
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 22 August 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.962173
 Ke Zhou1,2
Ke Zhou1,2 Jie Cao1,2
Jie Cao1,2 Huahang Lin1,2
Huahang Lin1,2 Linchuan Liang1,2
Linchuan Liang1,2 Zhongzhong Shen1
Zhongzhong Shen1 Lei Wang1
Lei Wang1 Zhiyu Peng1,2
Zhiyu Peng1,2 Jiandong Mei1,2*
Jiandong Mei1,2*Background: It remains controversial whether the platelet to lymphocyte ratio (PLR) serves as a potential indicator for the efficacy of immunotherapy in advanced lung cancer. This meta-analysis aimed to address this concern.
Methods: Up to March 2022, we searched PubMed, Embase, Web of Science and the Cochrane Library to retrieve potentially eligible articles. Combined hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated to assess the relationship between PLR and progression-free survival (PFS) as well as overall survival (OS), while the combined odds ratios (ORs) and 95% CIs were estimated to evaluate the relationship between PLR and the objective response rate (ORR) as well as the disease control rate (DCR). Subgroup analyses were further performed to detect the source of heterogeneity and potential predictive value of PLR in different groups in terms of OS and PFS.
Results: A total of 21 included studies involving 2312 patients with advanced lung cancer receiving immunotherapy were included. The combined results suggested that elevated PLR was associated with poorer OS (HR=2.24; 95% CI: 1.87-2.68; I² =44%; P=0.01) and PFS (HR=1.66; 95% CI: 1.36-2.04; I² =64%; P<0.01). Furthermore, elevated PLR showed a lower ORR (OR= 0.61; 95% CI: 0.43-0.87, I²=20%; P=0.29) and DCR (OR= 0.44; 95% CI: 0.27-0.72, I²=61%; P=0.02). In subgroup analyses, pretreatment PLR was significantly associated with adverse OS and PFS. The same results were observed in different PLRs in terms of cutoff value (>200 vs. ≤200). Furthermore, high PLR was significantly associated with poor OS and PFS in advanced non-small cell lung cancer (NSCLC); however, PLR was not associated with OS and PFS in advanced small cell lung cancer (SCLC). In addition, PLR predicted poor OS irrespective of regions and types of immune checkpoint inhibitors (ICIs).
Conclusion: On the whole, patients with low PLR had better OS and PFS, as well as higher ORR and DCR when receiving immunotherapy in advanced lung cancer especially for advanced NSCLC. And further investigations are warranted to confirm the prognostic value of PLR in advanced SCLC.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022315976.
Lung cancer, as one of the most common malignant tumors in the world, ranks first in both morbidity and mortality (1). Surgical resection remains the optimal choice for early-stage lung cancer (2). Unfortunately, most lung cancer patients are identified in advanced stages, and postoperative adjuvant therapies become necessary for these patients (3). To date, postoperative adjuvant therapies have enriched with targeted therapy and immunotherapy in addition to traditional chemotherapy and radiotherapy. In particular, immune checkpoint inhibitors (ICIs), such as nivolumab, have yielded encouraging results in advanced lung cancer by showing superior clinical benefits even after the failure of other treatments (4). However, individuals show a different response to ICIs, and not all advanced patients are able to benefit from ICIs (5). Multiple biomarkers found before, including tumor mutational burden (TMB) (6), neoantigens (7) and programmed cell death 1 ligand 1 (PD-L1) expression (8), have shown great potential predictive values for immunotherapy. However, some reports demonstrated that patients with negative PD-L1 expression or low TMB could benefit from ICIs (9). Furthermore, it is complicated to acquire these data with an additional economic cost for patients. Therefore, it is urgent to find inexpensive and practical biomarkers associated with immunotherapy outcomes.
The systemic inflammatory response in cancer patients exerts an important effect on the development of tumors via certain inflammatory factors (10). PLR is known as the ratio of platelets to lymphocytes, and elevated PLR was proven to be associated with poor survival in many cancer patients (11, 12). In recent years, several studies have explored the relationships between PLR and immunotherapy in advanced lung cancer. Some of them claimed that PLR could predict the outcome of immunotherapy (13), while others reported negative findings (14). The role of PLR in predicting the efficacy of immunotherapy in advanced lung cancer remains controversial. To address this concern, we collected relevant publications and conducted a meta-analysis to assess the correlation between PLR and the efficacy of immunotherapy in advanced lung cancer.
We strictly followed the PRISMA guidelines (15) to conduct this systematic review and meta-analysis and registered it on PROSPERO (registration number: CRD42022315976). The PRISMA checklist was provided in Supplementary Table S1.
Up to March 2022, we searched the PubMed, Cochrane Library, Web of Science and Embase databases to collect relevant articles evaluating the relationships between PLR and clinical outcomes of immunotherapy in advanced lung cancer. The search keywords were as follows: lung cancer, platelet lymphocyte ratio, immunotherapy, and immune checkpoint inhibitors. We provided a detailed online search strategy (Supplementary Table S2). Other eligible studies were retrieved from the references cited in the selected articles and relevant literature.
The inclusion criteria were as follows (1): advanced lung cancer including NSCLC or SCLC with recurrent, metastatic, unresectable situation or advanced stage (III or IV) (2); receiving any types of ICIs (3); reporting the value of the PLR before or after immunotherapy (4); study outcomes including overall survival (OS), progression-free survival (PFS), objective response rate (ORR) or disease control rate (DCR); and (5) the effect estimates and corresponding 95% CI were reported or could be estimated. The exclusion criteria were as follows (1): review, letter, meeting, abstract, full text unavailable; and (2) overlapping publications and repeated facilities.
According to PRISMA statements, we extracted the title, first author, study design, year of publication, sample size, sex, region, median follow-up time, histology, PLR cutoff value, time to record PLR, types of ICIs, presence of driver gene mutations, including epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK), number of patients with PD-L1+ (tumor proportion score [TPS]>1), HRs for OS and PFS and rate for ORR and DCR from each included study. When multivariate analysis and univariate analysis results both occurred in one study, we chose the multivariate analysis results since they accounted for confounding factors and were more accurate. The Newcastle–Ottawa Scale (NOS) (16) was used to evaluate the quality of the included studies. NOS evaluated the quality of articles from three main aspects: selection, comparability, and outcome. High-quality studies were defined to attain at least six NOS scores. Two investigators independently extracted data and conducted NOS evaluations. If there was any disagreement about the results, a third reviewer was consulted to resolve the concerns.
