
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 18 August 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.980736
This article is part of the Research TopicAdvances in Surgical Treatment of Hepatobiliary TumorsView all 13 articles
 Wen-qiang Wang1
Wen-qiang Wang1 Jian Li1
Jian Li1 Bin-yong Liang1
Bin-yong Liang1 Xing Lv1
Xing Lv1 Rong-hua Zhu1
Rong-hua Zhu1 Jin-lin Wang1
Jin-lin Wang1 Zhi-yong Huang1
Zhi-yong Huang1 Shu-hong Yang2*
Shu-hong Yang2* Er-lei Zhang1*
Er-lei Zhang1*Background: The efficacies of anatomical resection (AR) and non-anatomical resection (NAR) in the treatment of combined hepatocellular-cholangiocarcinoma (cHCC-CCA) remain unclear. This study aimed to compare the prognostic outcomes of AR with those of NAR for cHCC-CCA.
Method: Patients diagnosed with pathology-confirmed cHCC-CCA, and who underwent curative resection at Tongji hospital between January 2010 and December 2019 were included in this retrospective study. A one-to-one propensity score matching (PSM) analysis was used to compare the long-term outcomes of AR to those of NAR.
Results: A total of 105 patients were analyzed, of whom 48 (45.7%) and 57 (54.3%) underwent AR and NAR, respectively. There were no significant differences in short-term outcomes between the two groups, including duration of postoperative hospital stay, the incidence of perioperative complications, and incidence of 30-day mortality. However, both, the 5-year overall survival (OS) and recurrence-free survival (RFS) rates of AR were significantly better than those of NAR (40.5% vs. 22.4%, P=0.002; and 37.3% vs. 14.4%, P=0.002, respectively). Multivariate analysis showed that NAR, multiple tumors, larger-sized tumors (>5 cm), cirrhosis, lymph node metastasis, and vascular invasion were independent risk factors for poor prognoses. Stratified analysis demonstrated similar outcomes following AR versus NAR for patients with tumors > 5cm in diameter, while AR had better survival than NAR in patients with tumors ≤5 cm in diameter. After PSM, when 34 patients from each group were matched, the 5-year OS and RFS rates of AR were still better than those of NAR.
Conclusion: Patients with cHCC-CCA who underwent AR had better long-term surgical outcomes than those who underwent NAR, especially for those with tumors ≤5 cm in diameter. However, no differences in the risk of surgical complications were detected between the two groups.
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare type of primary liver cancer that exhibits both hepatocytic and cholangiocytic differentiation within the same tumor; cHCC-CCA has an incidence rate that ranges from 0.4–14.2% and is reported to be more common in men and those with chronic liver disease (1–3). cHCC-CCA is an aggressive malignancy, with clinical and biological patterns overlapping with those of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA) (1). Due to the low incidence of cHCC-CCA, there are few published studies (mostly with low sample sizes) on the treatment and prognosis of the condition (2, 4, 5). Furthermore, there are no detailed accounts of the clinical behavior, surgical outcomes, and prognostic factors for cHCC-CCA (5–7). Compared with HCC and iCCA, standardizing treatment for cHCC-CCA is difficult due to several factors. First, it is difficult to differentiate cHCC-CCA from HCC or iCCA through imaging. Second, the incidence of cHCC-CCA is relatively low, making it difficult for a single institution to have enough patients for detailed studies. The only curative option for patients with cHCC-CCA was found to be R0 resection with lymph node dissection; however, even after radical hepatectomy or liver transplantation, long-term survival remained low (2, 4, 8, 9). The 5-year tumor recurrence rate in cHCC-CCA patients was reported to be as high as 80%, and the 5-year overall survival (OS) rates were less than 30% (10–14). High incidence rates of postoperative recurrence in cHCC-CCA patients even after curative treatment is also a major issue in the treatment of this condition.
A nationwide study in China has indicated that although cHCC-CCA reflects the malignant behavior of iCCA, it should be characterized as a subtype of HCC due to similarities in mortality rates and long-term surgical outcomes between HCC and cHCC-CCA (15). The superiority of anatomical resection (AR) over non-anatomical resection (NAR) for surgical outcomes in HCC patients is an ongoing controversy. Since cHCC-CCA has characteristics of both HCC and iCCA, the tumors have a high propensity to invade intrahepatic pedicle structures, which allows the tumor to spread via the closest portal veins or bile ducts. Therefore, the complete removal of tumor-bearing hepatic pedicles is considered to be ideal for surgical eradication of potential micrometastases (16). Theoretically, AR in patients with cHCC-CCA could reduce the risk of local recurrence and may improve patient survival (17). However, no reports have proved that AR is superior to NAR in treating cHCC-CCA as yet.
Therefore, this study was undertaken to clarify which—AR or NAR—is the superior treatment option based on short-term and long-term outcomes for patients with cHCC-CCA.
