
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 July 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.975738
This article is part of the Research Topic Updates on Current Protocols for the Management of Brain and Spine Malignancies View all 15 articles
 Cinzia Baiano1*
Cinzia Baiano1* Teresa Somma1
Teresa Somma1 Raduan Ahmed Franca2
Raduan Ahmed Franca2 Marianna Di Costanzo1
Marianna Di Costanzo1 Maria Rosaria Scala1
Maria Rosaria Scala1 Pasquale Cretella2
Pasquale Cretella2 Felice Esposito1
Felice Esposito1 Luigi Maria Cavallo1
Luigi Maria Cavallo1 Paolo Cappabianca1
Paolo Cappabianca1 Domenico Solari1
Domenico Solari1Introduction: Endonasal endoscopic surgery has changed the treatment perspectives for different lesions of the hypothalamic–pituitary region. The metastases of the hypothalamic–pituitary region represent 0.4% of all intracranial metastatic tumors and account for only 1.8% of surgically managed pituitary lesions. The aim of tshis study is to describe a single-center institutional experience with 13 cases of hypothalamic–pituitary metastasis focused on presurgical workup, the evolution of the surgical technique, and postsurgical management according to our protocols, showing effects on progression-free and overall survival rates for this relatively uncommon location.
Material and Methods: We retrospectively reviewed the whole series of patients that received the endoscopic endonasal approach at the Division of Neurosurgery at the University of Naples “Federico II” undergoing surgery from January 1997 to December 2021. We identified 13 cases whose pathology reports revealed a metastatic lesion. Statistical analysis was performed to determine the Kaplan–Meier survival function and assess for log-rank differences in survival based on gender, surgical treatment, and postoperative therapy (p-value < 0.02*).
Results: The pathology report disclosed lung adenocarcinoma (six cases, 46%), breast adenocarcinoma (two cases, 15.4%), clear cell renal carcinoma (one case, 7%), melanoma (one case, 7%), colorectal adenocarcinoma (one case, 7%), uterine cervix carcinoma (one case, 7%), and follicular thyroid carcinoma (one case, 7%). A standard endoscopic endonasal approach was performed in 10 patients (76.9%), while an extended endonasal procedure was performed in only three cases (23%). Biopsy was the surgical choice in five patients with infiltrative and invasive lesions and a poor performance status (38%), while in the cases where neurovascular decompression was necessary, a subtotal resection was achieved in five patients (38%) and partial resection in three patients (23%). Recovery of visual field defect was observed in six of seven patients with visual loss (85.7%), improvement of oculomotor nerve palsy occurred in four of seven patients with this defect (57.1%), while the impairment of oculomotor palsy was observed in three patients (42.9%). Visual function was stable in the other patients. The median progression-free survival and overall survival were 14 and 18 months, respectively. There were statistically significant differences in PFS and OS in patients who underwent adjuvant radiotherapy (p=0.019 is referred to OS and p=0.017 to PFS, respectively; p-value = 0.02).
Conclusions: The endoscopic endonasal approach is a viable approach for the management of hypothalamic–pituitary metastases as this surgery provides an adequate opportunity to obtain tissue sample and neurovascular decompression, both being crucial for continuing the integrated adjuvant therapy protocols.
The inherent characteristics of endonasal endoscopic surgery have changed the treatment perspectives and operative nuances for different lesions of the hypothalamic–pituitary region (1).
This surgical route, in the standard version, has provided a more accurate distinction between healthy and pathological tissues in pituitary micro- and macroadenoma removal, thus ensuring pituitary gland preservation, an increase in the extent of resection, and an accurate histopathological characterization. Then, the extended approach, through access to the suprasellar, parasellar, retrosellar, clival, and retroclival spaces by shorter surgical corridors than the transcranial route, has revolutionized the management of complex and non-adenomatous midline lesions such as craniopharyngiomas, meningiomas, and clival chordomas (2–5). For the less common and more infiltrating lesions such as sarcomas, gliomas, metastases, and granulomatosis (sarcoidosis), it has enabled a minimally invasive biopsy and/or the identification of surgical removal limits, taking into consideration the principles of maximal safe resection (6).
The metastases of the hypothalamic–pituitary region represent 0.4% of all intracranial metastatic tumors and account for only 1.8% of surgically managed pituitary lesions (7). Metastatic tumor cells may involve the pituitary gland via different patterns of spread, including direct hematogenous, from the hypothalamus or stalk through the portal hypophyseal vessels or from the juxtasellar or skull base metastasis through the arachnoid of the suprasellar cistern (8, 9).
At our institution, in a dedicated tertiary center for hypothalamic–pituitary disorders, the possibility of observing more than one hundred endoscopic endonasal procedures per year has granted the wide series and also the variety with the inclusion of metastatic pathology of the aforementioned region (10–12).
In the circular process of update application in clinical complementary fields adjacent to the midline skull base surgery (endocrinology, neuroradiology, pathology, radiotherapy, and oncology), the new strategies of approach to hypothalamic–pituitary lesions are dependent on a multidisciplinary approach (13–18). Furthermore, advancements in target systemic radiosurgery and whole-brain radiotherapy and therapy for brain metastasis management have changed prognostic models (19–22). In this setting, the endoscopic endonasal approach is proposed as a valid tool for obtaining tissue for histological examination and determining consequently therapeutical steps in the treatment of metastatic patients (23–25).
The aim of this study is to describe a single-center institutional experience with 13 cases of sellar metastasis focused on presurgical workup, evolution of surgical technique, and postsurgical management according to our protocols, showing effects on progression-free and overall survival rates for this relatively uncommon location.
