- 1Pharmaceutical Department, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, China
Background: As an emerging immune checkpoint molecule, indoleamine 2,3-dioxygenase 1 (IDO1) is an immunosuppressive rate-limiting enzyme in metabolism of tryptophan to kynurenine. The expression of IDO1 affected the prognosis of patients in cancers by regulating the kynurenine pathway, inhibiting the proliferation of T cells. However, the association between IDO1 and solid tumor prognosis was controversial. To further investigate the role of IDO1 expression in solid tumors, we conducted the systematic review and meta-analysis.
Methods: We searched the Web of Science, PubMed, Embase, and Cochrane Library databases and China National Knowledge Infrastructure (CNKI) to identify studies evaluating the prognostic value of IDO1 in solid tumors. Overall survival (OS), progression-free survival (PFS), and disease-free survival (DFS) were extracted as the outcome. Pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated by using the fixed-effect/random-effect model, while heterogeneity, publication bias, and sensitivity between studies were also analyzed.
Results: Eighteen studies with 2,168 patients were included in this systematic review and meta-analysis. The results indicated that the high expression of IDO1 was associated with a shorter OS (n = 1926, HR = 1.60, 95% CI: 1.22–2.11, P = 0.001) and DFS (n = 327, HR = 2.65, 95% CI: 1.52–4.63, P = 0.001), while it was uncorrelated with PFS (n = 428, HR = 1.76, 95% CI: 0.99–3.14, P = 0.240). There was significant heterogeneity between studies on OS (I2 = 77.8%, P < 0.001). Subgroup analysis showed that age, gender, tumor type, follow-up period, and study quality were possible reasons for high heterogeneity. The result of the trim-and-fill method indicated that publication bias for OS had no impact on our results. Egger’s test suggested no publication bias for PFS (P = 0.553) and DFS (P = 0.273). Furthermore, sensitivity analysis indicated the result was stable.
Conclusion: High expression of IDO1 was associated with poor clinical outcomes, indicating that it could be a potential prognostic marker in various cancer types.
Introduction
In the last decade, cancer immunotherapy has made significant progress with the application of immune checkpoint inhibitors (ICs). Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed death 1 (PD-1), and programmed cell death 1 ligand 1 (PD-L1) inhibitors had shown good safety and efficacy in various tumor types, including genitourinary oncology, gastrointestinal oncology, and hematologic tumor (1). It is reported that high mutational burden and PD-L1 expression of urothelial carcinoma made it more sensitive to immunotherapy (2, 3), and several ICs had been approved to treat it (4). Similarly, ICIs had also shown good clinical efficacy in prostatic cancer (5), penile cancer (6), testicular cancer (7), colorectal cancer (8), and esophagus cancer (9). Additionally, nivolumab, pembrolizumab, camrelizumab, and tislelizumab had been approved to treat Hodgkin lymphoma (HL) (10).
With the success of PD-1, PD-L1, and CTLA-4 inhibitors, more and more emerging new immune checkpoints attract extensive attention, including lymphocyte activation gene 3, T-cell immunoglobulin- and mucin-domain-containing molecule 3, T-cell immunoglobulin and ITIM domain, and indoleamine 2,3-dioxygenase 1(IDO1) (11). IDO1, an intracellular enzyme, plays a vital role in converting tryptophan into downstream kynurenines, which could suppress T-cell proliferation in vitro, induce the T-cell apoptosis, and affect the natural killer (NK) cell function by depleting the tryptophan (12–14). In normal physiological conditions, IDO1 is mainly expressed in mucosal tissues, placenta, eye, pancreas, and some immune cell subsets (15). In tumors, high expression of IDO1 could cause overactivation of the kynurenine pathway, which could suppress the effector cells and promote the activation of the immunosuppressive cells, consequently forming an immunosuppressive environment in tumors (13, 14, 16). It was reported that IDO1 was an immune checkpoint which could be potentially exploited to improve treatment outcomes in various cancers (17, 18). Additionally, a large number of IDO1 inhibitors had been developed as anticancer drug in clinical trials, including NLG-8189, INCB024360, GDC-0919, PF-06840003, and BMS986205 (12, 18).
Numerous studies have reported that IDO1 is expressed and correlated with prognosis in various cancers, such as urothelial bladder cancer (17), non-small–cell lung cancer (NSCLC ) (19), esophageal squamous cell carcinoma (20), breast cancer (21), pancreatic cancer (22), endometrial cancer (23), and neuroendocrine skin cancer (24). However, the role of IDO1 in clinical cancer studies is still conflicting. Chen et al. reported that the overexpression of IDO1 was associated with poor survival of patients with diffuse large B-cell lymphoma (25). Inversely, the study of Ma et al. showed that high IDO1 expression was significantly associated with better overall survival (OS) for patients in adenosquamous lung carcinoma (26). It was also reported that the high expression of IDO1 was positively correlated with other immune checkpoints (17, 19, 27–29). Vienna’s study showed that high programmed cell death 1 ligand 2/IDO-1 co-expression levels were independent negative prognostic factors for survival in early NSCLC. Wei’s study suggested that progression-free survival (PFS) was associated with IDO/Foxp3 co-expression levels in breast cancer. In addition, many factors could have an effect on the expression of IDO1, such as pathological type, virus infection, and therapeutic straregies (14, 30). For example, triple-negative breast cancer (TNBC) patients were confirmed to have a higher IDO1 expression compared to non-TNBC patients (14). Higher IDO1 expressions were also found in human papillomavirus-positive head and neck squamous cell carcinoma patients (30) and hepatitis B virus-positive liver parenchymal cells (31). In terms of therapeutic straregies, chemotherapy regimens could regulate the immune microenvironment by inducing a period of transient lymphopenia and homeostatic recovery and further affecting the expression of IDO1 (32). Thus, it is necessary to investigate the correlation between IDO1 expression and tumor prognosis. Meanwhile, not only IDO1 expression but also single-nucleotide polymorphisms of IDO1 (rs9657182, rs3739319) were associated with outcome in tumor patients (33, 34).
The previous meta-analysis only involved studies prior to 2019 (35, 36). In recent years, numerous studies have been published related to IDO1 in cancer patients. Additionally, the expression of IDO1 in tumor tissue could be affected by different treatment methods, such as chemotherapy and immunotherapy. The previous meta-analysis ignored the effect of treatment before surgery or biopsy. In the systematic review, we would analyze the association of IDO1 expression in treatment-naive tissue with cancer survival.
Method
Literature search
We searched all relevant literature in Embase, PubMed, the Cochrane Library, Web of Science, and China National Knowledge Infrastructure (CNKI) on 3 April 2022, without language restriction. The keywords, including indoleamine 2,3-dioxygenase 1, tumor, prognosis, survival, treatment outcome, and their medical subject headings (MeSH) terms, were used to build a search strategy. This meta-analysis was carried out based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Study selection
Our systematic review addressed the following research question: In cancer patients, what is the relationship between IDO1 expression and cancer survival? The established Patients, Intervention, Comparison, Outcomes, and Study design (PICOS) framework was employed to the selection criteria. The PICOS strategy was defined as follows: “P” (patient)—patients with tumor, “I” (intervention)—not applicable, “C” (comparison)—comparison with a high expression group or positive groups according to IDO1 expression, “O” (outcome)—relevant indicators to evaluate the association between expression of IDO1 and prognostic outcomes in tumor tissue, and “S” (study design)—prospective studies, and retrospective study.
According to the PICOS principles, the inclusion criteria were as follows: 1) studies evaluated the association between expression of IDO1 and prognostic outcomes in tumor tissue; 2) the patients were divided into high and low expression groups, or positive and negative groups according to expression level of IDO1; 3) studies reported the prognosis of IDO1 expression using Kaplan–Meier survival analysis, univariate Cox regression analysis, or multivariate Cox regression analysis.
