4_pos/neg_pd1_pos) (P > 0.05) (Figure 9C). This finding suggests that the HLMscore groups in this risk score model may indicate a better prediction efficacy to predict insensitivity to PD1 blockade.
In this study, we performed bioinformatic analysis to evaluate the extensive regulatory mechanism of the HLM modification in PCa for the first time using the TCGA-PRAD dataset. We found that HLM modification patterns play a crucial role in PCa aggressiveness, prognosis, and prediction of immunotherapy sensitivity. Three representative HLM regulators (EZH2, NSD2, and KMT5C) were found to serve as highly valuable biomarkers in advanced PCa.
In general, the molecular mechanism of PCa progression remains unclear. Increasing evidence has demonstrated that epigenetic reprogramming through HLM can accelerate the development of PCa (14, 15). Epigenetic regulation by the degree of HLM occurs through alterations in gene transcription, chromatin structure, and mitosis (45). The most acknowledged HLM regulator is EZH2, a subunit of polycomb repressive complex 2 (PRC2) that silences gene expression via H3K37me3 methyltransferase activity and has been reported to be associated with the progression of CRPC and NEPC (16–19, 46). Unlike its role as a transcriptional repressor, the phosphorylation of EZH2 is associated with both coactivation (16, 47, 48) and corepression (15, 18) of AR transcriptional activity in PCa. Previous patient studies confirmed the value of EZH2 as a PCa prognosis parameter before (20) or after (21) treatment.
Moreover, as an H3K4 methyltransferase, SMYD3 can epigenetically upregulate AR expression by binding to the AR promoter region and is further associated with PCa tumorigenesis (25). R. Vatapalli et al. reported that the H3K79 methyltransferase DOT1L selectively regulates tumorigenicity and is associated with poor PCa outcomes by coordinating with AR and regulating MYC transcription (26). LSD1 acts as a transcriptional repressor by associating with FOXA1 through the demethylation of H3K4; it also acts as an AR coactivator, and an LSD1 inhibitor suppresses tumor growth synergy with enzalutamide in CRPC cells (22). NSD2 is a conserved driver of metastatic PCa progression, which robustly expressed in lethal PCa and its silencing inhibited PCa metastasis in vivo of mouse allografts (24). Our previous study also revealed that MLL5α inhibits PCa progression by forming a complex with AR and promotes the transcription of NDRG1 through H3K4me3 in the promoter region (28). All of these previous studies suggest that epigenetic regulation via HLM regulators plays a crucial role in PCa progression.
Nevertheless, the overall effect of HLM modification in PCa remains unclear. To our knowledge, only one preliminary study has been conducted to analyze the genetic abnormalities of HMT in TCGA-PRAD, but this study lacked integration with HDM and MLRP (13). The complexity and heterogeneity of the individual PCa microenvironment may be influenced by various HLM regulators that interact with each other and form a complex network to induce epigenetic reprogramming. Thus, we summarized the widely acknowledged HLM regulators, 51 writers (HMT), 21 erasers (HDM), and 19 readers (MLRP), and then comprehensively investigated the HLM modification patterns in PCa by assessing various clinicopathological characteristics and performing survival analysis using the TCGA-PRAD dataset.
The results revealed that more than half (51 of 91) of the HLM regulators are dysregulated in PCa, and the numbers of overexpressed and downregulated HLM regulators were approximately the same (26 vs. 25), suggesting various alterations of HLM regulators in PCa. The representative HLM regulator EZH2 was overexpressed, but negligible CNV was found in PCa. All HLM regulators showed a copy number gain of < 5%. The highest copy number loss (25.5%) was found in SETDB2, which was downregulated in PCa patients (P = 0.02). However, while HLM regulators, such as PRDM13, PRDM1, KDM6B, CHD1, PHF23, and PRDM7, all exhibited high levels of copy number loss (> 10%), no significant downregulation (even overexpression of PRDM13) was found in the PCa of TCGA-PRAD dataset. This finding implies no causal relationship between CNV and the expression levels of HLM regulators in PCa.
