
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 11 August 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.956372
This article is part of the Research TopicSmall Cell Lung Cancer: New Drugs and StrategiesView all 15 articles
A 50-year-old female patient presented with post-exercise dyspnea in September 2016, and was subsequently diagnosed with SCLC with multiple brain and spinal metastases. The first-line treatment was etoposide combined with cisplatin and synchronously performed radiotherapy for the brain and spinal cord metastases. She was treated with anlotinib after disease progression in December 2018 and continued to have clinical benefit for nearly 25 months. Unexpectedly, the patient can still benefit from further combination treatment with durvalumab after another disease progression in February 2021. Thus, it may be a potential option to use anlotinib along with immunotherapy after the anlotinib resistance in SCLC, but more clinical data are still needed to confirm it. Moreover, ctDNA dynamic monitoring was performed and reflected the outcome of the process of treatment.
Small cell lung cancer (SCLC) is a malignant tumor with aggressive, rapid progression, and metastatic potential, accounting for about 10%–15% of lung cancer cases. At present, chemotherapy is still the main treatment for SCLC, and only a small number of patients can receive second-line treatment with limited benefit. Immune checkpoint inhibitors have shown good clinical effects in the first-line and backward treatment of SCLC, but their absolute benefit for SCLC is still limited (1, 2). Besides, there have been attempts to research antiangiogenic agents for SCLC, but previous studies have demonstrated that most antiangiogenic agents and the combination drug regimens for treating first-line or posterior SCLC have failed (3, 4). Thus, it is still necessary to explore more effective and safe new drugs and therapeutic schedules for SCLC. As a novel multi-target tyrosine kinase inhibitor (TKI), anlotinib can inhibit vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR), and c-Kit at the same time, which can inhibit both angiogenesis and tumor growth. The study of ALTER1202 demonstrated that, compared with placebo, the progression-free survival (PFS) and overall survival (OS) were significantly improved in the third-line and above treatment of SCLC. Here, we present one case of an advanced SCLC patient who had received concurrent chemoradiotherapy (cCRT) and long-term benefit from anlotinib monotherapy after multiple lines of chemotherapy. Moreover, the combination of anlotinib and durvalumab still resulted in durable PFS and the tolerance was good enough after the disease progression.
A 50-year-old female was admitted to our hospital on 18 September 2016 due to post-exercise dyspnea and lower extremity parethesia. She had no cigarette history, no family history, but was allergic to sulfa. Enhanced chest CT indicated central lung cancer in the middle and lower hilum of the right lung with pulmonary atelectasis, invasion of the right hilar vessels, and mediastinal lymph node metastasis. Magnetic resonance imaging (MRI) of her brain and spinal cord revealed multiple brain and spinal metastases (Figures 1Ai,ii). Bronchoscopic biopsy pathology examination showed evidence of small cell carcinoma (Figure 2). An extensive stage of SCLC was diagnosed. The patient initially received inductive chemotherapy with “etoposide 100 mg/m2 (d1–d5) + cisplatin 120 mg/m2 (d1)” for two cycles on 23 September 2016 and 13 October 2016. Evaluation by CT scan showed a partial response (PR) based on the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Concurrent chemoradiotherapy (cCRT) was sequentially delivered, followed by two more cycles of adjuvant chemotherapy. The radiotherapy regimen including GTV/CTV 60/50 Gy/20 times for right lung and right hilar lymph node lesions, CTV 40 Gy/20 times for spinal cord metastasis at T2–3, CTV 30 Gy for intracranial metastasis of the whole brain (right frontal lobe, right paracentral lobule), and 50 Gy/15 times for GTV intracranial metastasis. During cCRT, the patient developed grade 1–2 gastrointestinal adverse reactions and grade 4 granulocytopenia and thrombocytopenia, so the adjuvant chemotherapy was suspended. The response was categorized as PR (Figures 1Bi,ii).
