
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 September 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.975485
This article is part of the Research Topic Advances in Prognosis and Treatment of Endometrial Cancers View all 12 articles
 Chengyu Shui1†
Chengyu Shui1† Lin Ran1†
Lin Ran1† Yong Tian1*
Yong Tian1* Li Qin1
Li Qin1 Xin Gu1
Xin Gu1 Hui Xu1
Hui Xu1 Cui Hu2,3
Cui Hu2,3 Lin-Lin Zhang2,3
Lin-Lin Zhang2,3 You Xu4
You Xu4 Chen Cheng5
Chen Cheng5 Wu Huan5
Wu Huan5Objective: To compare the long-term survival between laparoscopic surgery and open surgery in patients with apparent early-stage uterine clear cell carcinoma (UCCC).
Patients and methods: 254 patients with apparent early-stage UCCC were reviewed. Comparisons were made between patients who underwent laparoscopic surgery versus those who underwent open surgery. Baseline data, clinicopathological data, and oncological outcomes were analyzed. 5-year disease-free survival (DFS) rate and 5-year overall survival (OS) rate were estimated and compared using the Kaplan-Meier method and the Log-rank test. The Cox proportional hazard regression model was employed to control the confounding factors.
Results: 147 patients underwent laparoscopic surgery, and 107 patients were managed by open surgery. No differences in terms of recurrence rate (laparoscopy versus laparotomy: 10.9% versus 12.9%, P=0.842) and recurrence pattern were observed. For patients who underwent open surgery and patients who underwent laparoscopic surgery, the 5-year DFS rates and 5-year OS rate were 75.8% (95% CI: 65.8%-83.2%) and 69.1% (95% CI: 58.8%-77.4%), 66.0% (95% CI: 57.1%-73.5%) and 60.8% (95% CI: 52.0%-68.5%), respectively. The Cox proportional hazards regression model shown that for apparent early-stage UCCC, the approach of surgical staging was not an independent predictor for survival (laparoscopy versus laparotomy: for DFS, aHR=1.06, 95% CI=0.64-1.75, P=0.826; for OS, aHR=1.10, 95% CI=0.72-1.68, P=0.671).
Conclusion: For apparent early-stage UCCC, in terms of oncological survival, laparoscopic surgery was as safe as open surgery.
Generally, endometrial cancer (EC) can be broadly divided into type I tumors (approximately 80%) and type II tumors (approximately 20%) (1–3). Usually developing among the elderly, Type II EC has a hormone-independent pathogenesis and no identified precursor lesions (1, 3, 4). Including uterine serous carcinoma, uterine clear cell carcinoma (UCCC), and carcinosarcoma, type II EC typically has a worse prognosis when compared with type I EC (1–3). They are often present at advanced stages, have a high rate of extrauterine metastases, and are at high risk of recurrence after initial management (1, 2, 4).
For clinical early-stage EC, the primary management is surgical staging, at least including total hysterectomy, bilateral salpingo-oophorectomy, and the assessment of regional lymph nodes (1, 3, 4). Based on the results of two randomized prospective studies comparing minimally invasive surgery with traditional open surgery, the minimally invasive approach was recommended for early-stage EC by the European Society of Gynaecological Oncology, the European Society for Radiotherapy and Oncology, and the European Society of Pathology (5). Furthermore, pooled results of prospective studies and retrospective observational studies also support the employment of minimally invasive surgery for women with high-risk early-stage EC (including type II EC) (6–10). These studies concluded that when compared with those who were managed with open surgical staging, early-stage EC patients who were treated with minimally invasive surgery experienced similar survival, quicker recovery, and lower risk of perioperative complications (5–7, 9, 10). In 2018, however, two clinical studies reported that for women with early-stage cervical cancer, minimally invasive radical hysterectomy caused lower rates of disease-free survival (DFS) and overall survival (OS) than open radical hysterectomy (11, 12). Since then, the oncological safety of minimally invasive surgery for gynecologic malignancies has once again become a focus of attention in clinical studies.
UCCC accounts for less than 10% of all EC (1, 13, 14). Due to the rarity of UCCC, a large, powerful, and prospectively designed study regarding the management of UCCC is exceedingly difficult (14). Thus, the current data on the clinical practice of UCCC are usually from small and retrospective designed studies (5, 7, 13). In the aforementioned studies comparing minimally invasive surgery with traditional open surgery for high-risk endometrial cancer, the fraction of UCCC was fairly low (6, 7, 9, 10). Thus, the oncological safety of minimally invasive surgery for clinical early-stage UCCC needs further study.