Of the 6652 patients who underwent hepatectomy for primary hepatic malignancy between January 2010 and December 2019 at the Hepatic Surgery Center, Tongji Hospital, 118 (1.8%) were identified as having pathology-confirmed cHCC-CCA. Of these, eight were excluded due to incomplete data (including six patients who were lost to follow-up), three were excluded as exploration and biopsies confirmed that the tumors were not cHCC-CCA, and two were excluded as they had received preoperative anticancer treatments (Figure 1). The remaining 105 patients were divided into NAR (n=57) and AR groups (n=48) according to the hepatic resection they underwent.

Figure 1 Flowchart of steps taken for patient selection for this study. AR, anatomical resection; NAR, non-anatomical resection; PSM, propensity score matching.
Demographic and clinical data including age, sex, Eastern Cooperative Oncology Group-Performance status (ECOG-PS), Child classification, presence of underlying liver disease, positivity for hepatitis B viral surface antigen (HBsAg) and hepatitis C viral antibody (HCV-Ab), liver function, complete blood count, coagulation profile, tumor markers including serum α-fetoprotein (AFP), carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels were collected. Histopathological factors including the tumor size and number, vascular invasion, lymph node metastasis (LNM), and tumor stage according to the 8th edition of the Union for International Cancer Control TNM classification (8th TNM stage) were also recorded (18).
The main surgical procedure for AR involved complete identification of the target Couinaud segment(s), following which parenchymal dissection was performed along the segmental border. Next, landmark veins were exposed on the cut surface of the liver, and the corresponding portal branches were ligated for trisectionectomy, hemihepatectomy, sectionectomy, and segmentectomy (19). For NAR (also known as conventional limited resection), the surgical procedure focused on tumor resection with a negative tumor margin regardless of segment or section anatomy. Postoperative morbidity was defined as the occurrence of complications during the hospital stay or within 3 months of resection. Complication severity was graded as per the Clavien–Dindo classification system (20).
Postoperative follow-up consisted of abdominal ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) along with laboratory tests to check liver function. These included checking the levels of α-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) every 2–3 months during the first 2 years after surgery, and then every 4–6 months thereafter. Follow-up data were collected until February 28, 2022. Recurrence-free survival (RFS) was defined as the period after the operation when no tumor recurrence could be detected by imaging or biopsy. Overall survival (OS) was the time interval between the surgery and date of death (if any).
Propensity score matching (PSM) was performed to reduce biases arising from the different distributions of covariates among patients who underwent AR and those who underwent NAR. Of all the variables identified, several were significantly and independently different between the two groups. Based on these results, the following variables were included in the 1:1 PSM analysis: CEA, prothrombin time (PT), white blood cell (WBC) count, and presence of solitary tumor. To achieve the highest homogeneity, the caliper was set to 0.10.
For continuous variables, medians with inter-quartile ranges (IQR) have been reported. Such variables were compared using independent sample t-tests or Mann-Whitney U tests. Categorical variables were expressed as frequencies or percentages and compared using the Chi-square test or Fisher’s exact test. Kaplan-Meier (K-M) survival curves were used to compare survival rates between the AR and NAR groups using the log-rank test. Potential risk factors associated with OS and RFS were identified using univariate and multivariable Cox hazard regression models, and all variables with P<0.050 in the univariate analyses were utilized in multivariate analyses to determine independent risk factors. For all tests, P< 0.050 was considered statistically significant. All analyses were performed using the SSPS 24.0 software (IBM Corp., Armonk, NY, USA).
Of the 105 patients with cHCC-CCA included in this study, there were 90 (85.7%) men and 15 (14.3%) women; the mean age of the patients was 53 years (range, 28–83 years). Details regarding patient demographics, preoperative procedures, tumor characteristics, and operative procedures and care are reported in Table 1. A total of 57 patients underwent NAR (54.3%) while 48 underwent AR (45.7%). There were substantial differences in background variables between the two groups before PSM analysis. Patients in the AR group had significantly higher CEA levels and WBC counts, along with lower PT levels and smaller tumors than those in the NAR group. There were no significant differences in other clinicopathologic characteristics between the two groups. Details of the surgical procedures that the 48 patients who underwent AR are as follows: trisectionectomy (n=2); hemihepatectomy (n=11); sectionectomy (n=9); segmentectomy (n=16); combined resection of segments (n=10).
