
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 15 September 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.966534
This article is part of the Research TopicProstate Cancer Genomics: Application at Different Stages of a Patient’s JourneyView all 5 articles
 Umang Swami1†
Umang Swami1† Raquel Mae Zimmerman2†
Raquel Mae Zimmerman2† Roberto H. Nussenzveig1
Roberto H. Nussenzveig1 Edgar Javier Hernandez2
Edgar Javier Hernandez2 Yeonjung Jo3
Yeonjung Jo3 Nicolas Sayegh1
Nicolas Sayegh1 Sergiusz Wesolowski2
Sergiusz Wesolowski2 Lesli A. Kiedrowski4
Lesli A. Kiedrowski4 Pedro C. Barata5
Pedro C. Barata5 Gordon Howard Lemmon2
Gordon Howard Lemmon2 Mehmet A. Bilen6
Mehmet A. Bilen6 Elisabeth I. Heath7
Elisabeth I. Heath7 Lakshminarayan Nandagopal8
Lakshminarayan Nandagopal8 Hani M. Babiker9
Hani M. Babiker9 Sumanta K. Pal10
Sumanta K. Pal10 Michael Lilly11
Michael Lilly11 Benjamin L. Maughan1
Benjamin L. Maughan1 Benjamin Haaland3
Benjamin Haaland3 Mark Yandell2
Mark Yandell2 Oliver Sartor12
Oliver Sartor12 Neeraj Agarwal1*
Neeraj Agarwal1*BRCA1-mutated prostate cancer has been shown to be less responsive to poly (ADP-ribose) polymerase (PARP) inhibitors as compared to BRCA2-mutated prostate cancer. The reason for this differential response is not clear. We hypothesized this differential sensitivity to PARP inhibitors may be explained by distinct genomic landscapes of BRCA1 versus BRCA2 co-segregating genes. In a large dataset of 7,707 men with advanced prostate cancer undergoing comprehensive genomic profiling (CGP) of cell-free DNA (cfDNA), 614 men harbored BRCA1 and/or BRCA2 alterations. Differences in the genomic landscape of co-segregating genes was investigated by Fisher’s exact test and probabilistic graphical models (PGMs). Results demonstrated that BRCA1 was significantly associated with six other genes, while BRCA2 was not significantly associated with any gene. These findings suggest BRCA2 may be the main driver mutation, while BRCA1 mutations tend to co-segregate with mutations in other molecular pathways contributing to prostate cancer progression. These hypothesis-generating data may explain the differential response to PARP inhibition and guide towards the development of combinatorial drug regimens in those with BRCA1 mutation.
Poly (ADP-ribose) polymerase (PARP) inhibitors such as olaparib and rucaparib are currently approved for patients with metastatic castration-resistant prostate cancer (mCRPC) with BRCA1 and BRCA2 alterations (1, 2). However, multiple studies have noted that patients with prostate cancer harboring BRCA2 mutations are more responsive to PARP inhibitors compared to BRCA1 mutation-positive patients. In a pooled analysis of 5 studies, men with BRCA1 mutated prostate cancer compared to BRCA2 mutated had a lower PSA50 response rate (23.8% vs. 65.2%), lower overall response rate (26.3% vs. 50%) and a lower median radiographic progression-free survival (4.1 months vs. 10.1 months) (3). The reason for this differential efficacy is not clear. We hypothesized this differential efficacy may be explained by distinct genomic landscapes of prostate cancer harboring BRCA1 versus BRCA2 mutation.
All patients with advanced prostate cancer who underwent comprehensive genomic profiling (CGP) of cell-free DNA (cfDNA) by a Clinical Laboratory Improvement Amendments (CLIA)-certified, College of American Pathologists (CAP)-accredited laboratory (Guardant360, Redwood City, CA) between 11/2016 to 8/2020 were eligible. First available cfDNA CGP results from consecutive patients with advanced prostate cancer tested were evaluated for the presence of BRCA1/2 mutations. This included all cfDNA somatic alterations defined as reportable by clinical testing parameters. All variants of unknown significance were excluded from the analysis. Frameshift and nonsense mutations were included as pathogenic. Supplementary Figure 1 provides a graphical representation of the cohort selection process and number of patients excluded at each step.
