- 1The First Clinical School of Gansu University of Chinese Medicine, Lanzhou, China
- 2General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou, China
- 3Gansu Provincial Key Laboratory of Molecular Diagnosis and Precision Therapy of Surgical Tumors, Gansu Provincial Hospital, Lanzhou, China
Objective: We conducted a meta-analysis to evaluate the relationship between circulating tumor cells (CTC) and the prognosis of patients with gastric cancer.
Materials and methods: The cohort studies reporting on the relationship between CTC and prognosis of gastric cancer were collected from Pubmed, Cochrane, Embase, CNKI, WanFang Data, and VIP databases. The two researchers independently screened the literature, extracted the data, and evaluated the bias risk of the included literature. The data were analyzed by Revman software (Review Manager version 5.4).
Result: A total of 14 retrospective cohort studies with 1053 patients were included. The results showed that the overall survival time (OS) and progression-free survival time (PFS) of CTC-positive patients were shorter compared to CTC-negative patients. Taking into consideration the critical value of CTC positive patients, country of origin, sample size, treatment mode, and study time, the subgroup analysis showed that CTC-positive was related to the shortening of OS in patients with gastric cancer. Based on the subgroup analysis of the factors such as CTC positive critical value < 2.8, sample size ≥ 75, mixed therapy, longer study duration, country, and immunofluorescence detection of CTC, it was found that OS in CTC positive group was shorter than that in CTC-negative group (all P<0.05), while the critical value of positive CTC ≥ 2.8, sample size ≥ 75, choice of treatment only for operation or non-operation, short study time and molecular detection of CTC were not associated with OS (all P>0.05). In addition, CTC-positive patients had a more advanced TNM staging, poorer tumor differentiation, and earlier distant metastasis.
Conclusion: CTC can be used as a prognostic indicator of gastric cancer. Gastric cancer patients with positive CTC may have a poorer prognosis compared to those with CTC-negative tumors.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022323155.
Introduction
Gastric cancer is one of the most common malignant tumors (1) and the second deadliest tumor worldwide (2). Smoking tobacco, age over 60, Helicobacter pylori infection, alcohol consumption, and obesity are the main causes leading to a gastric tumor (3). Surgery is the most effective treatment, yet patients present with an advanced stage at the time of diagnosis, losing their chance to undergo surgical resection (4). Chemotherapy and immunotherapy are the most common treatment methods for advanced-stage gastric tumors (5). Still, most gastric patients develop metastasis after therapy and have a poor prognosis.
In recent years, with the development of liquid biopsy technology, several new biomarkers have been discovered for accurately predicting the prognosis of gastric cancer and effectively evaluating the efficacy of chemotherapy for gastric cancer. For example, circulating tumor cells (CTC), i.e., tumor cells that detach from the primary or metastatic focus of the tumor and enter the blood, have recently attracted interest as biomarkers of cancer metastases (6–9). At present, existing studies have shown that CTC has an important role in the diagnosis of early gastric cancer (10), the guidance of chemotherapy, and analysis of chemotherapy efficacy (11–15), chemotherapy resistance (16), and prognosis (17, 18). Clinically, CTC has incomparable potential value in evaluating the prognosis of tumors. Given the important value of CTC in evaluating the prognosis of malignant tumors, we performed a meta-analysis in order to determine the relationship between baseline CTC and the prognosis of patients with gastric cancer and objectively evaluate its prognostic value in gastric cancer.
Materials and methods
Retrieval strategy
Six electronic databases were explored: Pubmed, Cochrane, Embase, CNKI, WanFang Data, and VIP. The cohort studies reporting on the relationship between CTC and prognosis of gastric cancer were collected from the establishment of the database to December 26, 2021. The following key words were used (Pubmed database): Neoplasm Circulating Cells, Neoplasm Circulating Cell, Circulating Neoplastic Cells, Circulating Neoplastic Cell, Circulating Tumor Cells, Circulating Tumor Cell, Embolic Tumor Cell, Embolic Tumor Cells, Tumor Embolism, Tmor Embolisms, CTC, Stomach Neoplasms, Stomach Neoplasm, Gastric Neoplasms, Gastric Neoplasm, Cancer of Stomach, Stomach Cancers, Gastric Cancer, Gastric Cancers, Stomach Cancer, Cancer of the Stomach, Prognosis, Prognoses, Prognostic Factors, Prognostic Factor. Chinese keywords include: circulating tumor cells, CTC, gastric cancer, and prognosis. This study has been registered on PROSPERO platform (Registration number: CRD42022323155).
Inclusion and exclusion criteria
Inclusion criteria for research literature were (1): studies evaluating the relationship between CTC expression and prognosis of gastric cancer (2); dividing patients into high expression group and low expression group of CTC (3); describing effective prognostic indicators (OS, DFS, RFS, and PFS) or related clinicopathological parameters (tumor size, differentiation, depth of infiltration, lymph node metastasis, distant metastasis, and tumor stage) (4); enough data to calculate the hazard ratio (HR) or odds ratio (OR) and 95% confidence interval (95%CI) (5); patients did not receive any treatment at baseline (6); blood was collected and CTC were tested before treatment.
Exclusion criteria were (1): case reports, conference summaries, reviews, editorials, and non-human studies (2); repeated publication (3); lack of HR or Tumor and its 95%CI, or unable to estimate these parameters.
Literature screening and data extraction
Two researchers (ZL and MS) independently reviewed and analyzed the title and abstract of the study, screened the search results, and evaluated the full text of the research literature that met the inclusion criteria. All differences were resolved through group discussion or by inviting a third researcher (JY). Two researchers (ZL and MS) independently extracted the following data from each study: title, first author, year of publication, study time, country, sample size, sex, treatment, follow-up time, CTC positive threshold, outcome indicators, and outcome measurements.
Evaluation of research quality
The included study was independently assessed for bias risk according to the Newcastle-Ottawa quantity (NOS). A study with a score of 6 or more was defined as a high-quality study (19).
Statistical analysis
Revman software (Review Manager version 5.4) was used for statistical analysis, and Stata software (Stata12.0 version) was used for sensitivity analysis and publication bias. In order to evaluate the effect of CTC on the prognosis of gastric cancer, the standard errors of risk ratio (HR), OS, or PFS were extracted from the included literature. HR > 1 indicates that the prognosis of the positive group is worse than that of the negative group. The inverse variance method was used to combine HRs in the Revman software. Considering the heterogeneity between studies, the literature heterogeneity was judged by I2 statistics and the Q test. When P < 0.1 or I2 > 50%, the heterogeneity was significant, and the random effect model was used for meta-analysis; on the contrary, the fixed effect model was used for meta-analysis (20). Publication bias was tested by the Beg method and the Egger method (test level α = 0.05) (21).
