- 1Faculty of Electronics, Vilnius Gediminas Technical University (Vilnius TECH), Vilnius, Lithuania
- 2Department of Molecular and Cellular Biology, Faculty of Pharmacy, Wroclaw Medical University, Wroclaw, Poland
- 3Faculty of Medicine, Wroclaw Medical University, Wroclaw, Poland
Electric pulses are widely used in biology, medicine, industry, and food processing. Numerous studies indicate that electroporation (EP) is a pulse-dependent process, and the electric pulse shape and duration strongly determine permeabilization efficacy. EP protocols are precisely planned in terms of the size and charge of the molecules, which will be delivered to the cell. In reversible and irreversible EP applications, rectangular or sine, polar or bipolar pulses are commonly used. The usage of pulses of the asymmetric shape is still limited to high voltage and low voltage (HV/LV) sequences in the context of gene delivery, while EP-based applications of ultra-short asymmetric pulses are just starting to emerge. This review emphasizes the importance and role of the pulse shape for membrane permeabilization by EP.
The first waveforms for electroporation
Electroporation is a pulse-dependent phenomenon; therefore, the treatment can be controlled by changing the waveform, duration, a number of pulses, or burst delivery frequencies. Since the progress of using and developing new pulsing protocols depends on the electronics and the semiconductor technology behind the waveform generators, the respective evolution of electroporation in the past 50 years is also electronics-dependent.
Back in the day, the pulse generators (electroporators) frequently employed spark gaps as power switches to form the desired signal (1–4). As a result, in the case of capacitor discharge systems, the waveform featured an exponential decay form (1, 5) (see Figure 1A).
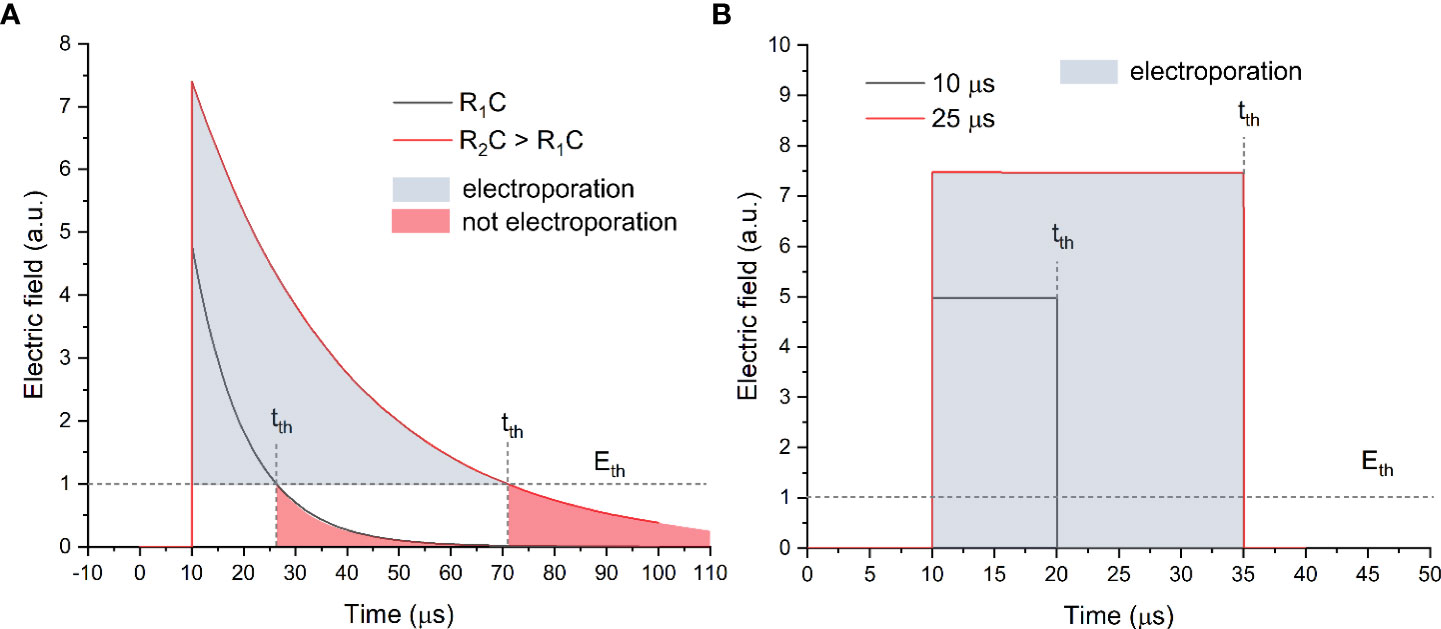
Figure 1 The exponential decay (A) and square wave (B) pulse waveforms, where tth is the time when the amplitude drop below the electric field value required for electroporation (Eth).
As can be seen in Figure 1A, the exponential decay waveform has a decaying tail, which depends on the RC parameters of the load. In order to control the duration of the pulse, a resistance (R) should be connected in series and/or the impedance of the biological load should be altered (which is not usually the case). Such an approach was used to generate pulses ranging from several microseconds to hundreds of milliseconds and, thus, trigger various rates of electroporation. Starting from the early 1980s, the exponential decay waveform electroporators feature solid-state electronics and employ silicon-controlled rectifiers to substitute the spark gap switches (6). However, the popularity of such a waveform in electroporation is constantly declining due to the complex control of the pulse duration and the energy losses. Electroporation is a threshold-type phenomenon; therefore, a certain electric field (Eth) is required for each type of cell to trigger plasma membrane permeabilization (7, 8). In the case of exponential decay pulses (refer to Figure 1A), the electric field amplitude drops below the Eth at a time moment tth, which implies that the energy is being used on other processes such as electrophoresis, electrolysis, and Joule heating rather than electroporation. Therefore, starting from the early 1990s, the tendency to employ square waves to trigger electroporation was observed (9–11). Indeed, due to sharp rise and fall times (refer to Figure 1B), the pulse resembles a square wave; therefore, the losses during the transient changes of the electric field are minimal; i.e., the electric field is above the Eth during the whole pulse. In the early days, the square wave pulsing was limited to transmission line systems (2, 12) until the mass availability of high power and high voltage transistors and the occurrence of commercial square wave electroporators.
Later, the square wave pulses became the dominant waveform to be used in electroporation studies. With the development of fast MOSFET and IGBT switches, the parametric protocol design started to feature unipolar and bipolar pulse delivery, duration control from nanoseconds to hundreds of milliseconds, and flexible frequency control. Some studies employ sine wave (13, 14) or bell-shape pulses (15) (16) to grasp the new opportunities in electroporation, but none of them have gone mainstream yet. So far, it has been established that square wave pulses are more effective for electroporation than the sinusoidal ones or other waveforms with slower rise and fall times (17).
Nevertheless, the square wave pulse features a square form only on paper. In reality, various transient processes occur, resulting in significant changes in the ideal shape. An example of transient processes for a 10-μs pulse is shown in Figure 2.
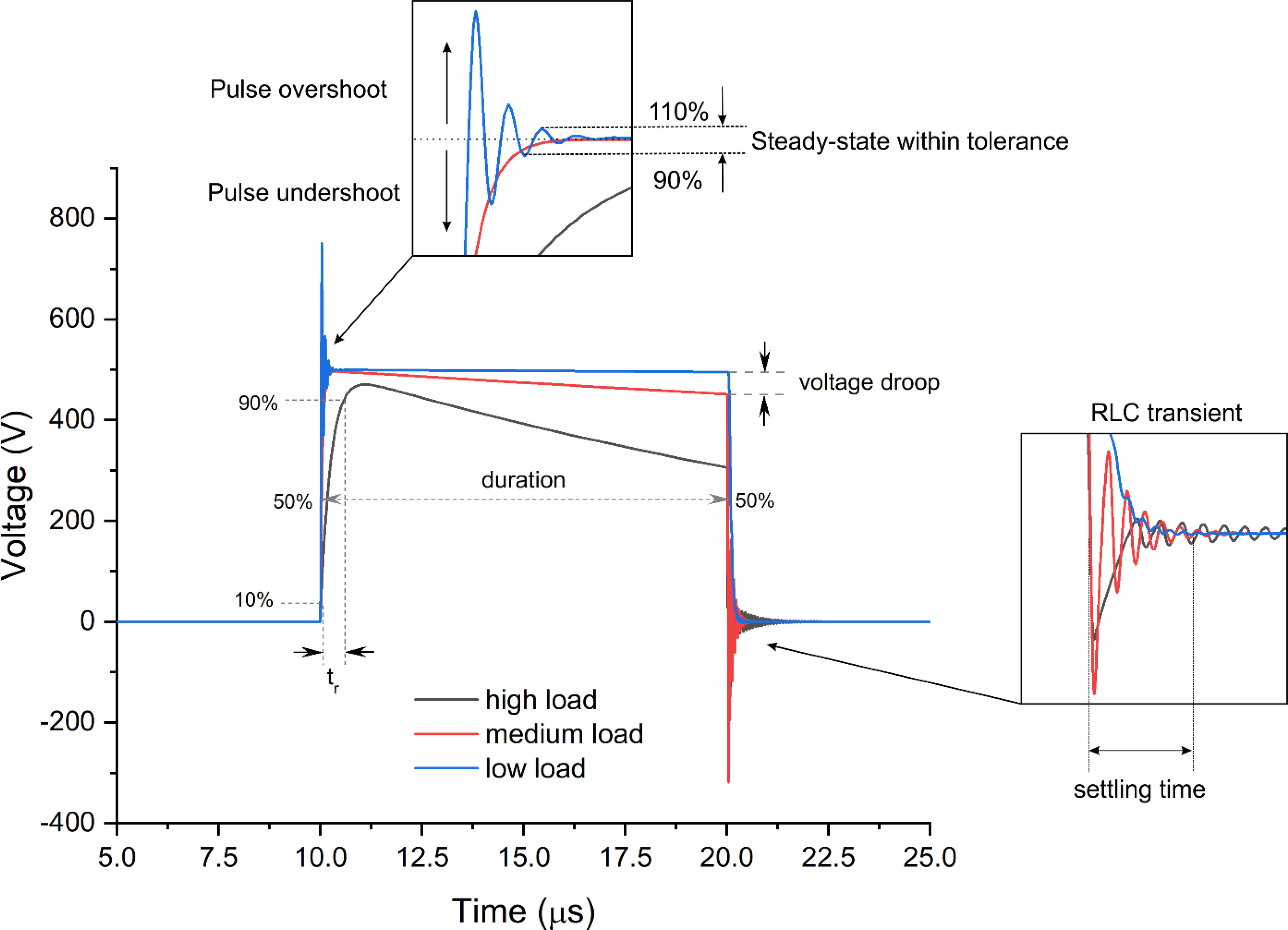
Figure 2 An example of typical transient processes occurrent in electroporation systems during pulse delivery, where tr is the pulse rise time and RLC is the resistor/inductor/capacitor circuit.