The prevalence of BRCA1 and BRCA2 mutations in our cohort of patients was compared to published reports by the chi-squared test. Pairwise associations of mutation-positive BRCA1 or BRCA2 genes with other mutated genes was independently assessed by Fisher’s exact test, and p-values were adjusted for false discovery rate* (FDR) to control multiple testing. Statistical significance was defined as a p-value ≤ 0.05.
Multilevel gene interdependencies between BRCA1 or BRCA2 were assessed using a combination of two probabilistic graphical model (PGM) machine learning approaches. To account for the high computational cost of the PGM dependence structure discovery, we identified the nearest BRCA1 or BRCA2 neighboring genes by an approximate PGM structure finding algorithm (4).
Once the candidate genes were identified, we used the “exact” DP-A* (5) structure search algorithm provided by the bnlearn R package (4). For the parameter learning and inference we used the default loopy belief propagation algorithm. Visualizations were done using LaTeX (https://texdoc.org/serve/pgfmanual.pdf/0). All significant relations were captured by the PGM.
CGP of cfDNA from 7,707 unique men with advanced prostate cancer was available to assess the presence of mutations in BRCA1 and/or BRCA2. The median age for the total cohort was 72 years (interquartile range 65-78 years). Pathogenic mutations in BRCA1/2 were found in 614 of 7,707 unique patients. The frequency of alterations in BRCA1 (4.6%) and BRCA2 (7.97%) detected in cfDNA was similar to what has been reported from CGP of primary tissue (Supplementary Table 1) (6, 7). The cfDNA mutational landscape of genes with alterations present in ≥5% of these 614 unique patients with advanced prostate cancer is presented in Figure 1.
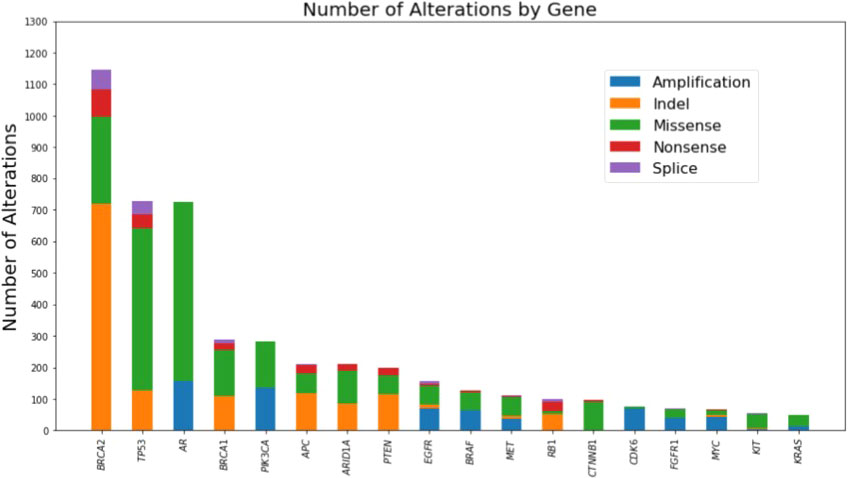
Figure 1 Mutational landscape of genes with alterations present in >=5% of the cohort as detected by comprehensive genomic profiling of cell-free DNA of 614 unique patients with advanced prostate cancer harboring BRCA1 or BRCA2 mutations.
A significant association between mutation-positive BRCA1 and alterations in 6 other genes (ERBB2, NOTCH1, AKT1, MTOR, ARID1A, and EGFR) was identified by Fisher’s exact test (Table 1). In contrast, there were no significant associations between mutation-positive BRCA2 and any other altered gene.