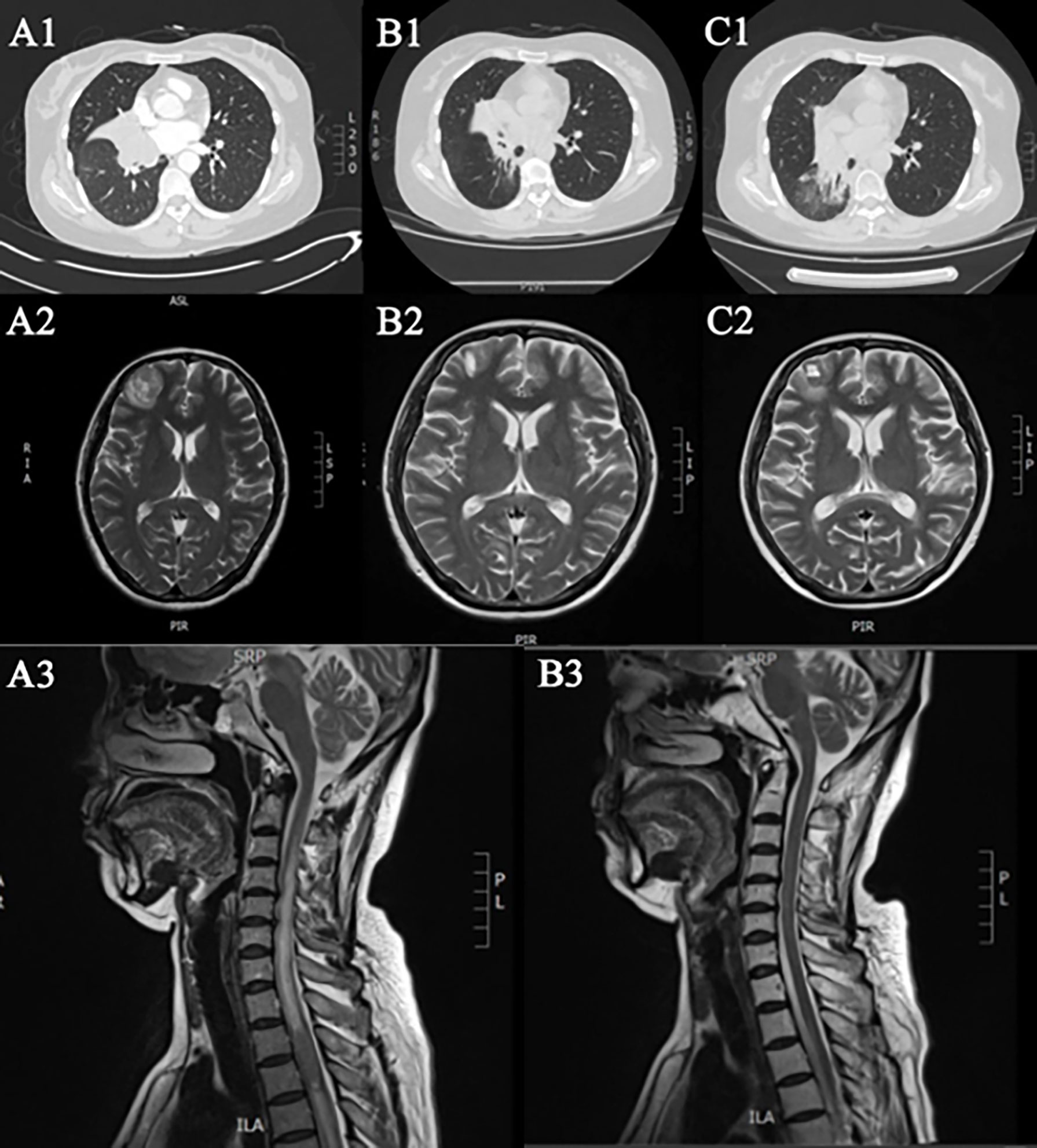
Figure 1 Imaging during chemotherapy. Ai-iii First diagnosis. Bi-iii After radiotherapy and chemotherapy. (Ci, ii) Progress after the second chemotherapy.
