- 1Key Laboratory of Cancer Prevention and Therapy, National Clinical Research Center for Cancer, Tianjin’s Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
- 2Graduate School of Tianjin Medical University, Tianjin, China
Objective: To evaluate the quality of clinical practice guidelines (CPGs) for nutrition management of patients with head and neck cancer (HNC) during peri-radiotherapy, as well as to summarize the nutrition recommendations fitting the subject.
Methods: CPGs published in English, Chinese and German were identified from databases, guideline networks, and websites of nutritional associations from the databases’ inception to March 8, 2022. Three independent appraisers used the Appraisal of Guidelines for Research and Evaluation II (AGREE II) Instrument to assess the quality of CPGs. The intraclass correlation coefficient (ICC) was used to calculate appraiser agreement.
Results: 769 records were identified. After removing duplicates, 470 articles were screened. 12 CPGs were identified with nutrition-specific recommendations. 67% of CPGs were rated as high quality, and 33% as low quality. Recommendations were categorized into nutritional risk screening, nutrition assessment, nutrition counseling, nutrition interventions, nutrition intake, swallowing function management, weight management, exercise, multidisciplinary team, post-discharge care, nutrients, and pharmacologic interventions.
Conclusion: We found discrepant recommendations in existing CPGs, including nutrition screening, nutrition assessment, nutrition intake, and nutrients. We also reported the absence of essential parts of CPGs, including the views of its target users, the statement of external review, the method to formulate the recommendations, strategies to improve uptake, and resource implications of applying the CPGs. CPGs with low quality should be improved in future updates based on currently available guideline development tools. Specialized CPGs on nutrition management for HNC patients during peri-radiotherapy should be developed.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/index.php, identifier CRD42022320322.
Introduction
Head and neck cancers (HNCs) arise from major anatomical sites: the oral cavity, paranasal sinuses, sinonasal cavity, pharynx, salivary glands, and larynx (1, 2). HNC was the seventh most common cancer worldwide in 2020 (930,000 new cases and 470,000 deaths) (3, 4). Radiotherapy (RT) has an integral role for HNC patients. In the primary treatment setting of HNC, RT can provide similar results to surgical treatment for specific early-stage HNCs. For most locally advanced HNCs, RT is a fundamental component of comprehensive therapy (5).
Malnutrition is a common complication of cancer and may reduce therapeutic effects. About two million cancer patients worldwide die yearly due to severe malnutrition, accounting for 30% of cancer patients (6). It is well known that HNC patients are frequently malnourished before starting treatment (7). RT may cause side effects, such as xerostomia, mucositis, nausea and vomiting, alteration or loss of taste, and consequent worsening malnutrition (8). A study by Abu et al. (9) reported that the mean weight loss of HNC patients was 7.4% during RT treatment and 2.1% post-treatment. Furthermore, deterioration of the nutritional status leads to an increase in RT-related toxicity, may decrease the responses to RT, and prolong treatment duration (8).
Healthcare professionals are responsible for providing patients with safe and high-quality treatment and care based on the best existing evidence (10). Guidelines promote high-quality cancer care. Clinical practice guidelines (CPGs) are formulated according to specific procedures, and high-quality CPGs serve as evidence-based resources to provide healthcare professionals with better decisions in clinical circumstances (11, 12). Many CPGs related to cancer nutrition management have been published, whereas those may vary dramatically in quality. Moreover, inconsistency across CPGs may dilute high-quality recommendations and increase confusion in clinical practice (13, 14). The Appraisal of Guidelines for Research and Evaluation II (AGREE II) was developed to evaluate the quality of reporting and the practice guideline development (15). The AGREE II Instrument has been widely used and validated since it was updated in 2010. Ng et al. (16) assessed the quality of HNC guidelines for complementary and alternative medicine recommendations and found that quality varied among the guidelines. Zhou et al. (17) evaluated the quality of CPGs of the nutritional risk for cancer patients, the majority of CPGs were rated as ‘strongly recommended’ or ‘recommended with modifications’.
Regarding our search, there has been no systematic review of CPGs on nutrition management for HNC population during peri-radiotherapy. This review appraised the quality of the relevant CPGs using the AGREE II Instrument and extracted nutrition recommendations for the target population to provide information for establishing nutritional care practice standards and developing or updating CPGs.
Materials and methods
Design
This review sought to identify nutrition management CPGs for HNC population using standard methods (18) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (19, 20). A protocol was registered with PROSPERO (CRD42022320322, May 7, 2022). Eligible CPGs were assessed with the AGREE II Instrument, which includes six domains: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence (21). Each item in six domains and the overall assessment item in the AGREE II Instrument are rated on a 7-point scale (1-strongly disagree to 7-strongly agree). The user’s manual states that each domain score is independent and should not be added to a single quality score (21). As no specific patient data was involved, ethics approval from the institutional review board was not applicable.
Search strategies
A comprehensive search was conducted from databases’ inception to March 8, 2022 by two independent reviewers (YK and XW) in the following data sources: (a) eight electronic databases, including PubMed, Web of Science, Excerpta Medica dataBASE (EMBASE), Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang Database China, Biomedical Literature Service System (SinoMed) and Weipu Information Chinese Periodical Service Platform (VIP); (b) eleven guideline databases, including National institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network (SIGN), National Comprehensive Cancer Network (NCCN), National Guideline Clearinghouse (NGC), Guideline International Network (GIN), New Zealand Guidelines Group (NZGG), Queensland Clinical Guidelines (QCG), Australian Government National Health and Medical Research Council (NHMRC), Association of the Scientific Medical Societies in Germany (AWMF), Medlive and MedSci; and (c) three professional nutrition society websites, including European Society for Clinical Nutrition and Metabolism (ESPEN), America Society for Parenteral and Enteral Nutrition (ASPEN) and Chinese Society of Parenteral and Enteral Nutrition (CSPEN).
We combined subject terms, for instance, MeSH terms, with entry terms for searches in four databases: PubMed, Cochrane Library, EMBASE, and SinoMed. In the remaining databases, free text terms (limited to title, abstract, or keywords) were used. Keywords included ‘nutrition*/diet*/malnutrition/sarcopenia/anorexia/cachexia’, ‘head and neck cancer*/neoplasm*/carcinoma*/tumor*’, ‘radiotherapy/radiation therapy/chemoradiotherapy’ and ‘guideline*/CPG*/clinical practice guideline*’.
All articles available as full-text versions were considered, and if the full-text or supplemental material was not available, an inquiry was sent to the author/guideline panel. The search strategy for each electronic database has been included in Supplementary Material 1. The literature search was repeated on May 1, 2022, and yielded no new CPGs eligible for this study.
