
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 14 November 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.968064
This article is part of the Research TopicPrimary and Acquired Resistance in Lung CancerView all 10 articles
 Sara Fancelli1,2
Sara Fancelli1,2 Enrico Caliman1,2
Enrico Caliman1,2 Francesca Mazzoni3
Francesca Mazzoni3 Luca Paglialunga2
Luca Paglialunga2 Marta Rita Gatta Michelet3
Marta Rita Gatta Michelet3 Daniele Lavacchi2
Daniele Lavacchi2 Rossana Berardi4
Rossana Berardi4 Giulia Mentrasti4
Giulia Mentrasti4 Giulio Metro5
Giulio Metro5 Ilaria Birocchi5
Ilaria Birocchi5 Angelo Delmonte6
Angelo Delmonte6 Ilaria Priano6
Ilaria Priano6 Camilla Eva Comin1,7
Camilla Eva Comin1,7 Francesca Castiglione8
Francesca Castiglione8 Caterina Bartoli8
Caterina Bartoli8 Luca Voltolini1,9
Luca Voltolini1,9 Serena Pillozzi3†
Serena Pillozzi3† Lorenzo Antonuzzo1,2,3*†
Lorenzo Antonuzzo1,2,3*†Background: KRAS is commonly mutated in non-small cell lung cancer (NSCLC); however, the prognostic and predictive impact of each G12 substitution has not been fully elucidated. The approval of specific G12C inhibitors has modified the idea of KRAS “undruggability”, and although the first-line standard consists of immune checkpoint inhibitors (ICIs) with or without chemotherapy, as suggested at ASCO 2022, the outcome in KRAS-mutated population is still controversial.
Methods: We retrospectively described the clinical and pathological characteristics of a homogeneous G12 mutated cohort of 219 patients treated in four Italian oncologic units. We evaluated the outcome (PFS at 18 months and OS at 30 months) of those who underwent standard first-line treatment according to PD-L1 status, focusing on differences across single mutations.
Results: In the study population, 47.9% of patients harbor the KRAS G12C mutation; 20.5%, G12V; 17.4%, G12D; and 8.2%, G12A. Smoking was a common behavior of patients harboring transversions and transition mutations. PD-L1 expression does not show particular distribution in the case series, although we recorded a prevalence of PD-L1 <1% in G12V (51.4%) compared to G12A (26.7%). ICIs alone was the clinician’s choice in 32.7% of patients, and the chemo-immune combination in 17.3% of patients. We described the independent prognostic role of young age (p = 0.007), female gender (p = 0.016), and an ICI-based regimen (p = 0.034) regardless of mutations. Overall, our data confirm the worst prognostic value of G12V mutation apart from treatment choice unlike the other major mutations (C, D, and A) that showed a favorable trend in PFS.
Conclusions: KRAS G12 mutations are confirmed to have different characteristics, and the outcome is influenced by ICI first-line regimen. This study provides valuable information for further analysis in the future.
RAS genes encode for a family of small membrane-bound guanosine-triphosphate (GTP) binding proteins involved in the regulation of cell proliferation, growth, and mobility, as well as in apoptosis mechanisms through several downstream effectors. RAS mutations lead to protein conformation changes resulting in the perpetual activation of downstream pathways and a complete independence from the upstream signaling (1). Fifteen percent to 25% of non-small cell lung cancer (NSCLC) patients harbor Kras mutations that, in 95% of cases, rely on base substitution in exon 2 (2). According to the COSMIC database, G12C, G12V, G12D, and G12A mutations are the most common KRAS single-amino acid substitutions in lung adenocarcinoma (LUAD) (3), unlike the squamous cell histotype in which KRAS mutations are rare (3%–5%) (4, 5). The prognostic and predictive value of Kras mutations is still controversial. Although the prognosis appears to be correlated to the KRAS’ codon damage and to the setting analyzed (6–8), the predictive value did not find a precise characterization. In fact, the clinical trials evaluating the use of different agents [TKI, chemotherapy (CT), antiangiogenics, or different combinations of these] were inconclusive in the KRAS-mutated population (9–11). Recent results from the phase 2 trials CodeBreaK 100 and KRYSTAL-1 (12, 13) led to FDA approval of KRAS G12C selective inhibitors, i.e., sotorasib in May 2021 and the new drug application for the use of adagrasib in February 2022, for patients with pretreated KRAS G12C-NSCLC (14, 15). Despite the encouraging results, neither sotorasib nor adagrasib is still recommended as a first-line treatment in advanced KRAS G12C LUAD, and results in this setting are awaited from ongoing clinical trials (e.g., KRYSTAL-7, CodeBreak201, and NCT04933695). Monoclonal antibodies targeting the PD1/PD-L1 axis and CTLA-4 (e.g., nivolumab, pembrolizumab, atezolizumab, and ipilimumab) induce T-cell reactivation in several neoplasms, although their efficacy is patchy due to existing mechanisms of immunosuppression (16, 17). However, immunotherapy has gradually become relevant in KRAS-mutated patients, both because it is currently a standard first-line treatment alone or in combination with CT (18, 19), and because of its efficacy in these patients, as described in numerous experiences including the Keynote-042 subgroup analysis (20–23). Moreover, as recently reported at ASCO 2022, the use of chemo-immune checkpoint inhibitors enhances overall survival (OS) and overall response rate (ORR) in a KRAS-mutated population (24). It is noteworthy that Kras has been reported to influence the peritumor immune microenvironment and the expression of PD-L1 (25, 26), and in vitro evidence suggests a difference in the enhancement of antitumor immunity caused by different punctiform mutations in KRAS (27, 28).
The above-mentioned background has inspired this Italian retrospective study to directly evaluate in an unselected KRAS G12-mutated population the real impact of the use of chemo- or immunotherapies alone or in combination, as a frontline treatment according to demographic characteristics and single-amino acid substitutions.
We enrolled all NSCLC patients with a confirmed diagnosis of LUAD, detected from January 2015 to December 2021 in four Italian Cancer Units [Clinical and Medical Oncology Units, Careggi University Hospital, Florence; Department of Medical Oncology, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona, Ancona; Medical Oncology Unit, Santa Maria della Misericordia Hospital, Perugia; and the Scientific Institute of Romagna for the Study and Treatment of Tumors (IRST) IRCCS, Meldola]. Eligibility criteria included the following: age >18 years and available KRAS G12 mutation status regardless of the expression of PD-L1, which was not mandatory. We collected demographic data in an electronic record including age, sex, ECOG PS, smoking habits, data of death or last follow-up, disease characteristics such as KRAS mutational status, and PD-L1 status (<1%; 1%–49% and >50%) when available. Details about first-line treatment [date of first and last dose treatment, and best response achieved according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1] were gathered. The measured clinical outcomes were progression-free survival (PFS) evaluated at 18 months and OS at 30 months. All data were collected and analyzed anonymously; all patients signed an informed consent prior to starting treatment. This study complies with the Declaration of Helsinki rules of the World Medical Association and has been reviewed and approved by the Regional Ethics Committee for Clinical Trials of the Tuscany Region (approval No.: 20039_oss).