Unfortunately, the patient suffered multifocal metastases on October 9, 2018. PET/CT found a new lesion in the right lower lobe with multiple new hypermetabolic mediastinal lymph nodes. Meanwhile, a plasma ctDNA test was performed and six gene missense mutations were found, including CREBBP, KIT, MUTYH, MYC, PREX2, and SMO. Blood tumor mutational burden (bTMB), defined as the number of somatic, coding, base substitution, and indel mutations per megabase (Mb) of genome examined, was calculated from the GENETRON OncoPanscan 825 Panel NGS platforms. “TMB high” was defined as cases with a TMB of ≥10 muts/Mb, and the bTMB of this patient was 23.33 muts/Mb. Since there were no approved immunotherapy drugs for SCLC, the patient just received another two cycles of “etoposide + lobaplatin” chemotherapy on 18 October 2018 and 12 November 2018. But the response was categorized as progressive disease (PD) (Figures 1Ci,ii).
The patient then started taking anlotinib (12 mg d1–d14/q3w) from December 2018 until the scale of the pulmonary lesion shrank and cavitated (Figure 3A). Later, the dose of anlotinib was reduced to 10 mg because of paronychia. The consolidation of lung lesions was reviewed on 14 May 2020 (Figure 3B), and the dose of anlotinib was increased to 12 mg considering the risk of disease progression. During this period, the best efficacy was PR (Figure 3C), and no adverse reactions were reported.
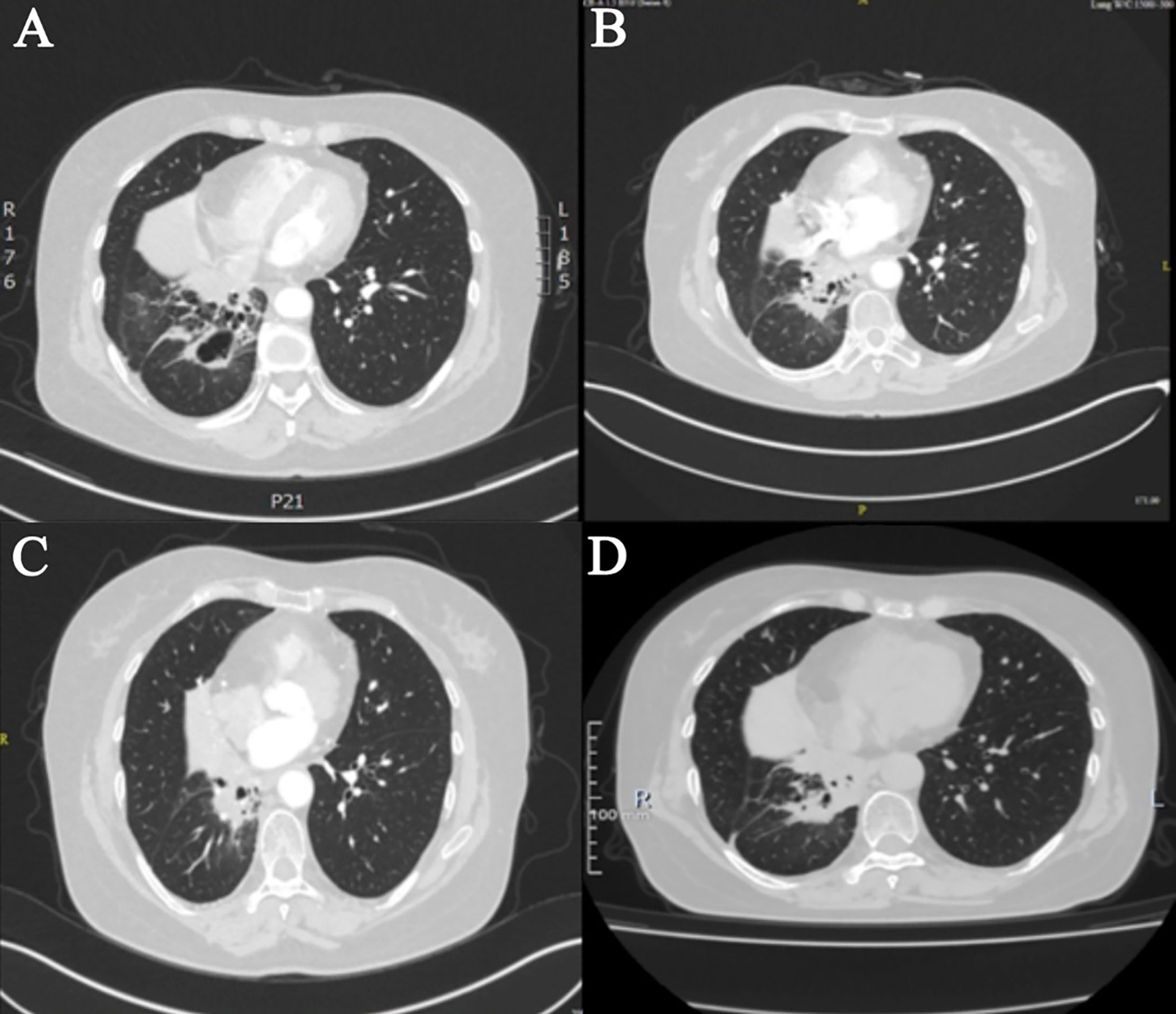
Figure 3 Imaging during Anlotinib treatment. (A) In December 2019, after anlotinib treatment, cavities formed. (B) In May 2020, void consolidation. (C) In November 2020, after the dose was increased, the lesions shrank again. (D) In October 2021, anlotinib combined with durvalumab shrink the lesion.