The overall incidence rates of postoperative complications and 30-day mortality were 34.3% (43/105) and 1% (1/105), respectively. The lengths of postoperative hospital stays and incidence rates of complications were similar between the two matched groups (Table 2). None of the patients experienced intraperitoneal bleeding within 72 hours after surgery. In the NAR group, one patient developed bile leakage after hepatectomy and underwent percutaneous catheter drainage for two months. One patient in each group developed postoperative hepatic failure; after conservative treatment, the AR patient recovered, whereas the NAR patient died 25 days after surgery. Postoperative infection (definite positive after bacterial culture) occurred in three patients in each group; however, these patients recovered after treatment with antibiotics and immune regulation. Other common complications included pleural effusion and ascites, which occurred at similar rates between both groups and required ultrasound-guided percutaneous drainage. There were no significant differences between the two groups in the severity of complications according to the Clavien–Dindo classification.
A total of 105 patients were followed up for various periods (range=0.8–97 months; median=42 months). The 1-year, 3-year, and 5-year OS rates for all patients were 88.6%, 59.8%, and 29.0%, respectively. Correspondingly, the 1-year, 3-year, and 5-year RFS rates for all patients were 75.2%, 42.9%, and 22.8%, respectively. The 1-year, 3-year, and 5-year OS rates were significantly higher in the AR group as compared to those in the NAR group (91.7% vs 86.0%; 70.0% vs 51.1%; 36.8% vs 22.3%, respectively; P=0.002; Figure 2A). The 1-year, 3-year, and 5-year RFS rates were also higher in the AR group as compared to those in the NAR group (79.2% vs 71.9%; 56.0% vs 31.0%; 32.6% vs 14.4%, respectively; P=0.002; Figure 2B). Tables 3, 4 show the results of the stratified analyses (Cox proportional hazard regression analysis and log-rank test) for the predictors of RFS and OS rates. Univariate analyses revealed that the presence of HBsAg (positive vs negative) and cirrhosis (yes vs no), tumor nodularity (multiple vs solitary), tumor size (>5 cm vs ≤5 cm), resection type (AR vs NAR), surgical margin (R1 vs R0), differentiation (poor vs moderate/well), and the presence of lymph node metastasis and vascular invasion (yes vs no) were prognostic factors for RFS. Multivariate analyses revealed that the presence of: multiple tumors (hazard ratio [HR]=2.560, 95% confidence interval [CI]=1.346–4.868, P=0.004), larger tumors (>5 cm) (HR=2.036, 95% CI=1.174–3.534, P=0.011), AR (HR=0.573, 95% CI=0.334–0.982, P=0.043), lymph node metastasis (HR=3.043, 95% CI=1.348-6.869, P=0.007), and vascular invasion (HR=2.325, 95% CI=1.220–4.432, P=0.010) were significant predictors of RFS. Similarly, univariate analyses