- 1Department of Otolaryngology- Head & Neck Surgery, Thomas Jefferson University, Philadelphia, PA, United States
- 2Department of Radiation Oncology, Thomas Jefferson University, Philadelphia, PA, United States
Background: Adjuvant radiotherapy (RT) following surgical resection confers a survival benefit for adult patients with locally advanced head and neck squamous cell carcinoma (HNSCC). We aim to investigate if adjuvant RT provides a similar survival advantage to patients ages 80+ through a national curated database.
Methods: This retrospective cohort study queried the National Cancer Database (NCDB) for all cases of HNSCC between 2004-2016. Patients treated with surgical resection alone were compared to those treated with surgery plus adjuvant RT. Overall survival (OS) was compared within adult (age <80 years) and senior adult (age ≥80 years) cohorts using Kaplan-Meier analysis. Hazard ratios (HR) were assessed using Cox proportional hazards to account for differences in patient characteristics, primary site, and HNSCC stage.
Results: NCDB identified 16,504 locally advanced HNSCC treated with definitive surgery with 9,129 (55.3%) also receiving adjuvant RT. The mean age was 63.8 years (SD = 12.0) with 88.7% of patients ages <80 years and 11.3% ages ≥80 years. In the adult cohort, adjuvant RT was associated with a significant increase in OS compared to surgery alone at 1 year (88.4% vs. 83.8%, p=<0.001), 3 years (64.0% vs. 59.2%, p=<0.001) and 5 years (52.8% vs. 47.2%, p=<0.001). Treatment with surgery alone remained a significant predictor of mortality risk at 1 year (HR 1.48, 95% CI 1.35-1.64, p<0.001), 3 years (HR 1.25, 95% CI 1.18-1.33, p<0.001), and 5 years (HR of 1.23, 95% CI 1.17-1.30, p=<0.001). In the senior adult cohort, there were no significant differences in OS between treatment groups at 1 year (73.4% vs. 74.8%, 0.296), 3 years (45.8% vs. 41.8%, p=0.465), or 5 years (28.2% vs. 27.7% p=0.759). Treatment with surgery alone was not a significant predictor of mortality risk at 1 year (HR 1.11, 95% CI 0.90-1.36, p=0.316), 3 years (HR 0.94, 95% CI 0.81-1.08, p=0.423), or 5 years (HR 0.95, 95% CI 0.83-1.08, p=0.476).
Conclusion: The addition of adjuvant RT in senior patients (age ≥80 years) may not provide a similar OS benefit to that observed in younger patients. Further research is needed to best guide shared-decision making in this population.
Introduction
According to the United States Census Bureau, the year 2030 will present a demographic transition for the population when all baby boomers will be older than 65 and nearly 20% of Americans will be retirement age (1). The country’s 65-and-older population is projected to double from 2016 to 2060, increasing to nearly 25% of the projected population, and the number of people 85 years and older is projected to double by 2035 and triple by 2060 (1). These demographic shifts are expected to bring with them a similar increase in cancer diagnoses, as cancer occurs most commonly in older adults (2). These impending changes will likely exert substantial stress on the health care system and portend the need to optimize treatments in the senior adult population. To date, senior adult patients have been largely under-represented in cancer clinical trials, making them vulnerable to treatment disparities (2–4). Additionally, aging confers physiological changes in nearly every body system, such as decreased cardiac output, immune system depression, impaired wound healing, and vascular changes, that increase the risk of morbidities associated with standard cancer treatments (5). Therefore, physicians will likely encounter increasing challenges in treating senior adult cancer patients whose goals of care may influence them to pursue treatment plans that deviate from accepted standards of care (6).
According to the Center for Disease Control and Prevention, approximately half of head and neck cancer cases are diagnosed in patients older than 65 years, with a large proportion of those cases being diagnosed in patients older than 70 years (7). For locally advanced HNSCC, the majority of patients are treated with combined treatment modalities, with approximately 40% of patients receiving primary surgery + adjuvant radiation +/- chemotherapy versus 50% receiving primary chemoradiotherapy (8). Moreover, the National Comprehensive Cancer Network (NCCN) provides evidence-based treatment guidelines for various cancer types based on tumor stage and characteristics. Guideline adherence is generally associated with improved survival outcomes, while deviance is associated with complications or recurrence (9–14). The NCCN guidelines recommend primary surgical resection with adjuvant radiation +/- chemotherapy based on final pathology for locoregionally advanced HNSCC (15). All treatments come with expected and possible side effects that effect quality of life. These sequalae are anticipated and tolerated when there is a clear oncologic advantage. Radiotherapy (RT) plays a key-role in curative-intent treatments of both early and late-stage disease but comes with the risk of early and late toxicities (16–18). When the sequela of treatment are compounded with the physiological changes of normal aging, clinicians must reevaluate the risks versus benefits of the treatment course in senior adult patients, which has been well-characterized in oncologic literature as shared decision making (6, 19–23). The current study used the National Cancer Database (NCDB) to analyze the survival benefits associated with adjuvant radiotherapy for senior adult patients with locally advanced HNSCC who undergo primary surgical resection.