Results
Data screening process and results
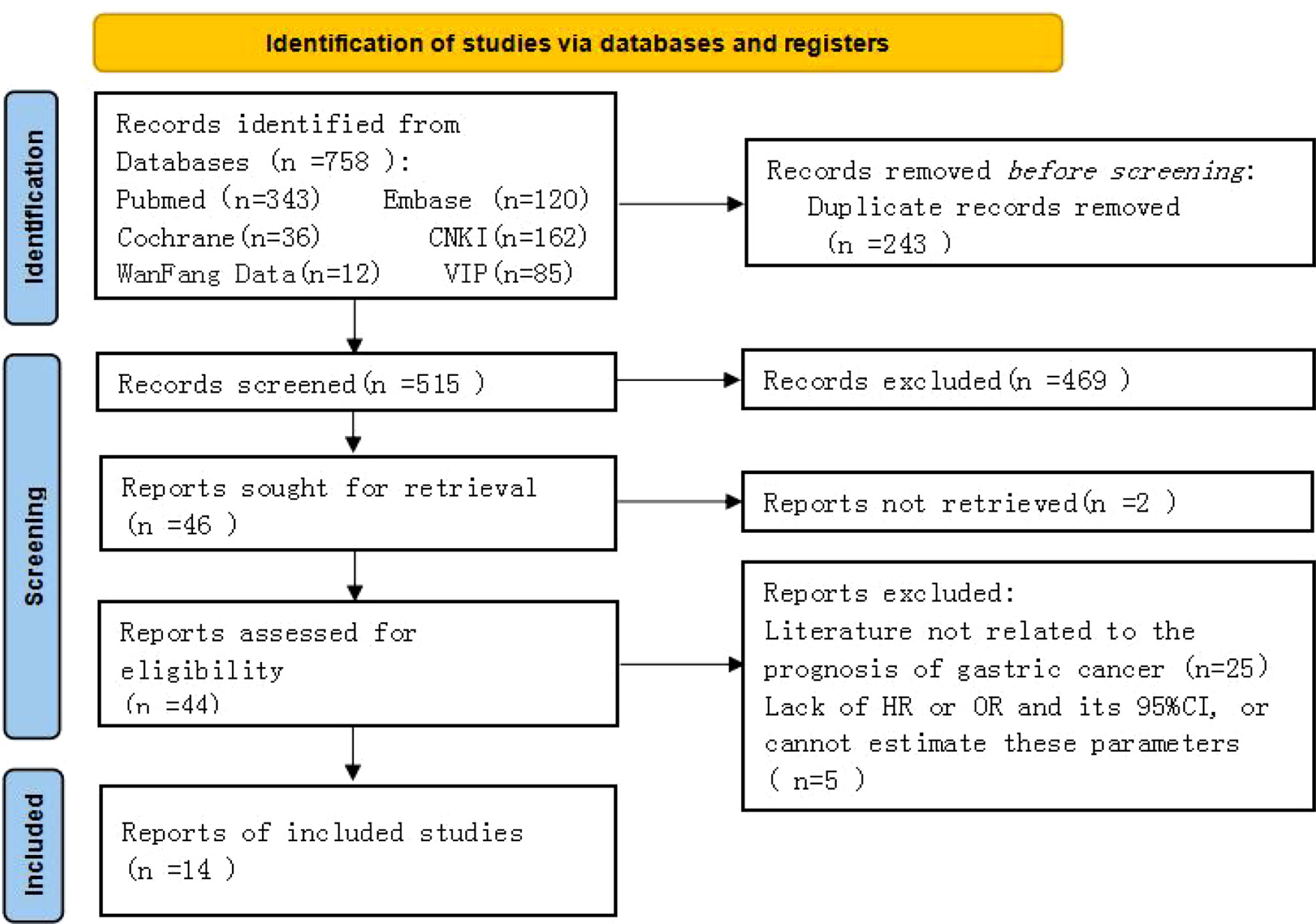
A total of 758 original studies were retrieved in the preliminary screening, 515 articles were obtained after deduplication, those that did not meet the inclusion criteria were excluded, and the full text was excluded after reading and evaluation. Finally, 14 retrospective cohort studies were included in the analysis (Figure 1).
Basic characteristics of the included study
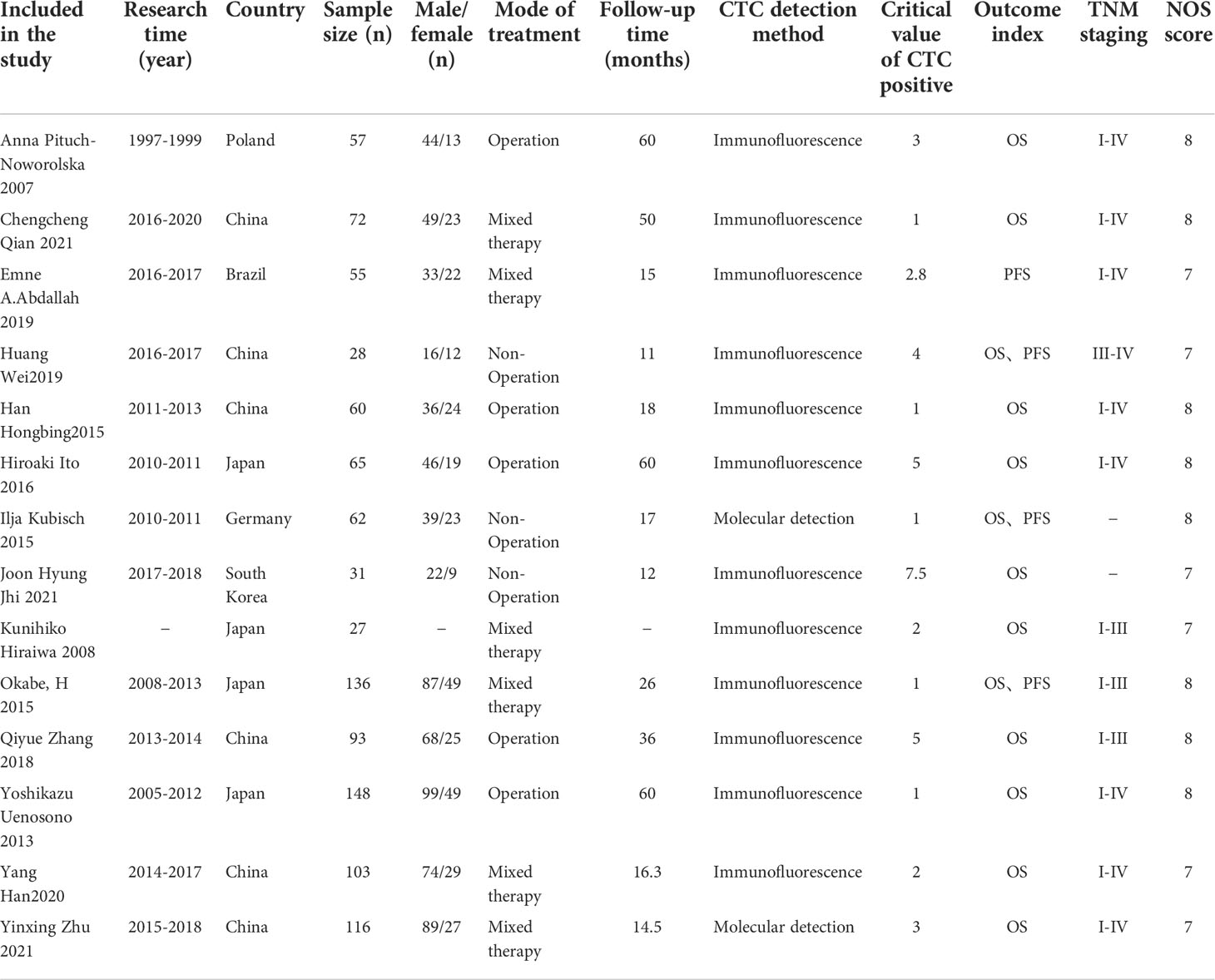
A total of 14 retrospective cohort studies were included (22–35), including 1053 patients. These studies were published from 2007 to 2021 (3 articles (23, 29, 35) were published in 2021). Six studies (23, 25, 26, 32, 34, 35) were conducted in China; others were carried out in Poland (22), Brazil (24), Germany (28), South Korea (29), Japan (27, 30, 31, 33) and other countries. Ten studies (22, 23, 26, 27, 29, 30, 32–35) reported the