
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 07 July 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.954759
This article is part of the Research TopicDifferential Diagnoses of Thyroid Neoplasms: Molecular and Histological Features and the Impact on Follow-UpView all 10 articles
 Antonio Mario Bulfamante1
Antonio Mario Bulfamante1 Eleonora Lori2*
Eleonora Lori2* Maria Irene Bellini2
Maria Irene Bellini2 Elisa Bolis3
Elisa Bolis3 Paolo Lozza1
Paolo Lozza1 Luca Castellani1
Luca Castellani1 Alberto Maria Saibene1
Alberto Maria Saibene1 Carlotta Pipolo1
Carlotta Pipolo1 Emanuela Fuccillo1
Emanuela Fuccillo1 Cecilia Rosso1
Cecilia Rosso1 Giovanni Felisati1
Giovanni Felisati1 Loredana De Pasquale4
Loredana De Pasquale4Differentiated thyroid cancers (DTCs) are slow-growing malignant tumours, including papillary and follicular carcinomas. Overall, prognosis is good, although it tends to worsen when local invasion occurs with bulky cervical nodes, or in the case of distant metastases. Surgery represents the main treatment for DTCs. However, radical excision is challenging and significant morbidity and functional loss can follow the treatment of the more advanced forms. Literature on advanced thyroid tumours, both differentiated and undifferentiated, does not provide clear and specific guidelines. This emerges the need for a tailored and multidisciplinary approach. In the present study, we report our single-centre experience of 111 advanced (local, regional, and distant) DTCs, investigating the rate of radical excision, peri-procedural and post-procedural complications, quality of life, persistence, recurrence rates, and survival rates. Results are critically appraised and compared to the existing published evidence review.
Thyroid cancer accounts for less than 1% of all malignancies, but for about 5% of all thyroid nodules (1). Among thyroid cancers, differentiated thyroid carcinoma (DTC) is the most common and includes papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and Hürthle cell thyroid carcinoma (HTC) (2). DTC has an annual incidence of 1/10,000 with a female-to-male ratio of 3:1 (3, 4). DTC incidence is increasing, most probably due to the continuous improvement offered by early diagnostic tools (4–6). DTCs have a favourable prognosis, with a 5-year survival rate of approximately 93% and 88% in women, and men, respectively (4), although unfortunately, 5% of DTCs are fatal (3). Surgery represents the initial treatment of choice (7, 8), followed in most cases by radioactive iodine (RAI) I131 to eradicate microscopical disease or distant metastases. In addition, multikinase inhibitors (MKIs), including sorafenib and lenvatinib, are rising as alternative medical treatments in the most advanced forms, especially in RAI-refractory differentiated thyroid cancer (RR-DTC). Advanced disease accounts for 13%–15% of DTCs and is characterized by a worse prognosis and more challenging management (5). Advanced DTC (ADTC) is defined by the existence of one or more of the following conditions: local invasion, bulky cervical nodes, and/or distant metastases. A recent review by Russel et al. describes all the issues associated with advanced thyroid cancer, from the lack of adequate guidelines and the need for a multidisciplinary and tailored approach. This is necessary to overcome the challenges of the distinct DTC subtypes, such as those related to surgical radicality, meaning not only total thyroidectomy and lymphadenectomy, but even tracheal ring resection, partial or total laryngectomy, or thoracic access. Based on the aforementioned study (4), the aim of the present manuscript is to critically review our single-centre experience of advanced thyroid cancer (local, regional, and distant), but focusing specifically on ADTC, thus not dealing with medullary, anaplastic carcinoma, or other undifferentiated cancers, because of their worst prognosis and the limited therapeutic options and surgical strategies currently available. Herein, we provided our DTC 30-year experience on therapeutic approach, peri-procedural and postsurgical management, quality of life disease recurrence, and survival rates.
A retrospective analysis of medical and electronic charts of a single-centre series comprised 656 thyroid tumours, 627 of which are DTCs (333 PTC, 92 FTC, 202 papillary microcarcinoma), 21 medullary thyroid carcinomas, 1 squamous cell carcinoma, and 7 anaplastic tumours. An exception from Institutional Review Board evaluation was granted due to the study’s retrospective nature. Our experience herein reported involved 111 consecutive patients diagnosed with ADTC who underwent a surgical assessment between May 1992 and September 2021 at the Outpatient Endocrine Surgery Service, Thyroid and Parathyroid Surgery Unit, in San Paolo Hospital, Milan, Italy. Before surgery, our protocol included blood tests, with particular attention to the TSH, calcium, PTH, 25 – OH vitamin D, and calcitonin blood levels. Neck ultrasound was always the first imaging examination, to evaluate for thyroid nodules and cervical lymph nodes. All patients with ultrasonographical suspicion of nodules and/or lymph node involvement underwent ultrasound-guided fine-needle aspiration (FNA) to confirm DTC cytologically. In case of suspicion of infiltration of surrounding tissues, further targeted radiological examinations, such as neck and chest CT, were performed.
All patients underwent a preoperative evaluation with video-laryngoscopy by an expert otolaryngologist. All ADTC cases were discussed pre- and postoperatively by a multidisciplinary team (MDT) consisting of an endocrinologist, an endocrine surgeon, an otolaryngologist, a pathologist, a radiologist, and a nuclear medicine physician (Figure 1).