Selection criteria
Inclusion criteria included (a) the eligible CPGs published in English, Chinese or German; (b) CPGs covering recommendations for nutrition management for HNC patients; (c) healthcare-professional-used CPGs; and (d) CPGs in the latest versions.
Exclusion criteria included (a) quick reference CPGs or abridged versions of the CPGs published alongside the CPGs; (b) publications in the form of statements, protocols, abstracts, conference proceedings, letters, editorials commentaries, or CPG interpretations; (c) consensus-based CPGs; (d) translated versions of original CPGs included before in this review; and (e) CPGs specific to one country or region.
Literature screening and data extraction
All records were imported to EndNote X9, and duplicates were removed automatically and by manual checking. The titles and abstracts of the outputs were screened independently by two reviewers (YK and XW) to select the potential CPGs. Then, the full text of each potential CPG was further assessed for eligibility. The reviewers also screened the reference lists of eligible CPGs to identify further relevant CPGs. All eligible CPGs were finally included after discussions between the reviewers.
Two reviewers (YK and XW) extracted the following information: author, year of publication or update, country or region, major development organization (academic institutions, government agencies, disease-specific foundations, or professional associations or societies), CPG topic, target group, grading system and whether there were nutritional experts in the CPG development panel.
Guideline quality assessment
All documents related to the CPGs (full CPG document, appendices, supplementary material, and journal publications) were collected for analysis. Three appraisers (YK, XW, and GW) assessed CPGs independently with the AGREE II Instrument. Before the assessment, a meeting was held to discuss the appraisal criteria according to the AGREE II manual and training tools. When the difference in score of a single item > 2 existed among the appraisers, discussions were conducted to agree until the difference achieved 2 or less.
Domain scores were calculated by summing up all the scores of included individual items and by scaling the total as a percentage of the maximum possible score for that domain. The scaled domain score (ranges from 0% to 100%) was calculated via the following formula: (obtained score – minimum possible score)/(maximum possible score − minimum possible score) × 100%. Domains were then compared based on these percentages. Higher scaled domain scores related to a higher quality of the guidelines.
The AGREE II Instrument does not offer the cut-off scores to determine quality of domains or whole of the guideline, so the decision on the quality level of CPGs was made by the appraisers’ evaluation of the scaled domain scores of the six domains. To determine the overall quality, the appraisers rated CPGs as high quality when five or more domains scored above 60%; average quality represented three to four domains scored above 60%; and two or fewer domains scored above 60% were considered low quality, according to previous literature (14, 22). In this study, the average score of 23 items indicates whether it is supposed to be recommended for clinical use. The average score above 6 suggests ‘yes’, with recommending the CPG for use; the score ranging from 4 to 6 presents ‘yes, with modifications’; and the score below 4 means ‘no’ for recommendation.
The Excel 2019 software was used to manage the data of the CPGs. The inter-rater agreement was presented by the intraclass correlation coefficient (ICC) via IBM SPSS 26.0 software. An ICC > 0.75 indicates satisfactory consistency, 0.5 ≤ ICC ≤ 0.75 is considered as generally acceptable consistency, and ICC < 0.5 suggests unsatisfactory consistency.
Recommendation extraction
Only the CPGs rated as high-quality or average-quality with the AGREE II Instrument were submitted to the recommendation extraction process by two reviewers (YK and XW). The chapters regarding nutrition management in CPGs were first identified and then screened for specific recommendations. The nutrition management spectrum included nutritional risk screening, nutrition assessment, nutrition interventions, nutrition intake, nutrition status, nutrients, dysphagia, enteral nutrition, parenteral nutrition, nutritional supplementary, feeding tube, oral nutrition, exercise, weight management, multidisciplinary team, follow-up, post-discharge care and so on.
Results
Literature search
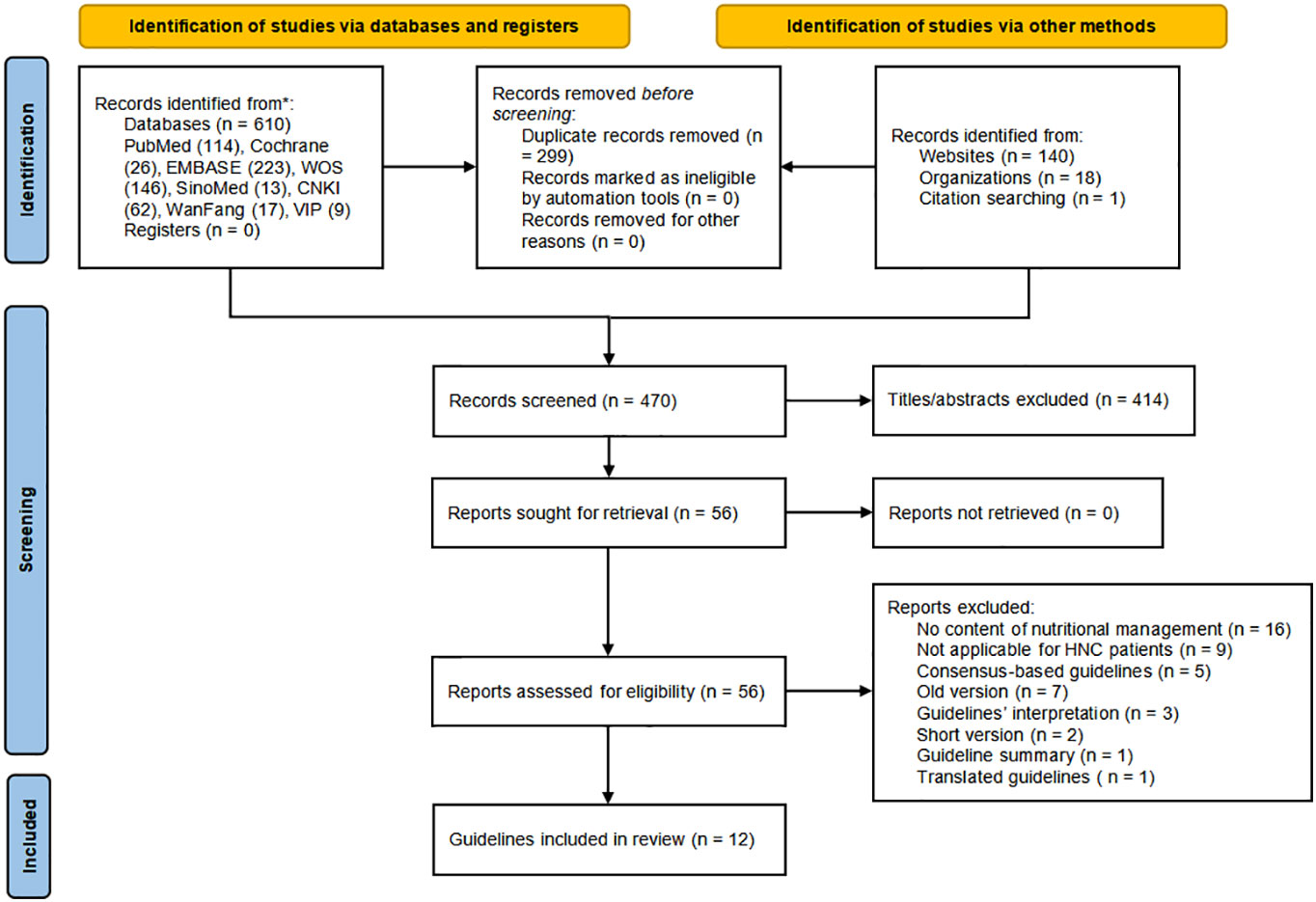
The PRISMA flowchart presents the literature retrieval and selection process in Figure 1. Supplemental Material 1 provides the database search findings which yielded 769 records, including 610 from electronic databases and 159 from relevant websites. After manual and software automatic duplicates removal, 470 articles were identified. The remaining articles were scrutinized in the titles and abstracts for covering nutrition recommendations for HNC patients, 414 articles were further excluded. Then 56 guidelines were sought for retrieval and assessed for eligibility, of which 12 CPGs (23–34) were finally included in the assessment.
