- 1Department of Neurosurgery and Brain and Nerve Research Laboratory, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Suzhou Medical College, Soochow University, Suzhou, China
Background: Pain relief is one of the main objectives of radiotherapy for cancer patients with bone metastases. Stereotactic body radiotherapy (SBRT) enables precise delivery of a higher dosage to the target area. Several trials have reported comparisons between SBRT and conventional radiotherapy (cRT) in patients with painful bone metastasis. However, the results of those investigations were inconsistent, and no systematic review or meta-analysis has been done till now.
Methods: We systematically searched MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and Clinicaltrials.gov up to May 1, 2022 for relevant studies. Patients with painful bone metastasis who received SBRT or cRT were included. The primary outcome was the patients’ pain response rate at three months. The secondary outcomes included the rate of pain responders at one month and six months, oral morphine equivalent dose (OMED) use, and any adverse events. STATA software 12.0 was used for the statistical analysis.
Results: We collected 533 patients’ data from 4 randomized controlled trials (RCTs), there was a significant difference of pain response rate at 3 months between two groups (RR = 1.41, 95% CI: 1.12-1.77, I2 = 0.0%, P = 0.003). However, no significant difference was found in pain response rate at 1 month (RR = 1.19, 95% CI: 0.91-1.54, I2 = 31.5%, P = 0.201) and 6 months (RR = 1.25, 95% CI: 0.93-1.69, I2 = 0.0%, P = 0.140). OMED consumption was not significantly different in patients treated with SBRT compared with control group (WMD = -1.11, 95% CI: -17.51-15.28, I2 = 0.0%, P = 0.894). For safety outcome, no statistical difference was found between SBRT and cRT (RR = 0.72, 95% CI: 0.46-1.14, I2=20.1%, P = 0.162).
Conclusion: This study shows that for painful bone metastases, patients with SBRT experienced better pain relief 3 months after radiation than patients with cRT, and SBRT did not increase the incidence of adverse events.
Systematic review registration: https://inplasy.com/inplasy-2022-6-0099/, identifier INPLASY202260099.
Introduction
Bones are a common site of metastasis for some advanced cancers, such as breast and prostate cancer or lung and kidney cancer, and they can often cause several complications such as pain, pathological fractures, hypercalcemia, hemorrhage, and spinal cord compression, with sensory disturbance and dyskinesia (1). The main goal of bone metastasis therapy is to improve patients’ prognoses and prolong survival. Therefore, improving the life quality of patients is an essential topic in bone metastasis therapy, especially for pain relief. The pain can be caused by mass effect on the spinal cord or nerve roots, pathological fractures with mechanical instability, inflammation-induced periosteal nociceptor stimulation, tumor-derived products (e.g., tumor necrosis factor), or tumor-induced cytokines (2).
Some percutaneous techniques like radiofrequency ablation, cryoablation, and vertebral augmentation have been reported to be effective in alleviating pain (3). But for a long time, external beam radiotherapy (EBRT) has been considered the primary treatment for oligometastatic disease and painful bone metastases (4, 5). Previous studies have shown that conventional radiotherapy (cRT) provides pain relief in approximately 60% of patients with painful bone metastases, and complete pain relief in approximately 30% (6, 7). In recent years, new progress has been made in radiation technology. Stereotactic body radiotherapy (SBRT) has been gradually applied in radiotherapy for bone metastasis, which is based on accurate real-time assessment of tumor target location and dose delivery of multiple beam irradiation. For selected extracranial lesions (such as lung, liver, and prostate lesions), compared with cRT, its accuracy allows delivery of high doses in limited fractions (8). Studies have shown that the tumor control rate of SBRT in the treatment of bone metastases is up to 80% (9, 10). And for pain relief, SBRT also showed a complete pain relief rate of up to 50% (11).
Previously, several trials and meta-analyses focused on determining the optimal radiation dose and fractionation for painful bone metastases. While the study by Rich et al. indicated that patients who received single-fraction treatments and those who received multiple-fraction treatments had similar overall response rates (12). Several trials have reported the comparison between SBRT and cRT for survival outcomes of diseases such as non-small cell lung cancer and hepatocellular carcinoma (13, 14). However, no meta-analysis has been conducted to verify the efficacy and safety of SBRT as compared to cRT for patients with painful bone metastases. Therefore, in this study, we included randomized controlled trials (RCTs) on SBRT versus cRT in patients with painful bone metastases, and conducted the systematic review and meta-analysis to compare the effects of the two treatments.
Methods
Study protocol
The study was registered on the INPLASY website (INPLASY202260099) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (15).
