
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 13 September 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.965719
Purpose: Induction chemotherapy followed by concurrent chemoradiotherapy (IC-CCRT) may be beneficial for nasopharyngeal carcinoma. However, the evidence on medium- and long-term effects of IC-CCRT is limited, and new randomized controlled trials (RCTs) have been published after 2018. Therefore, this systematic review and meta-analysis compared survival rates between patients with nasopharyngeal carcinoma receiving IC-CCRT or concurrent chemoradiotherapy (CCRT).
Methods: Four databases were searched for RCTs on this topic. Two authors independently selected studies, assessed evidence, and extracted data on progression-free survival, overall survival, metastasis-free survival, and local recurrence-free survival. Available data were pooled in a random-effects model and mainly presented in hazard ratio (HR). Heterogeneity and small study effects were also evaluated.
Results: Eleven RCTs (n = 3345) were deemed eligible. Pooled results revealed that patients receiving IC-CCRT had significantly improved progression-free survival (HR = 0.66, P < 0.05), overall survival (HR = 0.64, P < 0.05), metastasis-free survival (HR = 0.58, P < 0.05), and local recurrence-free survival (HR = 0.69, P < 0.05) at 3 years, but no significant difference in 5-year overall survival was noted between IC-CCRT and CCRT (HR = 0.84, P > 0.05). Most findings had low heterogeneity.
Conclusion: IC-CCRT may benefit patients with nasopharyngeal carcinoma in the medium term, although no significant difference was observed in 5-year survival compared with CCRT. All outcomes had decreased survival rate from the 3-years to 5-year follow-up. Differences in patient ethnicities and regimens of IC-CCRT may be sources of heterogeneity.
Nasopharyngeal carcinoma most commonly originates in the fossa of Rosenmüller, making it difficult to detect (1). According to Global Burden of Disease reports, nasopharyngeal carcinoma causes a decrease in lifespan by 13–15 years in terms of age-standardized years of life lost (2, 3). Peak incidence of nasopharyngeal carcinomas is middle age, and the male-to-female ratio is 3:1. However, because of the shortened lifespan of the middle-aged patient, even if cured, the quality of life is considerably affected.
Nasopharyngeal carcinoma is extremely common in the Asian population (4, 5), as well as in populations in North Africa, especially Tunisia and Algeria (6), and among Inuits in Alaska, North Canada, and Greenland (7). HLA subtypes A2, B14, and B46 are associated with an increased risk. The association of Epstein–Barr virus with nasopharyngeal carcinoma can be exploited in the diagnostic process, therapeutic strategies, and preventive treatment (8).
According to the 2018 National Comprehensive Cancer Network (NCCN) guidelines, the treatment algorithm for locoregionally advanced nasopharyngeal carcinoma (T1, N1–3; T2–4, any N) is concurrent chemoradiotherapy (CCRT) alone or followed by adjuvant chemotherapy or induction chemotherapy followed by CCRT. Induction chemotherapy can facilitate organ preservation, avoid morbid surgery, and improve the patient’s overall quality of life (9). The NCCN guidelines state that the intervention is appropriate based on any level of evidence in IC-CCRT in the advice of treatment of locoregionally advanced nasopharyngeal carcinoma; however, most of the relevant cited studies in the guidelines pertain to head and neck cancer.
Comparison of IC-CCRT and CCRT for nasopharyngeal carcinoma is worthy of further investigation due to incompleteness of evidence in previous syntheses although many head-to-head meta-analyses (10–16) and network meta-analyses have tried to provide conclusive evidence on this topic (17–24). Most studies have compared IC-CCRT with CCRT based on limited evidence, particularly the network meta-analyses. The most recent network meta-analyses were published in 2019 (22–24), whereas the largest network meta-analysis was published in 2017 (20). The most complete network meta-analysis included 27 trials, but direct evidence of IC-CCRT and CCRT in the network meta-analysis only relied on five trials (20). In fact, nine RCTs that had been published before the network meta-analysis was accepted for publication were not included in it (25–33).
In addition to the limited evidence in the previous syntheses, findings in the abovementioned studies are conflict with each other. For instance, one meta-analysis on the JAMA Network Open revealed that the addition of induction chemotherapy but not adjuvant chemotherapy to radiotherapy or CCRT can yield prolonged overall survival, progression-free survival, distance metastasis-free survival, and local recurrence-free survival (34). On the contrary, another meta-analysis on Journal of Clinical Oncology concluded that the addition of induction chemotherapy to CCRT does not present the highest survival benefit or consistent improvement for all end points (19). This inconclusive evidence may reduce clinicians’ confidence in applying the findings to clinical practice.
Thus, the benefit of IC-CCRT remains controversial, and most trials lack a sufficient sample size. Moreover, limited evidence has indicated the medium- and long-term benefits of IC-CCRT, and new randomized controlled trials (RCTs) have been published after 2018 (35–39). Therefore, this systematic review and meta-analysis included RCTs to compare survival rates between IC-CCRT and CCRT in treating nasopharyngeal carcinoma.
This systematic review and meta-analysis was conducted according to methodological guidance from the Cochrane handbook and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (40, 41). According to the study aim stated earlier, our research question in PICO form is as follows:
P: Patients with nasopharyngeal carcinoma receiving concurrent chemoradiotherapy
I: Induction chemotherapy
C: No induction chemotherapy
O: Overall survival, disease-free survival, local recurrence-free survival, and metastasis-free survival
To evidence the efficacy of induction chemotherapy in patients with nasopharyngeal carcinoma undergoing concurrent chemoradiotherapy, this systematic review and meta-analysis only included RCTs. The inclusion criteria were as follows: (a) RCTs that recruited only patients with nasopharyngeal carcinoma and (b) all patients received CCRT. The present systematic review did not exclude studies based on treatment protocol, regimen, or dose of induction chemotherapy.
