
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 13 July 2022
Sec. Cancer Molecular Targets and Therapeutics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.955670
This article is part of the Research TopicMolecular Markers and Targeted Therapy for Hepatobiliary TumorsView all 51 articles
Background: Cholangiocarcinoma (CCA) is a highly aggressive malignancy with extremely poor prognosis. Perihilar CCA (pCCA) is the most common subtype of CCA, but its biomarker study is much more lagged behind other subtypes. ZFAND5 protein can interact with ubiquitinated proteins and promote protein degradation. However, the function of ZFAND5 in cancer progression is rarely investigated, and the role of ZFAND5 in pCCA is never yielded.
Materials and Methods: In this study, we established a pCCA cohort consisting of 72 patients. The expression of ZFAND5 in pCCAs, and the paired liver tissues, intrahepatic bile duct tissues and common bile ducts (CBD) tissues were detected with IHC. ZFAND5 mRNA in pCCAs and CBDs was detected with qRT-PCR. The pCCA cohort was divided into ZFAND5low and ZFAND5high subsets according to the IHC score. The correlations between ZFAND5 expression and clinicopathological parameters were assessed bychi-square test. The prognostic significance of ZFAND5 expression and clinicopathological parameters was estimated by univariate analysis with Kaplan-Meier method, and by multivariate analysis with Cox-regression model.
Results: Expression of ZFAND5 in pCCAs was substantially higher than that in interlobular bile ducts and common bile ducts, but lower than that in liver tissues. The ZFAND5low and ZFAND5high subsets accounted for 44.4% and 55.6% of all pCCAs respectively. ZFAND5 high patients had much lower survival rates than the ZFAND5low patients, with the average survival time as 31.2 months and 19.5 months respectively. ZFAND5 was identified as an independent unfavorable prognostic biomarker of pCCA with multivariate analysis.
Conclusion: ZFAND5 expression was up-regulated in pCCAs compared with the CBDs. We identified ZFAND5 as an independent biomarker of pCCA, which could provide more evidence for the molecular classification of pCCA, and help stratify the high-risk patients based on the molecular features.
Cholangiocarcinoma (CCA) is a highly aggressive malignancy originating from bile duct (1). CCA has several clinical manifestations such as dormant symptoms, severe malignancy, rapid progression, early metastasis, easier invasion and recurrence (2). These clinical features attribute to the poor prognosis of CCA. The 5-year survival rate ranging from 5% to 20% (3, 4). Base on anatomical locations, CCA can be classified as three subtypes, which are the intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA) (5). pCCA has the highest prevalence among CCAs, taking up about 50% of all CCAs. pCCA is not sensitive to systemic chemotherapy and patients always suffer early recurrence (6). Till 2020, the first targeted drug of CCA, pemigtinib, just emerged for treatment of patients with FGFR2 fusion, which only accounted for 10%-15% iCCA and 5% iCCA/dCCA (7). The treatment options of CCA are very dismal, and pCCA is the most less studied CCA subtype in the field of biomarker and target therapy (8). Therefore, identifying new biomarkers and therapies to expand the pCCA treatment options and improve life span of pCCA is very important.
ZFAND5 (AN1-type zinc finger protein 5, also named ZNF216) is a 23-kD cytosolic protein with one A20 zinc finger domain and one AN1-type zinc finger domain (9). It is a member of the zinc finger AN1-type domain family, which are comprised of 8 members (10). Among the AN1-type domain family, ZFAND2a and ZFAND5 are featured by their ability to increase the overall protein degradation (11). ZFAND5 interacts with poly-ubiquitinated proteins with the N-terminal A20-type zinc finger domain (12). ZFAND5 is highly expressed in the brain and skeletal muscle, and is detectable in many tissues, and is reported to participate in muscle atrophy and osteoclasts differentiation (13). However, the expression of ZFAND5 in many other tissues are not clear.
The function of ZFAND5 in cancer progression is rarely investigated. Previous study suggested ZFAND5 as a biomarker of hepatocellular carcinoma indicating favorable prognosis (14). However, other studies showed that ZFAND5 was highly expressed in more aggressive nasopharyngeal carcinoma cells (15), and ZFAND5 may be associated with proliferation of colon cancer (16), indicating that ZFAND5 may have pro-tumor effects. Taken together, the current knowledge of ZFAND5 role in tumor progression is very poor and controversial. Here we investigated the expression of ZFAND5 in pCCA with immunohistochemistry (IHC), and estimated the clinical significance of ZFAND5 detection by investigating the correlation between ZFAND5 and clinicopathological parameters. Moreover, we estimated the prognostic value and the independent prognostic significance of ZFAND5 in pCCA.