In the prognosis validation by survival analysis, we conducted RFS instead of OS because of the low death rate (10 of 493) among TCGA-PRAD patients. We extracted the follow-up phenotype of “days_to_first_biochemical_recurrence” as “recurrence follow-up time” and confirmed “recurrence status”, as previously described (29). The Cox regression analysis showed that 10 HLM regulators (KMT5C, L3MBTL1, PRDM16, NSD2, KMT2B, SUV39H1, SETD4, DOT1L, EZH2, and PRDM15) were significant risk factors, with HR > 1 and P < 0.001.
We validated the oncogene status of HLM regulators via integration with DEGs associated with various clinicopathological characteristics (TN, GS, pT, and TP53 mutation) and RFS outcomes and found 6 intersecting genes, EZH2, NSD2, KMT5C, UHRF1, ORC1, and DNMT3A, which were highly expressed in association with all advanced-stage parameters (tumor, GS > 7, pT3, HR > 1, and TP53 mutation). When verifying these genes in clinical PCa tissues, six genes trended toward an increase in the GS > 7 group (compared with GS < 7), and 3 of them (EZH2, NSD2, and KMT5C) were statistically significant (P < 0.05). EZH2 and NSD2 are representative HLM regulators that were mentioned above and have been widely confirmed in PCa research. However, there is a lack of research focused on the role of KMT5C in PCa. KMT5C is an H4K20me3 methyltransferase and represses several key drivers of the epithelial state, further promoting epithelial to mesenchymal transition (EMT) in pancreatic cancer (49). UHRF1 promotes CRPC progression by triggering AR-regulated CDC6 transcription by binding to the CCAAT motif and recruiting KDM4C to demethylate H3K9me2/3 (50). UHRF1 could induce epigenetic inactivation of tumor suppressor genes in combination with SUV39H1, DNA methyltransferases, and EZH2 (51). ORC1 is a key subunit of the origin recognition complex, and few studies have focused on the role of ORC1 in PCa. DNMT3A epigenetically regulates EMT-associated key microRNAs to promote PCa metastasis (52); it is recruited to the promoters of these miRNAs and silences their transcription by increasing H3K9/27me3 and/or decreasing H3K4/36me3. In general, clinical PCa verification and previous research confirmed a certain level of accuracy of our bioinformatic analysis using the TCGA-PRAD dataset.
This study aimed to evaluate the integral HLM phenotypes in PCa patients. Here, we revealed three distinct HLM modification patterns based on 91 HLM regulators (HLMclusters) using consensus clustering analysis and investigated the effect of three HLMclusters on clinicopathological features and the prognosis of PCa patients; the results indicated no significant difference in these clusters in RFS (P = 0.194). This finding implies that clustering TCGA-PRAD patients based on 91 HLM regulators may not be suitable for involvement in a prognostic risk prediction model. We previously divided TCGA-PRAD patients into three clusters based on 25 N6-methyladenosine (m6A) regulators and found no significant differences in RFS analysis (29), which is discordant with the same analysis in gastric cancer (31). This finding suggests the heterogeneity of epigenetic regulation in diverse tumors.
Epigenetic modification of target genes to regulate their transcription is the primary function of HLM regulators. To predict genes downstream of HLM regulators, we identified 189 HLM phenotype-related genes that overlapped in the pairwise comparisons of HLMclusters (P value of Bayes test < 0.00001) and P < 0.01 in the Cox regression analysis. The patients were then classified into three subtypes (geneCluster) based on 189 HLM phenotype-related genes. These subtypes were significantly associated with different RFS rates (P = 0.007), suggesting that it may be an appropriate risk prediction model. In the results, geneCluster A exhibited the poorest RFS and recruited more of the clinicopathological parameters of PSA grade 3, pT4, pN1, and GS > 7.