relationship between CTC and OS in gastric cancer patients, 3 (25, 28, 31) reported the relationship between CTC and OS or PFS in patients with gastric cancer, and 1 study (24) reported the relationship between CTC and PFS in patients with gastric cancer. The critical value of CTC positive was between 1 and 7.5. The NOS scores of the included studies were all above 6, indicating that the quality of the included studies was high (Table 1).
Meta-analysis results
Relationship between CTC and OS in patients with gastric cancer
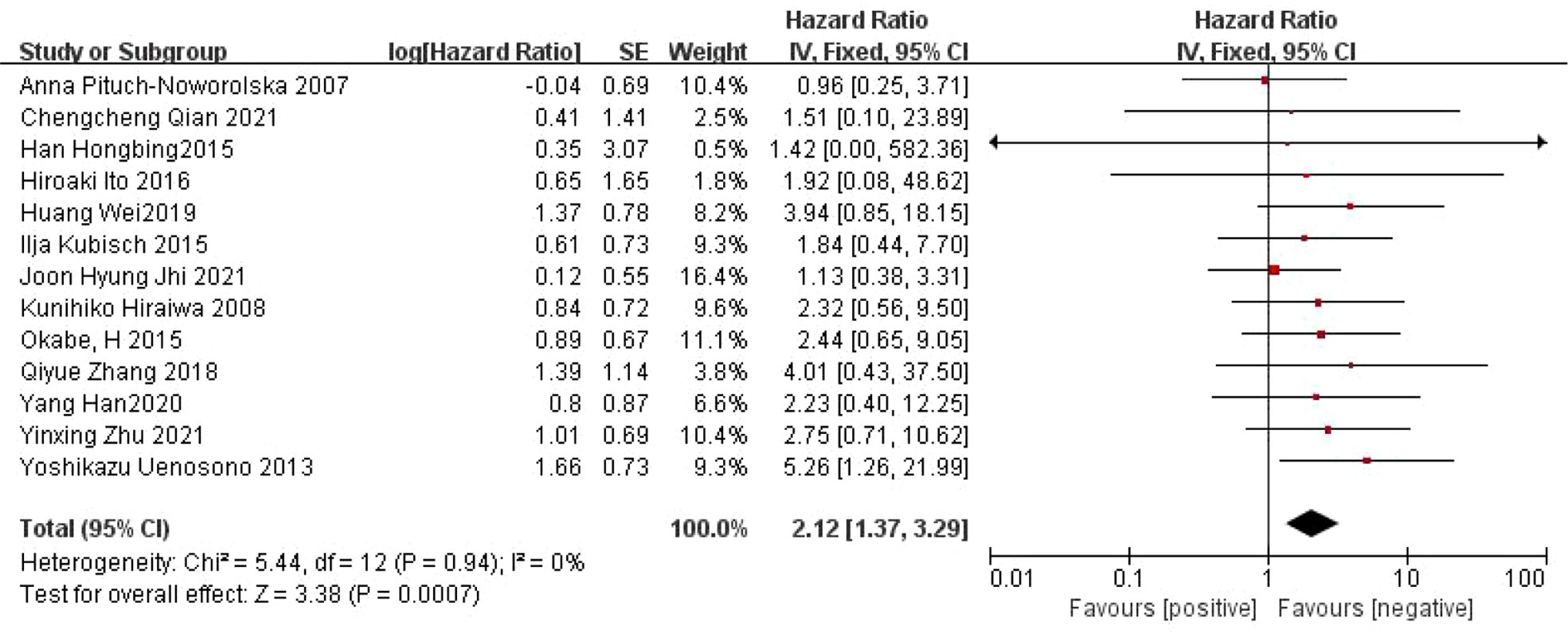
A total of 13 articles (22, 23, 25–35) reported on the relationship between CTC and OS in patients with gastric cancer. There was no heterogeneity between the studies (I2 =0%, P=0.94), and a fixed effect model was used. Meta-analysis showed that the OS was shorter in CTC-positive patients than in CTC-negative patients (HR=2.12, 95% CI=[1.37, 3.29], P=0.0007) (Figure 2).