The exclusion criteria were as follows: 1) reviews, case reports, letters, editorials, meeting abstracts; 2) full text was not available; 3) animal experiments or in vitro experiments rather than clinical studies; 4) IDO1 expression was not detected in tumor tissues; 5) IDO1 expression was only detected in RNA level by reverse transcription-polymerase chain reaction (RT-PCR); 6) patients who received antitumor therapy before surgery or biopsy or unclear; 7) hazard ratios (HR) and 95% confidence intervals (CI) were not directly provided.
Data extraction
Three investigators independently extracted the data according to the same criteria, and disagreement between the three investigators would be resolved by consensus. The collected data included publication year, the name of first author, country, tumor type, median of age, gender, number of patients, treatment method, detection method for IDO1 expression, outcome endpoints, median of follow-up period, and the method to estimate HRs and CIs. OS, progression-free survival (PFS), and disease-free survival (DFS) were chosen as outcome endpoints.
Quality assessment
Three investigators independently assessed the quality of included studies by the Quality In Prognostic Studies (QUIPS) tool (37, 38). Study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis were assessed. If more than four of these six criteria had a low risk of bias, the study was considered to be low risk of bias, and if two or more criteria had a high risk of bias, the study was considered to be high risk of bias. The remaining studies were classified as moderate risk of bias.
Statistical analysis
Stata version 14.0 (Stata Corporation, College Station, TX, USA) was used to carry out the statistical analysis. Pooled HRs and 95% CIs for OS, PFS, and DFS were used to assess the association between IDO1 expression and survival. Heterogeneity was assessed by the I2 value derived from the Q test. We considered P < 0.05 or I2 > 50% as significant heterogeneity. According to the I2 and P values, different effect models were used. When I2 > 50% or P < 0.05, a random-effect model was used. Otherwise, we used a fixed-effect model. Egger’s test and trim-and-fill method were used to assess publication bias. Sensitivity analysis was used to assess the stability of results by excluding one study.
Results
Study selection
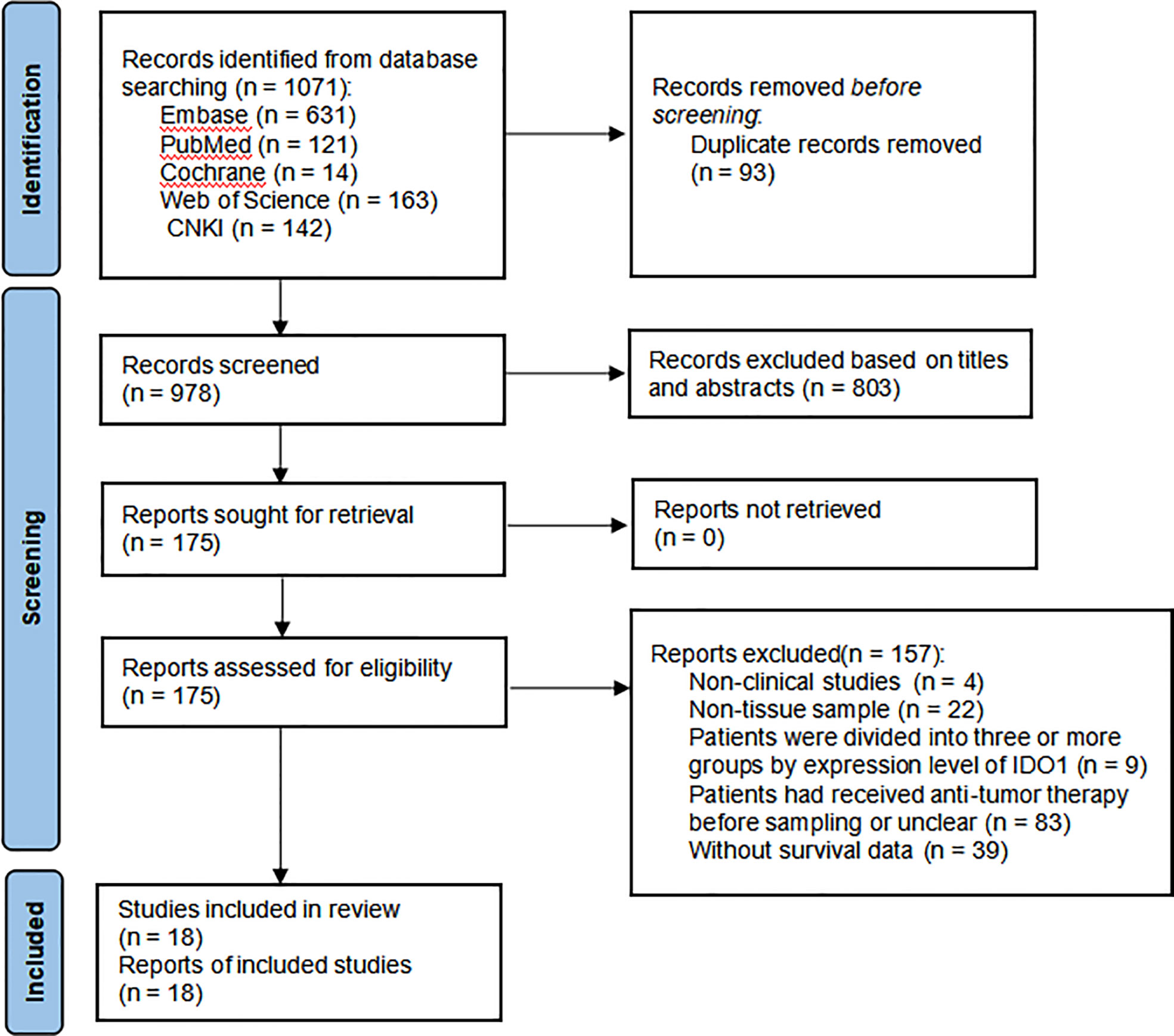
A total of 1,071 papers were initially identified. After removing duplicate literature and reading the title, abstract, and full text according to the study’s inclusion and exclusion requirements, inappropriate publications were excluded. Finally, 18 studies were identified, including 2,168 patients with malignant tumors (17, 25, 28, 39–53). These studies were published between 2008 and 2020. The flow diagram for the study selection is presented in Figure 1.
