- 1Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Cancer Research Institute, Seoul, South Korea
- 2Department of Radiology, Seoul National University College of Medicine, Seoul, South Korea
- 3Department of Pathology, Seoul National University College of Medicine, Seoul, South Korea
- 4Department of Orthopedic Surgery, Seoul National University College of Medicine, Seoul, South Korea
- 5Wide River Institute of Immunology, Hongcheon, South Korea
Background: A low-degree tumor necrosis after neoadjuvant chemotherapy is a poor prognostic factor for osteosarcoma (OSA). However, the role of high-dose chemotherapy (HDC) and autologous hematopoietic stem cell transplantation in OSA remains controversial. We analyzed the treatment outcomes and prognostic factors of nonmetastatic OSA and compared the HDC and conventional chemotherapy (CC) outcomes of patients with <90% necrosis after neoadjuvant chemotherapy.
Methods: We retrospectively evaluated patients with OSA treated at the Seoul National University Children’s Hospital from 2000 to 2020. Totally, 113 patients with non-metastatic OSA at diagnosis were included. The majority were treated with cisplatin, doxorubicin, and methotrexate as neoadjuvant chemotherapy. This was continued when the postoperative necrosis rate was >90% (good response [GR]), whereas most cases with <90% (poor response [PR]) were changed to chemotherapy. The HDC regimen was composed of melphalan, etoposide, and carboplatin.
Results: The median age at diagnosis was 12.6 years (range, 5.0–20.3), and 61.9% of patients were men. The 5-year event-free survival (EFS) and overall survival (OS) rates were 75.8% and 91.5%, respectively. Among these, 59 and 44 patients were included in the GR and PR groups, respectively. The GR group had a better 5-year EFS rate than the PR group (82.4% vs. 67.3%, p=0.071). Age at diagnosis, sex, tumor site, type of neoadjuvant chemotherapy, and degree of tumor necrosis were not different between the PR-HDC (n=24) and PR-CC (n=20) groups. The 5-year EFS and OS rates in the PR-HDC (n=24) and PR-CC (n=20) groups were 78.6% and 53.6% (p=0.065) and 100% and 76.9% (p=0.024), respectively. In the Cox regression analysis, the PR-CC group (hazard ratio, 4.95; p=0.004) and age ≥12 years (hazard ratio, 2.68; p=0.024) were significant risk factors for 5-year EFS.
Conclusions: HDC showed favorable outcomes in patients with non-metastatic OSA and <90% necrosis after neoadjuvant chemotherapy.
Introduction
Osteosarcoma (OSA) is a rare tumor that occurs mainly in adolescents and young adults, with 4.4 cases per million at the age of 0–24, accounting for approximately 5% of childhood and adolescent tumors (1, 2). In OSA, surgery without additional chemotherapy has been reported to recur in 90% of patients within 2 years, while systemic chemotherapy is very important in treatment, even if it is a local tumor (3, 4). The 5-year survival rate of OSA rose from approximately 40% to 76% in the 2010s in individuals under the age of 15. 5-year survival rate is 66% at the age of 15–19, which has not increased, particularly since the 1980s (1, 5). In Korea, the survival rate of patients diagnosed in 2007–2011 was 81.5%, although the survival rate was approximately 55.4% in the early 1990s, showing improved treatment outcomes (6).
Prognostic factors for OSA include tumor location, size, metastasis, possibility of surgical resection, and tumor necrosis after chemotherapy (7). It is well known that if the tumor necrosis rate is ≥90% after neoadjuvant chemotherapy, the prognosis is good (8, 9). There have been many studies on which postoperative treatment could improve the prognosis of patients if the tumor necrosis rate after neoadjuvant chemotherapy is <90%. In the EURAMOS-1 trial, patients with OSA who experience poor treatment response to preoperative chemotherapy were divided into a group that received postoperative high-dose methotrexate, cisplatin, and doxorubicin (MAP) and a group that received ifosfamide and etoposide plus MAP. However, the addition of ifosfamide and etoposide was associated with increased toxicity without improving the event-free survival (EFS) (10).
Although many studies have been conducted on high-dose chemotherapy (HDC) and autologous hematopoietic stem cell transplantation (ASCT) in OSA, there is still insufficient evidence to show a clear benefit compared to conventional chemotherapy (CC) (11–13). However, our institution has reported the results of HDC and ASCT using melphalan, etoposide, and carboplatin in high-risk OSA by determining indications based on three risk factors, including tumor necrosis <90% after neoadjuvant chemotherapy, metastasis, progression on therapy, or relapse, which showed promising outcomes (9, 14). Here, we retrospectively analyzed the treatment outcome of patients with local OSA without metastasis at the time of diagnosis and investigated the feasibility and safety of HDC and ASCT in patients with necrosis <90% after neoadjuvant chemotherapy.
Methods
Patients
We retrospectively reviewed the data of 113 patients diagnosed with non-metastatic OSA treated at Seoul National University Children’s Hospital from 2000 to 2020. Among them, seven patients who had undergone surgery and three patients who did not have information on the necrosis rate after neoadjuvant chemotherapy were excluded, and a total of 103 patients were included for further analysis (Figure 1). All the patients underwent a bone scan and chest computed tomography at the initial diagnosis, and no metastasis was found. The patient completed the planned neoadjuvant chemotherapy and underwent surgery, and there were no events for at least one month after the operation. Patients with a necrosis rate of ≥90% and <90% after neoadjuvant chemotherapy were defined as the good response (GR) and poor response (PR) groups. The overall patient classification and treatment scheme are shown in Figure 1. The Institutional Review Board approved the procedure for reviewing medical records, and obtaining consent was waived (H-1911-169-1082).