Search strategy
MEDLINE, EMBASE, the Clinical Trials.gov, and Cochrane Central Register of Controlled Trials (CENTRAL) has been systematically searched by two separate investigations to identify relevant studies published until May 1, 2022. The following keywords (in the title/abstract) were used: (Stereotactic body radiotherapy OR SBRT) AND (spinal metastases OR bone metastases) AND pain
Eligibility criteria
The following are the criteria we set: (1) patient: adult patients diagnosed with painful bone metastases (a worst pain score at least ≥2 of 10, according to the Brief Pain Inventory (BPI); (2) intervention: patients who received SBRT in Intent-to-Treat Population. (3) comparison: patients who received cRT in the Intent-to-Treat Population; (4) outcomes: The primary outcome was the rate of patients with pain response at 3 months. The secondary outcomes included the rate of pain responders at 1 month and 6 months, and oral morphine equivalent dose (OMED) use. Safety outcome is adverse events. (5) study type: RCT. Included studies were not required to provide all of the aforementioned outcomes.
Exclusion criteria
The following are the exclusion criteria we set: (1) study type: comment, letter, review, case report or case series, non-randomized; (2) language: non-English article; (3) no extractable data.
Study selection and data collection
All entries queried from the database, RCTs, and relevant systematic reviews or meta-analyses were examined separately by the two authors (ZLW and LYL), removing duplicates and abstract-only research papers. When differences between the two authors arose, a third author (ZQC), who was not involved in the data collection procedure, made the final choices about disputed data.
Risk of bias
The Review Manager 5.3 software was used to examine the risk of bias plot for individual research. The Cochrane Collaboration’s consistent criteria for assessing the risk of bias in RCTs were used, including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases. The bias criteria were categorized as “low,” “high,” or “unclear.”
Outcome measures