Risk factors in the patient’s medical history, such as family and personal history of thyroid disease, cancer, goitre endemics, and radiation exposures, were also analysed. Symptom onset was identified when the presence of local complaints such as dysphagia, dysphonia, dyspnoea, sensation of cervical encumbrance and cervical dysmorphism, lymphadenopathies, or distant metastases appeared. Patients were divided into the following classes according to the surgery performed: total thyroidectomy with adjacent structure resection and centra cervical lymphadenectomy (TT + CCL), total thyroidectomy and central and unilateral cervical lymphadenectomy (TT + CUCL), ;total thyroidectomy and central and bilateral cervical lymphadenectomy (TT + CBCL), unilateral cervical lymphadenectomy (UCL), and bilateral cervical lymphadenectomy (BCL) after previously performed thyroidectomy. “Cervical lymphadenectomy” was intended as modified radical neck dissection, including II, III, IV, and V levels. Only five patients, underwent additional resection of distant metastases. We systematically started the use of intraoperative inferior laryngeal nerve neuromonitoring in 2018. We also analysed the rate of complications and divided them into transient hypocalcaemia (TH), persistent hypocalcaemia (PH), recurrent laryngeal nerve transient unilateral palsy (RLNTUP), recurrent laryngeal nerve transient bilateral palsy (RLNTBP), recurrent laryngeal nerve persistent unilateral palsy (RLNPUP), recurrent laryngeal nerve persistent bilateral palsy (RLNPBP), superior laryngeal nerve deficit (SLD), and other complications. Moreover, we analysed the total excision rate and indications to perform postoperative RAI therapy or other adjuvant treatments. Of the 111 patients recruited, 53 were on regular follow-up (FU) at our Centre. Complete data were available on disease-free survival, total survival, and recurrence. Of the remaining 58 patients, we could not obtain up-to-date information, and they were therefore considered lost to follow-up. After treatment, all patients received levothyroxine TSH suppressive therapy and were entrusted to the attending endocrinologists for follow-up treatment. All ADTC patients were considered high-risk and underwent periodic blood checks consisting of TSH, thyroglobulin, and antithyroglobulin antibodies, as well as a cervical ultrasonography (US) every 6–12 months, for an average period of 15 years (range 2–30 years).
A total of 111 patients were enrolled in our study, 74 women and 37 men. The median age is 45.8 ± 19.5 SD years of age. Women were in the age ranges of 19 to 93, and men ranging between 11 and 81 years, with women’s mean age of 46.4 ± 20.4 and men’s 44.4 ± 17.9. We enrolled and treated exclusively in the abovementioned centre 96 patients, while 15 had previous thyroid surgery in other Italian centres.
Family history of cancer thyroid disease was present in 26/111 patients (23.42%), in particular 7 men (18.92%) and 19 women (25.68%). We identified 53 patients (47.75%) coming from areas known for endemic goitres; 17 are men (45.95%), and 36 are women (48.65%). Of our sample, 35 women (47.30%) were menopausal at the time of diagnosis. The clinical characteristics of the patients enrolled in the study are briefly summarized in Table 1.
At the time of diagnosis, 76/111 (68.5%) patients had lymph node metastases, and the presence of a cervical mass was the problem that initiated the diagnostic process, which then led to the discovery of DCTs.
In the other cases, at the time of diagnosis, 22/111 patients (19.8%) presented with a locally advanced tumour characterized by the involvement of adjacent anatomical structures. All of them presented with local symptomatology and thus were referred for clinical evaluation. In this group of 22 patients, 15 were women and 7 were men, respectively 18.92% and 21.62% of their groups. The most frequent symptoms were dysphonia (8/22 cases), dysphagia (3/22 cases), and cervical encumbrance sensation (19/22 cases).
For six out of 111 (5.4%) patients, the tumour was occasionally detected incidentally during clinical examination conducted for other reasons and they were asymptomatic at the time of diagnosis.
In our study, seven out of 111 (6.3%) patients were diagnosed with DCTs after clinical examinations for distant metastasis presence, initially unknown, but later identified as a repetition from DCT. The distant metastases included the following groups: bone metastasis of the mandible, liver metastasis, lung and liver metastasis, two isolated lung metastases, one multiple lung metastasis, and multiple bone metastasis of three ribs.
After preoperative blood tests, five out of 111 cases of reduced TSH levels were detected (normal range 0.15–3.5 U/ml); two out of 111 patients had increased TSH levels, while the remaining patients were within the normal range. Only four out of 111 patients were hypocalcemic, but three previously underwent TT at different Italian centres. Only a single patient was hypercalcemic.
Surgical procedures consisted of 21 (18.9%) TT and CCL, 70 (63.1%) TT and CUCL, 5 (4.5%) TT and CBCL, 10 (9.0%) UCL, and 5 (4.5%) BCL after previous thyroidectomy in other Italian centres. None of the patients enrolled in the study underwent hemithyroidectomy. In the case of local advanced tumours or distant metastases, patients received tailored treatments based on their individual clinical condition. For details, see Table 2.