found that the presence of cirrhosis (yes vs no), tumor nodularity (multiple vs solitary), tumor size (>5 cm vs ≤5 cm), resection type (AR vs NAR), surgical margin (R1 vs R0), and the presence of lymph node metastasis and vascular invasion (yes vs no) were prognostic factors for OS. Multivariate analysis revealed that cirrhosis (HR=1.921, 95% CI=1.101–3.352, P=0.022), the presence of larger tumors (>5 cm) (HR=1.793, 95% CI=1.015–3.165, P=0.044), AR (HR=0.548, 95% CI=0.316–0.950, P=0.032), the presence of lymph node metastasis (HR=3.108, 95% CI=1.429–6.761, P=0.004), and vascular invasion (HR=3.544, 95% CI=1.831–6.862, P=0.001) were significant predictors of OS.
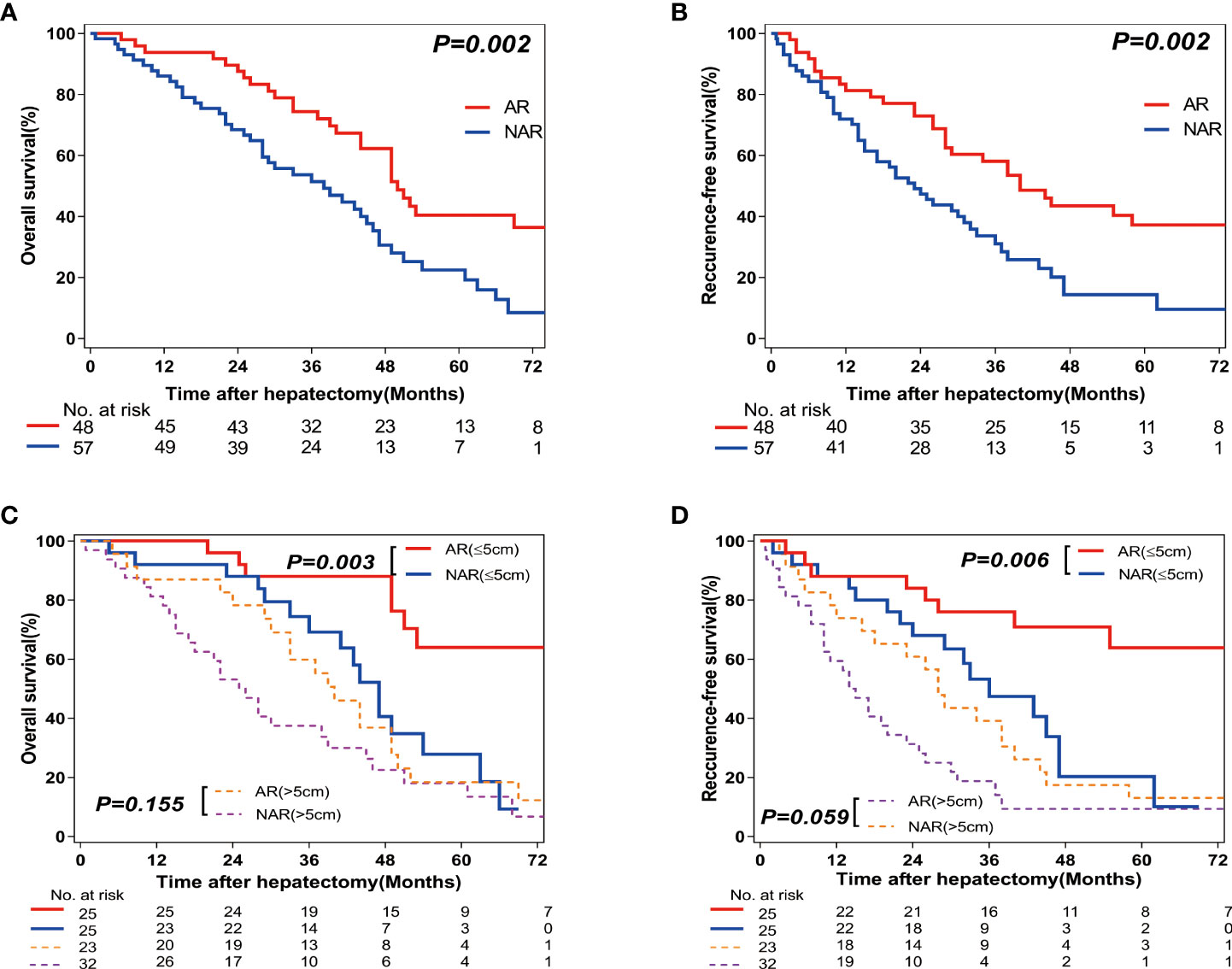
Figure 2 Overall survival (OS) and Recurrence-free survival (RFS) rates after Anatomic resection (AR) versus Non-anatomic resection (NAR) for combined hepatocellular-cholangiocarcinoma (cHCC-CCA) patients. (A) Overall survival (OS) and (B) recurrence-free survival (RFS) curves of cHCC-CCA patients in AR (n=57; P=0.002) and NAR (n=48; P=0.002) groups. (C) Overall survival (OS) and (D) recurrence-free survival (RFS) curves of cHCC-CCA patients with tumors ≤5 cm in size (n=50, P=0.006 and P=0.003, respectively) and >5 cm in size (n=55, P=0.059 and P=0.155, respectively).
Since tumor size may be associated with prognosis, the patients were further classified into subsets according to tumor size: tumor size <5 cm (n=50) and >5 cm (n=55). In the patients with smaller tumors (<5 cm), higher RFS and OS rates were observed in the AR (n=25) group as compared with those in the NAR (n=25) group (P=0.006 and P=0.003, respectively; Figures 2C, D). In the patients with larger tumors (>5 cm), there were no differences in RFS and OS rates between the AR (n=23) and NAR (n=32) groups (P=0.059 and P=0.155, respectively; Figures 2C, D).
After the 1:1 PSM, 68 patients were identified and classified into propensity-matched anatomical resection (PSM-AR) (n=34) and propensity-matched non-anatomical resection (PSM-NAR) groups (n=34) (Table 1). Except for the high laparoscopic resection rate in the PSM-AR group (26.5% vs 5.9%; P=0.045), there were no significant differences in demographic and clinicopathologic characteristics between the two groups after matching (Table 1). The operation time tended to be shorter in PSM-NAR (median time=180 in the PASM-NAR group as compared to 195 minutes for the PSM-NAR group; P=0.058).
Among the 68 patients included in this analysis, the 1-year, 3-year, and 5-year OS rates were higher in the PSM-AR group as compared to those in the PSM-NAR group (94.1% vs 88.2%; 65.9% vs 41.2%; 31.7% vs 14.0%, respectively; P=0.002; Figure 3A). The 1-year, 3-year, and 5-year RFS rates were also higher in the PSM-AR group as compared to those in the PSM-NAR group (79.4% vs 67.6%; 49.6% vs 32.4%; 30.2% vs 13.2%, respectively; P=0.010; Figure 3B).