The retrospective cohort was comprised of 122 patients who underwent the radical surgery of pCCA from 2017.1 to 2021.6 in Qilu Hospital of Shandong University and the Central Hospital of Shandong First Medical University, which constituted the initial cohort. 72 patients were enrolled from the initial cohort into the validation cohort if they (1) had available specimens for IHC detection and available follow-ups, (2) survived the perioperative period, (3)had no other malignancies. Moreover, another consecutive perspective cohort comprised of 10 patients was established if the patients underwent radical surgery and had enough pCCA tissues and adjacent CBD tissues for quantified real-time PCR (qRT-PCR). The pCCAs were staged as the 8th American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) Tumor-Node-Metastasis (TNM) classification system.
All specimens were obtained with informed consent from patients. All experiments were approved and supervised by the Ethics Committee of Qilu Hospital of Shandong University and the Central Hospital of Shandong First Medical University.
The slides were stained with hematoxylin and eosin staining for double confirmation of diagnosis and selection for proper tumor area to make TMA. The tumor area for TMA construction was determined by a senior pathologist. The core biopsies with 1.5 mm in diameter were taken from the selected area and arranged into TMA slides.
In the perspective cohort, total mRNA was purified with TRIzol reagent (Thermo Fisher, Waltham, MA, USA). The extracted total RNAs were reversely transcribed into cDNA with SuperScript Reverse Transcriptase (Thermo Fisher). The quantified real-time PCR was performed with QuantiFast Probe RT-PCR Plus Kit (Qiagen, Germany). mRNA level of ZFAND5 was standardized with GAPDH as an internal control by the 2-ΔΔCt method. All primers were designed as follows:
ZFAND5: forward primer 5’-GCTAGTGGTTCCAACAGTCCT-3’,
reverse primer 5’-TCGGGGTAGTTATTTTGTCCTCT-3’;
GAPDH: forward primer 5’-TGTGGGCATCAATGGATTTGG-3’,
reverse primer 5’-ACACCATGTATTCCGGGTCAAT-3’.
The streptavidin-peroxidase IHC was used to detect the ZFAND5 expression in pCCA tissues. In brief, the TMA was deparaffinized and rehydrated with xylene and graded alcohol, and then immersed in 3% hydrogen peroxide to inactivate the endogenous peroxidase. After that, the TMA slides were incubated in citrate buffer (pH = 6.0) and boiled using a microwave for optimal antigen retrieval. The unspecific antigen binding was blocked by bovine serum albumin incubation. TMA slides were incubated in the primary antibody of ZFAND5(NBP1-80609, Novus Biologicals, Minneapolis, MN, USA) at dilution as 1:100 overnight at 4°C, and then rinsed by phosphate buffered saline (PBS) for 3 times. After that, TMA was incubated in the secondary antibody labeled with biotin (Beyotime, Beijing, China) for 1 hour at room temperature. The antigen was finally visualized by peroxidase reaction with 3,3-diaminobenzidine (DAB) solution (Beyotime).
The IHC results were semi-quantified by IHC scores which were determined by the staining intensity as well as the positive cell percentage. The staining intensity was defined as negative (score 0), weak (score 1), moderate (score 2), or strong (score 3). The positive cell percentage was calculated as <25% (score 1), 25%-50% (score 2), 50%-75% (score 3) and >75% (score 4). The final IHC scores were the products of the score (staining intensity) multiplied by the score (positive cell percentage), varying from 0 to 12. The IHC scores were further divided into the ZFAND5low and ZFAND5high scores based on the cut-off in the receiver operating characteristic (ROC) curve, which was set as the cases with the highest specificity plus sensitivity. In our study, the cut-off was 3.5, representing that scores ≥4 were set as high ZFAND5.
All the statistical analyses were performed with software SPSS 22.0. The correlations between ZFAND5 and clinicopathological parameters were estimated by the chi-square test. The overall survival curves were plotted with Kaplan-Meier method and the statistical difference between different subsets were compared with the log-rank test. The Cox proportional hazard regression model was used to assess the independent prognostic significance of ZFAND5 and clinicopathological parameters. The statistical significance between IHC scores of different subtypes was analyzed with one-way ANOVA, and the statistical significance of qRT-PCR results was assessed with paired t test. P value less than 0.05 was considered as statistically significant.