All patients underwent frontline therapy with anti-PD-L1, CT, or a combination of them. The drugs that were mainly used were the following: four to six cycles of cisplatin 75 mg/m2 or carboplatin area under the concentration time curve, 5 mg per milliliter per minute intravenous (IV) D1 Q3W, pemetrexed 500 mg/m2 IV D1 Q3W continued until the radiographically confirmed PD or toxicity, and pembrolizumab 200 mg IV D1 Q3W until the radiographically confirmed progression disease, toxicity, or the conclusion of 35 planned cycles. Pembrolizumab was administered alone or in combination with the CT regimen previously described. Few patients underwent a carboplatin-based regimen with paclitaxel 175 mg/m2 and bevacizumab 7.5 mg/kg IV D1 Q3W.
DNA was extracted from formalin-fixed paraffin-embedded tissue using a MagCore® Genomic DNA FFPE One-Step Kit on MagCore® Automated Nucleic Acid Extractor HF16Plus. Mutational analysis was performed as per local practice with the following panels: Myriapod® NGS-LT 56G Onco panel on Ion Torrent Ion S5™ system, with Myriapod® NGS Cancer panel DNA on Illumina MiSeq® and with Myriapod® Lung status on MassARRAY®. The analysis of the results of the NGS sequencing was carried out using Myriapod NGS Data Analysis Software and mutations were selected using the online genetic databases Clinvar and COSMIC [a minimum variant allele frequency (VAF) of 5% was applied for variant filtering].
From each block, 4-µm sections were cut and stained with monoclonal antibody PD-L1 (clone SP263, Ventana Medical System, Ventana, Tucson, Arizona) on an automated staining platform (Benchmark ULTRA; Ventana). An OptiView DAB IHC detection kit (Ventana) and an OptiView Amplification kit (Ventana) were used according to the manufacturer’s recommendations for visualization of the immunoreaction. Positive and negative controls were set parallel to the analyzed section. The positive control used was a tonsil section, and the negative control used was ready-to-use mouse serum with no immunization (Ventana). Partial or complete membrane staining of vital malignant cells was considered positive regardless of intensity. For each positive case, the percentage of viable stained tumor cells over total tumor cells (TPS) was used to categorize PD-L1 expression in three groups: TPS < 1% (negative), 1%–49%, and ≥50%.
Demographic and clinical data, disease and treatment characteristics, treatment exposure, and outcomes were analyzed using descriptive statistics. Continuous variables were presented as median and range, and categorical data were presented as counts and percentages. The Kaplan–Meier analysis was used to estimate PFS and OS, and log-rank test was applied to test for statistical significance. Cox proportional hazards model analysis was used to generate point estimates of the hazard ratio (HR) and the corresponding 95% confidence interval (CI) to estimate the risk of each individual KRAS isoform with outcome. Survival distributions for specific subgroups of patients has been tested with log-rank test. A p-value of 0.05 or lower has been considered statistically significant According to the class of demographic/clinical variables, suitable multivariate models were constructed, consistent with the significance of each variable (significance identified through the respective p-values relating to the Student’s t-test of significance for each variable involved), as well as the possible significance of the interactions between the variables. All statistical analyses were performed using Jamovi (The Jamovi Project, 2021), and the creation of graphs and figures was carried out with R Statistical Software (v4.1.2; R Core Team 2021).
We evaluated 219 patients with single punctiform mutation on KRAS G12 treated in four Italian oncology units with CT or immune checkpoint inhibitors (ICIs) alone or their combination as first-line therapy for stage IV NSCLC. The baseline characteristics of the enrolled patients are described in Table 1. As expected, most of the patients enrolled were male (61.2%) and older than 65 years (64.8%). The majority of patients were ECOG PS 0–1 (94%). The harboring of G12 mutations was strictly related to smoking habits (p = 0.032). G12C, G12V, G12D, and G12A isoforms were depicted in 47.9%, 20.5%,17.4%, and 8.2% of our population, respectively, while the other isoforms (S, F, and I) were rare (lower than 6%). No particular distribution was observed regarding the expression of PD-L1 in our population. CT was the most used first-line regimen in half of the G12 mutated patients, followed by ICIs alone (32.7%). The recent (2020) approval of the chemo-immune combination in Italy has negatively influenced the sample size of this subgroup (17.3%). Data about demographic characteristics analyzed by G12 single mutation highlighted in the G12V subgroup a predominance of the elderly (80%), and an equal distribution between young (50%) and elderly (50%) in G12A. No differences in gender distribution were observed, as expected, with a prevalence of men in all mutations. However, smoking habit seems to be closely related to C isoform expression (100%), even though D and V isoforms also harbor in never-smokers (13.2% and 11.1%). We described in G12V a prevalence of PD-L1 < 1% (51.4%); conversely, G12C had 43% of PD-L1 > 50% (p = 0.042). Intriguingly, G12A had a higher PD-L1 > 1% expression (73.4%) than the other isoforms (Figures 1A–D). Even when analyzed for mutation, the most commonly used therapy was CT, unlike the chemo-immune combination (data not shown).
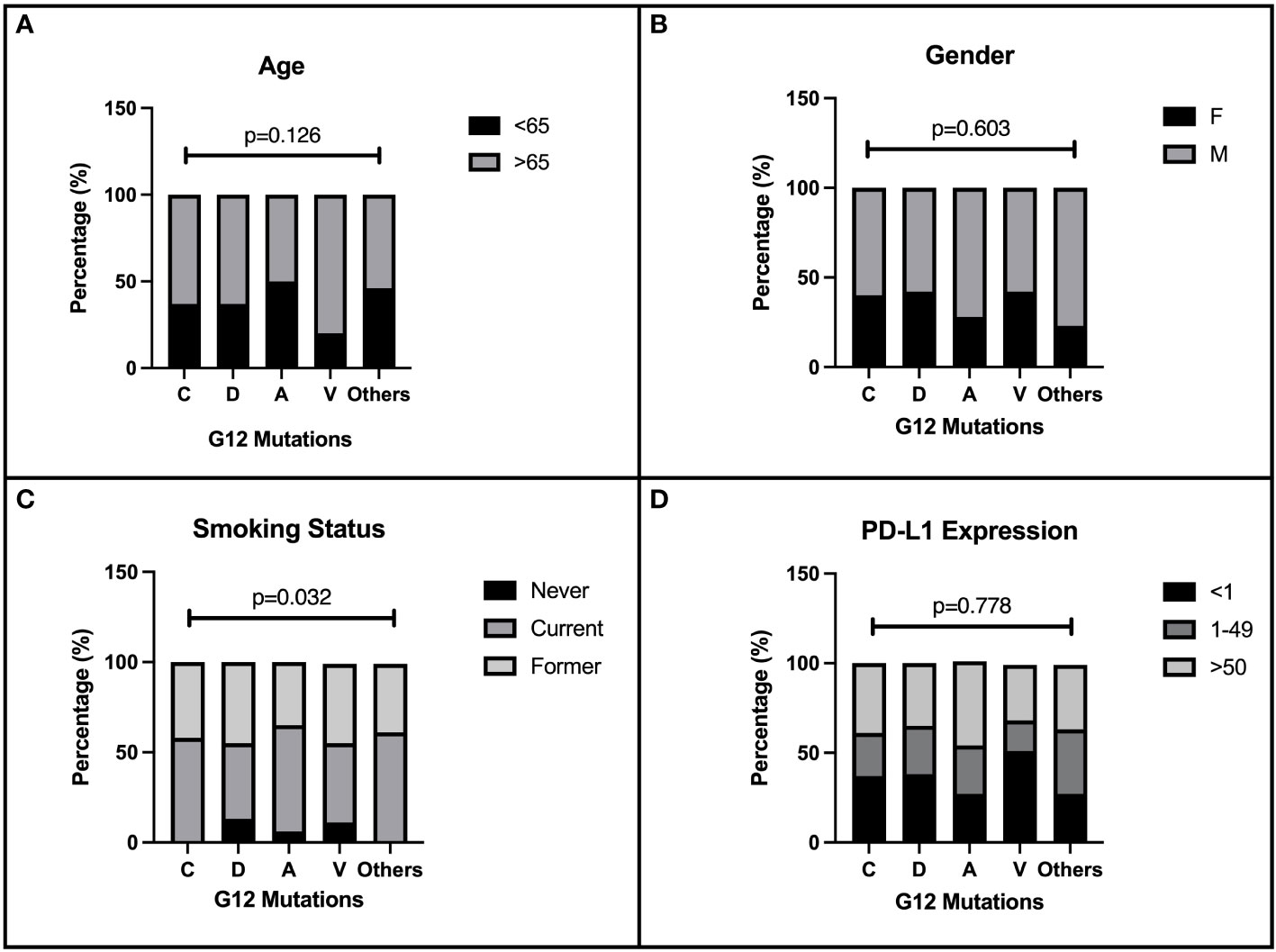
Figure 1 Correlation of patients’ characteristics and G12 bases. Baseline characteristics of 219 NSCLC patients enrolled in the present retrospective study. The distribution of demographic and immunohistochemical properties is shown in figure according to the single amino acid mutation. The distribution of G12 isoforms was correlated to: (A) age of patients at diagnosis (< 65 and ≥65 years), p=0.126. (B) gender p=0.603. (C) smoking habits p=0.032. (D) PD-L1 expression, p=0.778.