The endpoints in our study were OS and PFS, while the secondary endpoints were ORR and DCR. The definitions of these endpoints were described in previous articles (9, 17). We pooled all HRs of OS and PFS via fixed effects or random effects models. If the HRs were not reported directly, HRs with 95% CIs for OS and PFS were indirectly estimated from the Kaplan–Meier curves using the method reported by Tierney (18). The synthesis of ORs for ORR and DCR was conducted by the Mantel–Haenszel method via fixed effects or random effects models. Subgroup analyses were further conducted to detect the impact of the region, sample size, cutoff value, time point, median follow-up time, stage, ICIs types, the presence of mutations and proportion of patients with PD-L1+. All combined HRs in subgroup analyses were performed via a random effects model. Cochrane’s Q statistic and I² statistic were used to estimate heterogeneity between studies. A P value <0.05 for the Q-test and I² <50% were considered to indicate low heterogeneity. Sensitivity analyses were conducted by excluding studies one by one from the meta-analysis. Publication bias was assessed by Egger’s and Begg’s tests, and trim and fill funnel plots were further conducted to estimate the HRs after eliminating potential publication bias. P<0.05 was defined to indicate significant publication bias. For all combined results, P<0.05 was considered statistically significant. All statistical analyses were conducted by R software (version 4.0.5).
After the initial search, a total of 222 studies were identified. Eighty-four of them were removed due to duplication, and 138 studies were left for subsequent selection. Ultimately, 116 studies were excluded, and the remaining 21 retrospective studies (13, 19–38) were included for further combined analysis. The detailed flow diagram of the included study process is shown in Figure 1. There were 21 studies involving 2312 advanced lung cancer patients treated with immunotherapy published from 2017 to 2022. The cutoff value of PLR ranged from 119.2 to 441.8, and the cutoff values of 15 articles were within 200. The baseline characteristics of the included studies were listed in Table 1. The detailed NOS values of the included studies were listed in Supplementary Table S3.
Twelve articles reported the number of patients who achieved ORR and DCR after treatment with immunotherapy. In a total of 1332 patients, 238 patients were considered to achieve ORR and 628 to achieve DCR, with percentages of 17.86% and 47.14%, respectively. Furthermore, 62 patients (12.54%) achieved ORR in patients with high PLR, while it was 149 (21.18%) in patients with low PLR. In addition, 174 patients (35.22%) achieved DCR in patients with high PLR, while it was 351 (49.74%) in patients with low PLR (Supplementary Table S4). The pooled OR for ORR and DCR were shown in Figure 2. Compared to patients with low PLR, patients with high PLR showed a lower ORR (OR= 0.61; 95% CI: 0.43-0.87, I²=20%; P=0.29) (Figure 2A) and lower DCR (OR= 0.44; 95% CI: 0.27-0.72, I²=61%; P=0.02) (Figure 2B).
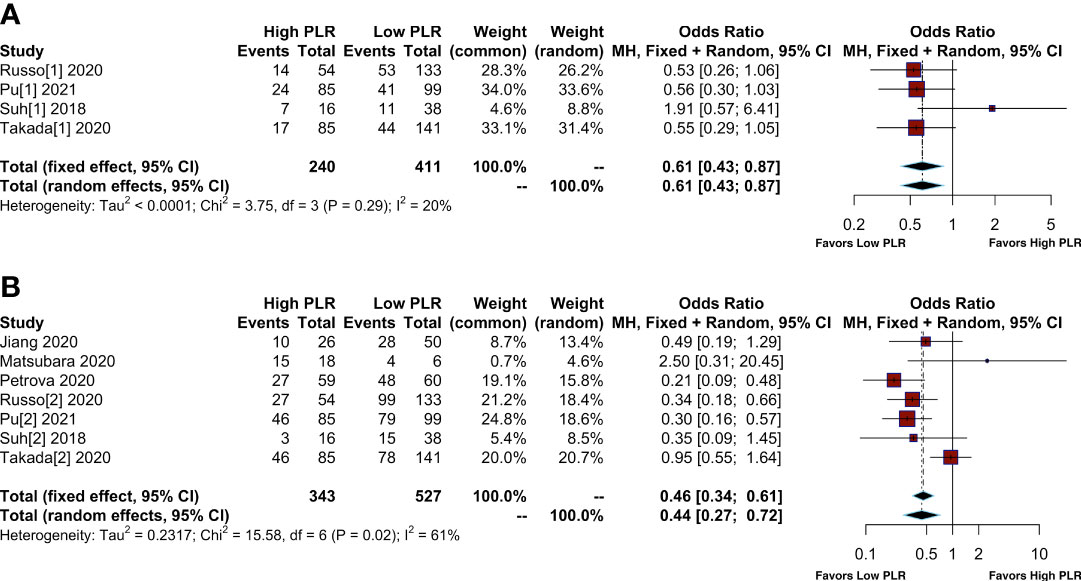
Figure 2 Meta-analysis of the relationship between different comparative models of the platelet to lymphocyte ratio (PLR) and response. (A) Meta-analysis of the relationship between PLR and objective response rate (ORR); (B) Meta-analysis of the relationship between PLR and disease control rate (DCR).
We collected OS/PFS data from each study. The overall median OS was 12 months (range, 8.4-NR months), while the median PFS was 5.25 months (range, 2-8.6 months). The median OS was 8.45 months (range, 2.99-14.7 months) in patients with high PLR and 15.2 months (range 10.6-36.4 months) in patients with low PLR. Furthermore, the median PFS was 3.4 months (range, 1.4-5.2 months) in patients with high PLR, while it was 6.95 months (range, 3-9.16 months) in patients with low PLR (Supplementary Table S5). After the synthesis of the HRs for OS from 20 studies, we observed that high PLR was associated with poorer OS (HR=2.24; 95% CI: 1.87-2.68; I² =44%, P=0.01) (Figure 3). After combining HRs for PFS from 15 studies, we observed that high PLR was also associated with adverse PFS (HR=1.66; 95% CI: 1.36-2.04; I²=64%, P<0.01) (Figure 4).