It can be seen that the shape of the pulse is load-dependent, which is dramatic in the context of electroporation. During electroporation, the bioimpedance is dynamic (18, 19), and the load of the generator both in vitro and in vivo depends on the experimental conditions (i.e., electrode gap, contact area, buffer or tissue conductivity, etc.). It implies that the same generator with the same pulse setting will deliver different pulses depending on the experiment or clinical setting. Considering the capacitive nature of the cell membrane, which is charged during the pulse to a specific threshold transmembrane potential (20), such differences in pulse shape may have a significant effect on the electroporation process and, in the end, on the treatment outcome. Therefore, adequate metrology for electroporation experiments and clinical application is required.
It should be noted that it is not the biological load that changes the pulse but rather the non-ideality of the inner electronic components of the generator (electroporator), which is employed in the treatment. Basically, the dominant majority of modern square wave electroporators will feature three major components highly influencing the pulse generation (1: the energy storage capacitor; 2: the semiconductor switch, and 3: the distributed parasitic capacitance and inductance of the circuit) (21, 22). The energy stored in the capacitor will influence how well the generator will handle various loads and pulse durations without a significant droop of voltage (refer to Figure 2). The bigger the capacitor, the smaller the droop; however, it is always a trade-off between the size of the electroporator, its price, and pulsing flexibility. Nowadays, a droop of voltage up to 10%–20% is usually tolerated in electroporation studies (23), while it should be understood that 10% is still a significant change in the electric field for a threshold-type phenomenon like electroporation.
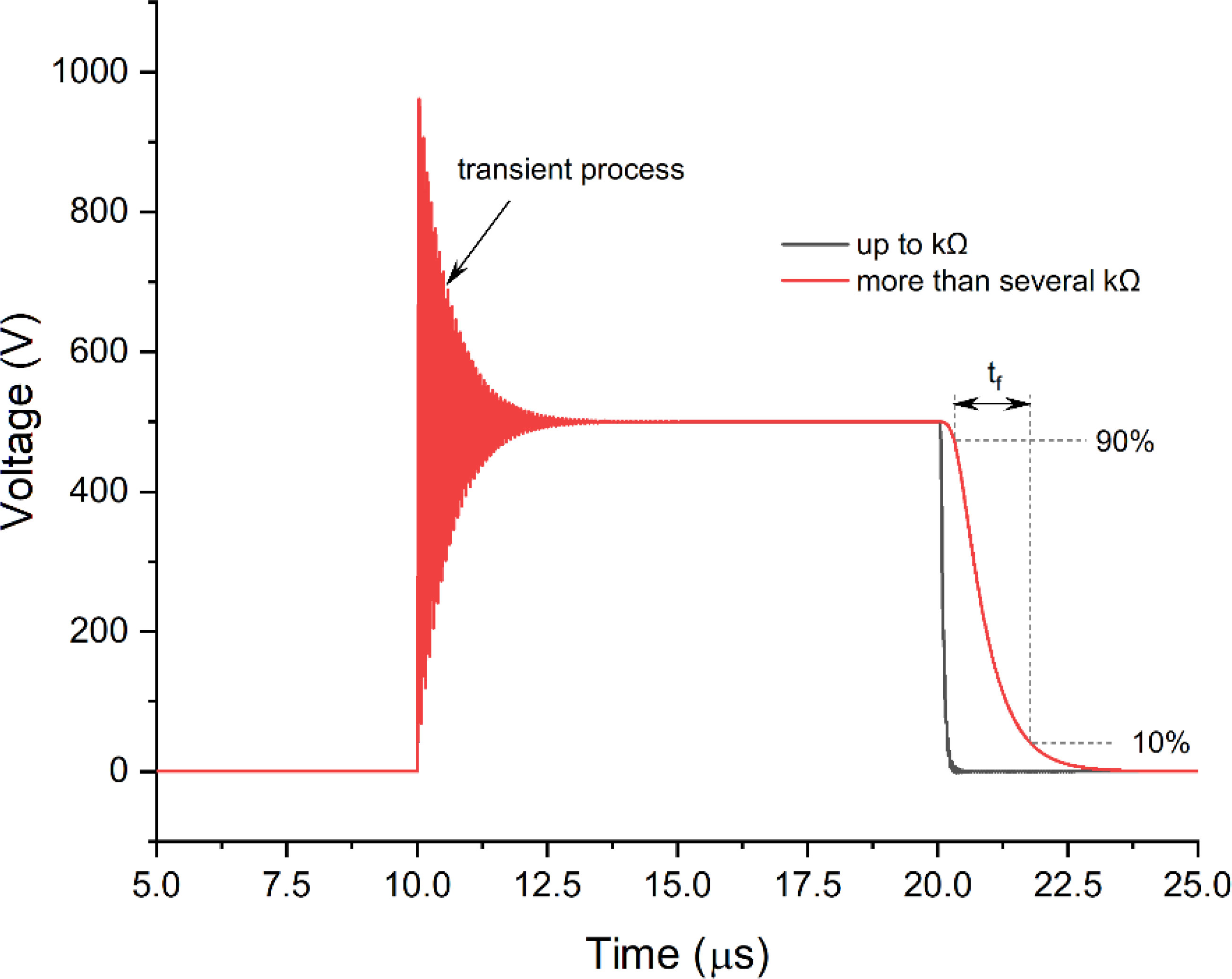
The semiconductors also play a role due to the dependence of the dynamic characteristics of the switches on the load. However, rise times (tr, Figure 2) and fall times (tf, Figure 3) are no longer an issue since modern transistors offer switching times in the sub-50-ns range, which is significantly faster than the polarization time of typical cells (24). An exception is the extremely low loads in the kOhm range, which may alter the rise time transient and fall times of the MOSFET or IGBT transistors (Figure 3).
A typical solution is to shunt the load with low parallel resistance (25) or introduce active circuitry to form the pulse independently on the load (26). In the first case, such a solution will influence higher voltage droop, and in the second case, the complexity of the circuit and its driving increase dramatically. However, it is obvious that if the high bioimpedance is left unmatched or uncompensated, the differences in pulse shape inevitably will alter the cellular response to the treatment.
The distributed parasitic RLC parameters are inevitable in any electrical circuit. The capacitive component is highly responsible for the rise-time transient (during low loads), while the parasitic inductance starts to alter the pulse significantly with high loads. In Figure 2, it can be seen that with the high load, the rise time becomes longer, and an RLC transient is present at the end of the pulse. Each generator has unique distributed parasitic parameters; therefore, the prediction of the exact pulse shape is complex and usually evaluated empirically. Lastly, it should be noted that the shorter the pulse, the harder it is to ensure a square waveform. It is typical for pulses in the sub-50-ns range that the waveform resembles a bell shape rather than a square wave (15), which is a limitation of state-of-the-art high-power semiconductors. Faster rise and fall times (sub-nanosecond range) can be ensured for low-power pulses, which are applicable in microscale electroporation studies (i.e., using lab-on-chip or microscope coverslips with micrometer-gap electrodes) (27).
With the understanding of the limitations of square wave pulse forming, the effects of various square wave pulses are further reviewed.
Unipolar square wave pulses
With the understanding of the benefits of square wave pulsing and considering the limitations of available semiconductors three decades ago, the micro-millisecond duration pulses started to dominate the field. In the context of cancer treatment, two main treatment approaches, including tissue ablation or irreversible electroporation (IRE) and electrochemotherapy, were heavily introduced into the clinics (28–31). In both cases, the microsecond and millisecond pulses were employed. Electrochemotherapy was more standardized, often featuring a series of 100-µs pulses to be used in the studies (32–34), later forming a base for ESOPE protocols (European Standard Operating Procedures of Electrochemotherapy) (35). In the case of IRE, the protocols were more flexible, including 100-µs procedures (36, 37). However, it also featured millisecond range pulses (29) and a variety of other microsecond and millisecond parametric protocols (38). It should be noted that depending on the applied pulse duration, the amplitude of the pulses should be adjusted, respectively. The ESOPE protocol with eight square wave pulses of 100 μs typically uses charging voltages of 100–1,000 V to trigger reversible electroporation in the 0.6–1.5 kV/cm electric field range. At the same time, IRE is an irreversible process; thus, the number of pulses and the amplitude of the pulse are increased. Frequently, sequences of more than 80 pulses are used with amplitudes at least two- to threefold higher than the ones used in ECT (39).
In the case of ECT, the ESOPE became a gold standard to be employed in research and clinics, which is still dominant in the context of electrochemotherapy (40–43). However, in the case of IRE, a tendency to reduce the duration of pulses nowadays is observed. Currently, typical unipolar IRE protocols deliver <200-μs pulses to prevent thermal damage and minimize tissue oxidation and muscle contractions (44–46).