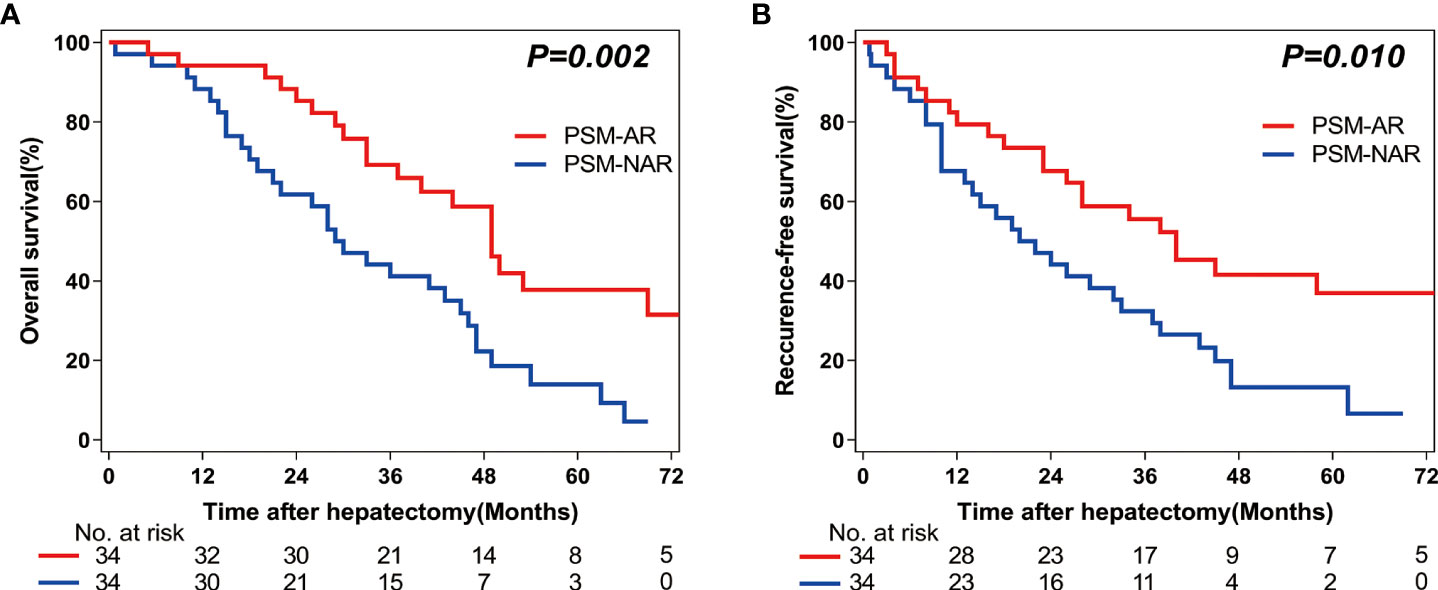
Figure 3 Overall survival (OS) and Recurrence-free survival (RFS) rates after Anatomic (AR) versus Non-anatomic resection (NAR) for combined hepatocellular-cholangiocarcinoma (cHCC-CCA) patients after propensity score matching (PSM). (A) Overall survival (OS) and (B) recurrence-free survival (RFS) curves of cHCC-CCA patients in PSM-AR (n = 34) and PSM-NAR (n = 34) groups after propensity score matching (PSM) (P = 0.002, P = 0.010, respectively).
The clinical significance of choosing AR or NAR in treating cHCC-CCA remains unclear because of the relative rarity of this primary liver malignancy, which has an incidence of 1.8% (118/6552; as observed in our study, which is consistent with previous reports) (6). In this single-center study, we have demonstrated that cHCC-CCA patients who underwent AR surgeries had longer DFS and OS times than those who underwent NAR surgeries, (both, before and after PSM analysis), especially for tumors <5 cm in diameter. To the best of our knowledge, this is the first report that compares the surgical outcomes of AR versus NAR in the treatment of cHCC-CCA; we find that patients who underwent AR, had better surgical outcomes than those who underwent NAR.
Although cHCC-CCA has features of both HCC and CC, several studies have observed that cHCC-CCA shares more etiological features with HCC than with iCCA, especially with respect to its epithelial characteristics (5, 15). In our study, the clinicopathological features of cHCC-CCA were more similar to those of patients suffering from HCC infected with HBV (hepatitis B virus), both of which are associated with elevated AFP levels in most patients. The results of this study are consistent with those of previous studies (15, 21). In clinical settings, cHCC-CCA is often misdiagnosed as either HCC or iCCA via imaging or hematology tests due to non-specific clinical manifestations, and a confirmed diagnosis of cHCC-CCA usually requires surgical resection (22). Since preoperative biopsy is not routinely used to diagnose cHCC-CCA (as large sampling areas are required and have low sensitivity of detection), some studies have explored the use of other risk factors to differentiate between cHCC-CCA and HCC or iCCA. Some of these factors include sex (men are more likely to develop cHCC-CCA than women), and the presence of chronic liver damage, cirrhosis, hepatitis infection, familial history of liver cancer, alcoholism, and diabetes (21, 23). Although both CA199 and AFP levels are expected to be higher than normal in cHCC-CCA patients, in this study, we found that elevated AFP levels were more common than elevated CA199 levels. Furthermore, 85.7% of cHCC-CCA patients in our study were men and 62.9% of them had HBV infections, which is consistent with previous reports (23, 24). Our results indicate that the clinicopathological characteristics of cHCC-CCA in the patients included in our study resemble those of HCC more than iCCA.
