
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 20 September 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.962473
This article is part of the Research TopicHallmark of Cancer: Replicative ImmortalityView all 8 articles
 Ido Laish1,2*
Ido Laish1,2* Zohar Levi2,3
Zohar Levi2,3 Hussein Mahajna1,2
Hussein Mahajna1,2 Ahmad Albshesh1,2
Ahmad Albshesh1,2 Nir Horesh2,4
Nir Horesh2,4 Efraim Katz2,4
Efraim Katz2,4 Dan Feldman2,5
Dan Feldman2,5 Nadav Shinar2,5
Nadav Shinar2,5 Orit Picard1,2
Orit Picard1,2 Miri Yavzori1,2
Miri Yavzori1,2 Ella Fudim1,2
Ella Fudim1,2 Pia Raanani2,6,7
Pia Raanani2,6,7 Tamar Berger2,6
Tamar Berger2,6 Hadar Goldvaser2,8
Hadar Goldvaser2,8 Einat Beery2,7
Einat Beery2,7 Orit Uziel2,7
Orit Uziel2,7Background: Human telomerase reverse transcriptase (hTERT)- mRNA was shown to be elevated in exosomes derived from the sera of a variety of hematological and solid cancer patients. We aimed to evaluate its role as a diagnostic marker in patients with newly diagnosed colon cancer and in hereditary syndromes with predisposition to colon cancer.
Methods: hTERT -mRNA levels were determined in serum-derived exosomes from 88 patients with colon cancer, 71 Lynch-syndrome carriers with unknown active malignancies and 50 healthy controls. Data, including demographics, background diseases, clinical data regarding tumor characteristics and genetic data, were retrieved data from medical files.
Results: Patients with colon cancer had both higher exosomal hTERT mRNA levels and a higher proportion of patients with positive exosomal hTERT mRNA than controls (29.5% vs. 4%, respectively, P values < 0.001). Within the cancer group, patients with a metastatic disease had higher levels of telomerase mRNA than non-metastatic disease patients, and these levels correlated with CEA levels. Likewise, Lynch syndrome carriers had a higher proportion of positive exosomal hTERT mRNA than controls (21.1% vs. 4%, respectively, P value 0.008) but only a trend towards higher exosomal hTERT mRNA levels. Higher telomerase mRNA levels were not correlated with the mutated gene.
Conclusions: Exosomal serum hTERT –mRNA levels are associated with metastatic colon cancer and were also demonstrated in a subset of Lynch syndrome carriers. Its significance as a biomarker for developing malignancy should be elucidated.
Colorectal carcinoma (CRC) is the second most common cause of cancer related death and the third most commonly detected cancer worldwide1 (1) . Currently, the only broadly used biomarker for CRC, chorio- embroginic antigen (CEA) has limited sensitivity and specificity and therefore there is an unmet need for novel biomarkers. Circulating mRNAs that encode human telomerase reverse transcriptase (hTERT) may be of particular interest in this setting. hTERT, the catalytic protein of the telomerase complex that synthetizes de novo telomere sequences, is considered a hallmark of cancer and is activated in more than 90% of cancer cells (2, 3). Its activity is essential for the endless proliferation and the perpetuation of the malignant clone and is correlated with hTERT mRNA (4). Several studies have demonstrated that hTERT mRNA level in tumors is an independent prognostic marker in various types of cancers (5, 6). Its serum levels are also increased in patients with various malignancies, including gastric (7), prostate (8), hepatocellular cancer (9) and also CRC (10), supporting the concept that circulating cell-free hTERT mRNA may be a promising non-invasive liquid biopsy related biomarker in clinical practice. Indeed, recent studies have shown its predictive and prognostic potential in CRC and in patients with rectal cancer undergoing chemo-radiotherapy (6, 11).
However, identifying of serum hTERT transcript is far from being accurate due to the unstable character of the transcripts. A more reliable, exosomal-based method, was recently reported by our group (12). Exosomes are small (30-150nm) membrane vesicles that originate from the endosomal membrane compartment. They contain mRNA, miRNA, DNA, proteins and lipids and are secreted by many cell types, including cancer cells, into the microenvironment and peripheral blood, thus fulfilling important roles in intercellular communications and perpetuation of carcinogenesis (13, 14). Cell-free mRNA is stabilized in exosomes and is considerably protected from digestion by extracellular RNases (15). We recently reported that hTERT mRNA is present in exosomes derived from all examined cancer cell lines (12), and it was also elevated in exosomes derived from the sera of many hematological and solid cancer patients with a variety of malignancies (16). Therefore, exosomal hTERT mRNA may potentially serve as a reliable ‘pan-cancer’ marker for the early detection of cancer.