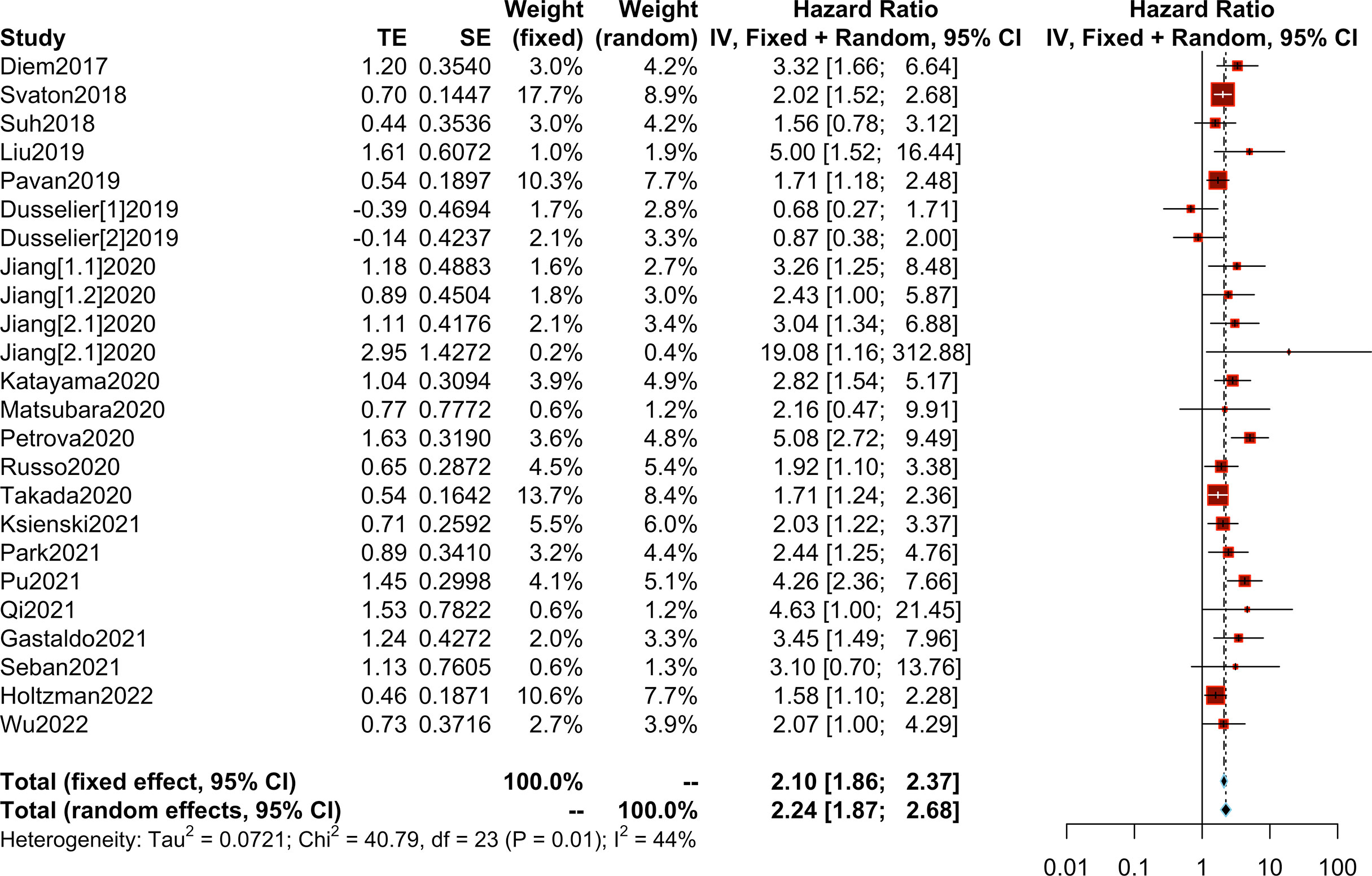
Figure 3 Meta-analysis of the relationship between different comparative models of the platelet to lymphocyte ratio (PLR) and overall survival (OS).
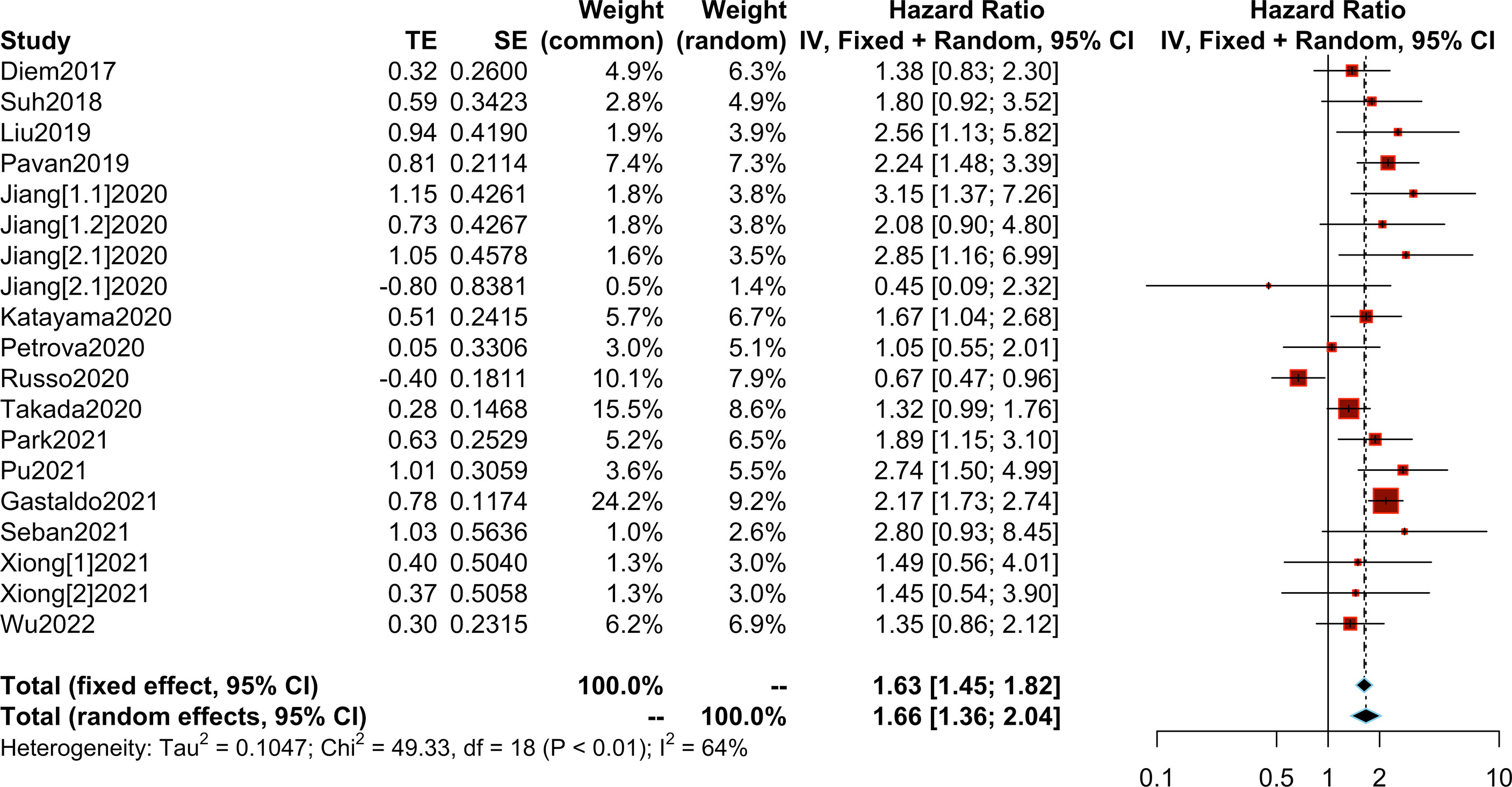
Figure 4 Meta-analysis of the relationship between different comparative models of the platelet to lymphocyte ratio (PLR) and progression-free survival (PFS).
In subgroup analyses, pretreatment high PLR was significantly associated with poorer OS (HR=2.32; 95% CI: 1.93-2.78; I² =43%; P=0.02) and PFS (HR=1.70; 95% CI: 1.34-2.15; I² =70%; P<0.01). Furthermore, high PLR in terms of different cutoff values correlated with poorer OS and PFS, but a lower PLR (cutoff <200) achieved lower heterogeneity (I² = 35% for OS and I² = 0% for PFS). High PLR was significantly associated with adverse OS (HR=2.22; 95% CI: 1.85-2.67; I² =45%; P=0.01) and PFS (HR=1.68; 95% CI: 1.35-2.09; I² =68%; P<0.01) in advanced NSCLC; in contrast, PLR was not associated with OS (HR=4.63; 95% CI: 1-21.45); or PFS (HR=1.47; 95% CI: 0.73-2.96; I² =0%; P<0.97) in advanced SCLC. In addition, low PLR could benefit OS irrespective of the regions, types of ICIs and proportion of patients with PD-L1+. However, high PLR from the European region was not significantly associated with poorer PFS (HR=1.48; 95% CI: 0.92-2.40, I² =86%; P<0.01), and the same negative results were observed for nivolumab (HR=1.62; 95% CI: 