We searched the Cochrane Central Register of Controlled Trials and Embase, New PubMed, and Web of Science databases for RCTs from inception until study date by using the following keywords in free-text and medical subject heading (MeSH): “nasopharyngeal carcinoma,” “chemoradiotherapy,” “chemotherapy,” “radiotherapy,” and “induction”. Next, the keywords were combined using Boolean operators “OR” and “AND.” Synonyms were connected by “OR,” and keyword sets of different concepts were connected by “AND” (Appendix 1). No filter was used for language and publication date. Reference lists of relevant systematic reviews and RCTs on this topic were also screened for retrieving potential evidence.
Two authors (TCH and CJC) independently reviewed titles and abstracts of the identified references; after exclusion of duplicated and irrelevant references, full-texts were retrieved for further review and eligibility judgment. Any disagreement was resolved through discussion with an experienced researcher.
TCH and CJC then independently extracted and double-checked information on study area; inclusion duration; sex; age; proportion of advanced stage; regimen, dose, and schedule of chemotherapy; protocol and dose of radiation therapy; study design; and overall, disease-free, local recurrence-free, and metastasis-free survival. Data on outcomes of interest were usually reported as HR (with CI) or events; if both were present, the authors extracted both.
The authors then evaluated the quality of the included RCTs byusing the Cochrane risk of bias tool (41). Generation of randomization, allocation concealment, blinding to investigators, blinding to participants, blinding to assessors, loss to follow-up, type of analysis, and selective reporting were evaluated. In case of disagreements, an experienced author (corresponding author) made the final decision.
Qualitative synthesis was performed through tabulation with relevant discussion, and quantitative synthesis was conducted using R 4.0.2 for Microsoft Windows. Log HR with standard error (SE) for outcomes of interest were derived from the extracted HR and CI. Primary outcomes were mainly based on pooled log HR with SE. Data on survival cases and total sample size were pooled for revealing risk ratio (RR) for each year after treatment because the included trials reported results at various time points. Both pooled HR and RR were estimated using the random-effects model due to heterogeneity in not only statistical findings but also clinical conditions. Statistical heterogeneity was tested with I² and P value for the heterogeneity test: values of >50% and <0.10, respectively, indicated high heterogeneity. Graphical display of study heterogeneity (GOSH) analysis was conducted using the “gosh” function for objects of class “rma” in library “metafor,” and a funnel plot was generated to assess potential publication bias.
We identified 3671 (2844) references in the initial search. Eventually, 15 references presented results of 11 RCTs comparing IC-CCRT with CCRT alone for management of nasopharyngeal carcinoma (25–29, 31–33, 35–39, 42, 43), and were considered for the present synthesis (Figure 1).
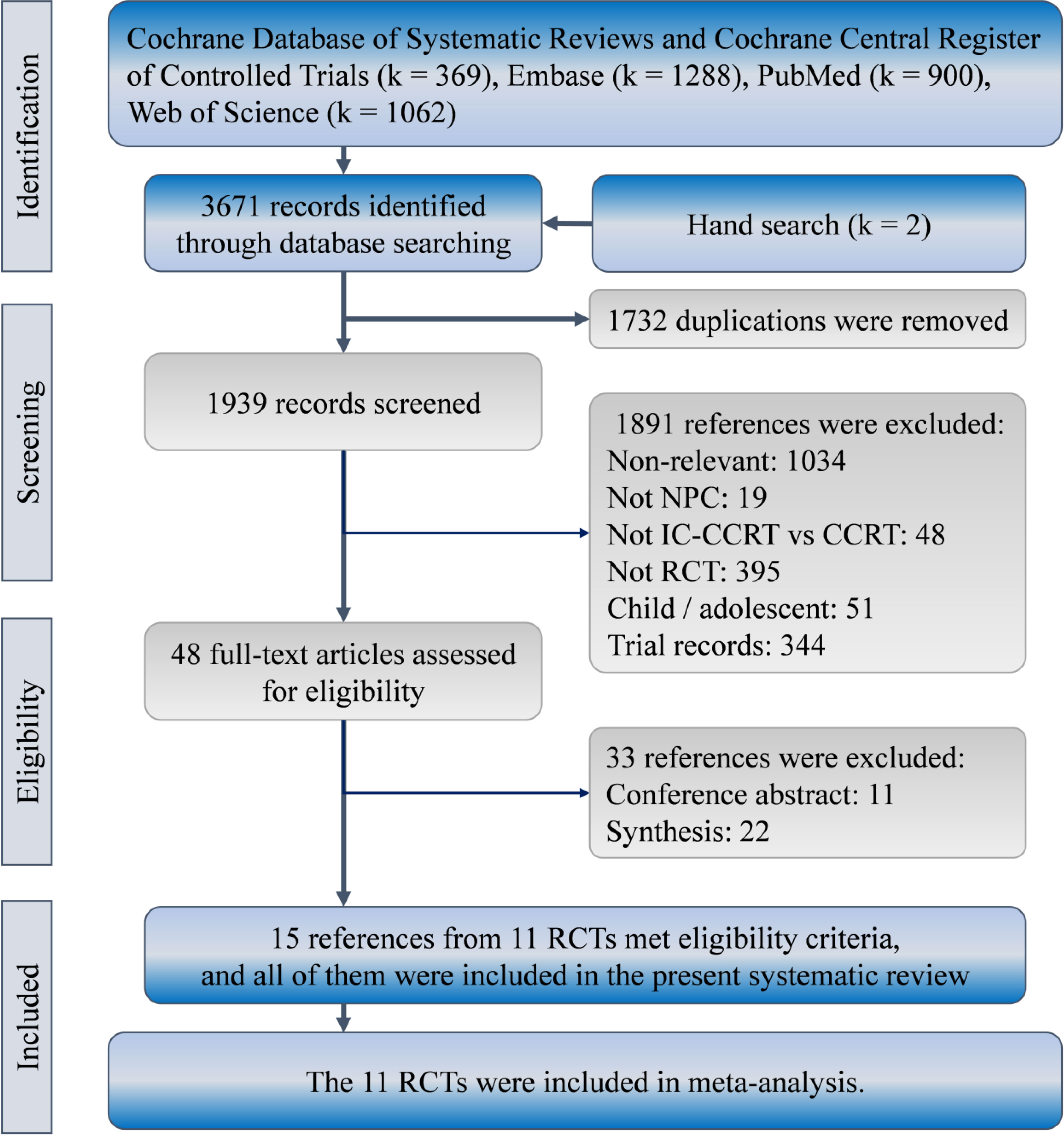
Figure 1 Patient selection flowchart according to PRISMA guidelines. IC-CCRT, induction chemotherapy; RCT, randomized controlled trial.