Relationship between CTC and PFS in patients with gastric cancer
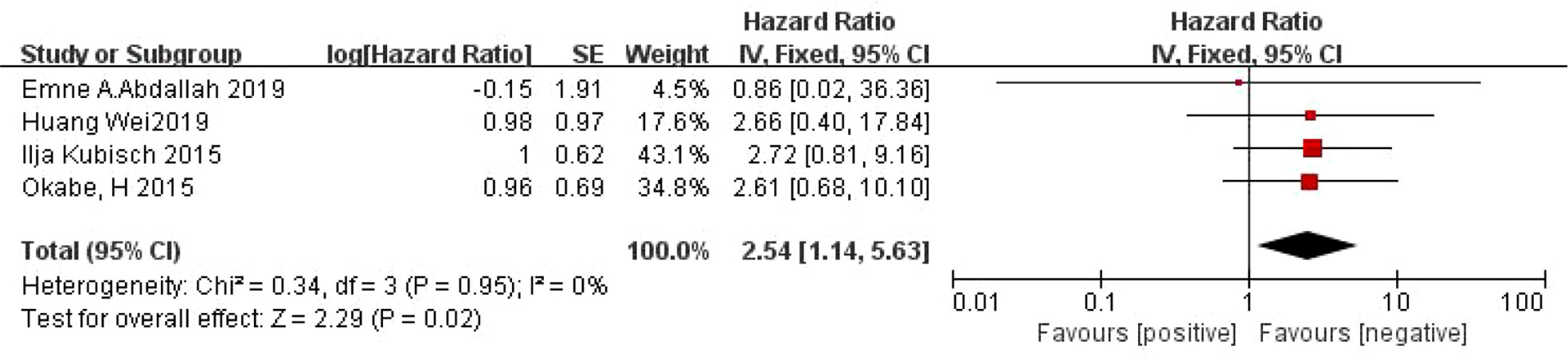
A total of 4 articles (24, 25, 28, 31) reported on the relationship between CTC and PFS in patients with gastric cancer. There was no heterogeneity between the studies (I2 =0%, P=0.95), and a fixed effect model was used. Meta-analysis showed that the PFS was shorter in CTC-positive patients than in CTC-negative patients (HR=2.54, 95% CI=[1.14, 5.63], P=0.02) (Figure 3).
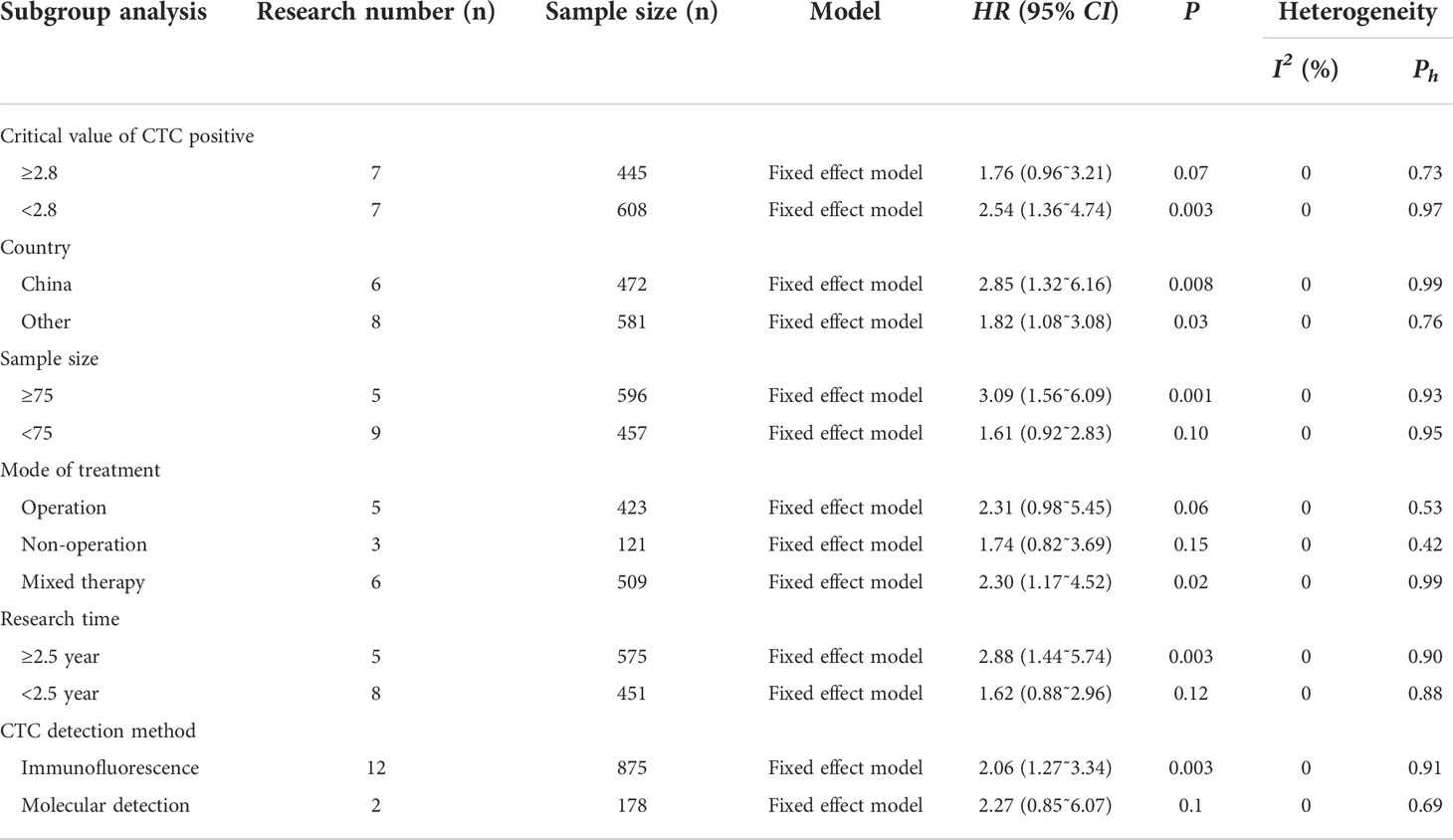
Subgroup analysis
In order to further analyze the prognostic effect of CTC on patients with gastric cancer, this study conducted a subgroup analysis taking into consideration the critical value, country, sample size, treatment mode, and research time of CTC positive. Subgroup analysis showed that when the critical value of CTC positive was < 2.8, the OS of the CTC positive group was shorter than that of a CTC-negative group; when the critical value of CTC was ≥ 2.8, there was no significant relationship between CTC and OS (all P<0.05). In addition, when the sample size was ≥ 75, the OS of the CTC-positive group was shorter than that of the CTC-negative group (P<0.05); when the sample size was < 75, there was no significant relationship between CTC and OS (P>0.05).
