
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol. , 23 December 2022
Sec. Cancer Molecular Targets and Therapeutics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.972322
 Darshana Patil1
Darshana Patil1 Dadasaheb Akolkar1
Dadasaheb Akolkar1 Rajnish Nagarkar2
Rajnish Nagarkar2 Navin Srivastava1
Navin Srivastava1 Vineet Datta1
Vineet Datta1 Sanket Patil1
Sanket Patil1 Sachin Apurwa1
Sachin Apurwa1 Ajay Srinivasan1*
Ajay Srinivasan1* Rajan Datar1
Rajan Datar1Purpose: The selection of safe and efficacious anticancer regimens for treatment of patients with broadly refractory metastatic cancers remains a clinical challenge. Such patients are often fatigued by toxicities of prior failed treatments and may have no further viable standard of care treatment options. Liquid Biopsy-based multi-analyte profiling in peripheral blood can identify a majority of drug targets that can guide the selection of efficacious combination regimens.
Patients and methods: LIQUID IMPACT was a pilot clinical study where patients with advanced refractory cancers received combination anticancer treatment regimens based on multi-analyte liquid biopsy (MLB) profiling of circulating tumor biomarkers; this study design was based on the findings of prior feasibility analysis to determine the abundance of targetable variants in blood specimens from 1299 real-world cases of advanced refractory cancers.
Results: Among the 29 patients in the intent to treat (ITT) cohort of the trial, 26 were finally evaluable as per study criteria out of whom 12 patients showed Partial Response (PR) indicating an Objective Response Rate (ORR) of 46.2% and 11 patients showed Stable Disease (SD) indicating the Disease Control Rate (DCR) to be 88.5%. The median Progression-Free Survival (mPFS) and median Overall Survival (mOS) were 4.3 months (95% CI: 3.0 – 5.6 months) and 8.8 months (95% CI: 7.0 – 10.7 months), respectively. Toxicities were manageable and there were no treatment-related deaths.
Conclusion: The study findings suggest that MLB could be used to assist treatment selection in heavily pretreated patients with advanced refractory cancers.
Clinical management of patients with advanced broadly refractory cancers faces challenges due to unavailability of standard of care systemic anticancer regimens as well as adverse impact on health due to accumulated toxicities of prior failed treatments. The National Comprehensive Cancer Network (NCCN) guidelines for several cancers, hence, often recommend palliative care or clinical trial in such cases. We have previously reported findings from the RESILIENT trial which showed that de novo multi-analyte profiling of tumor tissue and tumor derived component in blood can inform selection of safe and efficacious treatments even in heavily pre-treated advanced refractory cancer cases (1). The benefit of such treatments could not be extended to several patients who were screened for inclusion in the RESILIENT trial, but excluded since they were unable to undergo an invasive biopsy due to anatomical constraints (proximity of the lesion to vital organs) or co-morbidities (2, 3). In order to address this challenge, we designed a Multi-analyte Liquid Biopsy (MLB) which evaluated circulating tumor analytes in peripheral blood to determine targetable (molecular and functional) features and guide selection of patient-specific label-agnostic combination anticancer regimens. The rationale for combination regimens rather than monotherapies was based on the hypothesis that combination regimens can be potentially beneficial in targeting multiple vulnerabilities and/or blocking escape or resistance mechanisms. The design of MLB combined the strengths of a multi-analyte tumor profiling with the convenience and safety of a liquid biopsy. The MLB evaluated cell free DNA (cfDNA) and circulating tumor associated cells (CTACs). ctDNA was profiled for gene variants including gain of copy, point mutations and indels. C-TACs were profiled by immunocytochemistry (ICC) to determine overexpression of therapeutic target proteins as well as by in vitro chemo resistance/chemo response profiling against a panel of FDA approved anticancer agents. We first ascertained feasibility of MLB in an analysis of 1299 patient specimens, where circulating tumor analytes were profiled for the molecular and functional features detailed above. This feasibility analysis helped estimate the proportion of patients where an actionable indication may be observed. Subsequently, we conducted LIQUID IMPACT, a pilot basket trial which evaluated and established the clinical utility of MLB to inform selection of safe and efficacious patient-specific combination regimens in a cohort of heavily pre-treated advanced refractory cancers. Study participants provided blood specimens which were evaluated to select personalized combination regimens with targeted and cytotoxic anticancer agents. Patients were assigned to treatment baskets based on targetable molecular indication(s) and the performance of each basket was evaluated in addition to the overall performance to determine suitability for a future study with an expanded cohort.
For the feasibility study cohort of 1299 cancer patients, leftover peripheral blood specimens from the study sponsor’s (Datar Cancer Genetics, DCG) test services and prior research studies were utilized. All patients (whose samples were used) had previously consented to research use of deidentified leftover samples and publication of deidentified data. The feasibility study did not involve the collection of new specimens.
LIQUID IMPACT (Trial Registration number CTRI/2019/02/017548, http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=31265) was a pilot prospective, single arm, non-randomized interventional study for evaluation of MLB-guided personalized combination treatment regimens in patients with advanced refractory solid organ cancers. The trial was approved by the EC of the Study Sponsor as well as the trial site (HCG-Manavata Cancer Centre, HCG-MCC) and conducted in accordance with ethical guidelines and the Declaration of Helsinki. The single-arm design of the trial was based on earlier RESILIENT [1], NCI-MATCH and ASCO TAPUR basket trials where the pilot phase did not include a control arm (4–6). Considering the diversity in cancer types and unique treatment history of each patient, the trial acknowledged that there can be no accurate external control for each patient.
The sample size of the prospective clinical study was determined based on the assumption that the ORR in the study cohort is <10%. Simon’s 2-stage design was used to validate the adequacy of cohort size. The null hypothesis (10% true response rate) was tested against a one-sided alternative. Initially, at least 10 patients were required to accrue; if there was 1 or no response, the study was to be terminated. Otherwise, at least 15 additional patients were required to accrue. The null hypothesis would be rejected if 5 or more responses were observed in 25 patients. With 25 evaluable patients, this design yields a type I error rate of 5% and power of 86% when the true response rate is 20%. The 95% CI of ORR was constructed using a binomial distribution (Clopper-Pearson estimation method). Patient demographics were analysed with descriptive statistics. Contingency tables described the categorical data with counts and percentages. Continuous data was summarized using median and range. CONSORT diagram, Waterfall Plot and Bar Graphs were used to summarize the data. Kaplan-Meier estimator was used to estimate survival function.
