- Saint Petersburg Clinical Research and Practical Center of Specialized Types of Medical Care (Oncological), Saint Petersburg, Russia
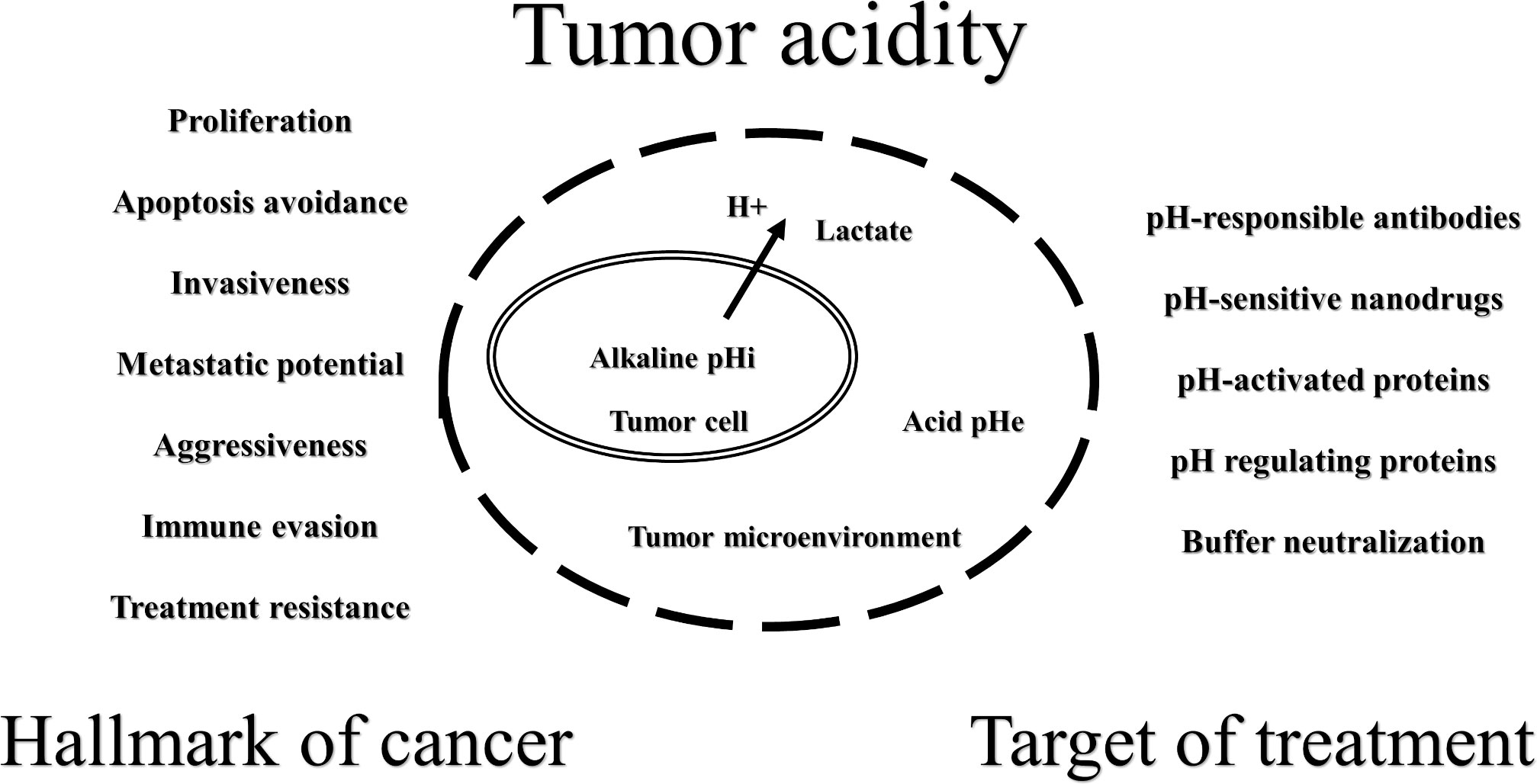
Tumor acidity is one of the cancer hallmarks and is associated with metabolic reprogramming and the use of glycolysis, which results in a high intracellular lactic acid concentration. Cancer cells avoid acid stress major by the activation and expression of proton and lactate transporters and exchangers and have an inverted pH gradient (extracellular and intracellular pHs are acid and alkaline, respectively). The shift in the tumor acid–base balance promotes proliferation, apoptosis avoidance, invasiveness, metastatic potential, aggressiveness, immune evasion, and treatment resistance. For example, weak-base chemotherapeutic agents may have a substantially reduced cellular uptake capacity due to “ion trapping”. Lactic acid negatively affects the functions of activated effector T cells, stimulates regulatory T cells, and promotes them to express programmed cell death receptor 1. On the other hand, the inversion of pH gradient could be a cancer weakness that will allow the development of new promising therapies, such as tumor-targeted pH-sensitive antibodies and pH-responsible nanoparticle conjugates with anticancer drugs. The regulation of tumor pH levels by pharmacological inhibition of pH-responsible proteins (monocarboxylate transporters, H+-ATPase, etc.) and lactate dehydrogenase A is also a promising anticancer strategy. Another idea is the oral or parenteral use of buffer systems, such as sodium bicarbonate, to neutralize tumor acidity. Buffering therapy does not counteract standard treatment methods and can be used in combination to increase effectiveness. However, the mechanisms of the anticancer effect of buffering therapy are still unclear, and more research is needed. We have attempted to summarize the basic knowledge about tumor acidity.
Introduction
Cancer cells have an inverted pH gradient: extracellular and intracellular pHs (pHe, pHi) are acid and alkaline, respectively (1). The acid shift in the tumor microenvironment (TME) is closely associated with hypoxia (2) but, more specifically, with highly activated glycolysis in tumor cells. Even in normoxia, about 80% of all malignant tumors use aerobic glycolysis, described as the Warburg effect (3), which is an integral part of metabolic reprogramming and sustaining biosynthetic pathways in cancer cells (4).
According to the present knowledge, the shift in the tumor acid-base balance promotes proliferation, apoptosis avoidance, invasiveness, metastatic potential, aggressiveness, immune evasion, and treatment resistance (5–8). On the other hand, inversion of the pH gradient in tumors could be a weakness that will allow for the development of new promising therapies (Figure 1). It is possible to create acid stress inside cancer cells by inhibiting proton release systems or by using drugs that decrease mitochondrial activity to increase lactate production (5, 9, 10). The acidity of the TME could be used for the drug delivery of cytotoxic agents and/or carriers that are more active and/or change physicochemical properties under such conditions (11–13). It is very attractive to increase the pHe by a combination of an alkaline diet and bicarbonate therapy (14–16) or by direct local isolated perfusion of the tumor with bicarbonate solutions (17, 18).
Obviously, the altered acid-base state of the tumor affects every stage of cancer development, from dysplasia to metastatic disease (1, 2). In this mini-review, we have attempted to summarize the basic knowledge about tumor acidity from hallmark of cancer to target of treatment.
Tumor acidity as a hallmark of cancer
One of the causes of tumor heterogeneity is altered tumor vasculature, which leads to different perfusion of nutrients and oxygen and to the accumulation of acidic metabolites (19, 20). Due to the reprogramming of metabolism in such conditions and the use of glycolysis as a major source of ATP production, tumor cells have an acidic pHe (6.4-7.1) and an alkaline pHi (7.1-7.8). For normal tissues, the pHe is around 7.4, and the pHi is around 7.2 (2, 21). Large amounts of lactate produced during glycolysis result in a significant increase in the intracellular proton (H+) concentration. It should be noted that glutaminolysis is another way for ATP production and an additional source of lactate and H+ in cancer cells (21–24). In addition, glutamine uptake and metabolism in oxidative cancer cells can be promoted by lactate (25). However, even in the presence of oxygen, glucose is almost completely converted into lactate. At the same time, glutamine is not fully respired, but it is rather fermented into lactate or pyruvate. Increased glutamine flux can enhance aerobic glycolysis and make it optimal for tumor proliferation (22, 26).