In January 2021, she was referred for combination therapy of anlotinib along with durvalumab. As she had side effects during the previous anlotinib treatment, when adding durvalumab on this basis, to avoid the aggravation of side effects, the dose of durvalumab was adjusted and reduced to 1,000 mg q4w. The current clinical effectiveness was PR (Figure 3D). The last follow-up time was 28 March 2022. A ctDNA test was performed to monitor the effect of the treatment with bTMB reduced to 0 muts/Mb, which indicated the continuous benefit of anlotinib plus durvalumab.
In summary, the complete treatment pathway for patients is demonstrated in Figure 4.
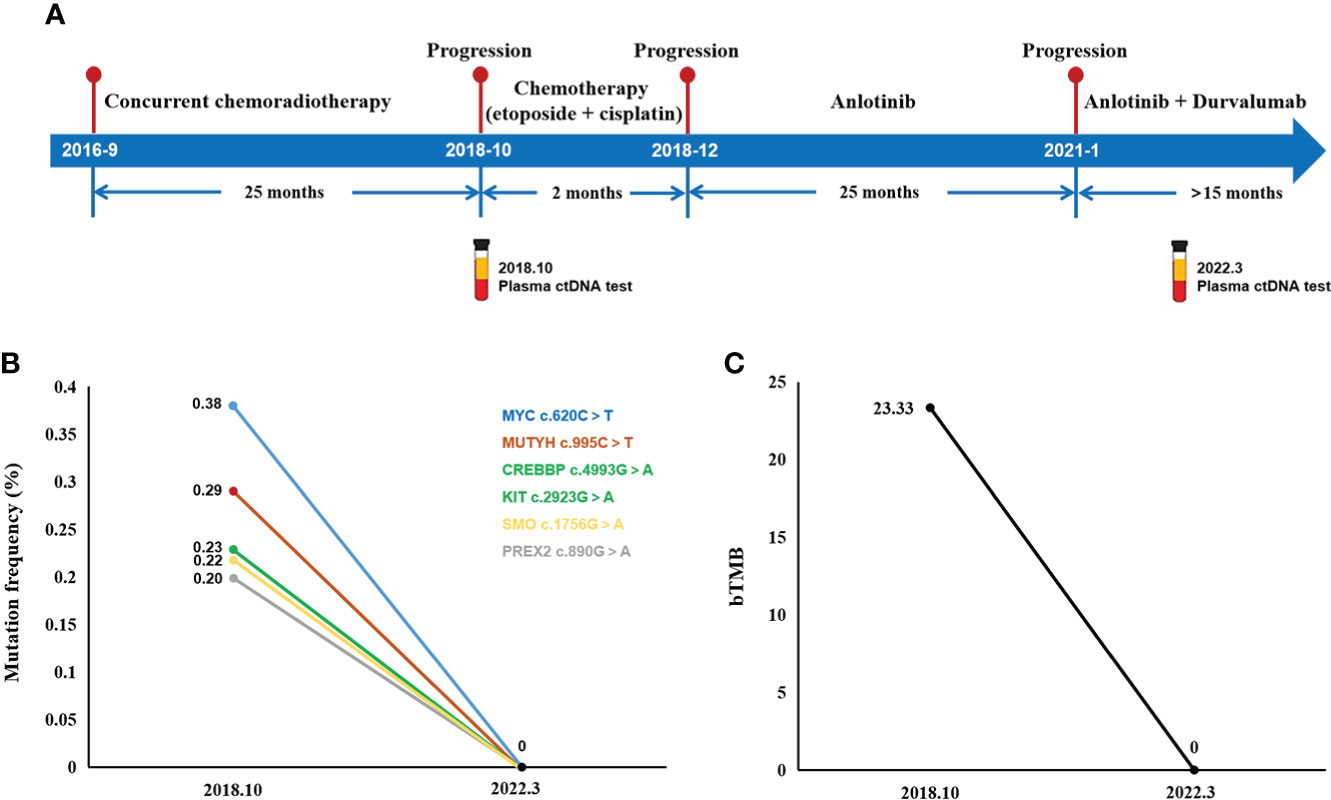
Figure 4 Treatment course and ctDNA NGS test results. (A) The complete treatment path of the patient. (B) Changes in gene mutation frequency and bTMB (C) between 2018 and 2022.
Extensive-stage SCLC accounts for about 60%–65% of SCLC (5). Previous studies have shown that the prognosis of extensive-stage SCLC is bleak, which has a median survival time of approximately 10 months and a less than 5% five-year survival rate with first-line chemotherapy regimens (6). Here, we report a case of a patient who achieved a response of nearly 25 months with the third-line treatment of anlotinib after the failure of second-line chemotherapy, and continued to achieve durable PFS and whose tolerance was satisfactory with subsequent anlotinib along with a durvalumab regimen after its progression.
Angiogenesis plays an important role in tumor growth, proliferation, and metastasis in SCLC (7). Thus, the anti-angiogenic drugs may play an important role in the treatment of SCLC as well. A study of 24 SCLC patients with the anti-angiogenic drug sunitinib, who had received at least one line of