This study was approved by the Institutional Review Board of the School of Medicine of the University of Naples “Federico II,” which waived the need for informed consent due to the retrospective nature of the study. Written informed consent was obtained from the patients prior to any invasive clinicodiagnostic and surgical procedure; indeed, it was obtained for the eventual publication—for scientific purposes—of any patient records/information anonymously.
We retrospectively reviewed the whole series of patients that received the endoscopic endonasal approach at the Division of Neurosurgery at the University of Naples “Federico II” undergoing surgery from January 1997 to December 2021. We identified 13 cases whose pathology report revealed a metastatic lesion.
Case history, histological diagnosis, endocrinological assessment, preoperative and postoperative radiological records, preoperative treatment, intraoperative surgical videos, and instrumental eye examinations were revised. All patients underwent a pituitary pre- and postsurgical function assessment, pre- and postoperative post-gadolinium magnetic resonance (MRI), and complete visual assessment (computerized visual field, Lancaster red-green test, visual acuity).
Statistical analysis was performed to determine the Kaplan–Meier survival function and assess for log-rank differences in survival based on gender, surgical treatment, and postoperative therapy (p-value < 0.02). All analyses were performed using the R environment software for statistical computing (Figure 1) (R Development Core Team, Vienna, Austria, 2013).
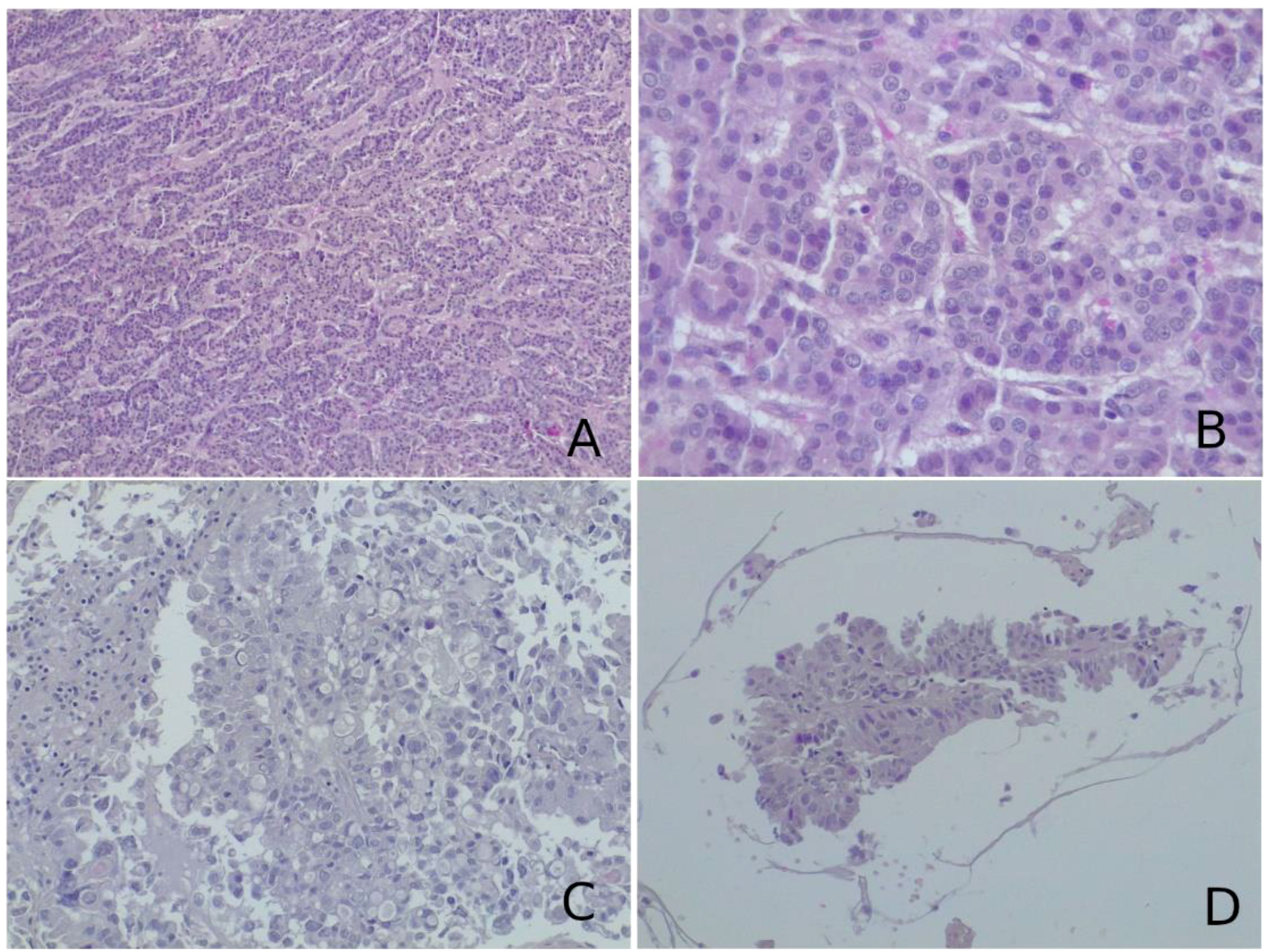
Figure 1 (A, B) Histological examination of the lesion biopsied revealed a neoplasm composed of cells arranged in follicular, cord-like, and nodular structures. On immunohistochemistry, neoplastic cells were positive for thyroglobulin, PAX8, TTF1, and cytokeratin 19 and negative for CD56 and CDX2. This morphological picture was suggestive of metastatic follicular thyroid carcinoma. (C, D) On histological slides, a glandular neoplasm, composed of pleomorphic, vacuolated cells with high-grade characteristics and papillary arrangement [most clear in (D)], was seen. Neoplastic cells were immunoreactive for cytokeratin 7 and TTF1 and negative for cytokeratin 20. These characteristics were more suggestive of adenocarcinoma metastasis from a lung primary tumor. (A–D) Hematoxylin–eosin, original magnification ×40.