However, the evaluation above was a qualitative analysis that highly depended on the population of patients and could not accurately predict the HLM modifications in individual PCa patients. Considering the heterogeneity of individual patients and complexity of HLM modifications, we constructed a set of scoring system (HLMscore) as described previously (31) based on the PCA of the 189 HLM phenotype-related genes to quantify the HLM modification pattern of individual PCa patients. The HLMscore progressively increased from geneCluster A–C, and a lower HLMscore resulted in a poorer prognosis in terms of RFS. The TMB score corresponds to tumor-related mutations that are sensitive to immune cell recruitment (41, 42). This study found no significant correlation between TMB and HLMscore (P = 0.21). However, the low-HLMscore group exhibited a higher proportion of TP53 mutations (13% vs. 7%) and fewer SPOP mutations (5% vs. 14%) than the high-HLMscore group. TP53 and SPOP were the most highly mutated genes in PCa (11% and 10%, respectively, in TCGA-PRAD). TP53 mutation was associated with a poor rPFS outcome, shorter time to CRPC progression, and higher aggressiveness (40, 53). SPOP mutation can enhance autophagy in PCa and respond to AR inhibition in various clinical settings (54, 55). Epigenetic modulation has been reported to play a vital role in antitumor immunity (56), but few studies have focused on HLM regulators and tumor immune cell infiltration. We found that 12 of 23 immune cell infiltrations were significantly correlated with HLMscore, and 8 of them were negatively correlated. The difference in the infiltrated subpopulations of immune-associated cells was also not substantial among the three HLMclusters (13 of 23) and three geneClusters (7 of 23), making it difficult to assess whether the HLM signature influences immune response patterns in PCa.
Immunotherapy with ICB (PD-1/L1 and CTLA-4) was demonstrated to have astounding efficacy in the positive response patients (43). Predicting the sensitivity to ICB in PCa patients is crucial for improving ICB therapy success and exploring novel immunotherapeutic strategies (42, 44). We found significant differences in the expression levels of PD-L1 and CTLA4 in the low- and high-HLMscore groups. Further assessment of immunotherapy responses showed that the IPS of CTLA4-positive and -negative responses accompanied by a PD1-negative response significantly differed between the two HLMscore groups (P < 0.05). This finding suggests that HLMscore estimation may have predictive value for immunotherapy sensitivity in PCa.
This study comprehensively evaluated the extensive HLM regulation mechanisms in the PCa microenvironment for the first time using the TCGA-PRAD dataset. HLM modification patterns play a crucial role in the complexity and heterogeneity of individual tumor microenvironments. Elucidating the overall effect of HLM regulators in PCa may contribute to the determination of a valuable risk model for predicting prognosis, aggressiveness, and immunotherapy strategies. We found 3 crucial HLM regulators (EZH2, NSD2, and KMT5C) that were consistently highly expressed in advanced PCa stages and associated with various clinicopathological characteristics (tumor, GS > 7, pT3, HR > 1, and TP53 mutation).
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Human prostate samples were provided by Beijing Tongren Hospital and Beijing Chaoyang Hospital. Ethical consent was approved by the Ethics Committee of Beijing Tongren Hospital and Beijing Chaoyang Hospital, affiliated with Capital Medical University.
YQ designed the study. YQ and MW performed the qPCR analysis. HP analyzed the data. XZ and HP provided the clinical prostate cancer samples. YQ wrote the manuscript, which was approved by all authors. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Grant Nos. 82072833 to HP) and the Open Research Fund from Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, Beijing Tongren Hospital, Beihang University & Capital Medical University (Grant No. BHTR-KFJJ-202005 to HP).
We thank Beijing Chaoyang Hospital, Capital Medical University for providing samples from PCa patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.981226/full#supplementary-material
Supplementary Figure 1 | CNV variation and mutation frequency of HLM regulators in PCa. (A) The location of CNV alterations of HLM regulators on 23 chromosomes. Red dot, copy number gain; blue dot, copy number loss. (B) The mutation frequency of HLM regulators in 484 TCGA-PRAD patients is shown in the waterfall plot. Columns represent individual patients, the upper bar plot shows TMB, the right bar plot shows the proportion of each variant type, and the stacked bar plot below shows the transformed fraction of each patient.