As acid stress triggers apoptosis (27), cancer cells use several ways to evade it (28). Activation and expression of H+ (and lactate) transporters and exchangers are the main mechanisms of tumor cell adaptation to intracellular acidification and of the inverted pH gradient phenomenon (29–32). It should be noted that not only H+ ejection systems lead to an increase in the pHi, but also a reduction of CO2 by decreased activity of the tricarboxylic acid cycle (TCA) and oxidative phosphorylation (OXPHOS) (1, 10). Carbonic anhydrases (CAs) additionally support the pH regulation of cancer cells by catalyzing the reversible hydration of CO2 to HCO3− and H+ (32).
Acidosis of the TME is an essential stage associated with high rates of tumor cell proliferation (33). Numerous studies have shown a role for tumor acidity in acquiring aggressive cancer characteristics, so it is recognized as a hallmark of cancer (21, 31, 34–36). For example, melanoma cells exposed to acidosis are characterized by a high invasive potential, high resistance to apoptosis and drug therapy, fixed independent growth, and a phenotype of epithelial to mesenchymal transition (37). Under growth factor limitations, alkaline pHi favors cancer cells survival (38). The acid adaptation of tumor cells leads to a gene expression response that correlates with human cancer tissue gene expression profiles and survival (39). Acidic TME improves the activity of regulatory T-cells and inhibits effector T-cells (40). In view of the foregoing, acidic TME could serve as an incubator that represses overabundant proliferation and cultures cells with a restricted growth rate but with strong proliferative potential (41). Clinicians should consider tumor acidity when diagnosing and determining optimal treatment, as it is also connected with poor cancer patients prognosis (39).
A wide range of non-invasive and minimally invasive imaging modalities have been studied preclinically for tumor pH monitoring, including magnetic resonance imaging (MRI) and spectroscopy, positron emission tomography, electron paramagnetic resonance, and optical and photoacoustic imaging (42). To date, among the methods used, MRI appears to be the most promising, particularly chemical exchange saturation transfer (CEST) MRI, which has good in vivo sensitivity for assessing tumor acidosis and changes in pH after therapeutic treatment, with a high spatial resolution to determine the heterogeneity of extracellular acidification. For example, CEST MRI has been used successfully to map tumor pH in a rabbit liver cancer model (43). In another study, tumor acidosis assessed by CEST MRI revealed the metastatic potential of breast cancer in mice (44). Translating the results of preclinical studies into clinical trials is only beginning to yield significant results. CEST MRI shows good results for measuring pH in ovarian cancer patients (45). In addition, CEST MRI has recently been shown to differentiate between benign and malignant liver tumors in patients (46). However, it is still difficult to routinely measure the pH of tumors in the clinic. In addition to direct measurements, tumor acidity can be assessed indirectly by determining the concentrations of bicarbonate (47) and lactate ions in the blood and using biopsy data (48). However, each clinical situation requires an individual approach.
Tumor acidity and tumor resistance
Cancer cell survival strategies in acidic TME promote resistance to radiation and chemotherapy. Radioresistance is closely related to hypoxia. Available clinical data show that the presence of large hypoxia areas in solid tumors is associated with a poor prognosis in cancer patients after radiotherapy (49). The cytotoxic effects of ionizing radiation are mainly due to damage to genomic DNA as a result of the indirect action of generated free radicals (50). Molecular oxygen must be present during irradiation, which is insufficient under hypoxic conditions. Hypoxia also prevents DNA repair and leads to the inhibition of the G1/S cell cycle checkpoint, an increase in DNA errors, and an increase in chromosomal instability. At the same time, the alkaline pHi of tumor cells prevents mitotic arrest initiated by activated checkpoints during DNA damage (21, 51). Thus, the inversion of the pH gradient of the tumor is a “partner” of hypoxia in creating conditions for radioresistance, and clinicians should consider acidic TME in the planning of radiation therapy.
Acidic TME itself can lead to chemoresistance due to ongoing physicochemical changes in the structure and charge of drugs. Weak-base chemotherapeutic agents, such as vincristine, mitoxantrone, doxorubicin, vinblastine, and paclitaxel, may have substantially reduced cellular uptake capacity due to neutralization or protonation [“ion trapping” (52)]. Therefore, the cytotoxic effects of these drugs may be reduced, resulting in a stable tumor phenotype. Interestingly, reversing the pH gradient may increase the intracellular concentrations of some weak-acid drugs, including cyclophosphamide and chlorambucil (53–56). Acidic TME induces p-glycoprotein (multiple drug resistance (MDR) protein) activity by promoting p38 mitogen-activated protein kinase (57–59). Tumor acidosis induces the expression of the transcription factor SOX2 by inhibiting vitamin D receptor-mediated transcription, which also results in drug resistance (60). Oxidation-induced lactic acidosis increases resistance to uprosertib, a serine/threonine protein kinase inhibitor, in colon cancer cells (61). To obtain the maximum effect of chemotherapy, the acidity of the TME must be considered.
Current knowledge strongly suggests that acidic TME inhibits the antitumor immune response, although the complication of experimentally measuring tumor acid-base status makes it difficult to obtain direct evidence (7, 62). For instance, a decrease in the pHe leads to a decrease in the activity and proliferation of T cells (63, 64). In an acidic environment, effector T cells require higher thresholds for full activation and co-stimulatory signals (e.g., CD28) and show increased negative regulatory signaling through upregulation of interferon gamma receptor 2 (IFN-γR2) and cytotoxic T cell-associated protein 4 (CTLA-4) (64). Acidic extracellular conditions reduce the expression of T-lymphocyte receptor components (65). Since the movement of lactate between the cytosol and the extracellular space depends on its concentration gradient, a high concentration of extracellular lactate in the TME prevents the export of lactate from T cells. This negatively affects the functions of activated T-lymphocytes dependent on glycolysis for ATP production (66). Notably, the functions of effector T- lymphocytes could be restored after normalization of pH (65–69), so the acidity does not have a cytotoxic effect. A significant effect of low acidity appears to be its negative effect on effector cytokines production by T cells, which is significantly reduced under acidic conditions (70–72). However, receptor interactions also play an important role. For example, in acidic TME, the V-domain Ig suppressor of T cell activation (VISTA), which is expressed by tumor-infiltrating myeloid suppressor cells, is activated and suppresses effector T cells (73). The inhibitory effect of acidic TME on dendritic cells is not related to the high concentration of H+, which actually stimulates antigen presentation (74). This inhibition can be explained by the accumulation of lactate, which modulates the dendritic cell phenotype and causes increased production of anti-inflammatory (e.g., IL-10) and decreased production of pro-inflammatory (e.g., IL-12) cytokines (75, 76). An acidic pHe and a high concentration of lactate together lead to a decrease in the activity of natural killers, including the depletion of interferon gamma (IFN-γ) and their ability to infiltrate the tumor (71, 77, 78). At the same time, for example, an acidic environment stimulates regulatory T cells (Tregs) activity by involving lactic acid in metabolism (79). In addition, lactic acid promotes Tregs’ expression of programmed cell death receptor 1 (PD-1) by absorption through monocarboxylate transporter 1 (MCT1). Thus, the PD-1 blockade activates PD-1-rich Tregs, resulting in treatment failure (80). Besides this, the acidity of the TME upregulates programmed death ligand 1 (PD-L1) in tumor cells (81).
It seems clear that the acidic conditions of the TME must be considered in monoclonal antibodies (mAbs) anticancer therapy. On the one hand, slightly acidic conditions are probably optimal for most mAbs (82), i.e., acidity in solid tumors may only slightly influence the deterioration of the therapeutic properties of mAbs. On the other hand, the possibility of the degradation of mAbs under such conditions cannot be excluded (7). For example, the rate of antibody Fc fragment oxidation and aggregation, which determines antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), has been shown to increase with decreasing pH (83, 84). Despite the fact that cancer immunotherapy uses immune checkpoint-blocking mAbs that are specifically modified to eliminate interactions with Fc receptors, fatal changes in other parts of mAbs that determine their activity are also possible at low pH values. For example, the chemical degradation of aspartic acid induced by acidic pH in the complementarity-determining region (CDR) of a monoclonal antibody against the epidermal growth factor receptor (EGFR) causes a loss of antibody-binding activity (85). The high structural and physicochemical affinity of mAbs to their targets is a condition for achieving a therapeutic effect. In particular, histidine residues in interacting sites can increase pH-mediated dissociation due to protonation under acidic conditions, favoring electrostatic repulsion between rigid domains in protein–protein interaction (86). The low pHe can also greatly affect the bioavailability of therapeutic mAbs. At the same time, the “useful” side of acidic TME is the possibility of creating therapeutic pH-selective mAbs (87, 88).