International Consensus on Palliative Radiotherapy Endpoints (ICPRE) defines a complete response (CR) as BPI = 0 with no associated increase in daily oral morphine equivalent (OME) consumption. And the partial response (PR) is defined as a reduction in the worst pain score of 2 or more points above baseline with no increase in OME, or no increase in the worst pain score and a reduction in daily OME consumption of at least 25%. Pain progression (PP) is defined as an increase from baseline in the worst pain score of 2 or more points without reduced daily OME consumption, or as no change in the worst pain score and an increase in daily OME consumption of at least 25%. Besides, any response not included in the above definition will be considered as intermediate pain (IP). CR and PR can be regarded as responders, and the others (PP and IP) are non-responders.
Statistical analysis
The data were evaluated using STATA software 12.0 (STATA Corp., College Station, Texas, USA) by the two authors (XYY and HYT). Disputed data was solved by a third author (XXW). A random-effects model was used to assess and generate the risk ratio (RR) with the 95% confidence interval (CI) for the dichotomous outcomes. The I2 statistic was used to measure heterogeneity; a value of less than 30% suggests “low heterogeneity,” a value between 30% and 50% indicates “moderate heterogeneity,” and a value of more than 50% indicates “severe heterogeneity.” The stability of the consolidated data was investigated using sensitivity analysis. Two-tailed tests were used for all of the analyses, and a P value of less than 0.05 was regarded as statistically significant.
Results
Search results and study characteristics
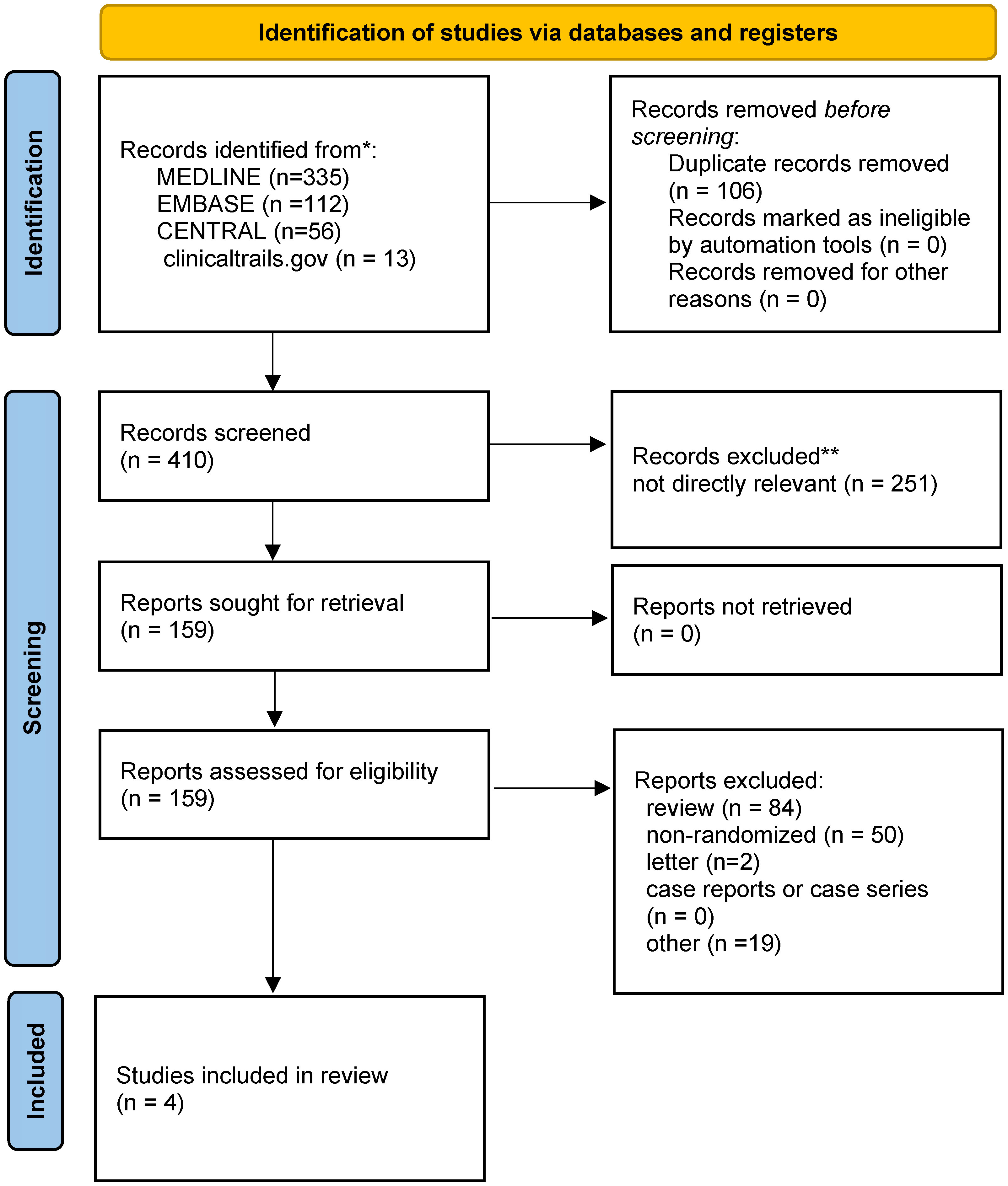
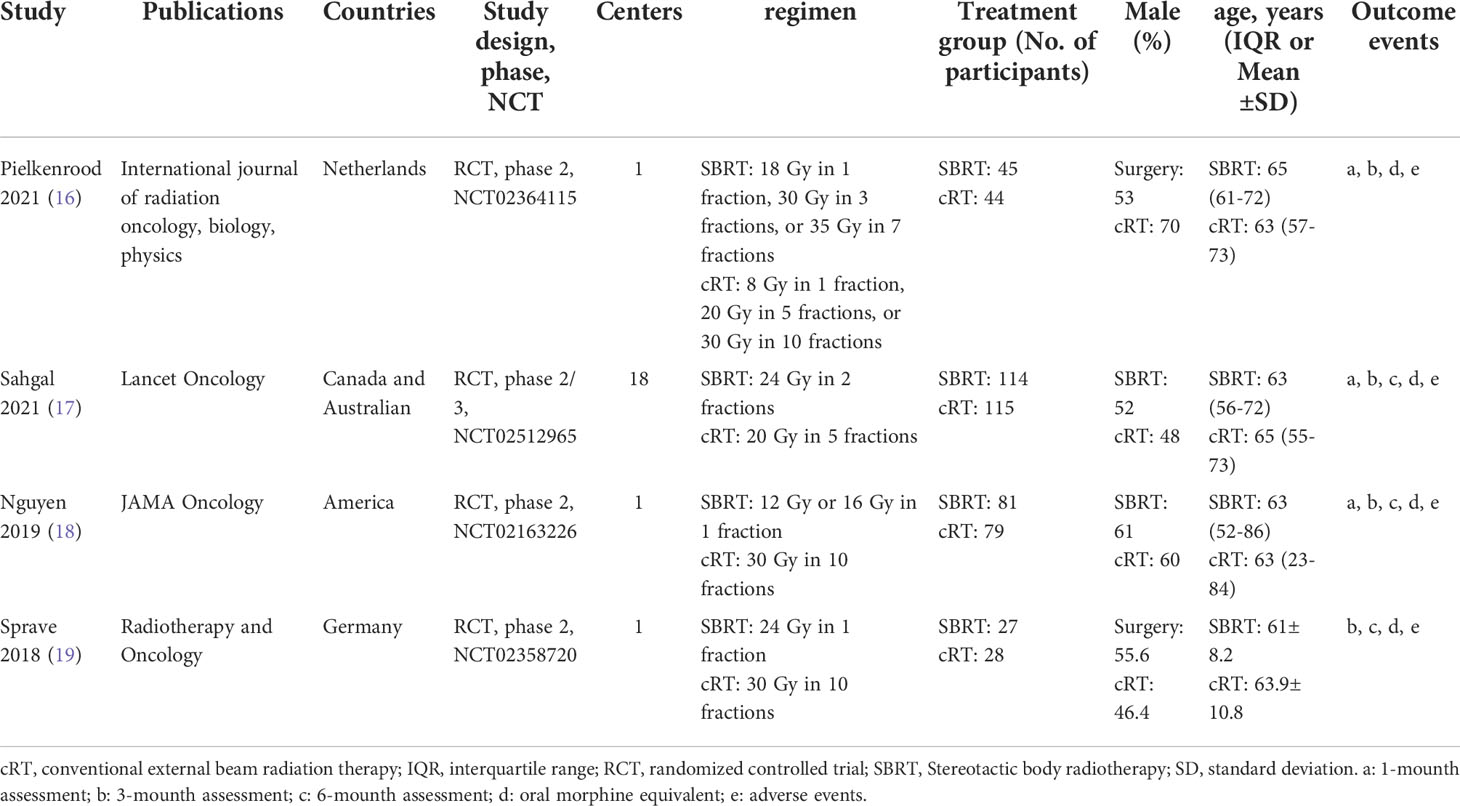
516 titles and abstracts were obtained for review from EMBASE, MEDLINE, CENTRAL, and ClinicalTrials.gov. We eliminated 106 duplicate articles and 251 irrelevant articles, leaving 159 articles, including 50 non-randomized clinical trials, 84 reviews, two letters, and 19 others. Finally, four RCTs (16–19) with 533 patients (267 in the SBRT group and 266 in the cRT group) were chosen for qualitative synthesis (Figure 1). Table 1 summarizes the main characteristics of the 4 included studies. The inclusion, exclusion criteria, and outcomes of each study were shown in the supplementary materials (Table S1).