All the patients underwent an endoscopic endonasal approach. For intrasellar lesion, a standard operative nuance was performed, while in the case of supradiaphragmatic lesion, the most suitable extended approach was chosen according to the techniques already described (2). In all cases, extemporaneous histological examination was decisive for subsequential surgical steps.
Unusual morphological patterns can cause diagnostic concern for other lesions (sinusoidal pattern and macronodular or festoon-like features, as well as lesions with diffuse epithelioid features). In these cases, a routine immunohistochemical panel was performed (ACTH, PRL, GH, TSH, FSH, LH, GH, Ki67), including reticulin staining, neuroendocrine differentiation using immunohistochemical markers (chromogranin, synaptophysin), and immunoreactivity for transcription factors (T-Pit, Pit1, SF1), which were directed toward a correct diagnosis.
The differential diagnosis between a pituitary adenoma and metastatic cancer is rarely a problem: mitotic activity and cellular pleomorphism are nearly always the hallmarks of a metastatic neoplasm, while these are rare in pituitary adenomas.
When a lesion is thought to be a metastasis with no primary tumor clearly diagnosed, additional markers should be performed to define the tumor lineage: LCA (CD45) positivity is seen in lymphoproliferative lesions; carcinomas are nearly always pan-cytokeratin-positive; TTF1 positivity suggests a pulmonary or thyroid origin (the latter being positive also for thyroglobulin and PAX8); CDX2 positivity suggests cancer originating in the gastroenteric tract; HMB45 along with MART1, SOX10, and S100 immunoreactivity is a feature of melanoma; GCDFP-15 and mammaglobin are markers of breast cancer; PSA positivity suggests a prostate primitivity; and PAX8 immunoreactivity supports a diagnosis of metastatic renal cell carcinoma.
A challenging differential diagnosis concerns distinguishing null cell adenoma from metastatic neuroendocrine carcinoma: indeed, both are tumors immunonegative for all the hypophysial markers (hormones and transcription factors) but positive for neuroendocrine markers. Morphology, mitotic activity, and lineage differentiation markers (TTF1, CDX2, CK, calcitonin, etc.) are useful to make a correct diagnosis. However, it must be kept in mind the possibility of the debated entity of primary intracranial neuroendocrine carcinoma arising in the sellar region (TTF1−) and small cell carcinoma of unknown primary (SCUP) (TTF1+) (Figure 2).
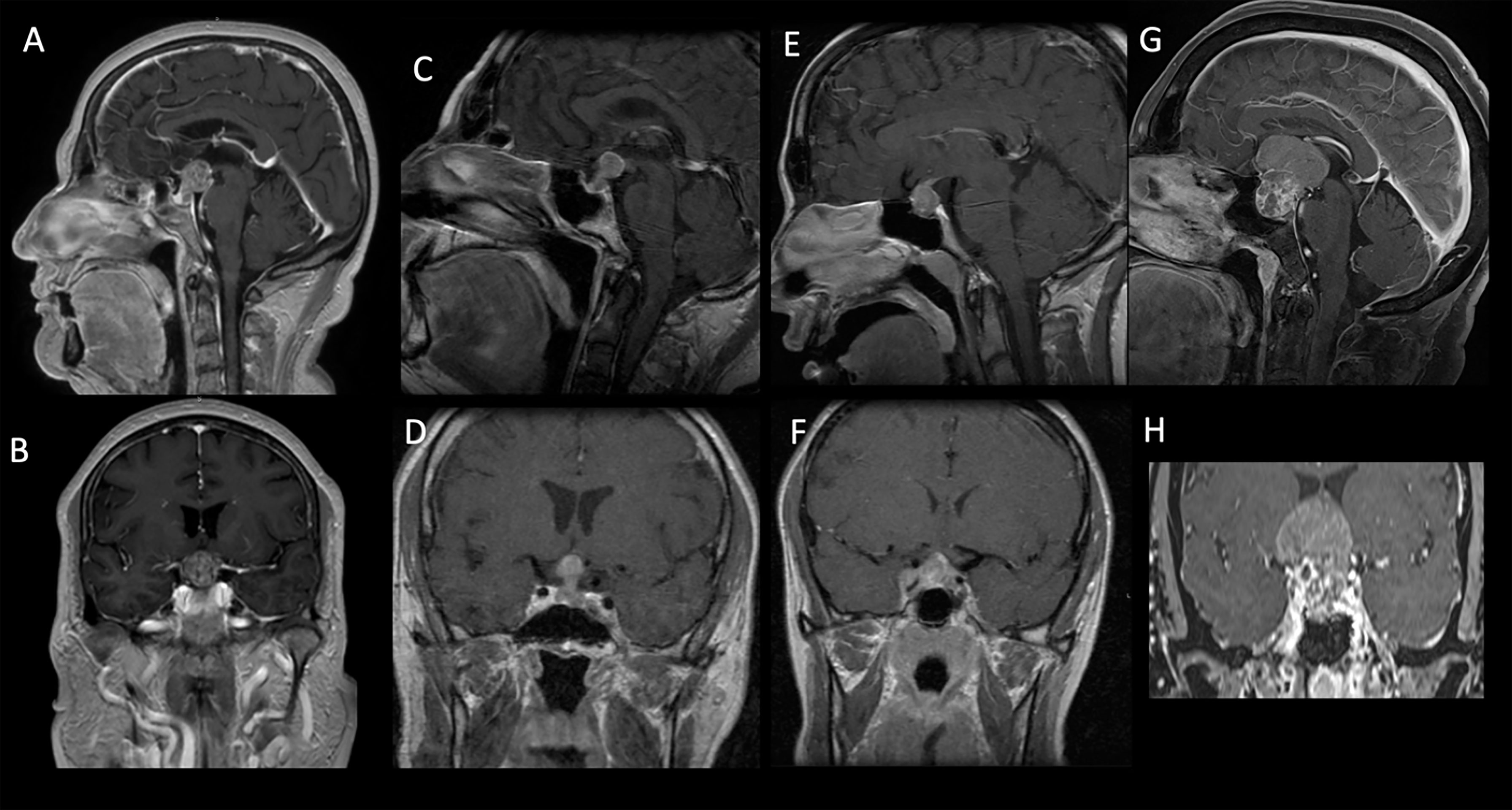
Figure 2 Sagittal and coronal preoperative MRI T1-weighted contrast-enhanced images of patients from our cohort showing intra- and suprasellar lesions with inhomogeneous enhancement. The pathology report disclosed breast adenocarcinoma (A, B), lung adenocarcinoma (C, D), clear cell renal carcinoma (E, F), and uterine cervix carcinoma (G, H).