Taken together, based on four Chinese high-volume teaching hospitals, we conducted this study to compare the risk of recurrence and death associated with minimally invasive surgery versus open surgery for clinical early-stage UCCC.
With four Chinese high-volume centers involved, this was a retrospectively designed and multi-institutional cohort study. Due to the retrospective nature and it did not report any identifiable private data, ethical approval and written informed consent for participation were not required for this study in accordance with the local legislation and institutional requirements. This study was conducted following the Declaration of Helsinki (15).
Data of consecutive patients with histologically proven EC who underwent surgical staging at the four Chinese tertiary referral centers (Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, West China Mianzhu Hospital, West China Second University Hospital, and the Second Affiliated Hospital of Chengdu Medical College) between January 1, 2011 and January 1, 2018 were reviewed. Patients were included in this study if they: (1) were between 18 and 75 years old, (2) had pathologically confirmed clear cell carcinoma, (3) had a clinical early-stage disease, (4) underwent comprehensive surgical staging at the participating hospitals, at least including total hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy, and (5) were consecutively followed up at these hospitals. In the current study, the clinical early-stage disease was defined as follows: cancer clinically confined to the uterus, no clinical evidence of bulky lymph nodes, and no clinical evidence of extrauterine macroscopic lesions. After surgical staging, all included cases were staged using the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system for EC.
Patients were excluded from this study if they: (1) were non-surgically managed, (2) underwent neoadjuvant therapies, (3) had a suspected advanced disease, (4) had synchronous cancer(s), (5) had a history of malignancy of the female reproductive system, (6) had a preoperative American Society of Anesthesiologists (ASA) physical status score of larger than III, (7) underwent assessment for regional lymph nodes by sentinel lymph node mapping, or (8) were lost to follow-up.
The collected data regarding clinicopathological characteristics were as follows: year of diagnosis, age at diagnosis, marital status at diagnosis, body mass index (BMI) at diagnosis, the preoperative ASA physical status score, the stage of disease (based on the 2009 FIGO staging system), the grade of tumor differentiation, the size of the primary tumor, whether there was lymphovascular space invasion (LVSI), and the result of peritoneal cytology.
The following data on treatment were collected: the approach of surgical staging (laparoscopy or laparotomy), the scope of regional lymphadenectomy (pelvic lymphadenectomy or combined pelvic and para-aortic lymphadenectomy), the protocol of postoperative adjuvant therapies (chemotherapy, radiation, or chemoradiation).
The following data regarding oncological outcomes were collected: the vital status of the patient, disease recurrence (site and date), date of death, and the cause of death. In this study, all included patients were followed up until death or January 1, 2022.
In the current study, the 5-year DFS rate and the 5-year OS rate were the primary outcomes of interest. DFS was defined as the time between the date of surgical staging for UCCC and the date of documented disease recurrence or death contributed by UCCC. OS was defined as the time from the date of surgical staging for UCCC to the date of documented death caused by any cause.
In this study, the secondary outcomes of interest were the independent predictors for the long-term survival of women with clinical early-stage UCCC.
Statistical analyses were performed using IBM SPSS version 25 (SPSS Inc., Chicago, IL, USA). The Kaplan-Meier survival curves were generated by Stata version 17 (Stata Corp., College Station, TX, USA).
Based on the type of surgical staging approach, the included cases were divided into the laparoscopy group and the laparotomy group. Data on the characteristics of the study cohort were reported using standard descriptive statistics. Comparisons were made between the two groups using the chi-squared test or Fisher exact test for categorical variables and the t-test or the Wilcoxon rank-sum test for continuous variables. The 5-year DFS rate and the 5-year OS rate of the two groups were estimated and compared using the Kaplan-Meier method and the log-rank test. Hazard ratio (HR) and 95% confidence interval (CI) were calculated. The Cox proportional hazard regression model was employed to control the confounding factors. Candidate variables that were with a P value of less than 0.05 on univariate analysis or that were considered clinically relevant were included in the Cox proportional hazard regression model.
In the study, A two-sided P value of less than 0.05 was considered statistically significant.