Fifteen altered genes were identified as nearest neighbors to mutation-positive BRCA1/2 by an approximate PGM structure finding algorithm (data not shown) and selected for further analysis by the costly, “exact” DP-A* (5) structure search algorithm. The PGM network analysis demonstrated positive interdependencies between pathogenic variants in BRCA1 and 6 other altered genes. A negative association between mutation-positive BRCA1 and BRCA2 was identified as indicated by a relative risk for co-segregation of 0.07 (Figure 2).
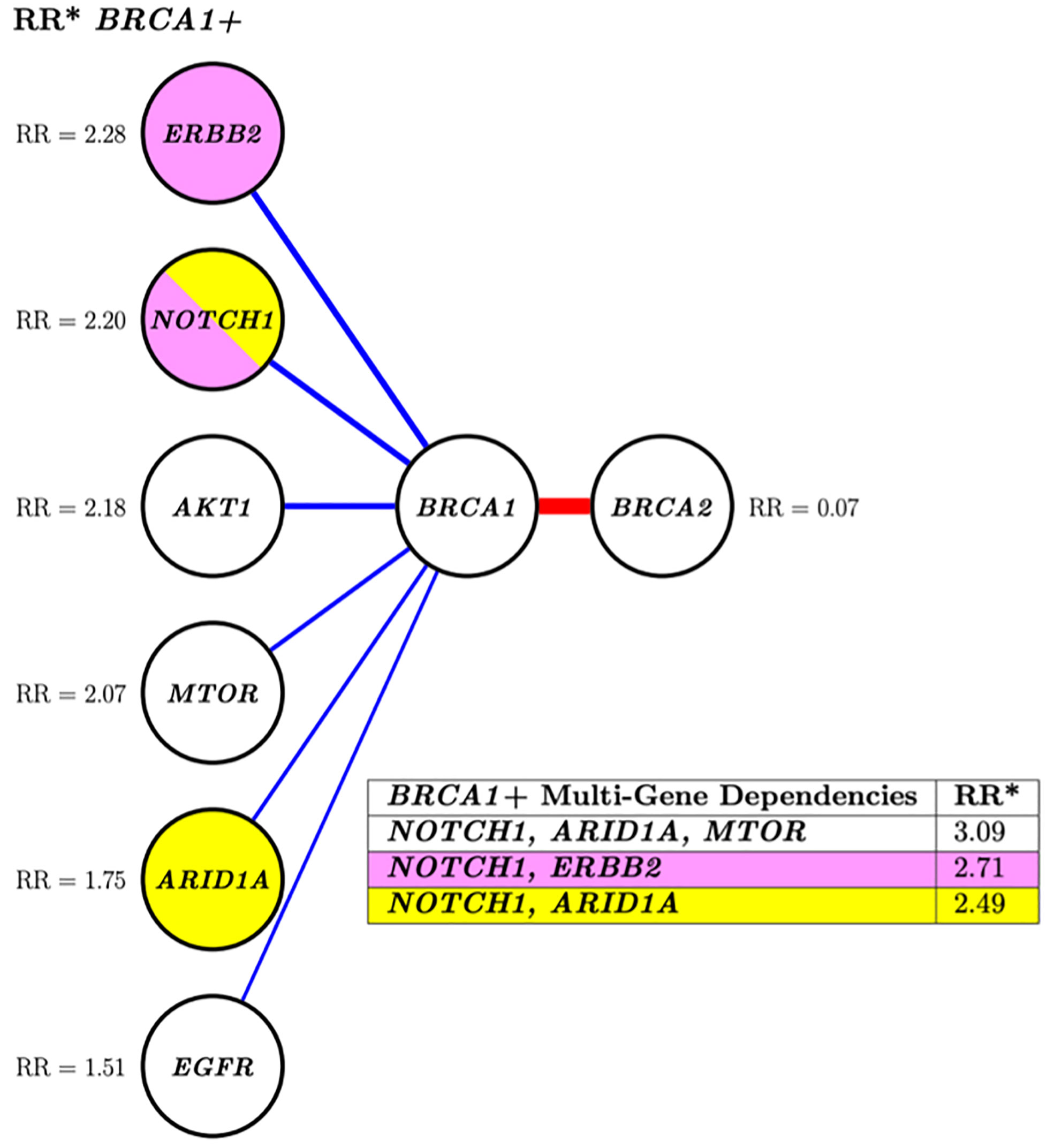
Figure 2 Conditional risk landscape visualization. There is an increased association of BRCA1 (left) versus BRCA2 (right) with somatically mutated genes with pathogenic variants. The table inset displays the risk of having mutations in the genes lkisted in the table if the patient also has a BRCA1 muted gene. *RR, Related risk of co-segregation of gene of interest and BRCA1 and/or BRCA2 compared to BRCA1 and/or BRCA2 in the absence of the gene. Each node represents a variable, and each edge (line) indicates a dependence between variables. Blue indicates increased risk of the outcome of co-segregation and red indicates decreased risk of the outcome of co-segregation. The width of the colored lines is scaled by strength of association. The pairs of yellow and pink shaded nodes correspond to the yellow and pink rows of the table (lower right) which states the associated relative risk of the pair in relation to the outcome.
Investigation of more complex multi-variant interactions, such as those between BRCA1 and the various mutated gene combinations of NOTCH1, ARID1A, and ERBB2 (Figure 2, table inset) revealed increased relative risks for these multi-level associations. For instance, the relative risk of patients with a BRCA1 mutation and a NOTCH1 alteration was 2.20, this risk increased to 3.09 if ARID1A and MTOR were also mutated. This type of multi-level dependency cannot be investigated by conventional pairwise statistics or regression analysis. Overall, both analyses showed a greater likelihood of multiple gene segregation with BRCA1 versus BRCA2.