Surgical resection is widely accepted as an optimal curative treatment for cHCC-CCA and can provide patients with a chance of long-term survival (13, 25).The main objectives of surgical resectioning in treating cHCC-CCA are to completely remove the tumor, preserve sufficient residual liver volume for survival, and ensure negative resection margins. Unfortunately, until now, the prognostic differences in treating cHCC-CCA with either AR or NAR surgeries have not been reported. Usually, treatment with AR reduces tumor recurrence as it involves the removal of tumor-bearing portal vein branches and corresponding liver parenchyma. Since this supports long-term survival, several studies have reported that AR is superior to NAR for the treatment of HCC or iCCA with resection (17, 26, 27). NAR is considered to be beneficial for patients with cirrhosis or poorly preserved liver function (28). Since cHCC-CCA resembles both, HCC and iCCA, the long-term outcomes of surgical resection may be similar to those of HCC and iCCA; however, it is important to have data-backed proof of differences in surgical outcomes of cHCC-CCA patients after AR or NAR surgeries. Although there were no significant differences in the occurrences or types of postoperative complications between the two surgical methods, we found that AR is prognostically superior to NAR for cHCC-CCA treatment. Our results (both, before and after PSM) show that AR significantly improved the RFS and OS times for cHCC-CCA patients.
Multivariate analyses also showed that tumor size and nodularity, as well as the presence of lymph node metastasis and vascular invasion were independent risk factors for postoperative survival of cHCC-CCA patients; these patterns are consistent with those for patients with HCC or iCCA (29–33). Tumor size may influence surgical outcomes for HCC patients (34); this was shown in a large-scale study from Japan, which found that the recurrence rates for HCC patients with tumors of diameter 2–5 cm were significantly lower for those who underwent AR surgery rather than for those who underwent NAR surgery. However, there were no significant differences in surgical outcomes after liver resection for HCC tumors ≤2 cm or ≥5 cm in size between the AR and NAR groups (35). Due to the similarities between HCC and cHCC-CCA, tumor size can be expected to be a crucial risk factor for surgical outcomes of AR or NAR surgeries in cHCC-CCA patients. In this study, stratified analysis showed that AR provides a better long-term survival benefit than NAR for patients with tumors ≤5 cm in size. However, there were no significant differences in RFS and OS at 1, 3, and 5 years after resection surgery for patients with tumors >5 cm in size. One reason for this inconsistency could be that the cHCC-CCA tumors in the patients included in this study were more similar to CCA tumors than to HCC tumors; for CCA tumors, AR surgery provides no extra survival benefits over NAR surgery. Furthermore, the diameters of all the tumor masses in this study were >2.2 cm. Our results, therefore, suggest that AR should be recommended for cHCC-CCA patients with small tumors.
Despite our clear-cut results, this study has several limitations. Of these, one is that this study has a small sample size with all samples drawn from a single center. Second, this is a retrospective study, which means that there is a high chance of it having selection biases despite our use of PSM analysis. Third, we have not analyzed the impact of postoperative therapy on the long-term outcomes in patients due to unavailable data. In addition, not all patients included in this study underwent lymph node dissection, as several had normal lymph nodes (as observed by preoperative imaging). We recommend that more prospective studies with larger sample sizes and RCT studies be performed to fully evaluate the relative merits of AR and NAR surgeries in treating cHCC-CCA.
In conclusion, the clinicopathologic characteristics of cHCC-CCA usually resemble those of HCC more than those of iCCA. Irrespective of the application of PSM, we found that AR was associated with better surgical outcomes as compared to NAR for patients with cHCC-CCA, especially for tumors of size ≤5 cm in diameter.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conception and design, W-QW, E-LZ, and S-HY. Analysis and interpretation of data, all authors. Drafting the article or revising it critically for important intellectual content, all authors. Final approval of manuscript, all authors. All authors contributed to the article and approved the submitted version.
This work was partially supported by the National Natural Science Foundation of China (No.81902839).