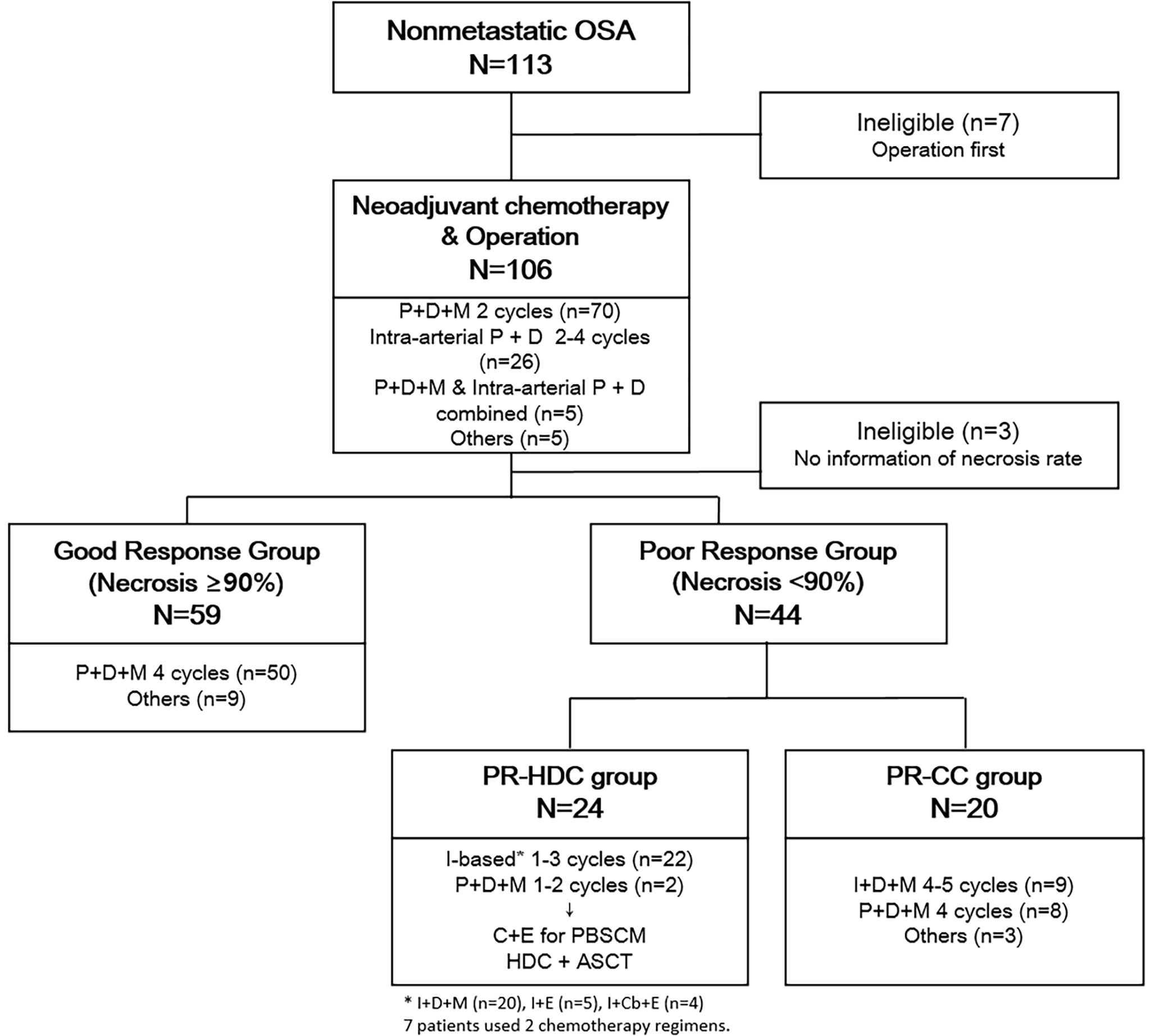
Figure 1 Overall treatment scheme and classification of patients. ASCT, autologous hematopoietic stem cell transplantation; C, cyclophosphamide; Cb, carboplatin; D, doxorubicin; E, etoposide; I, ifosfamide; P, cisplatin; PR-CC, poor response-chemotherapy only; PR-HDC, poor response-high dose chemotherapy.
Chemotherapy and treatment plan
Most patients (n=70) received intravenous cisplatin, doxorubicin, and high-dose methotrexate [Children’s Cancer Group-7921, regimen A (15)] as neoadjuvant chemotherapy, 26 received intra-arterial cisplatin plus intravenous doxorubicin, and 5 received combined intravenous and intra-arterial chemotherapy. Of the 103 patients who were able to confirm the postoperative necrosis rate, most of the GR group (n=50) were continuously administered regimen A. Among the PR group (n=44), the majority changed to ifosfamide-based chemotherapy [based on Children’s Cancer Group-7921, regimen B (15)]. From November 2007, HDC and ASCT were performed after peripheral blood stem cell (PBSC) mobilization if patients and guardians agreed. Otherwise, adjuvant CC was continued. An overview of the chemotherapy regimens is presented in Figure 1.
Autologous PBSCs were mobilized either with chemotherapy (cyclophosphamide plus etoposide) or plerixafor using granulocyte colony-stimulating factor (16). HDC consisted of melphalan 140 mg/m2 on day − 7 and 70 mg/m2 on day − 6, etoposide 200 mg/m2 and carboplatin 400 mg/m2 from days − 8 to − 5. Post-ASCT management was conducted according to institutional guidelines (14, 17).
Definition
Neutrophil engraftment and platelet engraftment days were calculated as the first three days with neutrophil count >0.5×109/L and platelet count >20×109/L, respectively, without transfusion for at least 7 days. The toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (v4.03).