A total of 3345 patients with nasopharyngeal carcinoma were recruited in the 11 RCTs for qualitative and quantitative synthesis. They participated in clinical trials between 2002 and 2016. Most patients were from Asia (n = 3123, 93%), and only 222 (7%) were from Europe. More than half of them were men (n = 2449, 73%), and the median age was between 42 and 51.6 years. More than 99% patients had advanced-stage carcinoma but no metastasis Table 1. Other information on regimen, dose, and treatment protocol is presented in Supplementary Table 1, and the quality of the included RCTs is indicated in Supplementary Table 2. Some concerns of risk of bias were raised due to the unclear bias of risk in allocation concealment. Biases from performance, detection, attrition, and selected reporting were low in most trials, except in the trial by Jin et al. (33).
Nine RCTs (n = 3013) reported HR and CI for disease-free survival. Seven of them reported 3-year disease-free survival (25, 26, 29, 32, 35, 37–39), and four reported 5-year disease-free survival (Figure 2) (33, 36–38). Compared with the CCRT group, the IC-CCRT group had lower HR in 3-year disease-free survival (HR = 0.66, 95% CI: 0.55–0.79; I2 = 26%, P for heterogeneity > 0.10) and lower HR in 5-year disease-free survival (HR = 0.75, 95% CI: 0.64–0.88; I2 = 39%, P for heterogeneity > 0.10). Small study effects may not seriously affect this finding.
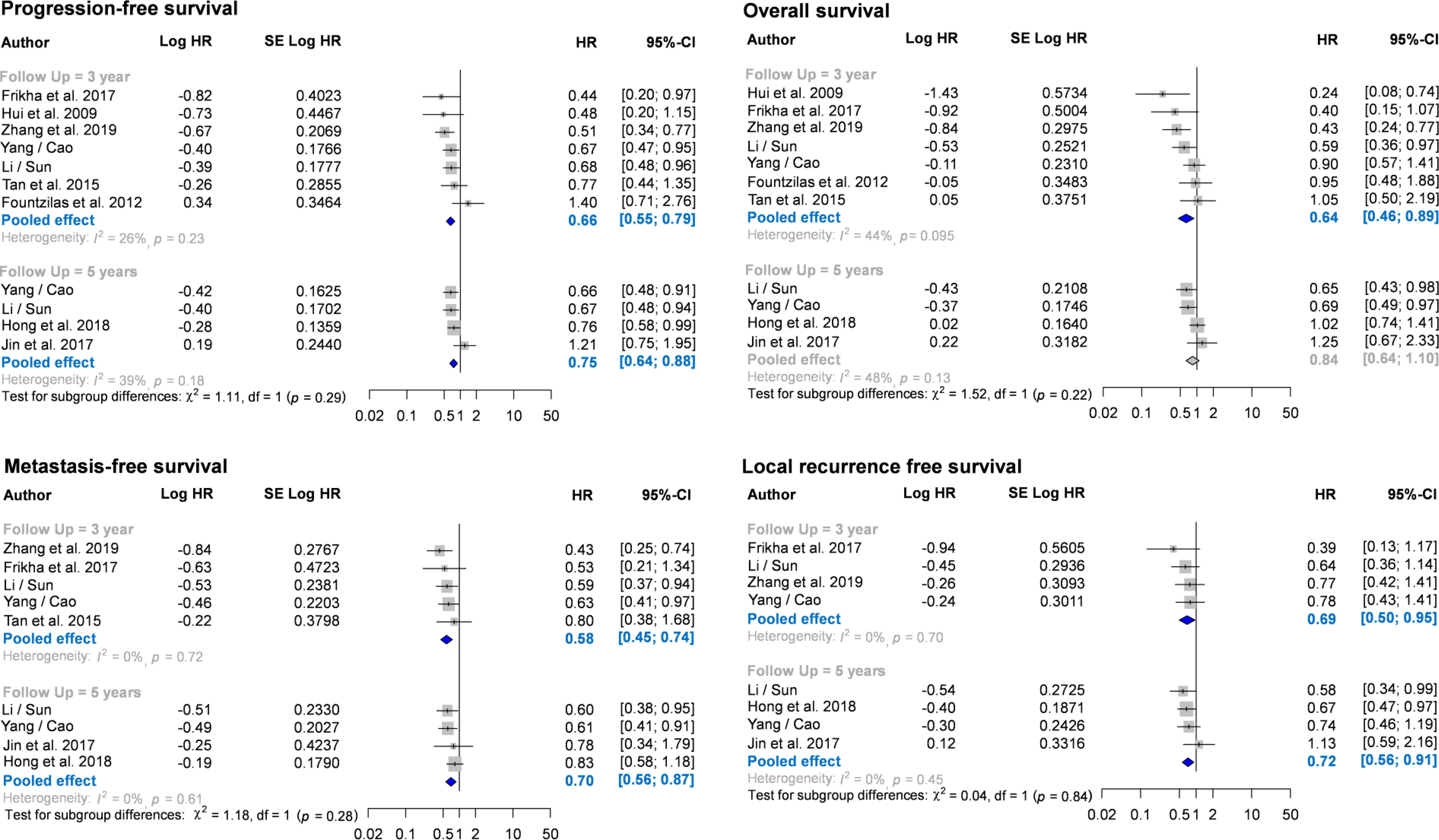
Figure 2 Forest plots for meta-analysis of hazard ratio of survival. CI, confidence interval; HR, hazard ratio.