Characteristics of included CPGs
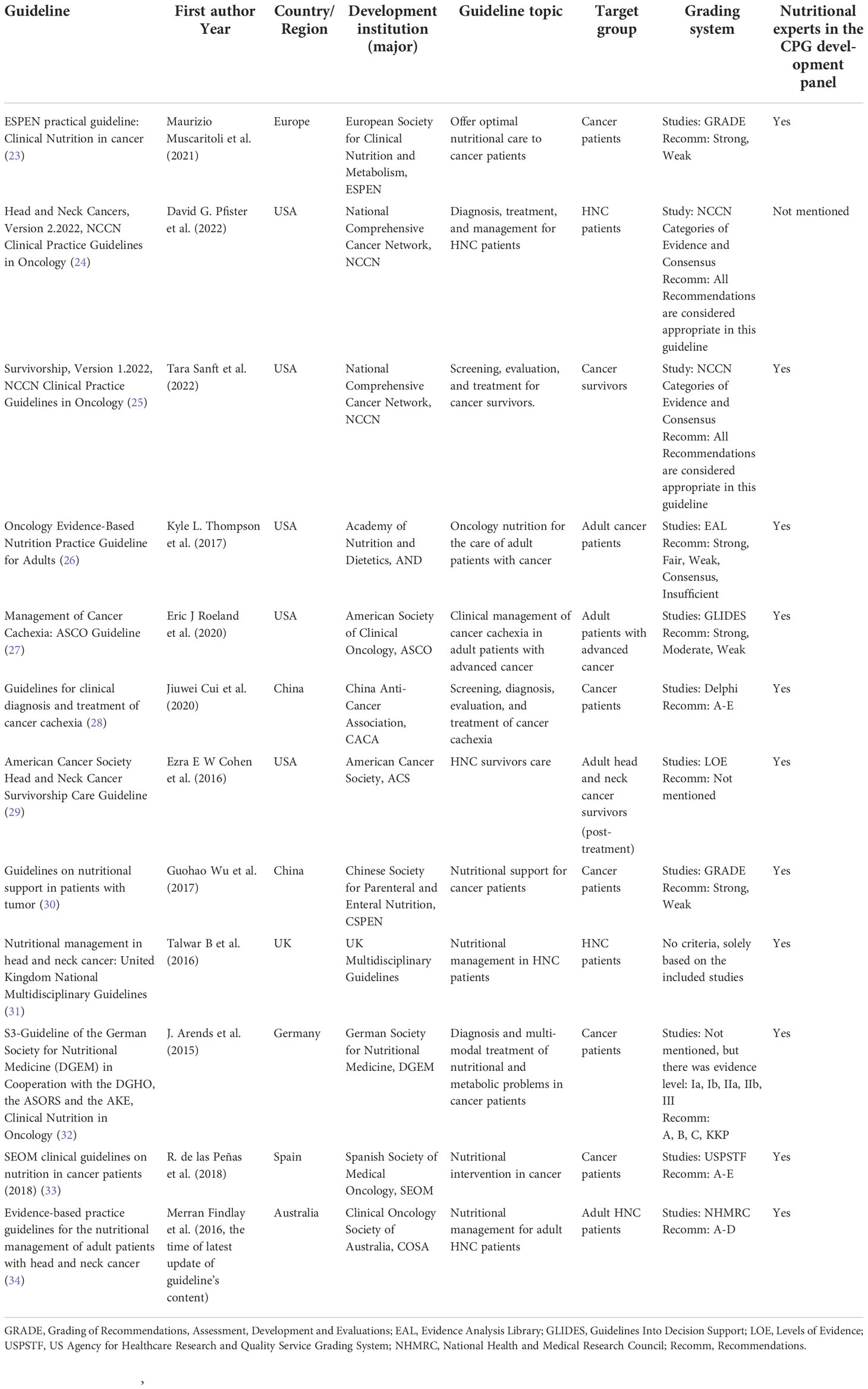
Eligible CPGs were published or updated from 2015 to 2022 (50% were in the last five years) in the Europe (n = 1), UK (n = 1), US (n = 5), Spain (n = 1), Australia (n = 1), China (n = 2) and Germany (n = 1). Four CPGs (24, 29, 31, 34) were developed for HNC patients, and eight CPGs (23, 25–28, 30, 32, 33) for cancer patients partly dedicated to nutrition management for HNC patients. The nutritional funding sources were found in four CPGs (23, 26, 32, 34). All CPGs included nutrition experts as part of the development panel except one (24). Table 1 shows the characteristics across the included CPGs.