chemotherapy or concurrent chemoradiotherapy, showed that the ORR was 19%, and the median PFS and OS were 1.4 months and 5.6 months, respectively (8). Pazopanib is an inhibitor of the tyrosine kinase VEGFR2, PDGFR, and c-kit. In the study by Koinis et al. (9), patients after first-line platinum-based chemotherapy were included and divided into platinum-sensitive groups and platinum-resistant groups. In the overall 58 patients, the ORR was 13.8%, and the median PFS and OS were 2.5 months and 6.0 months, respectively. It seems that anti-angiogenic drug therapy plays a role in the second/third and above-line treatment of SCLC. But the number of study cases is small among those studies, which needs to be verified by a larger sample size. Anlotinib, as a novel TKI, can inhibit VEGFR, FGFR, PDGFR, and c-Kit at the same time, as well as inhibit angiogenesis and tumor growth. The ALTER1202 study, a randomized, double-blind, placebo-controlled multicenter phase II study of anlotinib in third-line and above treatment of SCLC, has been conducted in 2018. The results of ALTER1202 showed (10, 11) that patients with progressive or recurrent SCLC who were treated with anlotinib after second-line treatment had significant clinical benefits compared with placebo. Patients had a favorable clinical benefit of 3.4-month improvement in PFS (HR = 0.19, p <0.0001) and 2.4-month improvement in OS (HR = 0.53, p = 0.0029) in the anlotinib group. In our case, anlotinib was prescribed for the patient after disease progression from chemotherapy. In August 2019, anlotinib was approved for the treatment of SCLC that has progressed or relapsed after at least two prior chemotherapy regimens in China, and it is the only approved anti-angiogenic drug for the treatment of SCLC.