Between around 1997 and December 2021, 2,303 patients underwent endonasal endoscopic surgery for the removal of different skull base lesions—mostly pituitary adenomas—at the Division of Neurosurgery at the University of Naples “Federico II.” Of these patients, 13 (0.6%) had a metastatic lesion (Table 1). Nine patients were women and four were men; the mean age was 58 years. Three patients presented a pure infradiaphragmatic intrasellar lesion (23%); four patients presented intra-, supra-, and parasellar lesions (30%); and six patients had intra-, supra-, and retrosellar lesions (46%). The most common presentation was headache in 10 patients (76.9%), followed by visual loss in 7 patients (53.8%), adenohypophysis dysfunction in 6 patients (46%), diabetes insipidus in 6 patients (46%), visual field defect in 3 patients (23%), and oculomotor nerve palsy in 3 patients (23%) (Table 2). In the five cases of lung adenocarcinoma, pituitary metastasis was the first presentation of neoplastic disease.
The surgical strategy was tailored based on the lesion extension and the performance status of the patients: in three cases, an extended endonasal approach was required; in five cases, only a biopsy was performed. The endoscopic endonasal standard approach was used in 10 patients; for the other three patients, an extended trans-planum approach was performed. Osteodural defect reconstruction was necessary in two cases (Table 3).
The pathology report disclosed lung adenocarcinoma (six cases, 46%), breast adenocarcinoma (two cases, 15.4%), clear cell renal carcinoma (one case, 7%), melanoma (one case, 7%) colorectal adenocarcinoma (one case, 7%), uterine cervix carcinoma (one case, 7%), and follicular thyroid carcinoma (one case, 7%) (Table 1 and Figure 1).
The standard endoscopic endonasal approach was performed in 10 patients (76.9%), while the extended endonasal procedure was used in only three cases (23%). Biopsy was the surgical choice in five patients with infiltrative and invasive lesions and a poor performance status (38%). On the other hand, in the cases where neurovascular decompression was necessary, a subtotal resection was achieved in five patients (38%) and a partial resection in three patients (23%).
Recovery of visual field defect was observed in six of seven patients with visual loss (85.7%), improvement of oculomotor nerve palsy occurred in four of seven patients with this defect (57.1%), while impairment of oculomotor palsy was observed in three patients (42.9%). Visual function was stable in the other patients.
Concerning complications, no infection and CSF leak were seen; we observed one patient developing transient diabetes insipidus.
Adjuvant therapy was used in all cases. Ten patients were treated with systemic chemotherapy (59%), two patients (20%) had stereotactic radiotherapy, and one patient with a clear cell renal carcinoma had a combination of radio- and chemotherapy. After adjuvant treatment, three patients (23%) developed pan-hypopituitarism.
The median progression-free survival and overall survival were 14 and 18 months, respectively (Table 4). There were statistically significant differences in PFS and OS in patients who underwent adjuvant radiotherapy (p=0.019 is referred to OS and p=0.017 to PFS, respectively; p-value = 0.02) (Figure 3).
