- 1Shandong Provincial Hospital, Shandong University, Jinan, China
- 2Shandong Provincial Hospital, Shandong First Medical University, Jinan, China
Background: The purpose of this meta-analysis was to evaluate the efficacy of lymph node dissection in patients with intrahepatic cholangiocarcinoma (ICC).
Methods: The literature from January 2009 to December 2021 was searched to determine the comparative study of lymph node dissection and non-lymph node dissection in patients with ICC.
Results: Seventeen studies were included in the analysis. There were no significant differences in 1-, 3-, and 5-year overall survival (OR = 0.80, p = 0.10; OR = 0.93, p = 0.71; OR = 0.80, p = 0.21) and 1-, 3-, and 5-year disease-free survival (OR = 0.89, p = 0.73; OR = 0.92, p = 0.81; OR = 0.85, p = 0.62).
Conclusions: Lymph node dissection does not seem to have a positive effect on the overall survival and disease-free survival.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a kind of primary liver cancer, and its incidence is second only to hepatocellular carcinoma. In particular, ICC accounts for 8%–10% of biliary tract cancers and 10%–20% of all primary liver tumors (1). Worldwide, the overall incidence of intrahepatic cholangiocarcinoma is on the rise (2). According to this trend, people have a growing interest in the management of ICC.
The onset of cholangiocarcinoma is hidden, and most patients are in advanced stage when the disease is found. Most patients with ICC are not candidates for curative resection because of advanced cancer at the time of initial presentation or underlying comorbidities (3). In the last decade, notable efforts have been made by the medical community in an attempt to improve clinical outcomes of patients with unresectable ICC with the development of various treatment methods, including immunotherapy (4), targeted therapy (5), chemotherapy (6), transarterial radioembolization, hepatic artery infusion, transarterial chemoembolization, and radiofrequency ablation (7, 8).{Brandi, 2020 #4;Massa, 2020 #7;Rizzo, 2021 #5;Rizzo, 2021 #6;Sommer, 2016 #3}{Brandi, 2020 #4;Massa, 2020 #7;Rizzo, 2021 #5;Rizzo, 2021 #6;Sommer, 2016 #3}{Rizzo, 2021 #5}{Rizzo, 2021 #5}{Rizzo, 2021 #5}{Brandi, 2020 #4;Massa, 2020 #7;Rizzo, 2021 #5;Rizzo, 2021 #6;Sommer, 2016 #3}{Brandi, 2020 #4;Massa, 2020 #7;Rizzo, 2021 #5;Rizzo, 2021 #6;Sommer, 2016 #3} A careful evaluation of tumor burden appears as a crucial element in choosing the best therapeutic strategy in unresectable ICC. For those with resectable disease, surgical resection is the best choice (9). However, the prognosis of ICC is not ideal. There are several independent factors associated with the worse long-term survival rate, including the presence of vascular invasion, symptomatic disease, regional lymph node metastasis, and multiple tumors (10).
It is reported that LNM is one of the most prominent adverse prognostic factors in patients with ICC (11), but the role of lymph node dissection (LND) in resectable ICC is still controversial. A recent guideline recommended that regional lymphadenectomy should be performed in patients undergoing resection (12). However, evidence of the benefits from lymphadenectomy does not seem sufficient (13). Therefore, it is difficult to reach a consensus on whether LND should be performed routinely. In this study, therefore, a meta-analysis of the published literature was performed to assess the effect of LND on prognosis for patients with resectable ICC.
Methods
The study protocol was published on PROSPERO, the international prospective register of systematic reviews (reference: CRD420223257). The search and analysis were performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement (14) and Cochrane Handbook for Systematic Review of Interventions (15).
Literature search and selection criteria
The online databases of PubMed/Medline, Cochrane Library, EMBASE, and Web of Science were searched for all levels of evidence published in print or electronically from January 2009 to December 2021. Search terms contain “intrahepatic cholangiocarcinoma”, “cholangiocarcinoma”, “lymph node excision”, “lymph node dissection”, and “lymphadenectomy”. The included articles have no language restrictions.
Only articles that meet the following conditions will be included: (1) population: patients were pathologically diagnosed as cholangiocarcinoma and underwent surgery; (2) intervention: lymph node dissection (LND+); (3) comparison: no lymph node dissection (LND−); (4) outcome: 1-, 3-, and 5-year overall survival (OS) and 1-, 3-, and 5-year disease-free survival (DFS); and (5) design: comparative studies, including retrospective and prospective investigations.
Articles were excluded if patients were diagnosed pathologically as hepatocellular carcinoma, mixed type ICC, or cancer in other parts of the bile duct; the information provided in the article was non-comparable or insufficient for data extraction or quality assessment; and the articles were conference abstracts, letters to the journal editors, and review articles.
Study selection
One investigator (Li F) performed the searching, inclusion, and exclusion of articles, which was subsequently double-checked by all other involved authors.
Data extraction
Two independent researchers (Li F and Yan) reviewed the studies; disagreements in eligibility, data extraction, and quality assessment were resolved through discussion and consultation by all involved authors. Data extracted from each article included the first author, year of publication, study design, number of patients, matching criteria, and reasons for and area of LND. The primary outcome was 1-, 3-, and 5-year overall survival and 1-, 3-, and 5-year disease-free survival. Data after matched were extracted if patients were matched in studies.
