
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 August 2022
Sec. Cancer Genetics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.960269
This article is part of the Research Topic 365 Days of Progress In Cancer Genetics View all 5 articles
This study evaluated the association between the microRNA (miRNA) gene polymorphisms and the susceptibility to soft tissue sarcomas (STSs). In this case–control study, DNA was extracted from leukocytes in peripheral blood, which was collected from 169 STSs patients and 170 healthy controls. Three SNPs for miR-210, five SNPs for miR-206, two SNPs for miR-485, two SNPs for miR-34b, two SNPs for miR-671, and three SNPs for miR-381 were investigated and genotyped using a Sequenom Mass ARRAY matrix-assisted laser desorption/ionization-time of flight mass spectrometry platform. Unconditional logistic regression analysis was used to analyze the association between miRNA gene polymorphisms and the susceptibility to STSs. The results showed that miR-671 rs1870238 GC + CC (OR = 1.963, 95% CI = 1.258–3.064, P = 0.003) and miR-671 rs2446065 CG + GG (OR =1.838, 95% CI = 1.178–2.868, P = 0.007) may be genetic risk factors for STSs after adjustment for age and smoking. Therefore, this study suggests that individuals carrying the GC + CC genotype for miR-671 rs1870238 or the CG + GG genotype for miR-671 rs2446065 are susceptible to STSs.
Soft tissue sarcomas (STSs) are a highly heterogeneous group of malignant tumors arising from mesenchymal tissues. Histologically speaking, they are composed of many subtypes with ambiguous clinical and histopathological features, which lead to great challenges in their diagnosis and therapy (1). One important clinical challenge is the lack of useful biomarkers. The identification of biomarkers that can be used for primary prevention or to detect tumor responses to chemotherapy or radiotherapy may provide more effective clinical management approaches for clinicians. A growing amount of evidence suggests that miRNA dysregulation in STSs plays an important role in its progression and prognosis (2). The evidence of microRNA in STSs promotes the potential application of microRNA as a clinical biomarker, giving us one potential solution for preventing STSs.
MiRNAs are single-stranded non-coding RNAs that contain 18–25 nucleotides (3). MiRNAs can mediate gene expression by binding to target genes and inhibiting translation and protein synthesis at the post-transcriptional level (4). Most miRNAs are found within keygenomic regions thought to be involved in carcinogenesis. Many miRNAs have also been abnormally expressed in STSs, and the knowledge of miRNA expression patterns in STSs could identify specific signatures for the histological subtypes (5, 6). Furthermore, the different profiling of miRNA expression in tumor tissue of STSs compared with adjacent tissue may provide a clue for the diagnosis of STSs (7–9). Many studies recently have demonstrated that the altered expression of miRNAs is associated with the occurrence of numerous diseases, which may have a significant possibility of being used as biomarkers and targets of treatment for human illnesses. Structural genetic alterations, including chromosomal abnormalities (10), mutations (11), and single-nucleotide polymorphisms (SNPs), frequently occur in cancers and can affect miRNA expression (12).
SNPs represent an alternate nucleotide that occurs on average every 100 to 1,000 base pairs in vertebrates. Several studies have shown that SNP provided an approach for identifying possible genetic loci associated with diseases, including cancer susceptibility (13). The SNPs in miRNA genes (miRNA-SNP) may work in three possible ways: altering transcription of the primary miRNA transcript; the processing of the pri-miRNA and pre-miRNA; and through their effects on the modulation of miRNA–mRNA interactions (14). Furthermore, a single nucleotide change in a primary miRNA can greatly influence its stability and maturation or alter its activity. Therefore, miRNA-SNPs are associated with many types of cancer (15), including chronic lymphocytic leukemia, thyroid, gastric, and lung cancer. However, the association between polymorphism in the miRNA gene and the risk of STSs has not been fully studied.
Therefore, we first screened the miRNAs associated with the initiation and development of STSs in the literature, including miR-206 (16), miR-671, miR-381 (17), miR-210, miR-485 (18), and miR-34b (19). Then, we detected three SNPs (rs10902173, rs12364149, rs7935908) in miR-210, five SNPs (rs1537670, rs2397080, rs16882131, rs17578851, rs6920648) in miR-206, two SNPs (rs4143957, rs12886869) in miR-485, two SNPs (rs2187473, rs4938723) in miR-34b, two SNPs (rs1870238, rs2446065) in miR-671, and three SNPs (rs2281610, rs7149890, rs34038694) in miR-381, and investigated the susceptible genotype of STSs.