The feasibility cohort consisted of 1299 patients diagnosed with cancers of the breast (n = 304), colo-rectum (n = 167), non-small cell lung cancer (n = 130), pancreas (n = 94), ovary (n = 88), sarcoma (n = 61), prostate (n = 60), head and neck (n = 49), esophagus (n = 43), melanoma (n = 33) and central nervous system (n = 32) (Refer Supplementary Table S1 for details). Evaluations included cfDNA analysis by Next-Generation Sequencing (NGS), gene expression profiling by immunocytochemistry (ICC) and in vitro chemo response profiling on Circulating Tumor Associated Cells (C-TACs).
The LIQUID IMPACT prospective trial recruited patients with refractory solid organ cancers who had either failed at least two prior lines of systemic Standard of Care (SoC) anticancer treatments or where (further) systemic SoC treatment options were unavailable/unviable, and where an invasive biopsy to obtain tumor tissue (for de novo tumor profiling) was not possible. Eligible patients had radiologically measurable lesions with an Eastern Co-operative Oncology Group (ECOG) performance status of ≤ 2 and fitness as ascertained by the treating clinician. Patients who fulfilled the above criteria were counselled regarding the potential benefits and risks of the trial. Thereafter, patients who provided signed informed consents were enrolled.
All study participants provided only peripheral blood specimens. The process of multi-analyte liquid biopsy and formulation of patient-specific therapy recommendations (TR) has been described previously (7, 8). The complete details of investigations under multi-analyte liquid biopsy are provided in Supplementary Methods. The study did not evaluate PD-L1, Microsatellite Instability (MSI) or Mismatch Repair (MMR) status in patients since these evaluations required tumor tissue (which was neither available nor feasible to obtain in this cohort) and at the time of study recruitment there were no approved non-invasive tests to determine the status of these biomarkers in blood samples. Consequently, immune checkpoint inhibitors (ICIs) were not considered for inclusion in the treatment regimens.
In all patients in the prospective (LIQUID IMPACT) cohort, the treatment regimens were initially administered at lower (≤50%) doses, and gradually escalated based on close monitoring of toxicity. Patient-specific regimens were administered until either disease progression, death or dose limiting toxicity was encountered. Patients with disease progression were excluded from the trial and shifted to other SoC treatment (if available) or physician’s choice treatment or considered for best supportive care alone.
Patients in the prospective study underwent an 18F-Fluorodeoxyglucose Positron Emission Tomography – Computed Tomography (FDG PET-CT) scan before initiation of treatment to determine baseline status of the disease. The response was evaluated as per RECIST 1.1 criteria (9) from follow-up scans following at least two treatment cycles or 60 days of treatment, except in cases where the treating clinician advised evaluation in the interim.
The primary endpoint of the prospective pilot study was Objective Response Rate (ORR) defined as the percentage of patients who achieved Complete Response (CR) or Partial Response (PR) during the active study phase evaluated as per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria. Secondary outcome measures included clinical benefit rate (CBR), i.e. the proportion of participants with CR, PR or stable disease (SD) for ≥ 60 days, Progression Free Survival (PFS, time from commencement of treatment to disease progression or death during the active study phase), Overall Survival (OS, time from commencement of treatment to death). Quality of Life (QoL) was evaluated based on patient’s feedback on symptomatic and functional status at baseline and at study termination or most recently available follow-up.
All patients in the prospective study underwent periodic clinical evaluations to assess fitness for treatment as per study protocol. Adverse events (AEs) were recorded every week either during patient admissions or by telephonic follow-up. All AEs were reported as per NCI-CTCAE v5 criteria (10). Grade 3 and above AEs, if any, were followed up till resolution. Patients were followed up until study termination or patient exclusion (death/loss to follow-up/withdrawal of consent), whichever was earlier to determine Progression Free Survival (PFS).
Specimens from different cancer patients availing the study sponsor’s test services along with specimens from prior research studies conducted by the study sponsor (n = 1299) were evaluated for the feasibility of multi-analyte liquid biopsy, patient stratification and selection of tailored anticancer treatment regimens. The dendrogram in Supplementary Figure S1 provides details of specimens in the feasibility study cohort, sourced from the test service and research study arms, respectively.
Figure 1 illustrates the snapshot of molecular features in the feasibility study cohort. In the analysis of 1299 cancer patients, we identified molecular/functional features in 807 (62.12%) patients. TP53 mutations were seen in 559 (43%) cases, KRAS mutations in 156 (12%) cases and NRAS, GNAS and MYC mutations were found in <3% of cases each. Copy Number Variations (CNV) as gain of copy were observed frequently in CCND, CDK, MYC, MET and FGFR. The multigene panel does not provide information on loss of gene copy. Overexpression of EGFR, VEGF, VEGFR and mTOR proteins as determined by ICC were seen in up to 805 (62%) cases indicating the possibility of selection of respective targeted therapies against EGFR, angiogenesis and mTOR pathways. Among other angiogenesis pathway targets, PDGFR and FGFR based indications for anti-angiogenesis agents were observed in 13 (1%) and 52 (4%) cases respectively while further indications for targeting mTOR pathway based on variants in PIK3CA were detected in 143 (11%) cases. Targetable indications in HER2 (ERBB) and ER (ESR) were identified in 52 (4%) and 39 (3%) cases.