Study characteristics and quality assessment
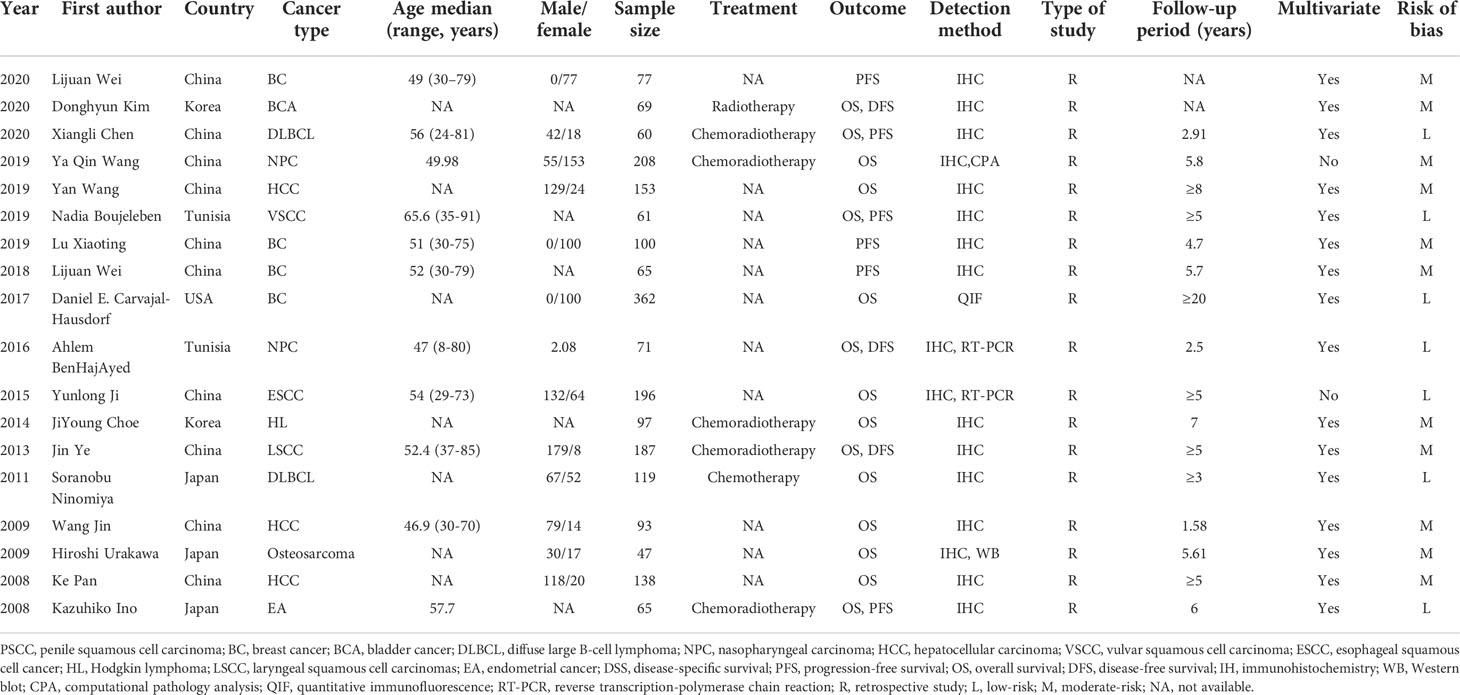
The characteristics of the studies involved in this meta-analysis are shown in Table 1. The study subjects were from Asia (China: 10, Korea: 2, Japan: 3, n = 15) and non-Asia (Tunisia: 2, USA: 1, n = 3). Cancer types included breast cancer (n = 4), bladder cancer (n = 1), diffuse large B-cell lymphoma (n = 2), nasopharyngeal carcinoma (n = 2), hepatocellular carcinoma (n = 3), vulvar squamous cell carcinoma (n = 1), esophageal squamous cell cancer (n = 1), HL(n = 1), laryngeal squamous cell carcinoma (n = 1), osteosarcoma (n = 1), and endometrial cancer (n = 1). The sample size of the included studies ranged from 47 to 362. There were 15 studies evaluating the effect of IDO1 expression on OS, six studies on DFS, and three on DFS. The expression of IDO1 was detected by immunohistochemistry (IHC), RT-PCR, Western blot (WB), quantitative immunofluorescence (QIF), or computational pathology analysis (CPA). Almost all the patients received chemoradiotherapy during the follow-up period. HRs were estimated by multivariate regression analysis in most studies (n = 16). According to the QUIPS checklist, seven studies had an overall low risk of bias and 11 studies had an overall moderate risk of bias.
The relation between IDO1 expression and OS in solid tumor patients
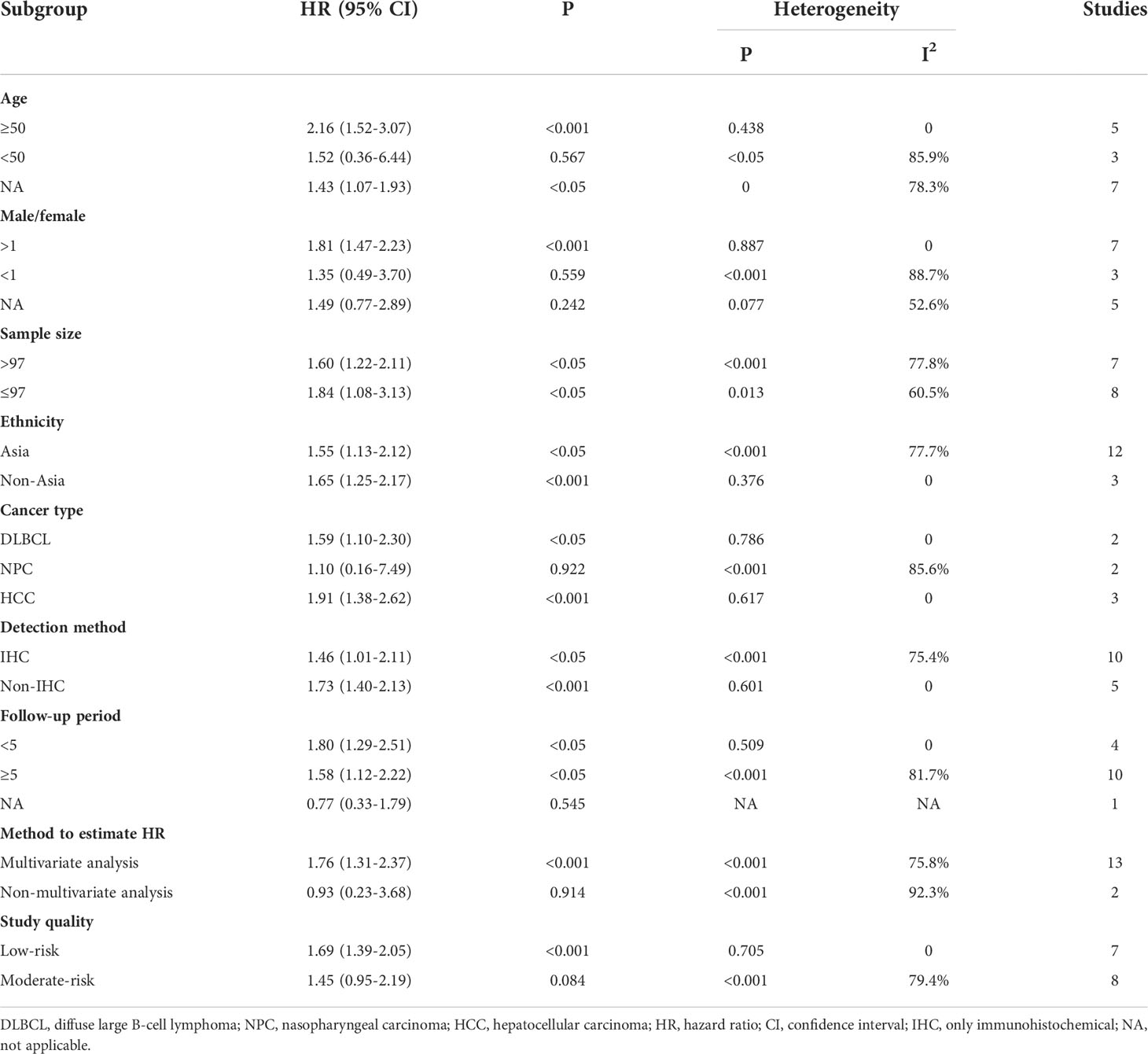
Fifteen studies were included in the meta-analysis of OS. A random-effect model was used to calculate the pooled HRs and 95% CIs, as the heterogeneity test reported a P value of 0 and an I2 value of 77.8%. The results showed that patients with a higher expression of IDO1 had a significant shorter OS (n = 1926, HR = 1.60, 95% CI: 1.22–2.11, P = 0.001) (Figure 2). Subgroup analyses of OS were further carried out according to multiple potential factors (age, gender, sample sizes, ethnicity, tumor type, detection method, follow-up periods, method to estimate HR, and study quality) to investigate the heterogeneity (Table 2). The result of the subgroup analysis showed that age, gender, follow-up period, tumor type, and the quality of the included studies were possible reasons for the high heterogeneity. The heterogeneity of age <50 (HR = 1.52, 95% CI: 0.36–6.44, P = 0.567), male/female <1 (HR = 1.35, 95% CI: 0.49–3.70, P = 0.559), and follow-up period ≥5 (HR = 1.58, 95% CI: 1.12–2.22, P < 0.05) were as high as I2 = 85.9%, I2 = 88.7%, and I2 = 81.7%, respectively, while no heterogeneity was found for age ≥50 (HR = 2.16, 95% CI: 1.52–3.07, P < 0.001), male/female >1 (HR = 1.81, 95% CI: 1.47–2.23, P < 0.001), and follow-up period <5 (HR = 1.80, 95% CI: 1.29–2.51, P < 0.05). In terms of tumor type, IDO1 expression was not associated with OS in nasopharyngeal carcinoma (n = 2, I2 = 85.6%, P = 0.922) but was related to worse OS in diffuse large B-cell lymphoma (n = 2, I2 = 0, P < 0.05) and hepatocellular carcinoma (n = 3, I2 = 0, P < 0.001). Subgroup analysis of other cancer types was not performed because only one study was involved. Studies with low-risk quality showed a tendency to increase the risk of shorter OS (HR = 1.69, 95% CI: 1.39–2.05, P < 0.001) without heterogeneity. In addition, the results of subgroup analyses by sample size, ethnicity, and detection method showed that the high expression of IDO1 was correlated with worse OS.