A total of 169 patients with histologically diagnosed STSs were recruited from the Henan Province Cancer Hospital in Henan Province, China. There were 33 synovial sarcomas, 32 undifferentiated pleomorphic sarcomas, 31 fibrosarcomas, 21 liposarcomas, 16 leiomyosarcomas, 11 rhabdomyosarcomas, eight Ewing sarcomas of soft tissues, five malignant peripheral nerve sheath tumors, five spindle cell sarcomas, three alveolar soft part sarcomas, three angiosarcomas, and one clear cell sarcoma. Besides, 170 population-based controls were enrolled during the same period of time as the individuals for physical examinations without a previous history of cancer. Participants who smoked no less than one cigarette per day or those who smoked for more than half a year were categorized as “smokers”, and those who drank more than two times a week and who drank continuously for more than 6 months were categorized as “drinkers”. The standard of the criteria of “smoking” or “drinking”, the method of questionnaire and peripheral blood collection were all described in more detail in our previous study (20). The study was approved by the ethical committee of Zhengzhou University, and all the participating patients signed informed consent.
The extraction and evaluation of genomic DNA from leukocytes in peripheral blood were described in our previous study (20). Genomic DNA was extracted from whole blood, using the Blood DNA Kit (Bioteke Corporation, Beijing, China) according to the protocol of the manufacturer, and stored at −80 °C until use. The DNA purity and concentration were determined by spectrophotometric measurement of absorbance at 260 and 280 nm using a Thermo Scientific NanoDrop™ 8000 UV–Vis Spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA).
Firstly, the miRNAs associated with STSs were screened in the literature, including miR-206 (16), miR-671, miR-381 (17), miR-210, miR-485 (18), and miR-34b (19). Next, we screened the functional region SNPs in the gene region, promoter proxy (TSS200), exon (missense and synonymous), and 3’ UTR region through the NCBI dbSNP database. Furthermore, we screened the validated and hot SNPs through the GWAS Catalog (https://www.ebi.ac.uk/gwas/), GWAS Atlas (https://atlas.ctglab.nl/), and GWAS Central (https://www.gwascentral.org/). Then identified with a cut-off value of r2 = 0.8 and a minor allele frequency greater than 0.05 in the Chinese population by 1,000 Genomes. Finally, 21 SNPs, including functional region SNPs and validated and hot SNPs, were selected for genotyping. Because the detection frequency of the rs11606481 genotype was different from the CHB&CHS 1,000 Genomes database and the detection rate of rs4467881, rs11246190, and rs28524679 was less than 95%, we further analyzed the other 17 loci after removing the four loci.
The SNPs were genotyped using a SequenomMassARRAY® matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry platform (Sequenom Inc., San Diego, CA, USA). The genotyping experiment mainly included a PCR amplification reaction and a single-base extension reaction. Among these, PCR amplification is to obtain gene fragments containing SNP loci. The 5 µl reaction system was conducted, including 4 µl PCR master mix and 1 µl DNA (20 ng/µl). In the PCR amplification conditions, an initial pre-denaturation was performed at 94°C for 5 min, followed by 45 cycles at 94°C for 20 s, 56°C for 30 s, and 72°C for 60 s, and then a final exposure to 72°C for 3 min. In the single-base extended, 9 μl PCR reaction systems were conducted, including 2 µl EXTEND mix (AgenaBiocience, Inc.), and 7 µl SAP (AgenaBiocience, Inc.) + PCR reaction (product of the PCR amplification). In the single-base extended PCR conditions, an initial pre-denaturation was performed at 94°C for 30 s, followed by 40 cycles at 94 °C for 5 s, 52 °C for 5 s and 80°C for 5 s, 5 cycles at 52°C for 5 s, 80°C for 5 s, and then a final exposure to 72°C for 3 min.
The primers for the PCR reaction were designed using Assay Designer 3.1 software and synthesized by a biotechnology company. Table 1 demonstrates the primer sequences of encoding miRNA genes polymorphic loci, and the genotype plots of seventeen SNPs are shown in Figure 1.

Figure 1 The genotype plots of miRNA polymorphisms. The X and Y axes represent the kurtosis of genotypes, respectively. The red square represents no call; the blue upright triangle, the green square, and the orange inverted triangle represent three different genotypes, respectively. In a good clustering plot, the two homozygous types are close to the horizontal axis and the vertical axis, while the heterozygous types are between them, showing a 45-degree angle. Clustering performance shows the evaluation of clustering efficiency, which ranges from 0 to 1.
SPSS 21.0 software was used to analyze all the data (SPSS Inc., Chicago, IL, USA). The distribution of population characteristics and genotypes between two groups was analyzed using a chi-square test or t-test. A logistic regression model was used to calculate adjusted odds ratios (OR) and 95% confidence intervals (CI). The level of statistical significance was set at two-sided α = 0.05.
The characteristics of the study population are presented in Table 2. In total, 170 controls and 169 cases were included in these analyses. The ages ranged from 18 to 85 years in cases, and the age in the cases (48.18 ± 15.16) was significantly younger than that in the controls (51.59 ± 11.08) (P= 0.019). The proportion of smokers in cases (16.6%) was less than that in controls (25.3%) (P = 0.048). The proportion of drinking status in cases (8.9%) was less than that in the controls (17.6%) (P = 0.017). There were no significant differences in gender between the two groups (P = 0.960).