Statistical analysis
Categorical variables were compared using the chi-square test, and continuous variables were compared using Student’s t-test or one-way analysis of variance. Events were defined as deaths or relapses. For survival analysis, overall survival (OS) was defined as the time from diagnosis to death from any cause, and EFS was defined as the time from diagnosis to first relapse or death. Patients who did not experience an event were censored at the last follow-up visit. Survival was analyzed using the Kaplan–Meier method. Differences in the survival rates were determined using the log-rank test. A Cox proportional hazard regression model was used for multivariate analysis of prognostic factors affecting survival; independent variables (p<0.2) were included in this model. Statistical significance was set at P < 0.05. Statistical analyses were conducted using the R version 3.2.2 (www.r-project.org) and SPSS 23.0 (IBM-SPSS, Armonk, NY, USA) softwares.
Results
Patient characteristics
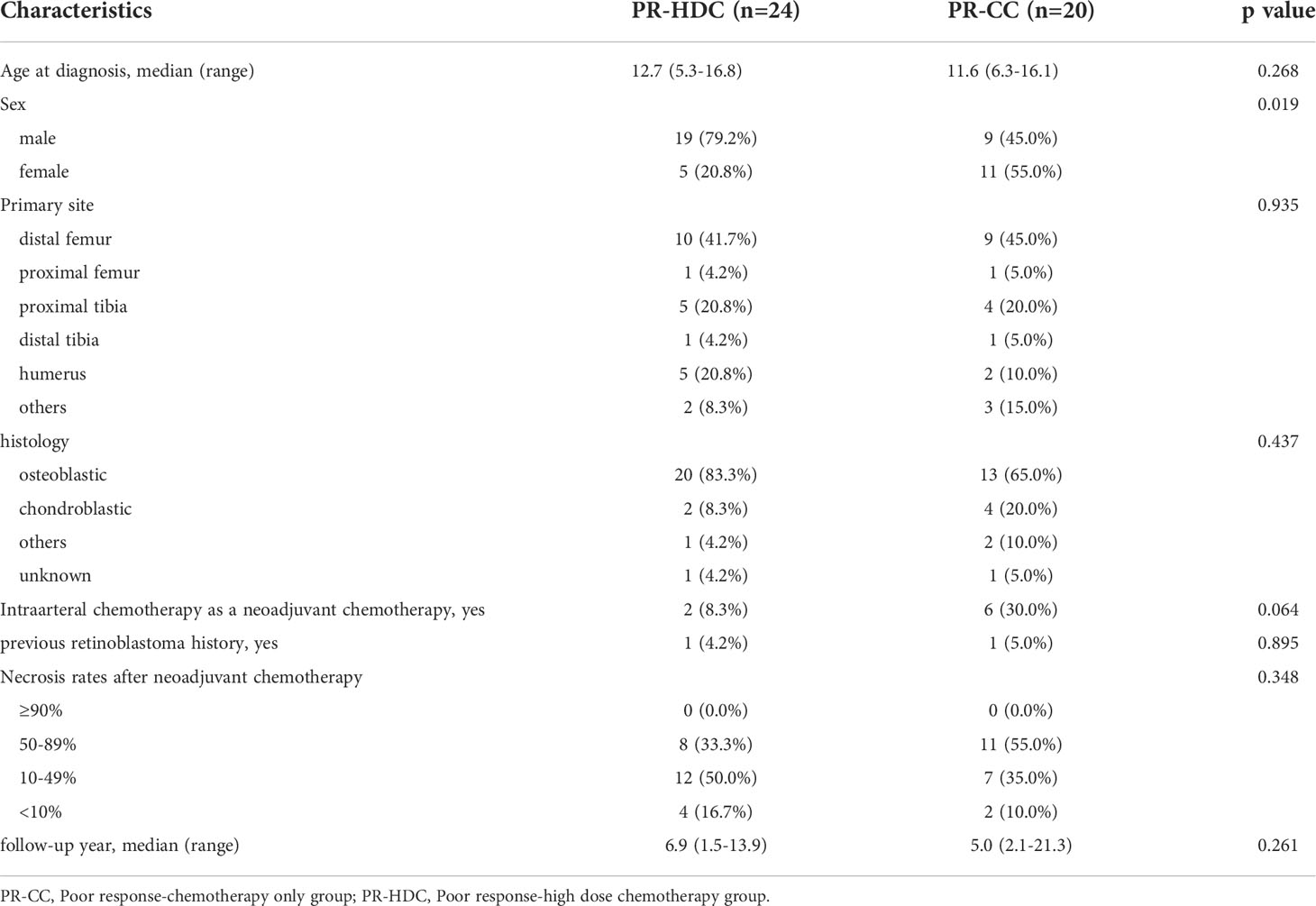
The clinical characteristics of the patients are summarized in Table 1. All patients were diagnosed with non-metastatic OSA, with a median age of 12.6 years (range, 5.0–20.3) and 61.9% were men. The median follow-up period was 7.7 years (range, 0.3–21.3 years). The most common tumor location was the distal femur (46.9%), followed by the proximal tibia (18.6%) and the humerus (12.4%). Thirty-one patients (27.4%) were treated at least once with neoadjuvant intra-arterial chemotherapy, while four patients had RB1 germline mutations and had previously been treated for retinoblastoma (Table 1).
Among them, seven patients underwent surgery first, and three had no information on necrosis rate. Therefore, the remaining 103 patients were included in further analysis based on the necrosis rate after neoadjuvant chemotherapy. Fifty-nine patients had a tumor necrosis rate of >90% after neoadjuvant chemotherapy, and they were classified into the GR group, while 44 patients were classified into the PR group. The median age at diagnosis, sex, tumor location, histology, and median follow-up time did not differ between the GR and PR groups. All patients, except for one, underwent wide excision or limb salvage surgery, and all margins were negative. One remaining patient had a tumor on T9, and only margin-positive tumor excision was performed. A comparison of the characteristics of the two groups is presented in Supplementary Table 1.