Materials and methods
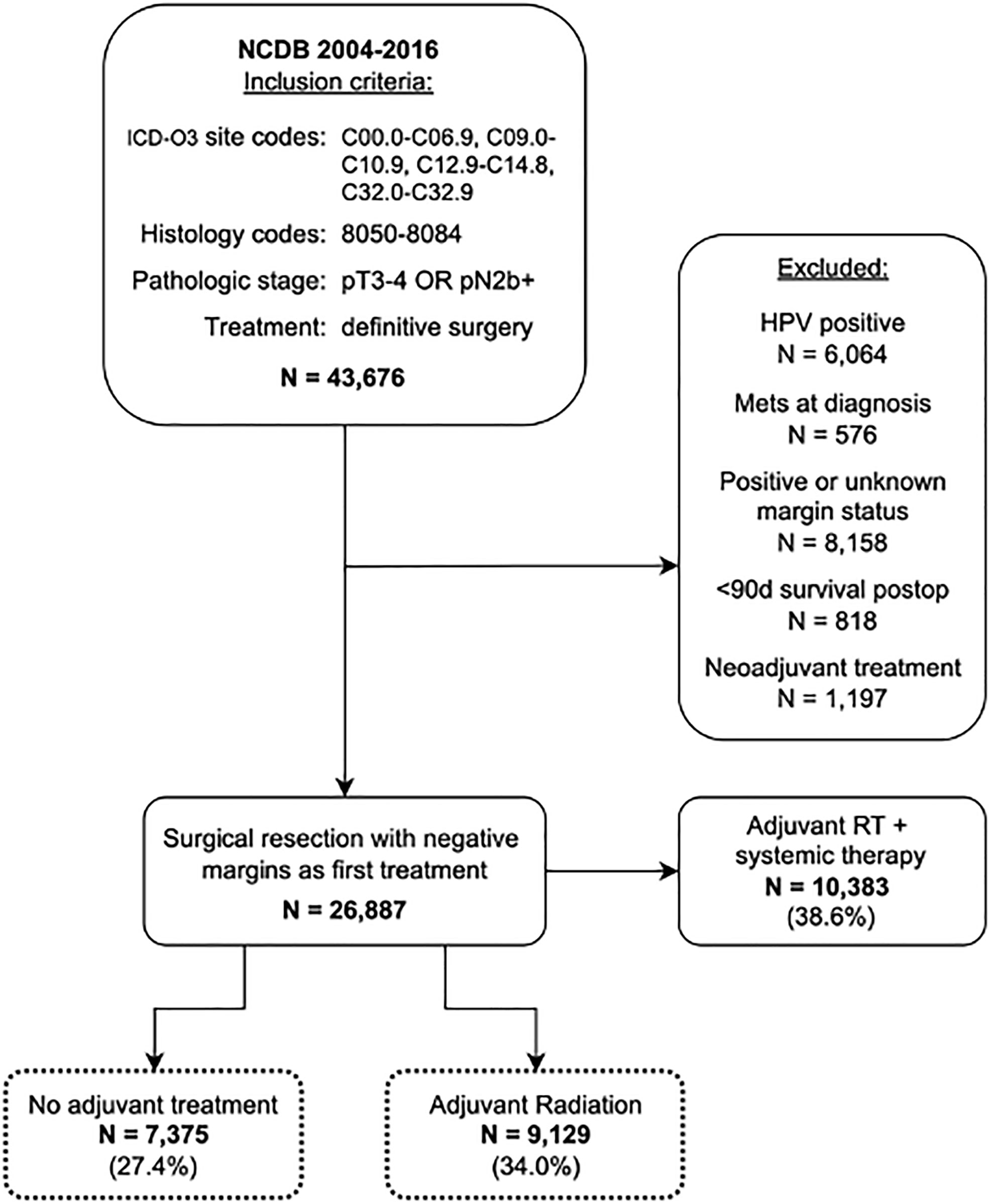
The National Cancer Database (NCDB) was queried for all cases of HNSCC from 2004-2016 using the International Classification of Disease for Oncology-3 (ICD-O3) topographical and morphological codes. Topographical codes included C00.0-C06.9, C09.0-10.9, C12.9-C14.8, and C32.0-32.9. Additional details on primary sites and ICD-O3 codes can be found in Supplemental Table 1. Squamous cell carcinoma histology was identified using ICD-O3 morphological codes 8050-8084. Patients were included if they were diagnosed with advanced locoregional disease, defined as pT3/T4 and/or N2b or greater without distant metastasis, as their first or only cancer. All included patients underwent definitive surgical resection with or without adjuvant RT. Exclusion criteria included HPV-positive disease, positive surgical margins, death within 90 days of surgical resection, administration of neoadjuvant treatment, treatment with adjuvant chemo- or immunotherapy, treatment with adjuvant RT with systemic therapy, or any missing variables pertaining to the American Joint Committee of Cancer (AJCC) clinical or pathological stage (Figure 1). Treatment with neoadjuvant therapy was defined as administration of chemotherapy or RT ≤90 days before surgery. Adjuvant RT was defined as a course of external beam radiation initiated after surgery as part of the planned first course of treatment. As per institutional guidelines, analysis of deidentified data does not require institutional review board approval. The final cohort included 16,504 patients.
Patients were grouped into adult (age <80 years) and senior adult (age ≥80 years) cohorts. The age 80 years was greater than one standard deviation from the mean population age (mean = 63.8 years, SD = 12.0) and was therefore chosen as the cutoff age for our senior adult cohort. Within each cohort, patients were divided into treatment groups based on whether they received adjuvant RT. Overall survival (OS) was compared between treatment groups within adult and senior adult cohorts and was assessed at 1, 3, and 5 years after surgery using Kaplan Meier estimates. The log-rank tests were used to assess significance. Cox proportional hazards analysis was used to further examine the impact of adjuvant RT on OS at these same time points while accounting for differences in cohort characteristics identified in Table 1. A Cox regression model was built with fixed covariates including patient age, sex, race, Charlson-Deyo comorbidity score, primary site, AJCC pathologic stage, and treatment type. Hazard ratios were assessed at 1, 3, and 5 year time points. The analysis was performed within IBM SPSS Statistics for Macintosh, version 27 (IBM Corp., Armonk, N.Y., USA) (24). Statistical significance was defined as a p-value<0.05.