In the early 2000s, the time for ultrashort pulsing in the context of electroporation came, and the scientific area of supra-electroporation was formed to develop non-thermal techniques for cell membrane permeabilization. Because of the ultra-short pulse duration, which is shorter than the plasma membrane charging time, the effects of sub-microsecond electric fields differ substantially from conventional micro-millisecond procedures. Due to the dramatic reduction of the pulse duration, the amplitude had to be increased (47). The ultra-short but high-intensity electric field pulses developed voltages across intracellular structures without destructive effects (48–50). It was noted that even short pulses as 3 ns can provoke poration of the lipid bilayer and the externalization of phosphatidylserine (PS), which was demonstrated experimentally and by MD simulations (51). Also, the significant differences in pore size, stability, and distribution between sub-microsecond and microsecond range pulses were indicated (52–55). Up to now, these features are mathematically predicted based on computer simulations, and further studies must be verified by experimental observations. The extremely high amplitude of the nanosecond pulses enabled more uniform permeabilization of the cell membrane, which is advantageous in heterogeneous tissues. Also, with the use of sub-microsecond pulses, modulation of cell death type became possible (i.e., trigger apoptosis), which can be employed to minimize inflammation of the tissue (56, 57). Finally, the potential to use nanosecond pulses in IRE applications for cancer treatment has been revealed (58–62). Nevertheless, the absolute majority of works featuring unipolar nanosecond pulses and electroporation were performed in vitro, which can be attributed to the lack of electroporators and technology to generate MV/m electric fields with bigger electrode gap systems required for in vivo or clinical applications (25, 63–66). Possibly due to the same reasons, the nanosecond electrochemotherapy was also hardly focused. Nevertheless, with the development of pulsed power technology, the potential of electrochemotherapy by nanosecond pulses has been highlighted in recent years, both in vitro, which can be attributed to the lack of electroporators and technology to generate MV/m electric fields with bigger and appropriate electrode gap systems required for in vivo or clinical applications. Thus, the electrode problem might also be the reason why nanosecond electrochemotherapy is still beyond the scope of practical application. Lastly, picosecond pulses are also in the field of interest. It is believed that the sub-nanosecond range of pulses (ps) can provoke mainly intracellular effects. Moreover, ultrashort pulse delivery is noninvasive and can be radiated by antennas (67, 68). Experimental studies demonstrated that the picosecond pulse induces the electroporation of intracellular structures while the cell membrane is unaffected (69). Semenov et al. studied picosecond pulses, which are shorter than channel activation time but can activate voltage-gated channels. It was shown that picosecond pulses could cause long-lasting opening of voltage-gated calcium channels by a mechanism different than electroporation (27). In the other study, intense picosecond electric pulses decreased mitochondrial transmembrane potential and induced apoptotic cell death, with caspase-3 and -9 activation (70). In turn, Zamponi et al. highlighted that picosecond electric pulses could be used to break down protein aggregates and affect neural stem cell differentiation, which can be used in neurodegeneration therapies (71). Thus, there is a high potential for ultrashort electric pulses, which can be used for the sensitive activation of the specific channels or organelles in cells.
Bipolar square wave pulses
In the 2000s, the electroporation techniques using bipolar, also called biphasic pulses, were focused on, while the first works were published in the early 1990s (72). The main motivation for using a more complex waveform (compared to a unipolar sequence) was to decrease the electrolysis and the contamination of the suspension with metal ions, improve the homogeneity of the treatment, and reduce the muscle contractions. It was shown that the bipolar pulses could be successfully used for cell membrane permeabilization, electrochemotherapy, and DNA transfection (73–77). However, considering the increased complexity and limited availability of the bipolar pulse generators until the early 2010s, the technique was hardly popular compared to ESOPE in electrochemotherapy. The benefits of using long (50+ µs) bipolar pulses (compared to ESOPE) were mainly to reduce the muscle electrical excitation, thus better accepted by patients (75, 78–82). Application of bipolar sequences in IRE was more common and used on a routine basis in many clinical applications (28, 83, 84). However, the true potential of bipolar square wave pulses is in the high-frequency domain using short pulses. Switching the pulse polarity with a frequency close to the charging time of plasma membranes can significantly improve the electroporation of heterogeneous tissues (85). Mitigation of impedance changes due to electroporation, and tissue heterogeneity is possible (18, 86, 87). Also, non-thermal ablation without muscle contraction can be ensured (88). With all the positive factors combined, a new electroporation-based technique called High-Frequency Irreversible Electroporation (H-FIRE) was born. However, soon it was determined that reversal of the pulses could weaken the effect (known as bipolar cancellation) due to the membrane depolarization (89, 90). Therefore, accurate tuning of the bipolar sequence is required, and modulation of the treatment via the interplay of delay between consequent opposite polarity pulses can be performed (91–93). The phenomenon of bipolar cancellation also formed a base for a new technique called CANCAN (cancellation of bipolar cancellation) when the nanosecond pulses can be structured and synchronized to achieve maximum effect remotely using interference targeting (94). It is particularly useful for targeting deep-seated tumors without collateral damage and is currently in the stage of development.
Nevertheless, the application of high-frequency bipolar pulses is still limited in the context of electrochemotherapy. Firstly, rapid bipolar pulses need larger amplitudes to disrupt cells, similar to longer monopolar pulses (95, 96). Secondly, the electrophoretic component of short bipolar pulses is negligible compared to ESOPE protocols (97). Thus, drug delivery is dominated by passive diffusion. However, when tuned right, the bipolar nanosecond pulse sequences can be as efficient as the standard electroporation protocols (25, 96).
CANCAN pulses
As it was mentioned above, the CANCAN effect stands for the cancellation of bipolar cancellation. The effect is achieved when two bipolar pulses overlap and the array overlay into a unipolar pulse between the system of electrodes (Figure 4A) (98). Most studies concerning the CANCAN technique focus on nanoseconds or short microsecond pulses (94). In general, unipolar nanosecond pulses exert several biological effects, i.e., cell death induction on the targeted cells, which is strictly related to membrane permeabilization, and ROS formation as a reaction due to stress (99). However, when the applied pulses remain bipolar, most of the mentioned effects do not occur due to the pulse cancellation from one phase by the pulse from the other phase (89, 90, 100–102). Studies by Sozer and Vernier proved that the mechanisms underlying the cancellation are the multistep process involving the charged species movement, the cellular homoeostatic response effectors, and cell repair mechanisms (103). Pakhomov et al. proved that the effects of bipolar cancellation remain the lowest or even absent at the lowest electric fields, close to the electroporation threshold (94). When the electroporation system requires the use of four electrodes in the specific array, two simultaneous bipolar pulses might be generated that would overlap each other (Figure 4B). In this case, the biological effect would arise from the combination of both pulses and not the stand-alone effect of a single bipolar pulsation (98).
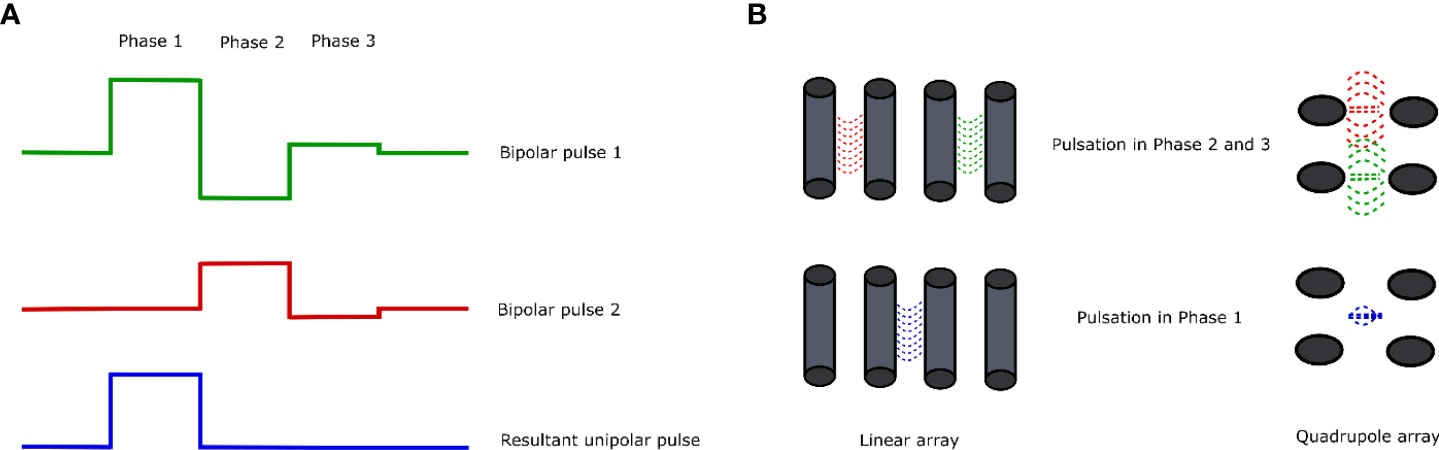
Figure 4 Overview of the CANCAN effect. (A) Summation of the bipolar pulses results in the formation of a unipolar pulse with the resultant pulse in Phase 1. (B) Two arrangements of electrodes for the study of the CANCAN effect and the effects of pulsation in each of the phases. The left side shows the linear arrangement of the needle electrodes; on the right side, the needle electrodes are arranged in a square—in each case, the distance between the neighboring needles remains constant.
Depending on the arrangement of electrodes for the generation of the CANCAN effect, the two most commonly used layouts could be distinguished (Figure 4B). The first one includes the quadrupole formation by inserting the electrodes in the rectangle array. In this way, two independent pulses might be generated that overlap in the rectangle’s middle. In this case, the biological effects of PEF treatment would be pronounced in the middle of the system—aside from the electrodes (98). Curiously, studies by Pakhomov et al. showed that even though the permeability of the cells towards Yo-Pro dye should not be highly exposed in the neighboring electrodes, indeed, it is (94). The quadrupole system is suitable for studies concerning targeting regions that do not directly neighbor the electrodes. The second layout of the electrodes is the linear one. This attempt is mainly used for research purposes. It was initially used to study the effects of bipolar pulses combined into a single unipolar pulse outside the electrodes.