Outcome
The 5- and 10-year OS rates in all patients were 91.5% (95% confidence interval [CI], 85.8–97.2) and 89.4% (95% CI, 82.5–96.3), respectively. Moreover, the 5- and 10-year EFS rates in all patients were 75.8% (95% CI, 67.4–84.2) and 74.4% (95% CI, 65.6–83.2), respectively (Figure 2). Only one case of a late event occurred five years after diagnosis in all patients. When all patients were divided into the GR (n=59) and PR (n=44) groups according to the necrosis rate, the 5-year EFS rates of the GR and PR groups were 82.4% (95% CI, 72.4–92.4) versus 67.3% (95% CI, 53.2–81.4; P=0.071), respectively, and the 5-year OS rates were 92.9% (95% CI, 86.2–99.6) versus 89.3% (95% CI, 79.6–99.6; P=0.766), respectively. Among the four patients with a history of retinoblastoma (2 GR and 2 PR groups, respectively), two patients were event-free at the data cut-off date (follow-up time 5.3 and 5.4 years, respectively), and one patient died due to septic shock during chemotherapy. The remaining patient relapsed after 3 years and received a second surgery and salvage chemotherapy.
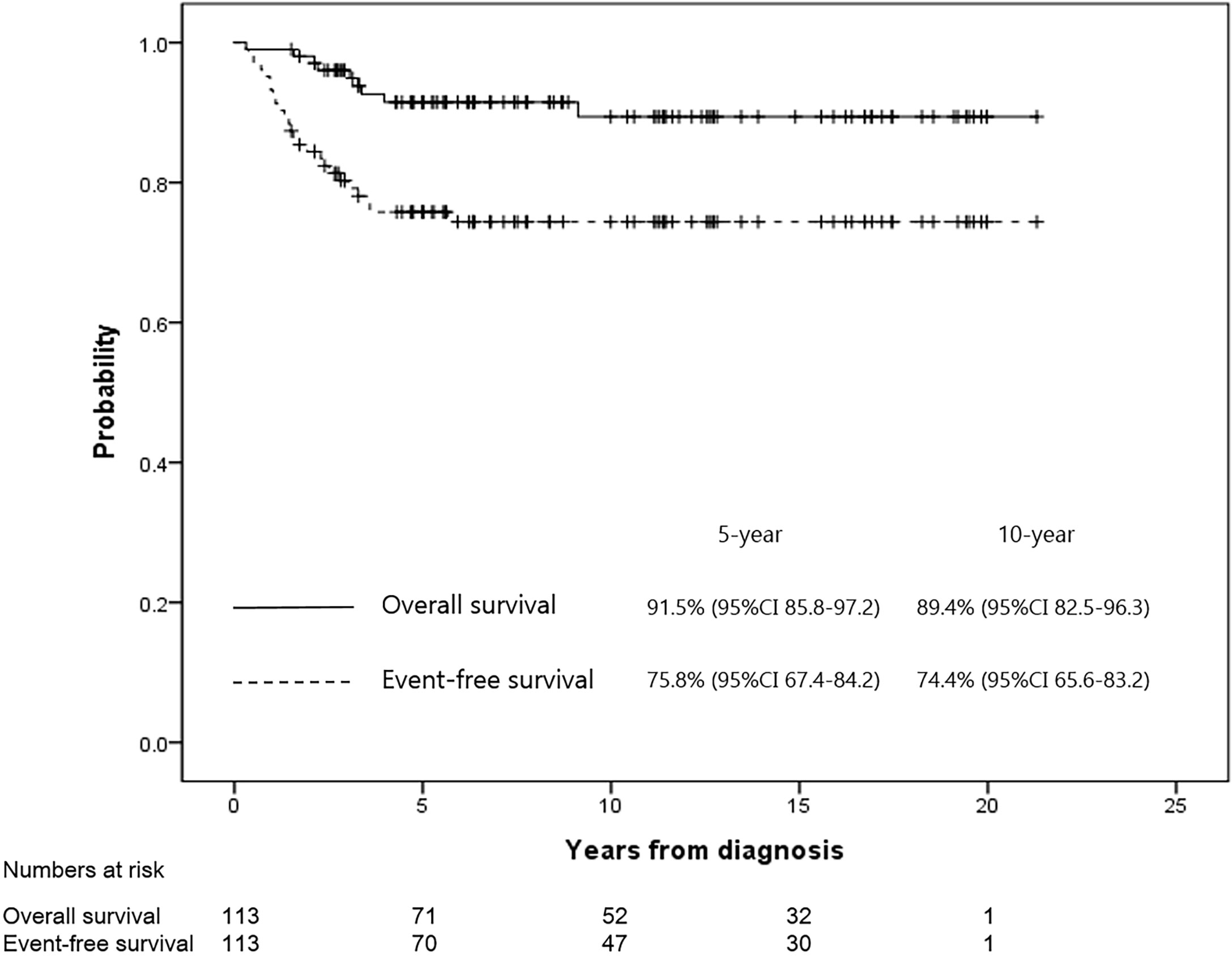
Figure 2 The EFS and OS rates for all patients were 75.8% and 91.5% at 5 years, and 74.4% and 89.4% at 10 years, respectively.
Sub-analysis of the PR group
In the PR group (n=44), 24 patients underwent HDC and ASCT (PR-HDC), and 20 patients received only adjuvant CC (PR-CC). A comparison of the groups is presented in Table 2. The median age at diagnosis, tumor location, histology, necrosis rate, and median follow-up time did not differ between the groups; however, more men were included in the PR-HDC group than women (P=0.019).