0.86-3.03, I² =81%; P<0.01), durvalumab (HR=1.29; 95% CI: 0.22-7.76, I² =73%; P=0.05) and a small proportion of patients with PD-L1+ (PDL1+1 ≤ 50% group) (HR=1.40; 95% CI: 0.97-2.01, I² =77%; P<0.01). Interestingly, in the group of patients with driver gene mutations, elevated PLR still showed a significant association with adverse OS (HR=1.94; 95% CI: 1.63-2.30; I² =0%; P=0.76) and PFS (HR=1.51; 95% CI: 1.10-2.06; I² =73%; P<0.01) (Figure 5).
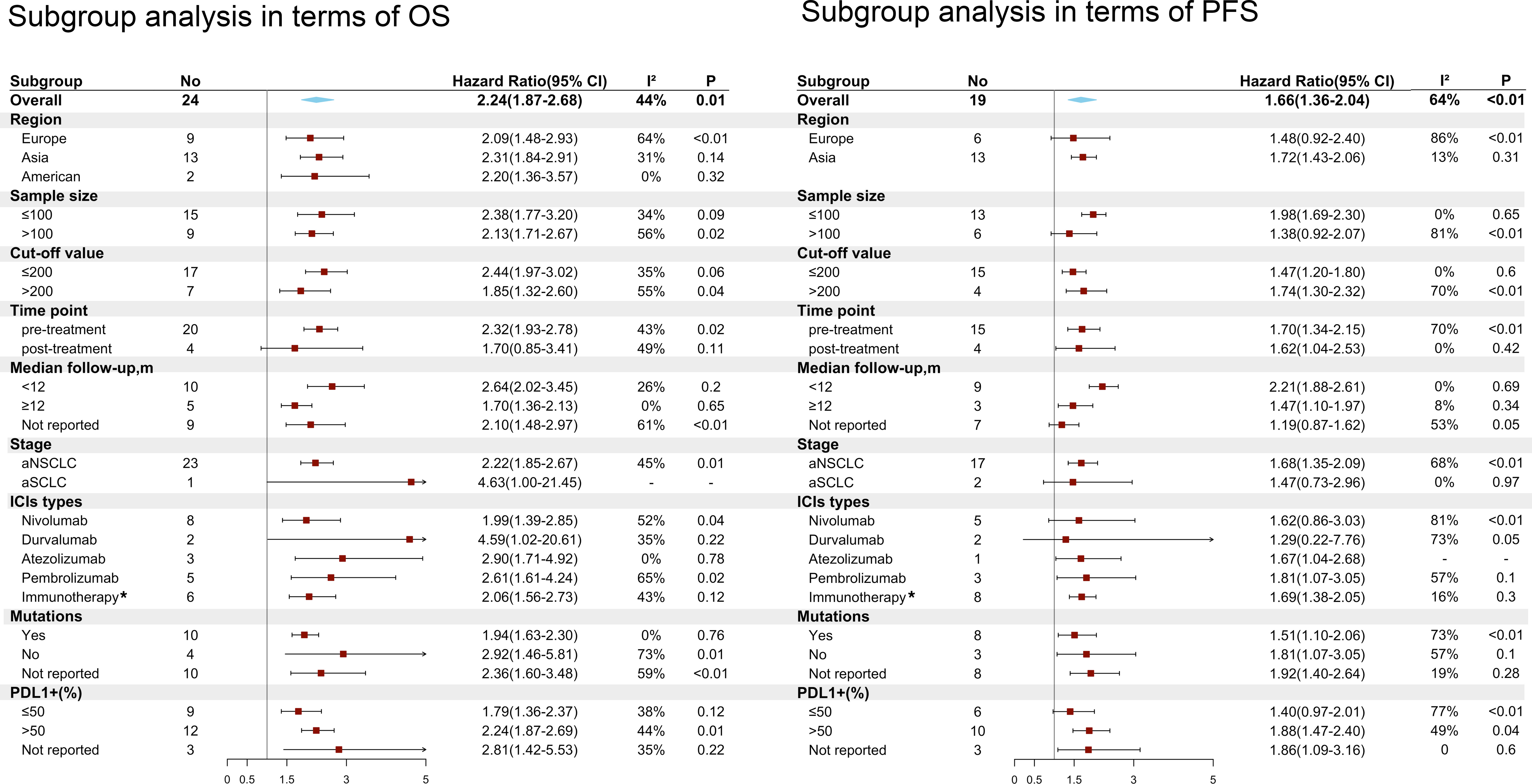
Figure 5 Subgroup analyses of the associations between the platelet to lymphocyte ratio (PLR) and overall survival (OS) and progression-free survival (PFS). *Immunotherapy means these articles reporting different types of ICIs or no detailed types of ICIs.
Sensitivity analysis showed that pooled HRs for OS and PFS were stable after excluding each of the included studies (HR>1, Figures 6A, C). No evidence of obvious publication bias was found in Begg’s test or Egger’s test (p>0.05, Supplementary Figure S1). Finally, trim and fill funnel plots of OS and PFS showed the same results after eliminating the potential publication bias, and the HR was 1.88 (95% CI: 1.53-2.33) for OS and 1.59 (95% CI: 1.30-1.94) for PFS (Figures 6B, D).
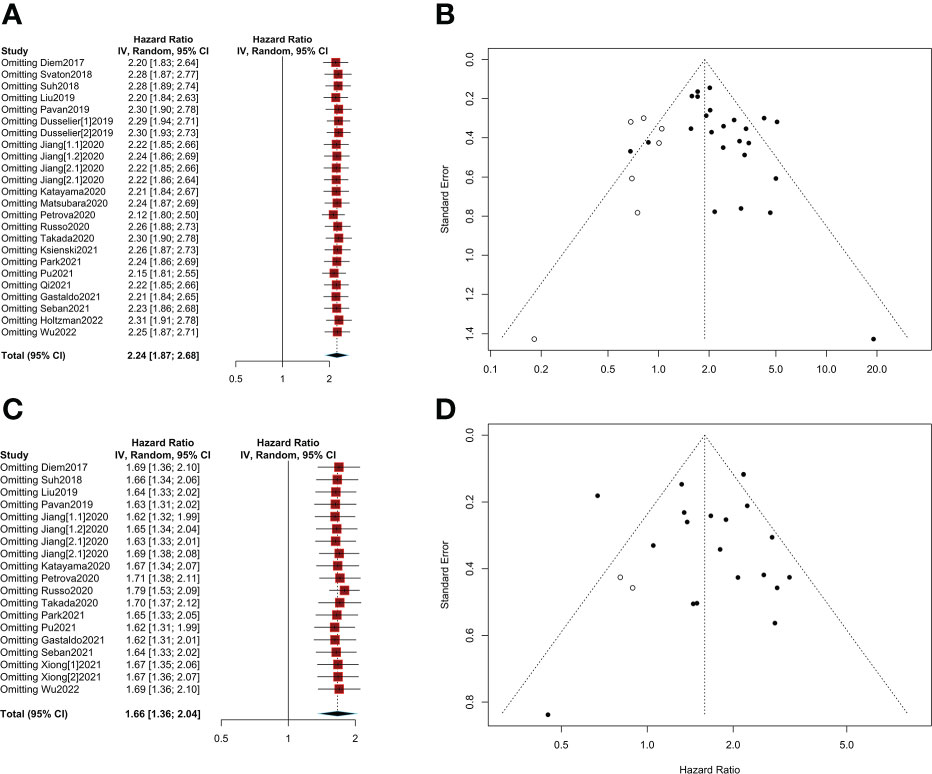
Figure 6 Sensitivity analyses and publication bias. (A) Sensitivity analysis of overall survival (OS); (B) A trim and fill funnel plot of overall survival (OS); (C) Sensitivity analysis of progression-free survival (PFS); (D) A trim and fill funnel plot of progression-free survival (PFS).
Since the role of the platelet-lymphocyte ratio (PLR) in predicting the efficacy of immunotherapy in advanced lung cancers is disputable, this study gathered 21 studies including 2312 patients with advanced lung cancer receiving ICIs to address this concern. Overall, the final meta-analysis showed that patients with high PLR were associated with poor OS and PFS, whereas patients with low PLR showed better OS and PFS. Furthermore, patients with high PLR achieved lower ORR and DCR than those with low PLR. Overall, these evidences showed that PLR was a practical prognostic biomarker for immunotherapy in advanced lung cancer.
Recently, several meta-analyses with respect to the prognostic value of PLR in cancer immunotherapy were published. Xu et al (39) reported that low PLR may be related to better survival for cancer patients with melanoma and NSCLC, which was consistent with our results. Another meta-analysis performed by Tan et al. (40) showed that PLR did not achieve a significant association with clinical outcomes in patients treated with immunotherapy. However, they included only four retrospective articles that evaluated the correlation between PLR and immunotherapy in cancer patients. High heterogeneity from their results was likely to reduce the reliability.