Immunotherapy has changed the treatment outcomes of advanced lung cancer. In the first-line treatment, atezolizumab combined with etoposide and carboplatin-improved OS from 10.3 months to 12.3 months compared with chemotherapy (1). The OS was also significantly better in the durvalumab + etoposide +/− carboplatin group than in the chemotherapy group (13.0 months vs 10.3 months) (12). Therefore, the above regimens have been approved for first-line treatment of extensive-stage SCLC in many countries. There are also case reports indicating that the PD-L1 antibody durvalumab can achieve a response of 7 months in third-line treatment of extensive-stage SCLC (13).
However, the clinical effectiveness of the anti-PD-1 inhibitors nivolumab and pembrolizumab in the third-line treatment of SCLC is still controversial. In the Checkmate032 study, the ORR was 10% in patients with relapsed SCLC treated with nivolumab 3 mg/kg, 23% in nivolumab (1 mg/kg) plus ipilimumab (3 mg/kg), and 19% in nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg) (2). In this study, the duration of response was 17.9 months, the PFS was 1.4 months, and the OS was 5.6 months in the analysis of the third-line treatment with nivolumab alone (14). Similar results were shown in the KEYNOTE-028/158 study, which showed that the median PFS was 2.0 months and the median OS reached 7.7 months in the third-line and above treatment for SCLC (15). Based on the poor outcomes, Bristol-Myers Squibb (BMS) announced the withdrawal of nivolumab for the SCLC indication in the United States, and pembrolizumab also voluntarily withdrew its application for the SCLC indication in consultation with the FDA in 2021.
In recent years, the synergistic anti-tumor effect of anti-angiogenic drugs combined with immune checkpoint inhibitors has been supported by several studies. In preclinical (16) studies, anti-angiogenic drugs can promote the normalization of tumor vessels and regulate the immune microenvironment in many ways, which in turn activates the immune system. The mechanisms include promoting the maturation of dendritic cells, restoring the mobilization and infiltration of T cells, influencing the adhesion of lymphocytes, and reducing the induction and proliferation of inhibitory immune cells. At the same time, various innate and acquired immune cells are involved in the formation of blood vessels in tumors, and immune checkpoint inhibitors can promote tumor vascular normalization (17). This combination regimen has shown some efficacy for treating advanced SCLC. In the PASSION study of second-line treatment of SCLC (18), the efficacy results showed that the ORR was 33.9% and the PFS was 2.8 months in the overall population of apatinib combined with camrelizumab. The analysis showed that the ORR in the chemoresistant population and chemosensitive population was similar to that in the overall population.
However, although anti-angiogenic or anti-angiogenic drugs combined with immune checkpoint inhibitors for treating SCLC have shown preliminary efficacy, there are still many issues to be discussed, such as the suitable treatment population and the dose of treatment. In the combination therapy, the appropriate dose of anti-angiogenic drugs and the medication regimen are still worth exploring. Lin et al. (19) found that low-dose anti-angiogenic drug therapy may play an immune-promoting role by enhancing M1 polarization of macrophages and enhancing CD8+ T-cell function, while high-dose anti-angiogenic drug therapy may lead to immunosuppression of the microenvironment.
Moreover, liquid biopsy refers to the analysis of tumor-derived components in body fluids, among which circulating tumor DNA (ctDNA) has been used for dynamic monitoring of tumor changes, therapeutic effects, and patient prognosis in many cancers, including NSCLC (20), melanoma (21), and colorectal cancer (22). Compared with traditional tissue biopsy, ctDNA was noninvasive and could solve the problem of tumor heterogeneity. In SCLC, a few studies have shown that high pre-treatment ctDNA levels were associated with a poor prognosis in PFS and OS (23, 24), and plasma ctDNA could monitor dynamically the effect of treatment (25). The detection of ctDNA in LS-SCLC patients after curative treatment predicts disease recurrence and death (26). Additionally, ctDNA is a prognostic determinant in patients with SCLC treated with atezolizumab, and ctDNA is strongly associated with prognosis in SCLC patients treated with second-line immunotherapy (27). Although patients benefited from immunotherapy regardless of bTMB status in the IM133 clinical trial, it was found that patients with bTMB ≥16 were more likely to benefit from immunotherapy (28). In conclusion, liquid biopsy methods provide effective baseline analysis and longitudinal surveillance of LS and ES disease and have now been included in expanded SCLC studies and trials. We look forward to the results of these studies, particularly prospective studies of the role of ctDNA in predicting the efficacy of immunotherapy in SCLC.