We investigate the prognostic role of KRAS G12 mutations according to patients and disease characteristics and treatment chosen. Median PFS and OS of the entire population were 5.0 months (4.0–6.0 months) and 16.0 months (16.0–19.0 months), respectively, with some differences, even not statistically significant among G12 isoforms (PFS p = 0.518 and OS p = 0.593). PFS at 18 months demonstrated to be better for young people (mPFS 6.0 months, 4.0–11.0 months; HR: 1.42, 95% CI 1.01–2.0, p = 0.044) and for women (mPFS 6.0 months, 4.0–11.0 months; HR: 1.54, 95% CI 1.10–2.15, p = 0.013). The advantage was preserved for gender (p = 0.020) despite age (p = 0.150) also in multivariate analysis. No significant differences in PFS were observed according to PD-L1 expression or G12 mutation; even a trend in favor of the C isoform was found compared to V (p = 0.145) and for PD-L1 ≥ 50% compared to PD-L1 <1% (p = 0.154). The subgroup of patients exposed to ICI with or without CT showed a benefit compared to chemotherapy alone (HR: 0.63, 95% CI 0.45–0.87, p = 0.005) in univariate and multivariate analysis (Table 2) (Figures 2A, B). In particular, we registered a mOS of 17.0 months (13.0–29.0 months) for the C isoform (n = 79), a mOS of 13.5 months (6.0–26.0 months) for D (n = 28), a mOS of 21.0 months (7.0–NR months) for the A substitution (n = 13), and a mOS of 12.0 months (8.0–18.0 months) for the V substitution (n = 35), which proved to be the mutation with the worst prognosis. Univariate analysis upheld a better OS for <65-year-old patients (mOS 20.5 months, 19.0–NR; HR: 1.71. 95% CI 1.15–2.55, p = 0.008) and female patients (mOS 23.0 months, 18.0–NR; HR: 1.78. 95% CI 1.21–2.64, p = 0.004) (Table 3) (Figures 2C, D). The benefit of young age and female gender was confirmed also in the multivariate analysis. As per PFS, our dataset supports a trend in OS in favor of C when compared to the V isoform (p = 0.130) and in patients with a lack of PD-L1 expression (p = 0.138) when compared to patients with PD-L1 overexpression. Immunotherapy in addition or not to CT demonstrates a survival benefit in the population regardless of the G12 isoform and PD-L1 expression as shown in Table 3 (mOS of 18.5 months, 15.0–NR; HR: 0.69. 95% CI 0.47–1.00, p = 0.048) benefit we also recorded in multivariate (p = 0.034).
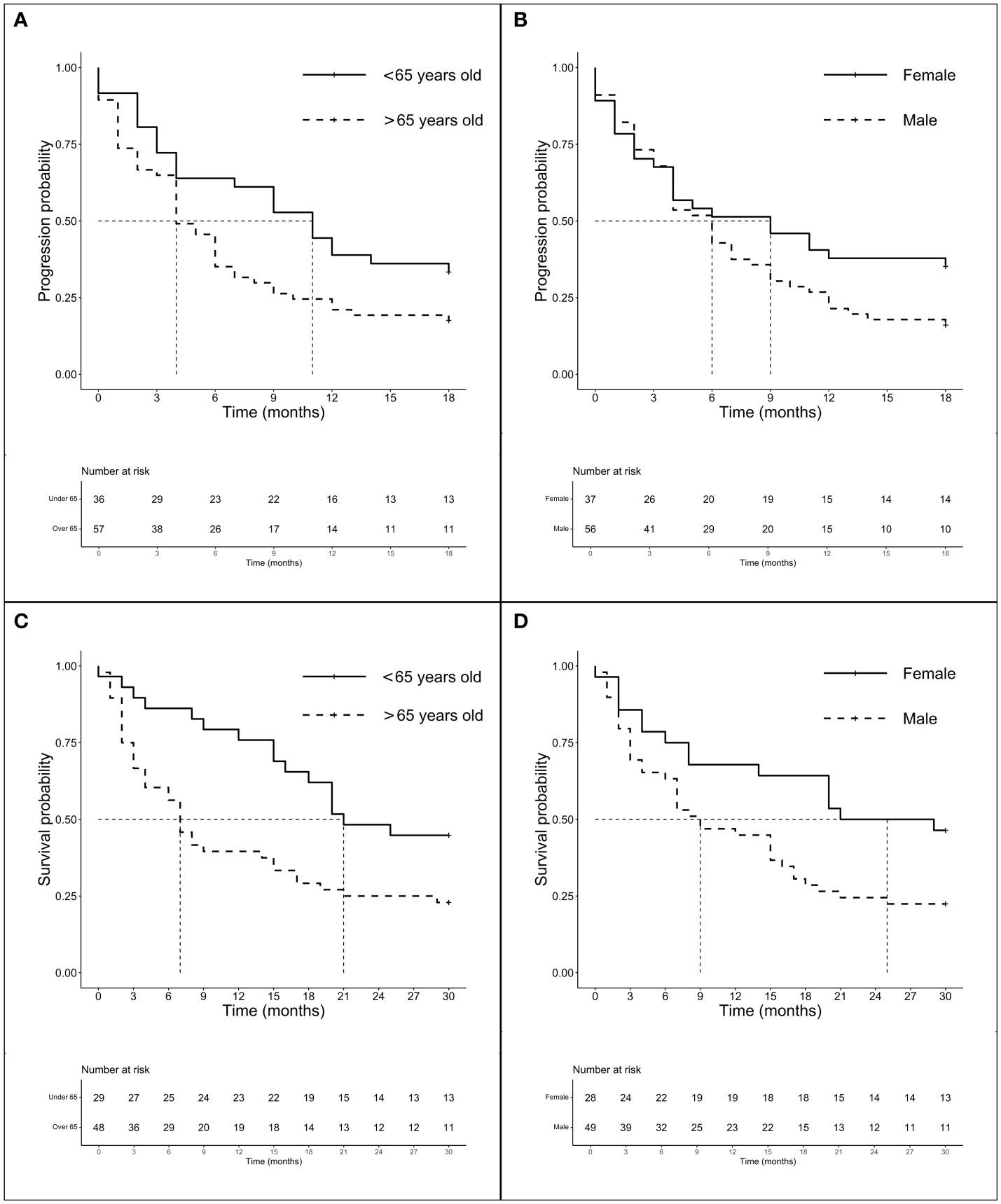
Figure 2 Survival analysis (PFS and OS) according to age and gender. Survival analysis (PFS and OS) of G12 patients according to age (panels A and C) and gender (panel B and D). (A) mPFS of patients < 65 years (n = 62) vs. patients > 65 years (n = 119): 6.0 months (95% CI 4.0–11.0 months) vs. 4.0 months (95% CI 4.0–6.0 months), p = 0.518. (B) mPFS of female (n = 71) vs. male (n = 110): 6.0 months (95% CI 4.0–11.0 months) vs. 4.0 months (95% CI 3.0–6.0 months), p = 0.013. (C) mOS of patients < 65years (n = 56) vs. patients > 65 years (n = 109): 20.5 months (95% CI 10.0–NR months) vs. 13.0 months (95% CI 9.0–17.0 months), p = 0.007. (D) mOS of female (n = 63) vs. male (n = 102): 23.0 months (95% CI 18.0–NR months) vs. 14.5 months (95% CI 9.0–17.0 months), p = 0.004.