Quality assessment
The methodological quality of the observational studies was assessed using the Newcastle–Ottawa scale (NOS) (16). This assessment scale consists of three factors: patient selection, comparability of groups, and assessment of outcomes. A score of 0–9 was allocated to each study, which was estimated by two independent reviewers. Disagreements were resolved through discussion and consultation by all involved authors. Observational studies with a score of 6 or more were considered to be of high quality.
Statistical analysis
Patients were divided into two groups according to whether lymph nodes were removed or not: non-lymph node dissection (LND−) group and lymph node dissection (LND+) group. Data analyses were performed using the Review Manager (RevMan, version 5.4.1) in accordance with the PRISMA guidelines. All variables were presented as dichotomous data to evaluate odds ratio (OR) and 95% confidence interval (CI). The OR of <1 favored the LND− group. P-value <0.05 was considered statistically significant. Statistical heterogeneity among studies was assessed using the χ² and I² statistics. A random-effects model was used if the heterogeneity among studies was considered present (P < 0.1 or I² > 50%). Otherwise, a fix-effects model was used.
Results
Study search results
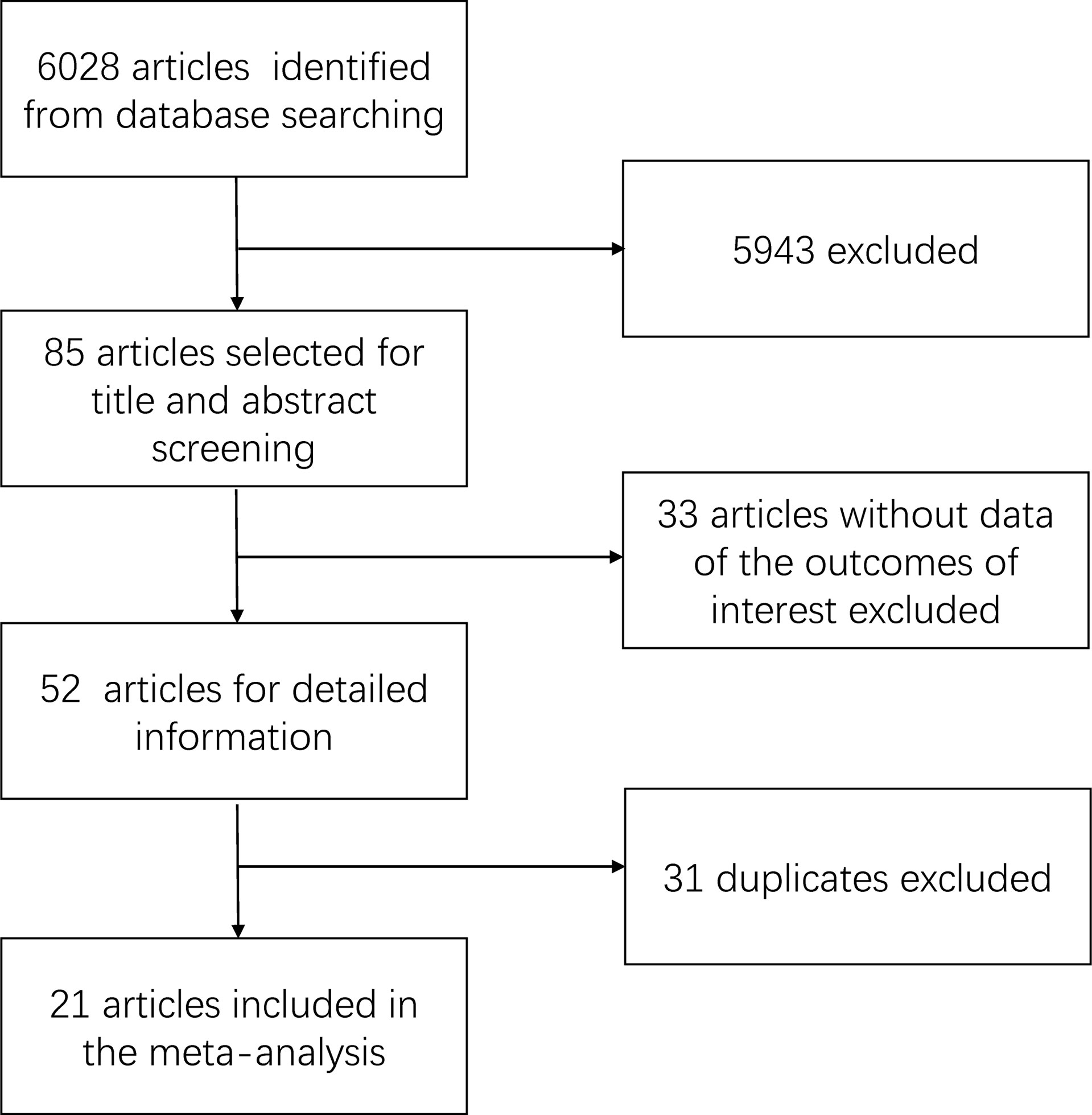
The summary of the search result is shown in Figure 1. We retrieved a total of 6,028 related articles. After preliminary screening, 85 articles met the inclusion criteria. We further excluded 33 articles because of the absent data in the outcome of interest. Finally, 31 duplicates were excluded. A total of 21 articles were included in this study, which included 3,796 patients (1,990 patients with LND+ and 1,806 patients with LND−).