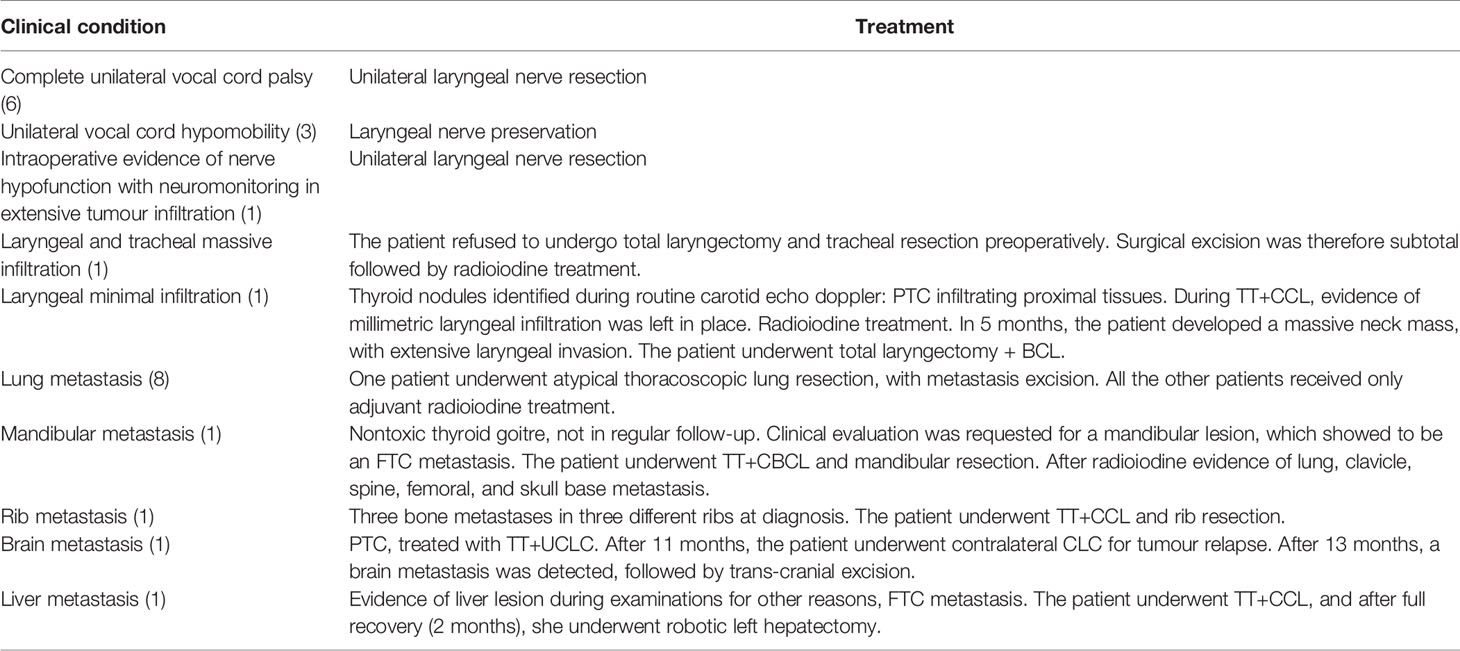
Table 2 Detailed treatment received by patients in case of local advanced tumour or distance metastases. In brackets are the reported number of cases.
The final histological examination on the samples obtained during surgery reported 9/111 (8.1%) cases of follicular carcinoma and 102/111 (91.9%) cases of papillary carcinoma.
After the surgery, 87/111 patients (78,3%) developed a temporary or persistent complication as summarized in Table 3.
A total of 110 patients received adjuvant radioiodine therapy after surgery; only one patient died 3 weeks after surgery, as reported in Table 3. They underwent from one to five cycles of treatment, with a mean of 2.4 ± 1.3, and of 98 ± 19 mCu total radiation dose. Of the 111 patients in our cohort, 53 remained in regular FU at our centre.
The three out of 53 patients that underwent TT and CCL presented peculiar conditions, such as liver, lung, or rib metastasis. After thyroid surgery, they all received surgical treatment for metastatic lesions (left robotic hepatectomy, atypical lung resection, and rib removal).
Of a total of 53 patients, 46 underwent TT and CUCL, 5 of them with LN resection, and 4 underwent TT and CBCL, 2 of which with LN resection. Moreover, one of these four patients presented mandible metastasis as symptom onset. She underwent mandible resection, with total metastasis gross excision.
Only 8/53 (15.1%) (3 men and 5 women, mean age 69 years old) are deceased, while 45/53 (84.9%) are currently alive (16 men and 29 women, mean age 42 years old). Patients currently still alive have a mean FU of 165 ± 101.2 months.
A total of 39/45 (86.7%) never developed recurrences; they all underwent TT and CUCL. Serum Tg and Ab-Tg never showed an increase during the follow-up period. Neck ultrasound always resulted negative. Five out of 45 (11.1%) have developed relapse, and one (2.2%) had persistence. The persistence was subjected to the neck radiotherapy cycle (5550 Mbq). The five relapses included heterogeneous cases (Table 4).
Three of eight patients deceased underwent TT and CCL. Three out of eight underwent TT and CUCL, and two out of eight underwent TT and CBCL. They had a mean survival of 47 months, ranging from 14 days to 151 months. All these patients presented distinctive characteristics as shown in Table 5.
In our series, only one patient refused surgery and was therefore not included in the above discussion. He underwent treatment with lenvatinib and is now in good clinical condition with tumour regression 20 months after diagnosis. The 45 patients still alive and in regular follow-up are in good clinical condition. During a telephone interview specifically for the present study, they reported living a normal life with good quality of life, taking into account age and other pathologies. They are all in thyroxine suppression therapy and present a mean TSH level of 0.54 µIU/ml (range 0.0015–3.61 µU/ml), mean Tg 0.07 ng/ml (range 0.04– 0.59 ng/ml), and Ab anti Tg 11.26 IU/ml (0–20 IU/ml), and all have neck ultrasound negative for thyroid bed and lymph node recurrence.
There is no standard definition for ADTC in the literature. In the ATA guidelines published in 2016 (9), there is a session entitled “DTC: Long-Term Management and Advanced Cancer Management Guidelines,” but the definition of ADTC is missing. In our series, we defined ADTC according to Russel et al. (5): invasion of thyroid adjacent structures (local invasion), laterocervical lymph nodes, metastasis, distant metastases, and more than one of the previously mentioned characteristics simultaneously.