The IC-CCRT group had a significantly higher cumulative disease-free survival rate than the CCRT group at 1 year (RR = 1.06, 95% CI: 1.04–1.09; I2 = 32%, P for heterogeneity > 0.10), 2 years (RR = 1.09, 95% CI: 1.05–1.14; I2 = 1%, P for heterogeneity > 0.10), 3 years (RR = 1.10, 95% CI: 1.05–1.16; I2 = 0%, P for heterogeneity > 0.10), and 4 years (RR = 1.11, 95% CI: 1.03–1.19; I2 = 0%, P for heterogeneity > 0.10) but not at 5 years (I2 = 36%, P for heterogeneity > 0.10; Figure 3; Appendix 2). Small study effects may not seriously affect disease-free survival (Figure 4; Appendix 3).
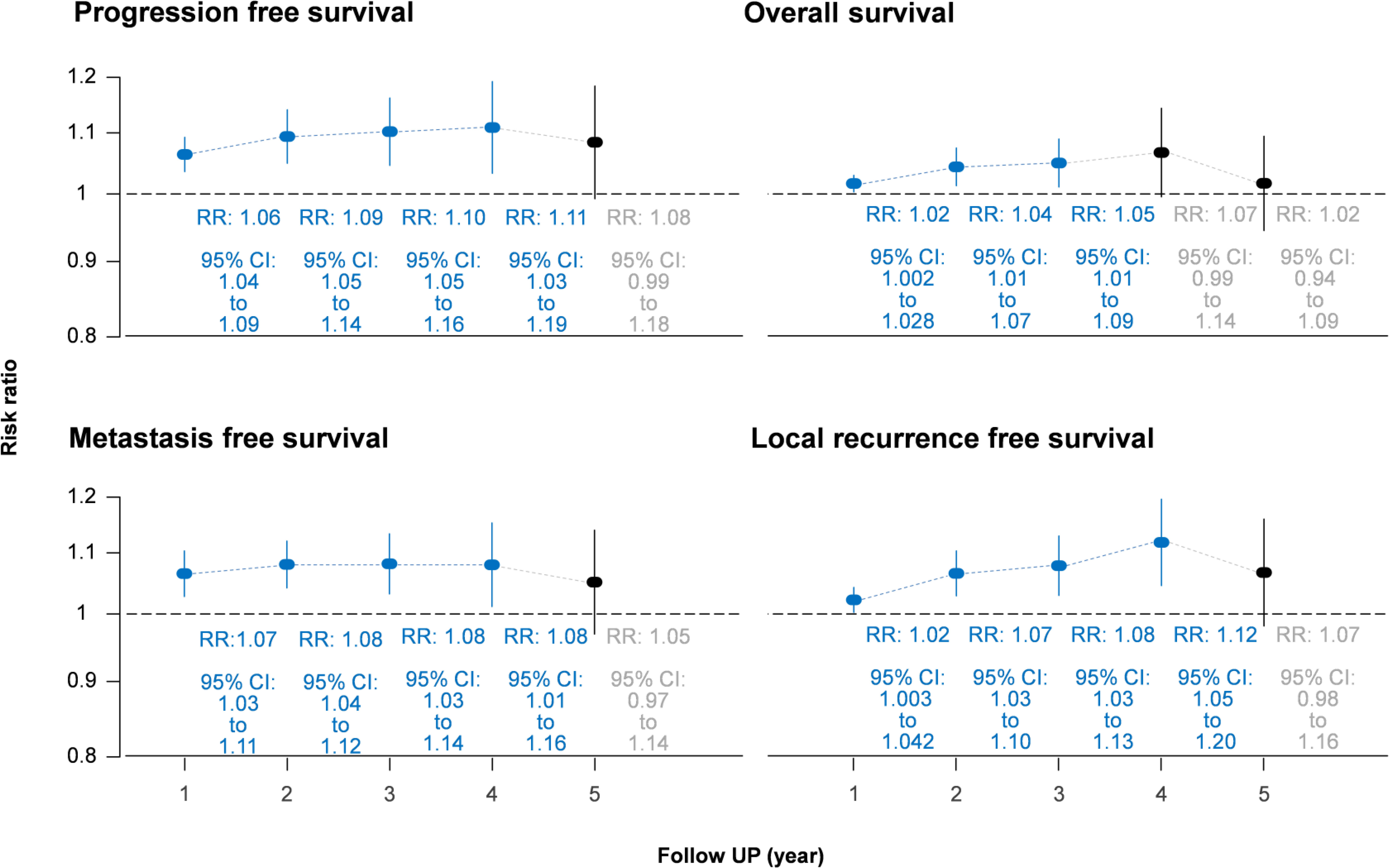
Figure 3 Forest plot of cumulative survival rate using risk ratio. CI, confidence interval; RR, risk ratio.

Figure 4 Funnel plots for meta-analysis of hazard ratio of survival. Bias, Egger’s regression intercept for publication bias test.
The nine RCTs (n = 3013) also reported HR and CI for overall survival. Seven of them reported 3-year overall survival (25, 26, 29, 32, 35, 37–39), and four reported 5-year overall survival (33, 36–38). The pooled results revealed that IC-CCRT significantly improved 3-year overall survival (HR = 0.64, 95% CI: 0.46–0.89; I2 = 44%, P for heterogeneity > 0.05), but no significant intergroup difference was observed in 5-year overall survival (I2 = 48%, P for heterogeneity > 0.10). Small study effects may not seriously affect 3-year overall survival.
In the measurement of cumulative rate, the overall survival rate in the IC-CCRT group was significantly higher than that in the CCRT group at 1 year (RR = 1.02, 95% CI: 1.002–1.028; I2 = 39%, P for heterogeneity < 0.10), 2 years (RR = 1.04, 95% CI: 1.01–1.07; I2 = 47%, P for heterogeneity < 0.10), and 3 years (RR = 1.05, 95% CI: 1.01–1.09; I2 = 0%, P for heterogeneity > 0.10; Appendix 4) but not at 4 and 5 years. Small study effects may not seriously affect overall survival (Appendix 5).