The small number of patients with CRC in the cohort, however, precluded us from testing specific associations between exosomal hTERT mRNA levels and CRC and to correlate it with disease staging. In addition, exosomal hTERT mRNA’s levels in pre-malignant conditions, such as colonic polyps and hereditary CRC syndromes, were not tested. Lynch syndrome (LS) is an autosomal-dominant disorder caused by germline mutations in the DNA mismatch repair (MMR) genes (17). Although characterized by early-onset CRC and increased risk for other malignancies, there is considerable variation in malignancy susceptibility (18), probably related to genetic modifiers as well as environmental factors. It was previously shown that cancer-free MMR-deficient and MMR-proficient non-polyposis hereditary cases show distinct patterns of blood telomere lengths (19), suggesting the importance of the MMR system’s status on telomere length in hereditary cases, and that a genetic variant in the telomerase gene can modify the cancer risk in Lynch carriers (20).
Due to its potential diagnostic and pathogenic importance, we aimed to evaluate the use of peripheral blood exosomal hTERT mRNA as a valid diagnostic marker in patients with newly diagnosed CRC and LS carriers compared to healthy controls, and to assess its correlation with patient- and tumor-related factors.
This was a case-control study conducted at the Sheba Medical Center (SMC) and Rabin Medical Center (RMC), both tertiary academic medical centers in Israel. We included patients with newly diagnosed biopsy-proven CRC, prospectively recruited between 6/2020 and 5/2021 in SMC (N=80), and CRC patients recruited between 5/2014 and 1/2015 in RMC (N=18), for which data was previously partially published (16). LS carriers, routinely followed by high risk clinics in these 2 medical centers, were recruited following a colonoscopy showing either no findings or benign polyps. Control patients were healthy individuals without a known hereditary cancer syndrome, who underwent colonoscopy for different etiologies (including screening, diagnostic, polyp surveillance or due to family history of cancer) showing either no findings or benign polyps. Both LS-carriers and controls were prospectively recruited in 2020-2021 at the same timeline of CRC patients. Baseline serum carcinoembryonic antigen (CEA) and exosomal hTERT mRNA were obtained from CRC patients before any surgical or oncological treatment.
Subsequent retrieved data from medical files included demographics, smoking habits, presence of diabetes, family history of CRC and aspirin use in all patients, and genetic information in LS carriers. For CRC patients, colonoscopy-, pathological- and cross-sectional imaging reports were explored for tumor localization, degree of differentiation and TMN staging, respectively. Immunohistochemistry for mismatch-repair (MMR) proteins was not available in all patients since routine screening for this was not yet included in Israel’s public health’s list of approved services (health basket). Immunohistochemistry for MMR was not performed on resected polyps in LS or control patients. Patients were excluded from the study if they were unable to provide informed consent or suffered from systemic active infectious diseases, autoimmune disorders (including inflammatory bowel disease) or other current known extra-colonic malignancies. Additionally, in order to avoid individuals with potential inherited polyposis syndrome, patients with multiple (≥ 10) adenomas were excluded. All patients signed an informed consent and the study was approved by the institutional ethics review boards.
Exosomes were isolated from patients’ sera by using the Total Exosome Isolation Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Exosomes concentration and sizes were analyzed by using the NanoSight tracking device as previously described (12) and by electron microscopy (EM). Usually, 3-1010 exosomes were used for each hTERT mRNA analysis. Exosomal markers CD81 and CD19 were detected by flow cytometry.
RNA from exosomes was purified with the Total Exosome RNA and Protein Isolation Kit (Invitrogen) according to the provided manual. mRNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions.
The expression of hTERT was measured relatively to that of the HPRT-1 as a reference gene. Gene amplification was executed using the following sets of primers (purchased from HyLabs, Rehovot, Israel). hTERT: forward, 5’- GTACTTTGTCAAGGTGGA-TGTGA-3’ and reverse, 5’-GCTGGAGGTCTGTCAAGGTAGAG-3’; HPRT-1: forward, 5’ TCAGGCAGTATAATCCAAAGATGGT-3’ and reverse, 5’-CTTCGTGGGGTCCTTTTCAC-3’. Polymerase chain reactions were prepared with the Taqman fluorophore-labelled primers (Applied Biosystems), run and analyzed on the Step One Detection System (Applied Biosystems). Reactions were performed for 50 cycles; a normal value (no expression of hTERT) was arbitrarily defined as 1 for further calculation purposes.
Exosomal hTERT mRNA was considered positive when Relative quantification (RQ) was > 1.2.
Categorical variables were described as frequency and percentage and compared using the Chi-square test or Fisher’s exact test. Continuous variables were described as median and interquartile range (IQR) and comparisons between categories were done using Kruskal-Wallis and Mann–Whitney. Associations between telomerase- mRNA levels and continuous variables were assessed using Spearman’s correlation coefficient. All statistical tests were two sided and p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (IBM SPSS Statistics for Windows, version 25, IBM Corp, Armonk, NY, USA).