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma. Cancer (2002) 94(7):2040–6. doi: 10.1002/cncr.10392
2. Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, et al. Combined hepatocellular-cholangiocarcinoma: A population-level analysis of an uncommon primary liver tumor. Liver Transpl (2014) 20(8):952–9. doi: 10.1002/lt.23897
3. Kassahun WT, Hauss J. Management of combined hepatocellular and cholangiocarcinoma. Int J Clin Pract (2008) 62(8):1271–8. doi: 10.1111/j.1742-1241.2007.01694.x
4. Ariizumi S, Kotera Y, Katagiri S, Nakano M, Yamamoto M. Combined hepatocellular-cholangiocarcinoma had poor outcomes after hepatectomy regardless of Allen and Lisa class or the predominance of intrahepatic cholangiocarcinoma cells within the tumor. Ann Surg Oncol (2012) 19(5):1628–36. doi: 10.1245/s10434-011-2150-0
5. Zhou YM, Sui CJ, Zhang XF, Li B, Yang JM. Influence of cirrhosis on long-term prognosis after surgery in patients with combined hepatocellular-cholangiocarcinoma. BMC Gastroenterol (2017) 17(1):25. doi: 10.1186/s12876-017-0584-y
6. Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: An update. J Hepatol (2021) 74(5):1212–24. doi: 10.1016/j.jhep.2021.01.035
7. Dageforde LA, Vachharajani N, Tabrizian P, Agopian V, Halazun K, Maynard E, et al. Multi-center analysis of liver transplantation for combined hepatocellular carcinoma-cholangiocarcinoma liver tumors. J Am Coll Surg (2021) 232(4):361–71. doi: 10.1016/j.jamcollsurg.2020.11.017
8. Kim KH, Lee SG, Park EH, Hwang S, Ahn CS, Moon DB, et al. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann Surg Oncol (2009) 16(3):623–9. doi: 10.1245/s10434-008-0278-3
9. Zhan Q, Shen BY, Deng XX, Zhu ZC, Chen H, Peng CH, et al. Clinical and pathological analysis of 27 patients with combined hepatocellular-cholangiocarcinoma in an Asian center. J Hepatobiliary Pancreat Sci (2012) 19(4):361–9. doi: 10.1007/s00534-011-0417-2
10. Renzulli M, Ramai D, Singh J, Sinha S, Brandi N, Ierardi AM, et al. Locoregional treatments in cholangiocarcinoma and combined hepatocellular cholangiocarcinoma. Cancers (Basel) (2021) 13(13):3336. doi: 10.3390/cancers13133336
11. Kim SH, Park YN, Lim JH, Choi GH, Choi JS, Kim KS. Characteristics of combined hepatocelluar-cholangiocarcinoma and comparison with intrahepatic cholangiocarcinoma. Eur J Surg Oncol (2014) 40(8):976–81. doi: 10.1016/j.ejso.2014.04.016
12. Yin X, Zhang B-H, Qiu S-J, Ren Z-G, Zhou J, Chen X-H, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: Clinical features, treatment modalities, and prognosis. Ann Surg Oncol (2012) 19(9):2869–76. doi: 10.1245/s10434-012-2328-0
13. Yoon Y-I, Hwang S, Lee Y-J, Kim K-H, Ahn C-S, Moon D-B, et al. Postresection outcomes of combined hepatocellular carcinoma-cholangiocarcinoma, hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Gastrointest Surg (2016) 20(2):411–20. doi: 10.1007/s11605-015-3045-3
14. Yamashita YI, Aishima S, Nakao Y, Yoshizumi T, Nagano H, Kuroki T, et al. Clinicopathological characteristics of combined hepatocellular cholangiocarcinoma from the viewpoint of patient prognosis after hepatic resection: High rate of early recurrence and its predictors. Hepatol Res (2020) 50(7):863–70. doi: 10.1111/hepr.13507
15. Chen PD, Chen LJ, Chang YJ, Chang YJ. Long-term survival of combined hepatocellular-cholangiocarcinoma: A nationwide study. Oncologist (2021) 26(10):e1774–e85. doi: 10.1002/onco.13893
16. Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet (1985) 161(4):346–50.