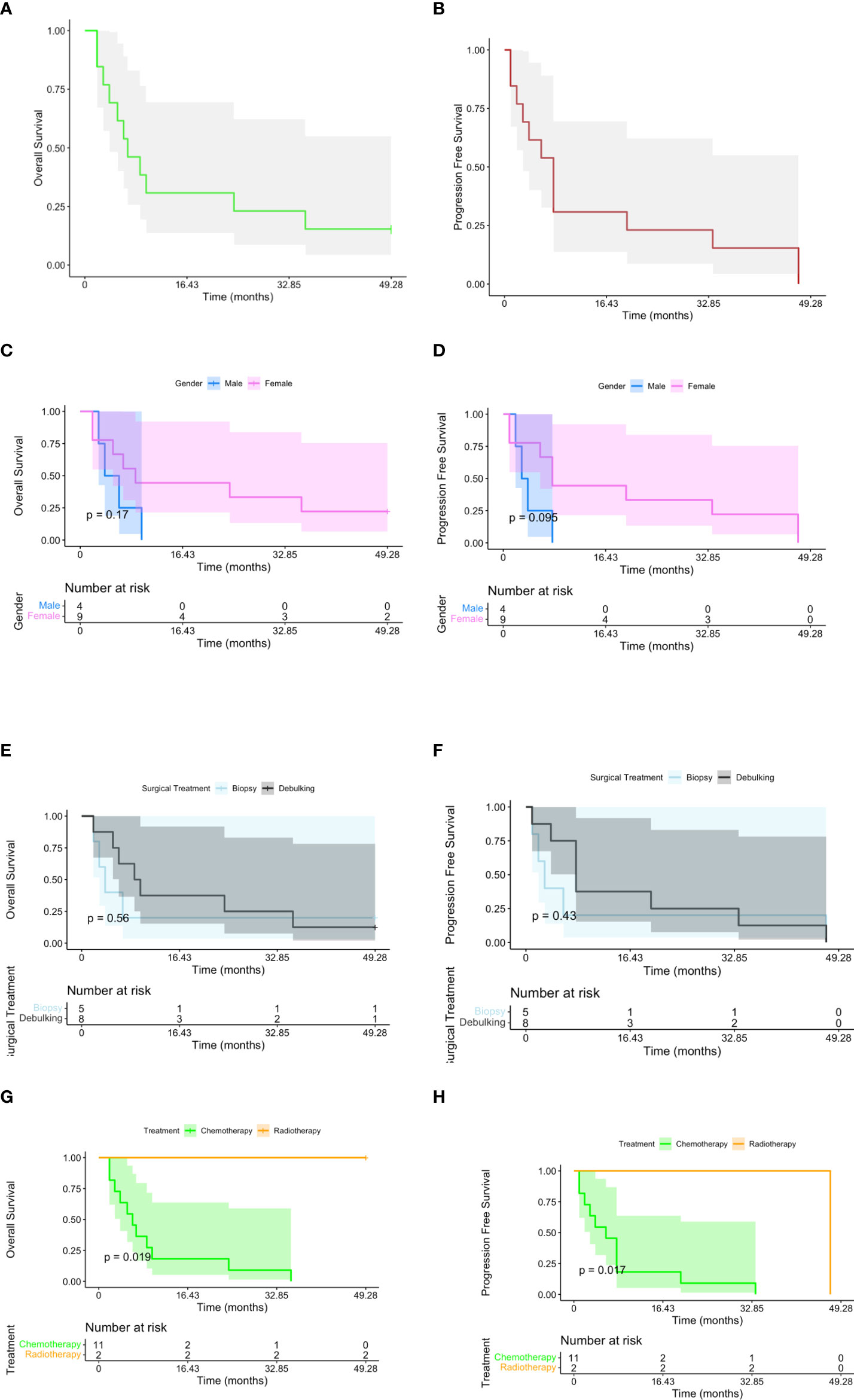
Figure 3 Kaplan–Meier plots reporting overall survival (OS) and progression-free survival (PFS) for all the subjects included in the study (A, B). OS and PFS have been stratified, respectively, by gender (C, D), surgical treatment (E, F), and postoperative therapy [G (p-value < 0.02), H (p-value < 0.02)].
Hypothalamic–pituitary metastatic lesions represent a very challenging diagnosis: clinical signs are not different from the other lesions affecting this area, and there are no pathognomonic signs at MRI or CT scan (6, 26). Moreover, the lack of a certain presence of tumor history jeopardizes the likelihood of a lesion of the hypothalamic–pituitary region being a metastasis (9). In the present series, we found five cases of hypothalamic–pituitary metastasis figured out as the first lesion of neoplastic disease.
A positive oncological anamnesis for the most common primary tumors associated with hypothalamic–pituitary metastasis (breast cancer in women, 40% of the cases; lung cancer in men, 24% of the cases) could verify the suspicion. Other primary tumors that are less common include gastrointestinal tract (6.3%), prostate (5%), melanoma (2.4%), and thyroid (2.2%) malignancies (8, 23, 27). Primitive tumors observed in this series were lung adenocarcinoma (six cases, 46%), breast adenocarcinoma (two cases, 15.4%), clear cell renal carcinoma (one case, 7%), melanoma (one case, 7%), colorectal adenocarcinoma (one case, 7%), cervical carcinoma (one case, 7%), and follicular thyroid carcinoma (one case, 7%) (Table 1).
In agreement with the current literature (8, 27–30), the main clinical manifestation was headache (76.9%), followed by visual loss (53.8%), adenohypophysis dysfunction (46%), diabetes insipidus (46%), visual field defect (23%), and oculomotor nerves palsy (23%). In this scenario, we have to consider diabetes insipidus as a typical feature of infiltrative non-adenomatous sellar lesions (sarcoidosis, hypophysitis, histiocytosis, craniopharyngiomas, ATRT) and, above all, when it is associated with visual loss and/or nerve palsy (6, 31).
Microsurgical trans-sphenoidal, open transcranial, and trans-facial surgery were the last common surgical routes to be reported in the management of pituitary metastasis, and the mortality and morbidity related to these procedures were not insignificant (28, 32).
Initially, the endoscopic endonasal technique was considered a two-handed technique, and similar to the microsurgical technique, the basic principles used are not far from those when operating using a microscope. The evolution of the four-handed technique, in a setting of close and dynamic cooperation between two operators, has granted a useful amplification of the surgical corridor and the augmentation of the angle of exposure by a continuous change of framing, focus, and distance view. The increase in surgical agility, allowing improvement in the dissection method, has made the endoscopic endonasal technique a valid tool for managing the entire ventral skull base, according to the same surgical principles of the open approaches, offering a possibility of treating a wide variety of median and paramedian lesions with satisfactory outcomes (2, 5, 33, 34).
The possibility to access the supra-, para-, and retrosellar spaces by extended procedures has changed the way of exploring and removing even more complex lesions. Extended approaches may be considered in selected cases, and it allows the removal of a vascularized tumor or suprasellar residual tumor in order to avoid postoperative hematoma. The benefit offered by the extended endonasal route is to allow an extracapsular dissection of the tumor beside the standard endosellar corridor (3, 5, 35). Advancements in surgical route reconstruction techniques have contributed to ameliorate postsurgical outcome and performance status (36, 37).