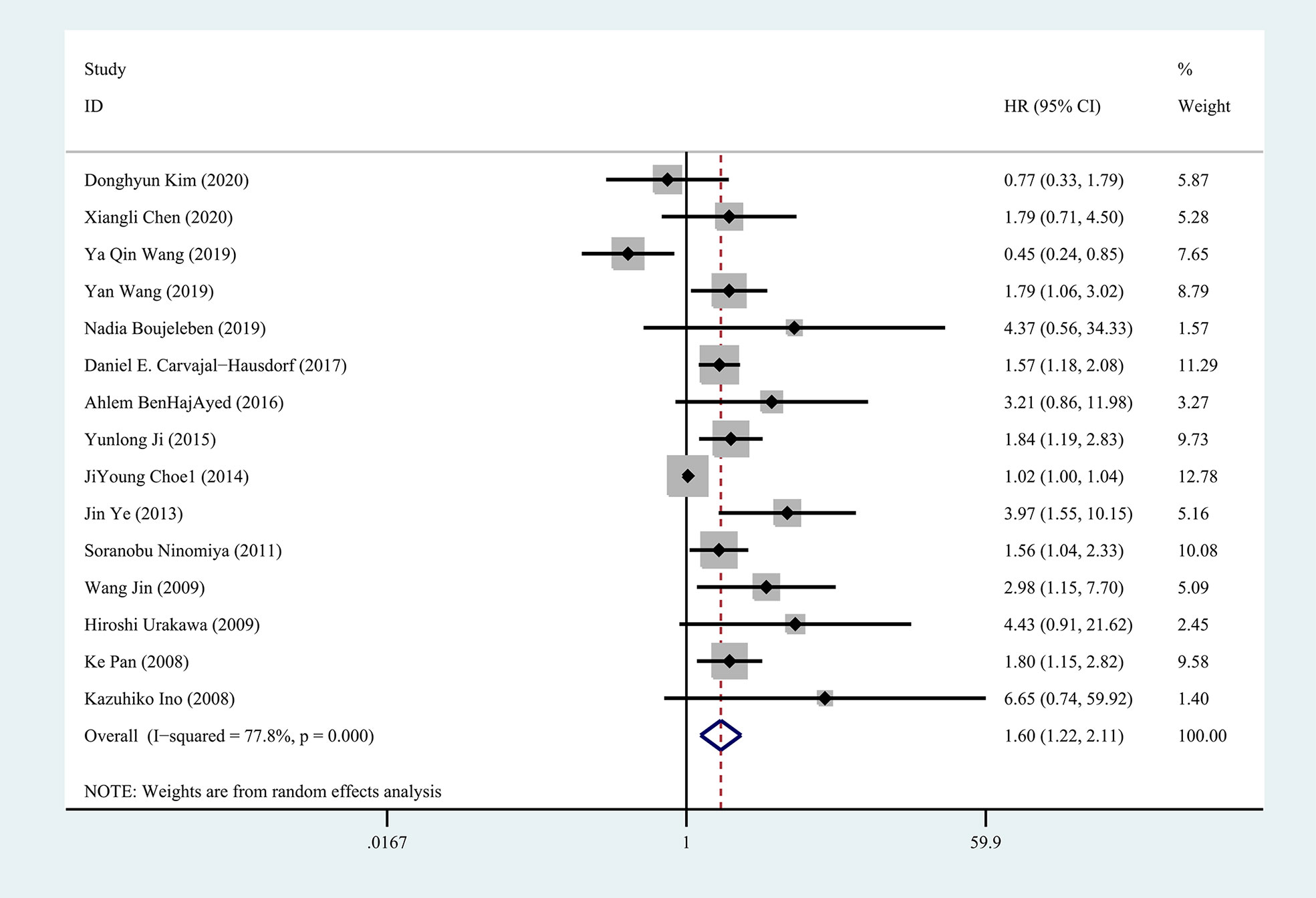
Figure 2 Forest plot of HR with 95% CI for correlation between expression of IDO1 and overall survival.
The relation between IDO1 expression and PFS and DFS in solid tumor patients
There were totally six and three studies that reported the effects of IDO1 expression on PFS and DFS involved in the meta-analysis, respectively. A fixed-effect model was used to calculate the pooled HRs and 95% CIs, as the lower heterogeneity of PFS (I2 = 26.0%, P = 0.240) and DFS (I2 = 0, P = 0.640), respectively. Our results demonstrated that a high expression of IDO1 was associated with poor DFS (n = 327, HR = 2.65, 95% CI: 1.52–4.63, P = 0.001), while no significant correlation was found between IDO1 and PFS (n = 428, HR = 1.76, 95% CI: 0.99–3.14, P = 0.054) (Figure 3).