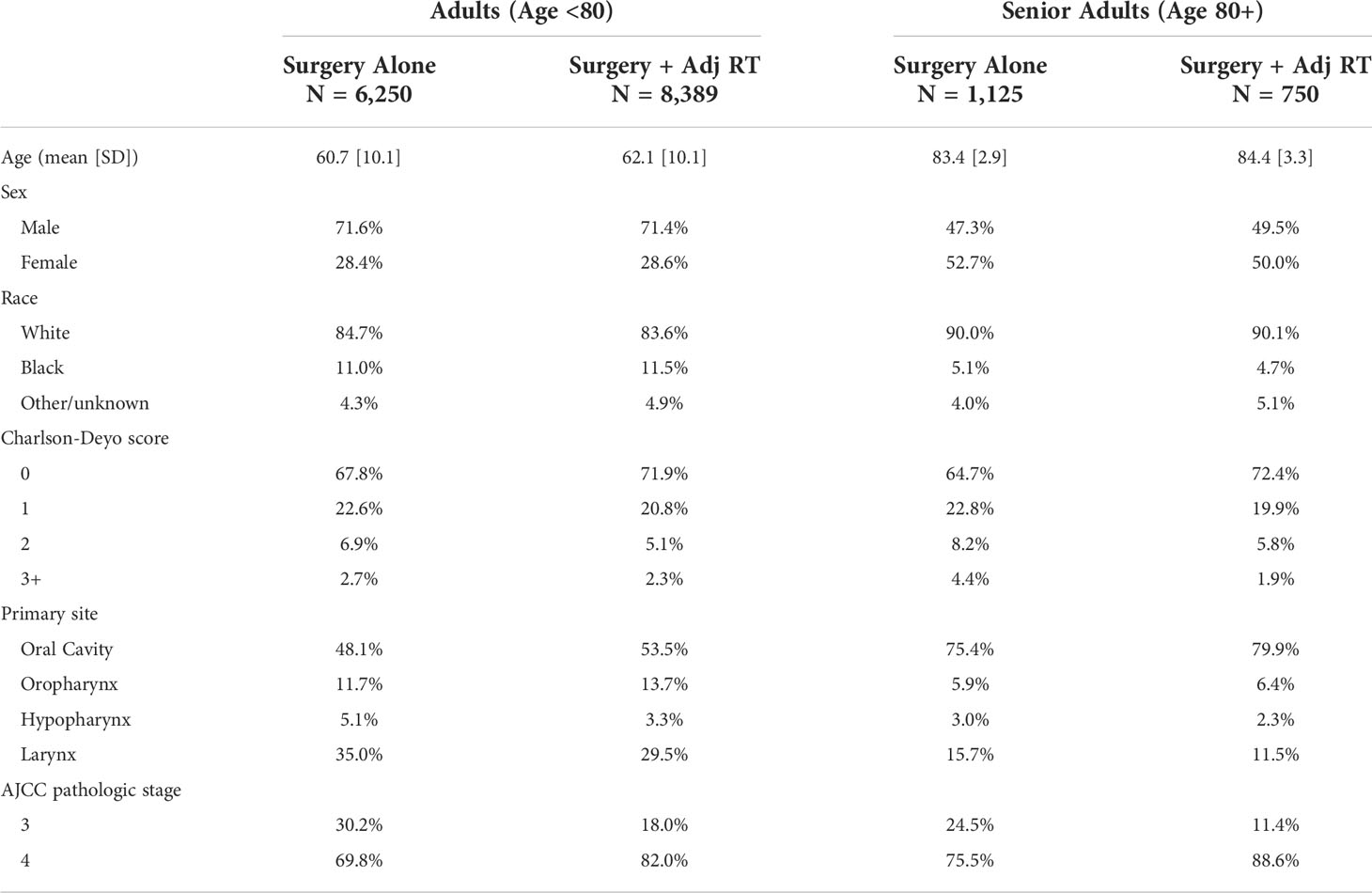
Table 1 Patient and tumor characteristics by age group and treatment type. additional analysis of cohort demographics can be found in Table 2 and Supplemental Tables 1, 2.
Results
Cohort characteristics
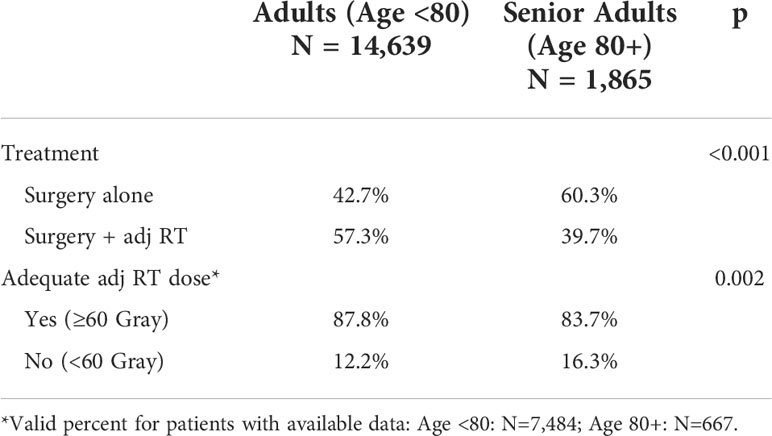
A total of 16,504 patients met inclusion criteria. Characteristics of each cohort are outlined in Table 1. The mean age of the total population was 63.8 years (SD 12.0 years). The adult cohort included 14,639 (88.7%) patients with a mean age of 61.3 years (SD 10.1 years). The senior adult cohort included 1,865 (11.3%) patients with a mean age of 84.0 years (SD 3.21 years). In both cohorts, patients undergoing adjuvant RT were identified for comparison to those treated with surgery alone. Adjuvant RT was utilized in a significantly higher proportion of patients <80 years old relative to senior adults (Table 2). Additionally, prior to exclusion of all patients receiving adjuvant systemic therapy, adjuvant RT was utilized in 72.6% of patients overall.