Supplementary Figure 2 | KEGG enrichment analysis in distinct HLMclusters. (A-C) Heatmap of KEGG enrichment analysis, including the activation states of biological pathways, in the respective comparisons of HLMclusters (A vs. B (A), A vs. C (B), and B vs. C (C).
Supplementary Figure 3 | Enrichment analysis of 3,297 intersecting DEGs in every pairwise comparison of HLMclusters. (A, B) GO enrichment of biological process terms (BP), cellular component terms (CC), and molecular function terms (MF) are shown as a barplot (A) and dotplot (B). (C, D) KEGG enriched terms in the activation states of biological pathways are shown as a barplot (C) and dotplot (D).
Supplementary Figure 4 | TME immune cell infiltration in three geneClusters and RFS prognosis based on HLMscore status among TCGA-PRAD patients stratified by various clinicopathological characteristics. (A) The abundance of infiltration of each immune cell type among the three geneClusters is shown in the boxplot. The values of the median ± interquartile range are shown in the graph. ns P > 0.05; ** P < 0.01; ***P < 0.001. (B) RFS analysis of the two HLMscore groups of TCGA-PRAD patients with GS 6 (left) and GS 7 (right). (C) RFS analysis of the two HLMscore groups of TCGA-PRAD patients with pT2 (left) and pT4 (right).
Supplementary Table 1 | Oligonucleotide primers of relative genes.
Supplementary Table 2 | The expression levels of 91 HLM regulators in TCGA-PRAD and normal tissues. a ns P > 0.05; * P < 0.05; ** P < 0.01; ***P < 0.001.
Supplementary Table 3 | Expression levels of HLM regulators in TCGA-PRAD data stratified by GS. a ns P > 0.05; * P < 0.05; ** P < 0.01; ***P < 0.001.
Supplementary Table 4 | Expression levels of HLM regulators in TCGA-PRAD data stratified by pT stage. a ns P > 0.05; * P < 0.05; ** P < 0.01; ***P < 0.001.
Supplementary Table 5 | Expression levels of HLM regulators in TCGA-PRAD data stratified by TP53 mutation. a ns P > 0.05; * P< 0.05; ** P< 0.01; ***P< 0.001.
Supplementary Table 6 | Abundance of each infiltrating immune cell among the three HLMclusters. a ns P > 0.05; * P < 0.05; ** P < 0.01; ***P < 0.001.
Supplementary Table 7 | Abundance of each infiltrating immune cell among the three geneClusters. a ns P > 0.05; ** P < 0.01; ***P < 0.001.
Supplementary Table 8 | Expression of HLM regulators among the three geneClusters. a ns P > 0.05; * P < 0.05; ** P < 0.01; ***P < 0.001.
PCa, prostate cancer; ADT, androgen-deprivation therapy; CRPC, castration-resistant prostate cancer; HLM, histone lysine methylation; NEPC, neuroendocrine prostate cancer; TCGA, The Cancer Genome Atlas; DEG, differentially expressed gene; TPM, transcripts per kilobase of exome per million; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; RFS, recurrence-free survival; PCA, principal component analysis; GSVA, gene set variation analysis; GSEA, gene set enrichment analysis; TMB, tumor mutational burden; TME, tumor microenvironment; ssGSEA, single-sample gene set enrichment analysis; IPS, immunophenoscore; GS, Gleason score; qPCR, quantitative real-time PCR; cDNA, complementary DNA; HR, hazard ratio; CNV, copy number variation; CDF, cumulative distribution function; EMT, epithelial to mesenchymal transition; ICB, immune checkpoint blockade; CI, confidence interval; pT, pathological T; pN, pathological N; rPFS, radiographic progression-free survival; pN, pathological N; OS, overall survival.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell (2007) 128(4):707–19. doi: 10.1016/j.cell.2007.01.015