Eighty eight patients with CRC [median age (range)- 68 (30–85)], 71 LS-carriers [median age (range)- 46 (27-77)], and 50 healthy controls [median age (range)- 68.5 (47-87)], were included in our cohort. There were 22 patients (44%) with adenomatous polyps in the control group and 17 LS-carriers (24%) with adenoma in their colonoscopy. Demographics and clinical characteristics are detailed in Table 1. LS- carriers were significantly younger and had lower rates of diabetes and aspirin use than the CRC patients and healthy controls, while having higher rate of family CRC history. There were no significant differences between CRC patients and healthy controls in these parameters.
Among the CRC patients, 25 (28.4%) had metastatic disease (stage IV) and most tumors (94.3%) had well/moderate differentiation. The MMR status was known for 40 tumors, of which 34 (85%) were MMR-proficient and 6 (15%) were MMR-deficient with MLH1/PMS2 loss of staining and positive BRAF mutation, which associates with a sporadic tumor. Among LS-carriers, the most common pathogenic variants were in the MSH2 (52.1%) and MSH6 (28.1%) genes, reflecting the high frequency of Ashkenazi-Jewish founder mutations in these genes (21).
There were 26 patients in the CRC group, 15 patients in the LS-carriers group and two patients in the control group, who had positive (RQ > 1.2) exosomal hTERT mRNA levels. As depicted in Figure 1, patients with CRC had both higher exosomal hTERT mRNA levels than controls [median (IQR) 0.22 (0.0- 1.48) vs. 0.08 (0.0- 0.34), respectively; P value – 0.01] and a higher proportion of patients with positive exosomal hTERT mRNA (29.5% vs. 4%, respectively, P values < 0.001). Likewise, LS carriers had higher proportions of positive exosomal hTERT mRNA than controls (21.1% vs. 4%, respectively, P value 0.008) but only a trend towards higher exosomal hTERT mRNA levels, [median (IQR) 0.21 (0.0- 0.96) vs. 0.08 (0.0- 0.34), respectively; P value – 0.11]. There were no statistically significant differences between CRC patients and LS carriers in both these measures (Figures 1A, B). None of the other demographic or clinical parameters in our cohort were associated with exosomal hTERT mRNA levels (Table 2).
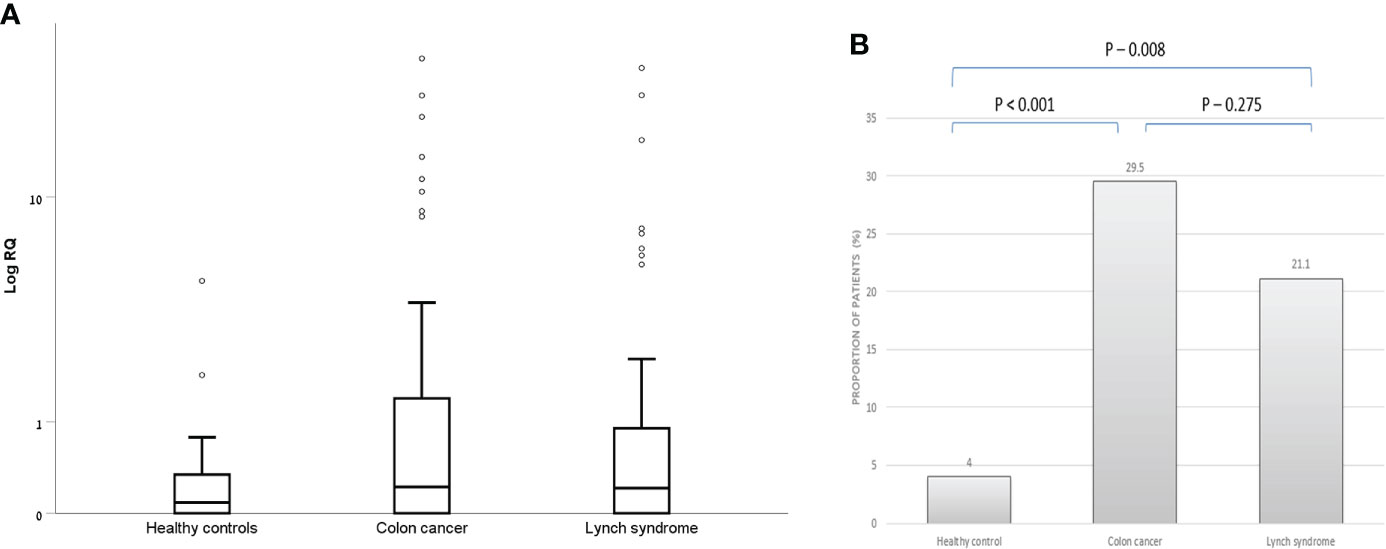
Figure 1 Exosomal hTERT mRNA levels in the study groups. (A) Box and Whisker plot of log hTERT mRNA median values and (B) percent of patients with positive hTERT mRNA values [Relative quantification (RQ) > 1.2].