The results of the Hardy–Weinberg balance shown in Table 3 demonstrate that the genotype distribution for each genetic polymorphism locus did not deviate (P >0.05), and the allele frequencies were similar to those of Asians in the International Human Genome HapMap Project, suggesting that the controls had representativeness. As shown in Table 4, there was a statistically significant difference in miR-206 rs17578851, miR-671 rs1870238, and miR-671 rs2446065 between the STSs and controls (P <0.05). Besides, there were no significant differences in other genotypic frequencies between these two groups.
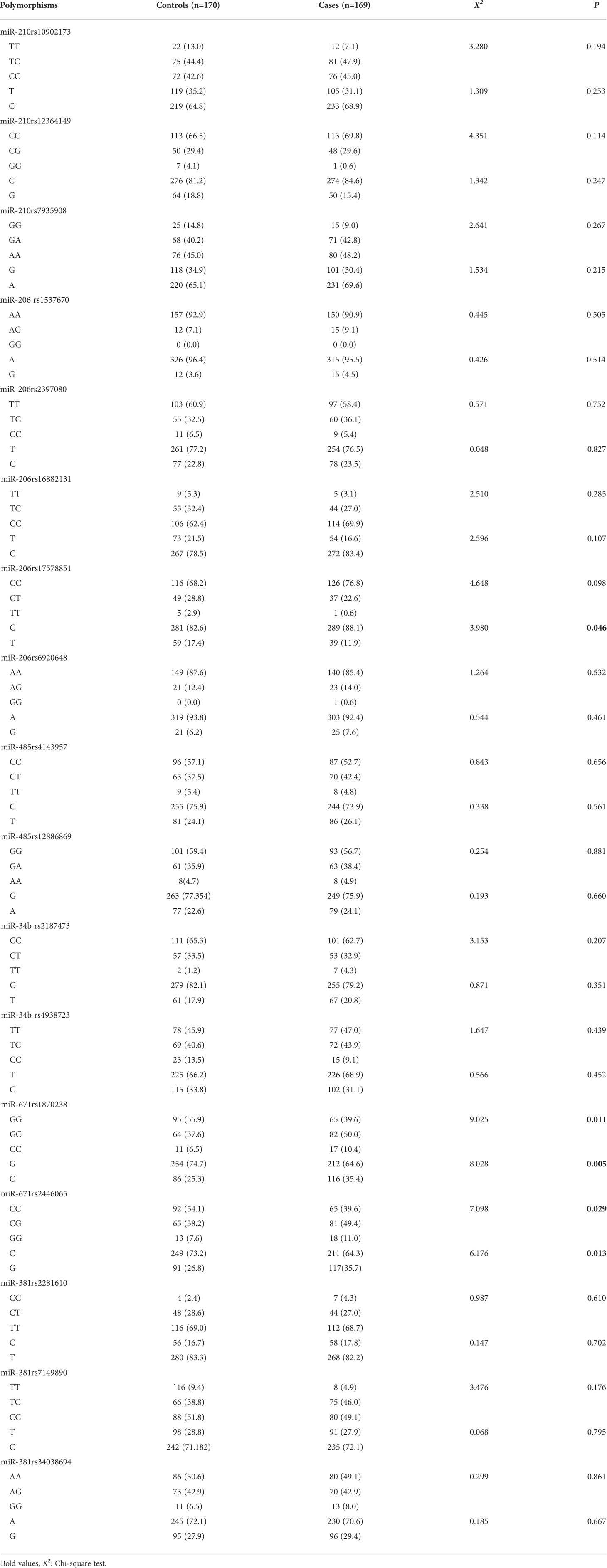
Table 4 The distribution of miRNA genetic polymorphism between the control group and the case group.
The polymorphisms for miRNA and their ORs and 95% CI in STSs are shown in Table 5. Regarded wild homozygous as the reference group, miR-671 rs1870238GC+CC had a 1.929-fold (OR = 1.929, 95% CI = 1.248–2.982, P = 0.003) increased risk of STSs, and a 1.963-fold (OR =1.963, 95% CI = 1.258–3.064, P = 0.003) increased risk of STSs after adjusting for age, smoking status, and drinking status. The results also showed that miR-671 rs2446065 CG+GG had a 1.796-fold (OR = 1.796, 95% CI = 1.163–2.774, P = 0.008) increased risk of STSs and a 1.838-fold (OR = 1.838, 95% CI = 1.178–2.868, P = 0.007) increased risk of STSs after adjusting for age, smoking status, and drinking status. Moreover, there was no association between other locus polymorphisms for miRNA and the risk of STSs.
This hospital-based case–control study showed a significant association between the miR-671 polymorphism (rs1870238 and rs2446065) and STS risk in humans from Henan Province, China. Individuals carrying the heterozygous GC genotype or with the homozygous CC genotype in rs1870238 had a 1.929-fold increased risk of developing STSs compared with individuals with the GG wild-type. Individuals carrying the heterozygous CG genotype or with the homozygous GG genotype in rs1870238 had a 1.796-fold increased risk of developing STSs compared with individuals with the CC wild-type.