Studies by Pakhomov et al. revealed several important pieces of information about the optimization of CANCAN protocol usage (94). The authors compared if the direction of the pulses in the quadrupole layout may modulate the occurrence of the CANCAN effect and found that the pulsation from both systems of electrodes should be delivered in the same direction. Only in this case was the CANCAN effect observed in the middle of the electrodes’ array. The authors also showed that the increase in the pulses’ delivery frequency exacerbated the system’s heating effect. Furthermore, the authors investigated the efficacy of CANCAN induction by the biphasic and triphasic pulses. The experiment showed that the effect is present when biphasic and triphasic pulses are combined without any time gap in their delivery. Namely, Pakhomov et al. delivered a sequence of pulses with a 50-ms gap and observed no CANCAN effect. Conversely, when the unipolar pulse formation arose from the overlap in the simultaneous pulsation with bi- and triphasic pulses, CANCAN was pronounced in the middle of the quadrupole electrodes’ system. The authors further investigated if the rotation of the electrodes would enhance the CANCAN effect and observed the symmetrical pattern of electroporation next to the pair of electrodes that produced the strong triphasic pulses. The amplification of the central CANCAN peak was observed as well. The authors also analyzed the effects of first phase pulse intensity and the effects of the pulse number on the uptake of YO-PRO-1 in the rotatory electrodes’ system. The study revealed that the increase in both parameters induces higher dye uptake in the center of the electrodes’ layout. Finally, the authors showed that the increase in the number of CANCAN stimuli induced an even cell killing area in the region encompassed by the electrodes and not only partially in the middle of the electrodes’ array. Gianulis et al. present several challenges in the application of CANCAN pulses (98). Namely, electric pulses can be distorted when traveling in various human fluid spaces, so the theoretical and realistic courses of electric pulses vary in time and space. Each medium in which we pass the electric current has a different conductivity (104); therefore, it is extremely hard to synchronize the pulses in time and space. Moreover, the electric field is higher near the electrodes; thus, tissue damage near the electrodes might occur at a lower applied voltage. The problem arises when we want to affect deep tissues while preventing tissues nearby electrodes from being damaged. Also, the effective distance from the electrodes for cell stimulation has to be enhanced. Recent advances came from Roth et al., who used deep H-coils, doubling the distance for deep tissue stimulation (105). The attempt, however, involves less precise targeting of the tissues. The other type of electrode setup involves the cathode ring surrounding an anode disc electrode. This arrangement may be used to stimulate brain tissue (106).
Asymmetric pulses
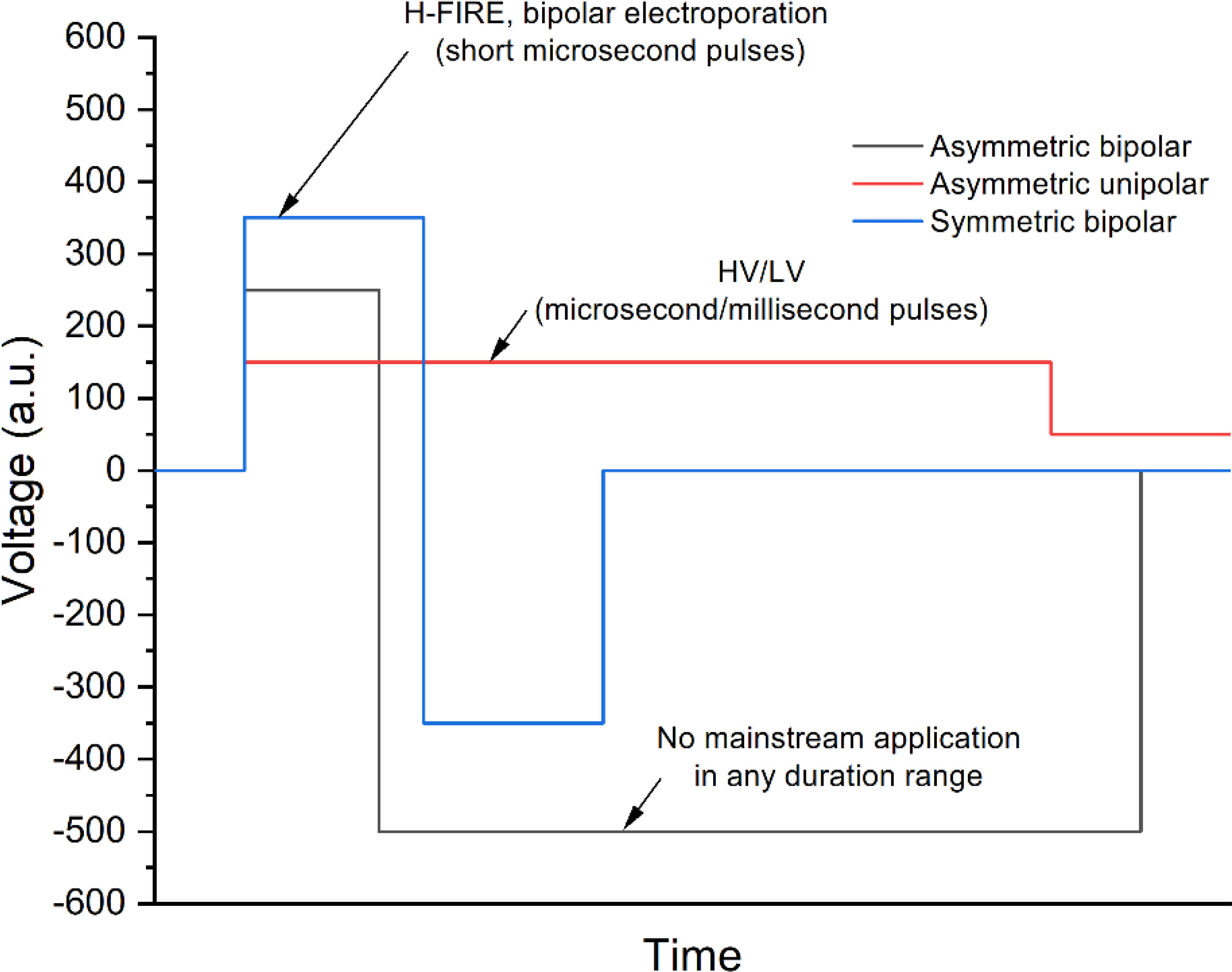
An asymmetric pulse means that the device can generate pulses of a positive and/or negative polarity where the pulse duration and pulse amplitude of a positive and/or negative polarity can be set independently, and the pause between both polarities can be changed (see Figure 5). The asymmetry can be ensured both in the intensity and in the duration space within the operation range of the device.
One of the most established techniques that employ asymmetric pulses is the high-voltage low-voltage methodology (HV/LV) for gene delivery (107–109). This technique uses the high-voltage microsecond range pulse (higher than the electroporation threshold) for permeabilization of cells and the low voltage (millisecond range pulse) for electrophoretic force induction. In such a way, the efficacy of transfection can be significantly improved since the delivery of DNA is dependent on the electrophoretic component of the treatment (110). The HV/LV method is hardly used in any other electroporation-based context. At the same time, the asymmetric bipolar pulses did not find any mainstream application yet. In the microsecond range, there are efforts to use such pulses for electrical isolation of cardiac tissue in atrial arrhythmias. Asymmetric high-frequency waveforms generate abrasions in cardiac tissue. Asymmetric high-frequency waveforms can create deeper lesions than a symmetric waveform with the same energy or a symmetric waveform with the same charge (111). Sano et al. demonstrated that the lethal threshold for H-FIRE treatments is also affected by the symmetry of the pulses. Asymmetric waveforms have significantly lower lethal thresholds than equivalent energy symmetric waveforms. Asymmetric high-frequency irreversible electroporation (H-FIRE) can be used to generate ablation volumes corresponding to standard IRE protocols (112). We speculate that the lack of works focusing on the asymmetric bipolar pulses is partly influenced by the lack of commercial and non-commercial generators able to generate such pulses. Nevertheless, the efforts to develop such technology are performed in a systemic manner. For example, Grainys et al. have developed a high-voltage, bipolar electroporator that generates single or multiple, symmetrical or asymmetrical, high-power square wave microsecond pulses up to ±1 kV and 100 A (113). Levkov et al. reported on a PEF system consisting of a high-voltage generator with an asymmetric voltage multiplying architecture and a treatment chamber with sliding electrodes for marine macroalgae electroporation (114). The system enables pulses of up to 4 kV and 1 kA with a pulse duration between 1 μs and 100 μs.
At the same time, the nanosecond asymmetric pulse range experimentally is hardly covered at all due to even more extreme technological challenges. However, in the past several years, at least three new systems have been reported. In 2021, Pirc et al. presented a prototype of the asymmetric bipolar pulse generator that can generate 4-kV pulses with 131-A maximal current, and duration as low as 200 ns (25). Kandratsyeu et al. presented a 6.5-kV asymmetric pulse generating device with a minimum pulse duration of 100 ns (115). Novickij et al. developed a device for 65 ns–100 µs asymmetric pulse generation in 2022 (26). It could be speculated that the high-energy asymmetric nanosecond pulses will elicit stronger and more selective effects on intracellular membranes than symmetrical pulses.