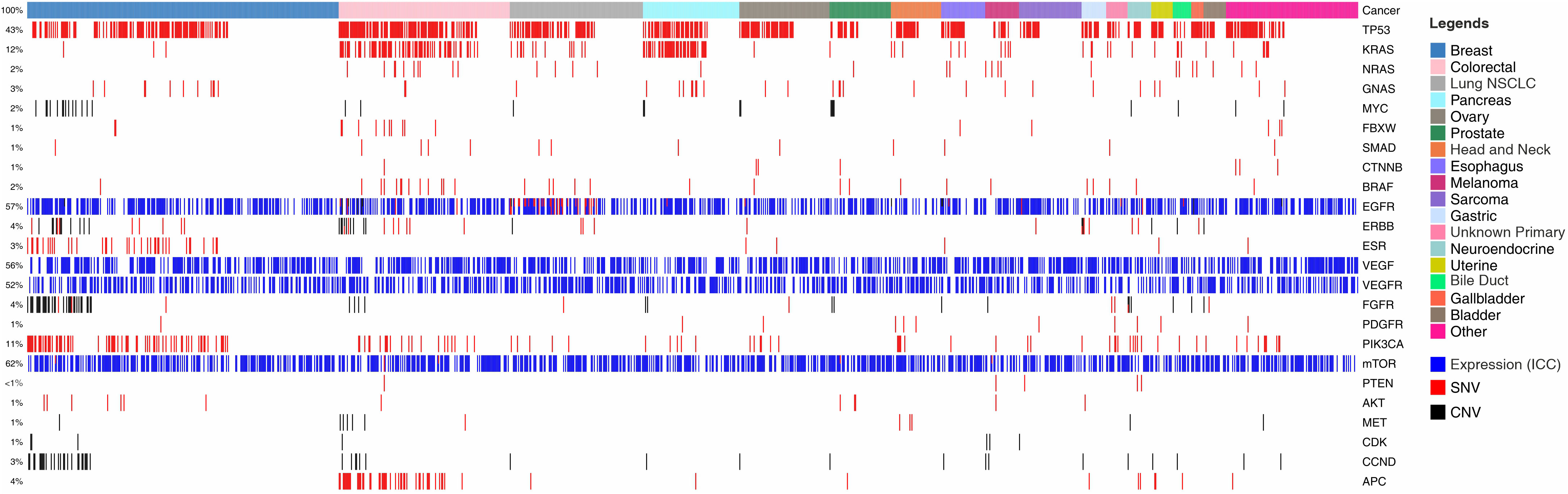
Figure 1 Landscape of Genomic Alterations in the Feasibility Study Cohort. Colour coded boxes in the topmost row indicate cancer types. Each vertical column indicates a single patient. Features such as Single Nucleotide Variations (SNVs) are represented by red colour; Copy Number Variations (CNVs; gain) by black; and ICC based gene expression analysis by blue. Gene names are enlisted on right Y-axis and the % of specimens harbouring the different features are enlisted on left Y-axis.
In vitro chemo response profiling data was also available for 1299 specimens where the C-TACs were treated with a panel of 30 anticancer drugs and the response determined. The panel included anticancer agents administered as SoC options (NCCN guidelines) as well as those which were ‘off-label’ drugs (non-SoC options). Among the 1299 specimens, C-TACs in 1087 (84%) showed response (cytotoxicity) to at least 1 SoC drug, C-TACs in 827 samples (64%) showed response to 2 or more drugs and C-TACs in 598 specimens (46%) showed response to 3 or more drugs. When the C-TACs were assessed for response to off-label (non-SoC) drugs, 1182 (91%) were responsive to ≥ 1 drug, 1039 (80%) were responsive to ≥ 2 drugs and 896 (69%) were responsive to ≥ 3 drugs respectively. The analysis thus makes available previously unexplored potential treatment options for heavily pretreated cancer patients where SoC options are either unavailable or unviable. The cancer-wise details of these sensitivities are presented in Supplementary Table S2, while Supplementary Figure S2 shows the % of specimens per cancer type which were responsive to each of the 30 anticancer agents tested. These findings suggested that treatment with either of these anticancer agents in a label agnostic manner may have potential clinical benefit.
For the prospective LIQUID IMPACT study cohort, 64 patients were screened between Feb 2019 and Oct 2019, of whom 56 were recruited, 43 were enrolled (received MLB guided therapy recommendations) and 29 patients eventually started treatment as per MLB. Thirty five patients were excluded between screening and start of treatment for various reasons including non-measurable lesions (n = 3), unfavourable or deteriorating Eastern Co-operative Oncology Group Performance Score (ECOG PS, n = 14), withdrawal of consent (n = 5) or absence of therapeutically targetable molecular indications (n = 13). Out of the 29 patients who started treatment, 3 were excluded within the first week (prior to any follow-up evaluation) for either ECOG PS deterioration (n = 1), withdrawal of consent (n = 1) and loss to follow up (n = 1), respectively. Twenty-six patients received MLB guided treatments and were evaluable as per study criteria. The CONSORT diagram (Figure 2) depicts the study structure and patient flow. Patient demographics, baseline status and prior treatments are provided in Supplementary Tables S3-S5 respectively.