Our results demonstrate that BRCA1 mutation in patients with advanced prostate cancer was significantly associated with 6 other genes, while BRCA2 was not significantly associated with any gene. The higher number of significant concurrently mutated genes in other molecular pathways may explain the decreased efficacy of PARP inhibitors in prostate cancer harboring BRCA1 mutations compared to those with BRCA2 mutation. For example, ERBB2 alterations co-segregate strongly with BRCA1 and are known to be associated with tumor aggressiveness in primary prostate cancer, tumor progression and invasion in advanced prostate cancer, and shorter time to castration-resistance (8). Similarly, alterations in NOTCH1 also co-segregate with BRCA1. Dysregulated activation of NOTCH1 promotes development of prostate cancer metastasis and castration-resistance by altering signaling through multiple oncogenic pathways including AKT, Myc, and Ras/Raf/MAPK pathway (9). Using both Fisher’s exact test and PGMs we demonstrate that the presence of mutated genes, such as ERBB2 and NOTCH1, are more likely to co-occur in patients harboring BRCA1 mutations. Furthermore, the use of PGMs, which capture multivariate, multi-level dependencies, revealed increased relative risks with the combination of two or more pathogenically mutated genes in BRCA1 mutation-positive patients.
Our results show a higher prevalence of BRCA1 alterations and a slightly lower prevalence of BRCA2 alterations than previously reported, as described in Supplementary Table 1. These differences may be due to three reasons. First, prior studies which have sequenced tumor tissue are mostly from primary prostate and less frequently from metastatic sites. Liquid biopsies in patients with prostate cancer are usually done in clinics once multiple therapies such as androgen-receptor axis targeting agents and taxanes have been utilized. In this situation, tissue biopsies are generally difficult due to bone predominant disease and there are not many studies to elucidate the tumor mutation profile in this scenario. Therefore, it is likely that patients with advanced disease after progression on standard therapies are enriched with BRCA1 mutations. Second, liquid biopsies combine the mutation profile of tumors across all metastatic sites. Again, it is currently unknown how the metastatic sites differ from primary in terms of mutations, and our results may be a reflection of it. Third, as acknowledged below in limitations we are unable to determine if the origin of mutations in cfDNA in our study is from tumor or germline and this may also have increased the incidence of BRCA1 alterations as compared to historical tissue somatic testing results.