Seven RCTs (n = 2807) also reported HR and CI for metastasis-free survival. Of them, five reported 3-year metastasis-free survival (29, 32, 35, 37–39), and four reported 5-year metastasis-free survival (33, 36–38). Pooled estimates revealed that IC-CCRT resulted in a significantly better metastasis-free survival than CCRT did at 3 years (HR = 0.58, 95% CI: 0.45–0.73; I2 = 0%, P for heterogeneity > 0.10) and 5 years (HR = 0.70, 95% CI: 0.56–0.87; I2 = 0%, P for heterogeneity > 0.10). Small study effects may not seriously affect this finding.
Cumulative metastasis-free survival rate in the IC-CCRT group was significantly higher than that in the CCRT group at 1 year (RR = 1.07, 95% CI: 1.03–1.11; I2 = 48%, P for heterogeneity > 0.05), 2 years (RR = 1.08, 95% CI: 1.04–1.12; I2 = 0%, P for heterogeneity > 0.10), 3 years (RR = 1.08, 95% CI: 1.03–1.14; I2 = 0%, P for heterogeneity > 0.10), and 4 years (RR = 1.08, 95% CI: 1.01–1.16; I2 = 0%, P for heterogeneity > 0.10) but not at 5 years (I2 = 28%, P for heterogeneity > 0.10; Appendix 6). Small study effects may not seriously affect cumulative metastasis-free survival (Appendix 7).
Six RCTs (n = 2506) also reported HR and CI for local recurrence-free survival. Of them, four reported 3-year local recurrence-free survival (32, 35, 37–39), and four reported 5-year local recurrence-free survival (33, 36–38). Pooled estimates indicated that IC-CCRT exhibited a significant improvement in local recurrence-free survival in both 3-year follow-up (HR = 0.69, 95% CI: 0.50–0.95; I2 = 0%, P for heterogeneity > 0.10) and 5-years follow-up (HR = 0.72, 95% CI: 0.56–0.91; I2 = 0%, P for heterogeneity > 0.10). Small study effects may not seriously affect the pooled estimate of local recurrence-free survival.
Similarly, the local recurrence-free survival rate in the IC-CCRT group was significantly higher than that in the CCRT group at 1 year (RR = 1.02, 95% CI: 1.003–1.042; I2 = 63%, P for heterogeneity < 0.05), 2 years (RR = 1.07, 95% CI: 1.03–1.10; I2 = 54%, P for heterogeneity > 0.05), 3 years (RR = 1.08, 95% CI: 1.03–1.13; I2 = 0%, P for heterogeneity > 0.10), and 4 years (RR = 1.12, 95% CI: 1.05–1.20; I2 = 0%, P for heterogeneity > 0.10) but not at 5 years (I2 = 7%, P for heterogeneity > 0.10; Appendix 8). GOSH analysis revealed that heterogeneity could be reduced by excluding the study by Frikha et al. (Figure 5 and Appendix 9). Small study effects may not seriously affect cumulative local recurrence-free survival (Appendix 10).
The results of our meta-analysis demonstrated significant improvement in medium-term (3-year) overall survival, progression-free survival, local-regional free survival, and metastasis-free survival in patients with advanced-stage nasopharyngeal carcinoma who receive induction chemotherapy additional to CCRT, whereas no such benefit was seen in long-term (5-year) overall survival, as evidenced by the trend observed in both HR and cumulative measurements. Moreover, some heterogeneity was observed in overall survival, cumulative measurements for metastasis-free survival at 1 year, and cumulative measurements for local recurrence-free survival at 1 and 2 years. Briefly, both time-to-event and cumulative measurements exhibit favorable trends toward IC-CCRT although the two measurements are inconsistent in statistical significance of 5-year progression-free survival, local-regional free survival, and metastasis-free survival. Overall, current evidence on relevant outcomes of 3-year survival is of moderate certainty, whereas evidence on 5-year survival outcomes has low to very low certainty (Supplementary Table 3).
The heterogeneity might be due to different ethnicities, patients’ baseline condition, or regimens of the induction chemotherapy. First, two studies were conducted in nonendemic areas (one in Tunisia and France and the other in Greece and Romania) (26, 35), and the outcomes were relatively poor. However, the association between human ethnicities and disease outcome remains unconfirmed due to the complexity of race and variations in regimens of the induction chemotherapies. Although no significant difference in overall survival and progression-free survival was noted among the induction chemotherapy of TPF, PF, and TP (22), some trials did not use common regimens of TPF, PF, and TP for induction chemotherapy (26, 29, 39). The regimen of induction chemotherapies in most of the included studies comprised two or all of docetaxel, cisplatin, and 5-flurouracine, except studies by Zhang et al. (gemcitabine and cisplatin) (39), Tan et al. (paclitaxel, gemcitabine, and carboplatin) (29), and Fountzilas et al. (epirubicin, paclitaxel, and cisplatin) (26). These differences may have led to heterogeneous estimates in data pooling, which was also indicated by GOSH analysis.
To be specific, poorer outcomes of progression-free survival and overall survival were indicated in the studies by Fountzilas et al. and Tan et al. (26, 29) and may have been due to more undesirable adverse effects caused by different regimens. In the trial by Fountzilas et al., for instance, more than 10% of cases in the IC-CCRT group discontinued due to toxicity (n = 2), withdrawal (n = 4), or no reason (n = 1) (26). Despite our finding that induction chemotherapy leads to better medium-term outcome, potential risks of adverse effects due to additional chemotherapy and poorer quality of life during therapy should be considered during clinical decision-making.