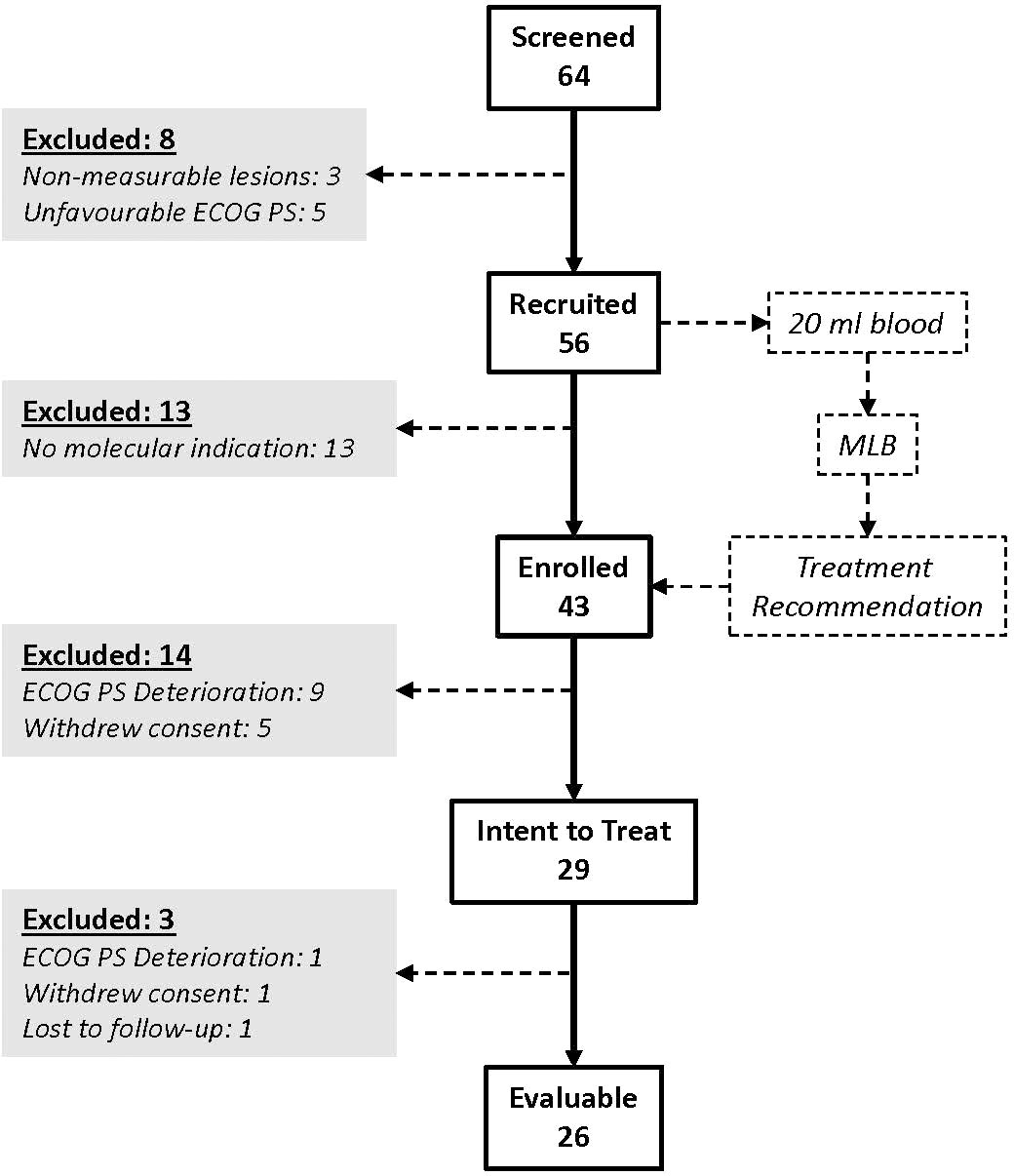
Figure 2 LIQUID IMPACT CONSORT Diagram. Among the 64 patients who were screened, 29 eventually started treatment (ITT population) and 26 were finally evaluable based on study criteria. Patients provided blood specimens at baseline which were used for multi-analyte liquid biopsy (MLB).
The landscape of molecular features in the prospective Intent to Treat (ITT) population is depicted in Figure 3. Single nucleotide variations (SNV) in TP53 were a frequently encountered (N = 11, 37.9%) feature followed by those in PIK3CA (N = 4, 13.8%) and KRAS/NRAS (N = 2, 6.9%). Among the ITT population, actionable SNVs were detected in 9 patients, of whom 8 were evaluable; actionable CNVs were detected in 2 patients, all of whom were evaluable, and gene overexpression was detected by ICC in 22 patients, of whom 19 were evaluable. Patient-wise actionable gene alterations that formed the basis for therapy selection are indicated in Supplementary Dataset.
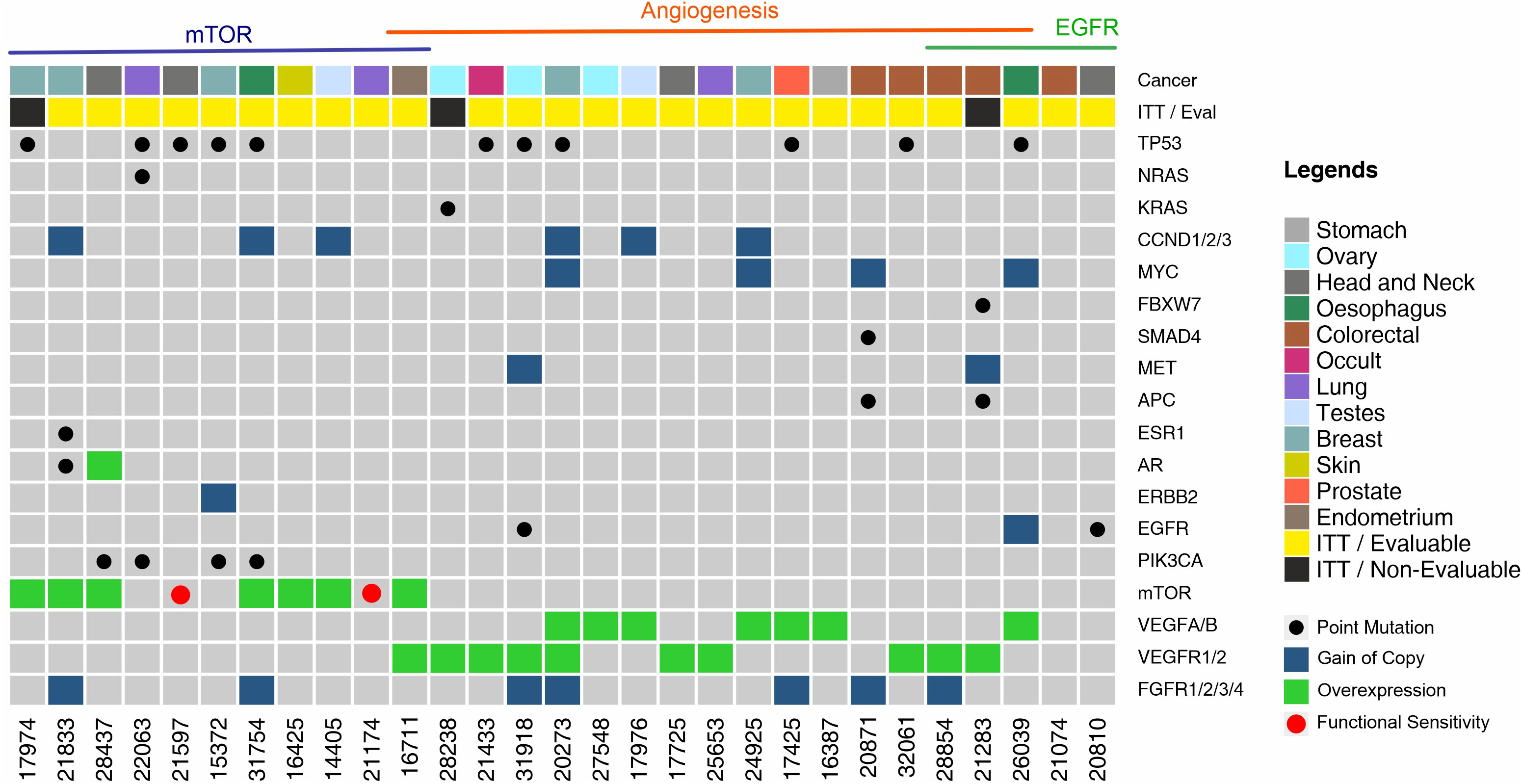
Figure 3 Landscape of Genomic Alterations in the Prospective Intent to Treat (ITT) Population. Each vertical column indicates a single patient (patient number in the bottom X-axis). Vertically stacked grey boxes in each column indicate individual genes (gene names on right Y-axis). Black dots within each box indicates a point mutation (single nucleotide variation), red dots indicate in vitro sensitivity (chemo response profiling, CRP), blue shaded boxes indicate gain of gene copy (CNV gain), green shaded boxes indicate gene overexpression. Patients are grouped by observed targetable molecular indications. Colour coded boxes in the topmost row indicate cancer types.