3. Jenuwein T, Allis CD. Translating the histone code. Sci (New York NY) (2001) 293(5532):1074–80. doi: 10.1126/science.1063127
4. Kouzarides T. Chromatin modifications and their function. Cell (2007) 128(4):693–705. doi: 10.1016/j.cell.2007.02.005
5. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res (2011) 21(3):381–95. doi: 10.1038/cr.2011.22
6. Khorasanizadeh S. The nucleosome: from genomic organization to genomic regulation. Cell (2004) 116(2):259–72. doi: 10.1016/S0092-8674(04)00044-3
7. Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol (2005) 6(11):838–49. doi: 10.1038/nrm1761
8. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet (2012) 13(5):343–57. doi: 10.1038/nrg3173
9. Berger SL. The complex language of chromatin regulation during transcription. Nature (2007) 447(7143):407–12. doi: 10.1038/nature05915
10. Di Lorenzo A, Bedford MT. Histone arginine methylation. FEBS Lett (2011) 585(13):2024–31. doi: 10.1016/j.febslet.2010.11.010
11. Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Molecular cell (2012) 48(4):491–507. doi: 10.1016/j.molcel.2012.11.006
12. Hyun K, Jeon J, Park K, Kim J. Writing, erasing and reading histone lysine methylations. Exp Mol Med (2017) 49(4):e324. doi: 10.1038/emm.2017.11
13. Zhang Y, Yan L, Yao W, Chen K, Xu H, Ye Z. Integrated analysis of genetic abnormalities of the histone lysine methyltransferases in prostate cancer. Med Sci Monit (2019) 25:193–239. doi: 10.12659/MSM.912294
14. Bai Y, Zhang Z, Cheng L, Wang R, Chen X, Kong Y, et al. Inhibition of enhancer of zeste homolog 2 (EZH2) overcomes enzalutamide resistance in castration-resistant prostate cancer. J Biol Chem (2019) 294(25):9911–23. doi: 10.1074/jbc.RA119.008152
15. Xiao L, Tien JC, Vo J, Tan M, Parolia A, Zhang Y, et al. Epigenetic reprogramming with antisense oligonucleotides enhances the effectiveness of androgen receptor inhibition in castration-resistant prostate cancer. Cancer Res (2018) 78(20):5731–40. doi: 10.1158/0008-5472.CAN-18-0941
16. Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is polycomb-independent. Sci (New York NY) (2012) 338(6113):1465–9. doi: 10.1126/science.1227604
17. Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med (2016) 22(3):298–305. doi: 10.1038/nm.4045
18. Dardenne E, Beltran H, Benelli M, Gayvert K, Berger A, Puca L, et al. N-myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell (2016) 30(4):563–77. doi: 10.1016/j.ccell.2016.09.005
19. Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Sci (New York NY) (2017) 355(6320):78–83. doi: 10.1126/science.aah4199
20. Matsika A, Srinivasan B, Day C, Mader SA, Kiernan DM, Broomfield A, et al. Cancer stem cell markers in prostate cancer: an immunohistochemical study of ALDH1, SOX2 and EZH2. Pathology (2015) 47(7):622–8. doi: 10.1097/PAT.0000000000000325
21. Li K, Chen MK, Situ J, Huang WT, Su ZL, He D, et al. Role of co-expression of c-myc, EZH2 and p27 in prognosis of prostate cancer patients after surgery. Chin Med J (2013) 126(1):82–7.