The first consideration in the analysis of the present study is treatment. The different therapeutic choices have been tailored to the characteristics of each patient, and this approach has led to encouraging results: 84.9% of the 45 patients with available follow-up are currently alive, with a median FU > 10 years, i.e., 165 months (range 12–345 months). All of them were in the group with regional lymph node metastases, more in detail, eight with locally advanced tumours. Among these 45, four (11.1%) have developed relapse, in all cases localized in the neck, that was subsequently successfully treated by re-surgery and adjuvant therapy. None of the 45 patients developed distant metastases. Two of these relapses had at the time of their first intervention one RLN infiltration and the other tracheal cartilage infiltration. Only one (2.2%) has persistence in the thyroid bed, without disease progression. For relapse and persistence, the histological result was PTC. Of the eight patients who died, only four died of neoplastic causes, two of them after more than 10 years, 151 months, and 111 months, both with histological results of PTC. The two patients with the worst evolution were, one, already diagnosed with multiple lung and bone metastases for follicular carcinoma, and the other, showed trachea and larynx involvement for papillary carcinoma.
According to literature (10, 11), local invasiveness, albeit with a limited number of local ADTC in our case series, had a greater impact on survival than cervical lymph node involvement. However, DTCs are generally slow-evolving tumours, even if advanced. This aspect should be the first to be considered in the therapeutic choice of ADTC. With regard to complications associated with surgery, we observed a higher percentage of definitive hypoparathyroidism and persistent RLNUP in thyroidectomies performed for diseases other than ADTC carcinoma (12, 13). This may be related to the fact that central neck dissection, always performed in all patients included in this study, is a well-known risk factor for persistent hypoparathyroidism. Regarding the definitive RLNUP, the locally advanced situation of the tumour, with the involvement of the RLN in 10 cases, preoperative palsy of the vocal cord and intraoperative nerve infiltration in 6, and intraoperative infiltration in 4 cases, leading to sacrifice in one, has increased the number of definitive vocal cord paralysis. In the case of locally advanced tumours (LADTC), the most commonly involved structure in our experience was the recurrent laryngeal inferior nerve (RLN), more specifically in 10 cases.
In agreement with the literature, treatment of the infiltrated RLN was based on the knowledge of preoperative vocal function (14, 15), magnitude of neural invasion, and occurrence of loco-regional or distant metastases not surgically removable. There is a general agreement that attempts should be made to preserve RLN when preoperative vocal cord function is normal, and intraoperatively it appears only to be superficially invaded. The location of the RLN invasion should be taken into account in the decision to preserve it or not: resection should be performed when neural invasion occurs near the point where the RLN enters the larynx. This is because an incomplete resection of the tumour at this point can lead to the progression of the tumour along the nerve and its spread into the larynx, with subsequent indications for laryngectomy (5). In addition to the location, the extent of neural invasion should influence intraoperative decision-making. When only the epineurium is infiltrated, a shave excision may be performed (14–16). Even if the nerve shaving may cause partial layer resection, most nerves demonstrate a long-term recovery of neural function (14). On the contrary, if the perineurium, endoneurium, and neural fibres are invaded, the resection should be considered. In this case, the simple shaving of the nerve without the complete removal of the tumour may be considered, as we did in the three cases abovementioned, that is, if adjuvant RAI therapy may treat the residual neoplastic tissue (14, 16–18). When intraoperative neuromonitoring (IONM) is routinely used during surgery, it is important to evaluate the possibility of proximally stimulating the nerve (18): the ability to stimulate the proximal side of an infiltrated RLN suggests some maintenance of neural function, even if paralysis of the vocal cord is documented preoperatively. As reported by Kamani et al., 60% of invaded nerves can be stimulated with IONM (19) and the resection of invaded RLN with preoperative paralysis of the vocal cord, according to some authors (20), may worsen glottic function. For this reason, the ability to stimulate the proximal segment of the invaded nerve may be a parameter for intraoperative decision to preserve or not the nerve (14). A very important aspect to consider about the treatment of an infiltrated RLN is that resecting or preserving it would not seem to change the prognosis (14, 17, 18, 21–23). For this reason, if possible, it would be best to preserve the sRLN, because the integrity of the nerve corresponds to the good functioning of the vocal cord, which has a great impact on the quality of life. A negative impact on the quality of life may occur when there is an infiltration of viscera adjacent to the thyroid, such as airways (larynx and/or trachea), oesophagus, and vascular structures. In our experience, among ADCT not lost at follow-up, five had invasions of the airway, two had laryngeal invasion, and three had tracheal invasion. One patient with laryngeal infiltration refused laryngectomy and died after 25. Another patient had infiltration of the larynx and the first two tracheal rings. She underwent laryngectomy 5 months after TT, and she died 12 months later. The others are alive respectively after 37, 77, and 101 months, without resecting trachea. They were treated with tracheal shaving during TT and CCL as well as postoperative RAI, referring to good quality of life. In the presence of visceral structure invasion, the objective of surgery should be to remove the majority of the tumour mass. ATA guidelines recommend surgery for tumours invading the upper aerodigestive tract along with 131I (RAI) and/or external beam radiation therapy (ERBT) (9, 24, 25). In some patients, surgery may represent a viable cure effort, while in others it represents a palliative approach to reduce symptoms such as asphyxia and haemoptysis (26, 27). In the case of tracheal infiltration, surgical techniques depend on the type of tumoral invasion. The extent of tracheal invasion has been classified by Shin, as well as others (21, 27, 28), in four stages: perichondrial invasion with adherence to the trachea (stage I), cartilaginous invasion but without mucosal involvement (stage II), mucosal infiltration (stage