Firstly, we established a cohort comprised of 72 pCCA patients. There were 31 male patients and 41 female patients, with an average age as 56.5 years old (Table 1). The expression of ZFAND5 in the 72 pCCAs, and their paired liver tissues, intrahepatic bile ducts tissues and common bile ducts (CBD) tissues were detected with IHC (Figure 1A). In these tissues, ZFAND5 was abundantly expressed in cell cytoplasm. Moreover, The IHC scores of pCCA were substantially higher than those of interlobular bile ducts tissues and CBD tissues, but lower than those of hepatic tissues(Figure 1B).
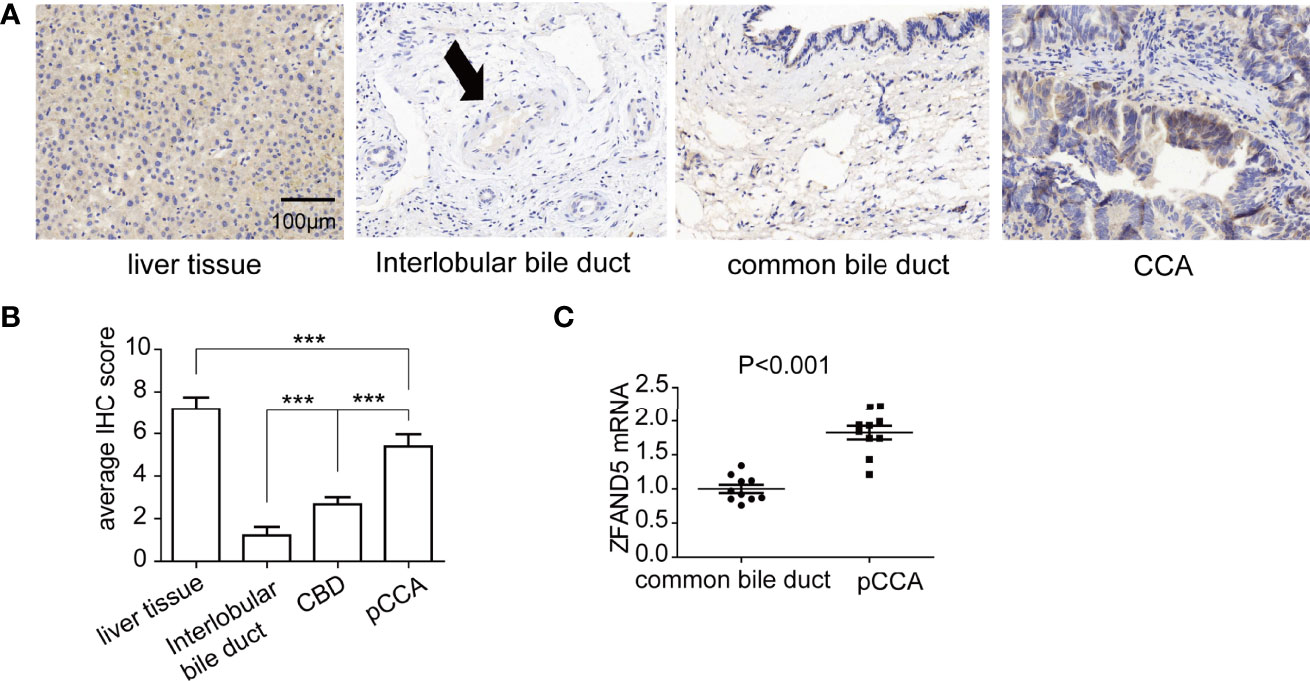
Figure 1 The expressions of ZFAND5 in pCCAs, liver tissues, interlobar bile ducts tissues, and common bile ducts tissues. (A) The expressions of ZFAND5 in pCCAs, liver tissues, interlobar bile ducts tissues, common bile ducts tissues were detected by IHC. Scale bar: 100 μm. The arrow indicates the interlobar bile duct. (B) The expressions of ZFAND5 in pCCAs, liver tissues, interlobar bile ducts tissues, and common bile ducts tissues were estimated by the IHC scores. *** represents P < with one-way ANOVA test. (C) mRNAs of ZFAND5 in pCCAs and common bile ducts were detected with qRT-PCR. The statistical significance was analyzed by paired t test.