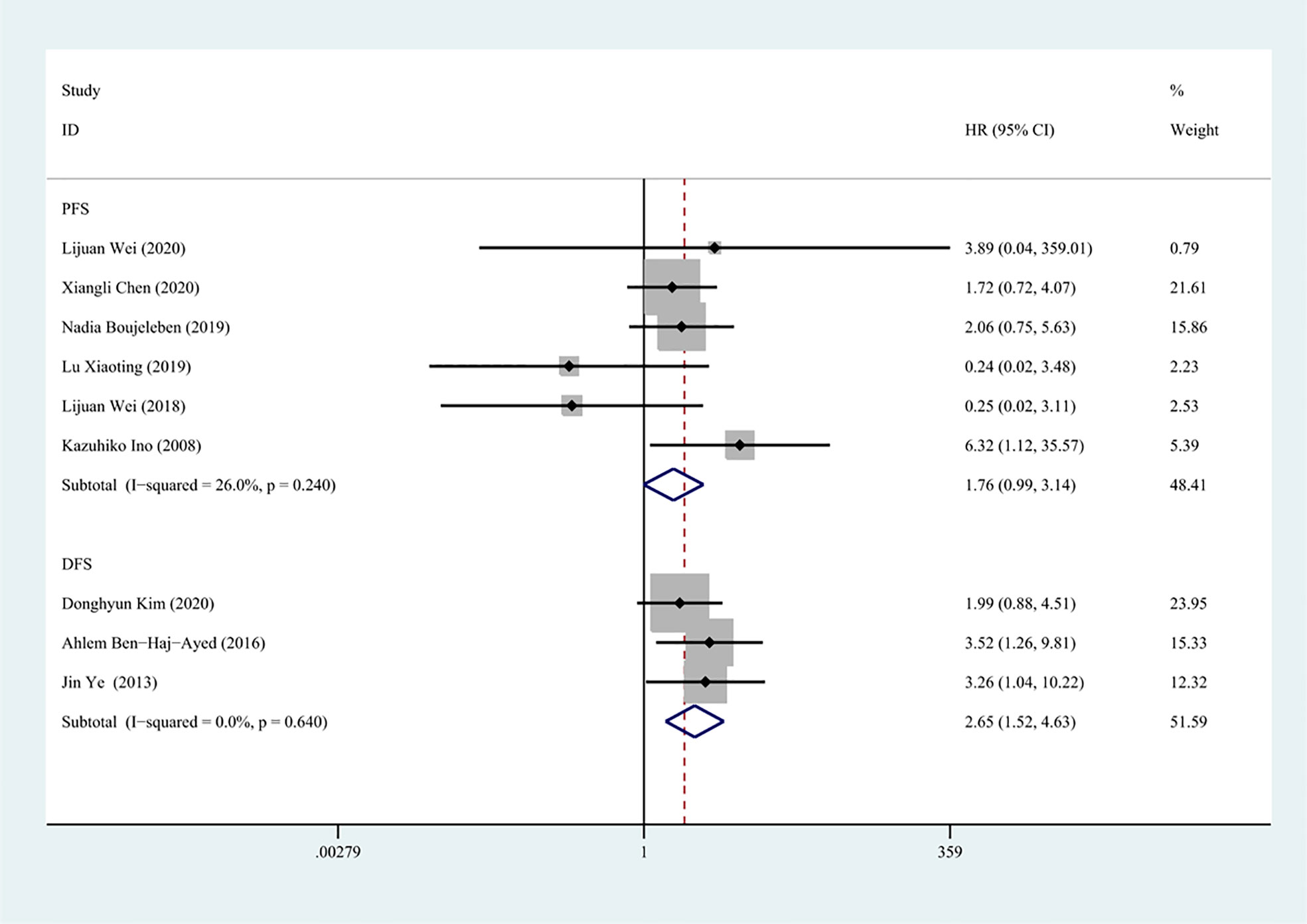
Figure 3 Forest plot of HR with 95% CI for correlation between expression of IDO1 and progression-free survival, disease-free survival.
Publication bias and sensitivity analysis
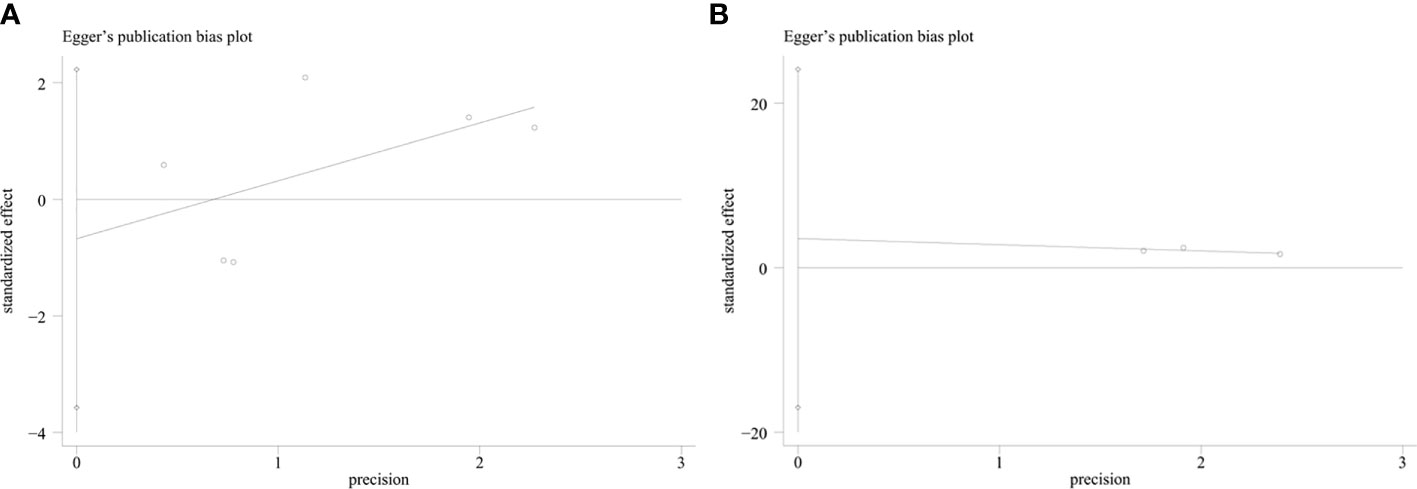
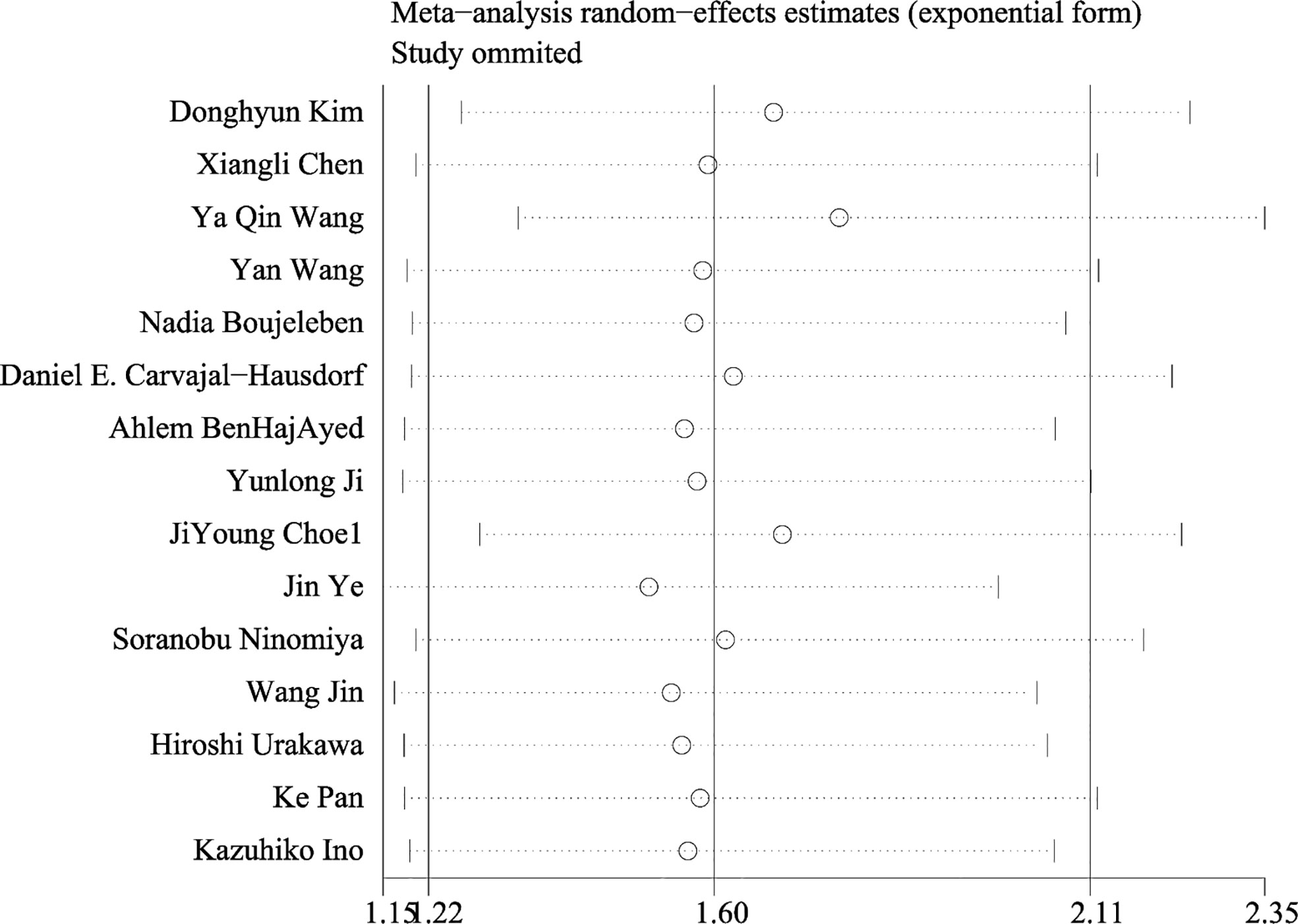
An evaluation of publication bias between studies was conducted using Egger’s test. Publication bias was found between IDO1 and OS (P = 0.002), but no publication bias was detected between IDO and PFS (P = 0.553), DFS (P = 0.273) (Figure 4). The trim-and-fill method was used to assess publication bias for OS. The overall effect was unchanged when four missing studies were added (HR = 1.418, 95% CI: 1.093–1.840, P = 0.009). Therefore, publication bias for OS had no impact on our results, which could be ignored. Sensitivity analysis showed that no single study had a significant impact on the conclusions of this meta-analysis (Figure 5), indicating that the results were stable.