Efficacy outcome
In this meta-analysis, we assessed rates of pain response at one month, three months, and six months after radiation, as well as OMED consumption. Three studies (16–18) reported rates of pain response at one months, three studies (16–18) reported rates of pain response at three months, and two studies (17, 18) reported rates of pain response at six months. At three months after radiotherapy, there was a significant difference of pain response rate between the SBRT group and the cRT group (RR = 1.41, 95% CI: 1.12-1.77, I2 = 0.0%, P = 0.003, Figure 2A). However, there was no significant difference in pain response rate at one month (RR = 1.19, 95% CI: 0.91-1.54, I2 = 31.5%, P = 0.201, Figure 2B) and six months (RR = 1.25, 95% CI: 0.93-1.69, I2 = 0.0%, P = 0.140, Figure 2C). Three studies (16, 17, 19) investigated the OMED consumption and found it was not significantly different in patients treated with SBRT compared with controls (WMD = -1.11, 95% CI: -17.51-15.28, I2 = 0.0%, P = 0.894, Figure 2D).
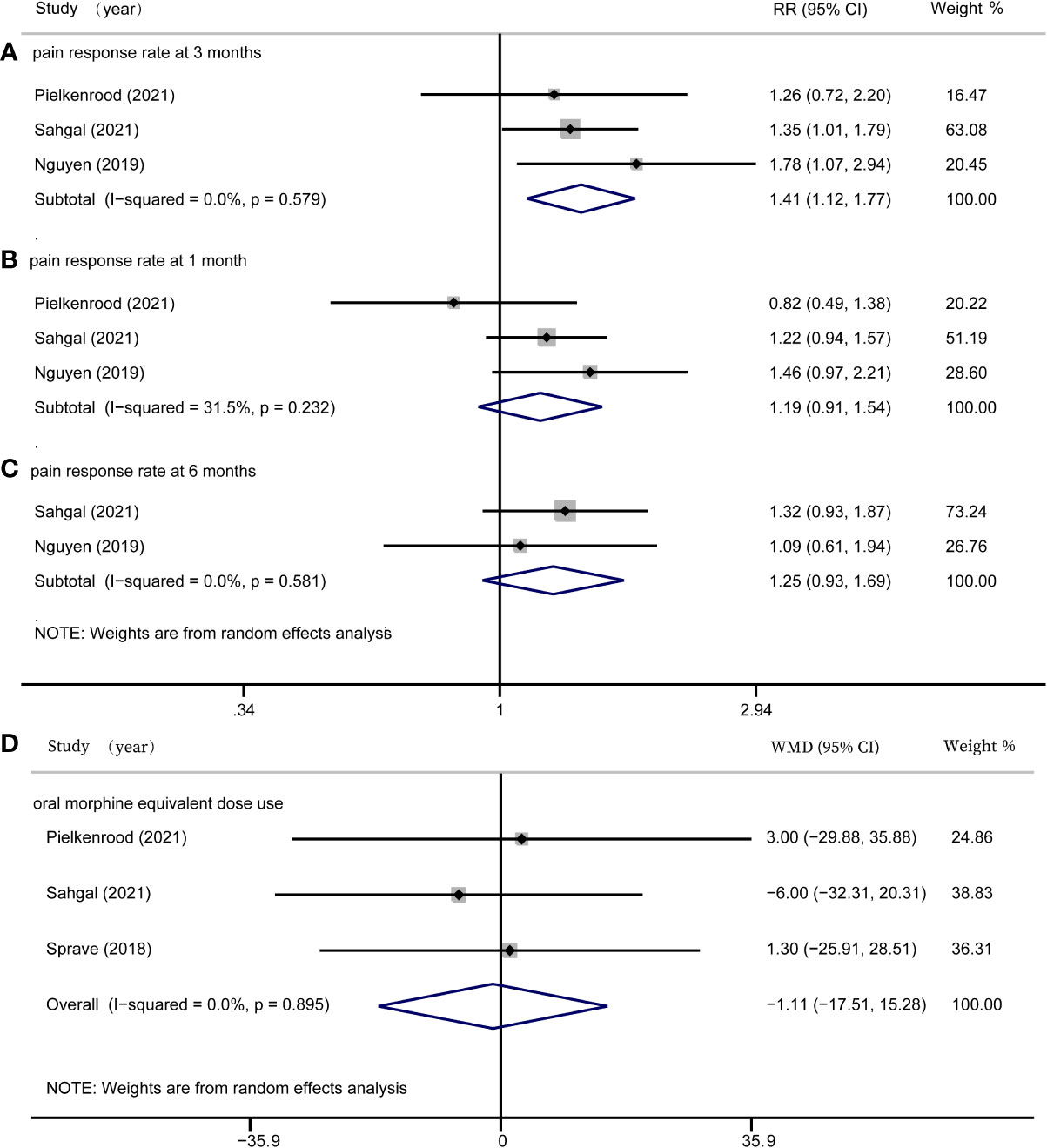
Figure 2 Forest plots for efficacy outcomes. (A) pain response rate at 3 months; (B) pain response rate at 1 month; (C) pain response rate at 6 months; (D) oral morphine equivalent dose use.