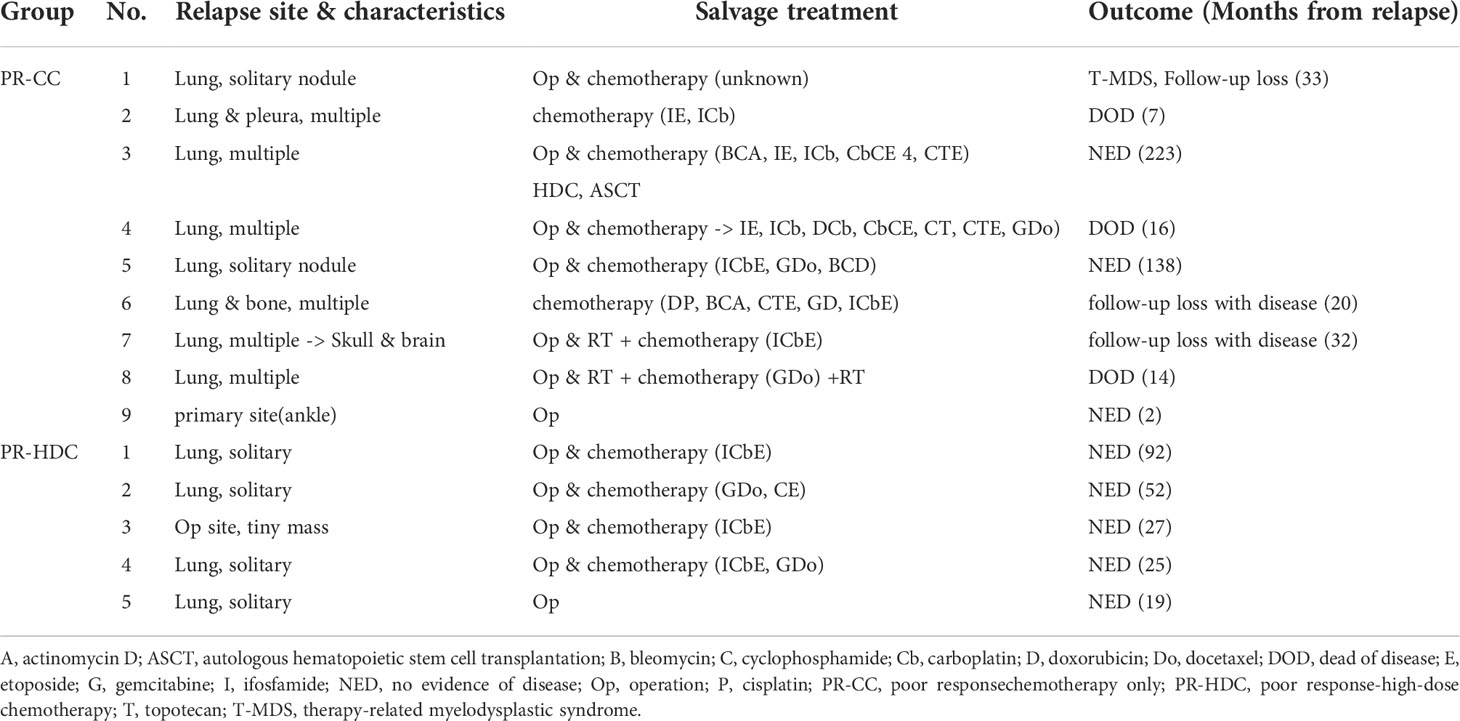
The 5-year EFS and OS rates of the PR-HDC and PR-CC groups were 78.6% (95% CI, 61.9–95.3) versus 53.6% (95% CI, 31.1–76.1; P=0.065) and 100% versus 76.9% (95% CI, 56.7–97.1; P=0.024), respectively (Figure 3A, B). In the PR group, there was no non-relapse mortality and all events were recurrences. Nine patients (45.0%) in the PR-CC group and five patients (20.8%) in the PR-HDC group had recurrence. The relapse sites and characteristics, an overview of salvage treatment, and outcomes are summarized in Table 3. Notably, most of the cases of recurrence in the PR-HDC group were solitary lesions, in contrast to the PR-CC group, and all relapsed patients in the PR-HDC group were successfully treated and survived without recurrence.
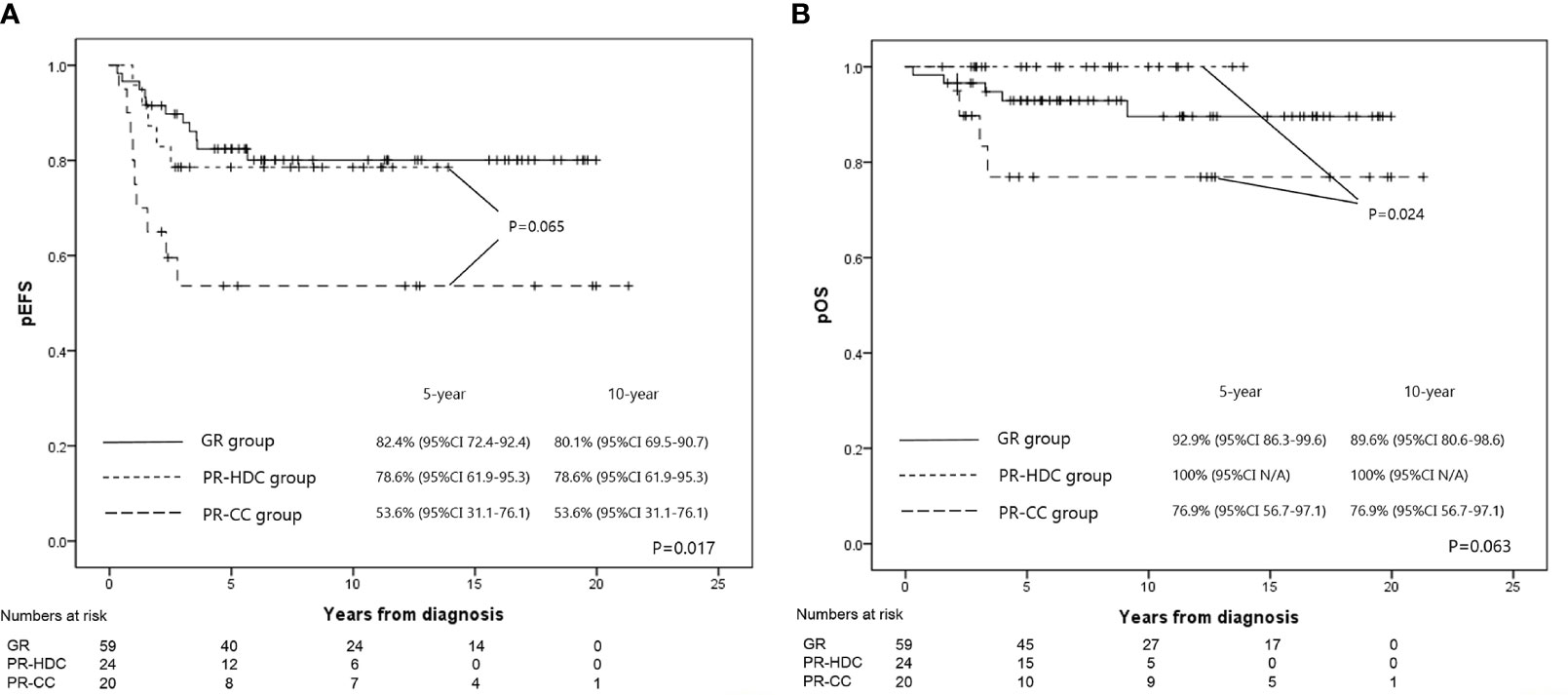
Figure 3 The EFS rates (A) and OS rates (B) at 5 years for GR, PR-HDC, and PR-CC groups were 82.4% versus 78.6% versus 53.6% (P=0.017), and 89.6% versus 100% versus 76.9% (P=0.063), respectively.