A total of 7127 women with EC were diagnosed and managed at these four participating hospitals between January 1, 2011 and January 1, 2018. After excluding 6873 patients who were not eligible for this study, a total of 254 women with apparent early-stage UCCC were eventually included in the current study. Among them, 147 patients underwent surgical staging by laparoscopy and were included in the laparoscopy group, the remaining 107 women underwent surgical staging by open approach and were included in the laparotomy group. Figure 1 shows the process of case selection.
For the entire study cohort, the mean age at diagnosis was 65.5 years with a standard deviation of 6.57, and the median duration of follow-up was 52.0 months (range: 4.0-131). Among the entire study cohort, 76 (29.9%) patients were identified with advanced diseases after surgical staging, 122 (48.0%) patients had primary tumors of larger than 4 centimeters, 58 (22.8%) patients were identified with LVSI, 36 (14.2%) patients had positive peritoneal cytology, and only 87 (34.3%) patients did not undergo any form of postoperative adjuvant therapy. In terms of the surgical-pathological stage of the disease, there was no statistical difference between the two groups (P=0.158).
Table 1 shows the comparisons of the characteristics of the two groups. Generally, there was good comparability between the laparoscopy group and the laparotomy group in terms of the baseline characteristics, the clinicopathologic data, and the treatment-related variables.
By January 1, 2022, 29 recurrences of UCCC were identified, and the rate of recurrence was 11.4% among the entire study cohort.
13 of the 107 patients (12.1%) in the laparotomy group had disease recurrence, and 16 cases of UCCC recurrence (10.9%) were identified in the laparoscopy group. In terms of the rate of disease recurrence, there was no statistical difference observed between the two groups (P=0.842). As for the patterns of disease recurrence, the most four common sites of recurrence were the abdomen (2.8%), the pelvis (2.4%), the lung (2.4%), and the vagina (1.6%). Also, there was no statistical difference observed between the two groups in terms of the patterns of disease recurrence. Table 2 presents the rates and the patterns of recurrence by laparoscopic surgery versus laparotomy.
For the patients who underwent surgical staging by open surgery and the patients who underwent laparoscopic surgery, the 5-year DFS rates by the Kaplan-Meier method were 75.8% (95% CI: 65.8%-83.2%) and 66.0% (95% CI: 57.1%-73.5%), respectively. For patients of apparent early-stage UCCC, surgical staging by laparoscopy was not associated with worse DFS when compared with traditional laparotomy (HR: 1.34, 95% CI: 0.85-2.11, P=0.213).
For the laparotomy group, the 5-year OS rate by the Kaplan-Meier method was 69.1% (95% CI: 58.8%-77.4%). Similarly, the 5-year OS rate for patients in the laparoscopy group was 60.8% (95% CI: 52.0%-68.5%). The comparison made by the Log-rank test indicated that for women with clinical early-stage UCCC, compared with open surgery, surgical staging by laparoscopy did not increase the risk of all-cause death (HR: 1.19, 95% CI: 0.81-1.76, P=0.372).
Figure 2 shows the Kaplan-Meier survival curves of the study cohort by laparoscopy versus laparotomy, Figure 2A for disease-free survival and Figure 2B for overall survival.
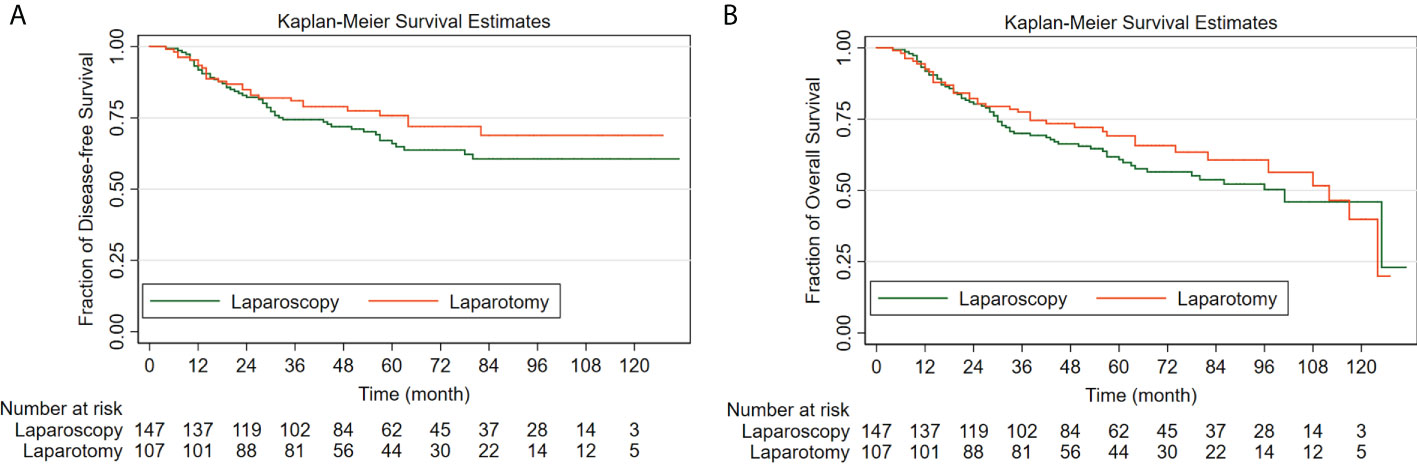
Figure 2 The Kaplan-Meier survival curves of the study cohort by laparoscopy versus laparotomy (A) for disease-free survival; (B) for overall survival.