STSs include more than 70 histological subtypes that may occur at any age. Among these different heterogeneous subtypes of STSs, aggressive high-grade malignancies often arise in adolescents and young adults, such as rhabdomyosarcoma, synovial sarcoma, Ewing sarcoma, and osteosarcoma (21).In this study, the age of the cases ranged from 18 to 85 years old, and the age of the cases (48.18 ± 15.16) was lower than the median diagnosis age of STSs, which is generally 60 years. We speculated that the difference between the actual mean age in the cases and the mean age at diagnosis of STSs may be due to the Berkson bias and lots of histological subtypes. Recently, a study also indicated that the peak or average age of STS onset varies with different histological subtypes. For example, Aaron et al. (2021) reported that synovial sarcoma presents at a younger mean age of 39 years at diagnosis (22). This study case had numerous synovial sarcomas, which may decrease the age of cases. Besides, a case–control study indicated that smoking and alcohol were potential risk factors for sarcomas (23). Therefore, we further analyzed the association between polymorphism in the miRNA gene and the susceptibility of STSs adjusted for age, smoking status, and drinking status and found that miR-671rs1870238GC+CC and miR-671 rs2446065 CG+GG may be risk factors for STSs (OR = 1.963, 95% CI = 1.258–3.064, P = 0.003 and OR = 1.838, 95% CI = 1.178–2.868, P = 0.007, respectively).
MiR-671, located at 7q36.1, serves as a suppressor or an oncogene in different tumors and plays a vital role in the biological process of many types of cancer, particularly in osteosarcoma (24), breast cancer (25), and non-small cell lung cancer (26). MiR-671 could directly target microfibril-associated glycoproteins (26), tripartite motif 14 (27), forkhead box protein P2 (28) and M1 (25), TNF receptor-associated factor 3 (29), and cyclin D2 (30), involved in the proliferation, migration, and invasion of tumor cells. Besides, miR-671 may also regulate the PI3k/Akt signaling (31) and the Wnt signaling pathways (32) that are closely related to the occurrence and development of cancers. miR-671 plays a crucial role in the oncogenesis of tumors by silencing SMARCB1 which manifests frequently with a loss-of-function mutation in malignant neoplasms (33). Furthermore, in previous studies, overexpression of miR-671 was found in epithelioid sarcomas (34), malignant peripheral nerve sheath tumors, and synovial sarcomas (17), implicating it as a promising therapeutic target for sarcomas. However, the association between genetic polymorphisms of miR-671 and the risk of STSs is ill-defined. In this study, we first identified that rs1870238 and rs2446065 of miR-671 were found to be associated with STS risk, which raised the most concerns and warranted further study. This evidence promotes the potential application of miR-671 polymorphisms as a clinical biomarker, giving us new hope for the early prevention and diagnosis of STSs.
MiR-206, located at 6p12.2, belongs to one of the muscle-specific miRNAs. MiR-206 could suppress myogenic differentiation and muscle cell proliferation through inhibition of DNA synthesis (35). Therefore, an inhibitory effect on cell growth can be observed following a forced expression of miR-206 in rhabdomyosarcoma, which is one kind of myogenic sarcoma, both in vitro and in vivo (36, 37). MiR-206, highly expressed in normal skeletal muscle, was downregulated in leiomyosarcoma, normal smooth muscle (38), and rhabdomyosarcoma (37, 39). In particular, lowmiR-206 expression correlated with poor overall survival in metastatic embryonal rhabdomyosarcoma and alveolar rhabdomyosarcoma cases without PAX3/7–FOXO1 fusion genes (40). However, MiR-206 was found to be overexpressed in epithelioid sarcomas (34) and sera of rhabdomyosarcoma patients (41). One possible reason for the controversial result is that the reference person or tissue was different in the above studies. Muscle-specific miRNA levels were usually lower in rhabdomyosarcoma compared with skeletal muscle but generally higher than in other normal tissues. A study provided evidence that miR-206 rs6920648 (HR = 0.77 (95% CI = 0.61–0.97, p-value = 0.02) was associated with breast cancer survival (42). But there is no study on the association of miR-206 gene polymorphisms with STS risk. In this study, the results showed the loci distribution of miR-206 rs17578851 between the cases and controls was a significant difference (P <0.05) through the chi-square test. However, there was no association between miR-206 rs17578851 and STS risk with logistic regression analysis.