Quality assessment
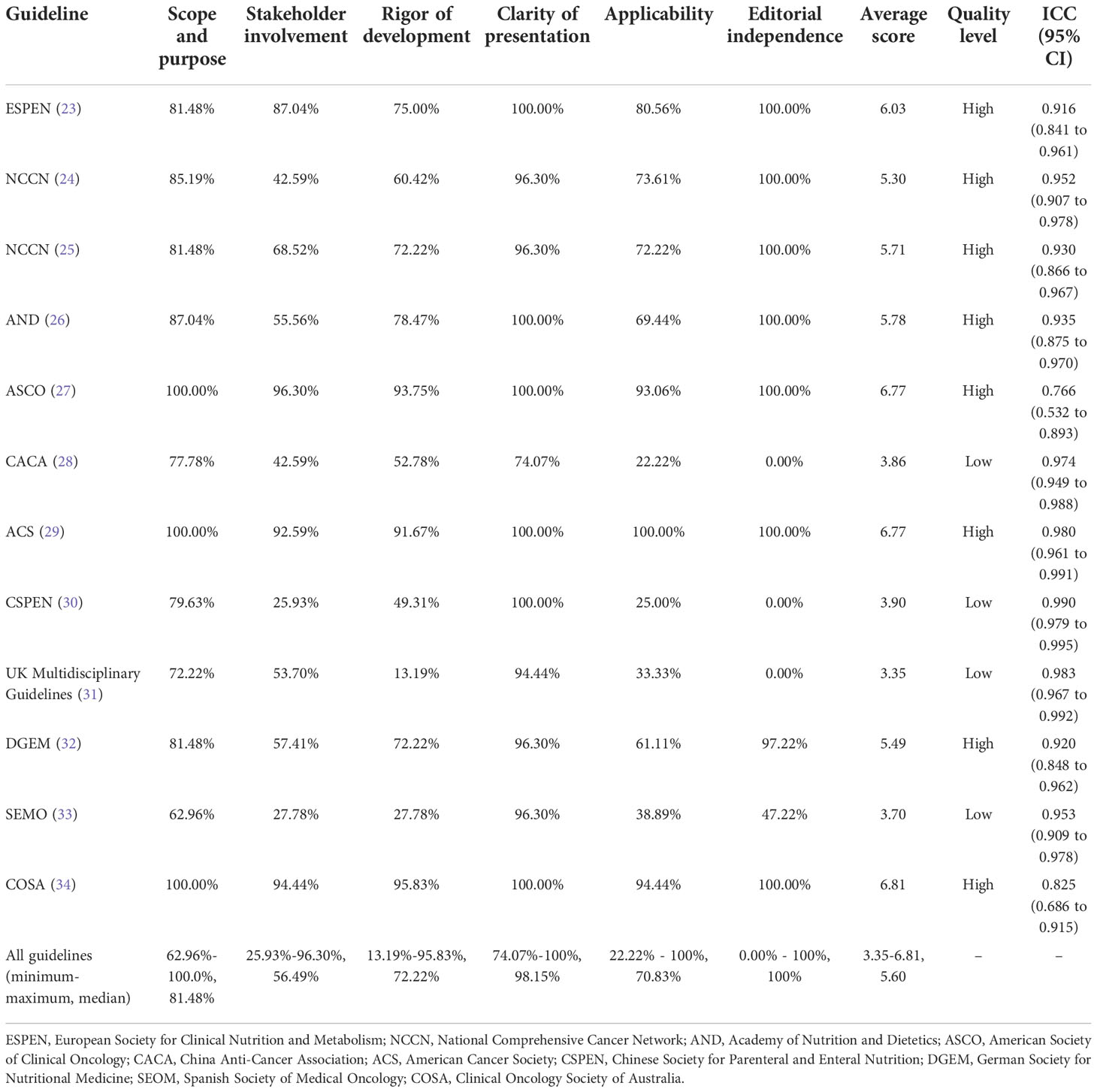
The results appraised with the AGREE II Instrument are presented in Supplementary Material 2. The average score, scaled domain scores, and the ICC are shown in Table 2. Results of average score showed that four CPGs (23, 27, 29, 34) scored above 6, with ‘yes’ for recommendation; four CPGs (24–26, 32) scored 4 ~ 6, with ‘yes, with modification’; and the remaining CPGs (28, 30, 31, 33) scored 3 ~ 4, with ‘no’ for recommendation. Based on the scaled scores of six domains, eight CPGs were rated as high quality (23–27, 29, 32, 34), and four as low quality (28, 30, 31, 33). The lowest domain score was for ‘stakeholder involvement’ (domain 2), with a median score of 56.48% (ranging from 25.93% to 96.30%). The ‘clarity of presentation’ (domain 4) got the highest scores and the minor variability with a median score of 98.15% (ranging from 74.07% to 100%). The lowest mean score (2.75) for an individual item was ‘The guideline has been externally reviewed by experts before its publication’ (Item 13). The ICC ranged from 0.766 to 0.990, indicating satisfactory consistency.
Scope and purpose
This domain involves the primary purpose, specific clinical issues, and target population of the guidelines (21). All the CPGs scored > 60%, and three CPGs scored 100% (27, 29, 34). All appraised CPGs made statements with reasonable clarity. The SEOM guideline (33) got the lowest score of 62.96%, for the descriptions of purpose, clinical questions, and intended audience, were not clear and concise enough without labeled sections or chapters.
Stakeholder involvement
This domain is concerned with the extent to which the guideline was developed by the appropriate stakeholders and whether the recommendations represent the views of its target users (21). Five CPGs (23, 25, 27, 29, 34) scored > 60%, one CPGs (30) received the lowest score (25.93%). Most CPGs mentioned some of the following information: members of the development group, institutional affiliation, geographical location, and subject discipline, but only two (27, 34) described members’ roles in the guideline development group. Five CPGs (23, 27, 29, 32, 34) provided involvement details of target population, of which the DGEM guideline’s (32) statement was found in the general standards of AWMF-S3 guidelines development on the website. The target users were poorly defined in three CPGs (30, 32, 33).
Rigor of development
This domain pertains to the process used to gather and synthesize the evidence, the methods to formulate the recommendations, and update them (21). Eight CPGs (23–27, 29, 32, 34) scored > 60%, and one (31) received the lowest score (13.19%). Two CPGs (31, 33) did not use systematic methods to search for evidence, and four (24, 30, 31, 33) did not mention the criteria for selecting evidence. The strengths and limitations of the body of evidence were clearly described for the majority of CPGs, but not for one (31). Eight CPGs (23–25, 27, 28, 30, 32, 34) elaborated on the method of formulating the recommendations, but two (31, 33) omitted it. For most CPGs, the health benefits, side effects, and risks were primarily considered in formulating the recommendations except one (31). All CPGs met the goal of an explicit link between the recommendations and the supporting evidence. Three CPGs (27, 29, 34) had been externally reviewed by experts before publication, whereas others had poor/no description. Eight CPGs (23–27, 29, 32, 34) included a procedure for updating, while four (28, 30, 31, 33) did not. The ESPEN guideline was developed based on the ESPEN-specific framework, which is available online (23). The method of DGEM guideline (32) was described in detail in the guideline report (including search strategy and evidence table et al.) on the website of the Association of Scientific Medical Societies (AWMF). The ASCO Guideline Methodology Manual provided the update process and a summary of literature search results in the Data Supplement (27). The ACS guideline (29) and COSA guideline (34) published a comprehensive list of evidence available online, and the COSA guideline (34) also provided search strategy on the website.