Safety outcome
As for the safety outcome, three out of the four studies (17–19) reported adverse events. According to our analysis, there were no statistically significant differences in adverse events such as vomiting, fatigue, or vertebral compression fracture in the SBRT group compared with the control group (RR = 0.72, 95% CI: 0.46-1.14, I2 = 20.1%, P = 0.162, Figure 3).
Risk of bias in included studies
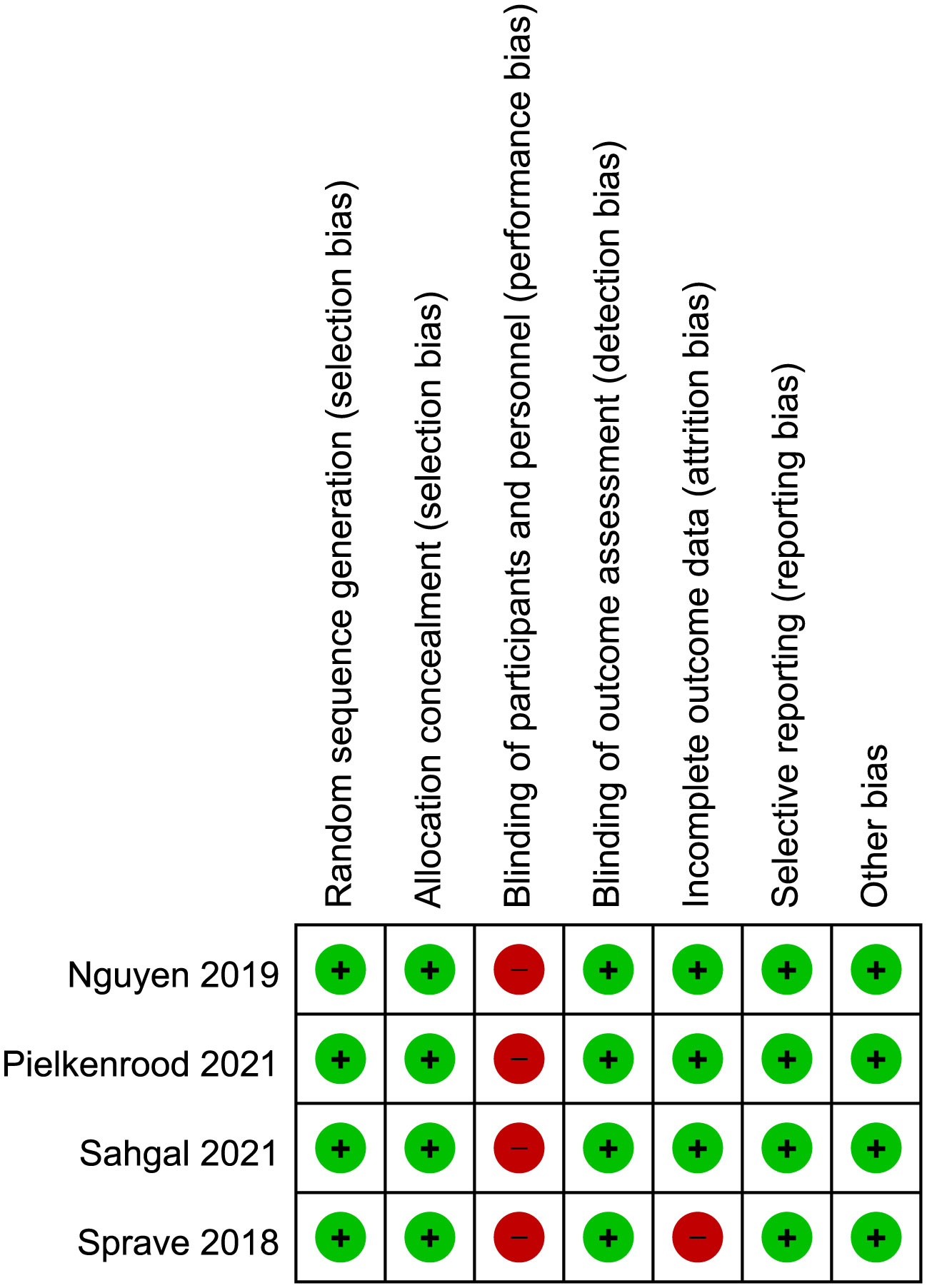
The risk of bias for included studies were shown in Figure 4. All included clinical trials had a low risk of bias in random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, and selective reporting. All included trials had a high risk in blinding of participants and personnel, as the differences in regimen of radiation therapy were obvious and required patient consent. The trial of Sprave et al. had a high risk in incomplete data as it only had data of the per-protocol cohort. Other items had not been found to have unclear or high risk of bias.