Discussion
IDO1 was first demonstrated by David Munn in 1998 to play crucial role in maintenance of maternal T-cell tolerance in the mouse placenta (54). Studies showed that IDO1 expression was strongly induced by interferon-γ in cell lines (55) and associated with lower CD3+ and CD8+ T lymphocyte infiltration and CD57+ NK-cell infiltration in tumor specimens (56), which could be the mechanism of immune escape. Many studies demonstrated that the expression of IDO1 in tumors was associated with prognosis (57, 58). However, the results were conflicting. Our meta-analysis included 2,168 patients with solid tumors from 18 studies. The results showed that a high expression of IDO1 was associated with a poor OS and DFS, which was consistent with previous meta-analyses (35, 36). Unexpectedly, no significant correlation was found between IDO1 expression and PFS. To the best of our knowledge, articles that have systematically evaluated the relationship between IDO1 expression and PFS in solid tumor are rare up to now, because of lack of enough data.
In this study, the heterogeneity of OS was obvious, which could be explained by several reasons. Firstly, 11 tumor types were included in this study and the role of IDO1 in different tumors may be inconsistent. Secondly, the cutoff value of IDO1 and treatment after surgery or biopsy could lead to the heterogeneity. To this end, subgroup analysis showed that age, gender, follow-up period, and study quality were possible reasons. The study of Stephane et al. showed that hyperprogressive disease was associated with a higher age and a worse OS in cancer patients treated by anti-PD-1/PD-L1, indicating a different immunological background in older patients (59). It was suggested that age could affect the immune microenvironment by altering the number, phenotype, and functions of neutrophils, macrophages, dendritic cells, and NK cells (60), further regulating IDO1 expression, which was similar to our study. Furthermore, Brandacher’s study showed that IDO-high expression was significantly correlated with better prognosis within the first 45 months, while the result was dramatically reversed after 45 months of follow-up (61), which attributed to the treatments during the follow-up period. This was why the follow-up period was a factor affecting heterogeneity in our study. In addition, the result of the subgroup analysis showed that the detection method could also lead to high heterogeneity, indicating the demand for more precise detection methods. Meanwhile, the studies involved in our meta-analysis were retrospective and the data identified were not comprehensive. It was supposed as the reason for the majority of studies suffering from a moderate risk of bias.
In a study of chemokine CXC motif ligand 12 (CXCL12), a prognosis factor similar to IDO1, a high CXCL12 expression was associated with a shorter OS in esophagogastric, pancreatic, or lung cancer, while the opposite was the case for breast cancer (62). Similarly, in our result, the relationship between IDO1 and PFS was different from OS and DFS, which may be explained by the included tumor types. Involving PFS, half of the included studies were about breast cancer. However, the exact cause of the different effects of IDO1 expression and outcome in breast was unclear. It was guessed that IDO1 could promote local invasion of cancer cells, which leads to a worse outcome in other cancers, but it was less affected in breast cancer, which was rarely fatal through local invasion unless metastasis occurs.
There were limitations in this meta-analysis. Firstly, we were unable to perform a subgroup analysis for each type of tumor, because of the limited number of included studies. Secondly, the cutoff values of IDO1 positivity and high expression were not completely consistent between studies, leading to the potential sources of heterogeneity. Thirdly, all the studies included were retrospective studies, lacking a prospective study. Additionally, the study was not registered in PROSPERO.
Conclusion
The study showed that a high expression of IDO1 was associated with a poor OS and DFS in cancers. There was no significant correlation between IDO1 expression and PFS. IDO1 appears to be a promising therapeutic target as well as a prognostic predictor in different cancers. IDO1 inhibitors in monotherapy or in combination with other ICs will be the promising treatment. However, the immune regulator function of IDO1 in different types of cancer could not be completely consistent. Meanwhile, the exact mechanism of IDO1 in immune escape remains unclear. Future prospective studies are required to validate the relationship between expression of IDO1 and immune escape.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
HZ searched and screened the literature according to the criteria, analyzed the data using Stata, and wrote the first draft of the manuscript. JL searched and screened the literature according to the criteria. QZ contributed to the conception and design of the study. All authors contributed to the manuscript revision and read and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Voskamp MJ, Li S, van Daalen KR, Crnko S, Ten Broeke T, Bovenschen N. Immunotherapy in medulloblastoma: Current state of research, challenges, and future perspectives. Cancers (2021) 13(21):5387. doi: 10.3390/cancers13215387
2. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature (2013) 500(7463):415–21. doi: 10.1038/nature12477
3. Barone B, Calogero A, Scafuri L, Ferro M, Lucarelli G, Di Zazzo E, et al. Immune checkpoint inhibitors as a Neoadjuvant/Adjuvant treatment of muscle-invasive bladder cancer: A systematic review. Cancers (Basel) (2022) 14(10):2545. doi: 10.3390/cancers14102545
4. Chu C, Pietzak E. Immune mechanisms and molecular therapeutic strategies to enhance immunotherapy in non-muscle invasive bladder cancer: Invited review for special issue “Seminar: Treatment advances and molecular biology insights in urothelial carcinoma”. Urol Oncol (2022). doi: 10.1016/j.urolonc.2022.05.013
5. Clanchy FIL, Huang YS, Ogbechi J, Darlington LG, Williams RO, Stone TW. Induction of Ido1 and kynurenine by serine proteases subtilisin, prostate specific antigen, Cd26 and htra: A new form of immunosuppression? Front Immunol (2022) 13:832989. doi: 10.3389/fimmu.2022.832989
6. Gu W, Zhu Y, Ye D. Beyond chemotherapy for advanced disease-the role of egfr and pd-1 inhibitors. Transl Androl Urol (2017) 6(5):848–54. doi: 10.21037/tau.2017.03.92