Clarity and presentation
This domain mainly concerns the guideline’s language, structure, and format (21). All the CPGs approached or came up to (23–27, 29–34) scores of 100% except one (28), which scored 74.07% because the key recommendations were not easily identifiable.
Applicability
This domain focuses on the potential facilitators and barriers to application, auditing criteria, measures to improve transmitting power, and resource implications of applying the guideline (21). Eight CPGs (23–27, 29, 32, 34) scored > 60%, two (28, 30) scored < 30% that only mentioned the monitoring/auditing criteria. Only three CPGs (27, 29, 34) provided complete description of applicability, by contrast, two (28, 30) had no mention of facilitators and barriers to application, four (28, 30, 31, 33) barely presented the tools and/or advice for applying the recommendations, and three (28, 30, 31) scarcely considered potential resource implications of applying the recommendations.
Editorial independence
This domain is to evaluate whether the formulation of recommendations was unduly biased by competing interests (21). Conflict of interest is the most common source of bias in guideline development (35). Eight CPGs (23–27, 29, 32, 34) approached or came up to a score of 100%. The SEMO guideline (33) scored 47.22% that reported competing interests without statements of funding bodies’ influence for recommendations, and three (28, 30, 31) received a score of 0.00% for no disclosure of editorial independence. Five CPGs (24–26, 32, 34) provided an explicit statement of interest disclosure and editorial independence on their official website.
Extraction of recommendations
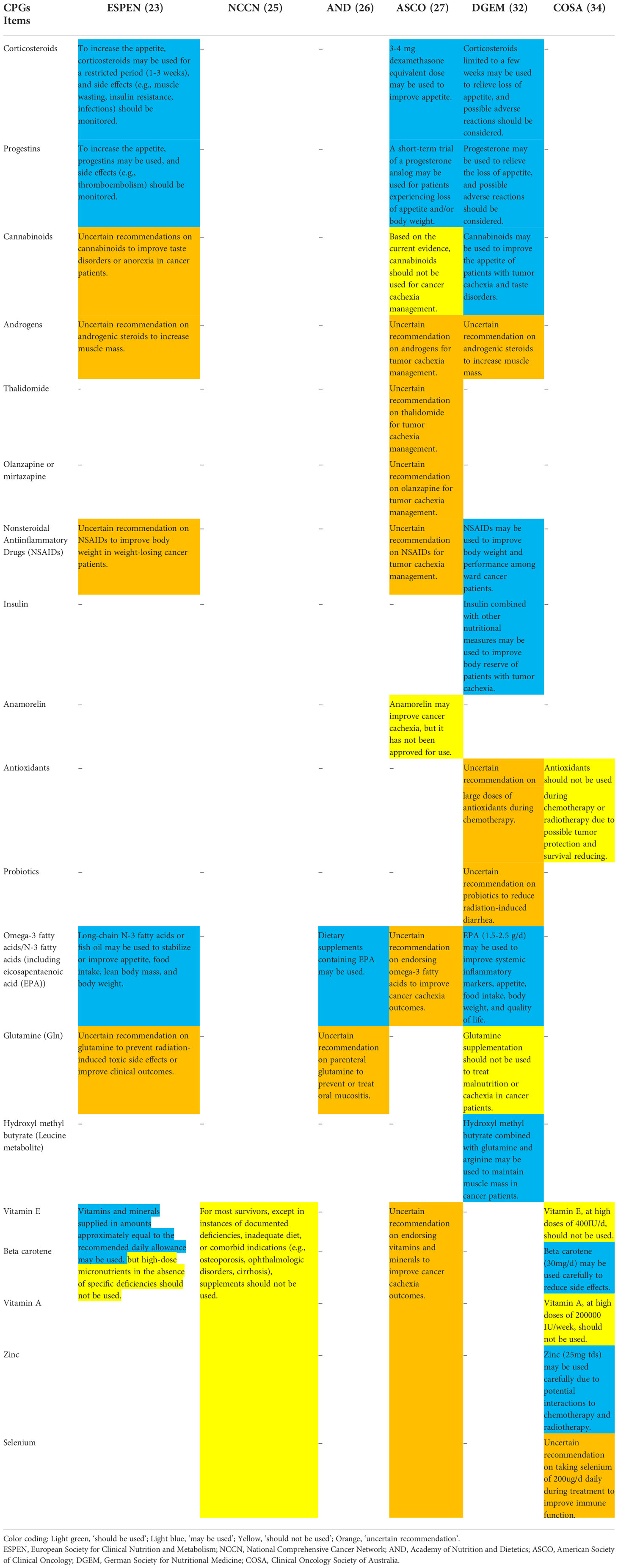
Classified recommendations for nutrition management were integrated into specific sections in Table 3, which included nutritional risk screening (24, 26, 32, 34), nutrition assessment (23, 24, 26, 32, 34), nutrition counseling (23, 24, 32, 34), nutrition interventions (23, 24, 32, 34), nutrition intake (23, 27, 32, 34), swallowing function management (23, 24, 29, 34), weight management (25, 34), exercise (23, 25, 27, 32), multidisciplinary team (24, 26, 27, 34) and post-discharge care (24, 25, 29, 34). The nutrients (23, 25–27, 32, 34) and pharmacologic interventions (23, 27, 32, 34) were summarized separately in Table 4 in view of the volume and complexity.
Discussion
HNC patients are at high risk of malnutrition during peri-radiotherapy (36). Clinicians have various obstacles to managing the nutrition status of patients better, such as using clinical judgment when facing ambiguous CPGs (37) or considering the applicability of the international CPGs in the local medical environment. Clinicians need guidance to aid them in making the present decision. Furthermore, clinicians were given quite different recommendations across even highly scoring CPGs sometimes. In that case, several strategies may be adopted, including to search for systematic reviews on quality assessment of related CPGs, which would provide the reference of the included CPGs’ quality level, to select up-to-date and authoritative CPGs if possible, or to use area-specific CPGs, although the latter may compromise the fairness of evidence citation (38). In this review, we also found that high-quality CPGs were mainly produced in developed countries and advanced research centers. It is worth considering whether these CPGs by developed countries apply to developing countries or regions or how to implement them properly in developing countries. Motivating developing countries to develop high-quality CPGs may be another area worth improving. We recommend developing multi-language versions of high-quality CPGs, and an English version must be available to facilitate communication.