The effects of pulse repetition rate
The importance of pulse amplitude and duration in the context of electroporation cannot be overestimated. However, the frequency of the delivered pulses forms an additional degree of freedom in parametric protocol design. In the microsecond range, frequency manipulation is rarely performed, with most protocols relying on low frequency (1–10 Hz). The main reason is the lack of electrotransfer effectiveness increase in the kilohertz range and the problems associated with excitation of the nerve fibers, which causes higher torque of muscle contraction (78, 116). Operating with extremely slow pulse delivery protocols (0.1–1 Hz) triggers a phenomenon known as “electrosensitization” when the long delays between the pulse bursts influence a higher uptake of molecules (117, 118). The improved effectiveness of the treatment was confirmed in vivo, too (119). However, the technique is not commonly used yet. With the increase of frequency from 1 to 10 Hz, the density of the pores on the cellular membrane increases (120), resulting in the higher uptake of fluorescent markers for nanosecond and microsecond pulses (78, 121). Romeo et al. have also shown that nanosecond pulses delivered at 1-kHz repetition frequency result in higher YO-PRO-1 uptake compared to the 1-Hz protocol (122), while Vernier et al. have confirmed that the increase of repetition frequency to 10 kHz further improves the molecular electrotransfer by nanosecond electric field pulses (123). Therefore, an increase of repetition frequency in the 1 Hz–10 kHz range is beneficial for nanosecond PEF-based procedures to ensure a faster and more efficient (electrotransfer-wise) treatment without significant changes in the intensity of muscle contractions. However, there are hardly any benefits for longer microsecond (i.e., 100 µs)-based procedures since the 1-Hz and kilohertz protocols can be used interchangeably with minor changes in the treatment outcome (124). The microsecond pulses are also hardly used in the middle kilohertz range (10–100 kHz), while the effectiveness of nanosecond pulse bursts is reduced (121, 125, 126). The exceptionally high-frequency (100 kHz–10 MHz) pulse repetition range is the least covered in scientific literature due to a lack of technological infrastructure. Nevertheless, a further increase in repetition frequency of nanosecond pulses triggers a new high-frequency (500 kHz–1 MHz) phenomenon of transmembrane potential accumulation resulting in a significantly increased electroporation efficiency (121, 125, 127). It was shown that it is possible to achieve a threshold repetition frequency when the discharging [transmembrane potential (TMP) relaxation] time of the membrane is higher than the delay between the pulses; thus, the TMP starts to accumulate throughout the burst (121, 128). The phenomenon in some studies is referred to as “MHz pulse compression” (129) and allows one to significantly lower the electroporation threshold using the pulse repetition frequency modulation. Based on in silico observations, the high repetitions rate pulses can modulate the size of the pores after electroporation, which is important both in drug delivery and in irreversible electroporation (130, 131). The 10-MHz+ range pulses in the context of the electroporation are not covered experimentally yet; however, the first works presenting theoretical guidance start to appear (132). It is predicted that the pore formation time becomes shorter when the symmetrical bipolar picosecond pulse train repetition frequency decreases (from the terahertz to megahertz range). The interfacial water (i.e., water dipole moment) is believed to play a key role in the high-frequency electroporation process (132). The same tendency is theoretically predicted for square wave unipolar bursts in the sub-terahertz repetition frequency region (133).
To summarize, the pulse repetition frequency is an important parameter for shorter pulses; however, for longer (i.e., 100 µs) pulses—the influence is minor and typically 1- or 10-Hz protocols are used.
Conclusions
Summarizing, we can conclude that pulse shape is crucial for electroporation, and accurate metrology is required for treatment planning and results interpretation. Depending on the pulse intensity and duration, the effects of other parameters such as pulse number or repetition frequency will be altered. The load of the generator should also be considered, to prevent a significant transient process, which, if not compensated, will significantly alter the cellular response. Considering all the factors and the different susceptibility of cells to electroporation, an individual approach should be performed depending on the application. Finally, it is concluded that there is a tendency to move toward the shorter pulse duration range, which is a logical evolution of microsecond range electroporation. However, shorter pulses are harder to generate, control, and measure, requiring higher interdisciplinary skills; thus, the electroporation community needs to adapt too.
Author contributions
JK and VN deigned the concept of the review, prepared and validated manuscript, NR and WS prepared Cancan and asymmetric pulses related description. All authors contributed to the article and approved the submitted version.
Funding
Funding was provided by the Polish National Centre of Science of DAINA 2 (2020/38/L/NZ7/00342; PI: JK) and the Research Council of Lithuania grant (Nr. S-LL-21-4, PI: VN).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol (1972) 10:279–90. doi: 10.1007/BF01867861
2. Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J (1982) 1:841–5. doi: 10.1002/j.1460-2075.1982.tb01257.x
3. Jamieson RD, Bodger MP, Bodger PS, Baran D. Transfection by electroporation: Cell gene transfer using electrical impulses, a new process in blood cancer research. IEE Proc A Phys Sci Meas Instrumentation Manag Educ Rev (1989) 136:41–4. doi: 10.1049/ip-a-2.1989.0006
4. Mishra KP, Joshua DC, Bhatia CR. In vitro electroporation of tobacco pollen. Plant Sci (1987) 52:135–9. doi: 10.1016/0168-9452(87)90115-4
5. Hama-Inaba H, Shiomi T, Ito A, Sato K, Takahashi M, Kasai M, et al. Optimum conditions for electric pulse-mediated gene transfer to mammalian cells in suspension. Cell Struct Funct (1987) 12:173–80. doi: 10.1247/csf.12.173
6. Bradshaw HD, Parson WW, Sheffer M, Lioubin PJ, Mulvihill ER, Gordon MP. Design, construction, and use of an electroporator for plant protoplasts and animal cells. Anal Biochem (1987) 166:342–8. doi: 10.1016/0003-2697(87)90583-5
7. Tsong TYY. Electroporation of cell membranes. Biophys J (1991) 60:297–306. doi: 10.1016/S0006-3495(91)82054-9
8. Marszalek P, Liu DS, Tsong TY. Schwan equation and transmembrane potential induced by alternating electric field. Biophys J (1990) 58:1053–8. doi: 10.1016/S0006-3495(90)82447-4
9. Saulis G, Venslauskas MS, Naktinis J. Kinetics of pore resealing in cell membranes after electroporation. J Electroanal Chem (1991) 321:1–13. doi: 10.1016/0022-0728(91)85564-6
10. Bliss JG, Harrison GI, Mourant JR, Powell KT, Weaver JC. Electroporation: the population distribution of macromolecular uptake and shape changes in red blood cells following a single 50 μs square wave pulse. J Electroanal Chem (1988) 254:57–71. doi: 10.1016/0022-0728(80)80334-2
11. Takahashi M, Furukawa T, Saitoh H, Aoki A, Koike T, Moriyama Y, et al. Gene transfer into human leukemia cell lines by electroporation: Experience with exponentially decaying and square wave pulse. Leuk Res (1991) 15:507–13. doi: 10.1016/0145-2126(91)90062-X
12. Pedrow PD, Zhang Q, Barbosa-C¡novas GV. Inactivation of microorganisms by pulsed electric fields of different voltage waveforms. IEEE Trans Dielectr Electr Insul (1994) 1:1047–57. doi: 10.1109/94.368658
13. García-Sánchez T, Merla C, Fontaine J, Muscat A, Mir LM. Sine wave electropermeabilization reveals the frequency-dependent response of the biological membranes. Biochim Biophys Acta - Biomembr (2018) 1860:1022–34. doi: 10.1016/j.bbamem.2018.01.018
14. Garcia-Sanchez T, Mercadal B, Polrot M, Muscat A, Sarnago H, Lucia O, et al. Successful tumor electrochemotherapy using sine waves. IEEE Trans BioMed Eng (2019) 67:1040–9. doi: 10.1109/tbme.2019.2928645
15. Pirc E, Miklavčič D, Reberšek M, Miklavcic D, Rebersek M. Nanosecond pulse electroporator with silicon carbide mosfets: Development and evaluation. IEEE Trans BioMed Eng (2019) 66:3526–33. doi: 10.1109/TBME.2019.2907165
16. Dimitrov JD. A bell-shape pulse generator. IEEE Trans Instrum Meas (1990) 39:667–70. doi: 10.1109/19.57255
17. Miklavcic D, Towhidi L. Numerical study of the electroporation pulse shape effect on molecular uptake of biological cells. Radiol Oncol (2010) 44:34–41. doi: 10.2478/v10019-010-0002-3
18. Bhonsle SP, Arena CB, Sweeney DC, Davalos RV. Mitigation of impedance changes due to electroporation therapy using bursts of high-frequency bipolar pulses. BioMed Eng Online (2015) 14:S3. doi: 10.1186/1475-925X-14-S3-S3
19. Beitel-White N, Lorenzo MF, Zhao Y, Aycock KN, Manuchehrabadi NM, Brock RM, et al. Comparison of analysis methods for determination of dynamic tissue conductivity during microseconds-long pulsed electric fields. BioMed Signal Process Control (2022) 72:103305. doi: 10.1016/j.bspc.2021.103305
20. Murovec T, Sweeney DC, Latouche E, Davalos RV, Brosseau C. Modeling of transmembrane potential in realistic multicellular structures before electroporation. Biophys J (2016) 111:2286–95. doi: 10.1016/j.bpj.2016.10.005
21. Butkus P, Murauskas A, Tolvaišiene S, Novickij V. Concepts and capabilities of in-house built nanosecond pulsed electric field (nsPEF) generators for electroporation: State of art. Appl Sci (2020) 10:4244. doi: 10.3390/app10124244
22. Abadi MRQR, Marzebali MH, Abolghasemi V, Anisi MH. High-voltage pulse generators for electroporation applications: A systematic review. IEEE Access (2022) 10:64933–51. doi: 10.1109/ACCESS.2022.3184015
23. Cvetkoska A, Piro E, Reberšek M, Magjarević R, Miklavčič D. Towards standardization of electroporation devices and protocols. IEEE Instrum Meas Mag (2020) 23:74–81. doi: 10.1109/MIM.2020.9062692
24. Krassowska W, Filev PD. Modeling electroporation in a single cell. Biophys J (2007) 92:404–17. doi: 10.1529/biophysj.106.094235
25. Pirc E, Miklavčič D, Uršič K, Serša G, Reberšek M. High-frequency and high-voltage asymmetric bipolar pulse generator for electroporation based technologies and therapies. Electron (2021) 10:1203. doi: 10.3390/electronics10101203
26. Novickij V, Staigvila G, Murauskas A, Rembialkowska N, Kulbacka J, Novickij J. High frequency bipolar electroporator with double-crowbar circuit for load-independent forming of nanosecond pulses. Appl Sci (2022) 12:1370. doi: 10.3390/APP12031370
27. Semenov I, Xiao S, Kang D, Schoenbach KH, Pakhomov AG, Semenov I, et al. Cell stimulation and calcium mobilization by picosecond electric pulses repository citation original publication citation cell stimulation and calcium mobilization by picosecond electric pulses. Bioelectrochemistry (2015) 105:65–71. doi: 10.1016/j.bioelechem.2015.05.013
28. Rubinsky B, Onik G, Mikus P. Irreversible electroporation: A new ablation modality - clinical implications. Technol Cancer Res Treat (2007) 6:37–48. doi: 10.1177/153303460700600106
29. Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: Irreversible electroporation. IEEE Trans BioMed Eng (2006) 53:1409–15. doi: 10.1109/TBME.2006.873745
30. Mir LM, Orlowski S. The basis of electrochemotherapy. Methods Mol Med (2000) 37:99–117. doi: 10.1385/1-59259-080-2:99
31. Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G, Snoj M. Electrochemotherapy in treatment of tumours. Eur J Surg Oncol (2008) 34:232–40. doi: 10.1016/j.ejso.2007.05.016
32. Sersa G. The state-of-the-art of electrochemotherapy before the ESOPE study; advantages and clinical uses. Eur J Cancer Suppl (2006) 4:52–59. doi: 10.1016/j.ejcsup.2006.08.007
33. Serša G, Štabuc B, Čemažar M, Miklavčič D, Rudolf Z. Electrochemotherapy with cisplatin: Clinical experience in malignant melanoma patients. Clin Cancer Res (2000) 6:863–7.