Kaplan Meier survival analysis
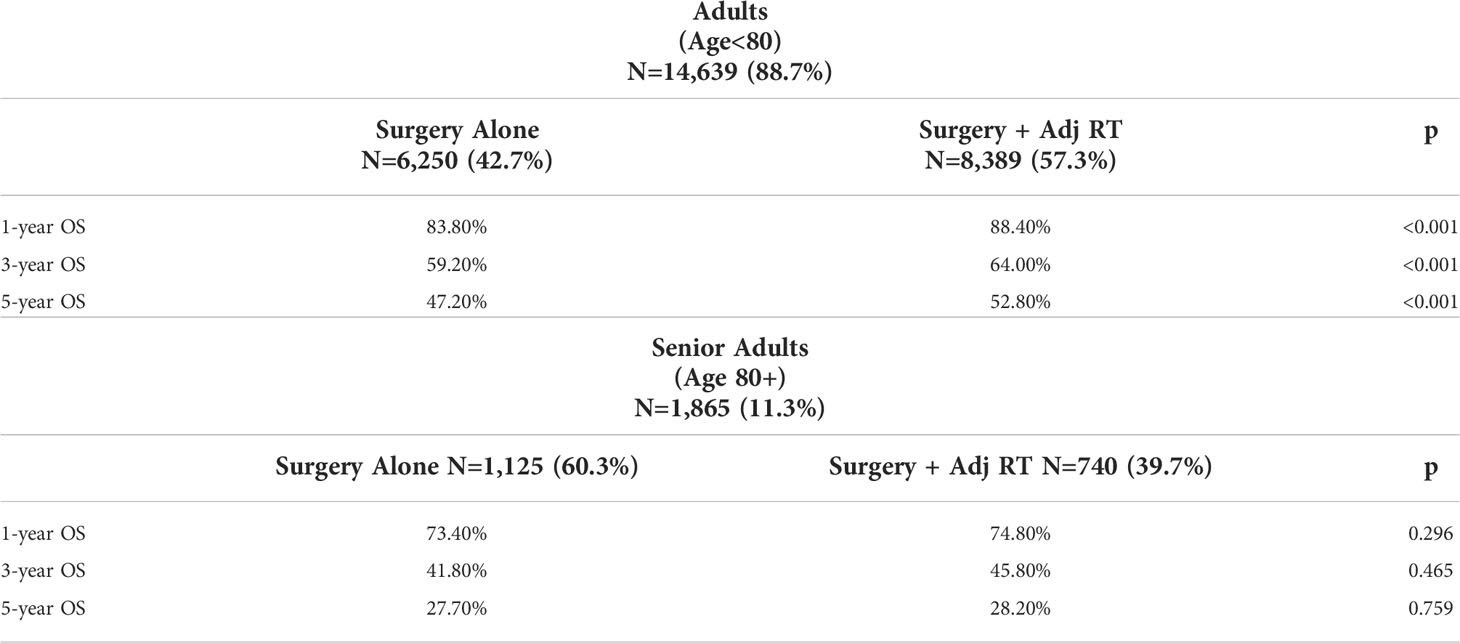
Within each age group, overall survival was compared between patients who underwent surgery alone versus those receiving adjuvant RT. Kaplan Meier OS curves were stratified by treatment groups within each age cohort (Figure 2). OS did not differ significantly between treatment groups in senior adults, whereas those ages <80 years demonstrated a loss of OS when they did not receive adjuvant RT. When assessed at specific time points, Kaplan Meier estimates of OS showed that adjuvant RT was associated with significantly higher OS in the adult cohort at 1-year (88.4% vs. 83.8%, p<0.001), 3-years (64.0% vs. 59.2%, p<0.001), and 5-years (52.8% vs. 47.2%, p<0.001) post-op compared to surgery alone. In the senior adult cohort, adjuvant RT was not associated with significantly different OS at 1-year (74.8% vs. 73.4%, p=0.296), 3-years (45.8% vs. 41.8%, p=0.465), and 5-years (28.2 vs. 27.7%, p=0.759) compared to surgery alone (Table 3).

Figure 2 Kaplan Meier OS curves stratified by treatment group within adult and senior adult patient cohorts. (A) OS for Adults (Ages<80) stratified by treatment type (B) OS for Senior Adults (Age 80+) stratified by treatment type.