To our knowledge, this is the first systematic review of CPGs on nutrition management for HNC population during peri-radiotherapy. Twelve CPGs were included and the recommendations for nutrition management were synthesized. Considering low domain scores with the AGREE II Instrument may be a risk factor for increasing the chance of making incorrect clinical decisions, only recommendations from CPGs of high and average quality were extracted and integrated while those from low quality CPGs were not synthesized, which would probably improve the quality of evidence derived from this study. Similar to other findings from previous studies focusing on CPG assessment of cancer nutrition or management of HNC (16, 17, 38–40), a common finding in this study is the included CPGs’ high heterogeneity of quality. In addition, some of the domains of CPGs, assessed by the AGREE II Instrument, such as ‘Applicability’, ‘Stakeholder involvement’, ‘Editorial independence’ and ‘Rigor of development’, scored lower due to incomprehensive description and need to be improved further.
Moreover, the AGREE II Instrument did not provide a cut-off and lacked detailed information to distinguish the quality of CPGs (41). There were several types of cut-offs as following: 1) high quality (five to six domains scoring > 60%), average quality (three to four domains scoring > 60%) and low quality (two or less domains scoring > 60%) (14, 22, 40); 2) grade A (strongly recommended, six domains scoring > 60%), grade B (recommended, more than three domains scoring ranged from 30% ~ 60%) and grade C (not recommended, more than three domains scoring < 30%) (17); 3) when the AGREE II score ≥ 45, CPGs were included in the final extraction (42); 4) the recommendation levels (‘yes’, ‘yes with modifications’ and ‘no’) were given without additional division of CPGs’ quality (16); 5) ‘high quality’, ‘moderate quality’ and ‘low quality’ were divided by tertile according to mean of AGREE II & AGREE REX overall scores (37); 6) domains or overall scores < 50% indicated lower quality (43, 44); 7) overall score > 60% was classified as ‘recommended’, score between 30% ~ 60% as ‘recommended with modifications’, and score < 30% as ‘not recommended’ (38, 39). It might be needed that clear distinction and specified cut-off of quality level in the update of the AGREE II Instrument (41).
The update, external review and editorial independence serve as significant parts for CPGs. In this study, the references of included CPGs indicated that CPGs were not always based on the latest systematic reviews or primary studies. Robin et al. (45) found that most of the CPG methodological handbooks (62.9%) included updating intervals of two to five years. CPGs are time-sensitive for knowledge development and changes (37). Recommendations become outdated quickly, with 20% of recommendations turning outdated after three years (46). Several CPGs within this review (29, 31, 32, 34) were not updated over five years which may lead to a lag of information. As for the external review, three CPGs (24, 25, 34) were not published in a journal but by academic organizations on websites. Although journal publications underwent rigorous peer review, academic organizations and professional societies have procedures that were not always described in the CPGs. Moreover, some CPG authors did not disclosed their editorial independence within the CPGs. Sometimes the relevant information can be found on websites despite difficulty finding it. CPGs did not report as the framework of the standard formulation may be short of important information, weakening the transparency and reliability.
The CPGs held different views, which were possibly based on population differences. At first, for the nutrition assessment, there were differences across CPGs. In the ESPEN guideline (23), nutrition assessment, including muscle mass, nutritional intake, the degree of systemic inflammation, physical performance, and nutrition impact symptoms, was recommended for patients at risk of malnutrition by nutrition screening. But in the DGEM guideline (32) and the SEOM guideline (33), the assessment items were not that comprehensive. Secondly, there were discrepancies among CPGs in terms of protein intake. The protein intake should be above 1.2 g/kg/day in patients receiving RT with or without chemotherapy, according to the ASCO guideline (27), the UK guideline (31) and the COSA guideline (34); in the ESPEN guideline (23) and the CACA guideline (28), aim for protein intake was at least 1 g/kg/day and, if possible, up to 1.5 g/kg/day; the CSPEN guideline (30) suggested the target of protein intake for cancer patients was 1.0 to 2.0 g/kg/day; and the DGEM guideline (32) recommended that the protein/amino acid intake be 1.2-1.5 g/kg/day and the requirement may be higher (up to 2 g/kg/day) in the case of obvious inflammation. Therefore, our prudent advice is that protein intake should be above 1.2 g/kg/day and achieve1.5 g/kg/day if available. Thirdly, the DGEM guideline (32) and the SEOM guideline (33) considered that cancer patients had similar nutritional needs as the healthy population, about 25-30 kcal/kg/day. Still, in three CPGs (27, 31, 34), recommended energy intake should not be less than 125 kJ/kg/day (30 kcal/kg/day). Consequently, it is appropriate for HNC patients to intake 30 kcal/kg/day.
Given ambiguity in guidance and an absence of further verification, nutrients, pharmacological interventions, nutritional risk screening and nutrition assessment, emphasized the need for robust evidences. Most nutrients or drugs to improve nutritional status (e.g., appetite, taste, and reducing weight loss) for cancer patients were not recommended, due to lack of high-quality and large randomized controlled or cohort studies, similar to the finding of a previous study (38). Further studies are needed to explore whether and how the nutrients or drugs play a role in HNC patients’ malnutrition. Moreover, there is no uniformly used or specialized scale of nutritional risk screening and assessment for HNC patients. The AND guideline (26) recommended the Malnutrition Screening Tool and the Malnutrition Universal Screening Tool for nutritional risk screening; the DGEM guideline suggested the Nutritional risk screening 2002 and the Malnutrition Universal Screening Tool for use (32); and the COSA guideline (34) considered the Malnutrition Screening Tool as recommendation. Although the Nutritional Risk Screening 2002 (NRS-2002) scale has been widely used, the sensitivity for HNC patients receiving RT is still uncertain. The Patient-Generated Subjective Global Assessment (PG-SGA) and Subjective Global Assessment (SGA) were recommended in the reviewed CPGs (26, 34), but their applicability for HNC patients still needs to be verified.
Limitations
There were several limitations to this study. Firstly, there were three appraisers to independently assess the CPGs while the AGREE II manual suggests that it be better if four appraisers are available. In the second place, the literature search was performed up to May 1, 2022, as the latest literature search was performed, there are no more new CPGs found. Thirdly, the AGREE II Instrument focuses on the comprehensiveness and intelligibility of the CPGs, not the quality of the recommendations or the clinical context (43). Besides, the overall assessment with the AGREE II Instrument is highly subjective (47). Last but not least, selection bias cannot be excluded in this study due to the language restrictions for Chinese, English, and German.