Discussion
We collected four RCTs to analyze, which mainly discussed the comparison of pain relief rates between SBRT and cRT. Our meta-analysis showed that at 3 months, SBRT is significantly superior to cRT for treating painful bone metastases pain. However, no significant difference has been found between SBRT and cRT group in the pain response at one month and six months. In addition, compared to the control group, SBRT group failed to reduce the use of OMED significantly.
Overall, according to our analysis, only pain response at 3 month was significantly improved in SBRT group. Several factors may attribute to the negative results at 1 month and 6 months between SBRT group and cRT group. Firstly, for pain response at 1 month, both Pelkenrood’s trial and Sahgal’s trial concluded that SBRT did not significantly improve pain response, while Nguyen’s trial drew the opposite conclusion. For pain response at 6 months, only Sahgal’s trial and Nguyen’s trial were included. As Nguyen’s trial mainly included patients with nonespinal metastases, it may interfere with the results’ extrapolation to the general population with spinal metastases. When evaluating which patients should be treated with SBRT, those RCTs also offered conflicting opinions. Sahgal et al. and Sparve et al. proposed that SBRT provides faster and better pain relief and should be recommended for patients with shorter expected survival (17, 19). Nguyen et al. proposed that SBRT has a persistent pain response rate, it should be the standard of care for patients with longer life expectancy and fewer bone metastases (18). Despite this disagreement, they agreed that SBRT was a better regimen for painful bone metastasis. Though no significant difference has been found for pain response at 1 month and 6 months, SBRT group tends to have a better efficacy outcome. Taking the above points into consideration, we hold the opinion that the application of SBRT to relief pain in patients with bone metastases of cancer has an anticipated clinical application prospect.
Radiation therapy has been considered as the main treatment for cancer with bone metastases. Radiation therapy uses externally generated electromagnetic and ion beams fired into the body to induce interactions between chemical free radicals and DNA, or other intracellular targets, leading to apoptosis (20). For conventional external radiation, there are two treatment options: single-fraction and multi-fraction radiotherapy, both has been widely used in clinical practice. Single-fraction is 8Gy in 1 fraction, multi-fraction adopts several regimens such as 30Gy in 10 fractions, 15Gy in 5 fractions, 20Gy in 5 fractions. Debate continues over which fractionation regimen is the best treatment for painful bone metastases. However, past studies have shown no significant difference in pain relief rates between single-fraction and multi-fraction (21–23).
In the past ten years, there has been a revolution in radiation therapy, SBRT is a novel treatment for cancer with spinal and bone metastases, as it can deliver higher doses precisely to the cancer cells in the target area (8). In 2012, the International Spinal Radiosurgery Consortium published a new standard for determining target volume of SBRT (24). Multiple prospective studies showed that SBRT could better control spinal metastasis tumors (9, 25). Thus, SBRT has been considered as a new treatment option that can bring benefits to patients. As for pain relief, one of the primary endpoints for palliative radiotherapy, a study by Owen et al. showed that the pain relief rate of SBRT in the treatment of non-spinal bone metastasis reached 88% (10), while Wang et al. and Chang et al. reported that the pain relief rate of SBRT in the treatment of spinal bone metastasis reached 50% (11, 26).
Studies with a higher level of evidence are urgently required to verify the efficacy and safety of SBRT for pain relief. In 2019, a prospective study compared SBRT with cRT plan, they put forward that SBRT can improve patients’ quality of life (27). Sakr et al. proposed that SBRT had better immediate pain relief than cRT (28). Results of an RCT in 2018 showed that palliative SBRT treatment for spinal metastatic tumors had a faster and better pain response rate (19). Subsequently, Sah et al. proposed that SBRT is superior to cRT for symptom control in patients with less metastatic disease (17). Based on these views, we conducted a meta-analysis to compare the pain relief rates between the two regimens. Considering the previous views and the result of our analysis, the effectiveness of SBRT in relieving pain of bone metastases might be promising, especially at three months after treatment.