To detect the source of heterogeneity as well as the potential predictive value of PLR in different groups, further subgroup analyses were performed in our meta-analysis. These results suggested that pretreatment high PLR was significantly associated with adverse OS and PFS. A recent meta-analysis performed by Liu et al. (41) also indicated a similar trend to ours, but the results of their subgroup analyses showed no significant correlation between posttreatment PLR and poor OS and PFS, which is inconsistent with our results. Our meta-analysis suggested that posttreatment PLR was significantly associated with PFS and with lower heterogeneity. Although high PLR in terms of different cutoff values correlated with poorer OS and PFS, a lower PLR (cutoff <200) achieved lower heterogeneity. This result indicated that the cutoff value of PLR set within 200 may be more reasonable. A meta-analysis conducted by Xu et al (39) suggested that PLR ≤ 170 was significantly correlated with PFS, which further supported our results.
In addition, high PLR was significantly associated with poor OS and PFS in advanced NSCLC; however, high PLR was not associated with adverse OS and PFS in advanced SCLC. Since only two included articles reported OS or PFS for advanced SCLC, the negative results for advanced SCLC may be biased. More investigations are warranted to provide evidences for the predictive value of PLR in advanced SCLC. Furthermore, PLR could predict poorer OS irrespective of regions, types of ICIs and proportion of patients with PD-L1+. However, high PLR from the European region was not associated with poorer PFS, and the same negative results were observed with nivolumab, durvalumab and a small proportion of patients with PD-L1+. These negative results may be the source of the high heterogeneity of PLR in predicting PFS, and these factors may potentially affect the prognostic value of PLR in PFS. Several factors, such as tumor mutation load and driver gene mutation, may affect the response to immunotherapy (42). Previous articles stated that driven gene mutations, including EGFR+, were associated with a poor response to immunotherapy (43). Our subgroup analysis showed that high PLR was associated with adverse OS/PFS in the presence of driven gene mutations in the cohort, which indicated that PLR was an effective marker for immunotherapy irrespective of driven gene mutations.
In summary, in contrast to the previous meta-analysis, we collected more original articles. Besides, more subgroup analyses were conducted in terms of clinical information to detect sources of heterogeneity and the potential predictive value of PLR in different groups.
In addition to PLR, it was reported that other inflammatory biomarkers (neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ratio (LMR), etc.) were associated with the prognosis of immunotherapy in advanced lung cancer. Elevated NLR was associated with poorer OS and PFS in patients with lung cancer receiving immunotherapy in a previous meta-analysis (9, 44). Furthermore, the meta-analysis from Liu et al (41) showed that a low LMR is related to unsatisfactory survival outcomes in NSCLC patients treated with ICIs. In addition, these inflammatory biomarkers were also reliable in predicting the outcome of other advanced tumors treated with immunotherapy. Elevated NLR was observed to be associated with adverse OS and PFS in patients with metastatic renal cell carcinoma (45) and metastatic melanoma (46) treated with ICIs in a previous meta-analysis. Of note, the predictive value of PLR was also observed in other advanced cancers (melanoma (47), renal cell carcinoma (48)) treated with immunotherapy. However, further investigations are warranted to confirm the prognostic value of PLR in other tumors.
Platelet elevation accelerates tumor progression by promoting the formation of new blood vessels and the production of adhesion molecules (49–51). In the latest research, Hinterleitner et al. (52) found that PD-L1 protein was able to be transferred from NSCLC tumor cells to platelets, and platelets expressing PD-L1 inhibited the infiltration of CD4+ and CD8+ T lymph cells. In contrast, lymphocytes play an important role in the antitumor process by releasing a range of cytokines that activate antitumor immunity (53). In summary, peripheral platelets may reflect the expression of PD-L1 on tumors, and high PLR may impair the efficacy of ICIs. These findings provide some evidence of the predictive value of immunotherapy for PLR. However, it is known that all individuals show a greatly different response to immunotherapy. Although PLR showed a potential predictive value in immunotherapy, this indicator should not be used alone. PLR combined with other biomarkers may improve the efficacy of predictive value, but further studies are needed to confirm the efficacy of predictive value of PLR alone or combined with other biomarkers.
There were some limitations in our study (1): the type of included studies was retrospective, which is not convincing compared with clinical randomized controlled trials (RCTs) (2). Because of the small number of samples, the heterogeneity of the combined results is inevitable. However, we tried to reduce this heterogeneity through various subgroup analyses. Except for the posttreatment value of PLR, the combined HRs for OS maintained good consistency. In contrast, we found that the heterogeneity of PFS may be related to regions, sample size, ICI types, median follow-up time and proportion of patients with PD-L1+. However, PLR still showed a robust correlation with PFS after sensitivity analysis (3). In addition, the cutoff value of PLR varied greatly. Although the cutoff values of PLR did not affect the ultimate result of OS/PFS in our study, the optimal cutoff value should be taken into priority consideration before the application of PLR.