7. Crocetto F, Arcaniolo D, Napolitano L, Barone B, La Rocca R, Capece M, et al. Impact of sexual activity on the risk of Male genital tumors: A systematic review of the literature. Int J Environ Res Public Health (2021) 18(16):8500. doi: 10.3390/ijerph18168500
8. Weng J, Li S, Zhu Z, Liu Q, Zhang R, Yang Y, et al. Exploring immunotherapy in colorectal cancer. J Hematol Oncol (2022) 15(1):95. doi: 10.1186/s13045-022-01294-4
9. Zou LQ, Yang X, Li YD, Zhu ZF. Immune checkpoint inhibitors: A new era for esophageal cancer. Expert Rev Anticancer Ther (2019) 19(8):731–8. doi: 10.1080/14737140.2019.1654379
10. Nakhoda S, Rizwan F, Vistarop A, Nejati R. Updates in the role of checkpoint inhibitor immunotherapy in classical hodgkin’s lymphoma. Cancers (Basel) (2022) 14(12):2936. doi: 10.3390/cancers14122936
11. Donini C, D’Ambrosio L, Grignani G, Aglietta M, Sangiolo D. Next generation immune-checkpoints for cancer therapy. J Thorac Dis (2018) 10:S1581–S601. doi: 10.21037/jtd.2018.02.79
12. Zhai L, Ladomersky E, Lenzen A, Nguyen B, Patel R, Lauing KL, et al. Ido1 in cancer: A Gemini of immune checkpoints. Cell Mol Immunol (2018) 15(5):447–57. doi: 10.1038/cmi.2017.143
13. Hornyak L, Dobos N, Koncz G, Karanyi Z, Pall D, Szabo Z, et al. The role of indoleamine-2,3-Dioxygenase in cancer development, diagnostics, and therapy. Front Immunol (2018) 9:151. doi: 10.3389/fimmu.2018.00151
14. Asghar K, Loya A, Rana IA, Tahseen M, Ishaq M, Farooq A, et al. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manage Res (2019) 11:475–81. doi: 10.2147/CMAR.S184221
15. Zhao Y, Wang B, Liu J, Sun P, Liu H. An overview on the methods of determining the activity of indoleamine 2, 3-dioxygenase 1. J Drug Targeting (2019) 27(7):724–31. doi: 10.1080/1061186X.2018.1523416
16. Jafarzadeh L, Khakpoor-Koosheh M, Mirzaei H, Mirzaei HR. Biomarkers for predicting the outcome of various cancer immunotherapies. Crit Rev Oncology/Hematol (2021) 157:103161. doi: 10.1016/j.critrevonc.2020.103161
17. Kim D, Kim JM, Kim JS, Kim S, Kim KH. Differential expression and clinicopathological significance of Her2, indoleamine 2,3-dioxygenase and pd-L1 in urothelial carcinoma of the bladder. J Clin Med (2020) 9(5):1265. doi: 10.3390/jcm9051265
18. Tang K, Wu YH, Song Y, Yu B. Indoleamine 2,3-dioxygenase 1 (Ido1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol (2021) 14(1):68. doi: 10.1186/s13045-021-01080-8
19. Ludovini V, Bianconi F, Siggillino A, Vannucci J, Baglivo S, Berti V, et al. High pd-L1/Ido-2 and pd-L2/Ido-1 Co-expression levels are associated with worse overall survival in resected non-small cell lung cancer patients. Genes (2021) 12(2):273. doi: 10.3390/genes12020273
20. Zhou S, Zhao L, Liang Z, Liu S, Li Y, Liu S, et al. Indoleamine 2,3-dioxygenase 1 and programmed cell death-ligand 1 Co-expression predicts poor pathologic response and recurrence in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. Cancers (2019) 11(2):169. doi: 10.3390/cancers11020169
21. Zhao Y, Wei L, Liu J, Li F. Chemoresistance was correlated with elevated expression and activity of indoleamine 2,3-dioxygenase in breast cancer. Cancer Chemother Pharmacol (2020) 85(1):77–93. doi: 10.1007/s00280-019-04009-8
22. Zhang T, Tan XL, Xu Y, Wang ZZ, Xiao CH, Liu R. Expression and prognostic value of indoleamine 2,3-dioxygenase in pancreatic cancer. Chin Med J (2017) 130(6):710–6. doi: 10.4103/0366-6999.201613
23. Wu D, Hacking S, Cao J, Nasim M. Understanding the role of indoleamine-2,3-Dioxygenase and stromal differentiation in rare subtype endometrial cancer. Rare Tumors (2021) 13:20363613211044690. doi: 10.1177/20363613211044690
24. Wardhani LO, Matsushita M, Iwasaki T, Kuwamoto S, Nonaka D, Nagata K, et al. Expression of the Ido1/Tdo2-ahr pathway in tumor cells or the tumor microenvironment is associated with merkel cell polyomavirus status and prognosis in merkel cell carcinoma. Hum Pathol (2019) 84:52–61. doi: 10.1016/j.humpath.2018.09.003
25. Chen X, Zang Y, Li D, Guo J, Wang Y, Lin Y, et al. Ido, tdo, and ahr overexpression is associated with poor outcome in diffuse Large b-cell lymphoma patients in the rituximab era. Medicine (2020) 99(21):e19883. doi: 10.1097/MD.0000000000019883
26. Ma W, Duan H, Zhang R, Wang X, Xu H, Zhou Q, et al. High expression of indoleamine 2, 3-dioxygenase in adenosquamous lung carcinoma correlates with favorable patient outcome. J Cancer (2019) 10(1):267–76. doi: 10.7150/jca.27507
27. Zhou QH, Han H, Lu JB, Liu TY, Huang KB, Deng CZ, et al. Up-regulation of indoleamine 2,3-dioxygenase 1 (Ido1) expression and catalytic activity is associated with immunosuppression and poor prognosis in penile squamous cell carcinoma patients. Cancer Commun (London England) (2020) 40(1):3–15. doi: 10.1002/cac2.12001
28. Wei L, Wu N, Wei F, Li F, Zhang Y, Liu J, et al. Prognosis significance of indoleamine 2, 3-dioxygenase, programmed death ligand-1 and tumor-infiltrating immune cells in microenvironment of breast cancer. Int Immunopharmacol (2020) 84:106506. doi: 10.1016/j.intimp.2020.106506
29. Ishihara S, Yamada Y, Iwasaki T, Yoshimoto M, Toda Y, Kohashi K, et al. Pd−L1 and Ido−1 expression in undifferentiated pleomorphic sarcoma: The associations with tumor infiltrating lymphocytes, dmmr and hla class I. Oncol Rep (2021) 45(1):379–89. doi: 10.3892/or.2020.7837
30. Lin DJ, Ng JC, Huang L, Robinson M, aO’Hara J, Wilson JA, et al. The immunotherapeutic role of indoleamine 2,3-dioxygenase (Ido) in head and neck squamous cell carcinoma: A systematic review. Clin Otolaryngol (2021) 46(5):919–34. doi: 10.1111/coa.13794
31. Iwamoto N, Ito H, Ando K, Ishikawa T, Hara A, Taguchi A, et al. Upregulation of indoleamine 2,3-dioxygenase in hepatocyte during acute hepatitis caused by hepatitis b virus-specific cytotoxic T lymphocytes in vivo. Liver Int (2009) 29(2):277–83. doi: 10.1111/j.1478-3231.2008.01748.x
32. Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest (2007) 117(5):1147–54. doi: 10.1172/JCI31178
33. Sunakawa Y, Cao S, Volz NB, Berger MD, Yang D, Parekh A, et al. Genetic variations in immunomodulatory pathways to predict survival in patients with locoregional gastric cancer. Pharmacogenom J (2017) 17(6):528–34. doi: 10.1038/tpj.2016.46
34. Stremitzer S, Sunakawa Y, Zhang W, Yang D, Ning Y, Stintzing S, et al. Variations in genes involved in immune response checkpoints and association with outcomes in patients with resected colorectal liver metastases. Pharmacogenom J (2015) 15(6):521–9. doi: 10.1038/tpj.2015.14