In accordance with previous results, we decided to analyze the amino acid substitutions’ outcomes according to treatments received. Among 106 CT-treated patients, global mPFS was 6.0 months (3.0–6.0 months) and no significant differences in mPFS were observed between the major mutations (A, C, D, and V) ranging from 3.5 to 4 months (p = 0.591). The mOS was 16.0 months (12.0–19.0 months) and none of the main isoforms analyzed seems to benefit from CT (p = 0.800). We then evaluated the outcome of treatment including ICI both on the total population (n = 94) and according to a major single mutation. Intriguingly, although the global mPFS was 6.0 months, isoforms C and V showed the same lower median of 4.0 months (3.0–12.0 months for V and 4.0–18.0 months for C). Mutations D and A instead showed higher mPFS (8.0 months, 6.0–NR and 9.0 months, 4.0–NR, respectively). Later, we chose to compare the outcomes of the better prognosis mutations C (n = 43), D (n = 16), and A (n = 10) with isoform V (n = 19) to evaluate the importance of the individual mutations in a population homogeneously exposed to ICI (Figures 3A–C). Interestingly, despite mPFS being similar in the two groups, we highlighted a favorable trend in PFS for C compared with the V isoform (p = 0.114) (Figure 3A). As shown in the figure, a similar trend was also described for D (p = 0.165) and A (p = 0.140) mutations when compared to V (Figures 3B, C). The mOS of the ICI-exposed subgroup (n = 78) was 15 months (8.0–20.0 months). As seen for PFS, V proved to be the mutation with the worst prognosis (mOS: 9.0 months, 4.0–NR) unlike the C (15.0 months, 6.0–NR), D (11.5 months, 7.0–NR), and A (20.0 months, 15.0–NR) mutations even if the comparison between better-survival mutations and the V isoform showed no significant differences. Finally, the PD-L1 expression seems to not have a prognostic role. We observed the worst outcome for those expressing PD-L1 > 50% (mOS 8.50 months, 4.0–21.0 months) who were exposed to ICI alone, while patients with PD-L1 <1% or 1%–49% who underwent CT-ICI had a mOS of 17.0 and 15.5 months, respectively (p = 0.404).
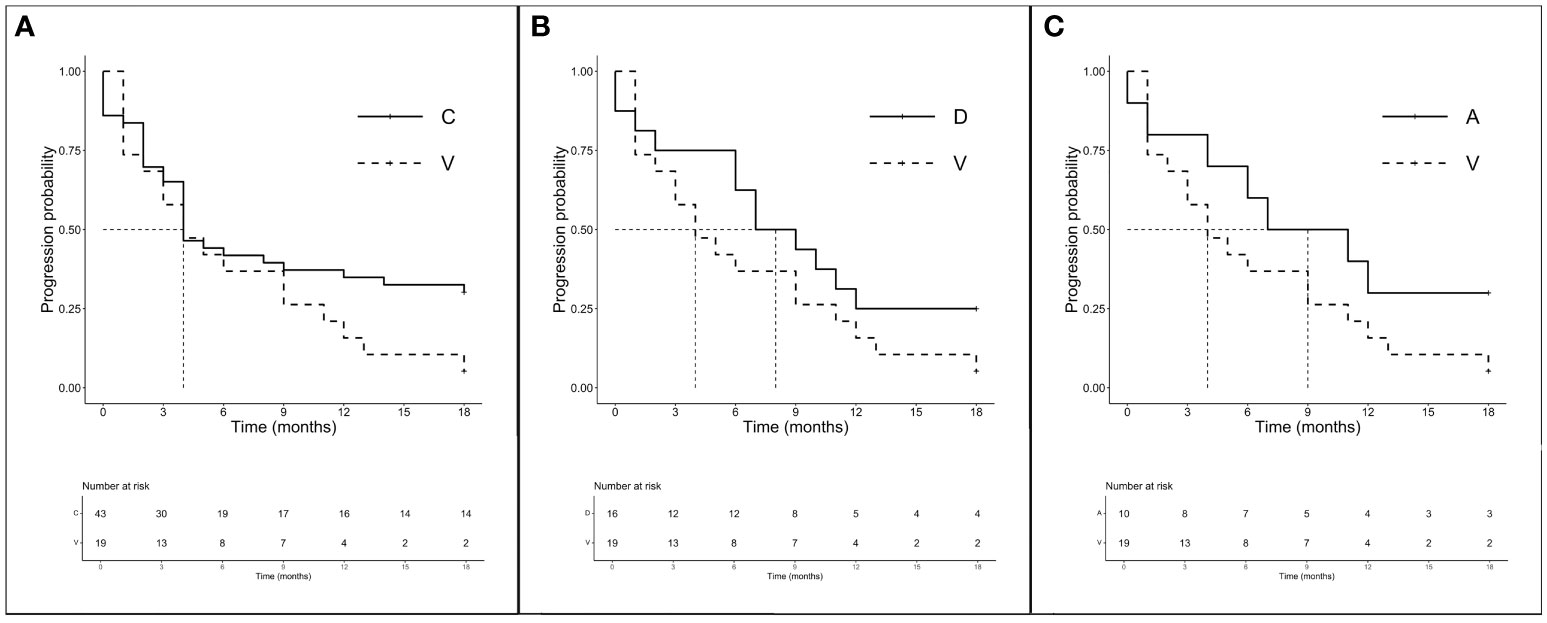
Figure 3 PFS according to single amino acid substitutions in patients treated with ICI ± CT (n = 94). (A) mPFS C (n = 43) vs. V (n = 19): 4.0 months (95% CI 4.0–18.0 months) vs. 4.0 months (95% CI 3.0–12.0 months), p = 0.114. (B) mPFS D (n = 16) vs. V (n = 16): 8.0 months (95% CI 6.0–NR months) vs. 4.0 months (95% CI 3.0–12.0 months), p = 0.165. (C) mPFS A (n = 10) vs. V (n = 16): 9.0 months (95% CI 4.0–NR months) vs. 4.0 months (95% CI 3.0–12.0 months), p = 0.140.