On the whole, our meta-analysis showed that patients with low PLR had better OS and PFS, as well as higher ORR and DCR when receiving immunotherapy in advanced lung cancer especially for advanced NSCLC. PLR showed a great potential value in predicting the outcome of immunotherapy in advanced NSCLC; further investigations are warranted to confirm the prognostic value of the PLR in advanced SCLC.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
KZ and JDM designed the study. KZ, JC, and HHL designed the statistical plan. KZ, LCL, and ZZS performed the key analyses. LW, ZYP, and KZ generated and collected the data. LW, ZYP, and LCL assisted in data interpretation. KZ wrote the manuscript. JDM and JC revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the 1.3.5 Project for Disciplines of Excellence (ZYJC18009), West China Hospital, Sichuan University to Dr. Mei.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.962173/full#supplementary-material
PLR, platelet-to-lymphocyte ratio; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; OS, HR, hazard ratio; OR, odds ratio; CI, confidence interval; PFS, progression-free survival; OS, overall survival; ORR, objective response rate; DCR, disease control rate; ICIs, immune checkpoint inhibitors; TMB, tumor mutational burden; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score; NOS, Newcastle–Ottawa Scale; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio. N, nivolumab; P, pembrolizumab; A, atezolizumab; T, toripalimab.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu Y-L, et al. Lung cancer: current therapies and new targeted treatments. Lancet (2017) 389:299–311. doi: 10.1016/S0140-6736(16)30958-8
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature (2018) 553:446–54. doi: 10.1038/nature25183
4. Remon J, Vilariño N, Reguart N. Immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): Approaches on special subgroups and unresolved burning questions. Cancer Treat Rev (2018) 64:21–9. doi: 10.1016/j.ctrv.2018.02.002
5. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med (2018) 50:1–11. doi: 10.1038/s12276-018-0191-1
6. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med (2017) 377:2500–1. doi: 10.1056/NEJMc1713444
7. Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov (2017) 7:264–76. doi: 10.1158/2159-8290.CD-16-0828
8. Marchetti A, Barberis M, Franco R, De Luca G, Pace MV, Staibano S, et al. Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J Thorac Oncol (2017) 12:1654–63. doi: 10.1016/j.jtho.2017.07.031
9. Cao D, Xu H, Xu X, Guo T, Ge W. A reliable and feasible way to predict the benefits of nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies. Oncoimmunology (2018) 7:e1507262. doi: 10.1080/2162402X.2018.1507262
10. Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol (2017) 116:134–46. doi: 10.1016/j.critrevonc.2017.06.002
11. Takenaka Y, Oya R, Kitamiura T, Ashida N, Shimizu K, Takemura K, et al. Platelet count and platelet-lymphocyte ratio as prognostic markers for head and neck squamous cell carcinoma: Meta-analysis. Head Neck (2018) 40:2714–23. doi: 10.1002/hed.25366
12. Oh D, Pyo J-S, Son BK. Prognostic roles of inflammatory markers in pancreatic cancer: Comparison between the neutrophil-to-Lymphocyte ratio and platelet-to-Lymphocyte ratio. Gastroenterol Res Pract (2018) 2018:9745601. doi: 10.1155/2018/9745601
13. Svaton M, Zemanova M, Skrickova J, Jakubikova L, Kolek V, Kultan J, et al. Chronic inflammation as a potential predictive factor of nivolumab therapy in non-small cell lung cancer. Anticancer Res (2018) 38:6771–82. doi: 10.21873/anticanres.13048
14. Zer A, Sung MR, Walia P, Khoja L, Maganti M, Labbe C, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-Small-Cell lung cancer. Clin Lung Cancer (2018) 19:426–434.e1. doi: 10.1016/j.cllc.2018.04.008
15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
16. Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed March 19, 2022).
17. Zhao Q, Zhang J, Xu L, Yang H, Liang N, Zhang L, et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: A systemic review and meta-analysis. Front Immunol (2021) 12:730320. doi: 10.3389/fimmu.2021.730320
18. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
19. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
20. Dusselier M, Deluche E, Delacourt N, Ballouhey J, Egenod T, Melloni B, et al. Neutrophil-to-lymphocyte ratio evolution is an independent predictor of early progression of second-line nivolumab-treated patients with advanced non-small-cell lung cancers. PloS One (2019) 14:e0219060. doi: 10.1371/journal.pone.0219060
21. Holtzman L, Moskovitz M, Urban D, Nechushtan H, Keren S, Reinhorn D, et al. dNLR-based score predicting overall survival benefit for the addition of platinum-based chemotherapy to pembrolizumab in advanced NSCLC with PD-L1 tumor proportion score >=50%. Clin Lung Cancer (2022) 23:122–34. doi: 10.1016/j.cllc.2021.12.006
22. Jiang M, Peng W, Pu X, Chen B, Li J, Xu F, et al. Peripheral blood biomarkers associated with outcome in non-small cell lung cancer patients treated with nivolumab and durvalumab monotherapy. Front Oncol (2020) 10:913. doi: 10.3389/fonc.2020.00913
23. Katayama Y, Yamada T, Chihara Y, Tanaka S, Tanimura K, Okura N, et al. Significance of inflammatory indexes in atezolizumab monotherapy outcomes in previously treated non-small-cell lung cancer patients. Sci Rep (2020) 10:17495. doi: 10.1038/s41598-020-74573-0
24. Ksienski D, Wai ES, Alex D, Croteau NS, Freeman AT, Chan A, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Trans Lung Cancer Res (2021) 10:355–67. doi: 10.21037/tlcr-20-541
25. Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal (2019) 33:e22964. doi: 10.1002/jcla.22964
26. Matsubara T, Takamori S, Haratake N, Toyozawa R, Miura N, Shimokawa M, et al. The impact of immune-inflammation-nutritional parameters on the prognosis of non-small cell lung cancer patients treated with atezolizumab. J Thorac Dis (2020) 12:1520–8. doi: 10.21037/jtd.2020.02.27
27. Park CK, Oh HJ, Kim MS, Koh BG, Cho HJ, Kim YC, et al. Comprehensive analysis of blood-based biomarkers for predicting immunotherapy benefits in patients with advanced non-small cell lung cancer. Trans Lung Cancer Res (2021) 10:2103–17. doi: 10.21037/tlcr-21-100
28. Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist (2019) 24:1128–36. doi: 10.1634/theoncologist.2018-0563