35. Yu CP, Fu SF, Chen X, Ye J, Ye Y, Kong LD, et al. The clinicopathological and prognostic significance of Ido1 expression in human solid tumors: Evidence from a systematic review and meta-analysis. Cell Physiol Biochem (2018) 49(1):134–43. doi: 10.1159/000492849
36. Wang S, Wu J, Shen H, Wang J. The prognostic value of ido expression in solid tumors: A systematic review and meta-analysis. BMC Cancer (2020) 20(1):471. doi: 10.1186/s12885-020-06956-5
37. Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med (2006) 144(6):427–37. doi: 10.7326/0003-4819-144-6-200603210-00010
38. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med (2013) 158(4):280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
39. Wang YQ, Zhang Y, Jiang W, Chen YP, Xu SY, Liu N, et al. Development and validation of an immune checkpoint-based signature to predict prognosis in nasopharyngeal carcinoma using computational pathology analysis. J Immunother Cancer (2019) 7(1):298. doi: 10.1186/s40425-019-0752-4
40. Wang Y, Yao R, Zhang L, Xie X, Chen R, Ren Z. Ido and intra-tumoral neutrophils were independent prognostic factors for overall survival for hepatocellular carcinoma. J Clin Lab Anal (2019) 33(5)::e22872. doi: 10.1002/jcla.22872
41. Boujelbene N, Ben Yahia H, Babay W, Gadria S, Zemni I, Azaiez H, et al. Hla-G, hla-e, and ido overexpression predicts a worse survival of Tunisian patients with vulvar squamous cell carcinoma. HLA (2019) 94(1):11–24. doi: 10.1111/tan.13536
42. Wei LJ, Zhu SS, Li MH, Li FX, Wei F, Liu JT, et al. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front Immunol (2018) 9:724. doi: 10.3389/fimmu.2018.00724
43. Carvajal-Hausdorf DE, Mani N, Velcheti V, Schalper KA, Rimm DL. Objective measurement and clinical significance of Ido1 protein in hormone receptor-positive breast cancer. J Immunother Cancer (2017) 5(1):81. doi: 10.1186/s40425-017-0285-7
44. Ben-Haj-Ayed A, Moussa A, Ghedira R, Gabbouj S, Miled S, Bouzid N, et al. Prognostic value of indoleamine 2,3-dioxygenase activity and expression in nasopharyngeal carcinoma. Immunol Lett (2016) 169:23–32. doi: 10.1016/j.imlet.2015.11.012
45. Jia Y, Wang H, Wang Y, Wang T, Wang M, Ma M, et al. Low expression of Bin1, along with high expression of ido in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patients. Int J Cancer (2015) 137(5):1095–106. doi: 10.1002/ijc.29481
46. Choe JY, Yun JY, Jeon YK, Kim SH, Park G, Huh JR, et al. Indoleamine 2,3-dioxygenase (Ido) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: A retrospective cohort study. BMC Cancer (2014) 14(1):335. doi: 10.1186/1471-2407-14-335
47. Ye J, Liu H, Hu YM, Li P, Zhang GH, Li Y. Tumoral indoleamine 2,3-dioxygenase expression predicts poor outcome in laryngeal squamous cell carcinoma. Virchows Archiv (2013) 462(1):73–81. doi: 10.1007/s00428-012-1340-x
48. Ninomiya S, Hara T, Tsurumi H, Hoshi M, Kanemura N, Goto N, et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse Large b-cell lymphoma treated with r-chop. Ann Hematol (2011) 90(4):409–16. doi: 10.1007/s00277-010-1093-z
49. Urakawa H, Nishida Y, Nakashima H, Shimoyama Y, Nakamura S, Ishiguro N. Prognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcoma. Clin Exp Metastasis (2009) 26(8):1005–12. doi: 10.1007/s10585-009-9290-7
50. Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol (2008) 134(11):1247–53. doi: 10.1007/s00432-008-0395-1
51. Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: Its association with disease progression and survival. Clin Cancer Res (2008) 14(8):2310–7. doi: 10.1158/1078-0432.CCR-07-4144
52. Xiaotong L, Yafen Z, Runfang G. The relationship between indoleamine 2, 3-dioxygenase and microvascular density-CD105 and clinicopathological features and prognosis of breast cancer. Chin Remedies Clinics (2019) 19(22):3869–72.
53. Jin W, Guohe L, Jun W, Li X, Yunfei Y, Sheng-ping L. Significance of indoleamine 2,3-dioxygenase expression in tumor-draining lymph node in patientswith hepatocellular carci-noma. Med J Of Chin People’s Liberation Army (2009) 34(7):844–7.
54. Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science (1998) 281(5380):1191–3. doi: 10.1126/science.281.5380.1191
55. Lin DJ, Ng JCK, Huang L, Robinson M, O’Hara J, Wilson JA, et al. The immunotherapeutic role of indoleamine 2,3-dioxygenase in head and neck squamous cell carcinoma: A systematic review. Clin Otolaryngol (2021) 46(5):919–34. doi: 10.1111/coa.13794
56. Pallotta MT, Rossini S, Suvieri C, Coletti A, Orabona C, Macchiarulo A, et al. Indoleamine 2,3-dioxygenase 1 (Ido1): An up-to-Date overview of an eclectic immunoregulatory enzyme. FEBS J (2021) 16086. doi: 10.1111/febs.16086
57. Toda Y, Kohashi K, Yamada Y, Yoshimoto M, Ishihara S, Ito Y, et al. Pd-L1 and Ido1 expression and tumor-infiltrating lymphocytes in osteosarcoma patients: Comparative study of primary and metastatic lesions. J Cancer Res Clin Oncol (2020) 146(10):2607–20. doi: 10.1007/s00432-020-03242-6
58. Nishi M, Yoshikawa K, Higashijima J, Tokunaga T, Kashihara H, Takasu C, et al. The impact of indoleamine 2,3-dioxygenase (Ido) expression on stage iii gastric cancer. Anticancer Res (2018) 38(6):3387–92. doi: 10.21873/anticanres.12605
59. Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-Pd-1/Pd-L1. Clin Cancer Res (2017) 23(8):1920–8. doi: 10.1158/1078-0432.CCR-16-1741
60. Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol (2012) 24(5):331–41. doi: 10.1016/j.smim.2012.04.008
61. Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: Effect on tumor-infiltrating T cells. Clin Cancer Res (2006) 12(4):1144–51. doi: 10.1158/1078-0432.CCR-05-1966
Keywords: IDO1, tumor, prognosis, survival, meta-analysis
Citation: Zhang H, Li J and Zhou Q (2022) Prognostic role of indoleamine 2,3-dioxygenase 1 expression in solid tumors: A systematic review and meta-analysis. Front. Oncol. 12:954495. doi: 10.3389/fonc.2022.954495
Received: 27 May 2022; Accepted: 23 August 2022;
Published: 23 September 2022.
Edited by:
Matteo Ferro, European Institute of Oncology (IEO), ItalyReviewed by:
Celeste Manfredi, University of Campania Luigi Vanvitelli, ItalyAchille Aveta, University of Naples Federico II, Italy
Copyright © 2022 Zhang, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Zhou, cWl6aG91MDkwOEAxNjMuY29t
 Haiyan Zhang
Haiyan Zhang Jing Li1
Jing Li1