All patients in the PR-HDC group achieved successful neutrophil and platelet engraftment. The median times of neutrophil and platelet engraftment were 9 (range, 8–11) and 15 (range, 11–40) days, respectively. The median infused post-thawing mononuclear and CD34+ cell counts were 8.68 × 108/kg and 4.04 × 106/kg, respectively. Complications associated with ASCT, such as hepatic veno-occlusive disease, thrombotic microangiopathy, or transfer to intensive care units, did not occur. More detailed information on the HDC-PR group is provided in Table 4. With regard to long-term complications, 3 premature ovarian failures, 2 hearing impaired (1 grade 1, 1 grade 2), 2 hypothyroidisms (grade 2), and 2 proteinurias (1 grade 1, 1 grade 2) occurred in the HDC group, and 2 Therapy-related myelodysplastic syndromes, 1 chronic kidney disease (grade 4), 2 proteinurias (grade 2), and 1 diabetes mellitus in the CC group.
Prognostic factors
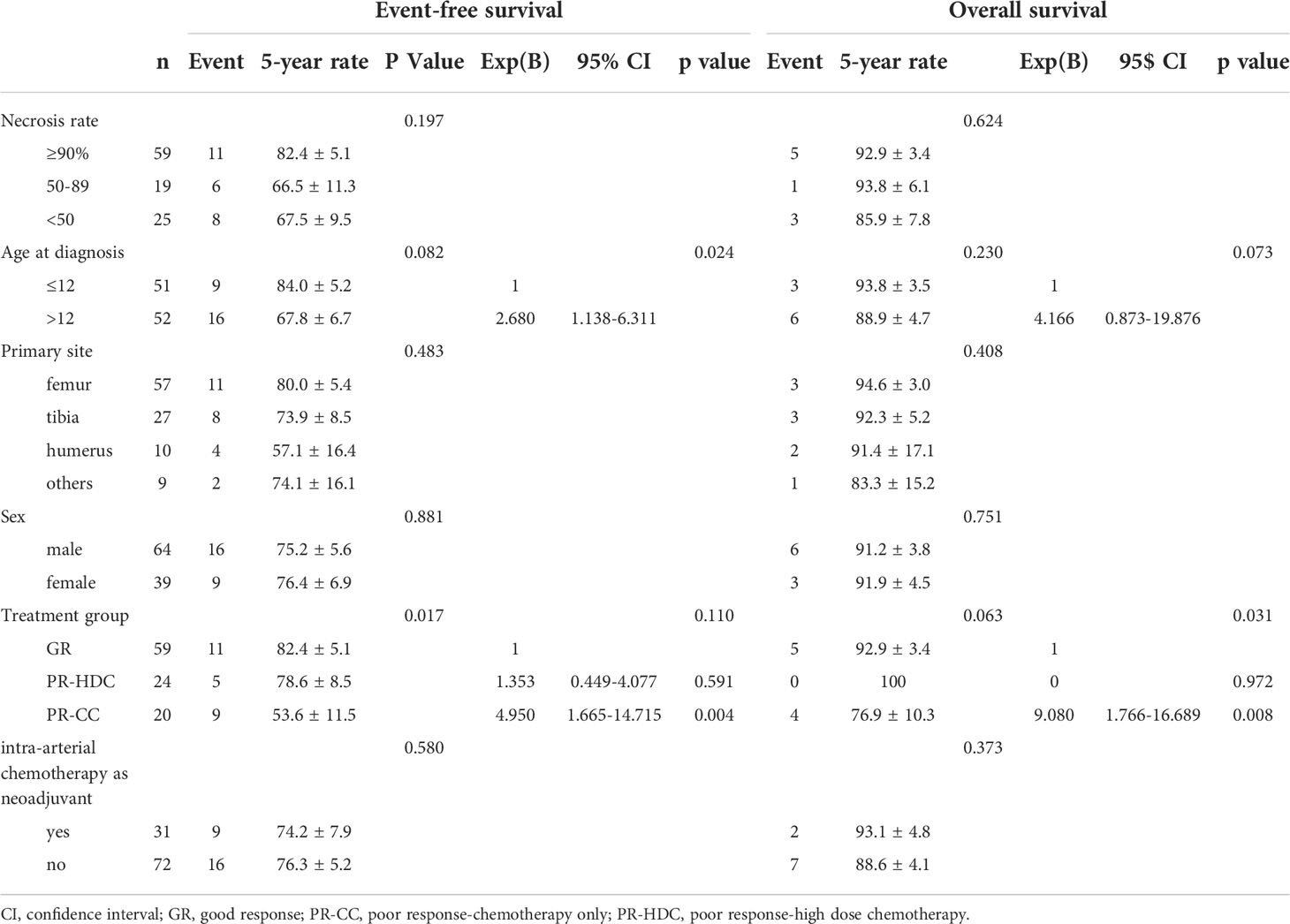
Among the variables, including necrosis rate (≥90%, 50–89%, and <50%), tumor sites, sex, and intra-arterial chemotherapy, there was no statistically significant difference in the univariate analyses of EFS and OS. However, the PR-CC group was associated with poor EFS (P=0.017). In the multivariate analysis, PR-CC was a significant poor prognostic factor for EFS (P=0.004) and OS (P=0.008), and age at diagnosis of >12 years was a significant poor prognostic factor for EFS (P=0.024) (Table 5).