34. Mir LM, Glass LF, Sersa G, Teissie J, Domenge C, Miklavcic D, et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer (1998) 77:2336–42. doi: 10.1038/bjc.1998.388
35. Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, et al. Electrochemotherapy - an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European standard operating procedures of electrochemotherapy) study. Eur J Cancer Suppl (2006) 4:3–13. doi: 10.1016/j.ejcsup.2006.08.002
36. Al-Sakere B, André F, Bernat C, Connault E, Opolon P, Davalos RV, et al. Tumor ablation with irreversible electroporation. PloS One (2007) 2. doi: 10.1371/journal.pone.0001135
37. Maor E, Ivorra A, Leor J, Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat (2007) 6:307–12. doi: 10.1177/153303460700600407
38. Jiang C, Davalos RV, Bischof JC. A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans BioMed Eng (2015) 62:4–20. doi: 10.1109/TBME.2014.2367543
39. Geboers B, Scheffer HJ, Graybill PM, Ruarus AH, Nieuwenhuizen S, Puijk RS, et al. High-voltage electrical pulses in oncology: Irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy. Radiology (2020) 295:254–72. doi: 10.1148/radiol.2020192190
40. Campana LG, Kis E, Bottyán K, Orlando A, de Terlizzi F, Mitsala G, et al. Electrochemotherapy for advanced cutaneous angiosarcoma: A European register-based cohort study from the international network for sharing practices of electrochemotherapy (InspECT). Int J Surg (2019) 72:34–42. doi: 10.1016/j.ijsu.2019.10.013
41. Borgognoni L, Pescitelli L, Gerlini G, Brandani P, Gelli R, Giannotti V, et al. Efficacy of electrochemotherapy in the treatment of cutaneous melanoma metastases and rare non-melanoma skin cancer. Anticancer Res (2020) 40:6485–92. doi: 10.21873/anticanres.14670
42. Djokic M, Cemazar M, Popovic P, Kos B, Dezman R, Bosnjak M, et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur J Surg Oncol (2018) 44:651–57. doi: 10.1016/j.ejso.2018.01.090
43. Rembiałkowska N, Dubińska-Magiera M, Sikora A, Szlasa W, Szewczyk A, Czapor-Irzabek H, et al. Doxorubicin assisted by microsecond electroporation promotes irreparable morphological alternations in sensitive and resistant human breast adenocarcinoma cells. Appl Sci (2020) 10:2765. doi: 10.3390/app10082765
44. Fesmire CC, Petrella RA, Kaufman JD, Topasna N, Sano MB. Irreversible electroporation is a thermally mediated ablation modality for pulses on the order of one microsecond. Bioelectrochemistry (2020) 135:107544. doi: 10.1016/j.bioelechem.2020.107544
45. Ruarus AH, Barabasch A, Catalano O, Leen E, Narayanan G, Nilsson A, et al. Irreversible electroporation for hepatic tumors: Protocol standardization using the modified Delphi technique. J Vasc Interv Radiol (2020) 31:1765–71.e15. doi: 10.1016/j.jvir.2020.02.030
46. Coelen RJS, Vogel JA, Vroomen LGPH, Roos E, Busch ORC, Van Delden OM, et al. Ablation with irreversible electroporation in patients with advanced perihilar cholangiocarcinoma (ALPACA): A multicentre phase I/II feasibility study protocol. BMJ Open (2017) 7:e015810. doi: 10.1136/bmjopen-2016-015810
47. Schoenbach K, Joshi R, Beebe S, Baum C. A scaling law for membrane permeabilization with nanopulses. IEEE Trans Dielectr Electr Insul (2009) 16:1224–35. doi: 10.1109/TDEI.2009.5293932
48. Vernier PT, Sun Y, Marcu L, Salemi S, Craft CM, Gundersen MA. Calcium bursts induced by nanosecond electric pulses. Biochem Biophys Res Commun (2003) 310:286–95. doi: 10.1016/j.bbrc.2003.08.140
49. Deng J, Schoenbach KH, Stephen Buescher E, Hair PS, Fox PM, Beebe SJ. The effects of intense submicrosecond electrical pulses on cells. Biophys J (2003) 84:2709–14. doi: 10.1016/S0006-3495(03)75076-0
50. Joshi RP, Schoenbach KH. Mechanism for membrane electroporation irreversibility under high-intensity, ultrashort electrical pulse conditions. Phys Rev E - Stat Physics Plasmas Fluids Relat Interdiscip Top (2002) 66:052901. doi: 10.1103/PhysRevE.66.052901
51. Vernier PT, Ziegler MJ, Sun Y, Gundersen MA, Tieleman DP. Nanopore-facilitated, voltage-driven phosphatidylserine translocation in lipid bilayers–in cells and in silico. Phys Biol (2006) 3:233–47. doi: 10.1088/1478-3975/3/4/001
52. Pakhomov AG, Kolb JF, White JA, Joshi RP, Xiao S, Schoenbach KH. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF). Bioelectromagnetics (2007) 28:655–63. doi: 10.1002/bem.20354
53. Gowrishankar TR, Weaver JC. Electrical behavior and pore accumulation in a multicellular model for conventional and supra-electroporation. Biochem Biophys Res Commun (2006) 349:643–53. doi: 10.1016/j.bbrc.2006.08.097
54. Vasilkoski Z, Esser AT, Gowrishankar TR, Weaver JC. Membrane electroporation: The absolute rate equation and nanosecond time scale pore creation. Phys Rev E - Stat Nonlinear Soft Matter Phys (2006) 74:021904. doi: 10.1103/PhysRevE.74.021904
55. Tieleman DP, Leontiadou H, Mark AE, Marrink SJ. Simulation of pore formation in lipid bilayers by mechanical stress and electric fields. J Am Chem Soc (2003) 125:6382–3. doi: 10.1021/ja029504i
56. Beebe SJ, Fox PM, Rec LJ, Somers K, Stark RH, Schoenbach KH. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. PPPS 2001 - Pulsed Power Plasma Sci 2001 (2015) 30:286–92. doi: 10.1109/PPPS.2001.01002030
57. Beebe SJ, White J, Blackmore PF, Deng Y, Somers K, Schoenbach KH. Diverse effects of nanosecond pulsed electric fields on cells and tissues. DNA Cell Biol (2003) 22:785–96. doi: 10.1089/104454903322624993
58. Nuccitelli R, Tran K, Sheikh S, Athos B, Kreis M, Nuccitelli P. Optimized nanosecond pulsed electric field therapy can cause murine malignant melanomas to self-destruct with a single treatment. Int J Cancer (2010) 127:1727–36. doi: 10.1002/ijc.25364
59. Chena X, James Swanson R, Kolb JF, Nuccitelli R, Schoenbach KH. Histopathology of normal skin and melanomas after nanosecond pulsed electric field treatment. Melanoma Res (2009) 19:361–71. doi: 10.1097/CMR.0b013e32832f1558
60. Beebe SJ, Chen X, Kolb J, Schoenbach KH. Non-ionizing radiation generated by nanosecond pulsed electric fields (nsPEFS) induce apoptosis in cancer in vivo. 2008 IEEE 35th International Conference on Plasma Science. (2008). p. 1–1.