Table 2 ssociation between patient- and tumor-characteristics and high exosomal hTERT mRNA in a univariate analysis.
Within the CRC group, patients with stage 4 had both higher exosomal hTERT mRNA levels than those in stage 1,2 and 3 [median (IQR) 1.38 (0.92- 7.41) vs. 0.29 (0.0- 1.64), 0.14 (0.0- 0.54) and 0.10 (0.0- 0.37), respectively; P value – 0.016] and a higher proportion of patients with positive exosomal hTERT mRNA (56.0% vs. 19.0% in stage 4 vs. 1-3, respectively, P values < 0.001) (Figures 2A, B). Higher CEA levels were also positively associated with higher exosomal hTERT mRNA levels [median (IQR) 0.71 (0.10- 3.03) vs. 0.15 (0.0- 0.78) in high and low CEA patients, respectively; P value – 0.005] and also with the proportion of patients with positive exosomal hTERT mRNA (48.1% vs. 21.7% in high and low CEA patients, respectively; P value – 0.013), although displaying a weak correlation (r- 0.328, P value - 0.02). Neither patient’s characteristics, including family history of cancer, nor tumor characteristics - including tumor localization, grade of differentiation and MMR status, were associated with exosomal hTERT mRNA levels (Table 2).
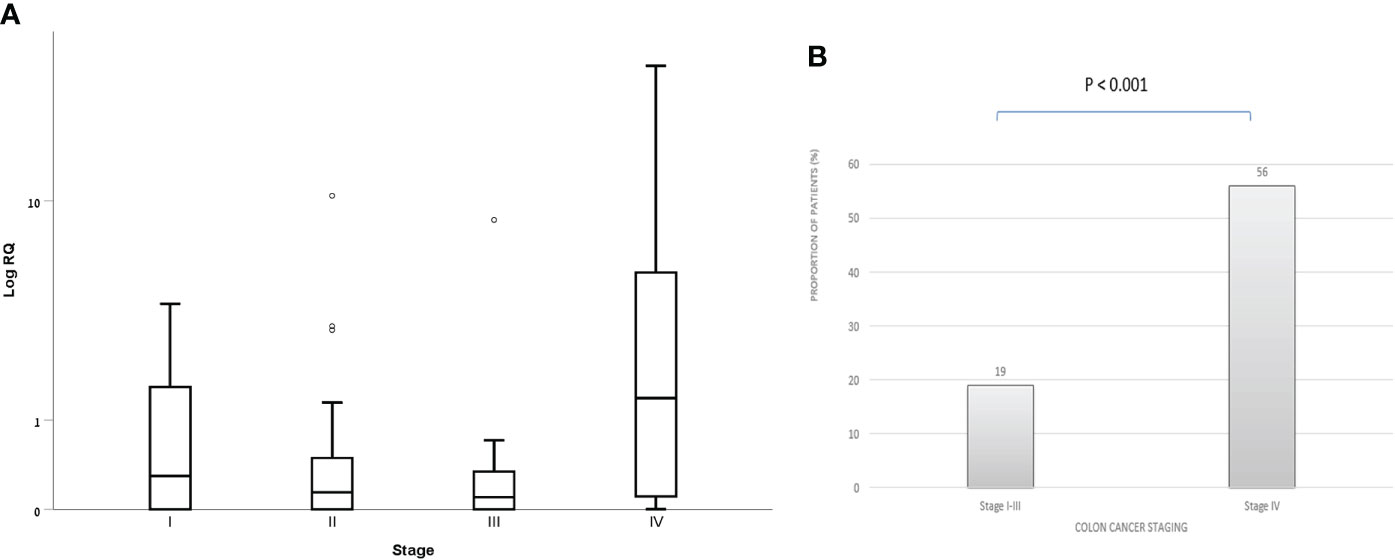
Figure 2 Exosomal hTERT mRNA levels in CRC patients according to disease staging (A) Box and Whisker plot of log hTERT mRNA median values and (B) percent of patients with positive hTERT mRNA values [Relative quantification (RQ) > 1.2].