When the gastric cancer patients were treated with mixed therapy, the OS of the CTC-positive group was shorter than that of the CTC-negative group. When surgery or a non-operative approach was used, the relationship between CTC and OS was not statistically significant (P>0.05). The positive rate of CTC was associated with shorter OS in longer study time (P<0.05). When the study time was short, the relationship between CTC and OS was not statistically significant (P>0.05).
In the subgroup analysis of national factors, it was found that CTC positive was associated with shorter OS. By performing a subgroup analysis of CTC assays, it was found that by applying immunofluorescence assays, CTC positivity was associated with a shorter OS (Table 2).
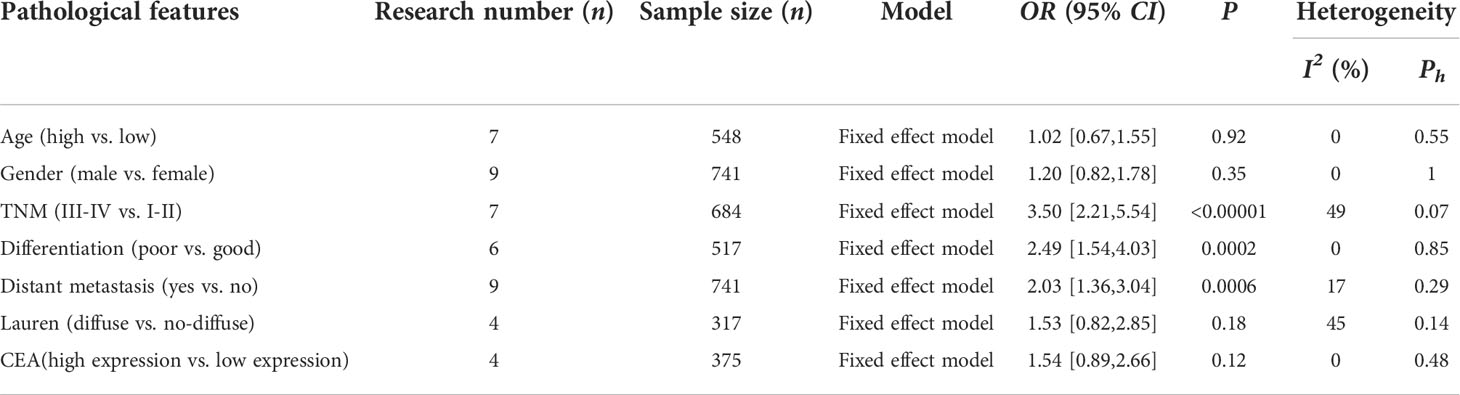
Relationship between CTC and clinicopathological features of patients with gastric cancer
This study explored the relationship between CTC and clinicopathological features of gastric cancer patients from the aspects of age, sex, TNM stage, tumor differentiation, distant metastasis, Lauren classification, and CEA (Table 3). The heterogeneity of each study was small. The results of the meta-analysis showed that the CTC-positive patients had a higher TNM stage (OR=3.50, 95% CI=[2.21,5.54], P<0.00001), poorer tumor differentiation (OR=2.49, 95% CI=[1.54,4.03], P=0.0002), and earlier distant metastasis (OR=2.03, 95% CI=[1.36,3.04], P=0.0006); while the positive rate of CTC was not related to age, sex, Lauren classification and CEA (P>0.05).
Sensitivity analysis
Single studies were excluded one by one for sensitivity analysis. The results showed that the results of a meta-analysis analyzing the relationship between CTC and OS or PFS were stable (OS: HR= 0.67- 0.86; PFS: HR=0.93- 1.10) (Figure 4).
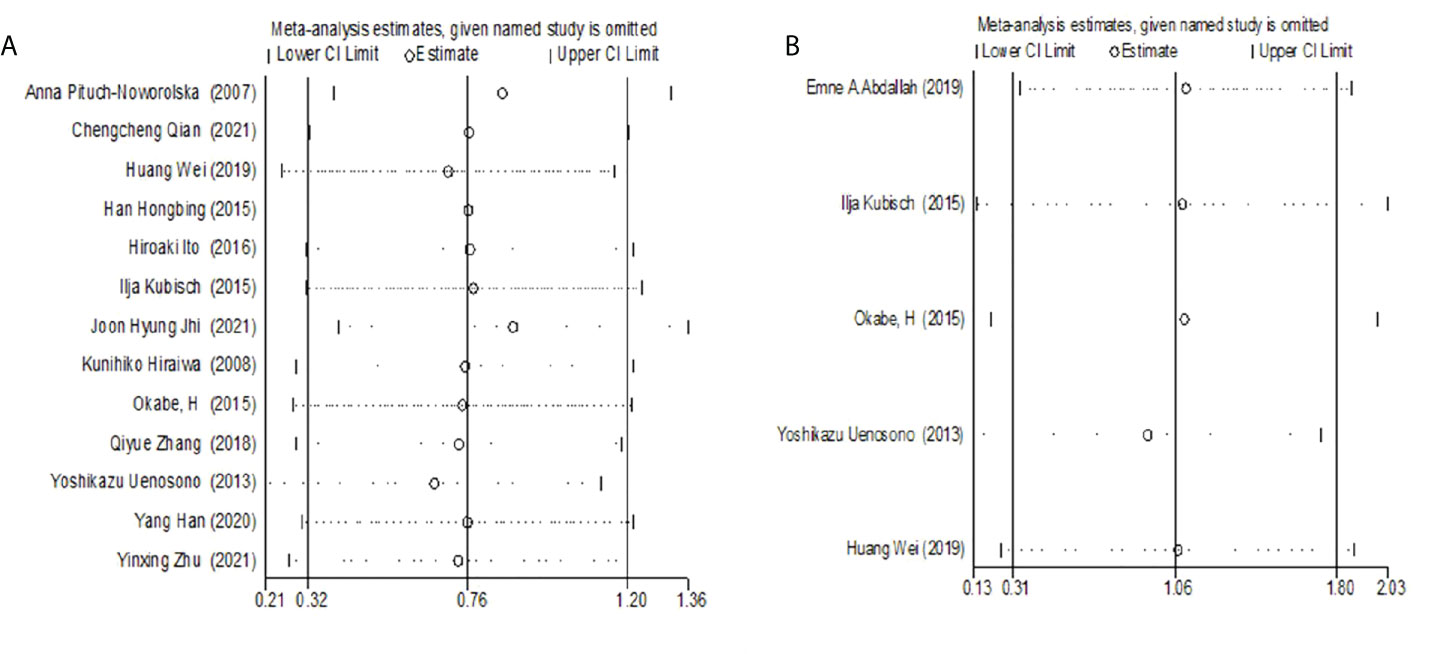
Figure 4 (A) Sensitivity Analysis of the relationship between CTC and OS. (B) Sensitivity analysis of the relationship between CTC and PFS.