17. Kaibori M, Kon M, Kitawaki T, Kawaura T, Hasegawa K, Kokudo N, et al. Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci (2017) 24(11):616–26. doi: 10.1002/jhbp.502
18. Chun YS, Pawlik TM, Vauthey JN. 8th edition of the ajcc cancer staging manual: Pancreas and hepatobiliary cancers. Ann Surg Oncol (2018) 25(4):845–7. doi: 10.1245/s10434-017-6025-x
19. Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg (2005) 242(2):252–9. doi: 10.1097/01.sla.0000171307.37401.db
20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
21. Lin CW, Wu TC, Lin HY, Hung CM, Hsieh PM, Yeh JH, et al. Clinical features and outcomes of combined hepatocellular carcinoma and cholangiocarcinoma versus hepatocellular carcinoma versus cholangiocarcinoma after surgical resection: A propensity score matching analysis. BMC Gastroenterol (2021) 21(1):20. doi: 10.1186/s12876-020-01586-4
22. Wakizaka K, Yokoo H, Kamiyama T, Ohira M, Kato K, Fujii Y, et al. Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol (2019) 34(6):1074–80. doi: 10.1111/jgh.14547
23. Cutolo C, Dell'Aversana F, Fusco R, Grazzini G, Chiti G, Simonetti I, et al. Combined hepatocellular-cholangiocarcinoma: What the multidisciplinary team should know. Diagnostics (Basel) (2022) 12(4):890. doi: 10.3390/diagnostics12040890
24. Chu KJ, Lu CD, Dong H, Fu XH, Zhang HW, Yao XP. Hepatitis b virus-related combined hepatocellular-cholangiocarcinoma: Clinicopathological and prognostic analysis of 390 cases. Eur J Gastroenterol Hepatol (2014) 26(2):192–9. doi: 10.1097/MEG.0b013e3283625df9
25. Leoni S, Sansone V, Lorenzo S, Ielasi L, Tovoli F, Renzulli M, et al. Treatment of combined hepatocellular and cholangiocarcinoma. Cancers (Basel) (2020) 12(4):794. doi: 10.3390/cancers12040794
26. Jiao S, Li G, Zhang D, Xu Y, Liu J, Li G. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? a meta-analysis. Int J Surg (2020) 80:243–55. doi: 10.1016/j.ijsu.2020.05.008
27. Si A, Li J, Yang Z, Xia Y, Yang T, Lei Z, et al. Impact of anatomical versus non-anatomical liver resection on short- and long-term outcomes for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol (2019) 26(6):1841–50. doi: 10.1245/s10434-019-07260-8
28. Zhang EL, Liang BY, Chen XP, Huang ZY. Severity of liver cirrhosis: A key role in the selection of surgical modality for child-pugh a hepatocellular carcinoma. World J Surg Oncol (2015) 13:148. doi: 10.1186/s12957-015-0567-9
29. Shim JH, Jun MJ, Han S, Lee YJ, Lee SG, Kim KM, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg (2015) 261(5):939–46. doi: 10.1097/sla.0000000000000747
30. Zheng J, Kuk D, Gönen M, Balachandran VP, Kingham TP, Allen PJ, et al. Actual 10-year survivors after resection of hepatocellular carcinoma. Ann Surg Oncol (2017) 24(5):1358–66. doi: 10.1245/s10434-016-5713-2
31. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. JAMA Surg (2014) 149(6):565–74. doi: 10.1001/jamasurg.2013.5137
32. Bartsch F, Baumgart J, Hoppe-Lotichius M, Schmidtmann I, Heinrich S, Lang H. Visceral infiltration of intrahepatic cholangiocarcinoma is most prognostic after curative resection - retrospective cohort study of 102 consecutive liver resections from a single center. Int J Surg (2018) 55:193–200. doi: 10.1016/j.ijsu.2018.05.027
33. Zhang E-L, Cheng Q, Huang Z-Y, Dong W. Revisiting surgical strategies for hepatocellular carcinoma with microvascular invasion. Front Oncol (2021) 11:691354. doi: 10.3389/fonc.2021.691354
34. Liang BY, Gu J, Xiong M, Zhang EL, Zhang ZY, Chen XP, et al. Tumor size may influence the prognosis of solitary hepatocellular carcinoma patients with cirrhosis and without macrovascular invasion after hepatectomy. Sci Rep (2021) 11(1):16343. doi: 10.1038/s41598-021-95835-5
35. Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery (2008) 143(4):469–75. doi: 10.1016/j.surg.2007.12.003
Keywords: anatomical resection, non-anatomical resection, combined hepatocellular carcinoma and cholangiocarcinoma, surgery, prognosis
Citation: Wang WQ, Li J, Liang BY, Lv X, Zhu RH, Wang JL, Huang ZY, Yang SH and Zhang EL (2022) Anatomical liver resection improves surgical outcomes for combined hepatocellular-cholangiocarcinoma: A propensity score matched study. Front. Oncol. 12:980736. doi: 10.3389/fonc.2022.980736
Received: 28 June 2022; Accepted: 29 July 2022;
Published: 18 August 2022.
Edited by:
Nikolaos Machairas, National and Kapodistrian University of Athens, GreeceReviewed by:
Maria Conticchio, Ospedale Generale Regionale Francesco Miulli, ItalyCopyright © 2022 Wang, Li, Liang, Lv, Zhu, Wang, Huang, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Er-lei Zhang, YmFpeXUxOTg2MTEwNEAxNjMuY29t; Shu-hong Yang, eWFuZ3NodWhvbmcwMTA4QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.