III), and extension into the lumen (stage IV). In the suspicion of tracheal involvement, besides CT scan, preoperative bronchoscopy is mandatory, to assess mucosal intraluminal involvement. Shave resection of the airway may be appropriated in cases of limited cartilage invasion, involving tiny segments. In the presence of more extensive or intraluminal tumour infiltration, tracheal resection is suggested (21, 29). In the case of larynx infiltration, shave excision is possible when the tumour does not extend into the larynx. Otherwise, more aggressive treatment, such as laryngectomy, may be required (27). In our series, patients with laterocervical lymph node metastases, not lost at follow-up, were 44. All had a functional cervical dissection, including II, III, IV, and V levels. Five of them had recurrences, and 32 were alive following a mean follow-up of 210 months. Treatment of distant metastases should consider that both morbidity and mortality are increased in patients with distant metastases and that individual prognosis is influenced by a number of factors, including histology of the primary tumour, distribution and number of metastatic sites (e.g., brain, bone, lung), age at diagnosis, and RAI avidity (30–38). Treatment of a specific metastasis should be grounded on patient status and on the presence of other sites of disease (39–42). According to ATA (9), the sequence of therapeutic choices for the treatment of metastatic disease should be in order:
i) surgical excision of loco-regional metastasis in potentially curable patients;
ii) 131I therapy for RAI-responsive disease;
iii)ERBT or other directed treatment modalities for metastases;
iv) TSH-suppressive thyroid hormone therapy for patients, with stable or slowly progressive asymptomatic disease;
v) systematic therapy with kinase inhibitors, especially for patients with significantly progressive macroscopic refractory disease.
In the management of the patient with lung metastases, therapeutic decisions should consider the following: size of metastatic lesions (i.e., macro-nodular detected by chest radiography, micro-nodular detected by CT, lesions below CT resolution); avidity for RAI; response to previous RAI therapy; stability or not of metastatic lesions. In view of the high rate of the observed complete remission, lung micro-metastases should be treated with RAI therapy and eventually be repeated every 6–12 months, if the disease continues to concentrate RAI and respond clinically (41, 42). Also, radioiodine-avid macro-nodular metastases should be treated with RAI when objective benefit is demonstrated (i.e., decrease in the size of the lesions, decreasing Tg). In the latter, however, complete remission is uncommon and survival remains poor. It is also worth considering the risks associated with repeated doses of RAI such as bone marrow suppression or pulmonary pneumonitis and fibrosis. Solitary lung metastases could be resected via surgery, even though the potential benefits compared with the risk of surgery remain unclear. With regard to bone metastasis, RAI therapy may be considered for those that are iodine-avid. In these cases, the therapy has been associated with improved survival, although RAI is rarely curative. Regardless, some patients with RAI-avid bone metastases may still benefit from this therapy (35–37). These patients may also be considered for directed therapy in cases where metastases are visible on anatomical imaging. Directed therapies include surgery, EBRT, and other focal treatment modalities discussed below. Systemic therapy with bone-targeted agents may also be considered in these patients. The therapeutic approaches to brain and liver metastases are similar to those previously mentioned for lungs and bone metastases (9). As mentioned, several focal treatments other than surgery, for lung, bone, liver, and brain DTC metastases, are available. In certain patients, these treatment modalities, alternative to surgery, may be effective for local tumour control as first-line treatment with similar efficacy to that of surgical resection. In general, such treatments may be indicated in case of high anaesthesiologic risk, lung metastasis in patients with insufficient respiratory reserve, multiple previous surgical resections, local recurrence after surgery, and refusal of additional surgery. In our experience, we treated seven metastatic patients: two had pulmonary metastases, three had bone metastases, one had liver metastases, and one had brain metastases. Of the two patients with lung metastases, one was a young patient for whom the diagnosis of DTC was made through the removal of a solitary pulmonary nodule, with pathological examination showing PTC metastasis as a result. Then, only a single sub-centimetre nodule was found during neck ultrasound and she underwent total thyroidectomy with central neck dissection. After these two interventions, she underwent RAI with no evidence of further lesions. She remains disease free at her 89-month follow-up. The other one showed multiple pulmonary areas of uptake at post-thyroidectomy 131I total body scan, after total thyroidectomy for follicular carcinoma. During a chest CT, she showed multiple micro-metastases. She underwent three cycles of RAI therapy. She is alive and persistent but reduced for many metastases at the 125-month follow-up. Of the three patients with bone metastases, two were treated: patients with ADTC are treated by RAI. The dose of RAI, the number of treatments, and the time between treatments should be personalized depending on unique patient characteristics such as disease response to therapy, patient’s age, and the onset of side effects (i.e., radiation lung damage, bone-marrow suppression, and salivary gland damage). Therapy should be repeated at least 6–12 months after the previous treatment (5). Finally, systemic therapy may be considered in patients with locally advanced and/or metastatic RAI refractory disease not suitable for local therapies with the purpose to reduce tumour growth and/or metastatic spread (43). The first-line systemic therapy is represented by the multikinase inhibitors lenvatinib and sorafenib, approved by FDA and EMA (44–49). In our series, we have two patients enrolled by endocrinologists for systemic therapy with lenvatinib, as described before: one for local advanced and metastatic disease who refused intervention and the other for multiple recurrent metastatic DTCs (4). Nowadays, traditional chemotherapy is indicated only in selected cases: when multikinase inhibitors are ineffective or cannot be used. If 131I-refractory, metastatic patients should be maintained only with TSH-suppressive thyroid hormone therapy if they are asymptomatic or stable or have a minimally progressive disease without clinically significant complications. These patients need serial radiographic imaging every 3–12 months (9).