Compared with trials by Fountzilas et al. and Tan et al., better survival outcomes could be found in the study by Zhang et al., (26, 29, 39) probably because it included fewer patients with N2 and N3 stage disease who have a higher risk of disease progression (39). Unfortunately, data are insufficient to analyze the interaction between the N stage and the effects of induction chemotherapy in patients with nasopharyngeal carcinoma. Future studies should attempt to evaluate this association. Furthermore, identification of an optimal strategy of IC-CCRT may help in the management of local nasopharyngeal carcinoma. The optimal combination strategies and indications for using induction chemotherapy warrant further evaluation.
Many relevant syntheses have been published in this decade, and meta-analysis of RCTs is worthy of a further discussion. Reduced certainty by methodological and statistical heterogeneity weakens the confidence of pooled results in meta-analyses using data from observational studies although those meta-analyses have more cases than the meta-analyses only using data from RCTs. For instance, a head-to-head meta-analysis by Tan et al. included 11 studies with 2802 cases (29), but only 6 were RCTs (25, 26, 29, 31, 32, 35). Inclusion of non-RCTs led to seemingly uncertain and unreliable pooled results due to highly heterogeneity (I2 = 62%) (12). Besides, our synthesis is also based on RCTs. In consequence, it would be appropriate to compare findings in the present synthesis with those in the meta-analyses using data from RCTs.
Our findings are consistent with recent meta-analyses of RCTs (13, 15, 16, 22). Meta-analysis by Wang et al. seems to be the largest synthesis of RCTs comparing IC-CCRT and CCRT for nasopharyngeal carcinoma amongst relevant syntheses on this topic (10–24), and it is a head-to-head meta-analysis of 10 RCTs with 2280 individuals (16). IC-CCRT appears to be an effective strategy for treating nasopharyngeal carcinoma since it improves progression-free survival (Peto’s odds ratio [POR] = 0.75, 95% CI: 0.65–0.87) and overall survival (POR = 0.7, 95% CI: 0.56–0.87) and based on cumulative measurement (16). However, their study used cumulative measurement only without separation of time points. Knowledge regarding time factors or trends in the relevant outcomes of nasopharyngeal carcinoma management is essential for clinicians. Time-to-event analysis is more informative than cumulative measurement with unclear time frames. Based on time-to-event measurement, IC-CCRT also significantly improves 3-year failure-free survival (HR = 0.67, 95% CI: 0.55–0.80), 5-year failure-free survival (HR = 0.70, 95% CI: 0.58–0.83), 3-year overall survival (HR = 0.70, 95% CI: 0.55–0.89), and 5-year overall survival (HR = 0.77, 95% CI: 0.62–0.94) (15). Nevertheless, these findings are only based on seven RCTs.
In addition to the abovementioned meta-analyses, a review of meta-analyses is also worthy of further discussion since it concludes oppositely after taking many meta-analyses on relevant topics into consideration (44). On the basis of the works of five earlier meta-analyses (10–12, 17, 45), the review indicates no concrete evidence in favor of routine addition of induction chemotherapy to CCRT in managing patients with locoregionally advanced nasopharynx cancer. Actually, we agree with their concerns because the earlier meta-analyses seem to have no effects of IC-CCRT on overall survival with relatively small sample size. Indeed, non-significant finding in 5-year overall survival might decrease patients’ willingness in receiving IC-CCRT; wherefore, CCRT alone might be still a good option for some patients.
The present meta-analysis complements the understanding of the effects of addition of induction chemotherapy to CCRT in nasopharyngeal carcinoma management. Our findings may be more reliable because we included 11 RCTs with more than 3000 cases in total. Primary outcomes, progression-free survival, and overall survival were not seriously affected by heterogeneity or small study effects. The quality and completeness of our findings are much higher than those of previous studies. Moreover, our study provides a clearer overview of the outcomes because we evaluated both time-to-event and cumulative measurements with separate time points. This study further provides a summary of findings according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Supplementary Table 3), and may thus improve knowledge translation from academic research to clinical practice.
For clinical practice, quality of life after both IC-CCRT and CCRT is critical point and may be worthy of further discussion although there is limited evidence on quality of life between these two treatment strategies for nasopharyngeal carcinoma (46). Induction chemotherapy appears to result in better quality of life as compared with those without induction chemotherapy, and the finding may be due to few toxicities in patients with nasopharyngeal carcinoma after IC-CCRT (46, 47). However, another study indicates that patient receiving IC-CCRT may have lower quality-adjusted life year and disability-adjusted life year than those receiving CCRT (48). Because quality of life might affect decision-making, further studies are warranted to investigate the relevant outcomes between IC-CCRT and CCRT.
This study has several limitations. First, most patients had advanced nasopharyngeal carcinoma, precluding the drawing of firm evidence for those with early-stage disease. More evidence is also required for the application of IC-CCRT to non-Asian ethnicities with nasopharyngeal carcinoma because >90% of the participants in this study were from Asia. GOSH analysis also indicated that heterogeneity was caused by a study from Europe. Second, differences in the regimens for induction chemotherapy were noted, but evidence consistently supported IC-CCRT. Clinical heterogeneity of the induction chemotherapy regimen is a threat to the internal validity of this synthesis. Future studies should investigate which regimen for IC-CCRT achieves the best outcomes.
According to the available data from RCTs, the present meta-analysis indicated that IC-CCRT may benefit patients with nasopharyngeal carcinoma, although no significant difference in 5-year survival was noted between IC-CCRT and CCRT. Due to the non-significance, clinicians might need to reconsider before the uses of IC-CCRT, or to have a shared-decision making for this situation. There are trends toward no differences in all measured outcomes between IC-CCRT and CCRTat the 5-year follow-up; however, some heterogeneity may exist due to differences in ethnicities and regimens. These sources of heterogeneity warrant further research.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conceptualization: Y-FD. and Y-NK. Data curation: T-CH and C-JC. Formal analysis: C-JC and Y-NK. Investigation: T-CH, C-JC, and Y-FD. Methodology: Y-NK. Interpretation: T-CH, C-JCand Y-FD. Supervision: Y-FD. Visualization: Y-NK. Writing – original draft: T-CH and C-JC. Writing – review & editing: Y-FD and Y-NK. All authors contributed to the article and approved the submitted version.