Within the LS-carriers group, the median (IQR) exosomal hTERT mRNA levels were 0.0 (0.0- 0.91), 0.21 (0.0- 0.98), 0.27 (0.0- 1.56) and 0.0 (0.0- 11.44) in MLH1, MSH2, MSH6 and PMS2 mutation carriers, respectively, without significant difference (P value – 0.77) (Figure 3A). The proportion of patients with positive exosomal hTERT mRNA were also comparable between the groups (Figure 3B). Among LS carriers with positive exosomal hTERT mRNA, 9/15 (60%) individuals had previous CRC and 3/15 (20%) had other Lynch-associated cancer. Carriers with (N=17) and without (N=54) adenoma had both the same exosomal hTERT mRNA levels [median (IQR) 0.20 (0.0-0.86) vs. 0.21 (0.0- 0.98), respectively; P value – 0.92] and proportion of positive patients for hTERT mRNA (17.6% vs. 22.2%, respectively; P value – 0.75).
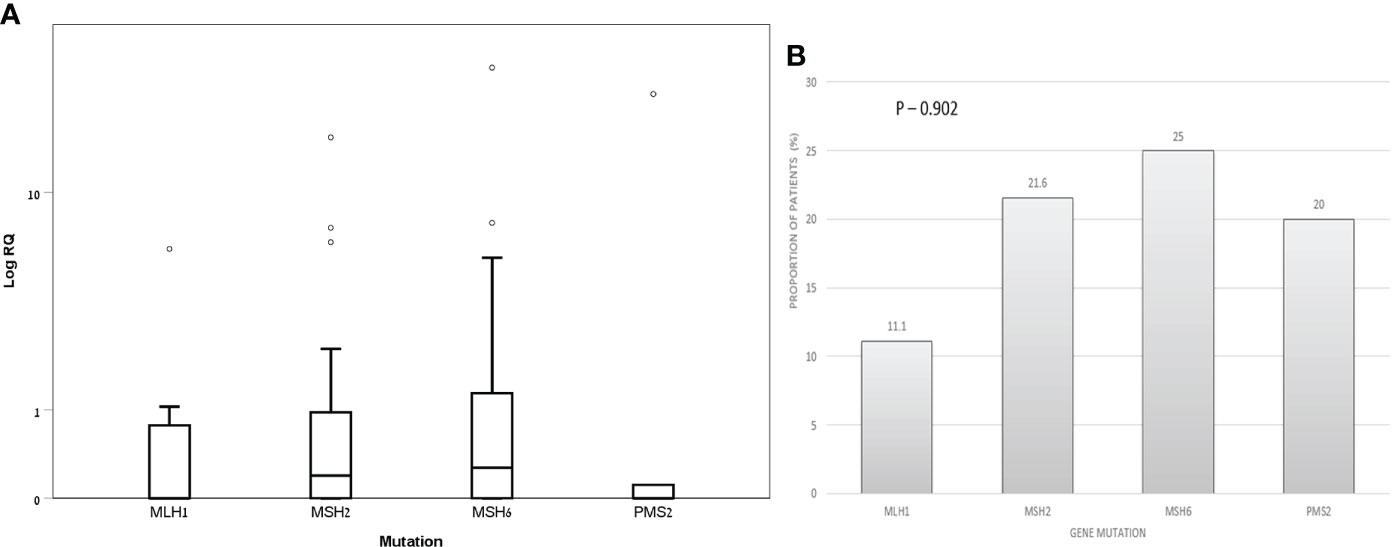
Figure 3 Exosomal hTERT mRNA levels in Lynch syndrome carriers according to genetic mutation (A) Box and Whisker plot of log hTERT mRNA median values and (B) percent of patients with positive hTERT mRNA values [Relative quantification (RQ) > 1.2].
The detection of cancer-related RNA molecules in plasma, specifically cell-free circulating TERT mRNA, can be potentially used as a disease marker. In this study we showed that CRC patients had increased concentrations of hTERT mRNA in exosomes derived from their sera and a higher percentage of them had positive exosomal hTERT mRNA than healthy controls. This elevation was accentuated in stage IV disease patients and correlated with CEA levels but not with MSI status, which is in agreement with many previous studies demonstrating that hTERT expression and/or telomerase activity increase with tumor progression and are positively correlated with disease staging and poor differentiation, but not MSI status (6, 22, 23). Studies evaluating both tumor and plasma hTERT levels found a correlation between the two, and also higher circulating hTERT levels in stage 4 tumors (10, 24). In addition, hTERT’s prognostic role and association with worse overall survival have been found in CRC patients across all pathologic stages (6) and it was recently shown to be a useful marker for monitoring the therapy response in patients with rectal cancer undergoing neo-adjuvant chemoradiotherapy (11).