Moreover, we established a perspective cohort including 10 consecutive pCCA patients, and detected the ZFAND5 mRNA of pCCAs and adjacent CBD tissues. As expected, the ZFAND5 mRNAs in pCCAs were extensively higher than those in CBDs, indicating a potential role of ZFAND5 in tumorigenesis and progression of pCCA(Figure 1C).
We divided the pCCA cohort into subsets with low or high expression of ZFAND5 based on the IHC score (Figure 2). There were 32 ZFAND5low patients and 40 ZFAND5high patients, accounting for 44.4% and 55.6% of all pCCAs respectively (Table 1). The correlations between ZFAND5 expression and clinicopathological parameters were further assessed with chi-square test. The enrolled clinicopathological parameters included the gender, age of patients, tumor size, histological grade, T stage, N stage and TNM stage. M stage was not included because all patients were present without metastasis. No significant correlation between ZFAND5 and these parameters was observed. The only potential ZFAND5-associated factor was tumor size. High expression of ZFAND5 appeared to be correlated with larger tumor size, though the statistical significance was not remarkable(P=0.155) (Table 2).
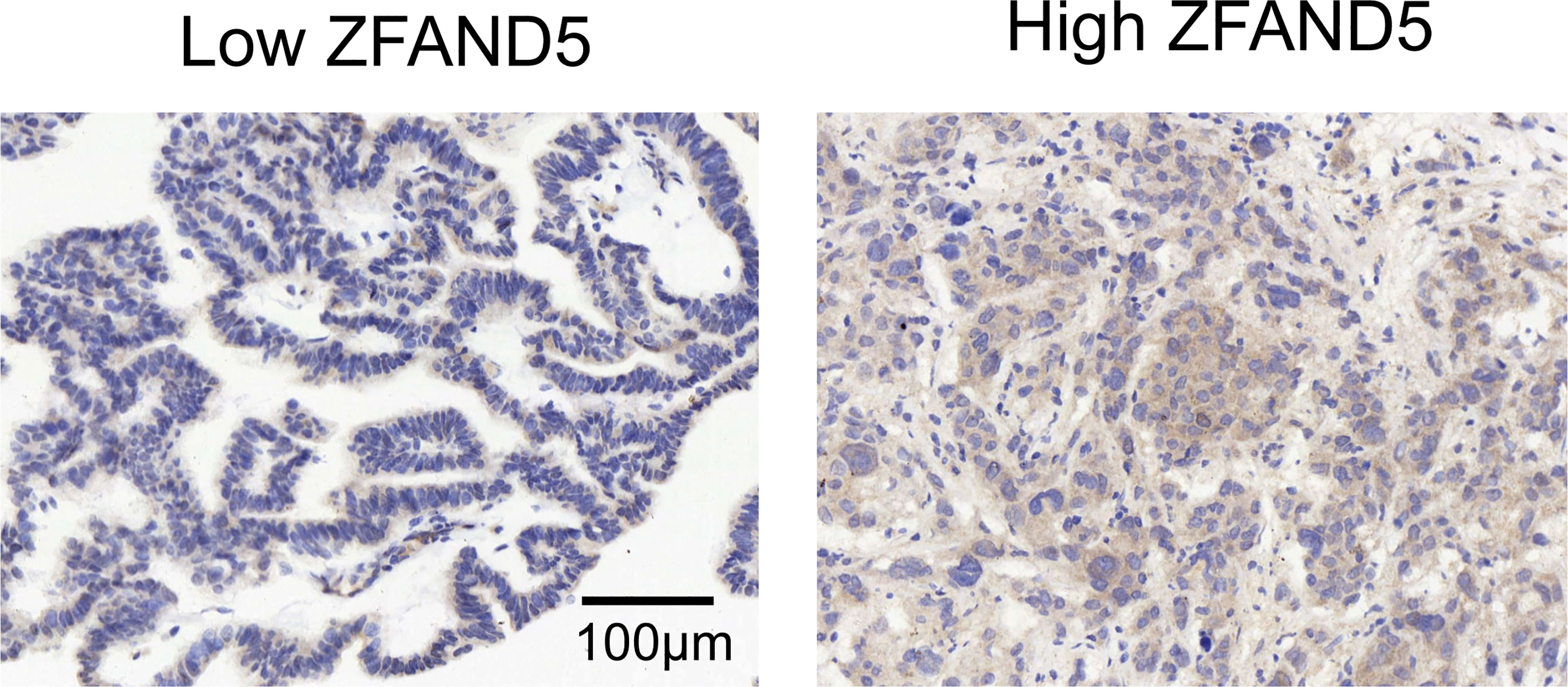
Figure 2 The cohort was divided into ZFAND5low and ZFAND5high subsets. The representative images of low and high ZFAND5 expression. Scale bar: 100 μm.