Characteristics of eligible studies
The included studies (17–37) were all retrospective cohort trials, and the publication dates ranged from 2009 to 2021. Eighteen studies (17–27, 30, 32–35, 37) were from East Asia, two studies (28, 29) were from Europe, one study (31) was from the US, and one study (36) was from Europe and East Asia. Seven multi-center studies (20, 23, 29–32, 36) were included. The largest multi-center study (31) involved 402 patients and used the Surveillance, Epidemiology, and End Results (SEER) database.
Three studies (22, 23, 32) had an overlapping population in the same center; therefore, only the study (32) with the largest time scope was included in the analysis. Two studies (19, 37) collected data in the same center, and there is an overlapping population in a certain part of the time. We only took the study (37) with a large number of patients into the analysis. Two other studies (26, 33) had the same condition, and only the study (26) with a relatively large number of patients was analyzed similarly.
Survival outcomes
Sixteen studies (17, 18, 20, 21, 24–27, 29–32, 34–37) were used to compare the 1- and 3-year OS of LND+ group and LND− group in the meta-analysis (Figures 2A, B). There was no significant difference between the two groups. Fourteen studies (17, 18, 20, 21, 24–27, 29–32, 34, 36) compared the 5-year OS of LND+ group and LND− group (Figure 2C). There was no significant difference between the two groups.
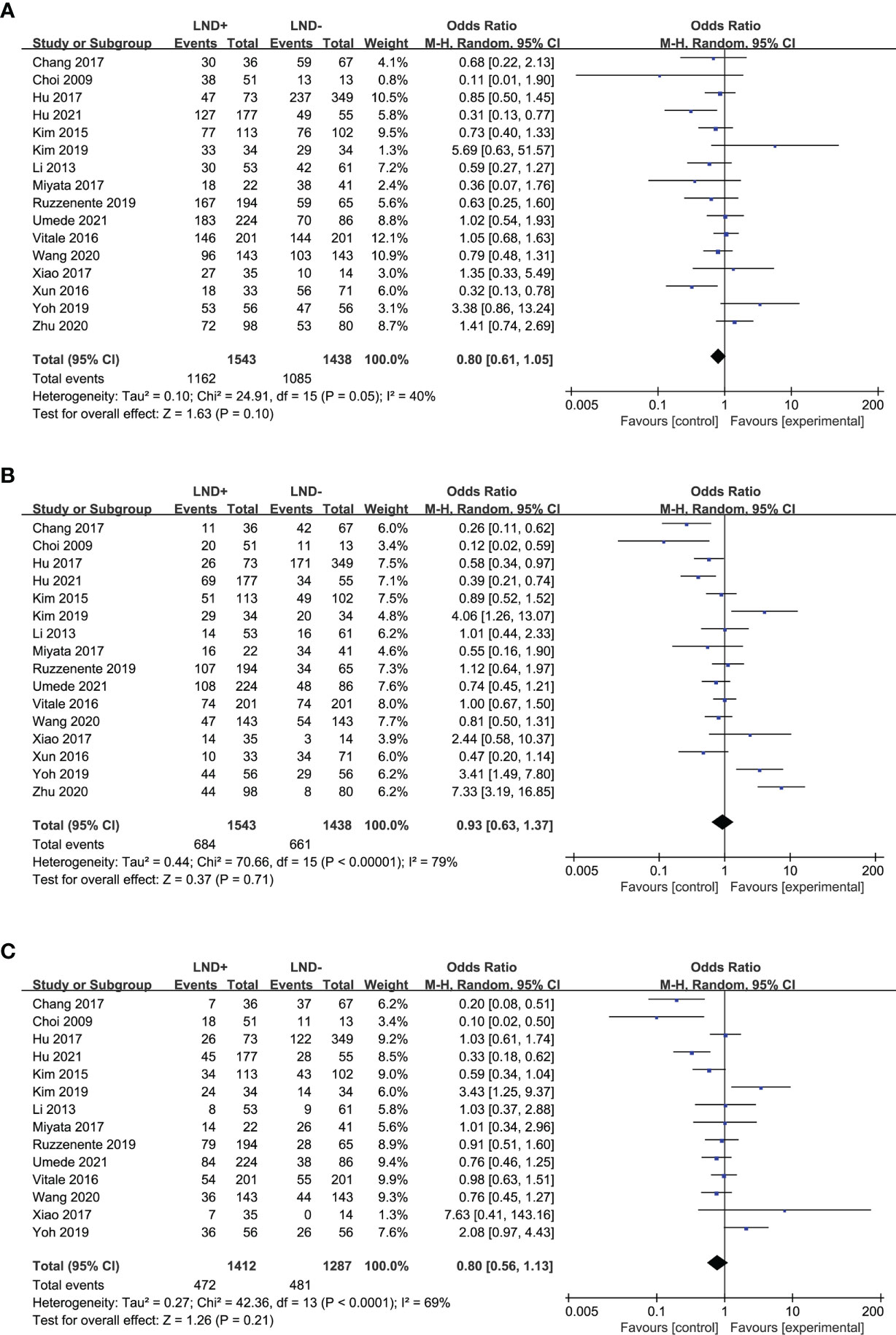
Figure 2 Forest plot comparing overall survival in LND+ and LND− groups. (A) 1-year OS; (B) 3-year OS; (C) 5-year OS.