In the case of locally and/or metastatic ADTC, there are many therapeutic options available. The best treatment modality must take into consideration various aspects related to the patient and the pathology at hand. Moreover, the benefits of surgical excision of a slow-evolving tumour, such as DTC, in terms of local control, risk of persistence/recurrence, and survival, must always be carefully weighed against the morbidity of surgery, which may require aggressive resection of extrathyroidal tissues. For these reasons, a single standardized approach for local and/or metastatic ADTC could not be established, but rather a tailored treatment must be defined for each patient. The best treatment certainly requires a multidisciplinary group, consisting of an endocrinologist, an expert endocrine surgeon, an otolaryngologist, a radiologist, a nuclear medicine doctor, and an oncologist, all operating in a high-volume centre. Multicentre studies from high-volume centres would be desirable, to optimize the choice of various therapeutic options discussed before, and to offer the best tailored treatment to the patients affected with locally and/or metastatic ADTC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: LP; methodology: LP, AB and MB; validation PL, LC and AS; investigation: AB, EB and LP; writing—original draft preparation: AB, LP, MB and EL; writing—review and editing: MB, EL, EF, CP, CR and GF. All authors have read and agreed to the published version of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
A special thanks to Denise Nemeth (OMS-II; University of the Incarnate Word - School of Osteopathic Medicine) for her assistance with the English language. She did extensive edits on this paper, making the work suitable for publication.
1. Van Den Heede K, Tolley NS, Di Marco AN, Palazzo FF. Differentiated Thyroid Cancer: A Health Economic Review. Cancers (Basel). (2021) 13(9):2253. doi: 10.3390/cancers13092253
2. Araque KA, Gubbi S, Klubo-Gwiezdzinska J. Updates on the Management of Thyroid Cancer. Horm Metab Res (2020) 52(8):562–77. doi: 10.1055/a-1089-7870 Orphanet
3. Differentiated Thyroid Carcinoma . Available at: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=GB&Expert=146 (Accessed 2 April 2022).
4. Russell MD, Kamani D, Randolph GW. Modern Surgery for Advanced Thyroid Cancer: A Tailored Approach. Gland Surg (2020) 9(Suppl 2):S105–19. doi: 10.21037/gs.2019.12.16
5. Pacini F, Basolo F, Bellantone R, Boni G, Cannizzaro MA, De Palma M, et al. Italian Consensus on Diagnosis and Treatment of Differentiated Thyroid Cancer: Joint Statements of Six Italian Societies. J Endocrinol Invest. (2018) 41(7):849–76. doi: 10.1007/s40618-018-0884-2
6. Ulisse S, Baldini E, Lauro A, Pironi D, Tripodi D, Lori E, et al. Papillary Thyroid Cancer Prognosis: An Evolving Field. Cancers (Basel). (2021) 13(21):5567. doi: 10.3390/cancers13215567
7. Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, et al. Thyroid Lobectomy for Papillary Thyroid Cancer: Long-Term Follow-Up Study of 1,088 Cases. World J Surg (2014) 38(1):68–79. doi: 10.1007/s00268-013-2224-1
8. Adam MA, Pura J, Gu L, Dinan MA, Tyler DS, Reed SD, et al. Extent of Surgery for Papillary Thyroid Cancer is Not Associated With Survival: An Analysis of 61,775 Patients. Ann Surg (2014) 260(4):601–7. doi: 10.1097/SLA.0000000000000925
9. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
10. Lirov R, Worden FP, Cohen MS. The Treatment of Advanced Thyroid Cancer in the Age of Novel Targeted Therapies. Drugs. (2017) 77(7):733–45. doi: 10.1007/s40265-017-0733-1
11. Jung YS, Oh CM, Kim Y, Jung KW, Ryu J, Won YJ. Long-Term Survival of Patients With Thyroid Cancer According to the Methods of Tumor Detection: A Nationwide Cohort Study in Korea. PloS One (2018) 13(4):e0194743. doi: 10.1371/journal.pone.0194743
12. De Pasquale L, Sartori PV, Vicentini L, Beretta E, Boniardi M, Leopaldi E, et al. Necessity of Therapy for Post-Thyroidectomy Hypocalcaemia: A Multi-Centre Experience. Langenbecks Arch Surg (2015) 400(3):319–24. doi: 10.1007/s00423-015-1292-0
13. Zhang D, Pino A, Caruso E, Dionigi G, Sun H. Neural Monitoring in Thyroid Surgery is Here to Stay. Gland Surg (2020) 9(Suppl 1):S43–6. doi: 10.21037/gs.2019.10.24
14. Wu CW, Dionigi G, Barczynski M, Chiang FY, Dralle H, Schneider R, et al. International Neuromonitoring Study Group Guidelines 2018: Part II: Optimal Recurrent Laryngeal Nerve Management for Invasive Thyroid Cancer-Incorporation of Surgical, Laryngeal, and Neural Electrophysiologic Data. Laryngoscope. (2018) 128(Suppl 3):S18–27. doi: 10.1002/lary.27360