Despite recent advances and the approval of multiple agents for the treatment of advanced prostate cancer, the disease remains lethal (10, 11). After disease progression on an androgen receptor targeted therapy, the median overall survival of these patients is only two years (12). Therefore, it is critical to discover molecular pathways of disease progression and develop novel drugs targeting these pathways to improve outcomes. One way to identify these pathways is to obtain tumor biopsies upon disease progression on a given therapy. However, in most patients with metastatic prostate cancer, bones remain the only site of metastatic disease, making these tumor biopsies impractical in the real-world setting. In many others, a metastatic site biopsy is considered unsafe, expensive, invasive, and not desirable. In this context, interrogation of cfDNA to identify these molecular pathways of disease resistance is an attractive alternative.
We and others have previously shown the feasibility of utilizing CGP of the cfDNA in patients with advanced prostate cancer (13, 14) to identify disease resistance pathways. Although two PARP inhibitors (olaparib and rucaparib) were only recently approved for mCRPC treatment, this field is expected to undergo a rapid evolution with more PARP inhibitors either as single agents or in novel combinations predicted to be approved shortly. Against this backdrop, it has become critical to identify mechanisms of differential response and disease resistance to PARP inhibitors concerning various underlying homologous recombination repair mutations. The current study attempts to elucidate the molecular mechanism of differential response to PARP inhibitors in men with advanced prostate cancer harboring BRCA1 versus BRCA2 mutation. Our results, upon external validation in independent cohorts with available cfDNA and tumor tissue DNA, may guide the development of novel treatment regimens for these patients.
The limitations of this study include the lack of clinical annotation such as the disease state and treatment exposure (including potential responses to PARP inhibitor therapy), as well as the inability to definitively determine the origin of mutations identified in cfDNA (e.g. tumor versus germline versus hematopoietic). Strengths of the study include the number of patients and centers included and the dataset’s real-world nature. These hypothesis-generating data reveal differential genomic signatures associated with BRCA1 as compared to BRCA2 which may translate in the development of novel combinatorial regimens for patients in the future.
The datasets generated and/or analyzed for the current study are not publicly available, as they are derived from commercial testing. This data may be made available under a fully executed data use agreement. Requests to access these datasets should be directed to Lesli Kiedrowski, bGtpZWRyb3dza2lAZ3VhcmRhbnRoZWFsdGguY29t.
The studies involving human participants were reviewed and approved by University of Utah IRB. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Conception and design: US, RN, NA. Acquisition of data: LK, RN. Analysis and interpretation of data: US, RZ, RN, EJH, BH, MY, NA. Drafting of the manuscript: US, RZ, RN, NA. Critical revision of the manuscript for important intellectual content: US, RZ, RN, EJH, YJ, NS, SW, LK, PB, GL, MB, EIH, LN, HB, SP, ML, BM, BH, MY, OS, NA. Statistical analysis: US, RZ, RN, EJH, YJ, BH. All authors contributed to the article and approved the submitted version.