In a term of recurrence, six studies (20, 22, 24, 25, 28, 36) allowed for pooling of the data to obtain the 1- and 3-year DFS (Figures 3A, B), which showed no significant difference between the LND+ and LND− groups. Five studies (20, 24, 25, 28, 36) that compared 5-year DFS between the LND+ and LND− groups were suitable for data merging (Figure 3C). There was no significant difference between the two groups.
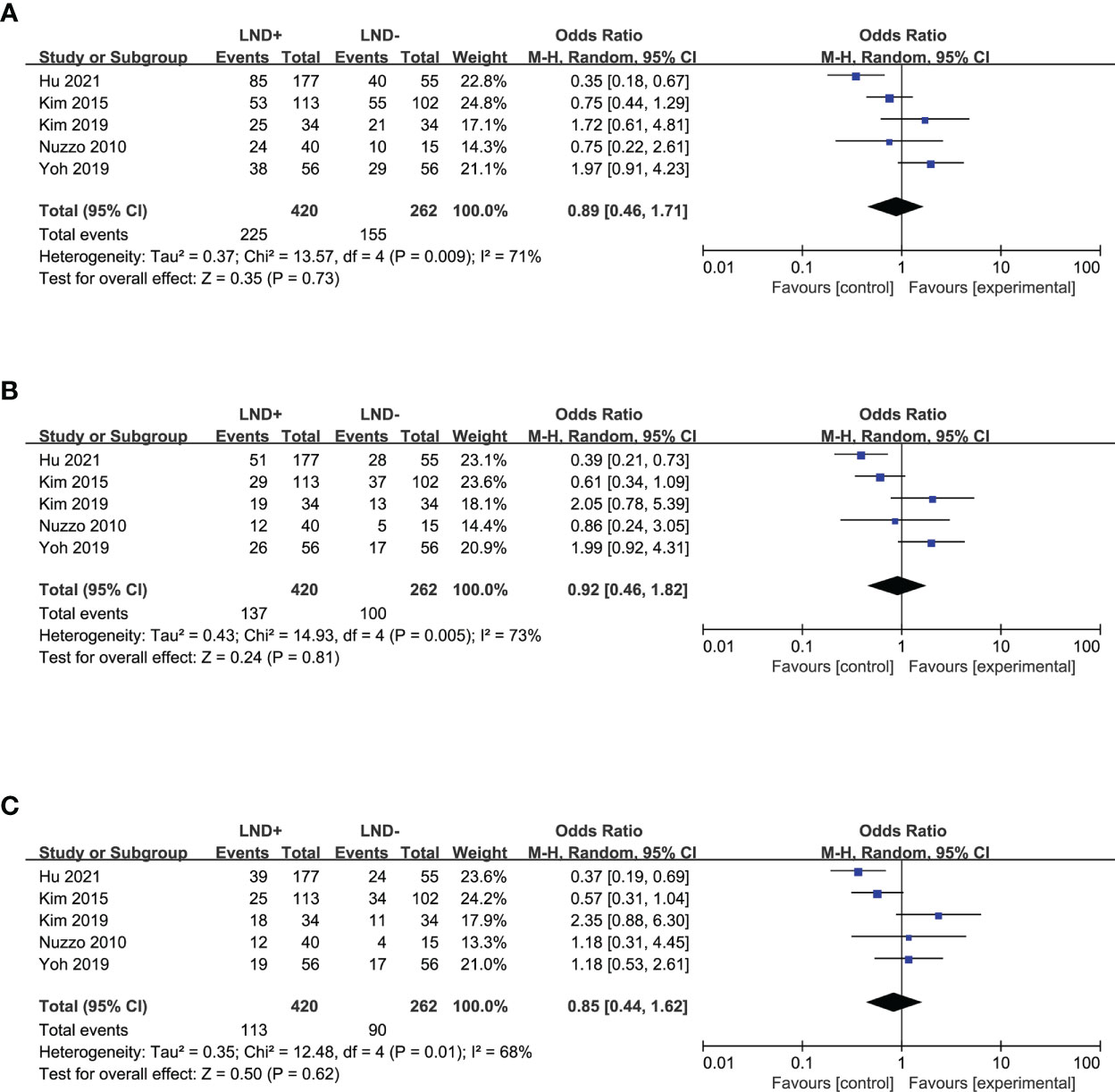
Figure 3 Forest plot comparing disease-free survival in LND+ and LND− groups. (A) 1-year DFS; (B) 3-year DFS; (C) 5-year DFS.