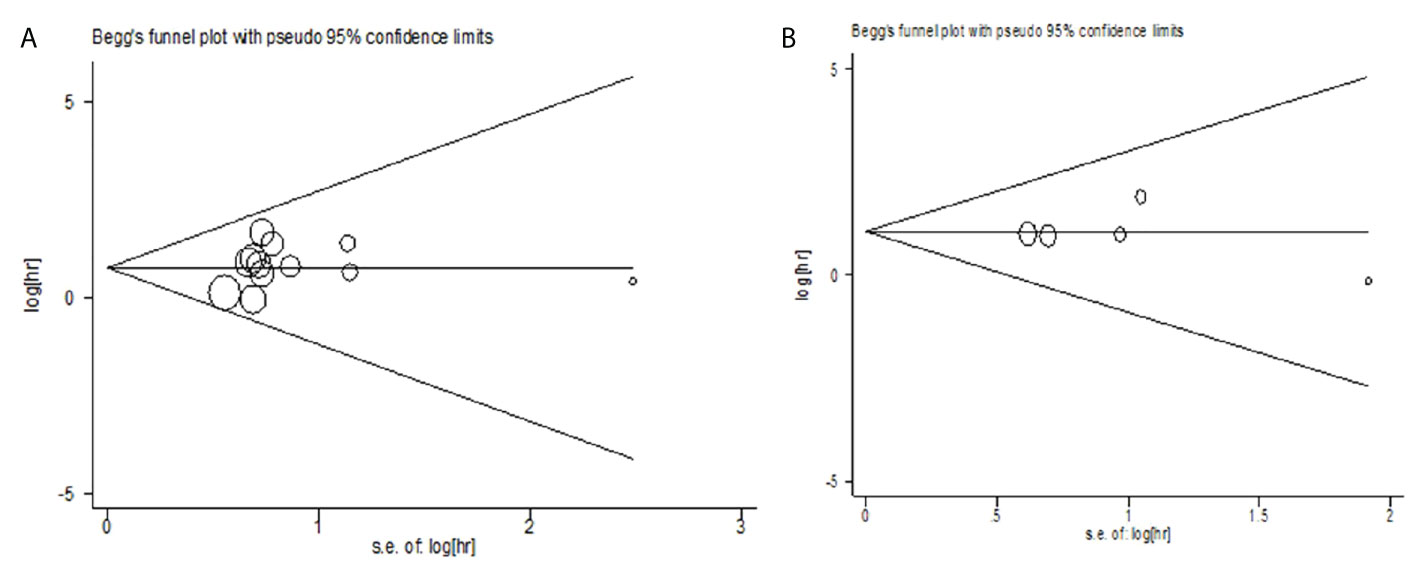
Publication bias
The publication bias of the relationship between CTC and OS was evaluated by the Begg test (Z=0.34, P=0.732) and Egger test (t=0.75, P=0.468). The publication bias of the relationship between CTC and PFS was analyzed by Begg test (Z =-0.24, P = 1.000) and Egger test (t = 0.33, P = 0.762). The results showed less possibility of publication bias in the included study (Figure 5).
Discussion
As a new prognostic marker, CTC has the biological characteristics of the primary tumor and strong invasive ability (7). The release of CTCs from the tumor into the circulating blood occurs through the epithelial-mesenchymal transition (EMT) and non-EMT-mediated invasion (36). As a non-invasive and simple “fluid biopsy” technique, CTC detection is a simple procedure (37). Herein, we conducted a meta-analysis to evaluate the relationship between circulating tumor cells (CTC) and the prognosis of patients with gastric cancer. This study included the original studies from Chinese and English databases, including South Korea, Japan, China, and other Asian countries with a high incidence of gastric cancer, in order to improve the scientific and reliable conclusion of the relationship between CTC and the prognosis of patients with gastric cancer. Fourteen retrospective cohort studies with 1053 patients were included to explore the prognostic role of CTC in gastric cancer. The results showed that the positive rate of CTC was associated with shorter OS and PFS. CTC are tumor cells that detach from the primary or metastatic focus of the tumor and enter the blood (38). In recent years, CTC has been used as an important prognostic marker for many solid tumors, including lung cancer (39), breast cancer (40), prostate cancer (41), nasopharyngeal cancer (42), rectal cancer (43), and so on. CTC detection can effectively make up for the deficiency of imaging, serum markers, and tissue samples in the evaluation of the prognosis of patients with gastric cancer, providing qualitative, specific, and dynamic evaluation, and avoiding temporal and spatial heterogeneity of tumors. CTC can be directly detected through blood samples, which is helpful for clinicians to systematically and effectively evaluate the progression of tumors so as to provide scientific and reasonable treatment.
In this study, subgroup analysis indicated that critical value of CTC positive < 2.8, sample size ≥ 75, mixed therapy, long study time, and using immunofluorescence assay were associated with shorter OS in CTC-positive patients. However, the critical value of positive CTC ≥ 2.8, sample size < 75, simple surgical or non-operative treatment, short research time, and molecular detection method had no significant relationship with OS. This may be the reason why the small sample size and short research time could not reveal the real results, but it also shows that mixed treatment is the best choice for patients with gastric cancer.
After analyzing the subgroups of different countries, it was found that the positive rate of CTC was related to the shorter OS. There was no heterogeneity in the whole subgroup analysis (I2 = 0%), ensuring the reliability of the research results. In addition, this study also explored the relationship between CTC and clinicopathological features of gastric cancer patients. CTC-positive patients had an advanced TNM stage, poorer tumor differentiation, and were more prone to distant metastasis than those with CTC-negative patients. These clinical parameters, which are closely related to the progression of malignant tumors, are correlated with CTC, which proves that the positive expression of CTC is an important index for evaluating the prognosis of patients with gastric cancer, which also indicates that the change of CTC in the process of tumor development may be the key factor causing tumor recurrence and metastasis, which is consistent with previous study (44).