Corrected survival analysis
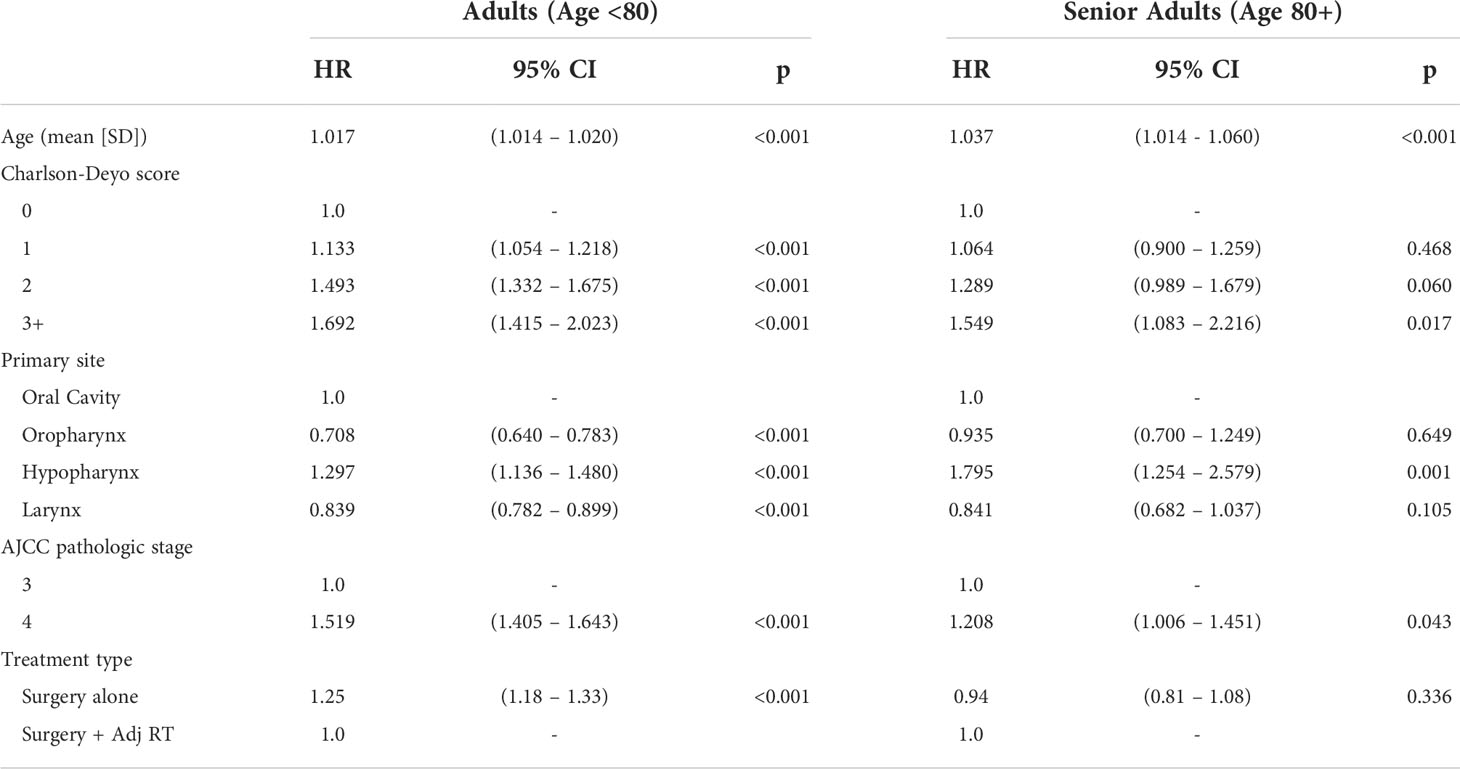
To account for significant differences in patient and tumor characteristics between treatment groups, Cox proportional hazards model was used to examine the impact of adjuvant RT on overall survival by analyzing the OS hazard ratio (HR) between treatment groups within the age cohorts. This was performed separately for the senior adult and adult cohorts at 1-, 3-, and 5-years post-op. All variables found to be significantly different between treatment groups in Table 1 were included in the regression model. Variables included age, sex, race, Charlson-Deyo comorbidity score, primary site, and AJCC pathologic stage. Surgery plus adjuvant therapy was used as the reference value. In the adult cohort, surgery alone remained a significant predictor of mortality risk at 1-year (HR 1.48, 95% CI 1.35-1.64, p<0.001), 3-years (HR 1.25, 95% CI 1.18-1.33, p<0.001), and 5-years (HR 1.23, 95% CI 1.17-1.30, p<0.001) post-op when accounting for other patient characteristics. In the senior adult cohort, adjuvant RT did not significantly impact mortality risk at 1-year (HR 1.11, 95% CI 0.90-1.36, p=0.316), 3-years (HR 0.94, 95% CI 0.81-1.08, p=0.423), or 5-years (HR 0.95, 95% CI 0.83-1.08, p=0.476) post-op relative to surgery alone (Table 4). Hazard ratios of OS did not significantly differ between treatment groups in senior adults, whereas the omission of adjuvant RT in those ages <80 years significantly increased mortality risk. The Cox proportional hazards model for 3-year mortality is shown in Table 5. Results of the 1-year and 5-year models can be found in Supplemental Tables 2 and 3, respectively.
Discussion
In this retrospective analysis of the NCDB, we found no significant difference in OS in senior adult patients with locally advanced HNSCC who underwent primary surgical resection with or without adjuvant RT, while adjuvant RT was associated with a significant increase in OS in adult patients. A significant difference in adjuvant RT utilization between senior adults (39.7%) and adults (57.3%) (p<0.001) likely represents a de-escalation of treatment as patients age (25). In senior adults, adjuvant RT did not significantly impact the adjusted mortality risk relative to surgery alone when accounting for differences in patient characteristics, primary site, and AJCC pathologic stage. In adults, treatment with surgery alone remained a significant predictor of mortality when accounting for the same variables.