Analyzing our metastasis series, three patients presented a pure intrasellar lesion (23%); four patients had intra-, supra-, and parasellar lesions (30%); and six patients had intra-, supra-, and retrosellar lesions (46%). Surgical strategy was tailored based on lesion extension, intraoperative histological examination, and performance status of the patients: in three cases, an extended endonasal approach was required; in five cases, only a biopsy was performed. The endoscopic endonasal standard approach was used in 10 patients; for the other three patients, an extended trans-planum approach was performed with good control of piecemeal resection.
Based on intraoperative histological examination, in the cases of intrasellar tumors, debulking could be smoother than supra- and retrosellar lesions, even if a poor performance status and a fibrous consistency with infiltrative pattern make biopsy a more reasonable choice. This principle is applied also in lesions extended to the supra-, para-, and retrosellar spaces in the cases which only a neurovascular decompression is possible (14) (15, 27, 30, 38).,
The proper management of a hypothalamic–pituitary metastasis requires a cogent balance between medical treatment, watchful waiting, surgery, and radiation therapy (1, 15, 23–25). Surgical resection can be complicated by fibrous consistency, irregular shape, and invasiveness of the tumor, which often lead to incomplete resections, increasing the risks of morbidity (27). Current literature shows one case report about a case of non-small cell lung cancer (NSCLC) with EGFR exon 19 deletion mutation, in which osimertinib eradicated the metastasis and prevented the need for radiation therapy (39).
In other studies, the main treatment for single brain metastasis is maximal safe surgical resection in combination with radio- and chemotherapy (16, 27, 30, 38, 40–42). In our series, this approach together with the combination of surgery and adjuvant therapy showed improvement in PFS and OS. Indeed, after multidisciplinary concertation, adjuvant therapy was used in all cases according to cogent protocols (24, 25). Ten patients were treated with systemic chemotherapy (59%), two patients (20%) had stereotactic radiotherapy on residual disease, and one patient with a clear cell renal carcinoma had a combination of radio- and chemotherapy. After adjuvant treatment, three patients (23%) developed pan-hypopituitarism.
In this study, the median progression-free survival and overall survival were 14 and 18 months, respectively (Table 3). Two patients are still alive 1 year after surgery. There were statistically significant differences in survival based on the type of adjuvant therapy on Kaplan–Meier analysis (i.e., radiotherapy was associated with a survival increase than chemotherapy). A limitation of this study is the reduced sample size. Before endonasal endoscopy introduction, the microsurgical transsphenoidal approach with partial resection and adjuvant treatment (local radiation) was associated with better symptom relief without effects on survival rates, which is less than 12 months in several studies; furthermore, the mean survival length in the clinical series was 6–7 months (28, 43–45). Zoli et al. reported a median survival of 11.8 months after transsphenoidal surgery followed by radiation therapy (46). In the series of anterior skull base metastases managed by the endoscopic endonasal approach reported by Zacharia et al., PFS and OS were 18 and 16 months and any correlation between survival and other variables was detected (41). In a similar multicentric study involving 12 patients, the mean OS was reported to be 17 months (30). The increase in survival is due to advancements in the surgical and oncological fields, and we do not speculate that only the endonasal approach has impacted survival.
Concerning outcomes, recovery of the visual field defect and impairment of oculomotor nerve palsy were both observed in three of four patients (75%). Recovery of visual field defect was observed in six of seven patients with visual loss (85.7%), improvement of oculomotor nerve palsy occurred in four of seven patients with this defect (57.1%), while impairment of oculomotor nerve palsy was observed in three patients (42.9%). These results validate the role of endoscopic surgery as a tool for a satisfying decompression of the optic pathways. Regarding postsurgical complications, no postoperative cerebrospinal fluid leak occurred in any of the patients; one patient developed transient diabetes insipidus. According to current literature about the cases of pituitary metastasis managed with endoscopic endonasal surgery, this strategy is associated with a few complications and does not have an impact on the performance status of patients (1, 30, 34, 40, 41, 46).
A correct balance between surgical indications and evaluation of functional recovery impact on quality of life is mandatory. Indeed, not being able to know a priori if a lesion is metastatic or not, presurgical workup combined with surgical endoscopic experience has allowed a better interpretation of intraoperative features guiding the diagnosis and the subsequent management of the lesions. Furthermore, an extemporaneous histological examination is crucial to determine the surgical procedure and level of resection for improving PFS and OS (24, 25, 41).
Pituitary metastasis surgery requires a cogent balance between medical treatment, watchful waiting, surgery, and radiation therapy; it requires cleverness, great versatility, and the collaboration of different specialists.
The endoscopic endonasal approach is a viable approach for the management of hypothalamic–pituitary metastases as this surgery provides adequate opportunity to obtain tissue sample and neurovascular decompression, both being crucial for continuing the integrated adjuvant therapy protocols.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
This study was approved by the institutional review board of the School of Medicine of University of Naples “Federico II”, which waived the need for informed consent due to the retrospective nature of the study. Written informed consent was obtained from the patients prior than any invasive clinico-diagnostic and surgical procedure; indeed, it was obtained for the eventual publication – for scientific purpose - of any patient records/information anonymously. The patients/participants provided their written informed consent to participate in this study.