Conclusion
In this study, the recommendations for nutrition of included oncology CPGs suggesting that nutrition is an essential component in peri-radiotherapy care. The quality of the included CPGs was highly heterogeneous. The discrepant recommendations from the whole process of nutrition management existed among CPGs. The CPG developers should adopt a multi-disciplinary approach, rely on evidence of high quality, and include the target population in the formulating recommendations. If the framework for CPG development and other supplementary materials cannot be reported within the main document, a trail or statement to its link should be listed. And beyond that, there are many kinds of evidence grading systems and recommendation strength methods, so we emphasize the need for comparing evaluation systems or developing a widely applicable system in the future. Using tools like the AGREE II Instrument to develop CPGs may be a good choice due to its scientific and unified criteria for quality reporting. At last, the development of CPGs should consider fundamental and medical resource allocation worldwide.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conception and design, JZ, YK, and SY. Literature retrieval, literature screening, and data extraction, YK and XW. Guideline quality assessment, YK, XW, and GW. Data analysis and interpretation, JZ, YK, XW, YB, and JL. Manuscript writing, all authors. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Ms. Ruishuang Zheng and Mr. Fangyuan Zhang for their cogitative guidance and perceptive comments on this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.974059/full#supplementary-material
References
2. Jiao Y, Liu D, Sun Y, Chen Z, Liu S. Survival benefit of metformin as an adjuvant treatment for head and neck cancer: A systematic review and meta-analysis. Front Pharmacol (2022) 13:850750. doi: 10.3389/fphar.2022.850750
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
4. Ferlay J EM, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, et al. Global cancer observatory: Cancer today . Lyon, France: International Agency for Research on Cancer (Accessed April 1, 2022).
5. Mody MD, Rocco JW, Yom SS, Haddad RI, Saba NF. Head and neck cancer. Lancet (2021) 398(10318):2289–99. doi: 10.1016/S0140-6736(21)01550-6
6. Planas M, Alvarez-Hernandez J, Leon-Sanz M, Celaya-Perez S, Araujo K, Garcia de Lorenzo A, et al. Prevalence of hospital malnutrition in cancer patients: A Sub-analysis of the Predyces(R) study. Support Care Cancer (2016) 24(1):429–35. doi: 10.1007/s00520-015-2813-7
7. Bossola M. Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: A narrative review. Nutrients (2015) 7(1):265–76. doi: 10.3390/nu7010265
8. Mulasi U, Vock DM, Jager-Wittenaar H, Teigen L, Kuchnia AJ, Jha G, et al. Nutrition status and health-related quality of life among outpatients with advanced head and neck cancer. Nutr Clin Pract (2020) 35(6):1129–37. doi: 10.1002/ncp.10476
9. Abu Zaid Z, Kay Neoh M, Mat Daud ZA, Md Yusop NB, Ibrahim Z, Abdul Rahman Z, et al. Weight loss in post-chemoradiotherapy head and neck cancer patients. Nutrients (2022) 14(3):548. doi: 10.3390/nu14030548
10. Guyatt G, Jaeschke R, Wilson MC, Montori VM, Richardson WS. What is evidence-based medicine? In: Guyatt G, Rennie D, Meade MO, Cook DJ, editors. Users' guides to the medical literature: A manual for evidence-based clinical practice, 3rd Ed. New York, NY: McGraw-Hill Education (2015).
11. Murad MH. Clinical practice guidelines: A primer on development and dissemination. Mayo Clin Proc (2017) 92(3):423–33. doi: 10.1016/j.mayocp.2017.01.001
12. Cabrera PA, Pardo R. Review of evidence based clinical practice guidelines developed in Latin America and Caribbean during the last decade: An analysis of the methods for grading quality of evidence and topic prioritization. Global Health (2019) 15(1):14. doi: 10.1186/s12992-019-0455-0
13. Alper BS, Price A, van Zuuren EJ, Fedorowicz Z, Shaughnessy AF, Oettgen P, et al. Consistency of recommendations for evaluation and management of hypertension. JAMA Netw Open (2019) 2(11):e1915975. doi: 10.1001/jamanetworkopen.2019.15975
14. Harrison CL, Teede H, Khan N, Lim S, Chauhan A, Drakeley S, et al. Weight management across preconception, pregnancy, and postpartum: A systematic review and quality appraisal of international clinical practice guidelines. Obes Rev (2021) 22(10):e13310. doi: 10.1111/obr.13310
15. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: Advancing guideline development, reporting, and evaluation in health care. Prev Med (2010) 51(5):421–4. doi: 10.1016/j.ypmed.2010.08.005
16. Ng JY, Dogadova E. The presence of complementary and alternative medicine recommendations in head and neck cancer guidelines: Systematic review and quality assessment. Curr Oncol Rep (2021) 23(3):32. doi: 10.1007/s11912-021-01024-y
17. Zhou H-J, Deng L-J, Wang T, Chen J-X, Jiang S-Z, Yang L, et al. Clinical practice guidelines for the nutritional risk screening and assessment of cancer patients: A systematic quality appraisal using the agree II instrument. Supportive Care In Cancer (2021) 29(6):2885–93. doi: 10.1007/s00520-021-06094-z
18. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions version 6.3. Available at: www.training.cochrane.org/handbook (Accessed March 8, 2022).
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
20. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
21. Agree next steps consortium, in: The agree II instrument (2017). Available at: http://www.agreetrust.org (Accessed January 1, 2022).
22. Messina C, Bignotti B, Tagliafico A, Orlandi D, Corazza A, Sardanelli F, et al. A critical appraisal of the quality of adult musculoskeletal ultrasound guidelines using the agree II tool: An euroaim initiative. Insights Imaging (2017) 8(5):491–7. doi: 10.1007/s13244-017-0563-4
23. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin Nutr (2021) 40(5):2898–913. doi: 10.1016/j.clnu.2021.02.005
24. National Comprehensive Cancer Network. Head and neck cancers (Version 2.2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (Accessed June 18, 2022).
25. National Comprehensive Cancer Network. Survivorship (Version 1.2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf (Accessed June 18, 2022).