This study received grant from Wan Fang Hospital, Taipei Medical University with grant number 111-wf-phd-04.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.965719/full#supplementary-material
CCRT, concurrent chemoradiotherapy; CI, confidence interval; IC, induction chemotherapy; GOSH, Graphical display of study heterogeneity; HR, hazard ratio; RCT, randomized controlled trial; RR, risk ratio; WMD, weighted mean difference.
2. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer group 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol (2017) 3:524–48. doi: 10.1001/jamaoncol.2016.5688
3. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer group 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol (2018) 4:1553–68. doi: 10.1001/jamaoncol.2018.2706
4. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev (2006) 15:1765–77. doi: 10.1158/1055-9965.EPI-06-0353
5. Fang W, Li X, Jiang Q, Liu Z, Yang H, Wang S, et al. Transcriptional patterns, biomarkers and pathways characterizing nasopharyngeal carcinoma of southern China. J Trans Med (2008) 6:32–2. doi: 10.1186/1479-5876-6-32
6. Zanetti R, Tazi MA, Rosso S. New data tells us more about cancer incidence in north Africa. Eur J Cancer (2010) 46:462–6. doi: 10.1016/j.ejca.2009.11.012
7. Forman D, Bray F, Brewster D, Gombe Mbalawa C, Kohler B, Piñeros M, et al. Cancer incidence in five continents. (2013). Geneva, Switzerland.
9. National Comprehensive Cancer Network. Cancer of the nasopharynx (2018). Available at: https://www.nccn.org/professionals/physician_gls/default.aspx (Accessed November 5, 2020).
10. Song Y, Wang W, Tao G, Zhou X. Survival benefit of induction chemotherapy in treatment for locally advanced nasopharyngeal carcinoma–a time-to-event meta-analysis. Oral Oncol (2015) 51:764–9. doi: 10.1016/j.oraloncology.2015.05.006
11. Wang M, Tian H, Li G, Ge T, Liu Y, Cui J, et al. Significant benefits of adding neoadjuvant chemotherapy before concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Oncotarget (2016) 7:48375–90. doi: 10.18632/oncotarget.10237
12. Tan TH, Soon YY, Cheo T, Ho F, Wong LC, Tey J, et al. Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: A systematic review and meta-analysis. Radiother Oncol (2018) 129:10–7. doi: 10.1016/j.radonc.2018.02.027
13. Ouyang PY, Zhang XM, Qiu XS, Liu ZQ, Lu L, Gao YH, et al. A pairwise meta-analysis of induction chemotherapy in nasopharyngeal carcinoma. Oncologist (2019) 24:505–12. doi: 10.1634/theoncologist.2018-0522
14. Tang M, Jia Z, Zhang J. The evaluation of adding induction chemotherapy to concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma: a meta-analysis. Eur Arch Otorhinolaryngol (2020) 278(5). doi: 10.1007/s00405-020-06218-x
15. Wang BC, Xiao BY, Lin GH, Wang C, Liu Q. The efficacy and safety of induction chemotherapy combined with concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in nasopharyngeal carcinoma patients: a systematic review and meta-analysis. BMC Cancer (2020) 20:393. doi: 10.1186/s12885-020-06912-3
16. Wang P, Zhang M, Ke C, Cai C. The efficacy and toxicity of induction chemotherapy plus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: A meta-analysis of randomized controlled trials. Med (Baltimore) (2020) 99:e19360. doi: 10.1097/MD.0000000000019360
17. Chen YP, Guo R, Liu N, Liu X, Mao YP, Tang LL, et al. Efficacy of the additional neoadjuvant chemotherapy to concurrent chemoradiotherapy for patients with locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis of randomized controlled trials. J Cancer (2015) 6:883–92. doi: 10.7150/jca.11814
18. Yu H, Gu D, He X, Gao X, Bian X. The role of induction and adjuvant chemotherapy in combination with concurrent chemoradiotherapy for nasopharyngeal cancer: a Bayesian network meta-analysis of published randomized controlled trials. Onco Targets Ther (2016) 9:159–70. doi: 10.2147/OTT.S96983
19. Ribassin-Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? an individual patient data network meta-analysis. J Clin Oncol (2017) 35:498–505. doi: 10.1200/JCO.2016.67.4119
20. You R, Cao YS, Huang PY, Chen L, Yang Q, Liu YP, et al. The changing therapeutic role of chemo-radiotherapy for loco-regionally advanced nasopharyngeal carcinoma from Two/Three-dimensional radiotherapy to intensity-modulated radiotherapy: A network meta-analysis. Theranostics (2017) 7:4825–35. doi: 10.7150/thno.21815
21. Liu M, You W, Song YB, Miao JD, Zhong XB, Cai DK, et al. The changing role of chemotherapy in locoregionally advanced nasopharyngeal carcinoma: A updated systemic review and network meta-analysis. Front Oncol (2018) 8:597. doi: 10.3389/fonc.2018.00597
22. He Y, Guo T, Wang J, Sun Y, Guan H, Wu S, et al. Which induction chemotherapy regimen followed by cisplatin-based concurrent chemoradiotherapy is the best choice among PF, TP and TPF for locoregionally advanced nasopharyngeal carcinoma? Ann Transl Med (2019) 7:104. doi: 10.21037/atm.2019.02.15
23. Li L, Liang W, Zhu JX, Dong CJ, Zou YM, Ye BC, et al. Evolutionary role of chemotherapy in advanced nasopharyngeal carcinoma: a literature-based network meta-analysis. Cancer Manag Res (2019) 11:501–12. doi: 10.2147/CMAR.S185932
24. Zhou R, Zhu J, Chen X, Liu Y, Wang Y, Zhang T. The efficacy and safety of docetaxel, cisplatin and fluorouracil (TPF)-based induction chemotherapy followed by concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis. Clin Transl Oncol (2019) 22(3). doi: 10.1007/s12094-019-02142-7
25. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol (2009) 27:242–9. doi: 10.1200/JCO.2008.18.1545
26. Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic cooperative oncology group (HeCOG) with biomarker evaluation. Ann Oncol (2012) 23:427–35. doi: 10.1093/annonc/mdr116
27. Huang S, Deng G, Huang G, Li Y, Meng Y, Chen J. Efficacy of induction chemotherapy combined with concurrent chemoradiotherapy for advanced nasopharyngeal carcinoma. Chin Oncol Clinics (2012) 39:788–91. doi: 10.3969/j.issn.1000-8179.2012.11.009
28. Gao J-Q, Gao T-S, Dong Z-R. A prospective and randomized study of induction chemotherapy combined with concurrent chemoradiotherapy in the treatment for nasopharyngeal carcinoma stage T 3∼4 n 2∼3 M0. J Chin Oncol (2013) 19:161–5.
29. Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK, et al. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys (2015) 91:952–60. doi: 10.1016/j.ijrobp.2015.01.002
30. Li WF, Chen L, Sun Y, Ma J. Induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer (2016) 35:94. doi: 10.1186/s40880-016-0157-4
31. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
32. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer (2017) 75:14–23. doi: 10.1016/j.ejca.2016.12.039
33. Jin YN, Yao JJ, Wang SY, Zhang WJ, Zhang F, Zhou GQ, et al. The effect of adding neoadjuvant chemotherapy to concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma and undetectable pretreatment Epstein-Barr virus DNA. Transl Oncol (2017) 10:527–34. doi: 10.1016/j.tranon.2017.03.007
34. Zhang B, Li MM, Chen WH, Zhao JF, Chen WQ, Dong YH, et al. Association of chemoradiotherapy regimens and survival among patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. JAMA Netw Open (2019) 2:e1913619. doi: 10.1001/jamanetworkopen.2019.13619
35. Frikha M, Auperin A, Tao Y, Elloumi F, Toumi N, Blanchard P, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol (2018) 29:731–6. doi: 10.1093/annonc/mdx770
36. Hong RL, Hsiao CF, Ting LL, Ko JY, Wang CW, Chang JTC, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan cooperative oncology group (TCOG) 1303 study. Ann Oncol (2018) 29:1972–9. doi: 10.1093/annonc/mdy249
37. Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, Sun Y, et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long-term results of phase 3 randomized controlled trial. Int J Cancer (2019) 145:295–305. doi: 10.1002/ijc.32099
38. Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer (2019) 119:87–96. doi: 10.1016/j.ejca.2019.07.007
39. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med (2019) 381:1124–35. doi: 10.1056/NEJMoa1905287
40. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj (2009) 339:b2535. doi: 10.1136/bmj.b2535
41. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken, New Jersey: John Wiley & Sons (2019). .
42. Zhang Y, Li WF, Liu X, Chen L, Sun R, Sun Y, et al. Nomogram to predict the benefit of additional induction chemotherapy to concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: Analysis of a multicenter, phase III randomized trial. Radiother Oncol (2018) 129:18–22. doi: 10.1016/j.radonc.2017.12.002
43. Zhang Y, Sun Y, Ma J. Induction gemcitabine and cisplatin in locoregionally advanced nasopharyngeal carcinoma. Cancer Commun (Lond) (2019) 39:39. doi: 10.1186/s40880-019-0385-5
44. Ahn YC. Less is more: role of additional chemotherapy to concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal cancer management. Radiat Oncol J (2019) 37:67–72. doi: 10.3857/roj.2019.00311
45. Chen YP, Tang LL, Yang Q, Poh SS, Hui EP, Chan ATC, et al. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: Individual patient data pooled analysis of four randomized trials. Clin Cancer Res (2018) 24:1824–33. doi: 10.1158/1078-0432.CCR-17-2656
46. Yang Q, Xia L, Lin M, Zhang M-X, Duan C-Y, Liu Y-P, et al. The impact of induction chemotherapy on long-term quality of life in patients with locoregionally advanced nasopharyngeal carcinoma: Outcomes from a randomised phase 3 trial. Oral Oncol (2021) 121:105494. doi: 10.1016/j.oraloncology.2021.105494
47. Xiang L, Zheng Y, Ren P, Lin S, Zhang J, Wen Q, et al. 5-fluorouracil combined with cisplatin via arterial induction for advanced T-stage nasopharyngeal carcinoma: A 10-year outcome of a phase I/II study. Front Oncol (2022) 12:868070. doi: 10.3389/fonc.2022.868070
48. Nittala MR, Kanakamedala MR, Mundra E, Woods WC 3rd, Smith ML, Hamilton RD, et al. Quality-adjusted life years and disability-adjusted life years are better with concurrent chemoradiation therapy than induction chemotherapy followed by chemoradiation therapy in nasopharyngeal carcinoma. Cureus (2021) 13:e13022. doi: 10.7759/cureus.13022
Keywords: neoadjuvant chemotherapy, concurrent chemoradiotherapy, nasopharynx cancer, induction chemotherapy, nasopharyngeal cancer
Citation: Huang T-C, Chen C-J, Ding Y-F and Kang Y-N (2022) Impact of induction chemotherapy with concurrent chemoradiotherapy on nasopharyngeal carcinoma: A meta-analysis of randomized controlled trials. Front. Oncol. 12:965719. doi: 10.3389/fonc.2022.965719
Received: 10 June 2022; Accepted: 17 August 2022;
Published: 13 September 2022.
Edited by:
Thorsten Fuereder, Medical University of Vienna, AustriaReviewed by:
Damian Tobias Rieke, Charité Universitätsmedizin Berlin, GermanyCopyright © 2022 Huang, Chen, Ding and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Fang Ding, MTAyMDA4QHcudG11LmVkdS50dw==; Yi-No Kang, YWNhZGVtaWNub25vQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.