With Kaplan-Meier methods, we plotted the survival curves of all clinical parameters and ZFAND5 (Table 3). Noticeably, high expression of ZFAND5 correlated with lower overall survival rates of pCCA, indicating that ZFAND5 was an effective prognostic biomarker of pCCA (Figure 3A). The average survival time of ZFAND5low and ZFAND5high patients were 31.2 months and 19.5 months, with the 5-year overall survival rates as 31.9% and 0% respectively. In addition to ZFAND5, the advanced histological grade could also predict a poor prognosis of pCCA (Figure 3B). Advanced T stage, TNM stage and tumor size tended to correlate with low survival rates, with a noteless significance (Figure 3C–E). Other parameters did not exhibit strong correlations with the overall survival rates.
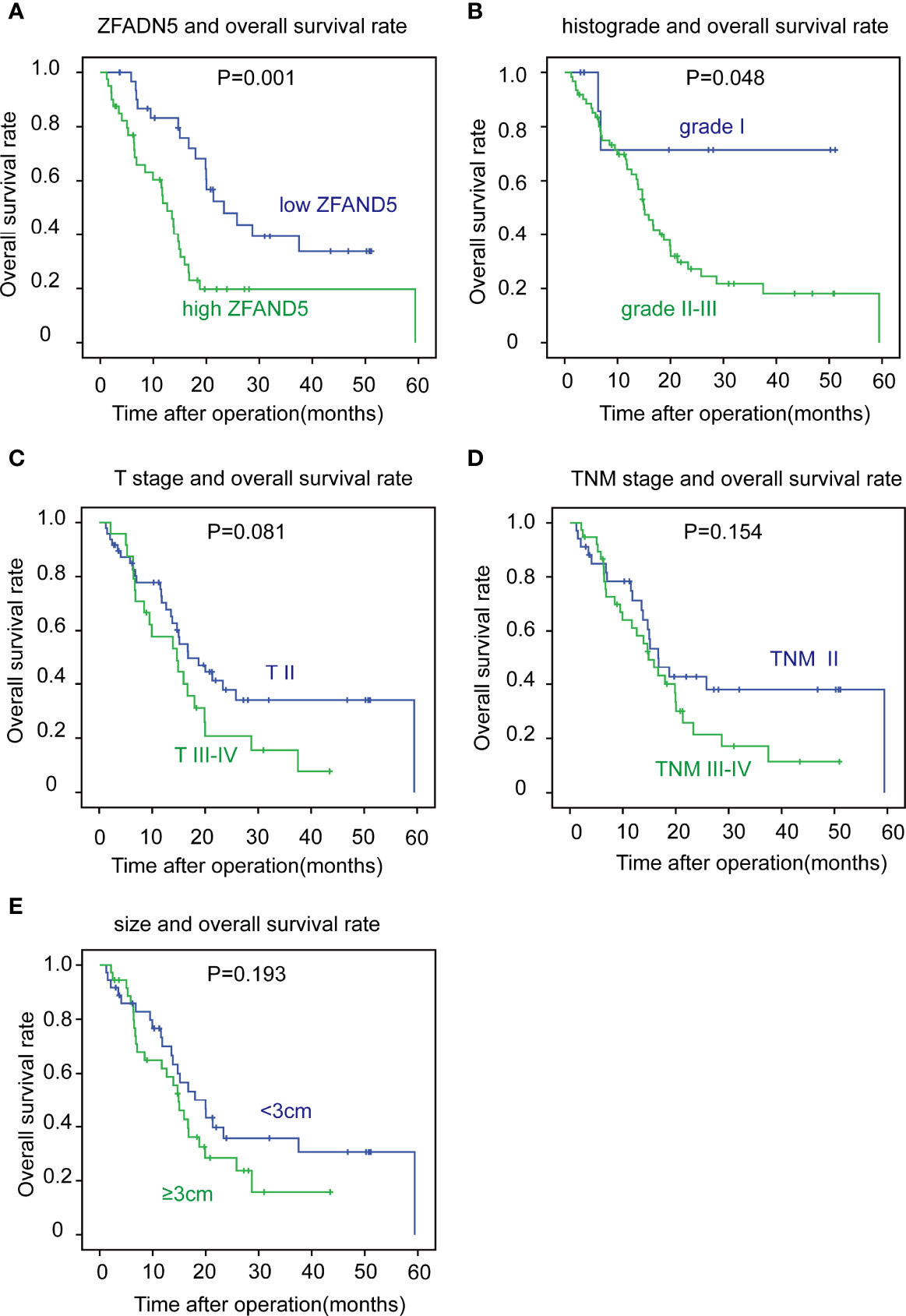
Figure 3 The correlation between clinicopathological factors and overall survival rates. The patients were divided into different subsets according to ZFAND5 expression (A), histological grade (B), T stage (C), TNM stage (D) and tumor size (E). The survival curves were plotted by Kaplan-Meier method, and the statistical significance was analyzed with the log-rank test.