Both miR-210 and miR-34b are located on chromosomal 11. MiR-210 is a well-known responder to hypoxia, which is expressed in a wide range of cells and involves numerous biological processes (43). A previous study showed that miR-210 could directly regulate HIF3α in hypoxia-responsive STSs (18). Besides, miR-210 was upregulated in malignant peripheral nerve sheath tumors compared with neurofibromas (44). Furthermore, hypoxia-induced miR-210 promoter demethylation enhances proliferation, autophagy, and angiogenesis of schwannoma cells (45). MiR-210 was also found to decrease expression in angiosarcoma cells both in vivo and in vitro, and was associated with angiosarcoma cell proliferation by target E2F3 and ephrin A3 (46). The expression levels of miR-210 were correlated with the prognosis and age of tumor onset in a gender-specific manner in STS patients (47). MiR-34b was silenced in numerous cancers by DNA methylation of its promoter (48) and became an important tumor suppressor in many sarcoma types. For instance, miR-34b is downregulated in synovial Sarcoma relative to other sarcomas (5), the methylation levels of miR-34b were associated with STSs clinical stage (19), and miR-34a levels inversely correlate with poor patient survival outcomes indicating its potential role as a diagnostic marker in Ewing sarcoma (49). However, a recent study showed that miR-34b expression levels were significantly higher in Ewing’s sarcoma tumors compared to normal tissue and acted as a tumor oncogene, promoting Ewing’s sarcoma cell proliferation, migration, and invasion by downregulating Notch1 (50). Furthermore, miR-34b polymorphisms have provided evidence of association with cancer risk and survival. For example, Jeannette et al. (2013) found that miR-34b rs4938723 was associated with breast cancer survival (42). Qi et al. (2014) suggested that miR-34b rs4938723 was a susceptible locus for hepatocellular cancer and colorectal cancer (51) and many other studies have also shown that miR-34b rs4938723 variant may be a disk factor for the development of prostate cancer (52) and acute lymphoblastic leukemia (53). However, there was no study on the association of miR-210 and miR-34b polymorphisms with STS risk. Our study did not find an association between miR-210 and miR-34b polymorphisms and the risk of STSs either.
Both miR-485 and miR-381 are located on chromosomal 14. Little is known about the relationship between miR-485 and miR-381 and STSs. In previous studies, miR-485 was reported to be decreased in osteosarcoma cells, and overexpression of miR-485-3p restrained osteosarcoma cell proliferation, migration, and sphere formation (54). Besides, miR-485 could indirectly regulate HIF3α in hypoxia-responsive STSs (18). MiR-485 has also been found to be associated with drug-resistant rhabdomyosarcoma (55). As for miR-381, one study reported that miR-381 overexpression in epithelioid sarcomas (34). There was no study on the association of miR-485 and miR-381 polymorphisms with STS risk. Our study did not find an association between miR-485 and miR-381 polymorphisms and STS risk either.
However, there are still some limitations to our study. Firstly, miRNA-SNPs in each pathological classification of STSs were not analyzed due to the limited sample of different pathological types. Secondly, further research is needed to elucidate the target gene and mechanism of miR-671 rs1870238 and rs1870238 in STSs. Thirdly, there are racial differences in gene polymorphism, and the results need to be further verified in other populations.
miR-671 rs1870238 GC+CC and miR-671 rs2446065 CG+GG may be genetic risk factors for STSs, suggesting that individuals carrying the GC+CC genotype for miR-671 rs1870238 or the CG+GG genotype for miR-671 rs2446065 are susceptible to STSs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was reviewed and approved by the Ethical Committee of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
PZ designed this research and wrote this paper. XL and FH analyzed data. LH and XN collected the blood samples. YS and WY instructed this study. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was financially supported by the grant of Scientific and Technological Innovation Outstanding Young Talent Training Project from the Health Commission of Henan Province (No. YXKC2021031).