The literature is mixed regarding which treatment modalities are best for senior adult patients with HNSCC. Roden et al. (2019) found no significant differences in OS in 159 older adult HNSCC patients (≥80 years) who received NCCN guideline-directed adjuvant therapy compared to those who deviated from the recommendations for adjuvant treatment (13). Moreover, Haehl et al. (2020) compared outcomes in 246 older adult HNSCC patients (age ≥75 years) who underwent definitive or adjuvant treatment with chemoradiation (CRT) or RT and found no significant differences in OS but noted prevalent treatment-associated toxicities (26). On the contrary, multiple other studies support that senior adults should be treated with conventional protocols if their baseline status allows (27–29). The ambivalence in recent literature could suggest an age-based transition point in outcomes where the risks associated with aggressive curative-intent therapy begin to mitigate its benefits.
While the benefits of adjuvant therapies are well-studied, so are the adverse effects. (13, 30, 31) Several studies report that physiologic changes contribute to age as a risk factor for complications from recommended therapy for advanced HNSCC, including aspiration pneumonia, dysphagia, mucositis, and dermatitis (5, 26, 27). Moreover, multiple unique studies of the SEER Medicare-linked database found that the development of treatment-associated morbidities was higher in older adults who received adjuvant RT compared to those who received surgery alone (32–34). However, the risk of adverse effects varies with tumor location and may influence shared decision-making regarding adjuvant therapy based on primary site. In addition to physiologic changes, several studies recognize increasing comorbidity burden as a contributor to increased susceptibility to treatment toxicity in older adult HNSCC patients, and geriatric assessment tools for treatment tolerability are yet to be optimized (35–37). Despite ambiguity about the role adjuvant therapy should play in senior adult patients, these data should encourage clinicians and patients to discuss the risks and benefits of the current standard of care based on patient goals, baseline functional status, and tumor primary site.
Our adjuvant RT treatment cohorts included patients who may have received partial courses to avoid introducing selection bias favoring healthier patients who are able complete adjuvant RT. Multiple studies suggest that early termination of adjuvant RT or prolonged interruptions have negative impacts on survival outcomes in older HNSCC patients, though advanced age itself is a debatable predictor of the likelihood of treatment completion (26, 37–40). This could represent an important distinction in the management of these patients by underscoring the importance of adjuvant RT course completion to receive survival benefits. Taken together, it is reasonable to recognize early termination or discontinuity of adjuvant RT as a risk factor for poorer outcomes and acknowledge that advanced age may pose increased challenges in treatment completion, be that from increased susceptibility to toxicity, increased comorbidity burden, or social factors limiting access to treatment.
One interpretation of our findings in the context of recent literature is that perhaps the survival benefit that adjuvant RT confers is limited to completed treatment courses, and interrupted or prematurely terminated adjuvant RT courses can cause adverse effects with negligible benefit. Our data captured both complete and incomplete treatment courses and concluded that adjuvant RT neither improves nor decreases OS compared to surgical resection alone, perhaps representing an average of the results seen in the aforementioned studies. Adjuvant RT should not be offered or withheld based on age, but great care should be taken to ensure that senior adult patients complete the entire course with minimal interruptions to reap survival benefits. If there is uncertainty regarding treatment tolerability or appropriate support to complete a full course, then it may be advisable to forego adjuvant RT to avoid survival curtailment from interruption/incompletion. Perhaps an “all or nothing” approach to adjuvant RT in senior adults can maximize OS for fit patients and minimize risks for those with greater frailty or fewer supportive resources.
Ultimately, there is a need for more comprehensive prospective data and clinical trials for senior adult HNSCC patients, as much of the current understanding is limited to large database studies or institutional retrospective analysis. We acknowledge several limitations to this study. The data contained in the NCDB do not contain the entire United States population and may be subject to biases regarding geography, urban versus rural reporting, and selection bias favoring patients who have access to a Commission on Cancer-approved hospital. The data’s reliability also depends on submission accuracy from participating hospitals. Additionally, analysis is limited to OS as the NCDB does not contain disease-specific mortality information, and in-depth analysis of specific treatment regimens is limited by the lack of specific radiation protocols and rationale for treatment selection included in the NCDB. Further research is needed to explore differences in disease-specific survival, disease-free survival, and quality of life measures to guide shared-decision making in this population. Prospective studies or clinical trials involving this population would provide a more comprehensive understanding of optimal treatments.
Conclusion
This study analyzed of the effects of adjuvant RT on OS in senior adult patients with locally advanced HNSCC using 16,504 cases from the NCDB. We found that the addition of adjuvant RT to definitive surgical treatment in senior adult patients (age ≥80 years) may not provide a similar OS benefit to that observed in younger patients.
Previous presentation statement
This article was presented at the ASTRO Multidisciplinary Head and Neck Cancers Conference, February 24-26, 2022, in Phoenix, AZ, and the Pennsylvania Academy of Otolaryngology- Head and Neck Surgery Annual Scientific Conference, June 17-18, 2022, in Hershey, PA.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The National Cancer Database Participant User Files are available through an application process. Requests to access these datasets should be directed to https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/puf/.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JB: study design, data collection, analysis, and manuscript writing/editing; MC: study design, data collection, analysis, and manuscript writing/editing; VB-A: manuscript writing/editing. AL: study design and manuscript writing/editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.973245/full#supplementary-material
References
1. United States Census Bureau. Demographic turning points for the united states: Population projections for 2020 to 2060 . Available at: https://www.census.gov/library/publications/2020/demo/p25-1144.html (Accessed Feb 17, 2022).
2. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the united state: Burdens upon an aging, changing nation. J Clin Oncol (2009) 27(17):2758–65. doi: 10.1200/JCO.2008.20.8983
3. Hutchins F, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med (1999) 341:2061–7. doi: 10.1056/NEJM199912303412706
4. Bouchardy C, Rapiti E, Blagojevic S, Vlastos A, Vlastos G. Older female cancer patients: Importance, causes, and consequences of undertreatment. J Clin Oncol (2007) 25(14):1858–69. doi: 10.1200/JCO.2006.10.4208
5. Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med (1981) 135(6):434–40.
6. VanderWalde NA, Fleming M, Weiss J, Chera BS. Treatment of older patients with head and neck cancer: A review. Oncologist (2013) 18(5):568–78. doi: 10.1634/theoncologist.2012-0427
7. Centers for Disease Control and Prevention. Leading cancers by age, sex, race, and ethnicity. Available at: https://gis.cdc.gov/Cancer/USCS/#/Demographics/ (Accessed Feb 17, 2022).
8. Lee YG, Kang EJ, Keam B, Choi J, Kim J, Park KU, et al. Treatment strategy and outcomes in locally advanced head and neck squamous cell carcinoma: a nationwide retrospective study (KCSG HN13-01). BMC Cancer (2020) 20(813):1–9. doi: 10.1186/s12885-020-07297-z
9. Lewis CM, Hessel AC, Roberts DB, Guo YZ, Holsinger FC, Ginsberg LE, et al. Prereferral head and neck cancer treatment: compliance with national comprehensive cancer network treatment guidelines. Arch Otolaryngol Head Neck Surg (2010) 136(12):1205–11. doi: 10.1001/archoto.2010.206
10. Boland GM, Chang GJ, Haynes AB, Chiang Y, Chagpar Y, Xing Y, et al. Association between adherence to national comprehensive cancer network treatment guidelines and improved survival in patients with colon cancer. Cancer (2013) 119(8):1593–601. doi: 10.1002/cncr.27935
11. Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol (2013) 121(6):1226–34. doi: 10.1097/AOG.0b013e3182922a17
12. Visser BC, Ma Y, Zak Y, Poultsides A, Norton JA, Rhoads KF. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB (Oxford) (2012) 14(8):539–47. doi: 10.1111/j.1477-2574.2012.00496.x
13. Roden D, Daniels K, Metkus J, Goldman R, Walsh A, Johnson J, et al. Evaluation of oncologic outcomes in head and neck cancer patients ≥80 years old base on adherence to NCCN guideline for postoperative adjuvant treatment. Head Neck (2019) 41:4128–35. doi: 10.1002/hed.25950
14. Moye VA, Chandramouleeswaran S, Zhao N, Muss HB, Weissler MC, Hayes DN, et al. Elderly patients with squamous cell carcinoma of the head and neck and the benefit of multimodality therapy. Oncologist (2015) 20(2):159–65. doi: 10.1634/theoncologist.2013-0325
15. National Comprehensive Cancer Network . Available at: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (Accessed March 1, 2022).
16. Alfouzan AF. Radiation therapy in head and neck cancer. Saudi Med J (2021) 42(3):247–54. doi: 10.15537/smj.2021.42.3.20210660
17. Alterio D, Marvaso G, Ferrari A, Volpe S, Orecchia R, Jereczek-Fossa BA. Modern radiotherapy for head and neck cancer. Sem Oncol (2019) 46(3):233–45. doi: 10.1053/j.seminoncol.2019.07.002
18. Eisbruch A, Foote RL, O’Sullivan B, Beitler JJ, Vikram B. Intensity-modulation radiation therapy for head and neck cancer: emphasis on the selection and delineation of the targets. Semin Radiat Oncol (2002) 12(3):238–49. doi: 10.1053/srao.2002.32435
19. Orlandi E, Bossi P. Toward personalized cancer care for elderly head and neck cancer patients. J Radiat Oncol Biol Phys (2017) 98(4):965–6. doi: 10.1016/j.ijrobp.2016.11.001
20. Coca-Pelaz A, Halmos GB, Strojan P, de Bree R, Bossi P, Bradford CR, et al. He role of age in treatment-related adverse events in patients with head and neck cancer: A systemic review. Head Neck (2019) 41(7):2410–9. doi: 10.1002/hed.25696
21. Mady LJ, Nilsen ML, Johnson JT. Head and neck cancer in the elderly: Frailty, shared decisions, and avoidance of low value care. Clin Geriatr Med (2018) 34(2):233–44. doi: 10.1016/j.cger.2018.01.003
22. Jabbour J, Dhillon HM, Shepherd HL, Sundaresan P, Milross C, Clark JR. The relationship between role preferences in decision-making and level of psychological distress in patients with head and neck cancer. Patient Educ Couns (2018) 101(10):1736–40. doi: 10.1016/j.pec.2018.05.023
23. Chi JJ. Reflections on shared decision making. Otolaryngol Head Neck Surg (2018) 159(5):809–10. doi: 10.1177/0194599818792212
25. Awan M, Akakpa KE, Shulka M, Graboyes EM, Pipkorn P, Puram SV, et al. The substantial omission of indivated postoperative radiotherapy in patients with advanced-stage oral cancer in the US-a call to action. JAMA Otolaryngol Head Neck Surg (2021) 147(10):907–9. doi: 10.1001/jamaoto.2021.1744