61. Nuccitelli R, Chen X, Pakhomov AG, Baldwin WH, Sheikh S, Pomicter JL, et al. A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int J Cancer (2009) 125:438–45. doi: 10.1002/ijc.24345
62. Guo F, Yao C, Li C, Mi Y, Peng Q, Tang J. In vivo evidences of nanosecond pulsed electric fields for melanoma malignancy treatment on tumor-bearing BALB/c nude mice. Technol Cancer Res Treat (2014) 337–44. doi: 10.7785/tcrt.2012.500385
63. Novickij V, Malyško V, Želvys A, Balevičiūte A, Zinkevičiene A, Novickij J, et al. Electrochemotherapy using doxorubicin and nanosecond electric field pulses: A pilot in vivo study. Molecules (2020) 25:4601. doi: 10.3390/molecules25204601
64. Vižintin A, Marković S, Ščančar J, Miklavčič D. Electroporation with nanosecond pulses and bleomycin or cisplatin results in efficient cell kill and low metal release from electrodes. Bioelectrochemistry (2021) 140:107798. doi: 10.1016/J.BIOELECHEM.2021.107798
65. Tunikowska J, Antończyk A, Rembiałkowska N, Jóźwiak Ł, Novickij V, Kulbacka J. The first application of nanoelectrochemotherapy in feline oral malignant melanoma treatment— case study. Animals (2020) 10:556. doi: 10.3390/ani10040556
66. Kulbacka J, Saczko J, Choromańska A, Rembiałkowska N, Dubinska-Magiera M, Surowiak P, et al. Transmembrane transport and anticancer activity of strontium ranelate delivered with nanosecond pulsed electric fields (nsPEFs) into human cells in vitro. IFMBE Proc (2015) 45:581–5. doi: 10.1007/978-3-319-11128-5_145
67. Semenov I, Xiao S, Semenov I, Xiao S. Electropermeabilization and electrostimulation by picosecond pulses. Handb Electroporation (2017) 1:171–86. doi: 10.1007/978-3-319-32886-7_89
68. Petrella RA, Schoenbach KH, Xiao S. A dielectric rod antenna for picosecond pulse stimulation of neurological tissue. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc (2016) 44:708–14. doi: 10.1109/TPS.2016.2537213
69. Mao Z, Liu L, Zhang Y, Zhang J, Liu N, Liu QH. Selective electroporation of organelles under an intense picosecond pulsed electric field. IEEE J Multiscale Multiphysics Comput Tech (2018) 3:235–45. doi: 10.1109/JMMCT.2018.2887000
70. Hua YY, Wang XS, Zhang Y, Yao CG, Zhang XM, Xiong ZA. Intense picosecond pulsed electric fields induce apoptosis through a mitochondrial-mediated pathway in HeLa cells. Mol Med Rep (2012) 5:981. doi: 10.3892/MMR.2012.780
71. Zamponi M, Petrella R, Mollica PA. Picosecond pulsed electric fields and promise in neurodegeneration research. Bioelectricity (2021) 3:176–85. doi: 10.1089/BIOE.2021.0005
72. Tekle E, Astumian RD, Chock PB. Electroporation by using bipolar oscillating electric field: An improved method for DNA transfection of NIH 3T3 cells. Proc Natl Acad Sci USA (1991) 88:4230–34. doi: 10.1073/pnas.88.10.4230
73. Kotnik T, Mir LM, Flisar K, Puc M, Miklavčič D. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses: Part i. increased efficiency of permeabilization. Bioelectrochemistry (2001) 54:83–90. doi: 10.1016/S1567-5394(01)00114-1
74. Kotnik T, Miklavčič D, Mir LM. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses: Part II. reduced electrolytic contamination. Bioelectrochemistry (2001) 54:91–5. doi: 10.1016/S1567-5394(01)00115-3
75. Daskalov I, Mudrov N, Peycheva E. Exploring new instrumentation parameters for electrochemotherapy: Attacking tumors with bursts of biphasic pulses instead of single pulses. IEEE Eng Med Biol Mag (1999) 18:62–6. doi: 10.1109/51.740982
76. Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther (1999) 6:508–14. doi: 10.1038/sj.gt.3300847
77. Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci USA (1999) 96:6417–22. doi: 10.1073/pnas.96.11.6417
78. Pucihar G, Mir LM, Miklavčič D. The effect of pulse repetition frequency on the uptake into electropermeabilized cells in vitro with possible applications in electrochemotherapy. Bioelectrochemistry (2002) 57:167–72. doi: 10.1016/S1567-5394(02)00116-0
79. Nikolova B, Tsoneva I, Peycheva E. Treatment of melanoma by electroporation of bacillus calmette-guerin. Biotechnol Biotechnol Equip (2011) 25:2522–24. doi: 10.5504/bbeq.2011.0059
80. Dotsinsky I, Nikolova B, Peycheva E, Tsoneva I. New modality for electrochemotherapy of surface tumors. Biotechnol Biotechnol Equip (2012) 26:3402–6. doi: 10.5504/bbeq.2012.0098
81. Pehlivanova VN, Tsoneva IH, Tzoneva RD. Multiple effects of electroporation on the adhesive behaviour of breast cancer cells and fibroblasts. Cancer Cell Int (2012) 12. doi: 10.1186/1475-2867-12-9
82. Fusco R, Di Bernardo E, D’Alessio V, Salati S, Cadossi M. Reduction of muscle contraction and pain in electroporation-based treatments: An overview. World J Clin Oncol (2021) 12:367–81. doi: 10.5306/wjco.v12.i5.367
83. Narayanan G, Hosein PJ, Arora G, Barbery KJ, Froud T, Livingstone AS, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol (2012) 23:1613–21. doi: 10.1016/j.jvir.2012.09.012
84. Pech M, Janitzky A, Wendler JJ, Strang C, Blaschke S, Dudeck O, et al. Irreversible electroporation of renal cell carcinoma: A first-in-man phase i clinical study. Cardiovasc Intervent Radiol (2011) 34:132–8. doi: 10.1007/s00270-010-9964-1
85. Arena CB, Sano MB, Rylander MN, Davalos RV. Theoretical considerations of tissue electroporation with high-frequency bipolar pulses. IEEE Trans BioMed Eng (2011) 58:1474–82.doi: 10.1109/TBME.2010.2102021
86. Sano MB, Arena CB, Bittleman KR, DeWitt MR, Cho HJ, Szot CS, et al. Bursts of bipolar microsecond pulses inhibit tumor growth. Sci Rep (2015) 5:14999. doi: 10.1038/srep14999
87. Bhonsle SP, Arena CB, Davalos RV. A feasibility study to mitigate tissue-tumor heterogeneity using high frequency bipolar electroporation pulses. 6th European Conference of the International Federation for Medical and Biological Engineering IFMBE Proc vol.45. Cham: Springer (2015). doi: 10.1007/978-3-319-11128-5_141
88. Arena CB, Sano MB, Rossmeisl JH, Caldwell JL, Garcia PA, Rylander MN, et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. BioMed Eng Online (2011) 10:102. doi: 10.1186/1475-925X-10-102
89. Pakhomov AG, Semenov I, Xiao S, Pakhomova ON, Gregory B, Schoenbach KH, et al. Cancellation of cellular responses to nanoelectroporation by reversing the stimulus polarity. Cell Mol Life Sci (2014) 71:4431–41. doi: 10.1007/s00018-014-1626-z
90. Gianulis EC, Lee J, Jiang C, Xiao S, Ibey BL, Pakhomov AG. Electroporation of mammalian cells by nanosecond electric field oscillations and its inhibition by the electric field reversal. Sci Rep (2015) 5:13818. doi: 10.1038/srep13818
91. Pakhomov AG, Grigoryev S, Semenov I, Casciola M, Jiang C, Xiao S. The second phase of bipolar, nanosecond-range electric pulses determines the electroporation efficiency. Bioelectrochemistry (2018) 122:123–33. doi: 10.1016/j.bioelechem.2018.03.014
92. Sano MB, Arena CB, DeWitt MR, Saur D, Davalos RV. In-vitro bipolar nano- and microsecond electro-pulse bursts for irreversible electroporation therapies. Bioelectrochemistry (2014) 100:69–79. doi: 10.1016/j.bioelechem.2014.07.010
93. Reberšek M, Connor RO, Miklavc D, JD C, Polajz T. Cancellation effect is present in high-frequency reversible and irreversible electroporation. Bioelectrochemistry (2020) 132:1–11. doi: 10.1016/j.bioelechem.2019.107442
94. Pakhomov AG, Gudvangen E, Xiao S, Semenov I. Interference targeting of bipolar nanosecond electric pulses for spatially focused electroporation, electrostimulation, and tissue ablation. Bioelectrochemistry (2021) 141:107876. doi: 10.1016/j.bioelechem.2021.107876
95. Sweeney DC, Reberšek M, Dermol J, Rems L, Miklavčič D, Davalos RV. Quantification of cell membrane permeability induced by monopolar and high-frequency bipolar bursts of electrical pulses. Biochim Biophys Acta - Biomembr (2016) 1858:2689–98. doi: 10.1016/j.bbamem.2016.06.024
96. Scuderi M, Rebersek M, Miklavcic D, Dermol-Cerne J. The use of high-frequency short bipolar pulses in cisplatin electrochemotherapy in vitro. Radiol Oncol (2019) 53:194–205. doi: 10.2478/raon-2019-0025
97. Schoenbach KH, Pakhomov AG, Semenov I, Xiao S, Pakhomova ON, Ibey BL. Ion transport into cells exposed to monopolar and bipolar nanosecond pulses. Bioelectrochemistry (2015) 103:44–51. doi: 10.1016/j.bioelechem.2014.08.015
98. Gianulis EC, Casciola M, Zhou C, Yang E, Xiao S, Pakhomov AG. Selective distant electrostimulation by synchronized bipolar nanosecond pulses. Sci Rep 2019 91 (2019) 9:1–10. doi: 10.1038/s41598-019-49664-2