Moreover, we enrolled all these clinicopathological parameters into the Cox-regression hazard model to identify the independent prognostic factors. In this multivariate analysis, ZFAND5 was identified as an independent prognostic biomarker of pCCA. The odds of ZFAND5high patients was 4.33 fold times higher than that of ZFAND5low patients to suffer from pCCA-related death (Table 4). Additionally, high histological grade was also an independent factor indicating the unfavorable outcome (P=0.039, HR=5.18).
CCA is a highly heterogeneous and complicated malignancy. The three subtypes of CCA, iCCA, pCCA and dCCA, have distinct clinical manifestations, treatment options and outcomes (6). However, their biological characters are not as more distinguished as their clinical features. In recent years, many molecular fingerprints of CCA have been identified, defining different molecular CCA patterns (1, 17). However, most studies on molecular CCA patterns are focused on iCCA, because iCCA specimens are much easier to get, compared with pCCA. pCCA and dCCA were separated as distinct subtypes in 2007 according to the 7th AJCC/UICC (18). But the understanding of the molecular differences between pCCA and dCCA is extremely poor. More studies on the molecular characters of CCA, especially pCCA, could deepen the understanding of differences among CCA subtypes, and help discover prognostic biomarkers.
Even in the field of CCA biology, the studies of pCCA are much fewer compared with iCCA and dCCA. Although pCCA has substantially higher incidence, the radical surgery of pCCA is more difficult than iCCA and dCCA. Hepatectomy and pancreaticoduodenectomy are the radical surgical approaches for iCCA and dCCA respectively, but the radical surgery of pCCA is extensively lower compared with iCCA and dCCA (19). The anatomical complexity of hepatic portal determines that pCCA is much easier to invade the hepatic artery and portal vein, making the radical surgery extremely difficult, which further results in that the specimen obtainment of pCCA is difficult, and that large pCCA cohort study is rare. Our cohort consisting 72 pCCA patients is a relatively large-sample-sized cohort. Our results demonstrated that ZFAND5 was a highly effective biomarker indicating the unfavorable prognosis of pCCA (P=0.001). Our study could provide more evidence for the molecular classification of pCCA, and help stratify the high-risk patients based on the molecular features.
Cancer cells have many hallmarks in cell metabolism and energy utilization (20). For example, in shortage of available nutrients, cancer cells can exert adaptive metabolic pathways to maintain their viability by the self-catabolic processes such as autophagy. Protein ubiquitination and degradation are the key segments in the self-catabolic processes, and the molecular characters of proteasome mechanisms and regulation need further studies. ZFAND5 interacts with both Ub and proteasomes, and it may function as a linker between substrates and 26S proteasome. Previous study showed that ZFAND5 could increase multiple proteasomes activities and extensively induce proteolysis by the Ub-proteasome pathway (12). However, the exact mechanism of ZFAND5-induced protein ubiquitination is still unknown. For example, whether ZFAND5 interacts with the Ub-binding substrates, and whether ZFAND5 functions as a E3 ligase is still unknown. We speculated that the high expression of ZFAND5 may be attributed to the enhanced autophagy of CCA, which is a promising topic but requires further experiments.