Discussion
Our study shows promising treatment outcomes for HDC and ASCT using melphalan, etoposide, and carboplatin in patients with non-metastatic osteosarcoma when the tumor necrosis rate is <90% after neoadjuvant chemotherapy. In this study, the PR group showed a tendency toward lower EFS than the GR group; however, the PR-HDC group showed 100% OS, which translated to improved overall treatment outcomes in the PR group. In particular, the PR-HDC group showed EFS and OS similar to those of the GR group, and the recurrences that occurred in the PR-HDC group were more successfully salvaged than those in the PR-CC group.
Various efforts have been made to improve the treatment outcomes of patients with localized OSA who show lower degrees of necrosis after preoperative chemotherapy. In a previous study, patients with lower degrees of necrosis were administered additional cisplatin after surgery; however, there was no significant difference in the outcome (18). The EURAMOS trial has shown that adding ifosfamide and etoposide does not improve prognosis (10). In line with this study, a report by the Children’s Oncology Group that added higher cumulative doses of doxorubicin or ifosfamide plus etoposide for patients with lower degrees of necrosis did not show improvement in outcomes (19). In addition, studies applying HDC using melphalan alone or carboplatin plus etoposide did not show an improved outcome (11, 13).
Our institution demonstrated the feasibility of using HDC in patients with OSA in a previous pilot study (14). We followed the same strategy for patients with lower degrees of necrosis after neoadjuvant chemotherapy. Eight patients (33.3%) failed PBSC mobilization after chemotherapy while PBSC were able to be collected successfully following treatment with plerixafor. All patients were safely engrafted after ASCT and recovered without serious ASCT-related adverse events. We used a melphalan/etoposide/carboplatin regimen, which was different from the studies that conducted HDC in OSA. This regimen has already been used for a variety of childhood cancers and is familiar to pediatricians (20–22). All of these medications are known to be effective in OSA, and we have confirmed through a previously reported pilot study that the melphalan/etoposide/carboplatin regimen showed good treatment results, especially in localized OSA patients with low-degree necrosis (14). Although selection bias should be considered, in which only patients with transplantable conditions can be included in the PR-HDC group, our study showed that HDC is feasible as a concept of consolidation treatment in OSA.
It is noteworthy that the relapsed patients in the PR-HDC group had only a solitary lesion, and thus, all of them survived with metastasectomy and salvage chemotherapy. Although EFS still needs to be improved, HDC and ASCT could improve the OS of localized OSA with lower degrees of necrosis.
However, this study was a retrospective analysis for 20 years, which is limited in that it included patients who received heterogeneous chemotherapies. In particular, 27.4% of the patients who received intra-arterial neoadjuvant chemotherapy were included. In this study, more patients who received intra-arterial chemotherapy were included in the PR-CC group, although there was no difference in EFS and OS compared with intravenous chemotherapy, as in previous studies (23). In addition, this study included only nonmetastatic OSA, and it was difficult to evaluate the role of HDC and ASCT in cases of recurrence or metastasis at initial diagnosis. In particular, attention should be paid to the interpretation of our study in consideration of previous studies that did not show improved outcomes even if HDC was used (11, 13). However, this study is different from the previous studies in that we used the melphalan/etoposide/carboplatin regimen and analyzed only nonmetastatic OSA patients who showed low-degrees necrosis after neoadjuvant chemotherapy.
In conclusion, this study reported 10-year EFS and OS rates of 74.4% and 89.4%, respectively, in 113 patients with non-metastatic OSA who were treated for >20 years in a single institution. Moreover, HDC with melphalan, etoposide, and carboplatin showed favorable outcomes in patients with non-metastatic osteosarcoma, with necrosis of <90% after neoadjuvant chemotherapy. Further prospective studies are required to confirm the role of HDC and ASCT in OSA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Seoul National University Hospital. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: the Institutional Review Board approved the procedure for reviewing medical records, and obtaining consent was waived.