15. Russell MD, Kamani D, Randolph GW. Surgical Management of the Compromised Recurrent Laryngeal Nerve in Thyroid Cancer. Best Pract Res Clin Endocrinol Metab (2019) 33(4):101282. doi: 10.1016/j.beem.2019.05.006
16. Schneider R, Randolph G, Dionigi G, Barczyński M, Chiang FY, Triponez F, et al. Prospective Study of Vocal Fold Function After Loss of the Neuromonitoring Signal in Thyroid Surgery: The International Neural Monitoring Study Group's POLT Study. Laryngoscope. (2016) 126(5):1260–6. doi: 10.1002/lary.25807
17. Kihara M, Miyauchi A, Yabuta T, Higashiyama T, Fukushima M, Ito Y, et al. Outcome of Vocal Cord Function After Partial Layer Resection of the Recurrent Laryngeal Nerve in Patients With Invasive Papillary Thyroid Cancer. Surgery. (2014) 155(1):184–9. doi: 10.1016/j.surg.2013.06.052
18. Lang BH, Lo CY, Wong KP, Wan KY. Should an Involved But Functioning Recurrent Laryngeal Nerve be Shaved or Resected in a Locally Advanced Papillary Thyroid Carcinoma? Ann Surg Oncol (2013) 20(9):2951–7. doi: 10.1245/s10434-013-2984-8
19. Kamani D, Darr EA, Randolph GW. Electrophysiologic Monitoring Characteristics of the Recurrent Laryngeal Nerve Preoperatively Paralyzed or Invaded With Malignancy. Otolaryngol Head Neck Surg (2013) 149(5):682–8. doi: 10.1177/0194599813504735
20. Chi SY, Lammers B, Boehner H, Pohl P, Goretzki PE. Is it Meaningful to Preserve a Palsied Recurrent Laryngeal Nerve? Thyroid. (2008) 18(3):363–6. doi: 10.1089/thy.2007.0124
21. Shindo ML, Caruana SM, Kandil E, McCaffrey JC, Orloff LA, Porterfield JR, et al. Management of Invasive Well-Differentiated Thyroid Cancer: An American Head and Neck Society Consensus Statement. AHNS Consensus Statement. Head Neck. (2014) 36(10):1379–90. doi: 10.1002/hed.23619
22. Nishida T, Nakao K, Hamaji M, Kamiike W, Kurozumi K, Matsuda H. Preservation of Recurrent Laryngeal Nerve Invaded by Differentiated Thyroid Cancer. Ann Surg (1997) 226(1):85–91. doi: 10.1097/00000658-199707000-00012
23. Falk SA, McCaffrey TV. Management of the Recurrent Laryngeal Nerve in Suspected and Proven Thyroid Cancer. Otolaryngol Head Neck Surg (1995) 113(1):42–8. doi: 10.1016/s0194-5998(95)70143-5
24. Kim JW, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Treatment Outcomes and Risk Factors for Recurrence After Definitive Surgery of Locally Invasive Well-Differentiated Papillary Thyroid Carcinoma. Thyroid. (2016) 26(2):262–70. doi: 10.1089/thy.2015.0433
25. McCaffrey JC. Evaluation and Treatment of Aerodigestive Tract Invasion by Well-Differentiated Thyroid Carcinoma. Cancer Control. (2000) 7(3):246–52. doi: 10.1177/107327480000700304
26. Musholt TJ, Musholt PB, Behrend M, Raab R, Scheumann GF, Klempnauer J. Invasive Differentiated Thyroid Carcinoma: Tracheal Resection and Reconstruction Procedures in the Hands of the Endocrine Surgeon. Surgery. (1999) 126(6):1078–88. doi: 10.1067/msy.2099.102267
27. Czaja JM, McCaffrey TV. The Surgical Management of Laryngotracheal Invasion by Well-Differentiated Papillary Thyroid Carcinoma. Arch Otolaryngol Head Neck Surg (1997) 123(5):484–90. doi: 10.1001/archotol.1997.01900050030003
28. Shin DH, Mark EJ, Suen HC, Grillo HC. Pathologic Staging of Papillary Carcinoma of the Thyroid With Airway Invasion Based on the Anatomic Manner of Extension to the Trachea: A Clinicopathologic Study Based on 22 Patients Who Underwent Thyroidectomy and Airway Resection. Hum Pathol (1993) 24(8):866–70. doi: 10.1016/0046-8177(93)90136-5
29. Merdad M, Eskander A, Kroeker T, Freeman JL. Predictors of Level II and Vb Neck Disease in Metastatic Papillary Thyroid Cancer. Arch Otolaryngol Head Neck Surg (2012) 138(11):1030–3. doi: 10.1001/2013.jamaoto.393
30. Bernier MO, Leenhardt L, Hoang C, Aurengo A, Mary JY, Menegaux F, et al. Survival and Therapeutic Modalities in Patients With Bone Metastases of Differentiated Thyroid Carcinomas. J Clin Endocrinol Metab (2001) 86(4):1568–73. doi: 10.1210/jcem.86.4.7390
31. Chiu AC, Delpassand ES, Sherman SI. Prognosis and Treatment of Brain Metastases in Thyroid Carcinoma. J Clin Endocrinol Metab (1997) 82(11):3637–42. doi: 10.1210/jcem.82.11.4386
32. Ronga G, Filesi M, Montesano T, Di Nicola AD, Pace C, Travascio L, et al. Lung Metastases From Differentiated Thyroid Carcinoma. A 40 Years' Experience. Q J Nucl Med Mol Imaging. (2004) 48(1):12–9.