26. Thompson KL, Elliott L, Fuchs-Tarlovsky V, Levin RM, Voss AC, Piemonte T, et al. Oncology evidence-based nutrition practice guideline for adults. J Acad Nutr Diet (2017) 117(2):297-310. doi: 10.1016/j.jand.2016.05.010
27. Roeland EJ, Bohlke K, Baracos VE, Bruera E, Del Fabbro E, Dixon S, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol (2020) 38(21):2438–53. doi: 10.1200/JCO.20.00611
28. Cui J-W, Li W, Xu H-X, Chen J-Q, L M, L T, et al. Guidelines for clinical diagnosis and treatment of cancer cachexia (2020 edition). Chin J Clin Oncol (2021) 48:379–85. doi: 10.3969/j.issn.1000-8179.2021.08.369
29. Cohen EEW, LaMonte SJ, Erb NL, Beckman KL, Sadeghi N, Hutcheson KA, et al. American Cancer society head and neck cancer survivorship care guideline. CA Cancer J Clin (2016) 66(3):203–39. doi: 10.3322/caac.21343
30. Wu G-H, Tan S-J. Guidelines on nutritional support in patients with tumor. Chin J Surg (2017) 55(11):801–29. doi: 10.3760/cma.j.issn.0529-5815.2017.11.001
31. Talwar B, Donnelly R, Skelly R, Donaldson M. Nutritional management in head and neck cancer: United kingdom national multidisciplinary guidelines. J Laryngol Otol (2016) 130(S2):S32–40. doi: 10.1017/S0022215116000402
32. Arends J, Bertz H, Bischoff SC, Fietkau R, Hermann HJ, Holm E, et al. S3-guideline of the German society for nutritional medicine (DGEM) in cooperation with the DGHO, the ASORS and the AKE clinical nutrition in oncology. Aktuelle Ernahrungsmedizin (2015) 40(5):e1–e74. doi: 10.1055/s-0035-1552741
33. de Las Penas R, Majem M, Perez-Altozano J, Virizuela JA, Cancer E, Diz P, et al. SEOM clinical guidelines on nutrition in cancer patients (2018). Clin Transl Oncol (2019) 21(1):87–93. doi: 10.1007/s12094-018-02009-3
34. Head and Neck Guideline Steering Committee. Evidence-based practice guidelines for the nutritional management of adult patients with head and neck cancer . Sydney: Clinical Oncological Society of Australia. Available at: https://wiki.cancer.org.au/australiawiki/index.php?oldid=215353 (Accessed March 21, 2022).
35. Traversa G, Venegoni M. [Conflict of interest and public health body: The bias and the rule]. Epidemiol Prev (2018) 42(2):105. doi: 10.19191/EP18.2.P105.032
36. Brewczyński A, Jabłońska B, Mrowiec S, Składowski K, Rutkowski T. Nutritional support in head and neck radiotherapy patients considering hpv status. Nutrients (2020) 13(1):57. doi: 10.3390/nu13010057
37. Gillespie BM, Latimer S, Walker RM, McInnes E, Moore Z, Eskes AM, et al. The quality and clinical applicability of recommendations in pressure injury guidelines: A systematic review of clinical practice guidelines. Int J Nurs Stud (2021) 115:103857. doi: 10.1016/j.ijnurstu.2020.103857
38. Zhao X-H, Yang T, Ma X-D, Qi Y-X, Lin Y-Y, Chen X-Z, et al. Heterogeneity of nutrition care procedures in nutrition guidelines for cancer patients. Clin Nutr (Edinburgh Scotland) (2020) 39(6):1692–704. doi: 10.1016/j.clnu.2019.08.022
39. Shen W-Q, Yao L, Wang X-Q, Hu Y, Bian Z-X. Quality assessment of cancer cachexia clinical practice guidelines. Cancer Treat Rev (2018) 70:9-15. doi: 10.1016/j.ctrv.2018.07.008
40. De Ravin E, Lu J, Salmon M, Venkatesh S, Romeo D, Moreira A, et al. Clinical practice guidelines for the management of recurrent head and neck cancer: A systematic review and quality appraisal. Eur Arch Otorhinolaryngol (2022). doi: 10.1007/s00405-022-07519-z
41. Hoffmann-Eßer W, Siering U, Neugebauer EAM, Lampert U, Eikermann M. Systematic review of current guideline appraisals performed with the appraisal of guidelines for research & evaluation II instrument-a third of agree II users apply a cut-off for guideline quality. J Clin Epidemiol (2018) 95:120–7. doi: 10.1016/j.jclinepi.2017.12.009
42. Stout NL, Santa Mina D, Lyons KD, Robb K, Silver JK. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J Clin (2021) 71(2):149–75. doi: 10.3322/caac.21639
43. Andrade R, Pereira R, van Cingel R, Staal JB, Espregueira-Mendes J. How should clinicians rehabilitate patients after ACL reconstruction? a systematic review of clinical practice guidelines (CPGs) with a focus on quality appraisal (Agree II). Br J Sports Med (2020) 54(9):512–9. doi: 10.1136/bjsports-2018-100310
44. Pincus D, Kuhn JE, Sheth U, Rizzone K, Colbenson K, Dwyer T, et al. A systematic review and appraisal of clinical practice guidelines for musculoskeletal soft tissue injuries and conditions. Am J Sports Med (2017) 45(6):1458–64. doi: 10.1177/0363546516667903
45. Vernooij RWM, Sanabria AJ, Solà I, Alonso-Coello P, Martínez García L. Guidance for updating clinical practice guidelines: A systematic review of methodological handbooks. Implement Sci (2014) 9:3. doi: 10.1186/1748-5908-9-3
46. Martínez García L, Sanabria AJ, García Alvarez E, Trujillo-Martín MM, Etxeandia-Ikobaltzeta I, Kotzeva A, et al. The validity of recommendations from clinical guidelines: A survival analysis. CMAJ: Can Med Assoc J (2014) 186(16):1211–9. doi: 10.1503/cmaj.140547
47. Hoffmann-Eßer W, Siering U, Neugebauer EAM, Brockhaus AC, McGauran N, Eikermann M. Guideline appraisal with agree II: Online survey of the potential influence of agree II items on overall assessment of guideline quality and recommendation for use. BMC Health Serv Res (2018) 18(1):143. doi: 10.1186/s12913-018-2954-8
Keywords: head and neck cancer, radiotherapy, nutrition management, nutrients, clinical practice guidelines
Citation: Zhao J, Kan Y, Wu X, Yang S, Wang G, Bao Y and Li J (2022) Nutrition management for patients with head and neck cancer during peri-radiotherapy: A systematic review and quality appraisal of clinical practice guidelines using the AGREE II instrument. Front. Oncol. 12:974059. doi: 10.3389/fonc.2022.974059
Received: 20 June 2022; Accepted: 07 November 2022;
Published: 29 November 2022.
Edited by:
Xian-Yang Qin, RIKEN, JapanReviewed by:
Jeremy Y. Ng, Ottawa Hospital Research Institute (OHRI), CanadaYumiko Kawashita, Nagasaki University, Japan
Copyright © 2022 Zhao, Kan, Wu, Yang, Wang, Bao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhao, ZnVzdWljdUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Jing Zhao
Jing Zhao Yajing Kan
Yajing Kan Xueting Wu1,2
Xueting Wu1,2