All the authors thank all the subjects who voluntarily joined this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brownstein JM, DeLaney TF. Malignant soft-tissue sarcomas. Hematol Oncol Clin North Am (2020) 34(1):161–75. doi: 10.1016/j.hoc.2019.08.022
2. Fujiwara T, Kunisada T, Takeda K, Uotani K, Yoshida A, Ochiya T, et al. Micrornas in soft tissue sarcomas: Overview of the accumulating evidence and importance as novel biomarkers. BioMed Res Int (2014) 2014:592868. doi: 10.1155/2014/592868
3. Lu TX, Rothenberg ME. Microrna. J Allergy Clin Immunol (2018) 141(4):1202–7. doi: 10.1016/j.jaci.2017.08.034
4. Nigita G, Acunzo M, Romano G, Veneziano D, Lagana A, Vitiello M, et al. Microrna editing in seed region aligns with cellular changes in hypoxic conditions. Nucleic Acids Res (2016) 44(13):6298–308. doi: 10.1093/nar/gkw532
5. Renner M, Czwan E, Hartmann W, Penzel R, Brors B, Eils R, et al. Microrna profiling of primary high-grade soft tissue sarcomas. Genes Chromosomes Cancer (2012) 51(11):982–96. doi: 10.1002/gcc.21980
6. Guled M, Pazzaglia L, Borze I, Mosakhani N, Novello C, Benassi MS, et al. Differentiating soft tissue leiomyosarcoma and undifferentiated pleomorphic sarcoma: A mirna analysis. Genes Chromosomes Cancer (2014) 53(8):693–702. doi: 10.1002/gcc.22179
7. Zhang P, Huang L, Ma P, Niu X. Altered expressions of Nf1 and Nf1-related micrornas as biomarkers in the diagnosis of undifferentiated pleomorphic sarcoma. Front Genet (2022) 13:870191. doi: 10.3389/fgene.2022.870191
8. Zhao H, Yan P, Wang J, Zhang Y, Zhang M, Wang Z, et al. Clinical significance of tumor mir-21, mir-221, mir-143, and mir-106a as biomarkers in patients with osteosarcoma. Int J Biol Markers (2019) 34(2):184–93. doi: 10.1177/1724600819843537
9. Yoshitaka T, Kawai A, Miyaki S, Numoto K, Kikuta K, Ozaki T, et al. Analysis of micrornas expressions in chondrosarcoma. J Orthop Res (2013) 31(12):1992–8. doi: 10.1002/jor.22457
10. Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- rna genes Mir15 and Mir16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A (2002) 99(24):15524–9. doi: 10.1073/pnas.242606799
11. Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, et al. Micrornas exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci U S A (2006) 103(24):9136–41. doi: 10.1073/pnas.0508889103
12. Mishra PJ, Mishra PJ, Banerjee D, Bertino JR. Mirsnps or mir-polymorphisms, new players in microrna mediated regulation of the cell: Introducing microrna pharmacogenomics. Cell Cycle (2008) 7(7):853–8. doi: 10.4161/cc.7.7.5666
13. Zhang Q, Xu X, Wu M, Qin T, Wu S, Liu H. Mirna polymorphisms and hepatocellular carcinoma susceptibility: A systematic review and network meta-analysis. Front Oncol (2020) 10:562019. doi: 10.3389/fonc.2020.562019
14. Lee HC, Yang CW, Chen CY, Au LC. Single point mutation of microrna may cause butterfly effect on alteration of global gene expression. Biochem Biophys Res Commun (2011) 404(4):1065–9. doi: 10.1016/j.bbrc.2010.12.114
15. Park JH, Jeong GH, Lee KS, Lee KH, Suh JS, Eisenhut M, et al. Genetic variations in microrna genes and cancer risk: A field synopsis and meta-analysis. Eur J Clin Invest (2020) 50(4):e13203. doi: 10.1111/eci.13203
16. Mihaly D, Papp G, Mervai Z, Reszegi A, Tatrai P, Szaloki G, et al. The oncomir face of microrna-206: A permanent mir-206 transfection study. Exp Biol Med (Maywood) (2018) 243(12):1014–23. doi: 10.1177/1535370218795406
17. Sapi Z, Papp G, Szendroi M, Papai Z, Plotar V, Krausz T, et al. Epigenetic regulation of Smarcb1 by mir-206, -381 and -671-5p is evident in a variety of Smarcb1 immunonegative soft tissue sarcomas, while mir-765 appears specific for epithelioid sarcoma. a mirna study of 223 soft tissue sarcomas. Genes Chromosomes Cancer (2016) 55(10):786–802. doi: 10.1002/gcc.22379
18. Gits CM, van Kuijk PF, de Rijck JC, Muskens N, Jonkers MB, van IWF, et al. Microrna response to hypoxic stress in soft tissue sarcoma cells: Microrna mediated regulation of Hif3alpha. BMC Cancer (2014) 14:429. doi: 10.1186/1471-2407-14-429
19. Xie Y, Zong P, Wang W, Liu D, Li B, Wang Y, et al. Hypermethylation of potential tumor suppressor mir-34b/C is correlated with late clinical stage in patients with soft tissue sarcomas. Exp Mol Pathol (2015) 98(3):446–54. doi: 10.1016/j.yexmp.2015.03.017