Using the log-rank test, we found that for women with apparent early-stage UCCC, age at diagnosis (≥ 65 years versus < 65 years: for DFS, HR=1.66, 95% CI=1.07-2.77, P=0.031; for OS, HR=1.84, 95% CI=1.18-2.88, P=0.007), BMI at diagnosis (≥ 24 kg/m2 versus < 24 kg/m2: for DFS, HR=1.52, 95% CI=1.17-2.29, P=0.019; for OS, HR=1.46, 95% CI=1.09-2.14, P=0.037), the preoperative ASA physical status score (III versus I/II: for DFS, HR=3.51, 95% CI=2.14-4.99, P=0.000; for OS, HR=3.25, 95% CI=1.89-5.28, P=0.000), the 2009 FIGO stage of the disease (III/IV versus I/II: for DFS, HR=6.34, 95% CI=4.43-8.30, P=0.000; for OS, HR=5.95, 95% CI=3.38-10.01, P=0.007), the tumor size (≥ 4 cm versus < 4 cm: for DFS, HR=2.05, 95% CI=1.29-3.24, P=0.002; for OS, HR=1.80, 95% CI=1.22-2.64, P=0.003), LVSI (Yes versus No: for DFS, HR=1.54, 95% CI=1.04-2.70, P=0.013; for OS, HR=1.43, 95% CI=1.17-2.36, P=0.015), and postoperative adjuvant therapy (chemotherapy/radiotherapy versus No: for DFS, HR=0.68, 95% CI=0.27-0.89, P=0.007; for OS, HR=0.62, 95% CI=0.23-0.92, P=0.008; combined chemotherapy and radiotherapy versus No: for DFS, HR=0.45, 95% CI=0.29-0.87, P=0.004; for OS, HR=0.37, 95% CI=0.25-0.73, P=0.000) were associated with the prognosis.
Table 3 shows the results of the univariate analyses.
Variables that have potential clinical relevance or that showed a univariate relationship (P < 0.05) with survival were included in the multivariate Cox proportional hazards regression model, they were as follows: age at diagnosis, BMI at diagnosis, the preoperative ASA physical status score, the 2009 FIGO stage of the disease, the tumor size, the status of LVSI, the approach of surgical staging, and postoperative adjuvant therapy.
The Cox proportional hazards regression model showed that for apparent early-stage UCCC, the approach of surgical staging was not an independent predictor for long-term survival (laparoscopy versus laparotomy: for DFS, aHR=1.06, 95% CI=0.64-1.75, P=0.826; for OS, aHR=1.10, 95% CI=0.72-1.68, P=0.671).