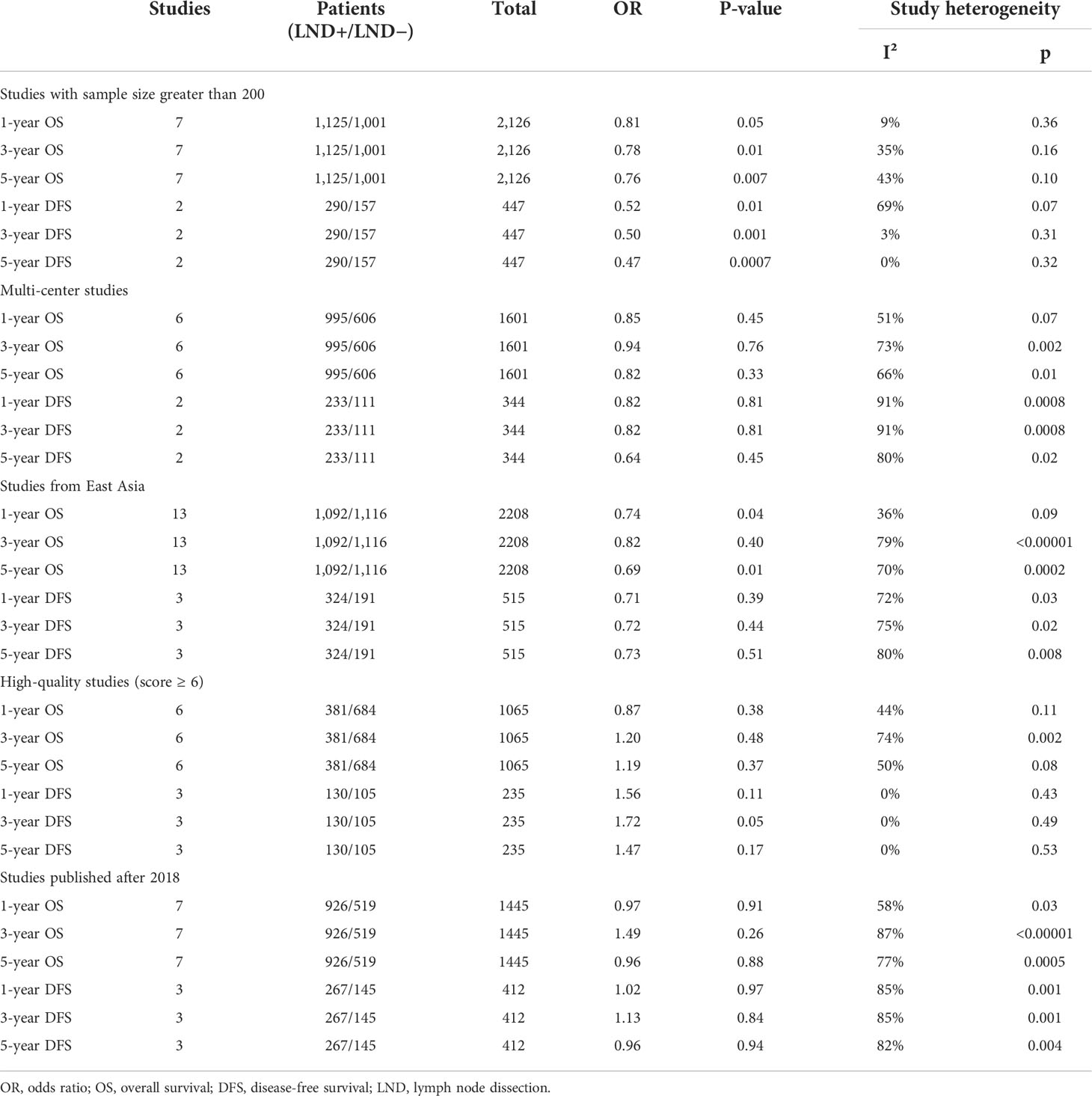
The sensitivity analysis result is shown in Table 1. Greater sample sizes (20, 21, 24, 29–32), multi-center (20, 29–32, 36), East Asia region (17, 18, 20, 21, 24–27, 30, 32, 34, 35, 37), high quality studies that score ≥6 on the NOS (21, 25, 26, 28, 31, 32, 36), and the publication date after 2018 (20, 25, 29, 30, 32, 36, 37) were assessed, respectively.
Discussion
This study quantitatively analyzed the effectiveness of LND in patients with resectable ICC. All of these patients were treated with radical surgery. Compared with patients who did not receive LND, patients who received LND had no survival benefit. Meanwhile, LND is not effective in reducing the probability of recurrence. In the sensitivity analysis to evaluate the number of patients included, a significant difference in support of LND− group was found. However, because of the significant differences between the two groups in baseline in these studies, these results should be carefully interpreted.