In addition, in terms of the safety of the two regimens, three of the four RCTs we included reported adverse events, including dysphagia, oesophagitis, nausea, pain, fatigue, radiodermatitis, and vertebral compression fracture. No statistical difference was found according to our meta-analysis, indicating the safety of SBRT as compared to cRT. However, the probability of spinal compression fractures after SBRT should not be ignored, a systematic review by Sahgal et al. analyzed risk factors for pathologic fracture after SBRT, and they put forward that the patients with SBRT have a higher risk of pathological fracture (29). To note, the RCT conducted by Sahgal et al. demonstrated that more patients in the cRT group had a vertebral compression fracture than SBRT group. Overall, despite the risk of pathological fractures and other adverse events exist, the safety of SBRT is still promising.
There are some limitations in this meta-analysis. Firstly, our analysis was based on limited data from four published RCTs to test the efficacy and safety of SBRT. And only three out of the four RCTs were included for the analysis of our primary outcome as Sprave et al.’s study did not reported data of the Intent-to-Treat analysis. Secondly, different extracorporeal radiotherapy segmentation schemes were adopted in the included RCTs. Thirdly, the study by Pelkenrood et al. only reported follow-up data within 3 months. Lastly, the response to SBRT and subsequent pain response may be impacted by the variations in tumor histology and molecular subtype. These are all possible reasons for heterogeneity.
In the future, defining the most effective and safe SBRT dose and segmentation schemes are new directions for SBRT development. Further larger randomized controlled trials are still required to determine the optimum treatment for patients with painful bone metastases.
Conclusion
In conclusion, this study shows that for painful bone metastases, patients with SBRT experienced better pain relief three months after radiation than patients with cRT, and did not increase the incidence of adverse events. For patients who expect better quality of life after palliative radiotherapy, SBRT seems to have a promising application prospect.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZLW and LL was the principal investigator. ZLW, LL and ZC designed the study and developed the analysis plan. XY, HT and XW analyzed the data and performed meta-analysis. ZLW and LL contributed in writing of the article. XY and HT revised the manuscript and polish the language. ZC, ZW and GC supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (Grants No. BK20200203) and the National Natural Science Foundation of China (Grant No. 81873741).
Acknowledgments
We appreciate the valuable and constructive suggestions and assistance from our Team of Neurosurgical study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.979201/full#supplementary-material
References
1. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer (2002) 2(8):584–93. doi: 10.1038/nrc867
2. Mannion RJ, Woolf CJ. Pain mechanisms and management: A central perspective. Clin J Pain (2000) 16(3 Suppl):S144–56. doi: 10.1097/00002508-200009001-00006
3. Greenwood TJ, Wallace A, Friedman MV, Hillen TJ, Robinson CG, Jennings JW. Combined ablation and radiation therapy of spinal metastases: A novel multimodality treatment approach. Pain Phys (2015) 18(6):573–81. doi: 10.36076/ppj.2015/18/573
4. Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (Royal Coll Radiol (Great Britain)) (2012) 24(2):112–24. doi: 10.1016/j.clon.2011.11.004
5. Sahgal A, Roberge D, Schellenberg D, Purdie TG, Swaminath A, Pantarotto J, et al. The Canadian association of radiation oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (Royal Coll Radiol (Great Britain)) (2012) 24(9):629–39. doi: 10.1016/j.clon.2012.04.006
6. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol (2007) 25(11):1423–36. doi: 10.1200/JCO.2006.09.5281
7. Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol phys (2011) 79(4):965–76. doi: 10.1016/j.ijrobp.2010.11.026
8. Lartigau E. Stereotactic body radiotherapy. BMJ (Clinical Res ed) (2011) 343:d4286. doi: 10.1136/bmj.d4286
9. Nguyen QN, Shiu AS, Rhines LD, Wang H, Allen PK, Wang XS, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol phys (2010) 76(4):1185–92. doi: 10.1016/j.ijrobp.2009.03.062
10. Owen D, Laack NN, Mayo CS, Garces YI, Park SS, Bauer HJ, et al. Outcomes and toxicities of stereotactic body radiation therapy for non-spine bone oligometastases. Pract Radiat Oncol (2014) 4(2):e143–e9. doi: 10.1016/j.prro.2013.05.006
11. Chang EL, Shiu AS, Mendel E, Mathews LA, Mahajan A, Allen PK, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine (2007) 7(2):151–60. doi: 10.3171/SPI-07/08/151