The Cox proportional hazards regression model also showed that for apparent early-stage UCCC, age at diagnosis (≥ 65 years versus < 65 years: for DFS, aHR=1.51, 95% CI=1.07-2.24, P=0.003; for OS, aHR=1.39, 95% CI=1.14-2.51, P=0.026), the preoperative ASA physical status score (III versus I/II: for DFS, aHR=1.98, 95% CI=1.14-3.27, P=0.021; for OS, aHR=2.02, 95% CI=1.10-3.09, P=0.016), the 2009 FIGO stage of the disease (III/IV versus I/II: for DFS, aHR=6.98, 95% CI=3.57-13.12, P=0.000; for OS, aHR=6.76, 95% CI=2.49-10.68, P=0.000), LVSI (Yes versus No: for DFS, aHR=2.14, 95% CI=1.11-2.57, P=0.010; for OS, aHR=2.09, 95% CI=1.27-2.92, P=0.001), and postoperative adjuvant therapy (chemotherapy/radiotherapy versus No: for DFS, aHR=0.64, 95% CI=0.28-0.97, P=0.012; for OS, aHR=0.67, 95% CI=0.32-0.89, P=0.033; combined chemotherapy and radiotherapy versus No: for DFS, aHR=0.49, 95% CI=0.23-0.78, P=0.018; for OS, aHR=0.55, 95% CI=0.27-0.90, P=0.025) were independently associated with the survival.
Table 4 shows the results of the multivariate Cox proportional hazards regression analyses.
By reviewing the data of 254 patients from four Chinese high-volume centers, the current study showed that for apparent early-stage UCCC, when compared with patients who underwent open surgical staging, patients who underwent surgical staging by laparoscopy experienced similar oncological outcomes.
The research topic on the employment of minimally invasive surgery among women with EC is not new. The Gynecologic Oncology Group (GOG) LAP2 study was a prospective randomized controlled clinical study with the purpose to study the feasibility and safety of minimally invasive surgery for clinical early-stage uterine cancer (16, 17). With 2616 patients included, the GOG LAP2 study preliminarily concluded that laparoscopic surgery for clinical early-stage EC was feasible and safe in terms of short-term outcomes and resulted in a lower risk of perioperative complications (16). In 2012, the GOG LAP2 reported its findings regarding oncological outcomes (17). It reported that the 3-year recurrence rates among patients who underwent laparoscopy and patients who underwent open surgery were 11.4% and 10.2%, respectively (17). The difference in recurrence rate by laparoscopy versus laparotomy was 1.14% (90% lower bound, -1.28; 95% upper bound, 4.0) (17). The Laparoscopic Approach to Cancer of the Endometrium (LACE) study, a multinational randomized equivalence study, also reported that for clinical early-stage uterine cancer, the employment of total laparoscopic hysterectomy compared with total open abdominal hysterectomy resulted in equivalent 4.5-year DFS rate (open surgery versus laparoscopic surgery: 81.6% versus 81.0%) and no difference in 4.5-year OS rate (open surgery versus laparoscopic surgery: 92.4% versus 92.0%) (18). Based on the evidence from the GOG LAP2 study, the LACE study, and other studies regarding this topic, minimally invasive surgery is recommended by many clinical practice guidelines as the preferred surgical approach for early-stage EC (5, 19–23).
However, one should note that in the aforementioned studies, the proportion of type II EC (including UCCC) was fairly low (16–18). Due to the rarity, prospectively designed clinical study regarding type II EC is difficult. So far, some retrospectively designed studies about the oncological safety of minimally invasive surgery for type II EC have been published. Including 295 patients from four Chinese teaching hospitals, the study conducted by Xu et al. found that for apparent early-stage uterine serous carcinoma, the approach of surgical staging was not an independent prognostic factor for oncological outcomes (laparoscopy versus open surgery: for DFS, aHR=1.16, 95% CI=0.63-2.12, P=0.636; for OS, aHR=1.11, 95% CI=0.52-2.38, P=0.794) (24). Comparing DFS between minimally invasive surgery and laparotomic surgery in patients with high-risk EC, the study conducted by Segarra-Vidal et al. included 626 patients (25). Among them, 468 women had type II EC (25). They found that there was no difference in 5-year DFS rate between the open surgery group (53.4%, 95% CI: 45.6%-60.5%) and the laparoscopy group (54.6%, 95% CI: 46.6%-61.8%) (25). They concluded that minimally invasive surgery was not associated with the deterioration of survival among patients with high-risk EC (25). Furthermore, the subgroup analysis showed that the employment of a uterine manipulator during laparoscopy surgery did not worsen the DFS (HR=1.01, 95% CI=0.65-1.58, P=0.960), the OS (HR=1.18, 95% CI=0.71-1.96, P=0.530), and the recurrence rate (HR=1.12, 95% CI=0.67-1.87, P=0.660) among patients with high-risk EC (25). To compare surgical and survival outcomes in patients with early-stage uterine carcinosarcoma managed by laparotomic surgery versus minimally invasive surgery, the study conducted by Corrado et al. included 170 patients and concluded that for women with early-stage uterine carcinosarcoma, there was no difference of oncologic outcome between the two approaches (26). The findings of our study were consistent with that of the aforementioned studies.