Comprehensive treatment based on surgery was recognized as the best mode to treat ICC, but the tumor recurred and metastasized early postoperatively, and the long-term survival rate is poor (38). The majority of studies considered that lymphatic metastasis was the most important prognostic determinant (13, 39, 40). The prevalence of LNM in ICC is as high as 17%–39.1% (41). Some surgeons point out that LND can improve ICC survival. However, some researchers have found that LND is only a staging operation, which has little effect on the prognosis (42, 43). The current findings suggest that LND may not prolong the survival of patients with ICC. The reasons may be as follows: (1) Patients mostly had intrahepatic recurrence after operation. If intrahepatic metastasis is not intervened at the same period, then it is difficult to effectively improve the prognosis of patients by LND alone (2). Once lymph node metastasis occurs, it indicates that the condition is late, and the lymph node metastasis may be beyond the scope of surgical dissection. Even if the scope of dissection is expanded, the effect is limited (44, 45).
Meanwhile, the extent of LND is still controversial. The 2021 NCCN Guidelines (12) and the consensus on American Association of Hepatobiliary and Pancreatic Surgery (9) recommend routine hilar lymph node dissection. In general, conventional or standard LND usually refers to the removal of lymph nodes along the hepatoduodenal ligament, and the area includes lymph node in lesser curvature or left gastric artery, when the tumors are located in the left lobe of the liver. The eighth edition of the AJCC cancer staging system (46) suggests routine LND and removal of at least six LNs. This system also clearly defines regional LNs. In addition to hilar nodes (common bile duct, hepatic artery, portal vein, and cystic duct nodes), regional LNs include the inferior phrenic and gastrohepatic lymph nodes in the left liver lobe. The right lobe covers the periduodenal and peripancreatic LN areas. However, the liver has multiple lymphatic drainage pathways, so further research is needed, possibly including the concept of sentinel lymph nodes.
As the results of surgical treatment of ICCs are generally poor, more and more attention has recently been paid to adjuvant therapy recently to further improve the surgical prognosis of ICC. While the clinical benefits of adjuvant therapy for ICC are still unclear, BILCAP randomized trial recently reported that capecitabine, an adjuvant, can improve the overall survival for biliary tract cancer (47). The potential survival benefits of adjuvant chemotherapy may be related to tumor subtypes, such as lymph node metastasis and the advanced tumor (48). In this perspective, LND is necessary to identify the node status.
The meta-analysis has several limitations. The included studies were almost retrospective, involving selection bias and data missing. The overall number of patients is low, which can easily lead to bias. The sample size of the study is not enough to offset the possible impact of individual differences on the results of the study, and there may be selective bias in the samples and treatment methods. Most of the included studies did not match the propensity score, and the baseline of patients was different. At the same time, some patients with ICC may have adopted other treatment methods such as radiotherapy, chemotherapy, or some molecular targeted drugs after surgery, and the influence of the treatment process on the research was not considered. All of these have led to the deviation and significant heterogeneity of the research results. The differences in patients’ physiological conditions, surgical methods and skills, indications and scope of LND, pathological characteristics of tumors, perioperative adjuvant therapy, and other factors may be the reasons for high heterogeneity.
In conclusion, the current evidence indicates that LND cannot significantly improve the survival of patients with ICC. Because of the uncertain survival benefit, routine or preventive LND cannot be recommended at present. Further prospective randomized clinical trials remain necessary to address this issue.
Author contributions
FL, YJ, and LJ contributed to conception and design of the study. FL organized the database. FL and XY performed the statistical analysis. FL wrote the first draft of the manuscript. QL, SH, JC, SY, YF and FL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gupta A, Dixon E. Epidemiology and risk factors: Intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr (2017) 6(2):101–4. doi: 10.21037/hbsn.2017.01.02
2. Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol (2013) 11(1):13–21.e1. doi: 10.1016/j.cgh.2012.09.009
3. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol (2014) 60(6):1268–89. doi: 10.1016/j.jhep.2014.01.021
4. Ricci AD, Brandi G, AJEooid R. Durvalumab: An investigational anti-Pd-L1 antibody for the treatment of biliary tract cancer. Expert Opin Investig Drugs (2021) 30-1/6:343–350. doi: 10.1080/13543784.2021.1897102
5. Rizzo A, Ricci AD, Brandi GJCT, Communications R. Pemigatinib: Hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat Res Commun (2021); 27:100337. doi: 10.1016/j.ctarc.2021.100337
6. Massa A, Varamo C, Vita F, Tavolari S, Peraldoneia C, Brandi G, et al. Evolution of the experimental models of cholangiocarcinoma. Cancers (Basel) (2020) 12(8):2308. doi: 10.3390/cancers12082308
7. Sommer CM, Kauczor HU, Pereira PLJVM. Locoregional therapies of cholangiocarcinoma. Visc Med (2016) 32(6):414. doi: 10.1159/000453010
8. Brandi G, Rizzo A, Dall'Olio FG, Felicani C, CJIJoH S. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: A retrospective single-center experience. Int J Hyperthermia (2020); 37(1):479–85. doi: 10.1080/02656736.2020.1763484
9. Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: Expert consensus statement. HPB (2015) 17(8):669–80. doi: 10.1111/hpb.12441
10. Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Makuuchi MA. New staging system for mass-forming intrahepatic cholangiocarcinoma. Cancer (2001) 92(9):2374–83. doi: 10.1002/1097-0142(20011101)92:9<2374::AID-CNCR1585>3.0.CO;2-L
11. Morine Y, Shimada M, Utsunomiya T, Imura S, Ikemoto T, Mori H, et al. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today (2012) 42(2):147. doi: 10.1007/s00595-011-0057-9
12. Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Darlow SD. Hepatobiliary cancers, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN (2021) 19(5):541–65. doi: 10.6004/jnccn.2021.0022
13. Endo I, Gonen M, Adam C, Dalal KM, Zhou Q, Klimstra D. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann Surg (2008) 248(1):84–96. doi: 10.1097/SLA.0b013e318176c4d3
14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
15. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell (2008). doi: 10.1002/9780470712184
16. The Newcastle-Ottawa scale (Nos) for assessing the quality of non-randomised studies in meta-analyses. Symposium Systematic Reviews: Beyond Basics (2014).