29. Petrova MP, Eneva MI, Arabadjiev JI, Conev NV, Dimitrova EG, Koynov KD, et al. Neutrophil to lymphocyte ratio as a potential predictive marker for treatment with pembrolizumab as a second line treatment in patients with non-small cell lung cancer. Bioscience Trends (2020) 14:48–55. doi: 10.5582/bst.2019.01279
30. Pu D, Xu Q, Zhou LY, Zhou YW, Liu JY, Ma XL. Inflammation-nutritional markers of peripheral blood could predict survival in advanced non-small-cell lung cancer patients treated with PD-1 inhibitors. Thorac Cancer (2021) 12:2914–23. doi: 10.1111/(ISSN)1759-771410.1111/1759-7714.14152
31. Qi WX, Xiang Y, Zhao S, Chen J. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer Immunology Immunotherapy (2021) 70:3199–206. doi: 10.1007/s00262-021-02926-3
32. Russano M, Franchina T, Migliorino MR, Aprile G, Mansueto G, Berruti A, et al. Neutrophil-to-Lymphocyte ratio (NLR), platelet-to-Lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (NSCLC): A Large retrospective multicenter study. Adv Ther (2020) 37:1145–55. doi: 10.1007/s12325-020-01229-w
33. Sanchez-Gastaldo A, Munoz-Fuentes MA, Molina-Pinelo S, Alonso-Garcia M, Boyero L, Bernabe-Caro R. Correlation of peripheral blood biomarkers with clinical outcomes in NSCLC patients with high PD-L1 expression treated with pembrolizumab. Trans Lung Cancer Res (2021) 10:2509–22. doi: 10.21037/tlcr-21-156
34. Seban RD, Assie JB, Giroux-Leprieur E, Massiani MA, Bonardel G, Chouaid C, et al. Prognostic value of inflammatory response biomarkers using peripheral blood and [18F]-FDG PET/CT in advanced NSCLC patients treated with first-line chemo- or immunotherapy. Lung Cancer (2021) 159:45–55. doi: 10.1016/j.lungcan.2021.06.024
35. Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother (2018) 67:459–70. doi: 10.1007/s00262-017-2092-x
36. Takada K, Takamori S, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, et al. Serum markers associated with treatment response and survival in non-small cell lung cancer patients treated with anti-PD-1 therapy. Lung Cancer (2020) 145:18–26. doi: 10.1016/j.lungcan.2020.04.034
37. Wu Y, Wu H, Lin M, Liu T, Li J. Factors associated with immunotherapy respond and survival in advanced non-small cell lung cancer patients. Trans Oncol (2022) 15:101268. doi: 10.1016/j.tranon.2021.101268
38. Xiong Q, Huang Z, Xin L, Qin B, Zhao X, Zhang J, et al. Post-treatment neutrophil-to-lymphocyte ratio (NLR) predicts response to anti-PD-1/PD-L1 antibody in SCLC patients at early phase. Cancer Immunol Immunotherapy (2021) 70:713–20. doi: 10.1007/s00262-020-02706-5
39. Xu H, He A, Liu A, Tong W, Cao D. Evaluation of the prognostic role of platelet-lymphocyte ratio in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int Immunopharmacol (2019) 77:105957. doi: 10.1016/j.intimp.2019.105957
40. Tan Q, Liu S, Liang C, Han X, Shi Y. Pretreatment hematological markers predict clinical outcome in cancer patients receiving immune checkpoint inhibitors: A meta-analysis. Thorac Cancer (2018) 9:1220–30. doi: 10.1111/1759-7714.12815
41. Liu N, Mao J, Tao P, Chi H, Jia W, Dong C. The relationship between NLR/PLR/LMR levels and survival prognosis in patients with non-small cell lung carcinoma treated with immune checkpoint inhibitors. Med (Baltimore) (2022) 101:e28617. doi: 10.1097/MD.0000000000028617
42. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-Small-Cell lung cancer. N Engl J Med (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
43. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol (2019) 30:1321–8. doi: 10.1093/annonc/mdz167
44. Jin J, Yang L, Liu D, Li W. Association of the neutrophil to lymphocyte ratio and clinical outcomes in patients with lung cancer receiving immunotherapy: a meta-analysis. BMJ Open (2020) 10:e035031. doi: 10.1136/bmjopen-2019-035031
45. Chen X, Meng F, Jiang R. Neutrophil-to-Lymphocyte ratio as a prognostic biomarker for patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front Oncol (2021) 11:746976. doi: 10.3389/fonc.2021.746976
46. Li Y, Meng Y, Sun H, Ye L, Zeng F, Chen X, et al. The prognostic significance of baseline neutrophil-to-Lymphocyte ratio in melanoma patients receiving immunotherapy. J Immunother (2022) 45:43–50. doi: 10.1097/CJI.0000000000000392
47. Khoja L, Atenafu EG, Templeton A, Qye Y, Chappell MA, Saibil S, et al. The full blood count as a biomarker of outcome and toxicity in ipilimumab-treated cutaneous metastatic melanoma. Cancer Med (2016) 5:2792–9. doi: 10.1002/cam4.878
48. De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res (2019) 25:3839–46. doi: 10.1158/1078-0432.CCR-18-3661
49. Jiang L, Luan Y, Miao X, Sun C, Li K, Huang Z, et al. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling. Br J Cancer (2017) 117:695–703. doi: 10.1038/bjc.2017.214
50. Menter DG, Kopetz S, Hawk E, Sood AK, Loree JM, Gresele P, et al. Platelet “first responders” in wound response, cancer, and metastasis. Cancer Metastasis Rev (2017) 36:199–213. doi: 10.1007/s10555-017-9682-0
51. Li Z, Riesenberg B, Metelli A, Li A, Wu BX. “The role of platelets in tumor growth, metastasis, and immune evasion.,”. In: Platelets. Elsevier (2019). p. 547–61. doi: 10.1016/B978-0-12-813456-6.00030-8
52. Hinterleitner C, Strähle J, Malenke E, Hinterleitner M, Henning M, Seehawer M, et al. Platelet PD-L1 reflects collective intratumoral PD-L1 expression and predicts immunotherapy response in non-small cell lung cancer. Nat Commun (2021) 12:7005. doi: 10.1038/s41467-021-27303-7
Keywords: platelet to lymphocyte ratio, advanced lung cancer, immune checkpoint inhibitor, prognosis, immunotherapy, biomarker, meta-analysis
Citation: Zhou K, Cao J, Lin H, Liang L, Shen Z, Wang L, Peng Z and Mei J (2022) Prognostic role of the platelet to lymphocyte ratio (PLR) in the clinical outcomes of patients with advanced lung cancer receiving immunotherapy: A systematic review and meta-analysis. Front. Oncol. 12:962173. doi: 10.3389/fonc.2022.962173
Received: 16 June 2022; Accepted: 20 July 2022;
Published: 22 August 2022.
Edited by:
Giuseppe Luigi Banna, United Lincolnshire Hospitals NHS Trust, United KingdomReviewed by:
Sara Elena Rebuzzi, San Martino Hospital (IRCCS), ItalyCopyright © 2022 Zhou, Cao, Lin, Liang, Shen, Wang, Peng and Mei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiandong Mei, amlhbmRvbmdtZWlAYWxpeXVuLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.