Twenty-six of the initial 29 patients who received MLB guided treatments were evaluable for response as per study criteria. Among 11 patients (ITT) who were assigned an mTOR inhibitor, 10 were eventually evaluable. Among 17 patients (ITT) who were assigned an angiogenesis inhibitor, 15 were evaluable. Finally, among 5 patients (ITT) who received an EGFR inhibitor based on EGFR/ERBB2 activation or KRAS wild type, 4 were evaluable. One patient received an mTOR inhibitor as well as an angiogenesis inhibitor and was evaluated in both treatment baskets. Among 3 patients who received angiogenesis inhibitor as well as EGFR inhibitor, 2 were evaluable and were evaluated in both respective treatment baskets. Endocrine therapy was administered to 2 patients in addition to cytotoxic and targeted agents. Details of cancer types, treatment baskets and treatments are provided in Supplementary Table S6. Patient-wise details of prior treatments, MLB-indications and treatments are provided in Supplementary Dataset.
Partial Response (PR) was observed in 12 of 26 evaluable patients yielding an ORR of 46.2% (41.4% in the ITT population of 29). Waterfall Charts depict the best response (Figure 4A) of all 26 patients. Patients in the evaluable subset were followed up for a median duration of 4.6 months (range: 1.3 months – 13.2 months). PRs were observed in 5 (50%) of 10 evaluable patients who received an mTOR inhibitor, 8 (53.3%) of 15 evaluable patients who received an angiogenesis inhibitor and 2 (50%) of 4 evaluable patients who received an EGFR inhibitor. Though not an endpoint of the study, Stable Disease (SD) for more than 60 days was observed in 11 of the 26 overall evaluable patients, yielding a Disease Control Rate (DCR) of 88.5% in the evaluable population.
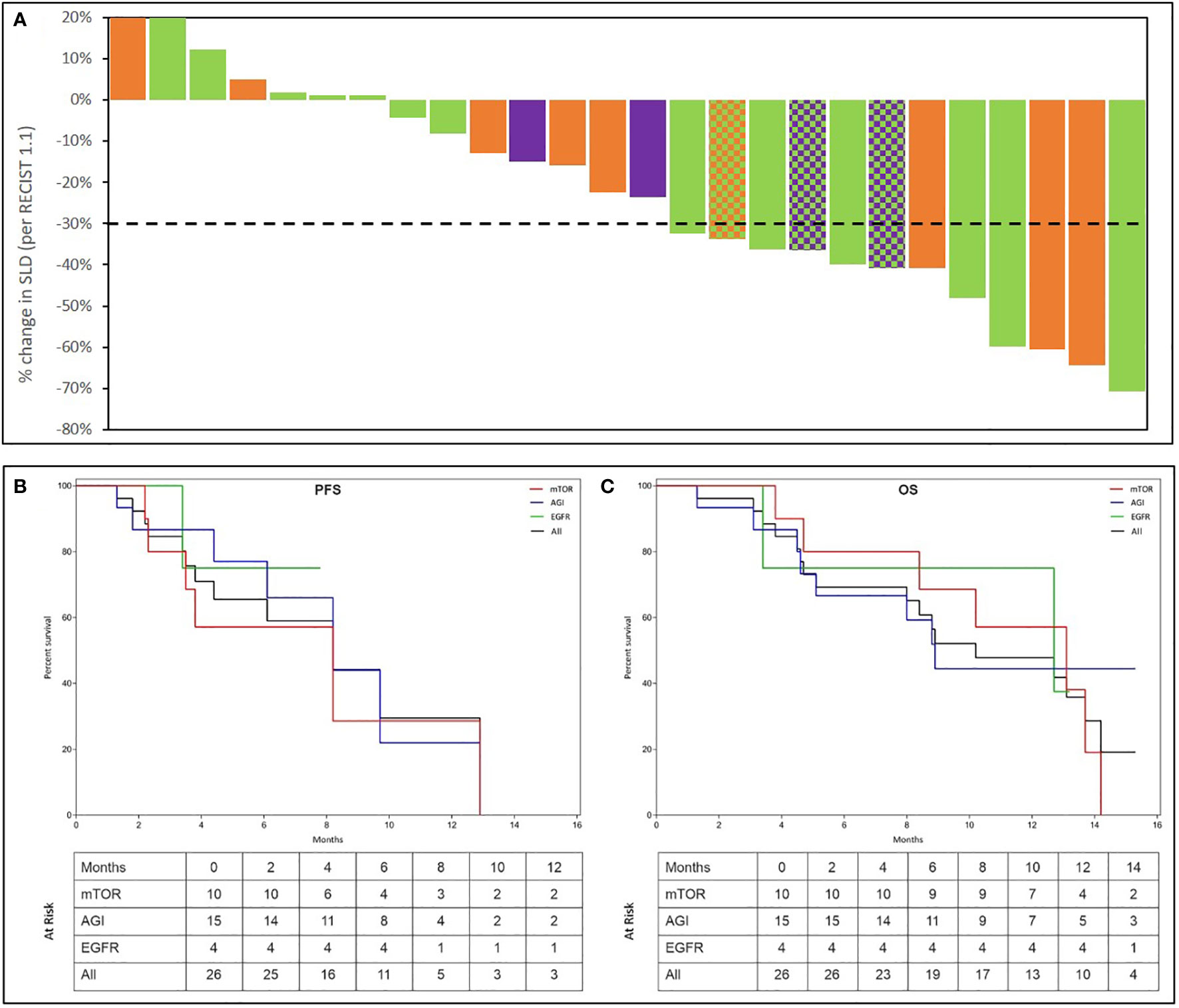
Figure 4 Summary of Outcomes. Treatment Response was evaluated as per RECIST 1.1. Percent change in dimensions of target lesions (Sum of Largest Diameters, SLD) between baseline and at best response are represented in the top panel (A). Each bar represents the response in a unique patient. Patients are arranged in descending order of change (%) in SLD. Bars are colour coded as per the molecular indication: Orange: mTOR; Green: Angiogenesis; Purple: EGFR. In 3 patients, 2 signalling pathways were detected which are represented by a pattern of 2-colours, each corresponding to the respective pathway. The Kaplan Meier plots in the bottom panel show the Progression Free Survival (PFS (B)) and Overall Survical (OS (C)) in all patients as well as in sub-cohorts where activation of EGFR, Angiogenesis (AGI) or mTOR signalling was observed. Patients at risk at each milestone are indicated in the inset table.