Here, we found that patients with extensive SCLC can benefit from anti-angiogenic therapy plus immunotherapy depending on the situation. The efficacy may be assessed by ctDNA or bTMB, but further research is also needed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Authors HYW, XNW, SXJ, and JNZ collected the clinical information, diagnostic information, therapeutic information, and images of the patients. HYW wrote the manuscript. YH identified the case and submitted the manuscript. JNZ and CHZ revised the manuscript. YJZ and JL proofread the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by the Wu Jieping Medical Foundation (No. 320.6750.2020-19-15).
We are thankful to HY, KW, and YL for their kindness help in this case. All the authors are thankful to the patients who participated in this case.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
2. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol (2016) 17:883–95. doi: 10.1016/S1470-2045(16)30098-5
3. Jalal S, Bedano P, Einhorn L, Bhatia S, Ansari R, Bechar N, et al. Paclitaxel plus bevacizumab in patients with chemosensitive relapsed small cell lung cancer: A safety, feasibility, and efficacy study from the Hoosier oncology group. J Thorac Oncol (2010) 5:2008–11. doi: 10.1097/JTO.0b013e3181f77b6e
4. Li H, Zeng J, Jin X, Yu X, Zhou G, Hong W. Apatinib for chemotherapy-refractory extensive-stage SCLC: a retrospective study. Cancer Chemother Pharmacol (2019) 83:1083–90. doi: 10.1007/s00280-019-03823-4
5. Bernhardt EB, Jalal SI. Small cell lung cancer. Cancer Treat Res (2016) 170:301–22. doi: 10.1007/978-3-319-40389-2_14
6. Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, et al. The international association for the study of lung cancer lung cancer staging project: Proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11:300–11. doi: 10.1016/j.jtho.2015.10.008
7. Lucchi M, Mussi A, Fontanini G, Faviana P, Ribechini A, Angeletti CA. Small cell lung carcinoma (SCLC): the angiogenic phenomenon. Eur J Cardiothorac Surg (2002) 21:1105–10. doi: 10.1016/s1010-7940(02)00112-4
8. Han JY, Kim HY, Lim KY, Han JH, Lee YJ, Kwak MH, et al. A phase II study of sunitinib in patients with relapsed or refractory small cell lung cancer. Lung Cancer (2013) 79:137–42. doi: 10.1016/j.lungcan.2012.09.019
9. Koinis F, Agelaki S, Karavassilis V, Kentepozidis N, Samantas E, Peroukidis S, et al. Second-line pazopanib in patients with relapsed and refractory small-cell lung cancer: a multicentre phase II study of the Hellenic oncology research group. Br J Cancer (2017) 117:8–14. doi: 10.1038/bjc.2017.137
10. Liu C, Liao J, Wu X, Zhao X, Sun S, Wang H, et al. A phase II study of anlotinib combined with etoposide and platinum-based regimens in the first-line treatment of extensive-stage small cell lung cancer. Thorac Cancer (2022) 13:1463–70. doi: 10.1111/1759-7714.14414
11. Cheng Y, Wang Q, Li K, Shi J, Liu Y, Wu L, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: A randomised, double-blind, placebo-controlled phase 2 study. Br J Cancer (2021) 125:366–71. doi: 10.1038/s41416-021-01356-3
12. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
13. Zhou Q, Zhao J, Wang J, Bao G, Gong LY. Durvalumab monotherapy as a third-line treatment for extensive-stage small-cell lung cancer: A case report. Ann Palliat Med (2020) 9:2386–92. doi: 10.21037/apm-20-1244
14. Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol (2019) 14:237–44. doi: 10.1016/j.jtho.2018.10.003
15. Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH Jr, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: Results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol (2020) 15:618–27. doi: 10.1016/j.jtho.2019.12.109
16. Liang H, Wang M. Prospect of immunotherapy combined with anti-angiogenic agents in patients with advanced non-small cell lung cancer. Cancer Manag Res (2019) 11:7707–19. doi: 10.2147/CMAR.S212238
17. Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med (2020) 52:1475–85. doi: 10.1038/s12276-020-00500-y
18. Fan Y, Zhao J, Wang Q, Huang D, Li X, Chen J, et al. Camrelizumab plus apatinib in extensive-stage SCLC (PASSION): A multicenter, two-stage, phase 2 trial. J Thorac Oncol (2021) 16:299–309. doi: 10.1016/j.jtho.2020.10.002
19. Lin YY, Tan CT, Chen CW, Ou DL, Cheng AL, Hsu C. Immunomodulatory effects of current targeted therapies on hepatocellular carcinoma: Implication for the future of immunotherapy. Semin Liver Dis (2018) 38:379–88. doi: 10.1055/s-0038-1673621
20. Fan G, Zhang K, Ding J, Li J. Prognostic value of EGFR and KRAS in circulating tumor DNA in patients with advanced non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget (2017) 8:33922–32. doi: 10.18632/oncotarget.15412
21. Herbreteau G, Vallée A, Knol AC, Théoleyre S, Quéreux G, Frénard C, et al. Circulating tumour DNA is an independent prognostic biomarker for survival in metastatic BRAF or NRAS-mutated melanoma patients. Cancers (Basel) (2020) 12:1871. doi: 10.3390/cancers12071871
22. Basnet S, Zhang ZY, Liao WQ, Li SH, Li PS, Ge HY. The prognostic value of circulating cell-free DNA in colorectal cancer: A meta-analysis. J Cancer (2016) 7:1105–13. doi: 10.7150/jca.14801
23. Almodovar K, Iams WT, Meador CB, Zhao Z, York S, Horn L, et al. Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J Thorac Oncol (2018) 13:112–23. doi: 10.1016/j.jtho.2017.09.1951
24. Nong J, Gong Y, Guan Y, Yi X, Yi Y, Chang L, et al. Circulating tumor DNA analysis depicts subclonal architecture and genomic evolution of small cell lung cancer. Nat Commun (2018) 9:3114. doi: 10.1038/s41467-018-05327-w
25. Zhang M, Huang C, Zhou H, Liu D, Chen R, Li X, et al. Circulating tumor DNA predicts the outcome of chemotherapy in patients with lung cancer. Thorac Cancer (2022) 13:95–106. doi: 10.1111/1759-7714.14230
26. Iams WT, Kopparapu PR, Yan Y, Muterspaugh A, Zhao Z, Chen H, et al. Blood-based surveillance monitoring of circulating tumor DNA from patients with SCLC detects disease relapse and predicts death in patients with limited-stage disease. JTO Clin Res Rep (2020) 1:100024. doi: 10.1016/j.jtocrr.2020.100024
27. Herbreteau G, Langlais A, Greillier L, Audigier-Valette C, Uwer L, Hureaux J, et al. Circulating tumor DNA as a prognostic determinant in small cell lung cancer patients receiving atezolizumab. J Clin Med (2020) 9:3861. doi: 10.3390/jcm9123861
28. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol (2021) 39:619–30. doi: 10.1200/JCO.20.01055
Keywords: extensive-stage small cell lung cancer, anlotinib, durvalumab, long survival, NGS, bTMB
Citation: Wang H, Wang X, Jiang S, Zhu J, Liu J, Zhou C, Zhu Y and Han Y (2022) Personalized treatment of extensive stage small cell lung cancer: A case report and literature review. Front. Oncol. 12:956372. doi: 10.3389/fonc.2022.956372
Received: 30 May 2022; Accepted: 19 July 2022;
Published: 11 August 2022.
Edited by:
Alessandro Morabito, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Hua Zhong, Shanghai Jiao Tong University, ChinaCopyright © 2022 Wang, Wang, Jiang, Zhu, Liu, Zhou, Zhu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Han, aGFueW9uZ190ZEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.