The role and characteristics of intracellular membrane proteins from the RAS family as the hub for signaling of receptor tyrosine kinases (RTK) (29, 30), G-protein-coupled receptors (GPCR) (31), and members of the integrin family (32) have been known for decades. The switch between an inactive GDP-bound and an active GTP-bound state, mediated by GTPase activating protein (GAP) and guanine nucleotide exchange factors (GEFs) (33, 34), was a well-known mechanism leading to several downstream pathways’ activation, especially RAF1 and PI3K (1). Somatic Ras mutations are prone to different switching times between an active and an inactive Ras state and to different GTP hydrolysis rates (27, 35), and the activation of different downstream pathways according to different single-amino acid substitutions in mutant KRAS tumors has been described (36–38). This evidence supports the renowned undruggability of KRAS directly or through several targets of up- or downstream signaling.
The recent discovery of drugs (sotorasib and adagrasib) able to selectively bind G12C with favorable efficacy/toxicity ratio has modified the concept of KRAS inhibition (39), and we are eager to know the results regarding their efficacy and clinical outcome (NCT04303780; NCT04685135). Despite the efforts and encouraging results of phase 2 trials in the post-first-line setting (14, 15), sotorasib and adagrasib are not recommended as frontline treatment in advanced KRAS G12C LUAD, and the final results of ongoing first-line clinical trials are still pending (e.g., KRYSTAL-7, CodeBreak201, and NCT04933695). Moreover, results on drugs targeting other mutations are still lacking even if several promising drug candidates emerge as inhibitors of other KRAS mutants as per EX185 designated to inhibit KRAS G12D, G12C, and G12V and to engage GNP-bound KRAS (40) or MRTX1133, which has shown efficacy in the KRAS G12D mutant xenograft mouse tumor model (41). Immunotherapy in combination or not with CT according to PD-L1 expression is still the standard in this wide population (18, 19, 42).
In our cohort, we analyzed a homogeneous population of KRAS G12-mutated LUAD patients (n = 219) undergoing first-line therapy with CT or ICI alone or their combination. The aim of the study was to characterize a possible unique profile to discriminate each single G12 mutation by treatment outcome or demographic characteristics. According to data from the COSMIC database, we observed comparable G12 amino acid substitution percentage in our population; in particular, the major detected substitutions were C (47.9%), V (20.5%), D (17.4%), and A (8.2%) (3), while the other isoforms (e.g., G, F, S, and I) were rare (less than 6%).
In accordance with Riely and colleagues (43) and preclinical evidence supporting the development of KRAS mutations due to epigenetic changes after tobacco exposure (44), we confirmed the prevalence of the KRAS G12 mutation in patients with smoking habit (95%), especially in transversions (G → T/C). Different from literature, we also described in transition mutations (G → A) a predominance of smokers (86.8%). Patients enrolled in our analysis were predominantly elderly (64.8%) and male (61.2%), which is comparable to the global LUAD distribution. Age and gender results are associated with outcome: female gender was an independent prognostic factor for longer PFS and OS and age <65 years correlated with better OS.
The different sensitivity of the specific mutant KRAS to different treatments (CT, TKIs, or anti-angiogenetics) has been widely described (45, 46), and previous in vitro and clinical data suggest that different KRAS mutations have different depths and durations of response to old-fashioned treatments (36, 47, 48). In addition, CT agents as platinum-derived drugs and the anti-metabolite pemetrexed have immunomodulatory properties, being able to increase MHC-I expression and recruitment of effectors (e.g., TILs, macrophages, and memory T cells) and to reduce the activity of players of the immunosuppressive microenvironment such as Tregs (49, 50). Nevertheless, in our analysis, the use of CT was globally unsatisfactory and none of the mutations analyzed had a solid benefit either in PFS or in OS. Based on the results, we investigated the efficacy of ICIs alone or in combination in our population, finding a 38% decreasing risk of disease progression and 34% death in patients receiving first-line treatment including ICI with or without CT.
Several studies attempted to determine the impact of KRAS mutations on the outcome of patients treated with ICI in the advanced setting with contradictory results (51–54). The gain from the addition of ICIs in KRAS-mutated patients is consistent with several retrospective analyses in real life (20–22) and supported by the results of the analysis of the mutated KRAS subgroup in the Keynote-042 trial, where pembrolizumab instead of CT had an ORR of 56.7% versus 18%, and similar benefits were registered in the G12C subgroup with PFS and OS similar to the entire population enrolled (23). Recently, at ASCO 2022, Nakajima and colleagues described an improved OS and ORR in a KRAS-mutated population treated with chemo-immune combination as frontline treatment, confirming our observations (24).
It should be noted that the outcome of KRAS-mutant treatments is dominated by co-occurring genetic events, as STK11/LKB1 and KEAP1 mutations define a subset of “cold” NSCLC resistant to ICI, while TP53 alterations increase the inflammatory microenvironment, leading to an efficient immune response. However, clinical reports and retrospective gene sequencing did not identify a specific association between certain comutations and single KRAS isoforms (55, 56). Furthermore, the use of gene panels including these comutations has a patchy distribution in clinical practice; thus, information regarding this in our retrospective case series is missing.
The PD-1/PD-L1 axis is involved in the inhibition of the immune system and more specifically in the self-tolerance and regulation of T lymphocyte activity (57). Chen and colleagues described in vitro the ability of KRAS to upregulate PD-L1 via p-ERK, inducing apoptosis of CD3+ T cells and resulting in immune escape, an unfavorable condition that can be reversed by anti-PD-1 antibody (25). The involvement of other signaling pathways supporting the expression of PD-L1 as MAPK, together with STAT3, but not PI3K, has also been suggested (58). Later, the role of the Akt-mTOR axis (59) and PD-L1 miRNA stabilization (60) offered further evidence of the complexity of the influence of KRAS to PD-L1 in NSCLC. Falk and colleagues (61) described a better prognosis in patients with PD-L1 TPS ≥ 1% in KRASmut than in KRASwt exposed to ICI, and several other clinical retrospective examples and literature review support Falk’s observations (52, 54, 62). It is evident that the KRAS mutational status could be a potential biomarker of favorable outcome for ICI treatments; however, pivotal ICI trials did not provide univocal data on either the efficacy of these treatments or the biomarker role of PD-L1. In contrast to Falk, our data suggest a trend towards a better prognosis for patients with PD-L1 <50%. These results were partially confirmed in the subgroup analysis of patients treated with ICI with/without CT where an absence or lower PD-L1 expression seems more advantageous than PD-L1 ≥50%. According to the Italian Medicine Agency, PD-L1 TPS ≥50% is the requirement to prescribe pembrolizumab while the combination with CT is allowed only in patients with expression of PD-L1 <50%, and this could explain the benefit of the population with PD-L1 <50% seen in our analysis. Despite the promising preclinical observations, pivotal clinical trials with the ambition to demonstrate the superiority of CT-ICI combinations did not allow the enrollment of patients with KRAS mutations; therefore, there is a lack of concrete efficacy results in this population.