CB: writing of the original draft, review, and editing. TS, DS, and LC: revisions. RF and PCr: responsible for the pathology methods section; MDC, RF, and PC: acquisition of data. MS: statistic analysis. DS, FE, LC, and PCa: supervision and validation. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Solari D, Zenga F, Angileri FF, Barbanera A, Berlucchi S, Bernucci C, et al. A survey on pituitary surgery in Italy. World Neurosurg (2019) 123:e440–9. doi: 10.1016/j.wneu.2018.11.186
2. Cappabianca P, Cavallo LM, Esposito F, De Divitiis O, Messina A, De Divitiis E. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg (2008) 33:151–99. doi: 10.1007/978-3-211-72283-1_4
3. Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. (2008) 63(1):36–52. doi: 10.1227/01.NEU.0000335069.30319.1E
4. Todeschini AB, Beer-Furlan A, Otto B, Prevedello DM, Carrau RL. Endoscopic endonasal approaches for anterior skull base meningiomas. Adv Otorhinolaryngol (2020) 84:114–23. doi: 10.1159/000457931
5. Koutourousiou M, Gardner PA, Tormenti MJ, Henry SL, Stefko ST, Kassam AB, et al. Endoscopic endonasal approach for resection of cranial base chordomas: outcomes and learning curve. Neurosurgery. (2012) 71(3):614–24. doi: 10.1227/NEU.0b013e31825ea3e0
6. Somma T, Solari D, Beer-Furlan A, Guida L, Otto B, Prevedello D, et al. Endoscopic endonasal management of rare sellar lesions: Clinical and surgical experience of 78 cases and review of the literature. World Neurosurg (2017) 100:369–80. doi: 10.1016/j.wneu.2016.11.057
7. Al-Aridi R, El Sibai K, Fu P, Khan M, Selman WR, Arafah BM. Clinical and biochemical characteristic features of metastatic cancer to the sella turcica: an analytical review. Pituitary. (2014) 17(6):575–87. doi: 10.1007/s11102-013-0542-9
8. Chiang MF, Brock M, Patt S. Pituitary metastases. Neurochirurgia (Stuttg). (1990) 33(4):127–31. doi: 10.1055/s-2008-1053571
9. Branch CL, Laws ER. Metastatic tumors of the sella turcica masquerading as primary pituitary tumors. J Clin Endocrinol Metab (1987) 65(3):469–74. doi: 10.1210/jcem-65-3-469
10. Casanueva FF, Barkan AL, Buchfelder M, Klibanski A, Laws ER, Loeffler JS, et al. Criteria for the definition of pituitary tumor centers of excellence (PTCOE): A pituitary society statement. Pituitary. (2017) 20(5):489–98. doi: 10.1007/s11102-017-0838-2
11. Zoli M, Milanese L, Faustini-Fustini M, Guaraldi F, Asioli S, Zenesini C, et al. Endoscopic endonasal surgery for pituitary apoplexy: Evidence on a 75-case series from a tertiary care center. World Neurosurg (2017) 106:331–8. doi: 10.1016/j.wneu.2017.06.117
12. McLaughlin N, Laws ER, Oyesiku NM, Katznelson L, Kelly DF. Pituitary centers of excellence. Neurosurgery. (2012) 71(5):916–24. doi: 10.1227/NEU.0b013e31826d5d06
13. Tortora F, Negro A, Briganti F, Del Basso De Caro ML, Cavallo LM, Solari D, et al. Pituitary magnetic resonance imaging. Gland Surg (2020) 9(6):2260–8. doi: 10.21037/gs-20-654
14. Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, et al. Updates in the management of brain metastases. Neuro Oncol (2016) 18(8):1043–65. doi: 10.1093/neuonc/now127
15. Rudà R, Franchino F, Soffietti R. Treatment of brain metastasis: current status and future directions. Curr Opin Oncol (2016) 28(6):502–10. doi: 10.1097/CCO.0000000000000326
16. Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European association of neuro-oncology (EANO). Neuro Oncol (2017) 19(2):162–74. doi: 10.1093/neuonc/now241
17. Asa SL. Practical pituitary pathology: what does the pathologist need to know? Arch Pathol Lab Med (2008) 132(8):1231–40. doi: 10.1043/1543-2165(2008)132[1231:PPPWDT]2.0.CO;2
18. Minniti G, Osti MF, Niyazi M. Target delineation and optimal radiosurgical dose for pituitary tumors. Radiat Oncol (2016) 11(1):135. doi: 10.1186/s13014-016-0710-y
19. Asa SL, Casar-Borota O, Chanson P, Delgrange E, Earls P, Ezzat S, et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): an international pituitary pathology club proposal. Endocr Relat Cancer. (2017) 04:C5–C8. doi: 10.1530/ERC-17-0004
20. Solari D, Pivonello R, Caggiano C, Guadagno E, Chiaramonte C, Miccoli G, et al. Pituitary adenomas: What are the key features? what are the current treatments? where is the future taking us? World Neurosurg (2019) 127:695–709. doi: 10.1016/j.wneu.2019.03.049
21. Ilie MD, Jouanneau E, Raverot G. Aggressive pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am (2020) 49(3):505–15. doi: 10.1016/j.ecl.2020.05.008
22. Marcus HJ, Khan DZ, Borg A, Buchfelder M, Cetas JS, Collins JW, et al. Pituitary society expert Delphi consensus: operative workflow in endoscopic transsphenoidal pituitary adenoma resection. Pituitary. (2021) 24(6):839–53. doi: 10.1007/s11102-021-01162-3
23. Cavallo LM, Solari D. Multimodality attitude for the treatment of a pituitary metastasis. World Neurosurg (2013) 79(5-6):673–4. doi: 10.1016/j.wneu.2013.01.073
24. Winther RR, Hjermstad MJ, Skovlund E, Aass N, Helseth E, Kaasa S, et al. Surgery for brain metastases-impact of the extent of resection. Acta Neurochir (Wien). (2022). doi: 10.1007/s00701-021-05104-7
25. Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. Jul (2007) 12(7):884–98. doi: 10.1634/theoncologist.12-7-884
26. Baiano C, Della Monica R, Franca RA, Del Basso De Caro ML, Cavallo LM, Chiariotti L, et al. Atypical teratoid rhabdoid tumor: A possible oriented female pathology? Front Oncol (2022) 12:854437. doi: 10.3389/fonc.2022.854437
27. Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, et al. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab (2004) 89(2):574–80. doi: 10.1210/jc.2003-030395
28. Morita A, Meyer FB, Laws ER. Symptomatic pituitary metastases. J Neurosurg (1998) 89(1):69–73. doi: 10.3171/jns.1998.89.1.0069
29. Koutourousiou M, Kontogeorgos G, Seretis A. Non-adenomatous sellar lesions: experience of a single centre and review of the literature. Neurosurg Rev (2010) 33(4):465–76. doi: 10.1007/s10143-010-0263-8
30. Castle-Kirszbaum M, Goldschlager T, Ho B, Wang YY, King J. Twelve cases of pituitary metastasis: a case series and review of the literature. Pituitary. (2018) 21(5):463–73. doi: 10.1007/s11102-018-0899-x
31. Paolini MA, Kipp BR, Sukov WR, Jenkins SM, Barr Fritcher EG, Aranda D, et al. Sellar region atypical Teratoid/Rhabdoid tumors in adults: Clinicopathological characterization of five cases and review of the literature. J Neuropathol Exp Neurol (2018) 77(12):1115–21. doi: 10.1093/jnen/nly091
32. Freda PU, Wardlaw SL, Post KD. Unusual causes of sellar/parasellar masses in a large transsphenoidal surgical series. J Clin Endocrinol Metab (1996) 81(10):3455–9. doi: 10.1210/jcem.81.10.8855784
33. Fernandez-Miranda JC, Zwagerman NT, Abhinav K, Lieber S, Wang EW, Snyderman CH, et al. Cavernous sinus compartments from the endoscopic endonasal approach: anatomical considerations and surgical relevance to adenoma surgery. J Neurosurg (2018) 129(2):430–41. doi: 10.3171/2017.2.JNS162214
34. Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg (2011) 114(6):1544–68. doi: 10.3171/2010.10.JNS09406
35. Di Maio S, Cavallo LM, Esposito F, Stagno V, Corriero OV, Cappabianca P. Extended endoscopic endonasal approach for selected pituitary adenomas: early experience. J Neurosurg (2011) 114(2):345–53. doi: 10.3171/2010.9.JNS10262
36. Wang EW, Zanation AM, Gardner PA, Schwartz TH, Eloy JA, Adappa ND, et al. ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol 07 (2019) 9(S3):S145–365. doi: 10.1002/alr.22326
37. Cavallo LM, Solari D, Somma T, Cappabianca P. The 3F (Fat, flap, and flash) technique for skull base reconstruction after endoscopic endonasal suprasellar approach. World Neurosurg (2019) 126:439–46. doi: 10.1016/j.wneu.2019.03.125
38. Doglietto F, Daffini L, Fazzari E, Cominelli M, Pagani F, Poliani PL. Sellar metastasis from clear cell sarcoma: Description of the first case. Clin Neuropathol. (2022) 41(3):122–7. doi: 10.5414/NP301448
39. Fan W, Sloane J, Nachtigall LB. Complete resolution of sellar metastasis in a patient with NSCLC treated with osimertinib. J Endocr Soc (2019) 3(10):1887–91. doi: 10.1210/js.2019-00217
40. Ravindran K, Zsigray BM, Wemhoff MP, Spencer JD, Borys E, Patel CR, et al. Sellar metastasis of cervical adenocarcinoma. Case Rep Neurol Med (2019) 2019:9769657. doi: 10.1155/2019/9769657
41. Zacharia BE, Romero FR, Rapoport SK, Raza SM, Anand VK, Schwartz TH. Endoscopic endonasal management of metastatic lesions of the anterior skull base: Case series and literature review. World Neurosurg (2015) 84(5):1267–77. doi: 10.1016/j.wneu.2015.05.061
42. Mormando M, Puliani G, Barnabei A, Lauretta R, Bianchini M, Chiefari A, et al. A rare case of pituitary melanoma metastasis: A dramatic and prolonged response to dabrafenib-trametinib therapy. Front Endocrinol (Lausanne). (2020) 11:471. doi: 10.3389/fendo.2020.00471
43. McCormick PC, Post KD, Kandji AD, Hays AP. Metastatic carcinoma to the pituitary gland. Br J Neurosurg (1989) 3(1):71–9. doi: 10.3109/02688698909001028
44. Sioutos P, Yen V, Arbit E. Pituitary gland metastases. Ann Surg Oncol (1996) 3(1):94–9. doi: 10.1007/BF02409058
45. Houck WA, Olson KB, Horton J. Clinical features of tumor metastasis to the pituitary. Cancer. (970) 26(3):656–9. doi: 10.1002/1097-0142(197009)26:3<656::aid-cncr2820260325>3.0.co;2-m
Keywords: hypothalamic–pituitary pathology, endoscopic endonasal surgery, brain metastasis, neuro-oncology-surgical, surgical procedures
Citation: Baiano C, Somma T, Franca RA, Di Costanzo M, Scala MR, Cretella P, Esposito F, Cavallo LM, Cappabianca P and Solari D (2022) Evolution in endoscopic endonasal approach for the management of hypothalamic–pituitary region metastasis: A single-institution experience. Front. Oncol. 12:975738. doi: 10.3389/fonc.2022.975738
Received: 22 June 2022; Accepted: 30 June 2022;
Published: 28 July 2022.
Edited by:
Luca Ricciardi, Sapienza University of Rome, ItalyReviewed by:
Pier Paolo Mattogno, Neurosurgery, Fondazione Policlinico Agostino Gemelli IRCCS, ItalyCopyright © 2022 Baiano, Somma, Franca, Di Costanzo, Scala, Cretella, Esposito, Cavallo, Cappabianca and Solari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cinzia Baiano, YmFpYW5vY2luemlhQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.