17. Chang ME, Lei HJ, Chen MH, Yeh YC, Li CP, Hung YP, et al. Evaluation of prognostic factors and implication of lymph node dissection in intrahepatic cholangiocarcinoma: 10-year experience at a tertiary referral center. J Chin Med Assoc (2017) 80(3):140–6. doi: 10.1016/j.jcma.2016.09.010
18. Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: Association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol (2009) 16(11):3048–56. doi: 10.1245/s10434-009-0631-1
19. Gao B, Zhao M, Zhang X, Meng B, Zhuang H, Cheng Z, et al. To study the extent of lymph mode dissection and prognostic factors in patients with intrahepatic cholangiocarcinoma. Chin J Hepatobiliary Surg (2021) 27(8):579–83. doi: 10.3760/cma.j.cn113884-20200810-00423
20. Hu H, Xu G, Du S, Luo Z, Zhao H, Cai J. The role of lymph node dissection in intrahepatic cholangiocarcinoma: A multicenter retrospective study. BMC Surg (2021) 21(1):359. doi: 10.1186/s12893-021-01363-4
21. Hu J, Chen F-Y, Zhou K-Q, Zhou C, Cao Y, Sun H-C, et al. Intrahepatic cholangiocarcinoma patients without indications of lymph node metastasis not benefit from lymph node dissection. Oncotarget (2017) 8(69):113817–27. doi: 10.18632/oncotarget.22852
22. Ji H, Si M, Kuang Y. Significance of lymphadenectomy in intrahepatic cholangiocarcinoma with radical resection. Chin J Pract Surg (2020) 40(6):703–9. doi: 10.19538/j.cjps.issn1005-2208.2020.06.18
23. Ke Q, Wang L, Lin Z, Lou J, Zheng S, Bi X, et al. Prognostic value of lymph node dissection for intrahepatic cholangiocarcinoma patients with clinically negative lymph node metastasis: A multi-center study from China. Front Oncol (2021) 11:585808. doi: 10.3389/fonc.2021.585808
24. Kim DH, Choi DW, Choi SH, Heo JS, Kow AW. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? a review of 17 years of experience in a tertiary institution. Surgery (2015) 157(4):666–75. doi: 10.1016/j.surg.2014.11.006
25. Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Oncologic impact of lymph node dissection for intrahepatic cholangiocarcinoma: A propensity score-matched study. J Gastrointest Surg (2019) 23(3):538–44. doi: 10.1007/s11605-018-3899-2
26. Li DY, Zhang HB, Yang N, Quan Y, Yang GS. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: Results of a monocentric series. World J Gastroenterol (2013) 19(47):9084–91. doi: 10.3748/wjg.v19.i47.9084
27. Miyata T, Yamashita YI, Yamao T, Umezaki N, Tsukamoto M, Kitano Y, et al. Clinical benefits of lymph node dissection in intrahepatic cholangiocarcinoma: A retrospective single-institution study. Anticancer Res (2017) 37(5):2673–7. doi: 10.21873/anticanres.11615
28. Nuzzo G, Giuliante F, Ardito F, De Rose AM, Vellone M, Clemente G, et al. Intrahepatic cholangiocarcinoma: Prognostic factors after liver resection. Updates Surg (2010) 62(1):11–9. doi: 10.1007/s13304-010-0007-x
29. Ruzzenente A, Conci S, Vigano L, Ercolani G, Manfreda S, Bagante F, et al. Role of lymph node dissection in small (</= 3 Cm) intrahepatic cholangiocarcinoma. J Gastrointest Surg (2019) 23(6):1122–9. doi: 10.1007/s11605-019-04108-0
30. Umeda Y, Mitsuhashi T, Kojima T, Satoh D, Sui K, Endo Y, et al. Impact of lymph node dissection on clinical outcomes of intrahepatic cholangiocarcinoma: Inverse probability of treatment weighting with survival analysis. J Hepatobiliary Pancreat Sci (2022) 29(2):217–29. doi: 10.1002/jhbp.1038
31. Vitale A, Moustafa M, Spolverato G, Gani F, Cillo U, Pawlik TM. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol (2016) 113(6):685–91. doi: 10.1002/jso.24213
32. Wang L, Lin Z, Yang T, Lou J, Zheng S, Bi X, et al. A multicenter retrospective study on clinical value of lymph node dissection in the radical resection of intrahepatic cholangiocarcinoma. Chin J Digestive Surg (2020) 19(1):72–80. doi: 10.3760/cma.j.issn.1673-9752.2020.01.012