Even more complex is the understanding of the predictive value of response to treatments when considering individual mutations. We have previously remarked on the lack of benefit across isoforms from the use of CT, which is consistent with the observation by Wiesweg and colleagues who described that isoforms have the same intermediate prognosis (48). Thereafter, we explored the prognostic value of G12C, V, D, and A undergoing ICI ± CT and we instead noted a more unfavorable trend in PFS for G12V compared to G12C, and although mOS between mutations was almost doubled for C (9.0 months vs. 15.0 months), the difference was not relevant. Furthermore, V has a worse prognosis than the remaining isoforms, particularly A and D. Differences in OS are observed in these subgroups, although not statistically significant. Ihle in 2012 (36) described a poor outcome affecting patients in the BATTLE-1 trial with isoforms C and V mutated, probably due to the differences in pathway activation, which are supported by in vitro analysis highlighting a predominance of p-Akt activation in the D isoform, and a predominance of RalA/B for C and V isoforms. In the BATTLE-1 trial, patients were exposed to several TKIs; however, this evidence suggested a modulation of the pathways when mutations are exposed to similar treatments. Similar to our data, the mPFS of C and V isoforms exposed to ICI was 4 months, but the authors highlighted a worsening PFS in those who have STK11/LKB1 comutations (63); unfortunately, this information is not available in our dataset. The disadvantageous weight of the V isoform has been extensively described (47, 64–66), and contrary to what has been reported in the literature (63, 67), our series was released from the high expression of PD-L1. As a matter of fact, in our case series and different from literature, the A isoform has a better outcome to ICI treatments, and this is the mutation with a higher rate of PD-L1 expression (>1%: 73.4%) and young patients, while the V mutation shows its worst prognosis in the elderly. Those results are in conflict with the study by Shen Mo et al. (68), according to which KRAS G12D and G12V mutations are better candidates for immunotherapy, whereas patients with KRAS G12A or G12C mutations are not. Intriguingly, and the same as what Jeanson and colleagues described (62), we noticed a better prognosis in those mutations as per A, which expressed a high rate of PD-L1, even if it is not possible to draw final conclusions given the small number of the subgroup.
Despite the limitations derived from the retrospective characteristics of the study and the relative lack of comutation assessment, we can affirm that, to our knowledge, this is the first multicenter, real-life study with this sample size aimed only at G12 mutations in first-line patients undergoing treatment including ICI. We confirmed the scarce efficacy of CT alone in this population, which instead benefits from the use of ICI alone or in combination with CT, a benefit not linked to PD-L1 overexpression. We also confirmed the benefit in some isoforms (C, D, and A) and the negative prognostic value of the V mutation, which maintains a poor prognosis regardless of the treatment chosen, probably related to an aged population and the relative lack of PD-L1 expression in the subgroup.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of Area Vasta Centro (CEAVC) part of the Regional Ethics Committee of the Tuscany Region. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: SF, EC, and SP. Collection of data: SF, GMen, IB, MM, GMet, IP, and CB. Interpretation of data: SF, SP, and EC. Data curation: LV, CC, FC, and CB. Writing—original draft preparation: SF. Writing—review and editing: FM, SP, LA, and LP. Supervision: AD, RB, and LA. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
The data collection and the writing of the entire project are partially supported by ERA PerMed, having participated in the call “Second Joint Transnational Call for Proposals (JTC) 2019 on Personalized Medicine: Multidisciplinary Research Towards Implementation within the framework of ERA PerMed”, passed the final selection, and obtained the required funding and funds of Professor Antonuzzo, Department of Experimental and Clinical Medicine, University of Florence.
The authors declare that the research was conducted in the absence of commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gimple RC, Wang X. RAS: Striking at the core of the oncogenic circuitry. Front Oncol (2019) 9:965. doi: 10.3389/fonc.2019.00965
2. Forbes SA, Beare D, Boutselakis H, Bamford S, Bindal N, Tate J, et al. COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res (2017) 45:D777–83. doi: 10.1093/nar/gkw1121
3. COSMIC database. (2022). Available at: https://cancer.sanger.ac.uk/cosmic/gene/analysis?all_data=&coords=AA%3AAA&dr=&end=190&gd=&id=398646&ln=KRAS&seqlen=190&sn=lung&start=1#ts (Accessed May 19, 2022).
4. Hammerman PS, Voet D, Lawrence MS, Voet D, Jing R, Cibulskis K, et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature (2012) 489:519–25. doi: 10.1038/nature11404
5. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. Cancer J Clin (2008) 58:71–96. doi: 10.3322/CA.2007.0010
6. Yu HA, Sima CS, Shen R, Kass S, Gainor J, Shaw A, et al. Prognostic impact of KRAS mutation subtypes in 677 patients with metastatic lung adenocarcinomas. J Thorac Oncol (2015) 10:431–7. doi: 10.1097/JTO.0000000000000432
7. Shepherd FA, Domerg C, Hainaut P, Jänne PA, Pignon J-P, Graziano S, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-Small-Cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol (2013) 31:2173–81. doi: 10.1200/JCO.2012.48.1390
8. Mellema WW, Masen-Poos L, Smit EF, Hendriks LEL, Aerts JG, Termeer A, et al. Comparison of clinical outcome after first-line platinum-based chemotherapy in different types of KRAS mutated advanced non-small-cell lung cancer. Lung Cancer (2015) 90:249–54. doi: 10.1016/j.lungcan.2015.09.012
9. Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol (2005) 23:5900–9. doi: 10.1200/JCO.2005.02.857
10. Zhu C-Q, da Cunha Santos G, Ding K, Sakurada A, Cutz J-C, Liu N, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in national cancer institute of Canada clinical trials group study BR.21. J Clin Oncol (2008) 26:4268–75. doi: 10.1200/JCO.2007.14.8924
11. Schneider CP, Heigener D, Schott-Von-Römer K, Gütz S, Laack E, Digel W, et al. Epidermal growth factor receptor-related tumor markers and clinical outcomes with erlotinib in non-small cell lung cancer: An analysis of patients from german centers in the trust study. J Thorac Oncol (2008) 3:1446–53. doi: 10.1097/JTO.0b013e31818ddcaa
12. Li B, Skoulidis F, Falchook G, Sacher A, Velcheti V, Dy G, et al. PS01.07 registrational phase 2 trial of sotorasib in KRAS p.G12C mutant NSCLC: First disclosure of the codebreak 100 primary analysis. J Thorac Oncol (2021) 16:S61. doi: 10.1016/j.jtho.2021.01.321
13. Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou S-HI, Pacheco JM, et al. Adagrasib in non–Small-Cell lung cancer harboring a KRASG12C mutation. New Engl J Med (2022) 33:1–12. doi: 10.1056/NEJMoa2204619
14. FDA Grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC (2021). Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sotorasib-kras-g12c-mutated-nsclc.