Still, most patients with non-metastatic CRC in our study did not secrete hTERT mRNA in their exosomes. Since the level of cellular telomerase activity in tumors and exosomal concentration of hTERT mRNA appear to be correlated (12), low levels of telomerase activity in early stage tumors, although not measured in this study, could have been be the cause of hTERT mRNA’s absence in exosomes. Alternatively, the content of exosomes is not merely a passive reflection of its constituents’ cellular concentrations. The involvement of the ‘endosomal sorting complex required for transport (ESCRT)’ complex in packaging nucleic acids in exosomes has been partially elucidated (14), and it may differ in various patients, depending on the cancer type and its expression.
Additionally, this study is the first to show that a significant subset of LS carriers without known active malignancies have elevated peripheral blood derived exosomal hTERT mRNA. In fact, both exosomal hTERT mRNA’s level and the proportion of patients with positive hTERT mRNA, were similar between LS- and non-metastatic CRC groups. There are several potential explanations for this interesting finding. First, although all LS patients had normal findings/benign polyps in their colonoscopy at the time of recruitment, and were all under routine follow-up for other at-risk organs, an occult malignancy could still occur, especially in organs which are not routinely surveilled, e.g., the urinary tract, small bowel or pancreas. Second, as recently reviewed by Ahadova et al., there are differences in the normal-appearing colonic mucosa between LS carriers and healthy individuals (25). Mismatch repair deficient crypt foci (MMR-DCF) can be found in the normal-looking colonic mucosa of LS carriers, which exhibit microsatellite instability (MSI) and carry mutations in microsatellite-bearing genes at very early tumorigenesis stages, even as an initiating event. These mutations result in the generation of frame-shift peptides (FSPs) that can elicit strong immune responses and elimination of FSP-expressing cells by T cells (26). Thus, continuous immunoediting, expressed by elevated mucosal T-cell infiltration and distinct immune profiles, constantly occur even in normal-appearing colonic mucosa (27). Moreover, systemic cellular immune responses to FSP have been found in the blood of healthy LS carriers (28). Exosomal telomerase mRNA can thus either reflect the perpetual generation and regression of MMR-deficient lesions, or originate from activated peripheral lymphocytes. Additionally, the status of the MMR system was previously shown to be important in defining telomere length, presenting by distinct patterns of peripheral blood telomere length in MMR-deficient and -proficient non-polyposis hereditary patients (19). It is possible that either high or low telomerase activity could be associated with shortened telomeres.
The significance and clinical consequences of high exosomal hTERT mRNA levels in LS-carriers are unknown. Although it is tempting to assume that these patients are at higher risk for malignancy, potentially needing stricter surveillance policy, we could not show this in our study since they were not followed up to see if they developed cancer. Moreover, exosomes can be a double-edged sword in cancer immunity. While they can transfer bioactive molecules between tumor cells to help cancer cells escape immune surveillance, those derived from immune cells can also inhibit tumor growth and proliferation (14). We did not find significant differences in the proportion of patients with positive hTERT mRNA among different MMR-gene variant carriers, despite the clinical and molecular differences between them, possibly due to the small sample size. A hint to hTERT’s potential clinical significance was shown in a large cohort of Dutch and Spanish LS carriers (20), in which polymorphism in the variant rs2075786 of the hTERT gene was associated with an increased risk of LS-related-cancers under 45 years of age, while not in the general population or in non-Lynch CRC families, making hTERT a potential cancer-risk modifier of LS.
In this study, two patients from the healthy control group had high exosomal telomerase. This could be attributed to an occult malignancy, either solid or hematological (e.g, lymphoma or chronic lymphocytic leukemia). Alternatively, other non-malignant conditions that are associated with activation of B- lymphocytes, e.g., metabolic syndrome (29, 30), systemic inflammatory disorders or mood disorders (31), could have been the cause of telomere attrition and the release of exosomal telomerase mRNA.
Our study has several limitations. First, the data from the medical files were collected retrospectively, which has an inherent bias. Second, the lack of recurrent measurements of exosomal- hTERT mRNA and long-term follow up of patients, precluded us from its evaluation as a prognostic marker or its association with treatment response. Third, cellular telomerase activity in tumors was not evaluated, thus we could not assess a tumor-exosome correlation of hTERT mRNA. Still, the findings of higher rate of positive exosomal hTERT mRNA in CRC patients which was stage-dependent, and in LS carriers without known malignancies, are innovative. While in CRC, it probably reflects the tumor burden and may have a prognostic effect, its significance and clinical applicability in LS is less clear, and more prospective studies with long-term follow-up and repeated telomerase testing should be performed to assess this.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The study was approved by the Sheba Medical Center ethics committee and written informed consent was obtained from each patient included in the study. Approval was granted for Helsinki protocol 6423-19. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee. The patients/participants provided their written informed consent to participate in this study.