20. Zhang P, Liu J, Li X, Gao M, Feng F, Wang W, et al. Nf1 and pten gene polymorphisms and the susceptibility to soft tissue sarcomas in a Chinese population: A case-control study. Exp Mol Pathol (2021) 118:104603. doi: 10.1016/j.yexmp.2021.104603
21. Ferrari A, Sultan I, Huang TT, Rodriguez-Galindo C, Shehadeh A, Meazza C, et al. Soft tissue sarcoma across the age spectrum: A population-based study from the surveillance epidemiology and end results database. Pediatr Blood Cancer (2011) 57(6):943–9. doi: 10.1002/pbc.23252
22. Gazendam AM, Popovic S, Munir S, Parasu N, Wilson D, Ghert M. Synovial sarcoma: A clinical review. Curr Oncol (2021) 28(3):1909–20. doi: 10.3390/curroncol28030177
23. Nabi S, Kahlon P, Kuriakose P. Analyzing multiple risk factors in patients with sarcomas. a case-control study. Indian J Cancer (2015) 52(3):337–42. doi: 10.4103/0019-509X.176752
24. Ma C, Nie ZK, Guo HM, Kong Y. Mir-671-5p plays a promising role in restraining osteosarcoma cell characteristics through targeting Tuft1. J Biochem Mol Toxicol (2020) 34(7):e22490. doi: 10.1002/jbt.22490
25. Tan X, Li Z, Ren S, Rezaei K, Pan Q, Goldstein AT, et al. Dynamically decreased mir-671-5p expression is associated with oncogenic transformation and radiochemoresistance in breast cancer. Breast Cancer Res (2019) 21(1):89. doi: 10.1186/s13058-019-1173-5
26. Ye J, Luo W, Luo L, Zhai L, Huang P. Microrna6715p inhibits cell proliferation, migration and invasion in nonsmall cell lung cancer by targeting Mfap3l. Mol Med Rep (2022) 25(1):30–7. doi: 10.3892/mmr.2021.12546
27. Wang WJ, Yuan Y, Zhang D, Liu P, Liu F. Mir-671-5p repressed progression of papillary thyroid carcinoma Via Trim14. Kaohsiung J Med Sci (2021) 37(11):983–90. doi: 10.1002/kjm2.12424
28. Li ZY, Zhang ZZ, Bi H, Zhang QD, Zhang SJ, Zhou L, et al. Upregulated Microrna6713p promotes tumor progression by suppressing forkhead box P2 expression in nonsmallcell lung cancer. Mol Med Rep (2019) 20(4):3149–59. doi: 10.3892/mmr.2019.10563
29. Liu Z, Chen S, Yang Y, Lu S, Zhao X, Hu B, et al. Microrna6713p regulates the development of knee osteoarthritis by targeting Traf3 in chondrocytes. Mol Med Rep (2019) 20(3):2843–50. doi: 10.3892/mmr.2019.10488
30. Yao Y, Zhou Y, Fu X. Mir6713p is downregulated in nonsmall cell lung cancer and inhibits cancer progression by directly targeting Ccnd2. Mol Med Rep (2019) 19(3):2407–12. doi: 10.3892/mmr.2019.9858
31. Zhu Q, Zhang X, Zai HY, Jiang W, Zhang KJ, He YQ, et al. Circslc8a1 sponges mir-671 to regulate breast cancer tumorigenesis via Pten/Pi3k/Akt pathway. Genomics (2021) 113(1 Pt 1):398–410. doi: 10.1016/j.ygeno.2020.12.006
32. Xiong DD, Chen H, He RQ, Lan AH, Zhong JC, Chen G, et al. Microrna-671-3p inhibits the development of breast cancer: A study based on in vitro experiments, in-house quantitative polymerase chain reaction and bioinformatics analysis. Int J Oncol (2018) 52(6):1801–14. doi: 10.3892/ijo.2018.4339
33. Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of Ini1 in atypical teratoid and rhabdoid tumors. Cancer Res (1999) 59(1):74–9. doi: 10.5336/pediatr.2019-72772
34. Papp G, Krausz T, Stricker TP, Szendroi M, Sapi Z. Smarcb1 expression in epithelioid sarcoma is regulated by mir-206, mir-381, and mir-671-5p on both mrna and protein levels. Genes Chromosomes Cancer (2014) 53(2):168–76. doi: 10.1002/gcc.22128
35. Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, et al. Microrna-1 and microrna-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol (2010) 190(5):867–79. doi: 10.1083/jcb.200911036
36. Taulli R, Bersani F, Foglizzo V, Linari A, Vigna E, Ladanyi M, et al. The muscle-specific microrna mir-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J Clin Invest (2009) 119(8):2366–78. doi: 10.1172/JCI38075
37. Yan D, Dong Xda E, Chen X, Wang L, Lu C, Wang J, et al. Microrna-1/206 targets c-met and inhibits rhabdomyosarcoma development. J Biol Chem (2009) 284(43):29596–604. doi: 10.1074/jbc.M109.020511
38. Subramanian S, Lui WO, Lee CH, Espinosa I, Nielsen TO, Heinrich MC, et al. Microrna expression signature of human sarcomas. Oncogene (2008) 27(14):2015–26. doi: 10.1038/sj.onc.1210836
39. Li L, Sarver AL, Alamgir S, Subramanian S. Downregulation of micrornas mir-1, -206 and -29 stabilizes Pax3 and Ccnd2 expression in rhabdomyosarcoma. Lab Invest (2012) 92(4):571–83. doi: 10.1038/labinvest.2012.10