This study has some limitations. First, only a few literature and retrospective cohort studies were included; also, there is a lack of data support for large samples of randomized controlled trials. At present, there is no unified positive standard of CTC in gastric cancer, which may lead to bias. In subgroup analysis, all heterogeneities could not be explored. Because only the baseline CTC count was collected in the included studies, the changes in CTC after an intervention such as surgery and chemotherapy were not analyzed, and it was impossible to evaluate the effect of treatment intervention on the prognosis of patients with gastric cancer. In their large sample size meta-analysis, Zou et al. (45) found that high CTC counts before and during chemotherapy were significantly correlated with poor OS, PFS, and disease control rates (DC) in patients with advanced gastric cancer. Moreover, Yue et al. (46) found that the dynamic changes in CTC and prognosis were also affected by the study of the relationship between gastrointestinal tumors and CTC.
Conclusion
The existing evidence shows that CTC can be used as an effective index to evaluate the prognosis of gastric cancer. However, due to the research quantity and quality limitation, larger, high-quality studies are needed to further verify the above conclusions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JY contributed to study design. ZL were responsible for literature search, data extraction and analysis and wrote the manuscript. MS, CJ and SH contributed to study selection. All authors contributed to the article and approved the submitted version.
Funding
Natural Science Foundation of Gansu provincial (No. 21JR1RA017); Natural Science Foundation of Gansu provincial hospital (No. 21GSSYB-5).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smyth EC, Lordick F, Nilsson M, Grabsch HI, van Grieken NCT, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
2. Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J (2014) 55(12):621. doi: 10.11622/smedj.2014174
3. Yuxia Y, Lili M, Yan G, Jufang B, Youhua Z, Lingling Q, et al. The relationship between hp infection and the expression of MUC2, MUC5AC, MUC6 and CD10 in gastric cancer and precancerous lesions. Chin J Hosp infect (2022) (05):721–5. doi: 10.11816/cn.ni.2022-210601
4. Leja M, Linē A. Early detection of gastric cancer beyond endoscopy-new methods. Best Pract Res Clin Gastroenterol (2021) 50:101731. doi: 10.1016/j.bpg.2021.101731
5. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci (2020) 21(11):4012. doi: 10.3390/ijms21114012
6. Vasseur A, Kiavue N, Bidard FC, Pierga JY, Cabel L. Clinical utility of circulating tumor cells: An update. Mol Oncol (2021) 15(6):1647–66. doi: 10.1002/1878-0261.12869
7. Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: Circulating tumor cell biology. Genes Dev (2017) 31(18):1827–40. doi: 10.1101/gad.305805.117
8. Schuster E, Taftaf R, Reduzzi C, Albert MK, Romero-Calvo I, Liu H. Better together: Circulating tumor cell clustering in metastatic cancer. Trends cancer. (2021) 7(11):1020–32. doi: 10.1016/j.trecan.2021.07.001
9. Francart ME, Lambert J, Vanwynsberghe AM, Thompson EW, Bourcy M, Polette M, et al. Epithelial–mesenchymal plasticity and circulating tumor cells: travel companions to metastases. Dev Dynamics. (2018) 247(3):432–50. doi: 10.1002/dvdy.24506
10. Lee M, Kim G, Jeon H, Park S. Clinical application of circulating tumor cells in gastric cancer. Gut liver. (2019) 13(4):394–401. doi: 10.5009/gnl18484
11. Franses JW, Philipp J, Missios P, Bhan I, Liu A, Yashaswini C, et al. Pancreatic circulating tumor cell profiling identifies LIN28B as a metastasis driver and drug target. Nat Commun (2020) 11(1):1–12. doi: 10.1038/s41467-020-17150-3
12. Gorges TM, Pantel K. Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol Immunother (2013) 62(5):931–9. doi: 10.1007/s00262-012-1387-1
13. Bidard F-C, Jacot W, Kiavue N, Dureau S, Kadi A, Brain E, et al. Efficacy of circulating tumor cell count–driven vs clinician-driven first-line therapy choice in hormone receptor–positive, ERBB2-negative metastatic breast cancer: The STIC CTC randomized clinical trial. JAMA Oncol (2021) 7(1):34–41. doi: 10.1001/jamaoncol.2020.5660
14. Chen Y, Zhu M, Shen J, He W, Qiu L, Xu R. Chemosensitivity evaluation using circulating tumor cells predicts chemotherapy outcomes in patients with advanced cancer. Clin Lab (2022) 68(1):10.7754/Clin.Lab.2021.210218. doi: 10.7754/Clin.Lab.2021.210218
15. Zhao Y, Han L, Zhang W, Shan L, Wang Y, Song P, et al. Preoperative chemotherapy compared with postoperative adjuvant chemotherapy for squamous cell carcinoma of the thoracic oesophagus with the detection of circulating tumour cells randomized controlled trial. Int J Surg (2020) 73:1–8. doi: 10.1016/j.ijsu.2019.11.005
16. Labib M, Kelley SO. Circulating tumor cell profiling for precision oncology. Mol Oncol (2021) 15(6):1622–46. doi: 10.1002/1878-0261.12901
17. Nanduri LK, Hissa B, Weitz J, Schoelch S, Bork U. The prognostic role of circulating tumor cells in colorectal cancer. Expert Rev Anticancer Ther (2019) 19(12):1077–88. doi: 10.1080/14737140.2019.1699065
18. Liang D, Hall C, Lucci A. Circulating tumor cells in breast cancer. Recent results Cancer Res Fortschr der Krebsforschung Progres dans les recherches sur le cancer. (2020) 215:127–45. doi: 10.1007/978-3-030-26439-0_7
19. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
20. Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: heterogeneity, random-or fixed-effects model analyses? Interact Cardiovasc Thorac Surg (2018) 27(3):317–21. doi: 10.1093/icvts/ivy163
21. Herrmann D, Sinnett P, Holmes J, Khan S, Koller C, Vassar M. Statistical controversies in clinical research: Publication bias evaluations are not routinely conducted in clinical oncology systematic reviews. Ann Oncol (2017) 28(5):931–7. doi: 10.1093/annonc/mdw691
22. Pituch-Noworolska A, Kolodziejczyk P, Kulig J, Drabik G, Szczepanik A, Czupryna A, et al. Circulating tumour cells and survival of patients with gastric cancer. Anticancer Res (2007) 27(1B):635–40.