Tumor acidity as a target of treatment
Tumor-targeted pH-sensitive antibodies should be screened for low pH activity, and antibody engineering should not be limited to finding molecules with activity over a wide pH range (87). For example, despite the pH-independent affinity of CTLA-4 for ipilimumab, an analog was developed with up to a 50-fold affinity for CTLA-4 at pH 6.0 compared to pH 7.4 (89). A bispecific pH-responsive anticarcinoembryonic antigen-related cell adhesion molecule (CEACAM) 5 antibody that binds pH-independently to CEACAM6 was generated (88). Likewise, acidic TME allows for pH-activated molecular targets, such as VISTA. A combination of anti-VISTA mAb with anti-PD-L1 therapy demonstrated a significant survival benefit in tumor-bearing mice (90). Nanotechnologies also provide a good tool for creating pH-responsible anticancer drugs based on pH-responsible polymer nanomaterials, nanogels, etc. (91, 92). It has recently been summarized that several types of pH-sensitive nanoparticle conjugates with paclitaxel, doxorubicin, or others enhance drug delivery and potentiate anticancer effects in various experimental cancer cell lines (93).
Another approach to influencing tumor aggressiveness and/or therapeutic response is the regulation of tumor pH levels. First, since glycolysis is the main source of lactate and H+, would it be possible to reduce lactate production by limiting glucose? Also considering that hyperglycemia is known to be associated with reduced survival rates in some types of cancer (94–97), although this is still controversial, for example, in pancreatic (98–100) or colorectal cancers (101–103). Indeed, glucose restriction can reverse the Warburg effect and decrease lactate production in vitro (104). However, cancer cells can also use glycogenolysis, glycogen synthesis, and gluconeogenesis to compensate for glucose starvation (105–107). Many therapies targeting glucose metabolism (e.g., targeting glucose transporters, glycogen phosphorylase, glycogen synthase kinase 3β, hexokinase 2, glucose-6-phosphate isomerase, etc.) have been developed, but have not yet been successful in clinical trials (107). Furthermore, glycolysis is the main metabolic pathway of neutrophils, M1 macrophages, dendritic cells, naive T cells, effector T cells, etc. (108). For example, glucose-deficient TME limits the anaerobic glycolysis of tumor-infiltrating T cells and thus suppresses tumor-killing effects (109). Nutritional deficiencies in the TME, especially glucose, impair the metabolism of NK cells and their antitumor activity (110). It is important to note that human glucose levels may be reduced to very low levels without causing harm (111), and ketone bodies can be used for energy production with benefits for the organism (112, 113). For instance, a ketogenic diet improves the function of T cells (114, 115) and possibly creates an unfavorable metabolic environment for cancer cells (116, 117). However, ketone bodies utilization or formation may be a promoter for tumor cells proliferation and metastasis (118–121). Therefore, limiting glucose or its metabolism to reduce lactate production can have a completely ambiguous effect.
A more optimal way to reduce lactate production seems to be the inhibition of lactate dehydrogenase A (LDHA). This approach provides the simultaneous restriction of lactate synthesis from both glycolysis and glutaminolysis. Indeed, the inhibition of LDHA in vivo redirects pyruvate to support OXPHOS (122, 123). To date, a large number of LDHA inhibitors have been studied preclinically, but unfortunately, the clinical utility of such inhibitors may be limited due to nonselective toxicity or complex interactions with other cellular components. Optimization of existing compounds and continued search and development of new LDHA inhibitors will be reasonable strategies to obtain direct antitumor effects or enhance, for example, immunotherapy results (48, 124, 125). For example, since the effect of immunotherapy can be prevented by lactate (79, 80) and high LDH levels before treatment are correlated with a poor response to immunotherapy (126, 127), inhibition of LDHA can improve the efficacy of anti-PD-1 therapy (128).
Alternate modality to regulate tumor acidity is the pharmacological inhibition of proteins responsible for regulating pHi or mitochondrial activity (5, 9, 10). For example, inhibition of mitochondrial pyruvate transporter (MPC) works to block lactate utilization while preventing oxidative glucose metabolism (129). Blocking the monocarboxylate transporter 1 (MCT1) (used to import lactate as an energy source in oxidative cancer cells) with the specific MCT1 inhibitor AZD3965 prevents lactate consumption, increases its concentration in the TME, and has an antiproliferative effect (130–132). Conversely, the inhibition of MCT4 (expressed to remove lactate in glycolytic tumor cells) causes intracellular lactate accumulation, a decrease in pHi, but also reduces tumor growth in vitro and in vivo (132, 133). The cooperative use of MCT1/MCT4 inhibitors or nonspecific MCT inhibitors has good therapeutic potential (125, 132, 134, 135). Also of great importance to decrease pHi values is the pharmacological inhibition of the proton pump H+-ATPase (136), sodium-hydrogen antiporter 1 (NHE1) (137), and carbonic anhydrase IX (CAIX) (138). For example, according to the results of a phase III clinical trial (NCT01069081), intermittent use of a high dose of the proton pump inhibitor esomeprazole potentiates the effects of docetaxel and cisplatin chemotherapy in metastatic breast cancer without causing further toxicity (139). In a retrospective study, omeprazole was found to have a synergistic effect with chemoradiotherapy and to significantly reduce the risk of rectal cancer recurrence (140). Other ion exchangers and transporters are involved in tumor pH regulation, but their role in cancer progression remains unclear (2).
Another way to affect tumor acidity is the use of buffer systems, such as sodium bicarbonate. Preclinical and some clinical studies suggest that “direct” tumor deacidification may slow progression or improve therapeutic response (34). Oral administration of sodium bicarbonate can increase the efficacy of doxorubicin and mitoxantrone in model experiments (52, 55). Furthermore, peroral administration of sodium bicarbonate and other buffer solutions significantly reduced the invasion and metastasis of various experimental (including spontaneous) tumors in genetically modified animals but had no effect on the growth of primary tumors (141–146). Neutralization of tumor acidity improved the antitumor response to anti-CTLA-4 and PD-1 mAbs, as well as the adoptive transfer of T-lymphocytes in experiments using the B16 melanoma model and Panc02 pancreatic cancer in mice (69).
At the same time, the first three clinical trials of oral sodium bicarbonate (NCT01350583, NCT01198821, NCT01846429) to improve outcomes and reduce pain in pancreatic adenocarcinoma failed due to poor taste sensation and gastrointestinal disturbances, resulting in bad compliance (147). However, a recent clinical study successfully examined the effect of alkalinization therapy (an alkaline diet supplemented with oral sodium bicarbonate) in combination with chemotherapy on the survival of patients with advanced pancreatic cancer (UMIN 000035659). The median overall survival rate in patients whose urine pH became high (>7.0) after the start of therapy was significantly greater than in patients with low urine pH (≤7.0) (16.1 vs 4.7 months; p<0.05) (14). In another study (UMIN000043056), the combination of alkalinization therapy with intravenous vitamin C was also associated with favorable outcomes in patients with small cell lung cancer (SCLC) receiving chemotherapy. The median overall survival for the intervention group was 44.2 months vs. 17.7 months for the control group (15).
Parenteral administration of buffer systems to directly neutralize tumor acidity is also of great importance, but it must be done under the close supervision of medical personnel and can have some serious side effects (148). The use of nanoobjects to deliver buffers due to the enhanced permeability and retention effect (EPR) can overcome such limitations (149). For example, the administration of sodium bicarbonate-loaded liposomes in combination with subtherapeutic doses of doxorubicin in mice with triple-negative breast cancer resulted in a superior therapeutic response compared to drug administration alone (150). Performing an isolated infusion or perfusion of the tumor with buffer solutions is another option. In the ChiCTR-IOR-14005319 clinical study, the efficacies of transarterial chemoembolization (TACE) with or without local administration of 5% sodium bicarbonate solution in patients with large-focal hepatocellular carcinoma were compared. In the case of sodium bicarbonate, the objective response rate (ORR) was 100% vs. 44.4% in the case of conventional TACE in a nonrandomized cohort and 63.6% in a randomized study (151). In a preclinical study, it was found that intraperitoneal perfusion with 1% sodium bicarbonate solution significantly prolonged overall survival in mice with the ascitic form of Ehrlich’s adenocarcinoma (median survival, 24 vs. 17 days; p < 0.05) when compared to 0.9% sodium chloride solution (18). In another study, perfusion was performed with a 4% sodium bicarbonate solution of rat limbs with a Pliss lymphosarcoma graft. The median survival in the sodium bicarbonate group was 17 days, while in the nonperfused group and in the isotonic saline group it was 13 days (17).