40. Missiaglia E, Shepherd CJ, Patel S, Thway K, Pierron G, Pritchard-Jones K, et al. Microrna-206 expression levels correlate with clinical behaviour of rhabdomyosarcomas. Br J Cancer (2010) 102(12):1769–77. doi: 10.1038/sj.bjc.6605684
41. Miyachi M, Tsuchiya K, Yoshida H, Yagyu S, Kikuchi K, Misawa A, et al. Circulating muscle-specific microrna, mir-206, as a potential diagnostic marker for rhabdomyosarcoma. Biochem Biophys Res Commun (2010) 400(1):89–93. doi: 10.1016/j.bbrc.2010.08.015
42. Bensen JT, Tse CK, Nyante SJ, Barnholtz-Sloan JS, Cole SR, Millikan RC. Association of germline microrna snps in pre-mirna flanking region and breast cancer risk and survival: The Carolina breast cancer study. Cancer Causes Control (2013) 24(6):1099–109. doi: 10.1007/s10552-013-0187-z
43. Bavelloni A, Ramazzotti G, Poli A, Piazzi M, Focaccia E, Blalock W, et al. Mirna-210: A current overview. Anticancer Res (2017) 37(12):6511–21. doi: 10.21873/anticanres.12107
44. Presneau N, Eskandarpour M, Shemais T, Henderson S, Halai D, Tirabosco R, et al. Microrna profiling of peripheral nerve sheath tumours identifies mir-29c as a tumour suppressor gene involved in tumour progression. Br J Cancer (2013) 108(4):964–72. doi: 10.1038/bjc.2012.518
45. Wang Z, Deng M, Liu Z, Wu S. Hypoxia-induced mir-210 promoter demethylation enhances proliferation, autophagy and angiogenesis of schwannoma cells. Oncol Rep (2017) 37(5):3010–8. doi: 10.3892/or.2017.5511
46. Nakashima S, Jinnin M, Kanemaru H, Kajihara I, Igata T, Okamoto S, et al. The role of mir-210, E2f3 and ephrin A3 in angiosarcoma cell proliferation. Eur J Dermatol (2017) 27(5):464–71. doi: 10.1684/ejd.2017.3084
47. Greither T, Wurl P, Grochola L, Bond G, Bache M, Kappler M, et al. Expression of microrna 210 associates with poor survival and age of tumor onset of soft-tissue sarcoma patients. Int J Cancer (2012) 130(5):1230–5. doi: 10.1002/ijc.26109
48. Vogt M, Munding J, Gruner M, Liffers ST, Verdoodt B, Hauk J, et al. Frequent concomitant inactivation of mir-34a and mir-34b/C by cpg methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch (2011) 458(3):313–22. doi: 10.1007/s00428-010-1030-5
49. Marino MT, Grilli A, Baricordi C, Manara MC, Ventura S, Pinca RS, et al. Prognostic significance of mir-34a in Ewing sarcoma is associated with cyclin D1 and ki-67 expression. Ann Oncol (2014) 25(10):2080–6. doi: 10.1093/annonc/mdu249
50. Lu Q, Lu M, Li D, Zhang S. Microrna34b promotes proliferation, migration and invasion of ewing's sarcoma cells by downregulating Notch1. Mol Med Rep (2018) 18(4):3577–88. doi: 10.3892/mmr.2018.9365
51. Liu Q, Yang G, Song XL, Wang Z, Shi G. Association between Rs4938723 functional polymorphism in the promoter region of mir-34b/C gene and cancer risk. Clin Res Hepatol Gastroenterol (2015) 39(4):526–33. doi: 10.1016/j.clinre.2014.10.007
52. Hashemi M, Danesh H, Bizhani F, Narouie B, Sotoudeh M, Nouralizadeh A, et al. Pri-Mir-34b/C Rs4938723 polymorphism increased the risk of prostate cancer. Cancer biomark (2017) 18(2):155–9. doi: 10.3233/CBM-160058
53. Tong N, Chu H, Wang M, Xue Y, Du M, Lu L, et al. Pri-Mir-34b/C Rs4938723 polymorphism contributes to acute lymphoblastic leukemia susceptibility in Chinese children. Leuk Lymphoma (2016) 57(6):1436–41. doi: 10.3109/10428194.2015.1092528
54. Du K, Zhang X, Lou Z, Guo P, Zhang F, Wang B, et al. Microrna485-3p negatively regulates the transcriptional Co-repressor Ctbp1 to control the oncogenic process in osteosarcoma cells. Int J Biol Sci (2018) 14(11):1445–56. doi: 10.7150/ijbs.26335
Keywords: miRNA-671, soft tissue sarcoma, rs1870238, rs2446065, gene polymorphisms
Citation: Zhang P, Li X, Huang L, Hu F, Niu X, Sun Y and Yao W (2022) Association between microRNA 671 polymorphisms and the susceptibility to soft tissue sarcomas in a Chinese population. Front. Oncol. 12:960269. doi: 10.3389/fonc.2022.960269
Received: 02 June 2022; Accepted: 18 July 2022;
Published: 09 August 2022.
Edited by:
Claudio Sette, Catholic University of the Sacred Heart, ItalyReviewed by:
Maria Anna Smolle, Medical University of Graz, AustriaCopyright © 2022 Zhang, Li, Huang, Hu, Niu, Sun and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Zhang, emR6cEB6enUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.