Median Progression Free Survival (mPFS) in the evaluable subset was 4.3 months (95% CI: 3.0 – 5.6 months). Similarly, mPFS was 3.8 months among 10 patients with mTOR activation, 5.2 months among 15 patients with angiogenesis activation and 7.0 months among 4 patients with EGFR activation. While true median Overall Survival (mOS) is indeterminate (several patients were lost to follow-up due to Covid pandemic), considering OS data censored at the most recent follow-up (Oct 2020), the mOS in the entire evaluable cohort was 8.8 months (95% CI: 7.0 – 10.7 months), 10.7 months among 10 patients with mTOR activation, 8.8 months among 15 patients with angiogenesis activation, 9.5 months among 4 patients with EGFR activation. The Kaplan Meier plots of PFS and OS are depicted in Figures 4B, C. Details of overall response and per study basket are provided in Supplementary Table S7.
All the 29 patients in the Intent to Treat (ITT) population were evaluated for Therapy Related Adverse Events (TR-AE). TR-AEs of any grade were reported in 25 (86.2%) of the overall population whereas Grade III and IV AEs were observed in 15 (51.7%) and 3 (10.3%) of the ITT cohort. Grade III TR-AEs which were considered as SAEs were observed in 34.5% of the overall population. All AEs were transient, acute (non-chronic), managed by standard procedures and followed up until resolution; most Grade IV AEs and Grade III SAEs were resolved between 48-72 hours. There were no treatment related deaths. Overall and basket-wise profiles of AEs are provided in Supplementary Table S8.
Quality of Life was measured based on the ECOG Performance Status as well as a brief questionnaire which evaluated the patients’ symptomatic status, ability to perform activities of daily living (ADL) and the ability to perform additional/more strenuous activities. Patients’ feedback was obtained at baseline and every month until study completion or exclusion. Within the evaluable cohort, 25 patients (96%) indicated stable to decreased symptomatic/ECOG status while 12 patients (46%) indicated stable to improved functional/ADL status.
We report the clinical potential of MLB-guided treatment selection in a cohort of heavily pre-treated cases of advanced refractory cancers. The significant ORR of 46.2% and manageable toxicities suggest that multi-analyte evaluations can identify multiple actionable vulnerabilities of a cancer, which can be treated with combination regimens of targeted and cytotoxic anticancer agents. The response rates reported in this pilot study justify the need for a larger cohort. The outcomes in our study are clearly superior to the outcomes reported in prior studies which attempted to personalize anticancer treatments based on univariate molecular profiling of the tumor for selection of label-agnostic monotherapies (4, 5, 11–17). The modest clinical benefits, if at all, reported by these prior trials highlight the limitations of their design.
MLB detected potentially targetable molecular indications in several patients, the abundance of these targets being generally concordant with what has been reported in other studies such as NCI-MATCH, SHIVA, MOSCATO and I-PREDICT (11, 12, 15, 17).
We show that in addition to gene variants (point mutations/copy number variations), MLB can provide information on upregulation of targetable pathways via ICC profiling of CTCs, akin to immunohistochemistry (IHC) on tumor tissue or cytology specimens. In a recent large (>30,000 specimens) cohort study, we have described the potential of ICC profiling of CTCs to provide diagnostically relevant information without requirement of tumor tissue (8). In the present paper, we describe the potential application of ICC towards theranostic guidance.
While molecular indications form the basis for selection of targeted and endocrine agents, there are fewer biomarkers for selection of cytotoxic anticancer agents which remain the mainstay of treatments in several cancers. We have previously evaluated and described the accuracy and utility of C-TACs in more than 5000 patients from peripheral blood for in vitro chemo response profiling (18). In vitro chemo response profiling of viable tumor cells from a tissue biopsy has previously demonstrated limited potential (19, 20), and has been unviable due to the inability to obtain viable tumor cells for de novo analysis. In these regards, the analysis of CTCs and C-TACs has shown some promise (18, 21–25). In the present study, in vitro chemo response profiling of C-TACs was used to guide selection of cytotoxic anticancer agents which were used in conjunction with targeted anticancer agents. MLB encompasses clonal variations arising from tumor heterogeneity and also captures the profile of the ‘leading edge’ tumour cells, which may well be the most aggressive elements for metastatic spread and invasiveness (26).
Studies have shown that combination regimens yield improved benefits over monotherapy (27, 28). Illustratively, the combination of Everolimus and Lenvatinib has higher efficacy over Everolimus monotherapy in metastatic RCC (29). The combination of Alpelisib and Fulvestrant has yielded higher response rates (~26%) in ER+/HER2- metastatic breast cancers than Alpelisib monotherapy (30, 31). It has been shown that monotherapy for blockade of VEGF/VEGFR signalling often yields transient responses followed by eventual resistance (32, 33), owing to which combination strategies to achieve tandem blockade of multiple signalling pathways have been proposed as a viable strategy (34–37).