The mechanisms of the anticancer effects of alkalization (buffering) therapy remain unclear. While the improved chemotherapeutic effect can be explained by “ion trapping” (53, 54), the antitumor, antimetastatic, and immunotherapy-enhancing effects of buffered therapy may be much more complex and have been studied predominantly as a phenomenon until now. Buffering of the TME can reduce the optimal conditions for enzymes involved in tumor invasion, such as cathepsins and matrix metalloproteases (MMPs) (152). Neutralization of acidity in the TME can result in a reduction of PD-L1 expression, which is increased at low pH through proton-sensing G protein-coupled receptors (81). Neutralization of lactic acid with sodium bicarbonate reactivates metabolically altered (in an acid environment) T cells, enabling extracellular lactate as an additional source for their energy production (153). More research is needed on the mechanisms of the effectiveness of sodium bicarbonate and other buffer solutions in cancer patients. Alkalization (buffering) therapy does not conflict with standard treatment methods but can be used in combination to increase effectiveness (154).
Conclusion
Despite extensive studies on the acid-base status of malignant tumors over the past decades, the mechanisms of tumor adaptation to acidity, induction of invasion and metastasis, and the mechanisms leading to evasion of immune surveillance are still poorly understood. Further research in this direction is needed, including the development of approaches and drugs that directly or indirectly increase the pH of the TME for use in conjunction with chemotherapy, radiation therapy, and immunotherapy. However, it is clear that clinical options already exist to counteract tumor acidosis in patients. Additionally, the selectivity of acidosis in tumors versus healthy tissues holds promise for pH-activated or pH-targeted drugs, which are safer than traditional chemotherapy and are applicable to more cancers than many targeted drugs. Regardless of the complexity of the clinical assessment of the TME acidity, clinicians should consider acidosis in practice, and the continued development of methods for clinical assessment of tumor pH should allow for accurate diagnosis and selection of personalized treatment regimens.
Author contributions
AlB and VM contributed to the conception of the mini-review. AlB and AnB wrote the first draft of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the Health Committee of Saint Petersburg state assignment for Saint Petersburg Clinical Research and Practical Center of Specialized Types of Medical Care (Oncological).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Corbet C, Feron O. Tumour acidosis: From the passenger to the driver’s seat. Nat Rev Cancer (2017) 17(10):577–93. doi: 10.1038/nrc.2017.77
2. Ward C, Meehan J, Gray ME, Murray AF, Argyle DJ, Kunkler IH, et al. The impact of tumour ph on cancer progression: Strategies for clinical intervention. Explor Targeted Anti-tumor Ther (2020) 1(2):71–100. doi: 10.37349/etat.2020.00005
3. Warburg O. On the origin of cancer cells. Science (1956) 123(3191):309–14. doi: 10.1126/science.123.3191.309
4. Vaupel P, Multhoff G. Revisiting the warburg effect: historical dogma versus current understanding. J Physiol (2021) 599(6):1745–57. doi: 10.1113/JP278810
5. Persi E, Duran-Frigola M, Damaghi M, Roush WR, Aloy P, Cleveland JL, et al. Systems analysis of intracellular ph vulnerabilities for cancer therapy. Nat Commun (2018) 9(1):2997. doi: 10.1038/s41467-018-05261-x
6. Zheng T, Jäättelä M, Liu B. Ph gradient reversal fuels cancer progression. Int J Biochem Cell Biol (2020) 125:105796. doi: 10.1016/j.biocel.2020.105796
7. Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, et al. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol (2017) 43:74–89. doi: 10.1016/j.semcancer.2017.03.001
8. Omran Z, Scaife P, Stewart S, Rauch C. Physical and biological characteristics of multi drug resistance (mdr): An integral approach considering ph and drug resistance in cancer. Semin Cancer Biol (2017) 43:42–8. doi: 10.1016/j.semcancer.2017.01.002
9. Harguindey S, Stanciu D, Devesa J, Alfarouk K, Cardone RA, Polo Orozco JD, et al. Cellular acidification as a new approach to cancer treatment and to the understanding and therapeutics of neurodegenerative diseases. Semin Cancer Biol (2017) 43:157–79. doi: 10.1016/j.semcancer.2017.02.003
10. Koltai T. Triple-edged therapy targeting intracellular alkalosis and extracellular acidosis in cancer. Semin Cancer Biol (2017) 43:139–46. doi: 10.1016/j.semcancer.2017.01.006
11. Du J-Z, Li H-J, Wang J. Tumor-acidity-cleavable maleic acid amide (tacmaa): A powerful tool for designing smart nanoparticles to overcome delivery barriers in cancer nanomedicine. Accounts Chem Res (2018) 51(11):2848–56. doi: 10.1021/acs.accounts.8b00195
12. Gong Z, Liu X, Zhou B, Wang G, Guan X, Xu Y, et al. Tumor acidic microenvironment-induced drug release of rgd peptide nanoparticles for cellular uptake and cancer therapy. Colloids Surf B Biointerfaces (2021) 202:111673. doi: 10.1016/j.colsurfb.2021.111673
13. Peng S, Xiao F, Chen M, Gao H. Tumor-microenvironment-responsive nanomedicine for enhanced cancer immunotherapy. Adv Sci (2022) 9(1):2103836. doi: 10.1002/advs.202103836
14. Hamaguchi REO, Narui R, Wada H. Effects of alkalization therapy on chemotherapy outcomes in metastatic or recurrent pancreatic cancer. Anticancer Res (2020) 40(2):873. doi: 10.21873/anticanres.14020
15. Hamaguchi R, Narui R, Morikawa H, Wada H. Improved chemotherapy outcomes of patients with small-cell lung cancer treated with combined alkalization therapy and intravenous vitamin C. Cancer Diagn Progn (2021) 1(3):157–63. doi: 10.21873/cdp.10021
16. Ando H, Emam SE, Kawaguchi Y, Shimizu T, Ishima Y, Eshima K, et al. Increasing tumor extracellular ph by an oral alkalinizing agent improves antitumor responses of anti-pd-1 antibody: Implication of relationships between serum bicarbonate concentrations, urinary ph, and therapeutic outcomes. Biol Pharm Bull (2021) 44(6):844–52. doi: 10.1248/bpb.b21-00076
17. Bogdanov AA, Egorenkov VV, Volkov NM, Moiseenko FV, Molchanov MS, Verlov NA, et al. Antitumor efficacy of an isolated hind legperfusion with a ph-increased solution in thepliss’ lymphosarcoma graft rat model. Almanac Clin Med (2021) 49(8):541–9. doi: 10.18786/2072-0505-2021-49-070541
18. Bogdanov AA, Verlov NA, Knyazev NA, Klimenko VV, Bogdanov AA, Moiseyenko VM. 46p intraperitoneal perfusion with sodium bicarbonate solution can significantly increase the lifespan of mice with ehrlich ascites carcinoma. Ann Oncol (2021) 32:S374–S5. doi: 10.1016/j.annonc.2021.08.324
19. Gillies RJ, Brown JS, Anderson ARA, Gatenby RA. Eco-evolutionary causes and consequences of temporal changes in intratumoural blood flow. Nat Rev Cancer (2018) 18(9):576–85. doi: 10.1038/s41568-018-0030-7
20. McDonald PC, Chafe SC, Dedhar S. Overcoming hypoxia-mediated tumor progression: Combinatorial approaches targeting ph regulation, angiogenesis and immune dysfunction. Front Cell Dev Biol (2016) 4:27. doi: 10.3389/fcell.2016.00027
21. Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated ph: A perfect storm for cancer progression. Nat Rev Cancer (2011) 11(9):671–7. doi: 10.1038/nrc3110
22. Damiani C, Colombo R, Gaglio D, Mastroianni F, Pescini D, Westerhoff HV, et al. A metabolic core model elucidates how enhanced utilization of glucose and glutamine, with enhanced glutamine-dependent lactate production, promotes cancer cell growth: The warburq effect. PloS Comput Biol (2017) 13(9):e1005758. doi: 10.1371/journal.pcbi.1005758
23. DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U.S.A. (2007) 104(49):19345–50. doi: 10.1073/pnas.0709747104
24. Zhu L, Zhu X, Wu Y. Effects of glucose metabolism, lipid metabolism, and glutamine metabolism on tumor microenvironment and clinical implications. Biomolecules (2022) 12(4):580. doi: 10.3390/biom12040580
25. Pérez-Escuredo J, Dadhich RK, Dhup S, Cacace A, Van Hée VF, De Saedeleer CJ, et al. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle (2016) 15(1):72–83. doi: 10.1080/15384101.2015.1120930
26. Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med (2020) 52(9):1496–516. doi: 10.1038/s12276-020-00504-8
27. Lagadic-Gossmann D, Huc L, Lecureur V. Alterations of intracellular ph homeostasis in apoptosis: origins and roles. Cell Death Differ (2004) 11(9):953–61. doi: 10.1038/sj.cdd.4401466
28. Koltai T. Cancer: Fundamentals behind ph targeting and the double-edged approach. Onco Targets Ther (2016) 9:6343–60. doi: 10.2147/ott.S115438
29. Spugnini EP, Sonveaux P, Stock C, Perez-Sayans M, De Milito A, Avnet S, et al. Proton channels and exchangers in cancer. biochimica et biophysica acta (BBA). Biomembranes (2015) 1848(10, Part B):2715–26. doi: 10.1016/j.bbamem.2014.10.015
30. Man CH, Mercier FE, Liu N, Dong W, Stephanopoulos G, Jiang L, et al. Proton export alkalinizes intracellular ph and reprograms carbon metabolism to drive normal and malignant cell growth. Blood (2022) 139(4):502–22. doi: 10.1182/blood.2021011563
31. Damaghi M, Wojtkowiak J, Gillies R. Ph sensing and regulation in cancer. Front Physiol (2013) 4:370. doi: 10.3389/fphys.2013.00370
32. Becker HM, Deitmer JW. Transport metabolons and acid/base balance in tumor cells. Cancers (2020) 12(4):899. doi: 10.3390/cancers12040899
33. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science (2009) 324(5930):1029–33. doi: 10.1126/science.1160809
34. Pillai SR, Damaghi M, Marunaka Y, Spugnini EP, Fais S, Gillies RJ. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev (2019) 38(1-2):205–22. doi: 10.1007/s10555-019-09792-7
35. Sharma M, Astekar M, Soi S, Manjunatha BS, Shetty DC, Radhakrishnan R. Ph gradient reversal: An emerging hallmark of cancers. Recent Pat Anticancer Drug Discovery (2015) 10(3):244–58. doi: 10.2174/1574892810666150708110608
36. Blaszczak W, Swietach P. What do cellular responses to acidity tell us about cancer? Cancer Metastasis Rev (2021) 40(4):1159–76. doi: 10.1007/s10555-021-10005-3
37. Andreucci E, Peppicelli S, Ruzzolini J, Bianchini F, Biagioni A, Papucci L, et al. The acidic tumor microenvironment drives a stem-like phenotype in melanoma cells. J Mol Med (2020) 98(10):1431–46. doi: 10.1007/s00109-020-01959-y
38. Kazyken D, Lentz SI, Fingar DC. Alkaline intracellular ph (phi) activates ampk–mtorc2 signaling to promote cell survival during growth factor limitation. J Biol Chem (2021) 297(4):101100. doi: 10.1016/j.jbc.2021.101100
39. Yao J, Czaplinska D, Ialchina R, Schnipper J, Liu B, Sandelin A, et al. Cancer cell acid adaptation gene expression response is correlated to tumor-specific tissue expression profiles and patient survival. Cancers (2020) 12(8):2183. doi: 10.3390/cancers12082183
40. Wang JX, Choi SYC, Niu X, Kang N, Xue H, Killam J, et al. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci (2020) 21(21):8363. doi: 10.3390/ijms21218363
41. Peppicelli S, Andreucci E, Ruzzolini J, Laurenzana A, Margheri F, Fibbi G, et al. The acidic microenvironment as a possible niche of dormant tumor cells. Cell Mol Life Sci (2017) 74(15):2761–71. doi: 10.1007/s00018-017-2496-y
42. Anemone A, Consolino L, Arena F, Capozza M, Longo DL. Imaging tumor acidosis: A survey of the available techniques for mapping in vivo tumor ph. Cancer Metastasis Rev (2019) 38(1-2):25–49. doi: 10.1007/s10555-019-09782-9
43. Coman D, Peters DC, Walsh JJ, Savic LJ, Huber S, Sinusas AJ, et al. Extracellular ph mapping of liver cancer on a clinical 3t mri scanner. Magn Reson Med (2020) 83(5):1553–64. doi: 10.1002/mrm.28035
44. Anemone A, Consolino L, Conti L, Irrera P, Hsu MY, Villano D, et al. Tumour acidosis evaluated in vivo by mri-cest ph imaging reveals breast cancer metastatic potential. Br J Cancer (2021) 124(1):207–16. doi: 10.1038/s41416-020-01173-0
45. Jones KM, Randtke EA, Yoshimaru ES, Howison CM, Chalasani P, Klein RR, et al. Clinical translation of tumor acidosis measurements with acidocest mri. Mol Imaging Biol (2017) 19(4):617–25. doi: 10.1007/s11307-016-1029-7
46. Tang Y, Xiao G, Shen Z, Zhuang C, Xie Y, Zhang X, et al. Noninvasive detection of extracellular ph in human benign and malignant liver tumors using cest mri. Front Oncol (2020) 10:578985. doi: 10.3389/fonc.2020.578985
47. Al-Kindi SG, Sarode A, Zullo M, Rajagopalan S, Rahman M, Hostetter T, et al. Serum bicarbonate concentration and cause-specific mortality: The national health and nutrition examination survey 1999-2010. Mayo Clin Proc (2020) 95(1):113–23. doi: 10.1016/j.mayocp.2019.05.036
48. de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol (2019) 9:1143. doi: 10.3389/fonc.2019.01143
49. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer (2004) 4(6):437–47. doi: 10.1038/nrc1367
50. Suwa T, Kobayashi M, Nam J-M, Harada H. Tumor microenvironment and radioresistance. Exp Mol Med (2021) 53(6):1029–35. doi: 10.1038/s12276-021-00640-9
51. Bristow RG, Hill RP. Hypoxia and metabolism. hypoxia, dna repair and genetic instability. Nat Rev Cancer (2008) 8(3):180–92. doi: 10.1038/nrc2344
52. Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. i. acid ph affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol (2003) 66(7):1207–18. doi: 10.1016/s0006-2952(03)00467-2
53. Gerweck LE, Vijayappa S, Kozin S. Tumor ph controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther (2006) 5(5):1275–9. doi: 10.1158/1535-7163.Mct-06-0024
54. Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer inst (2007) 99(19):1441–54. doi: 10.1093/jnci/djm135
55. Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. ii. ph-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol (2003) 66(7):1219–29. doi: 10.1016/s0006-2952(03)00468-4
56. Vukovic V, Tannock IF. Influence of low ph on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J Cancer (1997) 75(8):1167–72. doi: 10.1038/bjc.1997.201
57. Sauvant C, Nowak M, Wirth C, Schneider B, Riemann A, Gekle M, et al. Acidosis induces multi-drug resistance in rat prostate cancer cells (at1) in vitro and in vivo by increasing the activity of the p-glycoprotein via activation of p38. Int J Cancer (2008) 123(11):2532–42. doi: 10.1002/ijc.23818
58. Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of p-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia (2006) 8(2):143–52. doi: 10.1593/neo.05697
59. Thews O, Nowak M, Sauvant C, Gekle M. Hypoxia-induced extracellular acidosis increases p-glycoprotein activity and chemoresistance in tumorsin vivo via p38 signaling pathway. Adv Exp Med Biol (2011) 701:115–22. doi: 10.1007/978-1-4419-7756-4_16
60. Hu P-S, Li T, Lin J-F, Qiu M-Z, Wang D-S, Liu Z-X, et al. Vdr-sox2 signaling promotes colorectal cancer stemness and malignancy in an acidic microenvironment. Signal Transduct Target Ther (2020) 5(1):183–. doi: 10.1038/s41392-020-00230-7
61. Barnes EME, Xu Y, Benito A, Herendi L, Siskos AP, Aboagye EO, et al. Lactic acidosis induces resistance to the pan-akt inhibitor uprosertib in colon cancer cells. Br J Cancer (2020) 122(9):1298–308. doi: 10.1038/s41416-020-0777-y
62. Worsley CM, Veale RB, Mayne ES. The acidic tumour microenvironment: Manipulating the immune response to elicit escape. Hum Immunol (2022) 83(5):399–408. doi: 10.1016/j.humimm.2022.01.014
63. Huntington KE, Louie A, Zhou L, Seyhan AA, Maxwell AW, El-Deiry WS. Colorectal cancer extracellular acidosis decreases immune cell killing and is partially ameliorated by ph-modulating agents that modify tumor cell cytokine profiles. Am J Cancer Res (2022) 12(1):138–51
64. Bosticardo M, Ariotti S, Losana G, Bernabei P, Forni G, Novelli F. Biased activation of human t lymphocytes due to low extracellular ph is antagonized by b7/cd28 costimulation. Eur J Immunol (2001) 31(9):2829–38. doi: 10.1002/1521-4141(200109)31:9<2829::AID-IMMU2829>3.0.CO;2-U
65. Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating t lymphocytes. Cancer Res (2012) 72(11):2746–56. doi: 10.1158/0008-5472.Can-11-1272
66. Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human t cells. Blood (2007) 109(9):3812–9. doi: 10.1182/blood-2006-07-035972
67. Nakagawa Y, Negishi Y, Shimizu M, Takahashi M, Ichikawa M, Takahashi H. Effects of extracellular ph and hypoxia on the function and development of antigen-specific cytotoxic t lymphocytes. Immunol Lett (2015) 167(2):72–86. doi: 10.1016/j.imlet.2015.07.003
68. Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses ctl function by inhibition of p38 and jnk/c-jun activation. Int J Cancer (2012) 131(3):633–40. doi: 10.1002/ijc.26410
69. Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res (2016) 76(6):1381–90. doi: 10.1158/0008-5472.CAN-15-1743
70. Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D’Acquisto F, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of t cell migration and effector functions. PloS Biol (2015) 13(7):e1002202. doi: 10.1371/journal.pbio.1002202
71. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. Ldha-associated lactic acid production blunts tumor immunosurveillance by t and nk cells. Cell Metab (2016) 24(5):657–71. doi: 10.1016/j.cmet.2016.08.011
72. Wu H, Estrella V, Beatty M, Abrahams D, El-Kenawi A, Russell S, et al. T-Cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat Commun (2020) 11(1):4113. doi: 10.1038/s41467-020-17756-7
73. Johnston RJ, Su LJ, Pinckney J, Critton D, Boyer E, Krishnakumar A, et al. Vista is an acidic ph-selective ligand for psgl-1. Nature (2019) 574(7779):565–70. doi: 10.1038/s41586-019-1674-5
74. Vermeulen M, Giordano M, Trevani AS, Sedlik C, Gamberale R, Fernández-Calotti P, et al. Acidosis improves uptake of antigens and mhc class i-restricted presentation by dendritic cells. J Immunol (2004) 172(5):3196. doi: 10.4049/jimmunol.172.5.3196
75. Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood (2006) 107(5):2013–21. doi: 10.1182/blood-2005-05-1795
76. Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavölgyi E, Catrina AI, et al. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol (2013) 191(6):3090–9. doi: 10.4049/jimmunol.1300772
77. Loeffler DA, Juneau PL, Heppner GH. Natural killer-cell activity under conditions reflective of tumor micro-environment. Int J Cancer (1991) 48(6):895–9. doi: 10.1002/ijc.2910480617
78. Pötzl J, Roser D, Bankel L, Hömberg N, Geishauser A, Brenner CD, et al. Reversal of tumor acidosis by systemic buffering reactivates nk cells to express ifn-γ and induces nk cell-dependent lymphoma control without other immunotherapies. Int J Cancer (2017) 140(9):2125–33. doi: 10.1002/ijc.30646
79. Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory t cells by lactic acid. Nature (2021) 591(7851):645–51. doi: 10.1038/s41586-020-03045-2
80. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Y-t L, Togashi Y, et al. Lactic acid promotes pd-1 expression in regulatory t cells in highly glycolytic tumor microenvironments. Cancer Cell (2022) 40(2):201–18.e9. doi: 10.1016/j.ccell.2022.01.001
81. Mori D, Tsujikawa T, Sugiyama Y, Kotani SI, Fuse S, Ohmura G, et al. Extracellular acidity in tumor tissue upregulates programmed cell death protein 1 expression on tumor cells via proton-sensing g protein-coupled receptors. Int J Cancer (2021) 149(12):2116–24. doi: 10.1002/ijc.33786
82. Wang W, Singh S, Zeng DL, King K, Nema S. Antibody structure, instability, and formulation. J Pharm Sci (2007) 96(1):1–26. doi: 10.1002/jps.20727
83. Latypov RF, Hogan S, Lau H, Gadgil H, Liu D. Elucidation of acid-induced unfolding and aggregation of human immunoglobulin igg1 and igg2 fc. J Biol Chem (2012) 287(2):1381–96. doi: 10.1074/jbc.M111.297697
84. Ishikawa T, Ito T, Endo R, Nakagawa K, Sawa E, Wakamatsu K. Influence of ph on heat-induced aggregation and degradation of therapeutic monoclonal antibodies. Biol Pharm Bull (2010) 33(8):1413–7. doi: 10.1248/bpb.33.1413
85. Wang T, Kumru OS, Yi L, Wang YJ, Zhang J, Kim JH, et al. Effect of ionic strength and ph on the physical and chemical stability of a monoclonal antibody antigen-binding fragment. J Pharm Sci (2013) 102(8):2520–37. doi: 10.1002/jps.23645
86. Watanabe H, Matsumaru H, Ooishi A, Feng Y, Odahara T, Suto K, et al. Optimizing ph response of affinity between protein g and igg fc: how electrostatic modulations affect protein-protein interactions. J Biol Chem (2009) 284(18):12373–83. doi: 10.1074/jbc.M809236200
87. Klaus T, Deshmukh S. Ph-responsive antibodies for therapeutic applications. J BioMed Sci (2021) 28(1):11. doi: 10.1186/s12929-021-00709-7
88. Bogen JP, Hinz SC, Grzeschik J, Ebenig A, Krah S, Zielonka S, et al. Dual function ph responsive bispecific antibodies for tumor targeting and antigen depletion in plasma. Front Immunol (2019) 10:1892. doi: 10.3389/fimmu.2019.01892
89. Lee PS, MacDonald KG, Massi E, Chew PV, Bee C, Perkins P, et al. Improved therapeutic index of an acidic ph-selective antibody. MAbs (2022) 14(1):2024642–. doi: 10.1080/19420862.2021.2024642
90. Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, et al. Immune-checkpoint proteins vista and pd-1 nonredundantly regulate murine t-cell responses. Proc Natl Acad Sci U.S.A. (2015) 112(21):6682–7. doi: 10.1073/pnas.1420370112