NA reports consultancy to Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics; and additionally reports institutional research funding from Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. LK is an employee and stockholder of Guardant Health. PB declares grants or contracts from Merck, Seagen, Blue Earth Diagnostics, Pfizer, and EMD Serono; consulting fees from Dendreon, Pfizer, Caris Life Sciences, Astellas, Eisai, Janssen, EMD Serono, Seattle Genetics, Bristol-Myers Squibb, Bayer, and Guardant Health; payments or honoraria from Caris Life Sciences, Bayer, and Pfizer; and participation on boards for Bristol-Myers Squibb, Seagen, Astellas, Eisai, Janssen, EMD Serono, Dendreon, Pfizer, Seattle Genetics, Bayer, and Guardant Health. MB declares consulting fees from Exelixis, Bayer, Bristol-Myers Squibb, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, EMD Serono, Seagen, and Sanofi and institutional research support from Merck, Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, Seagen, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, and Pfizer for work performed outside the current study. EIH has received honoraria from Bayer, Sanofi, and Seattle Genetics; acted as a consultant/advisor for Astellas Pharma; is an Advisory Board and/or Speakers’ Bureau member for AstraZeneca, Bayer, Bristol-Myers Squibb, Sanofi; and has received paid travel from Astellas Pharma, Caris Life Sciences, Sanofi, and Seattle Genetics; in addition, her institution has received research funding from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Caris Life Sciences, Celgene, Celldex, Corcept Therapeutics, Curementa, Dendreon, eFFECTOR Therapeutics, Esanik, Fortis Therapeutics, Genetech/Roche, GlaxoSmithKline, Ignyta, Inovio Pharmaceuticals, Medivation, Merck Sharp & Dohme, Merck, Millennium, Oncolys BioPharma, Plexxicon, Seattle Genetics, Synta, Tokai Pharmaceuticals, and Zenith Epigenetics. HB Consulting or Advisory Role: Endocyte, Celgene, Idera, Myovant Sciences Speakers' Bureau: Guardant Health. SP reports personal fees from F. Hoffman-La Roche outside the submitted work, as well as research funding to his institution from Eisai, Genentech, Roche, Exelixis, and Pfizer; and reports a consulting/advisory role for Novartis, Medivation, Astellas Pharma, Pfizer, Aveo, Myriad, Genentech, Exelixis, and Bristol-Myers Squibb. US reports consultancy to Astellas, Exelixis and Seattle Genetics and research funding to institute from Janssen, Exelixis and Astellas/Seattle Genetics. OS is a consultant for Advanced Accelerator Applications, Astellas, AstraZeneca, Bayer Blue Earth Diagnostics Inc., Bavarian, Nordic, Bristol, Myers, Squibb, Clarity, Pharmaceuticals, Clovis, Constellation, Dendreon, EMD, Serono, Fusion, Janssen, Myovant, Myriad, Noria, Therapeutics, Inc., Novartis, Noxopharm, Progenics, POINT, Biopharma, Pfizer, Sanofi, Tenebio, Telix, Theragnostics, Dendreon, Endocyte, Innocrin, Invitae, Merck, and SOTIO; research funding from Advanced Accelerator Applications, AstraZeneca, Bayer, Invitae, and Merck. BH reports receiving travel assistance from Flatiron Health, and served as a consultant for AstraZeneca, Value Analytics, National Kidney Foundation, and Prometic Life Sciences. MY is a stock holder or has received stock option awards from Fabric Genomics Inc. and has received consulting fees from Fabric Genomics Inc. BM has consulted for Janssen Oncology, Exelixis, Tempus, Peloton Therapeutics and Astellas. RN has consulted for Tempus.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.966534/full#supplementary-material
1. Hussain M, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med (2020) 383(24):2345–57. doi: 10.1056/NEJMoa2022485
2. Abida W, Patnaik A, Campbell D, Shapiro J, Bryce AH, McDermott R, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol (2020) 38(32):3763–72. doi: 10.1200/JCO.20.01035
3. Markowski MC, Antonarakis ES. BRCA1 versus BRCA2 and PARP inhibitor sensitivity in prostate cancer: More different than alike? J Clin Oncol (2020) 38(32):3735–9. doi: 10.1200/JCO.20.02246
4. Scutari M. Learning Bayesian networks with thebnlearnRPackage. J Stat Software (2010) 35(3):1–22. doi: 10.18637/jss.v035.i03
5. Yuan C, Malone B, Wu X. Learning optimal Bayesian networks using a* search. In: Proceedings of the twenty-second international joint conference on artificial intelligence - volume volume three. Barcelona, Catalonia, Spain: AAAI Press (2011). p. 2186–91.