22. Gao S, Chen S, Han D, Wang Z, Li M, Han W, et al. Chromatin binding of FOXA1 is promoted by LSD1-mediated demethylation in prostate cancer. Nat Genet (2020) 52(10):1011–7. doi: 10.1038/s41588-020-0681-7
23. Omarjee S, Carroll JS. Targeting LSD1 and FOXA1 in prostate cancer. Nat Genet (2020) 52(10):1002–3. doi: 10.1038/s41588-020-0702-6
24. Aytes A, Giacobbe A, Mitrofanova A, Ruggero K, Cyrta J, Arriaga J, et al. NSD2 is a conserved driver of metastatic prostate cancer progression. Nat Commun (2018) 9(1):5201. doi: 10.1038/s41467-018-07511-4
25. Liu C, Wang C, Wang K, Liu L, Shen Q, Yan K, et al. SMYD3 as an oncogenic driver in prostate cancer by stimulation of androgen receptor transcription. J Natl Cancer Inst (2013) 105(22):1719–28. doi: 10.1093/jnci/djt304
26. Vatapalli R, Sagar V, Rodriguez Y, Zhao JC, Unno K, Pamarthy S, et al. Histone methyltransferase DOT1L coordinates AR and MYC stability in prostate cancer. Nat Commun (2020) 11(1):4153. doi: 10.1038/s41467-020-18013-7
27. Thomas T. DOT1L in prostate cancer. Nat Rev Urol (2020) 17(10):544. doi: 10.1038/s41585-020-0374-0
28. Quan Y, Cui Y, Wahafu W, Liu Y, Ping H, Zhang X. MLL5α activates AR/NDRG1 signaling to suppress prostate cancer progression. Am J Cancer Res (2020) 10(5):1608–29.
29. Quan Y, Zhang X, Ping H. Construction of a risk prediction model using m6A RNA methylation regulators in prostate cancer: comprehensive bioinformatic analysis and histological validation. Cancer Cell Int (2022) 22(1):33. doi: 10.1186/s12935-021-02438-1
30. Zhao Y, Sun H, Zheng J, Shao C. Analysis of RNA m(6)A methylation regulators and tumour immune cell infiltration characterization in prostate cancer. Artif Cells Nanomed Biotechnol (2021) 49(1):407–35. doi: 10.1080/21691401.2021.1912759
31. Zhang B, Wu Q, Li B, Wang D, Wang L, Zhou YL. m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer (2020) 19(1):53. doi: 10.1186/s12943-020-01170-0
32. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf (2013) 14:7. doi: 10.1186/1471-2105-14-7
33. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
34. Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep (2017) 18(1):248–62. doi: 10.1016/j.celrep.2016.12.019
35. Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature (2009) 462(7269):108–12. doi: 10.1038/nature08460
36. Jiang Q, Chen H, Tang Z, Sun J, Ruan Y, Liu F, et al. Stemness-related LncRNA pair signature for predicting therapy response in gastric cancer. BMC Cancer (2021) 21(1):1067. doi: 10.1186/s12885-021-08798-1
37. Ma X, Guo J, Liu K, Chen L, Liu D, Dong S, et al. Identification of a distinct luminal subgroup diagnosing and stratifying early stage prostate cancer by tissue-based single-cell RNA sequencing. Mol Cancer (2020) 19(1):147. doi: 10.1186/s12943-020-01264-9
38. Eggener SE, Scardino PT, Walsh PC, Han M, Partin AW, Trock BJ, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol (2011) 185(3):869–75. doi: 10.1016/j.juro.2010.10.057
39. Kozminski MA, Tomlins S, Cole A, Singhal U, Lu L, Skolarus TA, et al. Standardizing the definition of adverse pathology for lower risk men undergoing radical prostatectomy. Urol Oncol (2016) 34(9):415.e411–416. doi: 10.1016/j.urolonc.2016.03.019
40. Deek MP, van der Eecken K, Phillips R, Parikh NR, Isaacsson Velho P, Lotan TL, et al. The mutational landscape of metastatic castration-sensitive prostate cancer: The spectrum theory revisited. Eur Urol (2021) 80(5):632–40. doi: 10.1016/j.eururo.2020.12.040
41. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
42. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med (2013) 19(11):1423–37. doi: 10.1038/nm.3394
43. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. Engl J Med (2012) 366(26):2443–54. doi: 10.1056/NEJMoa1200690
44. Ali HR, Chlon L, Pharoah PD, Markowetz F, Caldas C. Patterns of immune infiltration in breast cancer and their clinical implications: A gene-Expression-Based retrospective study. PloS Med (2016) 13(12):e1002194. doi: 10.1371/journal.pmed.1002194
45. Tian X, Zhang S, Liu HM, Zhang YB, Blair CA, Mercola D, et al. Histone lysine-specific methyltransferases and demethylases in carcinogenesis: new targets for cancer therapy and prevention. Curr Cancer Drug Targets (2013) 13(5):558–79. doi: 10.2174/1568009611313050007
46. Cai H, Memarzadeh S, Stoyanova T, Beharry Z, Kraft AS, Witte ON. Collaboration of kras and androgen receptor signaling stimulates EZH2 expression and tumor-propagating cells in prostate cancer. Cancer Res (2012) 72(18):4672–81. doi: 10.1158/0008-5472.CAN-12-0228
47. Kim J, Lee Y, Lu X, Song B, Fong KW, Cao Q, et al. Polycomb- and methylation-independent roles of EZH2 as a transcription activator. Cell Rep (2018) 25(10):2808–2820 e2804. doi: 10.1016/j.celrep.2018.11.035
48. Liu Q, Wang G, Li Q, Jiang W, Kim JS, Wang R, et al. Polycomb group proteins EZH2 and EED directly regulate androgen receptor in advanced prostate cancer. Int J Cancer (2019) 145(2):415–26. doi: 10.1002/ijc.32118
49. Viotti M, Wilson C, McCleland M, Koeppen H, Haley B, Jhunjhunwala S, et al. SUV420H2 is an epigenetic regulator of epithelial/mesenchymal states in pancreatic cancer. J Cell Biol (2018) 217(2):763–77. doi: 10.1083/jcb.201705031
50. Gao Y, Liu Y, Liu Y, Peng Y, Yuan B, Fu Y, et al. UHRF1 promotes androgen receptor-regulated CDC6 transcription and anti-androgen receptor drug resistance in prostate cancer through KDM4C-mediated chromatin modifications. Cancer Lett (2021) 520:172–83. doi: 10.1016/j.canlet.2021.07.012
51. Babbio F, Pistore C, Curti L, Castiglioni I, Kunderfranco P, Brino L, et al. The SRA protein UHRF1 promotes epigenetic crosstalks and is involved in prostate cancer progression. Oncogene (2012) 31(46):4878–87. doi: 10.1038/onc.2011.641
52. Mancini M, Grasso M, Muccillo L, Babbio F, Precazzini F, Castiglioni I, et al. DNMT3A epigenetically regulates key microRNAs involved in epithelial-to-mesenchymal transition in prostate cancer. Carcinogenesis (2021) 42(12):1449–60. doi: 10.1093/carcin/bgab101
53. Maxwell KN, Cheng HH, Powers J, Gulati R, Ledet EM, Morrison C, et al. Inherited TP53 variants and risk of prostate cancer. Eur Urol (2022) 81(3):243–50. doi: 10.1016/j.eururo.2021.10.036
54. Gao K, Shi Q, Liu Y, Wang C. Enhanced autophagy and NFE2L2/NRF2 pathway activation in SPOP mutation-driven prostate cancer. Autophagy (2022) 18(8):2013–5. doi: 10.1080/15548627.2022.2062873
55. Bernasocchi T, Theurillat JP. SPOP-mutant prostate cancer: Translating fundamental biology into patient care. Cancer Lett (2022) 529:11–8. doi: 10.1016/j.canlet.2021.12.024
Keywords: prostate cancer, histone lysine methylations, The Cancer Genome Atlas (TCGA), recurrence-free survival, HLMcluster, geneCluster, HLMscore
Citation: Quan Y, Zhang X, Wang M and Ping H (2022) Histone lysine methylation patterns in prostate cancer microenvironment infiltration: Integrated bioinformatic analysis and histological validation. Front. Oncol. 12:981226. doi: 10.3389/fonc.2022.981226
Received: 29 June 2022; Accepted: 29 August 2022;
Published: 28 September 2022.
Edited by:
Darren Poon, The Chinese University of Hong Kong, ChinaReviewed by:
Bin Xu, Shanghai Jiao Tong University, ChinaCopyright © 2022 Quan, Zhang, Wang and Ping. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Ping, cGluZ2hhb3RyaEBjY211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.