Author contributions
KTH performed the analysis and wrote and reviewed the article. HJP, BKK, HYA, and JYC collected the data and reviewed the article. J-EC, S-HP, and H-SK reviewed the article. HJK supervised, wrote, and reviewed the article. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by a grant (20182MFDS443) from Ministry of Food and Drug Safety in 2020 and supported by grant no 0320200110 from the SNUH Research Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.978949/full#supplementary-material
References
1. Gorlick R, Janeway K, Lessnick S, Randall RL, Marina N, Committee COGBT. Children's oncology group's 2013 blueprint for research: bone tumors. Pediatr Blood Cancer (2013) 60(6):1009–15. doi: 10.1002/pbc.24429
2. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer (2009) 115(7):1531–43. doi: 10.1002/cncr.24121
3. Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Eng J Med (1986) 314(25):1600–6. doi: 10.1056/NEJM198606193142502
4. Link MP. The multi-institutional osteosarcoma study: an update. Cancer Treat Res (1993) 62:261–7. doi: 10.1007/978-1-4615-3518-8_31
5. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol (2015) 33(27):3029–35. doi: 10.1200/JCO.2014.59.4895
6. Park HJ, Moon EK, Yoon JY, Oh CM, Jung KW, Park BK, et al. Incidence and survival of childhood cancer in Korea. Cancer Res Treat (2016) 48(3):869–82. doi: 10.4143/crt.2015.290
7. Pakos EE, Nearchou AD, Grimer RJ, Koumoullis HD, Abudu A, Bramer JA, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer (2009) 45(13):2367–75. doi: 10.1016/j.ejca.2009.03.005
8. Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol (2002) 20(3):776–90. doi: 10.1200/JCO.2002.20.3.776
9. Lee JW, Kim H, Kang HJ, Kim HS, Park SH, Kim IO, et al. Clinical characteristics and treatment results of pediatric osteosarcoma: the role of high dose chemotherapy with autologous stem cell transplantation. Cancer Res Treat (2008) 40(4):172–7. doi: 10.4143/crt.2008.40.4.172
10. Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol (2016) 17(10):1396–408. doi: 10.1016/S1470-2045(16)30214-5
11. Boye K, Del Prever AB, Eriksson M, Saeter G, Tienghi A, Lindholm P, et al. High-dose chemotherapy with stem cell rescue in the primary treatment of metastatic and pelvic osteosarcoma: final results of the ISG/SSG II study. Pediatr Blood Cancer (2014) 61(5):840–5. doi: 10.1002/pbc.24868
12. Marec-Berard P, Dalban C, Gaspar N, Brugieres L, Gentet JC, Lervat C, et al. A multicentric randomized phase II clinical trial evaluating high-dose thiotepa as adjuvant treatment to standard chemotherapy in patients with resectable relapsed osteosarcoma. Eur J Cancer (2020) 125:58–68. doi: 10.1016/j.ejca.2019.11.007
13. Venkatramani R, Murray J, Helman L, Meyer W, Hicks MJ, Krance R, et al. Risk-based therapy for localized osteosarcoma. Pediatr Blood Cancer (2016) 63(3):412–7. doi: 10.1002/pbc.25808
14. Hong CR, Kang HJ, Kim MS, Ju HY, Lee JW, Kim H, et al. High-dose chemotherapy and autologous stem cell transplantation with melphalan, etoposide and carboplatin for high-risk osteosarcoma. Bone Marrow Transpl (2015) 50(10):1375–8. doi: 10.1038/bmt.2015.145
15. Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol (2005) 23(9):2004–11. doi: 10.1200/JCO.2005.06.031
16. Hong KT, Kang HJ, Kim NH, Kim MS, Lee JW, Kim H, et al. Successful mobilization using a combination of plerixafor and G-CSF in pediatric patients who failed previous chemomobilization with G-CSF alone and possible complications of the treatment. J Hematol Oncol (2012) 5:14. doi: 10.1186/1756-8722-5-14
17. Hong KT, Park HJ, Kim BK, An HY, Choi JY, Kang HJ. PTCy-based haploidentical vs matched unrelated donor peripheral blood HSCT using myeloablative targeted busulfan-based conditioning for pediatric acute leukemia. Transplant Cell Ther (2022) 28(4):195.e1–7. doi: 10.1016/j.jtct.2022.01.002
18. Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the memorial Sloan-Kettering experience. J Clin Oncol (1992) 10(1):5–15. doi: 10.1200/JCO.1992.10.1.5
19. Schwartz CL, Wexler LH, Krailo MD, Teot LA, Devidas M, Steinherz LJ, et al. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: A report from the children's oncology group. Pediatr Blood Cancer (2016) 63(1):54–61. doi: 10.1002/pbc.25753
20. Choi JY, Kang HJ, Hong KT, Hong CR, Lee YJ, Park JD, et al. Tandem high-dose chemotherapy with topotecan-thiotepa-carboplatin and melphalan-etoposide-carboplatin regimens for pediatric high-risk brain tumors. Int J Clin Oncol (2019) 24(12):1515–25. doi: 10.1007/s10147-019-01517-8
21. Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. Effect of tandem autologous stem cell transplant vs single transplant on event-free survival in patients with high-risk neuroblastoma: A randomized clinical trial. JAMA (2019) 322(8):746–55. doi: 10.1001/jama.2019.11642
22. Ussowicz M, Mielcarek-Siedziuk M, Musial J, Stachowiak M, Weclawek-Tompol J, Sega-Pondel D, et al. Melphalan, etoposide, and carboplatin megatherapy with autologous stem cell transplantation in children with relapsing or therapy-resistant extracranial germ-cell tumors-a retrospective analysis. Cancers (Basel) (2020) 12(12):3841. doi: 10.3390/cancers12123841
Keywords: osteosarcoma, high-dose chemotherapy, autologous hematopoietic stem cell transplantation, low-degree necrosis, nonmetastatic
Citation: Hong KT, Park HJ, Kim BK, An H Y, Choi JY, Cheon J-E, Park S-H, Kim H-S and Kang HJ (2022) Favorable outcome of high-dose chemotherapy and autologous hematopoietic stem cell transplantation in patients with nonmetastatic osteosarcoma and low-degree necrosis. Front. Oncol. 12:978949. doi: 10.3389/fonc.2022.978949
Received: 27 June 2022; Accepted: 22 August 2022;
Published: 13 September 2022.
Edited by:
Francois Lamoureux, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Deepam Pushpam, All India Institute of Medical Sciences, IndiaSebastian Dorin Asaftei, Citta della Salute e della Scienza, Italy
Copyright © 2022 Hong, Park, Kim, An, Choi, Cheon, Park, Kim and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyoung Jin Kang, a2FuZ2hqQHNudS5hYy5rcg==
 Kyung Taek Hong
Kyung Taek Hong Hyun Jin Park1
Hyun Jin Park1 Hyoung Jin Kang
Hyoung Jin Kang