99. Kiełbik A, Szlasa W, Novickij V, Szewczyk A, Maciejewska M, Saczko J, et al. Effects of high-frequency nanosecond pulses on prostate cancer cells. Sci Rep 2021 111 (2021) 11:1–10. doi: 10.1038/s41598-021-95180-7
100. Xiao S, Yamada R, Zhou C. Quadrupoles for remote electrostimulation incorporating bipolar cancellation. Bioelectricity (2020) 2:382–90. doi: 10.1089/BIOE.2020.0024
101. Ibey BL, Ullery JC, Pakhomova ON, Roth CC, Semenov I, Beier HT, et al. Bipolar nanosecond electric pulses are less efficient at electropermeabilization and killing cells than monopolar pulses. Biochem Biophys Res Commun (2014) 443:568–73. doi: 10.1016/J.BBRC.2013.12.004
102. Gianulis EC, Casciola M, Xiao S, Pakhomova ON, Pakhomov AG. Electropermeabilization by uni- or bipolar nanosecond electric pulses: The impact of extracellular conductivity. Bioelectrochemistry (2018) 119:10–9. doi: 10.1016/j.bioelechem.2017.08.005
103. Sözer EB, Vernier PT. Modulation of biological responses to 2 ns electrical stimuli by field reversal. Biochim Biophys Acta - Biomembr (2019) 1861:1228–39. doi: 10.1016/J.BBAMEM.2019.03.019
104. Ruzgys P, Jakutavičiūtė M, Šatkauskienė I, Čepurnienė K, Šatkauskas S. Effect of electroporation medium conductivity on exogenous molecule transfer to cells. vitro Sci Rep (2019) 9:1436. doi: 10.1038/s41598-018-38287-8
105. Roth Y, Amir A, Levkovitz Y, Zangen A. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep h-coils. J Clin Neurophysiol (2007) 24:31–8. doi: 10.1097/WNP.0B013E31802FA393
106. Datta A, Elwassif M, Battaglia F, Bikson M. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng (2008) 5:163. doi: 10.1088/1741-2560/5/2/007
107. Čepurniene K, Ruzgys P, Treinys R, Šatkauskiene I, Šatkauskas S. Influence of plasmid concentration on DNA electrotransfer in vitro using high-voltage and low-voltage pulses. J Membr Biol (2010) 236:81–5. doi: 10.1007/s00232-010-9270-5
108. Haberl S, Kandušer M, Flisar K, Hodžić D, Bregar VB, Miklavčič D, et al. Effect of different parameters used for in vitro gene electrotransfer on gene expression efficiency, cell viability and visualization of plasmid DNA at the membrane level. J Gene Med (2013) 15:169–81. doi: 10.1002/jgm.2706
109. Kandušer M, Miklavčič D, Pavlin M. Mechanisms involved in gene electrotransfer using high- and low-voltage pulses - an in vitro study. Bioelectrochemistry (2009) 74:265–71. doi: 10.1016/j.bioelechem.2008.09.002
110. Šatkauskas S, André F, Bureau MF, Scherman D, Miklavčič D, Mir LM. Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum Gene Ther (2005) 16:1194–201. doi: 10.1089/hum.2005.16.1194
111. Van Es R, Konings MK, Du Pré BC, Neven K, Van Wessel H, Van Driel VJHM, et al. High-frequency irreversible electroporation for cardiac ablation using an asymmetrical waveform. BioMed Eng Online (2019) 18. doi: 10.1186/s12938-019-0693-7
112. Sano MB, Fan RE, Xing L. Asymmetric waveforms decrease lethal thresholds in high frequency irreversible electroporation therapies. Sci Rep (2017) 7:1–13. doi: 10.1038/srep40747
113. Grainys A, Novickij V, Novickij J. High-power bipolar multilevel pulsed electroporator. Instrum Sci Technol (2016) 44:65–72. doi: 10.1080/10739149.2015.1060607
114. Levkov K, Linzon Y, Mercadal B, Ivorra A, González CA, Golberg A. High-voltage pulsed electric field laboratory device with asymmetric voltage multiplier for marine macroalgae electroporation. Innov Food Sci Emerg Technol (2020) 60:102288. doi: 10.1016/J.IFSET.2020.102288
115. Kandratsyeu A, Sabaleuski U, Redondo L, Pakhomov AG. Four channel 6.5 kV, 65 a, 100 ns–100 µs generator with advanced control of pulse and burst protocols for biomedical and biotechnological applications. Appl Sci (2021) 11. doi: 10.3390/app112411782
116. Miklavčič D, Pucihar G, Pavlovec M, Ribarič S, Mali M, MačEk-Lebar A, et al. The effect of high frequency electric pulses on muscle contractions and antitumor efficiency in vivo for a potential use in clinical electrochemotherapy. Bioelectrochemistry (2005) 65:121–8. doi: 10.1016/j.bioelechem.2004.07.004
117. Dermol J, Pakhomova ON, Pakhomov AG, Miklavčič D. Cell electrosensitization exists only in certain electroporation buffers. PloS One (2016) 11:e0159434. doi: 10.1371/journal.pone.0159434
118. Pakhomova ON, Gregory BW, Khorokhorina VA, Bowman AM, Xiao S, Pakhomov AG. Electroporation-induced electrosensitization. PloS One (2011) 6:e17100. doi: 10.1371/journal.pone.0017100
119. Muratori C, Pakhomov AG, Heller L, Casciola M, Gianulis E, Grigoryev S, et al. Electrosensitization increases antitumor effectiveness of nanosecond pulsed electric fields In vivo. Technol Cancer Res Treat (2017) 16:987–96. doi: 10.1177/1533034617712397
120. Lamberti P, Romeo S, Sannino A, Zeni L, Zeni O. The role of pulse repetition rate in nsPEF-induced electroporation: A biological and numerical investigation. IEEE Trans BioMed Eng (2015) 62:2234–43. doi: 10.1109/TBME.2015.2419813
121. Novickij V, Ruzgys P, Grainys A, Šatkauskas S. High frequency electroporation efficiency is under control of membrane capacitive charging and voltage potential relaxation. Bioelectrochemistry (2018) 119. doi: 10.1016/j.bioelechem.2017.09.006
122. Romeo S, Wu Y-H, Levine ZA, Gundersen MA, Vernier PT. Water influx and cell swelling after nanosecond electropermeabilization. Biochim Biophys Acta - Biomembr (2013) 1828:1715–22. doi: 10.1016/j.bbamem.2013.03.007
123. Vernier PT, Sun Y, Gundersen MA, Vernier PT, Sun Y, Marcu L, et al. Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. BMC Cell Biol (2006) 7:37. doi: 10.1186/1471-2121-7-37
124. Shankayi Z, Firoozabadi SM. Antitumor efficiency of electrochemotherapy by high and low frequencies and repetitive therapy in the treatment of invasive ductal carcinoma in balb/c mice. Cell J (2012) 14:110–5.
125. Steelman ZA, Tolstykh GP, Beier HT, Ibey BL. Cellular response to high pulse repetition rate nanosecond pulses varies with fluorescent marker identity. Biochem Biophys Res Commun (2016) 478:1261–7. doi: 10.1016/j.bbrc.2016.08.107
126. Silve A, Guimerà Brunet A, Al-Sakere B, Ivorra A, Mir LM. Comparison of the effects of the repetition rate between microsecond and nanosecond pulses: Electropermeabilization-induced electro-desensitization? Biochim Biophys Acta - Gen Subj (2014) 1840:2139–51. doi: 10.1016/j.bbagen.2014.02.011
127. Semenov I, Casciola M, Ibey BL, Xiao S, Pakhomov AG. Electropermeabilization of cells by closely spaced paired nanosecond-range pulses. Bioelectrochemistry (2018) 121:135–41. doi: 10.1016/j.bioelechem.2018.01.013
128. Murauskas A, Staigvila G, Girkontaitė I, Zinkevičienė A, Ruzgys P, Šatkauskas S, et al. Predicting electrotransfer in ultra-high frequency sub-microsecond square wave electric fields. Electromagn Biol Med (2020) 39:1–8. doi: 10.1080/15368378.2019.1710529
129. Pakhomov AG, Xiao S, Novickij V, Casciola M, Semenov I, Mangalanathan U, et al. Excitation and electroporation by MHz bursts of nanosecond stimuli. Biochem Biophys Res Commun (2019) 518:759–64. doi: 10.1016/j.bbrc.2019.08.133
130. Mi Y, Xu J, Liu Q, Wu X, Zhang Q, Tang J. Single-cell electroporation with high-frequency nanosecond pulse bursts: Simulation considering the irreversible electroporation effect and experimental validation. Bioelectrochemistry (2021) 140:107822. doi: 10.1016/j.bioelechem.2021.107822
131. Mi Y, Xu J, Yao C, Li C, Liu H. Electroporation modeling of a single cell exposed to high-frequency nanosecond pulse bursts. IEEE Trans Dielectr Electr Insul (2019) 26:461–8. doi: 10.1109/TDEI.2018.007777
132. Tang J, Ma J, Guo L, Wang K, Yang Y, Bo W, et al. Interpretation of the molecular mechanism of the electroporation induced by symmetrical bipolar picosecond pulse trains. Biochim Biophys Acta Biomembr (2020) 1862. doi: 10.1016/J.BBAMEM.2020.183213
Keywords: electric pulse, pulse shape, electroporation, permeabilization, pulse frequency
Citation: Novickij V, Rembiałkowska N, Szlasa W and Kulbacka J (2022) Does the shape of the electric pulse matter in electroporation? Front. Oncol. 12:958128. doi: 10.3389/fonc.2022.958128
Received: 31 May 2022; Accepted: 15 August 2022;
Published: 14 September 2022.
Edited by:
Richard Heller, University of South Florida, United StatesCopyright © 2022 Novickij, Rembiałkowska, Szlasa and Kulbacka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vitalij Novickij, dml0YWxpai5ub3ZpY2tpakB2aWxuaXVzdGVjaC5sdA==; Julita Kulbacka, anVsaXRhLmt1bGJhY2thQHVtdy5lZHUucGw=
 Vitalij Novickij1*
Vitalij Novickij1* Julita Kulbacka
Julita Kulbacka