15. U.S. food and drug administration (FDA) accepts mirati therapeutics’ new drug application for adagrasib as treatment of previously treated KRASG12C-mutated non-small cell lung cancer (2022). Available at: https://ir.mirati.com/press-releases/press-release-details/2022/U.S.-Food-and-Drug-Administration-FDA-Accepts-Mirati-Therapeutics-New-Drug-Application-for-Adagrasib-as-Treatment-of-Previously-Treated-KRASG12C-Mutated-Non-Small-Cell-Lung-Cancer/default.asp.
16. Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS -mutant lung adenocarcinoma. Cancer Discovery (2018) 8:822–36. doi: 10.1158/2159-8290.CD-18-0099
17. Helena JJ van R, Azad T, Ling M, Hao Y, Snetsinger B, Khanal P, et al. The hippo pathway component taz promotes immune evasion in human cancer through PD-L1. Cancer Res (2018) 78:1457–70. doi: 10.1158/0008-5472.CAN-17-3139
18. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Correction to: “Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up”. Ann Oncol (2019) 30:863–70. doi: 10.1093/annonc/mdy474
19. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
20. Amanam I, Mambetsariev I, Gupta R, Achuthan S, Wang Y, Pharaon R, et al. Role of immunotherapy and co-mutations on KRAS-mutant nonsmall cell lung cancer survival. J Thorac Dis (2020) 12:5086–95. doi: 10.21037/jtd.2020.04.18
21. Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett (2020) 470:95–105. doi: 10.1016/j.canlet.2019.10.027
22. Dietrich M, Hunis B, Raez L. P2.07-052 detection of KRAS mutation in blood predicts favorable response to immunotherapy in NSCLC. J Thorac Oncol (2017) 12:S2149. doi: 10.1016/j.jtho.2017.09.1308
23. Herbst RS, Lopes G, Kowalski DM, Kasahara K, Wu Y-L, de Castro G, et al. LBA4 association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in keynote-042. Ann Oncol (2019) 30:xi63–4. doi: 10.1093/annonc/mdz453.001
24. Nakajima E, Ren Y, Vallejo J, Al. E. Outcomes of first-line immune checkpoint inhibitors with or without chemotherapy according to KRAS mutational status and PD-L1 expression in patients with advanced NSCLC: FDA pooled analysis. JCO (2022) 40:9001. doi: 10.1200/JCO.2022.40.16_suppl.9001
25. Chen N, Fang W, Lin Z, Peng P, Wang J, Zhan J, et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother (2017) 66:1175–87. doi: 10.1007/s00262-017-2005-z
26. Incecco AD, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. British Journal of Cancer (2015) 112:95–102. doi: 10.1038/bjc.2014.555
27. Koltun E, Cregg J, Rice MA, Whalen DM, Freilich R, Jiang J, et al. Abstract 1260: First-in-class, orally bioavailable KRAS<sup<G12V</sup<(ON) tri-complex inhibitors, as single agents and in combinations, drive profound anti-tumor activity in preclinical models of KRAS<sup<G12V</sup< mutant cancers. Cancer Res (1260) 2021) 81:1260. doi: 10.1158/1538-7445.AM2021-1260
28. Glorieux C, Xia X, He YQ, Hu Y, Cremer K, Robert A, et al. Regulation of PD-L1 expression in K-ras-driven cancers through ROS-mediated FGFR1 signaling. Redox Biol (2021) 38:101780. doi: 10.1016/j.redox.2020.101780
29. Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and sos nucleotide exchange factor. Cell (1993) 73:611–20. doi: 10.1016/0092-8674(93)90146-H
30. Arvidsson AK, Rupp E, Nånberg E, Downward J, Rönnstrand L, Wennström S, et al. Tyr-716 in the platelet-derived growth factor beta-receptor kinase insert is involved in GRB2 binding and ras activation. Mol Cell Biol (1994) 14:6715–26. doi: 10.1128/mcb.14.10.6715
31. Wan Y, Kurosaki T, Huang X-Y. Tyrosine kinases in activation of the MAP kinase cascade by G-protein-coupled receptors. Nature (1996) 380:541–4. doi: 10.1038/380541a0
32. Clark EA, Hynes RO. Ras activation is necessary for integrin-mediated activation of extracellular signal-regulated kinase 2 and cytosolic phospholipase a 2 but not for cytoskeletal organization. J Biol Chem (1996) 271:14814–8. doi: 10.1074/jbc.271.25.14814
33. Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Publishing Group (2010) 10(12):842–57. doi: 10.1038/nrc2960
34. Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev (2013) 93:269–309. doi: 10.1152/physrev.00003.2012
35. Mccormick F. Sticking it to KRAS : Covalent inhibitors enter the clinic. Cancer Cell (2020) 37:3–4. doi: 10.1016/j.ccell.2019.12.009
36. Ihle NT, Byers LA, Kim ES, Saintigny P, Lee JJ, Blumenschein GR, et al. Effect of KRAS oncogene substitutions on protein behavior: Implications for signaling and clinical outcome. J Natl Cancer Inst (2012) 104:228–39. doi: 10.1093/jnci/djr523
37. Hunter JC, Manandhar A, Carrasco MA, Gurbani D, Gondi S, Westover KD. Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol Cancer Res (2015) 13:1325–35. doi: 10.1158/1541-7786.MCR-15-0203
38. Virtudes Céspedes M, Josep Sancho F, Guerrero S, Parreñ M, Casanova I, Angel Pavón M, et al. K-Ras Asp12 mutant neither interacts with raf, nor signals through erk and is less tumorigenic than K-ras Val12. Carcinogenesis (2006) 27(11):2190-200. doi: 10.1093/carcin/bgl063
39. Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras (G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature (2013) 503:548–51. doi: 10.1038/nature12796
40. Vasta JD, Peacock DM, Zheng Q, Walker JA, Zhang Z, Zimprich CA, et al. KRAS is vulnerable to reversible switch-II pocket engagement in cells. Nat Chem Biology (2022) 18:596–604. doi: 10.1101/2021.10.15.464544
41. Wang X, Allen S, Blake JF, Bowcut V, Briere DM, Calinisan A, et al. Identification of MRTX1133, a noncovalent, potent, and selective KRAS G12D inhibitor. Cite This: J Med Chem (2021) 2022:3133. doi: 10.1021/acs.jmedchem.1c01688
42. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, de Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–Small-Cell lung cancer. New Engl J Med (2018) 378:2078–92. doi: 10.1056/nejmoa1801005
43. Riely GJ, Kris MG, Rosenbaum D, Marks J, Li A, Chitale DA, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res (2008) 14:5731–4. doi: 10.1158/1078-0432.CCR-08-0646
44. Vaz M, Hwang SY, Kagiampakis I, Phallen J, Patil A, O’Hagan HM, et al. Chronic cigarette smoke-induced epigenomic changes precede sensitization of bronchial epithelial cells to single-step transformation by KRAS mutations. Cancer Cell (2017) 32:360–376.e6. doi: 10.1016/j.ccell.2017.08.006