33. Lin JD, Chao TC, Chou SC, Hsueh C. Papillary Thyroid Carcinomas With Lung Metastases. Thyroid. (2004) 14(12):1091–6. doi: 10.1089/thy.2004.14.1091
34. Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF, et al. Prognostic Indicators of Outcomes in Patients With Distant Metastases From Differentiated Thyroid Carcinoma. J Am Coll Surg (2003) 197(2):191–7. doi: 10.1016/S1072-7515(03)00332-6
35. Zettinig G, Fueger BJ, Passler C, Kaserer K, Pirich C, Dudczak R, et al. Long-Term Follow-Up of Patients With Bone Metastases From Differentiated Thyroid Carcinoma – Surgery or Conventional Therapy? Clin Endocrinol (Oxf). (2002) 56(3):377–82. doi: 10.1046/j.1365-2265.2002.01482.x
36. Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, et al. Bone Metastases From Thyroid Carcinoma: Clinical Characteristics and Prognostic Variables in One Hundred Forty-Six Patients. Thyroid. (2000) 10(3):261–8. doi: 10.1089/thy.2000.10.261
37. Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, et al. Radioactive Iodine Treatment and External Radiotherapy for Lung and Bone Metastases From Thyroid Carcinoma. J Nucl Med (1996) 37(4):598–605.
38. Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID. Distant Metastases in Papillary Thyroid Carcinoma: 100 Cases Observed at One Institution During 5 Decades. J Clin Endocrinol Metab (1995) 80(7):2041–5. doi: 10.1210/jcem.80.7.7608252
39. Pak H, Gourgiotis L, Chang WI, Guthrie LC, Skarulis MC, Reynolds JC, et al. Role of Metastasectomy in the Management of Thyroid Carcinoma: The NIH Experience. J Surg Oncol (2003) 82(1):10–8. doi: 10.1002/jso.10189
40. Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, et al. Immediate Causes of Death in Thyroid Carcinoma: Clinicopathological Analysis of 161 Fatal Cases. J Clin Endocrinol Metab (1999) 84(11):4043–9. doi: 10.1210/jcem.84.11.6115
41. Ilgan S, Karacalioglu AO, Pabuscu Y, Atac GK, Arslan N, Ozturk E, et al. Iodine-131 Treatment and High-Resolution CT: Results in Patients With Lung Metastases From Differentiated Thyroid Carcinoma. Eur J Nucl Med Mol Imaging. (2004) 31(6):825–30. doi: 10.1007/s00259-004-1460-x
42. Hod N, Hagag P, Baumer M, Sandbank J, Horne T. Differentiated Thyroid Carcinoma in Children and Young Adults: Evaluation of Response to Treatment. Clin Nucl Med (2005) 30(6):387–90. doi: 10.1097/01.rlu.0000162602.48653.54
43. Baldini E, Presutti D, Favoriti P, Santini S, Papoff G, Tuccilli C, et al. In Vitro and In Vivo Effects of the Urokinase Plasminogen Activator Inhibitor WX-340 on Anaplastic Thyroid Cancer Cell Lines. Int J Mol Sci (2022) 23(7):3724. doi: 10.3390/ijms23073724
44. Elia G, Ferrari SM, Ragusa F, Paparo SR, Mazzi V, Ulisse S, et al. Advances in Pharmacotherapy for Advanced Thyroid Cancer of Follicular Origin (PTC, FTC). New Approved Drugs and Future Therapies. Expert Opin Pharmacother. (2022) 23(5):599–610. doi: 10.1080/14656566.2022.2030704
45. Gabillard JC, Ulisse S, Baldini E, Sorrenti S, Cremet JY, Coccaro C, et al. Aurora-C Interacts With and Phosphorylates the Transforming Acidic Coiled-Coil 1 Protein. Biochem Biophys Res Commun (2011) 408(4):647–53. doi: 10.1016/j.bbrc.2011.04.078
46. Ferrari SM, Politti U, Spisni R, Materazzi G, Baldini E, Ulisse S, et al. Sorafenib in the Treatment of Thyroid Cancer. Expert Rev Anticancer Ther (2015) 15(8):863–74. doi: 10.1586/14737140.2015.1064770
47. Ferrari SM, Bocci G, Di Desidero T, Elia G, Ruffilli I, Ragusa F, et al. Lenvatinib Exhibits Antineoplastic Activity in Anaplastic Thyroid Cancer In Vitro and In Vivo. Oncol Rep (2018) 39(5):2225–34. doi: 10.3892/or.2018.6306
48. Baldini E, Tuccilli C, Prinzi N, Sorrenti S, Antonelli A, Gnessi L, et al. Effects of Selective Inhibitors of Aurora Kinases on Anaplastic Thyroid Carcinoma Cell Lines. Endocr Relat Cancer. (2014) 21(5):797–811. doi: 10.1530/ERC-14-0299
Keywords: thyroid, thyroid cancer, papillary carcinoma, follicular carcinoma, differentiated carcinoma, thyroidectomy, lymph node dissection
Citation: Bulfamante AM, Lori E, Bellini MI, Bolis E, Lozza P, Castellani L, Saibene AM, Pipolo C, Fuccillo E, Rosso C, Felisati G and De Pasquale L (2022) Advanced Differentiated Thyroid Cancer: A Complex Condition Needing a Tailored Approach. Front. Oncol. 12:954759. doi: 10.3389/fonc.2022.954759
Received: 27 May 2022; Accepted: 13 June 2022;
Published: 07 July 2022.
Edited by:
Pietro Giorgio CALO’, University of Cagliari, ItalyReviewed by:
Alessandro Sanguinetti, Università degli Studi Perugia, ItalyCopyright © 2022 Bulfamante, Lori, Bellini, Bolis, Lozza, Castellani, Saibene, Pipolo, Fuccillo, Rosso, Felisati and De Pasquale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleonora Lori, ZWxlb25vcmEubG9yaUB1bmlyb21hMS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.