91. Chu S, Shi X, Tian Y, Gao F. Ph-responsive polymer nanomaterials for tumor therapy. Front Oncol (2022) 12:855019. doi: 10.3389/fonc.2022.855019
92. Li Z, Huang J, Wu J. Ph-sensitive nanogels for drug delivery in cancer therapy. Biomater Sci (2021) 9(3):574–89. doi: 10.1039/D0BM01729A
93. Morarasu S, Morarasu BC, Ghiarasim R, Coroaba A, Tiron C, Iliescu R, et al. Targeted cancer therapy Via ph-functionalized nanoparticles: A scoping review of methods and outcomes. Gels (2022) 8(4):232. doi: 10.3390/gels8040232
94. Villarreal-Garza C, Shaw-Dulin R, Lara-Medina F, Bacon L, Rivera D, Urzua L, et al. Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Exp Diabetes Res (2012) 2012:732027. doi: 10.1155/2012/732027
95. Iarrobino NA, Gill BS, Bernard M, Klement RJ, Werner-Wasik M, Champ CE. The impact of serum glucose, anti-diabetic agents, and statin usage in non-small cell lung cancer patients treated with definitive chemoradiation. Front Oncol (2018) 8:281. doi: 10.3389/fonc.2018.00281
96. Li W, Zhang X, Sang H, Zhou Y, Shang C, Wang Y, et al. Effects of hyperglycemia on the progression of tumor diseases. J Exp Clin Cancer Res (2019) 38(1):327. doi: 10.1186/s13046-019-1309-6
97. Ramteke P, Deb A, Shepal V, Bhat MK. Hyperglycemia associated metabolic and molecular alterations in cancer risk, progression, treatment, and mortality. Cancers (2019) 11(9):1402. doi: 10.3390/cancers11091402
98. Zhang M, Hu X, Kang Y, Xu W, Yang X. Association between fasting blood glucose levels at admission and overall survival of patients with pancreatic cancer. BMC Cancer (2021) 21(1):131. doi: 10.1186/s12885-021-07859-9
99. Wang X, Xu W, Hu X, Yang X, Zhang M. The prognostic role of glycemia in patients with pancreatic carcinoma: A systematic review and meta-analysis. Front Oncol (2022) 12:780909. doi: 10.3389/fonc.2022.780909
100. Zhang P, Xiao Z, Xu H, Zhu X, Wang L, Huang D, et al. Hyperglycemia is associated with adverse prognosis in patients with pancreatic neuroendocrine neoplasms. Endocrine (2022) 77(2):262–71. doi: 10.1007/s12020-022-03100-0
101. Cui G, Zhang T, Ren F, Feng WM, Yao Y, Cui J, et al. High blood glucose levels correlate with tumor malignancy in colorectal cancer patients. Med Sci Monit (2015) 21:3825–33. doi: 10.12659/msm.894783
102. Lee SJ, Kim JH, Park SJ, Ock SY, Kwon SK, Choi YS, et al. Optimal glycemic target level for colon cancer patients with diabetes. Diabetes Res Clin Pract (2017) 124:66–71. doi: 10.1016/j.diabres.2016.12.009
103. Yang IP, Miao ZF, Huang CW, Tsai HL, Yeh YS, Su WC, et al. High blood sugar levels but not diabetes mellitus significantly enhance oxaliplatin chemoresistance in patients with stage iii colorectal cancer receiving adjuvant folfox6 chemotherapy. Ther Adv Med Oncol (2019) 11:1758835919866964. doi: 10.1177/1758835919866964
104. Tahtouh R, Wardi L, Sarkis R, Hachem R, Raad I, El Zein N, et al. Glucose restriction reverses the warburg effect and modulates pkm2 and mtor expression in breast cancer cell lines. Cell Mol Biol (Noisy-le-grand) (2019) 65(7):26–33. doi: 10.14715/cmb/2019.65.7.6
105. Khan T, Sullivan MA, Gunter JH, Kryza T, Lyons N, He Y, et al. Revisiting glycogen in cancer: A conspicuous and targetable enabler of malignant transformation. Front Oncol (2020) 10:592455. doi: 10.3389/fonc.2020.592455
106. Grasmann G, Smolle E, Olschewski H, Leithner K. Gluconeogenesis in cancer cells - repurposing of a starvation-induced metabolic pathway? Biochim Biophys Acta Rev Cancer (2019) 1872(1):24–36. doi: 10.1016/j.bbcan.2019.05.006
107. Bose S, Zhang C, Le A. Glucose metabolism in cancer: The warburg effect and beyond. In: Le A, editor. The heterogeneity of cancer metabolism. Cham: Springer International Publishing (2021). p. 3–15.
108. Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: Insights into metabolism and lymphocyte function. Science (2013) 342(6155):1242454. doi: 10.1126/science.1242454
109. Cao Y, Rathmell JC, Macintyre AN. Metabolic reprogramming towards aerobic glycolysis correlates with greater proliferative ability and resistance to metabolic inhibition in cd8 versus cd4 t cells. PloS One (2014) 9(8):e104104. doi: 10.1371/journal.pone.0104104
110. Cong J, Wang X, Zheng X, Wang D, Fu B, Sun R, et al. Dysfunction of natural killer cells by fbp1-induced inhibition of glycolysis during lung cancer progression. Cell Metab (2018) 28(2):243–55.e5. doi: 10.1016/j.cmet.2018.06.021
111. Drenick EJ, Alvarez LC, Tamasi GC, Brickman AS. Resistance to symptomatic insulin reactions after fasting. J Clin Invest (1972) 51(10):2757–62. doi: 10.1172/JCI107095
112. Kolb H, Kempf K, Röhling M, Lenzen-Schulte M, Schloot NC, Martin S. Ketone bodies: From enemy to friend and guardian angel. BMC Med (2021) 19(1):313. doi: 10.1186/s12916-021-02185-0
113. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab (2017) 25(2):262–84. doi: 10.1016/j.cmet.2016.12.022
114. Hirschberger S, Gellert L, Effinger D, Muenchhoff M, Herrmann M, Briegel J-M, et al. Ketone bodies improve human cd8+ cytotoxic t-cell immune response during covid-19 infection. Front Med (2022) 9:923502. doi: 10.3389/fmed.2022.923502
115. Hirschberger S, Strauß G, Effinger D, Marstaller X, Ferstl A, Müller MB, et al. Very-low-carbohydrate diet enhances human t-cell immunity through immunometabolic reprogramming. EMBO Mol Med (2021) 13(8):e14323. doi: 10.15252/emmm.202114323
116. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer - where do we stand? Mol Metab (2020) 33:102–21. doi: 10.1016/j.molmet.2019.06.026
117. Ferrere G, Tidjani Alou M, Liu P, Goubet A-G, Fidelle M, Kepp O, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of pd-1 blockade. JCI Insight (2021) 6(2):e145207. doi: 10.1172/jci.insight.145207
118. Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Ketone body utilization drives tumor growth and metastasis. Cell Cycle (2012) 11(21):3964–71. doi: 10.4161/cc.22137
119. Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Lisanti MP, Sotgia F. Ketone bodies and two-compartment tumor metabolism: Stromal ketone production fuels mitochondrial biogenesis in epithelial cancer cells. Cell Cycle (2012) 11(21):3956–63. doi: 10.4161/cc.22136
120. Gouirand V, Gicquel T, Lien EC, Jaune-Pons E, Da Costa Q, Finetti P, et al. Ketogenic hmg-coa lyase and its product β-hydroxybutyrate promote pancreatic cancer progression. EMBO J (2022) 41(9):e110466. doi: 10.15252/embj.2021110466
121. Sperry J, Condro MC, Guo L, Braas D, Vanderveer-Harris N, Kim KKO, et al. Glioblastoma utilizes fatty acids and ketone bodies for growth allowing progression during ketogenic diet therapy. iScience (2020) 23(9):101453. doi: 10.1016/j.isci.2020.101453
122. Oshima N, Ishida R, Kishimoto S, Beebe K, Brender JR, Yamamoto K, et al. Dynamic imaging of ldh inhibition in tumors reveals rapid in vivo metabolic rewiring and vulnerability to combination therapy. Cell Rep (2020) 30(6):1798–810.e4. doi: 10.1016/j.celrep.2020.01.039
123. Yeung C, Gibson AE, Issaq SH, Oshima N, Baumgart JT, Edessa LD, et al. Targeting glycolysis through inhibition of lactate dehydrogenase impairs tumor growth in preclinical models of ewing sarcoma. Cancer Res (2019) 79(19):5060–73. doi: 10.1158/0008-5472.CAN-19-0217