Conceptualization, IL and OU; methodology, IL, TB, and OU.; software, HM.; validation, HM and AA; formal analysis, HG.; investigation, OU; resources ZL, NH, EK, DF, and NS; data curation, OP, MY, EF, and EB; writing—IL, and OU; writing—review and editing, ZL, DF, HG, TB, and PR; visualization, PR; supervision, OU; project administration, IL; funding acquisition, IL and OU. All authors have read and agreed to the published version of the manuscript.
This study was supported by research grants obtained from the Israeli Gastroenterology Society and from the Germanis Research Foundation affiliated with the CBRC at Tel-Aviv University, Israel.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
3. Phatak P, Burger AM. Telomerase and its potential for therapeutic intervention. Br J Pharmacol (2007) 152:1003–11. doi: 10.1038/sj.bjp.0707374
4. Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction intumorigenesis. Oncogene (2002) 21(4):619. doi: 10.1038/sj.onc.1205061
5. Giunco S, Rampazzo E, Celeghin A, Petrara MR, De Rossi A. Telomere and telomerase in carcinogenesis: their role as prognostic biomarkers. Curr Pathobiol Rep (2015) 3(4):315–28. doi: 10.1007/s40139-015-0087-x
6. Bertorelle R, Briarava M, Rampazzo E, Biasini L, Agostini M, Maretto I, et al. Telomerase is an independent prognostic marker of overall survival in patients with colorectal cancer. Br J Cancer (2013) 108(2):278–84. doi: 10.1038/bjc.2012.602
7. Kang Y, Zhang J, Sun P, Shang J. Circulating cell-free human telomerase reverse transcriptase mRNA in plasma and its potential diagnostic and prognostic value for gastric cancer. Int J Clin Oncol (2013) 18(3):478–86. doi: 10.1007/s10147-012-0405-9
8. March-Villalba JA, Martı´nez-Jabaloyas JM, Herrero MJ, Santamaria J, Alin˜o SF, Dası´ F. Cell-free circulating plasma hTERT mRNA is a useful marker for prostate cancer diagnosis and is associated with poor prognosis tumor characteristics. PloS One (2012) 7(8):e43470. doi: 10.1371/journal.pone.0043470
9. Miura N, Osaki Y, Nagashima M, Kohno M, Yorozu K, Shomori K, et al. A novel biomarker TERT mRNA is applicable for early detection of hepatoma. BMC Gastroenterol (2010) 10:46. doi: 10.1186/1471-230X-10-46
10. Terrin L, Rampazzo E, Pucciarelli S, Agostini M, Bertorelle R, Esposito G, et al. Relationship between tumor and plasma levels of hTERT mRNA in patients with colorectal cancer: implications for monitoring of neoplastic disease. Clin Cancer Res (2008) 14(22):7444–51. doi: 10.1158/1078-0432.CCR-08-0478
11. Rampazzo E, Del Bianco P, Bertorelle R, Boso C, Perin A, Spiro G, et al. The predictive and prognostic potential of plasma telomerase reverse transcriptase (TERT) RNA in rectal cancer patients. Br J Cancer (2018) 118(6):878–86. doi: 10.1038/bjc.2017.492
12. Gutkin A, Uziel O, Beery E, Nordenberg J, Pinchasi M, Goldvaser H, et al. Tumor cells derived exosomes contain hTERT mRNA and transform nonmalignant fibroblasts into telomerase positive cells. Oncotarget (2016) 7:59173–88. doi: 10.18632/oncotarget.10384
13. Ramachandran S, Palanisamy V. Horizontal transfer of RNAs: exosomes as mediators of intercellular communication. Wiley Interdiscip Rev RNA (2012) 3:286–93. doi: 10.1002/wrna.115
14. Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B (2021) 11(9):2783–97. doi: 10.1016/j.apsb.2021.01.001
15. Garcia JM, Garcı´a V, Pen˜a C, Domı´nguez G, Silva J, Diaz R, et al. Extracellular plasma RNA from colon cancer patients is confined in a vesicle-like structure and is mRNAenriched. RNA (2008) 14:1424–32. doi: 10.1261/rna.755908
16. Goldvaser H, Gutkin A, Beery E, Edel Y, Nordenberg J, Wolach O, et al. Characterisation of blood-derived exosomal hTERT mRNA secretion in cancer patients: a potential pan-cancer marker. Br J Can (2017) 117:353–7. doi: 10.1038/bjc.2017.166
17. Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medico-legal ramifications. Clin Genet (2009) 76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x
18. Møller P, Seppälä TT, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer risk and survival in path MMR carriers by gene and gender up to 75 years of age: a report from the prospective lynch syndrome database. Gut (2018) 67:1306–16. doi: 10.1136/gutjnl-2017-314057