26. Haehl E, Ruhle A, David H, Kalckreuth T, Sprave T, Stoian R, et al. Radiothearpy for geriatric head-and-neck cancer patients: what is the value of standard treatment in the elderly? Rad Onc (2020) 15(31):1–12. doi: 10.1186/s13014-020-1481-z
27. Sarini J, Fournier C, Lefebvre JL, Bonafos G, Van JT, Coche-Dequeant B. Head and neck squamous cell carcinoma in elderly patients: A long-term retrospective review. Arch Otolaryngol Head Neck Surg (2020) 127(9):1089–92. doi: 10.1001/archotol.127.9.1089
28. Hosohawa S, Takahashi G, Okamura J, Imai A, Mochizuki D, Ishikawa R, et al. Management of elderly patients with head and neck carcinoma: analysis of outcomes for radical versus palliative treatment. Int J Clin Oncol (2020) 25:432–8. doi: 10.1007/s10147-019-01531-w
29. Jang IJH, Skanthakumar T, Tan HK, Tan NC, Soo KC, Iyer NG. Elderly patients with advanced head and neck carcinoma: Does aggressive treatment result in better outcomes? Otolaryngol Head Neck Surg (2019) 160(4):642–50. doi: 10.1177/0194599818815065
30. Clayman GL, Eicher SA, Sicard MW, Goepfert H. Surgical outcomes in head and neck cancer patients 80 years of age and older. Head Neck (1998) 20(3):216–23. doi: 10.1002/(SICI)1097-0347(199805)20:3<216::AID-HED6>3.0.CO;2-3
31. Van der Schroeff MP, Derks W, Hordijk GJ, de Leeuw RJ. The effect of age on survival and quality of life in elderly head and neck cancer patients: a long-term prospective study. Eur Arch Otorhinolaryngol (2007) 264(4):415–22. doi: 10.1007/s00405-006-0203-y
32. Hutcheson KA, Nurgalieva Z, Zhao H, Gunn GB, Giordano SH, Bhayani MK, et al. Two-year prevalence of dysphagia and related outcomes in head and neck cancer survivors: An updated SEER-Medicare analysis. Head Neck (2019) 41(2):479–87. doi: 10.1002/hed.25412
33. Francis DO, Weymuller EA Jr, Parvathaneni U, Merati AL, Yueh B. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol (2010) 119(6):391–7. doi: 10.1177/000348941011900605
34. Eytan DF, Blackford AL, Eisele DW, Fakhry C. Prevalence of comorbidities among older head and neck cancer survivors in the united states. Otolaryngol Head Neck Surg (2019) 160(1):85–92. doi: 10.1177/0194599818796163
35. Eytan DF, Blackford AL, Eisele DW, Fakhry C. Prevalence of comorbidities and effect on survival in survivors of human papillomavirus-related and human papillomavirus-unrelated head and neck cancer in the united states. Cancer (2019) 125:249–60. doi: 10.1002/cncr.31800
36. Maggiore R, Zumsteg ZS, BrintzenhofeSzoc K, Gajra KM, Korc-Grodzicki B, Epstein JB, et al. The older adult with locoregionally advanced head and neck squamous cell carcinoma: Knowledge gaps and future direction in assessment and treatment. Int J Radiat Oncol Biol Phys (2017) 98(4):868–83. doi: 10.1016/j.ijrobp.2017.02.022
37. Cervenka BP, Rao S, Bewley AF. Head and neck cancer and the elderly patient. Otolaryngol Clin North Am (2018) 51(4):741–51. doi: 10.1016/j.otc.2018.03.004
38. Mayer A, Wenzel W, Wallschlager D, Bostel T, Kruger M, Matthias C, et al. Adjuvant chemoradtiotherapy in elderly patients with head and neck cancer: a monoinstitutional, two-to-one pair-matching analysis. Strahlentherapie und Onkologie (2022) 198:159–70. doi: 10.1007/s00066-021-01890-2
39. Fesinmeyer MD, Mehta V, Blough D, Tock L, Ramsey SD. Effect of radiotherapy interruptions on survival in Medicare enrollees with local and regional head-and-Neck cancer. Interat J Rad Onc Biol Phys (2010) 78(3):675–81. doi: 10.1016/j.ijrobp.2009.08.004
Keywords: senior adult, adjuvant radiotherapy, head and neck cancer, survival, National Cancer Database
Citation: Butkus JM, Crippen M, Bar-Ad V and Luginbuhl A (2022) Impact of adjuvant radiation therapy after definitive surgery in senior adults >80 years old with advanced head and neck squamous cell carcinoma on overall survival. Front. Oncol. 12:973245. doi: 10.3389/fonc.2022.973245
Received: 19 June 2022; Accepted: 16 September 2022;
Published: 30 September 2022.
Edited by:
Dan Zandberg, University of Pittsburgh Medical Center, United StatesReviewed by:
Henry Soo-Min Park, Yale University, United StatesBeat Bojaxhiu, University Hospital Bern, Switzerland
Copyright © 2022 Butkus, Crippen, Bar-Ad and Luginbuhl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joann M. Butkus, Sm9hbm4uYnV0a3VzQHN0dWRlbnRzLmplZmZlcnNvbi5lZHU=
 Joann M. Butkus
Joann M. Butkus Meghan Crippen1
Meghan Crippen1 Adam Luginbuhl
Adam Luginbuhl