45. Renaud S, Guerrera F, Seitlinger J, Reeb J, Voegeli C, Legrain M, et al. KRAS-specific amino acid substitutions are associated with different responses to chemotherapy in advanced non-small cell lung cancer. Clin Lung Cancer (2018) 1:e919-e931. doi: 10.1016/j.cllc.2018.08.005
46. Metro G, Chiari R, Duranti S, Siggillino A, Fischer MJ, Giannarelli D, et al. Impact of specific mutant KRAS on clinical outcome of EGFR-TKI-treated advanced non-small cell lung cancer patients with an EGFR wild type genotype. Lung Cancer (2012) 78:81–6. doi: 10.1016/J.LUNGCAN.2012.06.005
47. Garassino M, Marabese M, Rusconi P, Rulli E, Martelli O, Farina G. Different types of K-ras mutations could affect drug sensitivity and tumour behaviour in non-small-cell lung cancer. Ann Oncol (2011) 24:92–6. doi: 10.5791/0882-2875-24.2.92
48. Wiesweg M, Kasper S, Worm K, Herold T, Reis H, Sara L, et al. Impact of RAS mutation subtype on clinical outcome–a cross-entity comparison of patients with advanced non-small cell lung cancer and colorectal cancer. Oncogene (2019) 38:2953–66. doi: 10.1038/s41388-018-0634-0
49. Cavazzoni A, Digiacomo G, Alfieri R, Monica S, Fumarola C, Galetti M, et al. Pemetrexed enhances membrane PD-L1 expression and potentiates T cell-mediated cytotoxicity by anti-PD-L1 antibody therapy in non-Small-Cell lung cancer. Cancers (Basel) (2020) 12:666. doi: 10.3390/cancers12030666
50. de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clin Cancer Res (2014) 20(21):5384–91. doi: 10.1158/1078-0432.CCR-14-1298
51. Torralvo J, Friedlaender A, Achard V, Addeo A. The activity of immune checkpoint inhibition in kras mutated non-small cell lung cancer: A single centre experience. Cancer Genomics Proteomics (2019) 16:577–82. doi: 10.21873/cgp.20160
52. Passiglia F, Cappuzzo F, Alabiso O, Bettini AC, Bidoli P, Chiari R, et al. ARTICLE efficacy of nivolumab in pre-treated non-small-cell lung cancer patients harbouring KRAS mutations. Br J Cancer (2019) 120:57–62. doi: 10.1038/s41416-018-0234-3
53. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann Oncol (2019) 30:1321–8. doi: 10.1093/annonc/mdz167
54. Kim JH, Kim HS, Kim BJ. Prognostic value of KRAS mutation in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors: A metaanalysis and review. Oncotarget (2017) 8:48248–52. doi: 10.18632/oncotarget.17594
55. Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-Occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discovery (2015) 5:861–78. doi: 10.1158/2159-8290.CD-14-1236
56. Arbour KC, Jordan E, Kim HR, Dienstag J, Yu HA, Sanchez-Vega F, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res (2018) 24:334–40. doi: 10.1158/1078-0432.CCR-17-1841
57. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Publishing Group (2012) 12:252–64. doi: 10.1038/nrc3239
58. Sumimoto H, Takano A, Teramoto K, Daigo Y. RAS-Mitogen-Activated protein kinase signal is required for enhanced PD-L1 expression in human lung cancers. PLOS ONE (2016) 11(11):e0166626. doi: 10.1371/journal.pone.0166626
59. Lastwika KJ, Iii WW, Li QK, Norris J, Xu H, Ghazarian SR, et al. Microenvironment and immunology control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res (2016) 76(2):227–38. doi: 10.1158/0008-5472.CAN-14-3362
60. Coelho MA, de Carné Trécesson S, Rana S, Zecchin D, Moore C, Molina-Arcas M, et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity (2017) 47:1083–99.e6. doi: 10.1016/j.immuni.2017.11.016
61. Falk AT, Yazbeck N, Guibert N, Chamorey E, Paquet A, Ribeyre L, et al. Effect of mutant variants of the KRAS gene on PD-L1 expression and on the immune microenvironment and association with clinical outcome in lung adenocarcinoma patients. Lung Cancer (2018) 121:70–5. doi: 10.1016/j.lungcan.2018.05.009
62. Jeanson A, Tomasini P, Souquet-Bressand M, Brandone N, Boucekine M, Grangeon M, et al. Efficacy of immune checkpoint inhibitors in KRAS-mutant non-small cell lung cancer (NSCLC). J Thorac Oncol (2019) 14:1095–101. doi: 10.1016/j.jtho.2019.01.011
63. Tamiya Y, Zenke Y, Matsumoto S, Furuya N, Sakamoto T, Kato T, et al. Therapeutic impact of mutation subtypes and concomitant STK11 mutations in KRAS–mutated non-small cell lung cancer (NSCLC): A result of nationwide genomic screening project (LC-SCRUM-Japan). J Clin Oncol (2020) 38:9589. doi: 10.1200/JCO.2020.38.15_suppl.9589
64. Renaud S, Falcoz PE, Schaëffer M, Guenot D, Romain B, Olland A, et al. Prognostic value of the KRAS G12V mutation in 841 surgically resected Caucasian lung adenocarcinoma cases. Br J Cancer (2015) 113:1206–15. doi: 10.1038/bjc.2015.327
65. Nadal E, Chen G, Prensner JR, Shiratsuchi H, Sam C, Zhao L, et al. KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol (2014) 9:1513–22. doi: 10.1097/JTO.0000000000000305
66. Wu SG, Liao WY, Su KY, Yu SL, Huang YL, Yu CJ, et al. Prognostic characteristics and immunotherapy response of patients with nonsquamous NSCLC with kras mutation in East Asian populations: A single-center cohort study in Taiwan. JTO Clin Res Rep (2021) 2:100140. doi: 10.1016/j.jtocrr.2020.100140
67. Pan LN, Ma YF, Li Z, Hu JA, Xu ZH. KRAS G12V mutation upregulates PD-L1 expression via TGF-β/EMT signaling pathway in human non-small-cell lung cancer. Cell Biol Int (2021) 45:795–803. doi: 10.1002/cbin.11524
Keywords: KRAS mutations, KRAS G12 isoforms, treatment responses, immune check-point inhibitors, PD-L1
Citation: Fancelli S, Caliman E, Mazzoni F, Paglialunga L, Gatta Michelet MR, Lavacchi D, Berardi R, Mentrasti G, Metro G, Birocchi I, Delmonte A, Priano I, Comin CE, Castiglione F, Bartoli C, Voltolini L, Pillozzi S and Antonuzzo L (2022) KRAS G12 isoforms exert influence over up-front treatments: A retrospective, multicenter, Italian analysis of the impact of first-line immune checkpoint inhibitors in an NSCLC real-life population. Front. Oncol. 12:968064. doi: 10.3389/fonc.2022.968064
Received: 13 June 2022; Accepted: 17 October 2022;
Published: 14 November 2022.
Edited by:
Iacopo Petrini, University of Pisa, ItalyReviewed by:
Marcello Tiseo, University Hospital of Parma, ItalyCopyright © 2022 Fancelli, Caliman, Mazzoni, Paglialunga, Gatta Michelet, Lavacchi, Berardi, Mentrasti, Metro, Birocchi, Delmonte, Priano, Comin, Castiglione, Bartoli, Voltolini, Pillozzi and Antonuzzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenzo Antonuzzo, bG9yZW56by5hbnRvbnV6em9AdW5pZmkuaXQ=
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.