19. Seguí N, Guinó E, Pineda M, Navarro M, Bellido F, Lázaro C, et al. Longer telomeres are associated with cancer risk in MMR-proficient hereditary non-polyposis colorectal cancer. PloS One (2014) 9(2):e86063. doi: 10.1371/journal.pone.0086063
20. Bellido F, Guinó E, agmohan-Changur S, Seguí N, Pineda M, Navarro M, et al. Genetic variant in the telomerase gene modifies cancer risk in lynch syndrome. Eur J Hum Genet (2013) 21:511–6. doi: 10.1038/ejhg.2012.204
21. Goldberg Y, Kedar I, Kariiv R, Halpern N, Plesser M, Hubert A, et al. Lynch syndrome in high risk ashkenazi jews in Israel. Familial Cancer (2014) 13:65–73. doi: 10.1007/s10689-013-9675-2
22. Ghori A, Usselmann B, Ferryman S, Morris A, Fraser I. Telomerase expression of malignant epithelial cells correlates with dukes’ stage in colorectal cancer. Colorectal Dis (2002) 4:441–6. doi: 10.1046/j.1463-1318.2002.00378.x
23. Sanz-Casla MT, Vidaurreta M, Sanchez-Rueda D, Maestro ML, Arroyo M, Cerdán FJ. Telomerase activity as a prognostic factor in colorectal cancer. Onkologie (2005) 28:553–7. doi: 10.1159/000088525
24. Safont MJ, Gil M, Sirera R, Jantus-Lewintre E, Sanmartín E, Gallach S, et al. The prognostic value of hTERT expression levels in advanced-stage colorectal cancer patients: a comparison between tissue and serum expression. Clin Transl Oncol (2011) 13:396–400. doi: 10.1007/s12094-011-0673-2
25. Ahadova A, Seppälä TT, Engel C, Gallon R, Burn J, Holinski-Feder E, et al. The “unnatural” history of colorectal cancer in lynch syndrome: Lessons from colonoscopy surveillance. Int J Cancer (2021) 148:800–11. doi: 10.1002/ijc.33224
26. Ballhausen A, Przybilla MJ, Jendrusch M, Haupt S, Pfaffendorf E, Seidler F, et al. The shared frameshift mutation landscape of microsatellite-unstable cancers suggests immunoediting during tumor evolution. Nat Commun (2020) 11:4740. doi: 10.1038/s41467-020-18514-5
27. Bohaumilitzky L, Kluck K, Hüneburg R, Gallon R, Nattermann J, Kirchner M, et al. The different immune profiles of normal colonic mucosa in cancer-free Lynch syndrome carriers and Lynch syndrome colorectal cancer patients. Gastroenterology (2021) 907–19. doi.: 10.1053/j.gastro.2021.11.029
28. Kloor M, von Knebel Doeberitz M. The immune biology of microsatelliteunstable cancer. Trends Cancer (2016) 2:121–31. doi: 10.1016/j.trecan.2016.02.004
29. Cheng F, Carroll L, Joglekar MV, Januszewski AS, Wong KK, Hardikar AA, et al. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol (2021) 9:117–26. doi: 10.1016/S2213-8587(20)30365-X
30. Rentoukas E, Tsarouhas K, Kaplanis I, Korou E, Nikolaou M, Marathonitis G, et al. Connection between telomerase activity in PBMC and markers of inflammation and endothelial dysfunction in patients with metabolic syndrome. PloS One (2012) 7:e35739. doi: 10.1371/journal.pone.0035739
31. Bürhan-Çavuşoğlu P, İscan E, Güneş A, Atabey N, Alkın T. Increased telomerase activity in major depressive disorder with melancholic features: Possible role of pro-inflammatory cytokines and the brain-derived neurotrophic factor. Brain Behav Immun Health (2021) 14:100259. doi: 10.1016/j.bbih.2021.100259
Keywords: telomerases, exosomes, colon cancer, Lynch syndrome, genetic syndromes
Citation: Laish I, Levi Z, Mahajna H, Albshesh A, Horesh N, Katz E, Feldman D, Shinar N, Picard O, Yavzori M, Fudim E, Raanani P, Berger T, Goldvaser H, Beery E and Uziel O (2022) Characterization of blood-derived exosomal hTERT mRNA as a biomarker for colon cancer and Lynch syndrome. Front. Oncol. 12:962473. doi: 10.3389/fonc.2022.962473
Received: 06 June 2022; Accepted: 26 August 2022;
Published: 20 September 2022.
Edited by:
Hussain Gadelkarim Ahmed, University of Khartoum, SudanReviewed by:
Carmen Guillen Ponce, Ramón y Cajal University Hospital, SpainCopyright © 2022 Laish, Levi, Mahajna, Albshesh, Horesh, Katz, Feldman, Shinar, Picard, Yavzori, Fudim, Raanani, Berger, Goldvaser, Beery and Uziel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ido Laish, ido.laish@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.