Prior meta-analyses have shown that it is possible to safely administer de novo drug combinations in patients without increasing the risk of adverse events (AEs) (38–40). Where the cancer has progressed following failure of multiple prior systemic lines of therapy (as in the current study cohort), patients tend to be at an inherently higher risk of AEs due to cumulative toxicities from prior treatments. Hence, all patients were administered initial doses at ≤50% and with controlled dose escalation. This strategy effectively controlled AEs; while Grade III and IV AEs were observed, they were limited, transient and manageable. Significantly, there were no treatment related mortalities. This pilot study provides evidence of the efficacy and reasonable safety of patient specific label-agnostic combination anticancer regimens when selected on the basis of MLB. The concept of MLB-guided personalized anticancer regimens may have potential in therapy naïve patients recently diagnosed with difficult to treat cancers. However, the present study cohort did not include treatment naïve patients, and may be evaluated in a separate future study. The study findings are encouraging and justify a future larger multi-arm trial with various molecular subtype baskets for treatment of broadly refractory cancers.
The LIQUID IMPACT trial was intended to be a pilot study to evaluate MLB for its potential and clinical utility to inform selection of safe and efficacious patient-specific combination regimens in advanced refractory cancers. Owing to the pilot nature of the trial, the size of each basket as well as the entire cohort was limited and the study design excluded a traditional control arm; this design was based on the aims and design of the NCI-MATCH and ASCO TAPUR basket trials where the pilot phase did not include a control arm (4–6). The outcome of the study shows promise for and also justifies the need for a future larger cohort study in the intended use population and also evaluate the potential of this approach in treatment naïve patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Datar Cancer Genetics Limited Institutional Ethics Committee and Manavata Clinical Research Institute Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: RN, DP, DA, VD, AS, RD; Data curation: DA, SP, SA, AS; Formal analysis: DP, DA, NS, SP, SA, AS; Funding acquisition: RD; Investigation: RN, DA, NS, SP, SA; Methodology: RN, DP, DA, NS, SA, AS, RD; Project administration: RN, DP, DA, NS, VD; Resources: DA, RD; Software: DA, SA, AS; Supervision: RN, DP, DA, VD, AS, RD; Visualization; DP, DA, AS, RD; Writing – original draft: AS, RD; Writing – review and editing: RN, DP, DA, NS, VD, AS, RD. All authors contributed to the article and approved the submitted version.
The study was wholly supported by the Sponsor, Datar Cancer Genetics Pvt Ltd. The funder was not involved in the study design, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors are grateful towards all study participants and their caregivers. The contributions of HCG Manavata Cancer Centre as well as the staff of the Study Sponsor (DCG) towards managing various clinical, operational and laboratory aspects of the study are also acknowledged with gratitude.
DP, DA, NS, VD, SP, SA and AS are employees of the Study Sponsor DCG. RD is the founder of the Study Sponsor.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.972322/full#supplementary-material
1. Nagarkar R, Patil D, Crook T, Datta V, Bhalerao S, Dhande S, et al. Encyclopedic tumor analysis for guiding treatment of advanced, broadly refractory cancers: results from the RESILIENT trial. Oncotarget (2019) 10(54):5605–21. doi: 10.18632/oncotarget.27188
2. Sennerstam RB, Franzén BSH, Wiksell HOT, Auer GU. Core-needle biopsy of breast cancer is associated with a higher rate of distant metastases 5 to 15 years after diagnosis than FNA biopsy. Cancer Cytopathol (2017) 125(10):748–56. doi: 10.1002/cncy.21909
3. Hiley CT, Le Quesne J, Santis G, Sharpe R, de Castro DG, Middleton G, et al. Challenges in molecular testing in non-small-cell lung cancer patients with advanced disease. Lancet (London England) (2016) 388(10048):1002–11. doi: 10.1016/S0140-6736(16)31340-X
4. Rodon J, Soria J-C, Berger R, Miller WH, Rubin E, Kugel A, et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat Med (2019) 25(5):751–8. doi: 10.1038/s41591-019-0424-4
5. Harris L, Chen A, Dwyer P, Flaherty K, Hamilton S, McShane L, et al. Abstract B080: Update on the NCI-molecular analysis for therapy choice (NCI-MATCH/EAY131) precision medicine trial. Mol Cancer Ther (2018) 17(1 Supplement):B080–0. doi: 10.1158/1535-7163.TARG-17-B080
6. ASCO TAPUR. Available at: https://www.tapur.org/.
7. Akolkar D, Patil D, Crook T, Limaye S, Page R, Datta V, et al. Circulating ensembles of tumor-associated cells: A redoubtable new systemic hallmark of cancer. Int J Cancer (2020) 146(12):3485–94. doi: 10.1002/ijc.32815
8. Gaya A, Crook T, Plowman N, Ranade A, Limaye S, Bhatt A, et al. Evaluation of circulating tumor cell clusters for pan-cancer noninvasive diagnostic triaging. Cancer Cytopathol (2021) 129(3):226–38. doi: 10.1002/cncy.22366
9. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
10. Division of Cancer Treatment and Diagnostics, National Cancer Institute. Common terminology criteria for adverse events v5.0. (2017). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50. Last accessed on 12-Dec-2022.