Till now, the studies of ZFAND5 physiological function are very insufficient, and the reports on the oncogenic role of ZFAND5 are extremely poor. In addition to an activator of proteasome, other functions of ZFAND5 were also reported. Previous study demonstrated that ZFAND5 could bind and stabilize mRNAs with AU-rich elements in 3’-untranslated regions (21). In addition, ZFAND family are mainly stress-associated proteins. For example, expression of ZFAND2a (also named AIRAP) is increased in cases that misfolded proteins are accumulated, such as arsenite exposure, heat shock, or proteasome inhibition (22). ZFAND2a could promote proteins degradation by binding to proteasomes and thus increased their peptidase activity (21, 22). The exact mechanism of ZFAND5 up-regulation in pCCA, and the function of ZFAND5 in pCCA progression is worthy of further investigation.
The role of ZFAND5 in tumor immunity is not investigated. On one hand, ZFAND5 can be induced by cytokines including RANKL, TNFα, and IL-1β, on the other hand, ZFAND5 can inhibit the activation of transcription by NF-κB, TNFα, or IL-1β (13, 23). However, the exact mechanism of how ZFAND5 influences the cytokine and how it participates in inflammation is still unknown. Given that ZFAND5 affects cytokine expression and is involved in immunology, it would be a very interesting topic to further study the role of ZFAND5 in tumor response to immune checkpoint inhibitors.
In summary, here we established a pCCA cohort consisting of 72 patients, and identified ZFAND5 as an independent biomarker of pCCA. The odds of ZFAND5high patients was 4.33 fold times higher than that of ZFAND5low patients to suffer from pCCA-related death. Our results provide more evidence for the molecular classification of pCCA, and help stratify the high-risk patients based on the molecular features.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
All experiments were approved and supervised by the Ethics Committee of Qilu Hospital of Shandong University and the Central Hospital of Shandong First Medical University. The patients/participants provided their written informed consent to participate in this study.
PL and YW performed the experiments, PL and LD designed the study, collected the specimens and wrote the paper. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - Evolving Concepts and Therapeutic Strategies. Nat Rev Clin Oncol (2018) 15:95–111. doi: 10.1038/nrclinonc.2017.157
2. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet (2014) 383:2168–79. doi: 10.1016/S0140-6736(13)61903-0
3. Chen T, Li K, Liu Z, Liu J, Wang Y, Sun R, et al. WDR5 Facilitates EMT and Metastasis of CCA by Increasing HIF-1alpha Accumulation in Myc-Dependent and Independent Pathways. Mol Ther (2021) 29:2134–50. doi: 10.1016/j.ymthe.2021.02.017
4. Li Z, Liu J, Chen T, Sun R, Liu Z, Qiu B, et al. HMGA1-TRIP13 Axis Promotes Stemness and Epithelial Mesenchymal Transition of Perihilar Cholangiocarcinoma in a Positive Feedback Loop Dependent on C-Myc. J Exp Clin Cancer Res (2021) 40:86. doi: 10.1186/s13046-021-01890-1
5. Liu Z, Liu J, Chen T, Wang Y, Shi A, Li K, et al. Wnt-TCF7-SOX9 Axis Promotes Cholangiocarcinoma Proliferation and Pemigatinib Resistance in a FGF7-FGFR2 Autocrine Pathway. Oncogene (2022) 41:2885–96. doi: 10.1038/s41388-022-02313-x
6. Sun R, Liu Z, Qiu B, Chen T, Li Z, Zhang X, et al. Annexin10 Promotes Extrahepatic Cholangiocarcinoma Metastasis by Facilitating EMT via PLA2G4A/PGE2/STAT3 Pathway. EBioMedicine (2019) 47:142–55. doi: 10.1016/j.ebiom.2019.08.062
7. Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol (2020) 21:671–84. doi: 10.1016/S1470-2045(20)30109-1