12. Rich SE, Chow R, Raman S, Liang Zeng K, Lutz S, Lam H, et al. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol (2018) 126(3):547–57. doi: 10.1016/j.radonc.2018.01.003
13. Geng Y, Zhang Q, Feng S, Li C, Wang L, Zhao X, et al. Safety and efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Med (2021) 10(4):1222–39. doi: 10.1002/cam4.3718
14. Zheng J, Cai J, Tao L, Kirih MA, Shen Z, Xu J, et al. Comparison on the efficacy and prognosis of different strategies for intrahepatic recurrent hepatocellular carcinoma: A systematic review and Bayesian network meta-analysis. Int J Surg (2020) 83:196–204. doi: 10.1016/j.ijsu.2020.09.031
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed) (2021) 372:n71. doi: 10.1136/bmj.n71
16. Pielkenrood BJ, van der Velden JM, van der Linden YM, Bartels MMT, Kasperts N, Verhoeff JJC, et al. Pain response after stereotactic body radiation therapy versus conventional radiation therapy in patients with bone metastases-a phase 2 randomized controlled trial within a prospective cohort. Int J Radiat Oncol Biol phys (2021) 110(2):358–67. doi: 10.1016/j.ijrobp.2020.11.060
17. Sahgal A, Myrehaug SD, Siva S, Masucci GL, Maralani PJ, Brundage M, et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol (2021) 22(7):1023–33. doi: 10.1016/S1470-2045(21)00196-0
18. Nguyen QN, Chun SG, Chow E, Komaki R, Liao Z, Zacharia R, et al. Single-fraction stereotactic vs conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: A randomized phase 2 trial. JAMA Oncol (2019) 5(6):872–8. doi: 10.1001/jamaoncol.2019.0192
19. Sprave T, Verma V, Förster R, Schlampp I, Bruckner T, Bostel T, et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol (2018) 128(2):274–82. doi: 10.1016/j.radonc.2018.04.030
20. Pisansky TM. External-beam radiotherapy for localized prostate cancer. New Engl J Med (2006) 355(15):1583–91. doi: 10.1056/NEJMct055263
21. Kaasa S, Brenne E, Lund JA, Fayers P, Falkmer U, Holmberg M, et al. Prospective randomised multicenter trial on single fraction radiotherapy (8 gy x 1) versus multiple fractions (3 gy x 10) in the treatment of painful bone metastases. Radiother Oncol (2006) 79(3):278–84. doi: 10.1016/j.radonc.2006.05.006
22. Hartsell WF, Scott CB, Bruner DW, Scarantino CW, Ivker RA, Roach M 3rd, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst (2005) 97(11):798–804. doi: 10.1093/jnci/dji139
23. Wu JS, Wong R, Johnston M, Bezjak A, Whelan T. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys (2003) 55(3):594–605. doi: 10.1016/S0360-3016(02)04147-0
24. Cox BW, Spratt DE, Lovelock M, Bilsky MH, Lis E, Ryu S, et al. International spine radiosurgery consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol phys (2012) 83(5):e597–605. doi: 10.1016/j.ijrobp.2012.03.009
25. Deodato F, Cilla S, Macchia G, Torre G, Caravatta L, Mariano G, et al. Stereotactic radiosurgery (SRS) with volumetric modulated arc therapy (VMAT): interim results of a multi-arm phase I trial (DESTROY-2). Clin Oncol (Royal Coll Radiol (Great Britain)) (2014) 26(12):748–56. doi: 10.1016/j.clon.2014.08.005
26. Wang XS, Rhines LD, Shiu AS, Yang JN, Selek U, Gning I, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol (2012) 13(4):395–402. doi: 10.1016/S1470-2045(11)70384-9
27. Moon DH, Basak RS, Usinger DS, Dickerson GA, Morris DE, Perman M, et al. Patient-reported quality of life following stereotactic body radiotherapy and conventionally fractionated external beam radiotherapy compared with active surveillance among men with localized prostate cancer. Eur Urol (2019) 76(3):391–7. doi: 10.1016/j.eururo.2019.02.026
28. Sakr A, Hashem WB, Ebrahim N, Mashhour KN. Randomized pilot study of 20 gy in 5 fractions versus 27 gy in 3 fractions radiotherapy for treating painful bone metastases: A single institution experience. Asian Pac J Cancer Prevent: APJCP (2020) 21(6):1807–11. doi: 10.31557/APJCP.2020.21.6.1807
Keywords: bone metastases, conventional external radiation, meta-analysis, pain relief, stereotactic body radiotherapy
Citation: Wang Z, Li L, Yang X, Teng H, Wu X, Chen Z, Wang Z and Chen G (2022) Efficacy and safety of stereotactic body radiotherapy for painful bone metastases: Evidence from randomized controlled trials. Front. Oncol. 12:979201. doi: 10.3389/fonc.2022.979201
Received: 27 June 2022; Accepted: 28 September 2022;
Published: 19 October 2022.
Edited by:
Francesco Deodato, Università Cattolica del Sacro Cuore, Roma, ItalyReviewed by:
Francesco Cellini, Università Cattolica del Sacro Cuore, ItalyRossella Di Franco, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2022 Wang, Li, Yang, Teng, Wu, Chen, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhouqing Chen, enFjaGVuNkAxNjMuY29t; Zhong Wang, d2FuZ3pob25nNzYxQDE2My5jb20=
†These authors have contributed equally to this work
 Zilan Wang
Zilan Wang Longyuan Li1†
Longyuan Li1† Zhouqing Chen
Zhouqing Chen Zhong Wang
Zhong Wang Gang Chen
Gang Chen