11. Flaherty KT, Gray RJ, Chen AP, Li S, McShane LM, Patton D, et al. Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National cancer institute molecular analysis for therapy choice (NCI-MATCH). J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(33):3883–94. doi: 10.1200/JCO.19.03010
12. Le Tourneau C, Delord J-P, Gonçalves A, Gavoille C, Dubot C, Isambert N, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol (2015) 16(13):1324–34. doi: 10.1016/S1470-2045(15)00188-6
13. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: Results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(6):536–42. doi: 10.1200/JCO.2017.75.3780
14. Von Hoff DD, Stephenson JJJ, Rosen P, Loesch DM, Borad MJ, Anthony S, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28(33):4877–83. doi: 10.1200/JCO.2009.26.5983
15. Massard C, Michiels S, Ferté C, Le Deley M-C, Lacroix L, Hollebecque A, et al. High-throughput genomics and clinical outcome in hard-to-Treat advanced cancers: Results of the MOSCATO 01 trial. Cancer Discovery (2017) 7(6):586–95. doi: 10.1158/2159-8290.CD-16-1396
16. Tsimberidou A-M, Iskander NG, Hong DS, Wheler JJ, Falchook GS, Fu S, et al. Personalized medicine in a phase I clinical trials program: the MD Anderson cancer center initiative. Clin Cancer Res an Off J Am Assoc Cancer Res (2012) 18(22):6373–83. doi: 10.1158/1078-0432.CCR-12-1627
17. Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med (2019) 25(5):744–50. doi: 10.1038/s41591-019-0407-5
18. Crook T, Gaya A, Page R, Limaye S, Ranade A, Bhatt A, et al. Clinical utility of circulating tumor-associated cells to predict and monitor chemo-response in solid tumors. Cancer Chemother Pharmacol (2021) 87(2):197–205. doi: 10.1007/s00280-020-04189-8
19. Schrag D, Garewal HS, Burstein HJ, Samson DJ, Von Hoff DD, Somerfield MR. American Society of clinical oncology technology assessment: chemotherapy sensitivity and resistance assays. J Clin Oncol Off J Am Soc Clin Oncol (2004) 22(17):3631–8. doi: 10.1200/JCO.2004.05.065
20. Burstein HJ, Mangu PB, Somerfield MR, Schrag D, Samson D, Holt L, et al. American Society of clinical oncology clinical practice guideline update on the use of chemotherapy sensitivity and resistance assays. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29(24):3328–30. doi: 10.1200/JCO.2011.36.0354
21. Ignatiadis M, Lee M, Jeffrey SS. Circulating tumor cells and circulating tumor DNA: Challenges and opportunities on the path to clinical utility. Clin Cancer Res an Off J Am Assoc Cancer Res (2015) 21(21):4786–800. doi: 10.1158/1078-0432.CCR-14-1190
22. Rawal S, Yang Y-P, Cote R, Agarwal A. Identification and quantitation of circulating tumor cells. Annu Rev Anal Chem (Palo Alto Calif) (2017) 10(1):321–43. doi: 10.1146/annurev-anchem-061516-045405
23. Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res an Off J Am Assoc Cancer Res (2006) 12(14 Pt 1):4218–24. doi: 10.1158/1078-0432.CCR-05-2821
24. Balakrishnan A, Koppaka D, Anand A, Deb B, Grenci G, Viasnoff V, et al. Circulating tumor cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci Rep (2019) 9(1):7933. doi: 10.1038/s41598-019-44404-y
25. Khoo BL, Lee SC, Kumar P, Tan TZ, Warkiani ME, Ow SGW, et al. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget (2015) 6(17):15578–93. doi: 10.18632/oncotarget.3903
26. Maffeis V, Nicolè L, Cappellesso RRAS, Plasticity C. And tumor budding in colorectal cancer. Front Oncol (2019) 9:1255. doi: 10.3389/fonc.2019.01255
27. Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-Patient variability without drug additivity or synergy. Cell (2017) 171(7):1678–1691.e13. doi: 10.1016/j.cell.2017.11.009
28. Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, et al. Combination therapy in combating cancer. Oncotarget (2017) 8(23):38022–43. doi: 10.18632/oncotarget.16723
29. Leonetti A, Leonardi F, Bersanelli M, Buti S. Clinical use of lenvatinib in combination with everolimus for the treatment of advanced renal cell carcinoma. Ther Clin Risk Manage (2017) 13:799–806. doi: 10.2147/TCRM.S126910
30. Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, et al. Phosphatidylinositol 3-kinase α-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: Results from the first-in-Human study. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(13):1291–9. doi: 10.1200/JCO.2017.72.7107
31. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med (2019) 380(20):1929–40. doi: 10.1056/NEJMoa1813904
32. Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist (2015) 20(6):660–73. doi: 10.1634/theoncologist.2014-0465
33. Haibe Y, Kreidieh M, El Hajj H, Khalifeh I, Mukherji D, Temraz S, et al. Resistance mechanisms to anti-angiogenic therapies in cancer. Front Oncol (2020) 10:221. doi: 10.3389/fonc.2020.00221
34. Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L, et al. Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer (2020) 9(3):338–57. doi: 10.1159/000505695
35. Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol (2019) 12(1):27. doi: 10.1186/s13045-019-0718-5
36. Gotink KJ, Verheul HMW. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis (2010) 13(1):1–14. doi: 10.1007/s10456-009-9160-6
37. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer (2019) 18(1):60. doi: 10.1186/s12943-019-0974-6
38. Liu S, Nikanjam M, Kurzrock R. Dosing de novo combinations of two targeted drugs: Towards a customized precision medicine approach to advanced cancers. Oncotarget (2016) 7(10):11310–20. doi: 10.18632/oncotarget.7023
39. Nikanjam M, Liu S, Kurzrock R. Dosing targeted and cytotoxic two-drug combinations: Lessons learned from analysis of 24,326 patients reported 2010 through 2013. Int J Cancer (2016) 139(9):2135–41. doi: 10.1002/ijc.30262
Keywords: encyclopedic tumor analysis, liquid biopsy, multi-analyte liquid biopsy, precision oncology, combination regimens
Citation: Patil D, Akolkar D, Nagarkar R, Srivastava N, Datta V, Patil S, Apurwa S, Srinivasan A and Datar R (2022) Multi-analyte liquid biopsies for molecular pathway guided personalized treatment selection in advanced refractory cancers: A clinical utility pilot study. Front. Oncol. 12:972322. doi: 10.3389/fonc.2022.972322
Received: 18 June 2022; Accepted: 01 December 2022;
Published: 23 December 2022.
Edited by:
Zhijie Wang, National Cancer Center of China, ChinaReviewed by:
Lucia Guadalupe Taja Chayeb, National Institute of Cancerology (INCAN), MexicoCopyright © 2022 Patil, Akolkar, Nagarkar, Srivastava, Datta, Patil, Apurwa, Srinivasan and Datar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ajay Srinivasan, YWpheXNAZGF0YXJwZ3gub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.