124. Pérez-Tomás R, Pérez-Guillén I. Lactate in the tumor microenvironment: An essential molecule in cancer progression and treatment. Cancers (Basel) (2020) 12(11):3244. doi: 10.3390/cancers12113244
125. Wang Z-H, Peng W-B, Zhang P, Yang X-P, Zhou Q. Lactate in the tumour microenvironment: From immune modulation to therapy. eBioMedicine (2021) 73:103627. doi: 10.1016/j.ebiom.2021.103627
126. Zhang Z, Li Y, Yan X, Song Q, Wang G, Hu Y, et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med (2019) 8(4):1467–73. doi: 10.1002/cam4.2024
127. Zhao L, Liu Y, Zhang S, Wei L, Cheng H, Wang J, et al. Impacts and mechanisms of metabolic reprogramming of tumor microenvironment for immunotherapy in gastric cancer. Cell Death Dis (2022) 13(4):378. doi: 10.1038/s41419-022-04821-w
128. Qiao T, Xiong Y, Feng Y, Guo W, Zhou Y, Zhao J, et al. Inhibition of ldh-a by oxamate enhances the efficacy of anti-Pd-1 treatment in an nsclc humanized mouse model. Front Oncol (2021) 11:632364. doi: 10.3389/fonc.2021.632364
129. Corbet C, Bastien E, Draoui N, Doix B, Mignion L, Jordan BF, et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat Commun (2018) 9(1):1208. doi: 10.1038/s41467-018-03525-0
130. Silva A, Antunes B, Batista A, Pinto-Ribeiro F, Baltazar F, Afonso J. In vivo anticancer activity of azd3965: A systematic review. Molecules (2021) 27(1):181. doi: 10.3390/molecules27010181
131. Guan X, Morris ME. In vitro and in vivo efficacy of azd3965 and alpha-cyano-4-hydroxycinnamic acid in the murine 4t1 breast tumor model. AAPS J (2020) 22(4):84. doi: 10.1208/s12248-020-00466-9
132. Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab (2020) 33:48–66. doi: 10.1016/j.molmet.2019.07.006
133. Todenhöfer T, Seiler R, Stewart C, Moskalev I, Gao J, Ladhar S, et al. Selective inhibition of the lactate transporter mct4 reduces growth of invasive bladder cancer. Mol Cancer Ther (2018) 17(12):2746–55. doi: 10.1158/1535-7163.Mct-18-0107
134. Puri S, Juvale K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: A review with structure-activity relationship insights. Eur J Medicinal Chem (2020) 199:112393. doi: 10.1016/j.ejmech.2020.112393
135. Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, et al. Dual inhibition of the lactate transporters mct1 and mct4 is synthetic lethal with metformin due to nad+ depletion in cancer cells. Cell Rep (2018) 25(11):3047–58.e4. doi: 10.1016/j.celrep.2018.11.043
136. Eaton AF, Merkulova M, Brown D. The h(+)-atpase (v-atpase): From proton pump to signaling complex in health and disease. Am J Physiol Cell Physiol (2021) 320(3):C392–c414. doi: 10.1152/ajpcell.00442.2020
137. Hu Y, Lou J, Jin Z, Yang X, Shan W, Du Q, et al. Advances in research on the regulatory mechanism of nhe1 in tumors (review). Oncol Lett (2021) 21(4):273. doi: 10.3892/ol.2021.12534
138. McDonald PC, Chafe SC, Supuran CT, Dedhar S. Cancer therapeutic targeting of hypoxia induced carbonic anhydrase ix: From bench to bedside. Cancers (2022) 14(14):3297. doi: 10.3390/cancers14143297
139. Wang BY, Zhang J, Wang JL, Sun S, Wang ZH, Wang LP, et al. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res (2015) 34(1):85. doi: 10.1186/s13046-015-0194-x
140. Zhang J-L, Liu M, Yang Q, Lin S-Y, Shan H-B, Wang H-Y, et al. Effects of omeprazole in improving concurrent chemoradiotherapy efficacy in rectal cancer. World J Gastroenterol (2017) 23(14):2575–84. doi: 10.3748/wjg.v23.i14.2575
141. Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, et al. Bicarbonate increases tumor ph and inhibits spontaneous metastases. Cancer Res (2009) 69(6):2260–8. doi: 10.1158/0008-5472.CAN-07-5575
142. Ibrahim-Hashim A, Wojtkowiak JW, de Lourdes Coelho Ribeiro M, Estrella V, Bailey KM, Cornnell HH, et al. Free base lysine increases survival and reduces metastasis in prostate cancer model. J Cancer Sci Ther (2011) Suppl 1(4):JCST-S1-004. doi: 10.4172/1948-5956.S1-004
143. Ibrahim-Hashim A, Robertson-Tessi M, Enriquez-Navas PM, Damaghi M, Balagurunathan Y, Wojtkowiak JW, et al. Defining cancer subpopulations by adaptive strategies rather than molecular properties provides novel insights into intratumoral evolution. Cancer Res (2017) 77(9):2242–54. doi: 10.1158/0008-5472.Can-16-2844
144. Ibrahim-Hashim A, Cornnell HH, Abrahams D, Lloyd M, Bui M, Gillies RJ, et al. Systemic buffers inhibit carcinogenesis in tramp mice. J Urol (2012) 188(2):624–31. doi: 10.1016/j.juro.2012.03.113
145. Ibrahim Hashim A, Cornnell HH, Coelho Ribeiro Mde L, Abrahams D, Cunningham J, Lloyd M, et al. Reduction of metastasis using a non-volatile buffer. Clin Exp Metastasis (2011) 28(8):841–9. doi: 10.1007/s10585-011-9415-7
146. Ibrahim-Hashim A, Abrahams D, Enriquez-Navas PM, Luddy K, Gatenby RA, Gillies RJ. Tris-base buffer: A promising new inhibitor for cancer progression and metastasis. Cancer Med (2017) 6(7):1720–9. doi: 10.1002/cam4.1032
147. Pilot C, Mahipal A, Gillies R. Buffer therapy–buffer diet. J Nutr Food Sci (2018) 8:1000685. doi: 10.4172/2155-9600.1000685
148. Sarno J. Prevention and management of tumor lysis syndrome in adults with malignancy. J Adv Pract Oncol (2013) 4(2):101–6. doi: 10.6004/jadpro.2013.4.2.4
149. Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun (2018) 9(1):1410. doi: 10.1038/s41467-018-03705-y
150. Abumanhal-Masarweh H, Koren L, Zinger A, Yaari Z, Krinsky N, Kaneti G, et al. Sodium bicarbonate nanoparticles modulate the tumor ph and enhance the cellular uptake of doxorubicin. J Control Release (2019) 296:1–13. doi: 10.1016/j.jconrel.2019.01.004
151. Chao M, Wu H, Jin K, Li B, Wu J, Zhang G, et al. A nonrandomized cohort and a randomized study of local control of large hepatocarcinoma by targeting intratumoral lactic acidosis. Elife (2016) 5:e15691. doi: 10.7554/eLife.15691
152. Robey IF, Nesbit LA. Investigating mechanisms of alkalinization for reducing primary breast tumor invasion. BioMed Res Int (2013) 2013:485196. doi: 10.1155/2013/485196
153. Uhl FM, Chen S, O’Sullivan D, Edwards-Hicks J, Richter G, Haring E, et al. Metabolic reprogramming of donor t cells enhances graft-versus-leukemia effects in mice and humans. Sci Trans Med (2020) 12(567):eabb8969. doi: 10.1126/scitranslmed.abb8969
Keywords: cancer, metabolism, acidity, hallmark, treatment target
Citation: Bogdanov A, Bogdanov A, Chubenko V, Volkov N, Moiseenko F and Moiseyenko V (2022) Tumor acidity: From hallmark of cancer to target of treatment. Front. Oncol. 12:979154. doi: 10.3389/fonc.2022.979154
Received: 27 June 2022; Accepted: 08 August 2022;
Published: 29 August 2022.
Edited by:
Reo Hamaguchi, Juntendo University, JapanReviewed by:
Thomas N. Seyfried, Boston College, United StatesCopyright © 2022 Bogdanov, Bogdanov, Chubenko, Volkov, Moiseenko and Moiseyenko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexey Bogdanov, QS5Cb2dkYW5vdkBvbmNvY2VudHJlLnJ1
 Alexey Bogdanov
Alexey Bogdanov Andrey Bogdanov
Andrey Bogdanov Viacheslav Chubenko
Viacheslav Chubenko Fedor Moiseenko
Fedor Moiseenko