Our study also found that some of the classic risk factors that can be applied to predict the prognosis of type I EC were also useful for apparent early-stage UCCC. These risk factors were as follows: age at diagnosis, the preoperative ASA physical status score, the stage of cancer, and the status of LVSI (1, 3, 4, 27–31). However, unlike for low-risk early-stage type I EC, postoperative adjuvant therapy was beneficial to apparent early-stage UCCC (32–35). This was because when compared with patients of clinical early-stage type I EC, patients with clinical early-stage type II EC are at higher risk of extrauterine metastases and disease recurrence (1–4). In our study, nearly one-third of clinical early-stage UCCC patients were eventually confirmed to have extrauterine metastases after surgery. Among them, the most common site of extrauterine metastases was regional lymph nodes. These findings were consistent with that of previously published studies (34–37). The postoperative adjuvant therapy can reduce the risk of disease recurrence among patients of high-risk EC (including UCCC) (33–35).
Our study included 254 patients with apparent early-stage UCCC, this was a large sample in consideration of the rarity of UCCC. Also, almost all included patients in our study underwent guidelines-based management and a long-term follow-up, this can reduce the effect of confounding factors (such as protocol of treatment) on patients’ prognosis as much as possible and enable us to identify the outcomes of interest. However, this study still suffers from some limitations. First, due to the retrospective nature of the study design, this study was at risk of inevitable biases, such as information bias, selection bias, et al. To reduce the possibility of these biases as much as possible, we pre-set inclusion and exclusion criteria and strictly followed them to screen eligible patients, and excluded those cases that lack relevant data. Second, because robotic-assisted minimally invasive surgery for EC is relatively new in these participating institutions, our study failed to explore its impact on oncological outcomes of apparent early-stage UCCC. However, according to the findings of the study conducted by Segarra-Vidal et al, the robotic-assisted minimally invasive surgery was oncological safe as open surgery for type II EC (25). Third, because of the limited resources, the pathological diagnoses of UCCC were not reviewed again by experts in pathology. The last, some variables of clinical significance, such as the protocol and the number of cycles of postoperative adjuvant therapy, comorbidities, etc. were not included in the statistical analysis, mainly due to the difficulty in obtaining these data. This was potentially representing a bias in our analysis.
In summary, there was no difference in recurrence rate, recurrence pattern, DFS, and the risk of all-cause death when comparing laparoscopic surgery and open surgical staging among women with apparent early-stage UCCC. Although our study has some limitations, the findings of our study support the assertion that surgical staging by laparoscopy did not compromise the survival of women with apparent early-stage UCCC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conceptualization: CS, LR, and YT. Methodology: LQ, XG, Hui Xu, CH, L-LZ, and YT. Data collection: all authors. Project administration: CS, LR, and YT. Supervision: YT. Writing - original draft: CS, LR, and YT. Writing - review and editing: all authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med (2020) 383(21):2053–64. doi: 10.1056/NEJMra1514010
2. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol (1983) 15(1):10–7. doi: 10.1016/0090-8258(83)90111-7
3. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet (London England) (2022) 399(10333):1412–28. doi: 10.1016/S0140-6736(22)00323-3
4. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet (London England) (2016) 387(10023):1094–108. doi: 10.1016/S0140-6736(15)00130-0
5. Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J gynecol Cancer Off J Int Gynecol Cancer Soc (2021) 31(1):12–39. doi: 10.1136/ijgc-2020-002230
6. Vardar MA, Gulec UK, Guzel AB, Gumurdulu D, Khatib G, Seydaoglu G. Laparoscopic surgery for low, intermediate and high-risk endometrial cancer. J gynecol Oncol (2019) 30(2):e24. doi: 10.3802/jgo.2019.30.e24
7. Favero G, Anton C, Le X, Silva ESA, Dogan NU, Pfiffer T, et al. Oncologic safety of laparoscopy in the surgical treatment of type II endometrial cancer. Int J gynecol Cancer Off J Int Gynecol Cancer Soc (2016) 26(9):1673–8. doi: 10.1097/IGC.0000000000000803
8. Bennich G, Rudnicki M, Lassen PD. Laparoscopic surgery for early endometrial cancer. Acta Obstet Gynecol Scand (2016) 95(8):894–900. doi: 10.1111/aogs.12908
9. Monterossi G, Ghezzi F, Vizza E, Zannoni GF, Uccella S, Corrado G, et al. Minimally invasive approach in type II endometrial cancer: Is it wise and safe? J Minim Invasive Gynecol (2017) 24(3):438–45. doi: 10.1016/j.jmig.2016.12.022
10. Koskas M, Jozwiak M, Fournier M, Vergote I, Trum H, Lok C, et al. Long-term oncological safety of minimally invasive surgery in high-risk endometrial cancer. Eur J Cancer (Oxford Engl 1990) (2016) 65:185–91. doi: 10.1016/j.ejca.2016.07.001
11. Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med (2018) 379(20):1905–14. doi: 10.1056/NEJMoa1804923
12. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med (2018) 379(20):1895–904. doi: 10.1056/NEJMoa1806395
13. Abeler VM, Kjørstad KE. Clear cell carcinoma of the endometrium: A histopathological and clinical study of 97 cases. Gynecol Oncol (1991) 40(3):207–17. doi: 10.1016/0090-8258(90)90279-t
14. Olawaiye AB, Leath CA 3rd. Contemporary management of uterine clear cell carcinoma: A society of gynecologic oncology (SGO) review and recommendation. Gynecol Oncol (2019) 155(2):365–73. doi: 10.1016/j.ygyno.2019.08.031
15. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA (2013) 310(20):2191–4. doi: 10.1001/jama.2013.281053
16. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic oncology group study LAP2. J Clin Oncol Off J Am Soc Clin Oncol (2009) 27(32):5331–6. doi: 10.1200/JCO.2009.22.3248
17. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic oncology group LAP2 study. J Clin Oncol Off J Am Soc Clin Oncol (2012) 30(7):695–700. doi: 10.1200/JCO.2011.38.8645
18. Janda M, Gebski V, Davies LC, Forder P, Brand A, Hogg R, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: A randomized clinical trial. Jama (2017) 317(12):1224–33. doi: 10.1001/jama.2017.2068
19. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN (2018) 16(2):170–99. doi: 10.6004/jnccn.2018.0006
20. Abu-Rustum NR, Yashar CM, Bradley K, Campos SM, Chino J, Chon HS, et al. NCCN guidelines® insights: Uterine neoplasms, version 3.2021. J Natl Compr Cancer Netw JNCCN (2021) 19(8):888–95. doi: 10.6004/jnccn.2021.0038
21. Santaballa A, Matías-Guiu X, Redondo A, Carballo N, Gil M, Gómez C, et al. SEOM clinical guidelines for endometrial cancer (2017). Clin Trans Oncol Off Publ Fed Spa Oncol Soc Natl Cancer Inst Mex (2018) 20(1):29–37. doi: 10.1007/s12094-017-1809-9
22. Ribeiro R, Fontes Cintra G, Barrozo A, Tieko Tsunoda A, Pupo Nogueira A, Andreazza Laporte G, et al. Brazilian Society of surgical oncology guidelines for surgical treatment of endometrial cancer in regions with limited resources. J Surg Oncol (2020) 121(5):730–42. doi: 10.1002/jso.25797
23. Barretina-Ginesta MP, Quindós M, Alarcón JD, Esteban C, Gaba L, Gómez C, et al. SEOM-GEICO clinical guidelines on endometrial cancer (2021). Clin Trans Oncol Off Publ Fed Spa Oncol Soc Natl Cancer Inst Mex (2022) 24(4):625–34. doi: 10.1007/s12094-022-02799-7
24. Xu Y, Shen J, Zhang Q, He Y, Chen C, Tian Y. Oncologic safety of laparoscopic surgery for women with apparent early-stage uterine serous carcinoma: A multi-institutional retrospective cohort study. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet (2022) 158(1):162–71. doi: 10.1002/ijgo.13942