23. Qian C, Cai R, Zhang W, Wang J, Hu X, Zhang Y, et al. Neutrophil-lymphocyte ratio and circulating tumor cells counts predict prognosis in gastrointestinal cancer patients. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.710704
24. Abdallah EA, Braun AC, Flores BC, Senda L, Urvanegia AC, Calsavara V, et al. The potential clinical implications of circulating tumor cells and circulating tumor microemboli in gastric cancer. oncol (2019) 24(9):e854–e63. doi: 10.1634/theoncologist.2018-0741
25. Huang W, Xuan Q, Wu Y, Liu D, Xue J. Study on the relationship between peripheral circulating tumor cells and blood hypercoagulable state and prognosis in advanced gastric cancer. J Oncol (2019) 25(08):691–7 .doi: 10.11735/j.issn.1671-170X.2019.08.B003
26. Han H, Li Z, Liu S, Dong S. Detection of circulating tumor cells in peripheral blood of patients with gastric cancer and its clinical significance. Chin J basic Clin Gen Surg (2015) 22(07):840–3. doi: 10.7507/1007-9424.20150217
27. Ito H, Sato J, Tsujino Y, Yamaguchi N, Kimura S, Gohda K, et al. Long-term prognostic impact of circulating tumour cells in gastric cancer patients. World J gastroenterol (2016) 22(46):10232. doi: 10.3748/wjg.v22.i46.10232
28. Kubisch I, de Albuquerque A, Schuppan D, Kaul S, Schaich M, Stölzel U. Prognostic role of a multimarker analysis of circulating tumor cells in advanced gastric and gastroesophageal adenocarcinomas. Oncology (2015) 89(5):294–303. doi: 10.1159/000437373
29. Jhi JH, Kim GH, Park SJ, Kim DU, Lee MW, Lee BE, et al. Circulating tumor cells and TWIST expression in patients with metastatic gastric cancer: A preliminary study. J Clin Med (2021) 10(19):4481. doi: 10.3390/jcm10194481
30. Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa Y, Suda K, Ando T, et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol (2008) 15(11):3092–100. doi: 10.1245/s10434-008-0122-9
31. Okabe H, Tsunoda S, Hosogi H, Hisamori S, Tanaka E, Tanaka S, et al. Circulating tumor cells as an independent predictor of survival in advanced gastric cancer. Ann Surg Oncol (2015) 22(12):3954–61. doi: 10.1245/s10434-015-4483-6
32. Zhang Q, Shan F, Li Z, Gao J, Li Y, Shen L, et al. A prospective study on the changes and clinical significance of pre-operative and post-operative circulating tumor cells in resectable gastric cancer. J Trans Med (2018) 16(1):1–12. doi: 10.1186/s12967-018-1544-1
33. Uenosono Y, Arigami T, Kozono T, Yanagita S, Hagihara T, Haraguchi N, et al. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer (2013) 119(22):3984–91. doi: 10.1002/cncr.28309
34. Han Y, Yizhang C, Xiaowen C, Nan C, Cuiju T. Study on circulating tumor cells, blood coagulation and prognosis in patients with gastric cancer. J Pract Cancer (2020) 35(04):615–9. doi: 10.3969/j.issn.1001-5930.2020.04.025
35. Zhu Y, Chen N, Chen M, Cui X, Yang H, Zhu X, et al. Circulating tumor cells: A surrogate to predict the effect of treatment and overall survival in gastric adenocarcinoma. Int J Biol Markers (2021) 36(1):28–35. doi: 10.1177/1724600820981972
36. Chiang SP, Cabrera RM, Segall JE. Tumor cell intravasation. Am J Physiol-Cell Physiol (2016) 311(1):C1–C14. doi: 10.1152/ajpcell.00238.2015
37. Jacot W, Mazel M, Mollevi C, Pouderoux S, D'Hondt V, Cayrefourcq L, et al. Clinical correlations of programmed cell death ligand 1 status in liquid and standard biopsies in breast cancer. Clin Chem (2020) 66(8):1093–101. doi: 10.1093/clinchem/hvaa121
38. Castro-Giner F, Aceto N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med (2020) 12(1):1–12. doi: 10.1186/s13073-020-00728-3
39. Maly V, Maly O, Kolostova K, Bobek V. Circulating tumor cells in diagnosis and treatment of lung cancer. vivo. (2019) 33(4):1027–37. doi: 10.21873/invivo.11571
40. Agrawal N, Punia RS, Handa U, Attri AK. Isolation and morphology of circulating tumor cells by cell block technique in breast cancer. Indian J Pathol Microbiol (2021) 64(2):329. doi: 10.4103/IJPM.IJPM_855_20
41. Hayes B, Brady L, Sheill G, Baird A-M, Guinan E, Stanfill B, et al. Circulating tumour cell numbers correlate with platelet count and circulating lymphocyte subsets in men with advanced prostate cancer: Data from the ExPeCT clinical trial (CTRIAL-IE 15-21). Cancers (2021) 13(18):4690. doi: 10.3390/cancers13184690
42. Yu Y, Lin Z-X, Li H-W, Luo H-Q, Yang D-H, Zhou H-C, et al. Circulating tumor cells and fibronectin 1 in the prognosis of nasopharyngeal carcinoma. Technol Cancer Res Treat (2020) 19:1533033820909911. doi: 10.1177/1533033820909911
43. Shen F, Zhu Y, Wang F, Cai X, Ding H, Zhou F, et al. Clinical significance of circulating tumour cells and tumour marker detection in the chemotherapeutic evaluation of advanced colorectal cancer. Colorectal Dis (2022) 24(1):68–76. doi: 10.1111/codi.15939
44. Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell (2019) 176(1-2):98–112. e14. doi: 10.1016/j.cell.2018.11.046
45. Zou K, Yang S, Zheng L, Wang S, Xiong B. Prognostic role of the circulating tumor cells detected by cytological methods in gastric cancer: a meta-analysis. BioMed Res Int (2016) 2016:2765464. doi: 10.1155/2016/2765464
Keywords: gastric cancer, circulating tumor cell, CTC, prognosis, meta-analysis
Citation: Li Z, Song M, Han S, Jin C and Yang J (2022) The prognostic role of circulating tumor cells in gastric cancer: A meta-analysis. Front. Oncol. 12:963091. doi: 10.3389/fonc.2022.963091
Received: 07 June 2022; Accepted: 29 August 2022;
Published: 13 October 2022.
Edited by:
Chiara Bazzichetto, Regina Elena National Cancer Institute (IRCCS), ItalyReviewed by:
Baoguang Hu, Binzhou Medical University Hospital, ChinaKai Cui, Shandong Cancer Hospital, Shandong University, China
Copyright © 2022 Li, Song, Han, Jin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yang, MjE2MzQ2MDRAcXEuY29t
 Zuxi Li
Zuxi Li Meijuan Song1,2,3
Meijuan Song1,2,3 Jing Yang
Jing Yang