6. Mateo J, Seed G, Bertan C, Rescigno P, Dolling D, Figueiredo I, et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J Clin Invest (2020) 130(4):1743–51. doi: 10.1172/JCI132031
7. Chung JH, Dewal N, Sokol E, Mathew P, Whitehead R, Millis SZ, et al. Prospective comprehensive genomic profiling of primary and metastatic prostate tumors. JCO Precis Oncol (2019) 3. doi: 10.1200/PO.18.00283
8. Rossini A, Giussani M, Ripamonti F, Aiello P, Regondi V, Balsari A, et al. Combined targeting of EGFR and HER2 against prostate cancer stem cells. Cancer Biol Ther (2020) 21(5):463–75. doi: 10.1080/15384047.2020.1727702
9. O’Brien R, Marignol L. The notch-1 receptor in prostate tumorigenesis. Cancer Treat Rev (2017) 56:36–46. doi: 10.1016/j.ctrv.2017.04.003
10. Sayegh N, Swami U, Agarwal N. Recent advances in the management of metastatic prostate cancer. JCO Oncol Pract (2021) 18(1):45–55. doi: 10.1200/OP.21.00206
11. Swami U, Sinnott JA, Haaland B, Sayegh N, McFarland TR, Tripathi N, et al. Treatment pattern and outcomes with systemic therapy in men with metastatic prostate cancer in the real-world patients in the united states. Cancers (Basel) (2021) 13(19):4951–4951. doi: 10.3390/cancers13194951
12. Sayegh N, Tripathi N, Nussenzveig RH, Thomas VM, Tandar C, Goel D, et al. Survival of Patients with Metastatic Prostate Cancer After Disease Progression on an Androgen Receptor Axis-Targeted Therapy Given in the Metastatic Castration-Sensitive Versus Metastatic Castration-Resistant Prostate Cancer Setting. Eur Urol Focus (2022) S2405–4569(22):00145–6. doi: 10.1016/j.euf.2022.06.015
13. Lin E, Hahn AW, Nussenzveig RH, Wesolowski S, Sayegh N, Maughan BL, et al. Identification of somatic gene signatures in circulating cell-free DNA associated with disease progression in metastatic prostate cancer by a novel machine learning platform. Oncologist (2021) 26(9):751–60. doi: 10.1002/onco.13869
Keywords: BRCA1 vs. BRCA2 landscape by cfDNA BRCA1, BRCA2, ctDNA, advanced prostate cancer, machine learning
Citation: Swami U, Zimmerman RM, Nussenzveig RH, Hernandez EJ, Jo Y, Sayegh N, Wesolowski S, Kiedrowski LA, Barata PC, Lemmon GH, Bilen MA, Heath EI, Nandagopal L, Babiker HM, Pal SK, Lilly M, Maughan BL, Haaland B, Yandell M, Sartor O and Agarwal N (2022) Genomic landscape of advanced prostate cancer patients with BRCA1 versus BRCA2 mutations as detected by comprehensive genomic profiling of cell-free DNA. Front. Oncol. 12:966534. doi: 10.3389/fonc.2022.966534
Received: 11 June 2022; Accepted: 25 August 2022;
Published: 15 September 2022.
Edited by:
Rosa M. Nadal, National Heart, Lung, and Blood Institute (NIH), United StatesReviewed by:
Benjamin A. Teply, University of Nebraska Medical Center, United StatesCopyright © 2022 Swami, Zimmerman, Nussenzveig, Hernandez, Jo, Sayegh, Wesolowski, Kiedrowski, Barata, Lemmon, Bilen, Heath, Nandagopal, Babiker, Pal, Lilly, Maughan, Haaland, Yandell, Sartor and Agarwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neeraj Agarwal, bmVlcmFqLmFnYXJ3YWxAaGNpLnV0YWguZWR1
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.