25. Segarra-Vidal B, Dinoi G, Zorrilla-Vaca A, Mariani A, Student V, Garcia NA, et al. Minimally invasive compared with open hysterectomy in high-risk endometrial cancer. Obstet Gynecol (2021) 138(6):828–37. doi: 10.1097/AOG.0000000000004606
26. Corrado G, Ciccarone F, Cosentino F, Legge F, Rosati A, Arcieri M, et al. Role of minimally invasive surgery versus open approach in patients with early-stage uterine carcinosarcomas: a retrospective multicentric study. J Cancer Res Clin Oncol (2021) 147(3):845–52. doi: 10.1007/s00432-020-03372-x
27. Koual M, Ngo C, Girault A, Lécuru F, Bats AS. Endometrial cancer in the elderly: does age influence surgical treatments, outcomes, and prognosis? Menopause (New York NY) (2018) 25(9):968–76. doi: 10.1097/GME.0000000000001119
28. Hag-Yahia N, Gemer O, Eitan R, Raban O, Vaknin Z, Levy T, et al. Age is an independent predictor of outcome in endometrial cancer patients: An Israeli gynecology oncology group cohort study. Acta Obstet Gynecol Scand (2021) 100(3):444–52. doi: 10.1111/aogs.14015
29. Bosse T, Peters EE, Creutzberg CL, Jürgenliemk-Schulz IM, Jobsen JJ, Mens JW, et al. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer–a pooled analysis of PORTEC 1 and 2 trials. Eur J Cancer (Oxford Engl 1990) (2015) 51(13):1742–50. doi: 10.1016/j.ejca.2015.05.015
30. Ørtoft G, Lausten-Thomsen L, Høgdall C, Hansen ES, Dueholm M. Lymph-vascular space invasion (LVSI) as a strong and independent predictor for non-locoregional recurrences in endometrial cancer: a Danish gynecological cancer group study. J Gynecol Oncol (2019) 30(5):e84. doi: 10.3802/jgo.2019.30.e84
31. Tortorella L, Restaino S, Zannoni GF, Vizzielli G, Chiantera V, Cappuccio S, et al. Substantial lymph-vascular space invasion (LVSI) as predictor of distant relapse and poor prognosis in low-risk early-stage endometrial cancer. J Gynecol Oncol (2021) 32(2):e11. doi: 10.3802/jgo.2021.32.e11
32. Deleon MC, Ammakkanavar NR, Matei D. Adjuvant therapy for endometrial cancer. J Gynecol Oncol (2014) 25(2):136–47. doi: 10.3802/jgo.2014.25.2.136
33. Michalak M, Warenczak Florczak Z, Staszewska-Nowak A, Roszak A. Adjuvant therapy for early endometrial cancer - who benefits the most from a radiation therapy? Ginekologia Polska (2020) 91(1):6–12. doi: 10.5603/GP.2020.0003
34. Xu KM, Gill BS, Balasubramani GK, Sukumvanich P, Kelley JL, Beriwal S. Utilization and role of adjuvant radiotherapy and chemotherapy for uterine clear cell carcinoma: A national cancer data base analysis. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc (2016) 26(3):472–82. doi: 10.1097/IGC.0000000000000640
35. Hong JC, Foote J, Broadwater G, Gaillard S, Havrilesky LJ, Chino JP. Impact of chemotherapy and radiotherapy on management of early stage clear cell and papillary serous carcinoma of the uterus. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc (2017) 27(4):720–9. doi: 10.1097/IGC.0000000000000926
36. Sarı ME, Meydanlı MM, Türkmen O, Cömert GK, Turan AT, Karalök A, et al. Prognostic factors and treatment outcomes in surgically-staged non-invasive uterine clear cell carcinoma: a Turkish gynecologic oncology group study. J Gynecol Oncol (2017) 28(4):e49. doi: 10.3802/jgo.2017.28.e49
Keywords: uterine clear cell carcinoma, laparoscopy, surgical staging, overall survival, disease-free survival
Citation: Shui C, Ran L, Tian Y, Qin L, Gu X, Xu H, Hu C, Zhang L-L, Xu Y, Cheng C and Huan W (2022) Survival after laparoscopy versus laparotomy for apparent early-stage uterine clear cell carcinoma: Results of a large multicenter cohort study. Front. Oncol. 12:975485. doi: 10.3389/fonc.2022.975485
Received: 22 June 2022; Accepted: 16 August 2022;
Published: 05 September 2022.
Edited by:
Shannon Neville Westin, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Martina Arcieri, University of Messina, ItalyCopyright © 2022 Shui, Ran, Tian, Qin, Gu, Xu, Hu, Zhang, Xu, Cheng and Huan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Tian, dGlhbnlvbmdfb2JneW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.