8. Liu Z, Sun R, Zhang X, Qiu B, Chen T, Li Z, et al. Transcription Factor 7 Promotes the Progression of Perihilar Cholangiocarcinoma by Inducing the Transcription of C-Myc and FOS-Like Antigen 1. EBioMedicine (2019) 45:181–91. doi: 10.1016/j.ebiom.2019.06.023
9. Hishiya A, Iemura S, Natsume T, Takayama S, Ikeda K, Watanabe K. A Novel Ubiquitin-Binding Protein ZNF216 Functioning in Muscle Atrophy. EMBO J (2006) 25:554–64. doi: 10.1038/sj.emboj.7600945
10. Enesa K, Evans P. The Biology of A20-Like Molecules. Adv Exp Med Biol (2014) 809:33–48. doi: 10.1007/978-1-4939-0398-6_3
11. Garner TP, Strachan J, Shedden EC, Long JE, Cavey JR, Shaw B, et al. Independent Interactions of Ubiquitin-Binding Domains in a Ubiquitin-Mediated Ternary Complex. Biochemistry (2011) 50:9076–87. doi: 10.1021/bi201137e
12. Lee D, Takayama S, Goldberg AL. ZFAND5/ZNF216 is an Activator of the 26S Proteasome That Stimulates Overall Protein Degradation. Proc Natl Acad Sci USA (2018) 115:E9550–9. doi: 10.1073/pnas.1809934115
13. Hishiya A, Ikeda K, Watanabe K. A RANKL-Inducible Gene Znf216 in Osteoclast Differentiation. J receptor Signal transduction Res (2005) 25:199–216. doi: 10.1080/10799890500240781
14. Zheng Y, Yu K, Huang C, Liu L, Zhao H, Huo M, et al. Integrated Bioinformatics Analysis Reveals Role of the LINC01093/miR-96-5p/ZFAND5/NF-KappaB Signaling Axis in Hepatocellular Carcinoma. Exp Ther Med (2019) 18(5):3853–60. doi: 10.3892/etm.2019.8046
15. Yang XY, Ren CP, Wang L, Li H, Jiang CJ, Zhang HB, et al. Identification of Differentially Expressed Genes in Metastatic and Non-Metastatic Nasopharyngeal Carcinoma Cells by Suppression Subtractive Hybridization. Cell Oncol (2005) 27:215–23. doi: 10.1155/2005/108490
16. Cao H, Sethumadhavan K, Cao F, Wang TTY. Gossypol Decreased Cell Viability and Down-Regulated the Expression of a Number of Genes in Human Colon Cancer Cells. Sci Rep (2021) 11:5922. doi: 10.1038/s41598-021-84970-8
17. Kam AE, Masood A, Shroff RT. Current and Emerging Therapies for Advanced Biliary Tract Cancers. Lancet Gastroenterol Hepatol (2021) 6:956–69. doi: 10.1016/S2468-1253(21)00171-0
18. Xu YF, Yang XQ, Lu XF, Guo S, Liu Y, Iqbal M, et al. Fibroblast Growth Factor Receptor 4 Promotes Progression and Correlates to Poor Prognosis in Cholangiocarcinoma. Biochem Biophys Res Commun (2014) 446:54–60. doi: 10.1016/j.bbrc.2014.02.050
19. Qiu B, Chen T, Sun R, Liu Z, Zhang X, Li Z, et al. Sprouty4 Correlates With Favorable Prognosis in Perihilar Cholangiocarcinoma by Blocking the FGFR-ERK Signaling Pathway and Arresting the Cell Cycle. EBioMedicine (2019) 50:166–77. doi: 10.1016/j.ebiom.2019.11.021
20. Pavlova NN, Zhu J, Thompson CB. The Hallmarks of Cancer Metabolism: Still Emerging. Cell Metab (2022) 34:355–77. doi: 10.1016/j.cmet.2022.01.007
21. He G, Sun D, Ou Z, Ding A. The Protein Zfand5 Binds and Stabilizes mRNAs With AU-Rich Elements in Their 3'-Untranslated Regions. J Biol Chem (2012) 287:24967–77. doi: 10.1074/jbc.M112.362020
22. Stanhill A, Haynes CM, Zhang Y, Min G, Steele MC, Kalinina J, et al. An Arsenite-Inducible 19S Regulatory Particle-Associated Protein Adapts Proteasomes to Proteotoxicity. Mol Cell (2006) 23:875–85. doi: 10.1016/j.molcel.2006.07.023
Keywords: ZFAND5, prognosis, biomarker, perihilar cholangiocarcinoma, cohort
Citation: Liu P, Wang Y and Duan L (2022) ZFAND5 Is an Independent Prognostic Biomarker of Perihilar Cholangiocarcinoma. Front. Oncol. 12:955670. doi: 10.3389/fonc.2022.955670
Received: 29 May 2022; Accepted: 13 June 2022;
Published: 13 July 2022.
Edited by:
Xuesong Gu, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesReviewed by:
Qi Sun, The First Affiliated Hospital of Xi’an Jiaotong University, ChinaCopyright © 2022 Liu, Wang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingling Duan, bGluZ2xpbmdkbGxkQDEyNi5jb20=; ZHVhbmxsMjAwNUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.