33. Wu ZF, Wu XY, Zhu N, Xu Z, Li WS, Zhang HB, et al. Prognosis after resection for hepatitis b virus-associated intrahepatic cholangiocarcinoma. World J Gastroenterol (2015) 21(3):935–43. doi: 10.3748/wjg.v21.i3.935
34. Xiao J, Zhu J, Liu Z, Wan R, Li Y, Xiao W. Role of surgical treatment for hepatolithiasis-associated intrahepatic cholangiocarcinoma: A retrospective study in a single institution. J Cancer Res Ther (2017) 13(5):756–60. doi: 10.4103/jcrt.JCRT_356_17
35. Xun X, Li Q, Chen X. Surgical treatment of intrahepatic cholangiocarcinoma: A retrospective study of 104 cases. Chin J Hepatobiliary Surg (2016) 22(6):382–5. doi: 10.3760/cma.j.issn.1007-8118.2016.06.008
36. Yoh T, Cauchy F, Le Roy B, Seo S, Taura K, Hobeika C, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery (2019) 166(6):975–82. doi: 10.1016/j.surg.2019.06.025
37. Zhu M, Zhang X, Han F, Nie C. Prognostic factors of radical resection on patients with intrahepatic cholangiocarcinoma and impact of abdominal lymph node dissection on prognosis. Chin J Hepatobiliary Surg (2020) 26(1):48–52. doi: 10.3760/cma.j.issn.1007-8118.2020.01.011
38. Mosconi S, Beretta GD, Labianca R, Zampino MG, Heinemann V. Cholangiocarcinoma. Crit Rev Oncology/hematology (2008) 69(3):259–70. doi: 10.1016/j.critrevonc.2008.09.008
39. Miwa S, Miyagawa S, Kobayashi A, Akahane Y, Nakata T, Mihara M, et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol (2006) 41(9):893–900. doi: 10.1007/s00535-006-1877-z
40. Uchiyama K, Yamamoto M, Yamaue H, Ariizumi S, Aoki T, Kokudo N, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: A multicenter analysis by the study group for hepatic surgery of the Japanese society of hepato-Biliary-Pancreatic surgery. J Hepatobiliary Pancreat Sci (2011) 18(3):443–52. doi: 10.1007/s00534-010-0349-2
41. Bartsch F, Hahn F, Müller L, Baumgart J, Lang H. Relevance of suspicious lymph nodes in preoperative imaging for resectability, recurrence and survival of intrahepatic cholangiocarcinoma. BMC Surg (2020) 20(1):75. doi: 10.1186/s12893-020-00730-x
42. Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver international: Off J Int Assoc Study Liver (2019) 39(Suppl 1):143–55. doi: 10.1111/liv.14089
43. Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg (2005) 29(6):728. doi: 10.1007/s00268-005-7761-9
44. Shima Da M, Yamashita Y, Aishima S, Shira Be K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg (2001) 88(11):1463–6. doi: 10.1046/j.0007-1323.2001.01879.x
45. Yoshikazu M, Yasuhiro T, Toshinori I, Masaaki N, Hiroyuki N, Toshiro N. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J Hepato-Biliary-Pancreatic Surg (2003) 10(6):432–40. doi: 10.1007/s00534-002-0842-3
46. Yun SC, Pawlik TM, Vauthey JN. 8th edition of the ajcc cancer staging manual: Pancreas and hepatobiliary cancers. Ann Surg Oncol (2018) 25(4):1–3. doi: 10.1245/s10434-017-6025-x
47. Primrose J, Fox R, Palmer D, Prasad R, Mirza D, Anthoney D, et al. Adjuvant capecitabine for biliary tract cancer: The bilcap randomized study. J Clin Oncol (2017) 35(15_suppl):4006. doi: 10.1200/JCO.2017.35.15_suppl.4006
Keywords: intrahepatic cholangiocarcinoma, lymph node, prognosis, overall survival, disease-free survival
Citation: Li F, Jiang Y, Jiang L, Li Q, Yan X, Huang S, Chen J, Yuan S, Fu Y and Liu J (2022) Effect of lymph node resection on prognosis of resectable intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Front. Oncol. 12:957792. doi: 10.3389/fonc.2022.957792
Received: 31 May 2022; Accepted: 09 September 2022;
Published: 27 September 2022.
Edited by:
Zhaohui Tang, Shanghai Jiao Tong University, ChinaReviewed by:
Mikel Prieto, Cruces University Hospital, SpainGiovanni Brandi, University of Bologna, Italy
Copyright © 2022 Li, Jiang, Jiang, Li, Yan, Huang, Chen, Yuan, Fu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, RHJfbGl1anVuMTk2N0AxMjYuY29t
 Feiyu Li
Feiyu Li Yong Jiang
Yong Jiang Liyong Jiang
Liyong Jiang Qingbin Li2
Qingbin Li2 Xiangyu Yan
Xiangyu Yan Yingda Fu
Yingda Fu Jun Liu
Jun Liu