
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 29 December 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.954345
This article is part of the Research Topic Efficacy and Safety of CAR-T Cell Therapy in Hematologic Malignancies View all 8 articles
Background: CD22 single and CD19/CD22 bispecific targeted chimeric antigen receptor T (CAR-T) cell therapy are promising immunotherapy modalities for the treatment of hematologic malignancies. The aim of this study was to assess the efficacy and safety of CD22 and CD19/CD22 targeted CAR-T cell therapy by summarizing the existing evidence.
Methods: Electronic databases including PubMed, Embase, and Scopus were comprehensively searched from inception up to November 30, 2022. Pooled response rates and minimal residual disease (MRD) negative response rates, cytokine release syndrome (CRS) rates and neurotoxicity rates were calculated. Subgroup analysis was performed based on the type of immunotherapy.
Results: Ten clinical studies including 194 patients with hematologic malignancies were included after a systematical screening of literature. The pooled complete response (CR) rates of CD22 and CD19/CD22 CAR-T cell therapy for relapsed or refractory B-cell lymphoblastic leukemia (B-ALL) were 0.75 (95% CI: 0.60 - 0.88) and 0.87 (95% CI: 0.76 - 0.96). The overall MRD negative response rates of CD22 and CD19/CD22 CAR-T were 0.54 (95% CI: 0.42 - 0.66) and 0.91 (95% CI: 0.47 - 0.88). Pooled CRS rates of CD22 targeted and CD19/CD22 targeted immunotherapy were 0.92 (95% CI: 0.82 - 0.98) and 0.94 (95% CI: 0.82 - 1.00), respectively.
Conclusion: Both CD22 and CD19/CD22 CAR-T immunotherapy demonstrated favorable efficacy and acceptable adverse events in the treatment of hematologic malignancies. Well-designed and large sample-sized clinical trials are warranted.
High dose chemotherapy and hematopoietic stem cell transplantation are standard curative strategies for patients with hematological malignancies including myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), Hodgkin’s lymphoma and diffuse large B cell lymphoma (DLBCL) (1). For example, treatment options for AML included either intensive chemotherapy with anthracycline and cytarabine-based regimens (7 + 3) or lower intensity regimens including hypomethylating agents or low dose cytarabine, followed by either allogeneic stem cell transplant or consolidation chemotherapy (2). Treatment for pediatric ALL typically consists of induction therapy with steroids, vincristine, and asparaginase with or without anthracycline, followed by multi-agent consolidation including high-dose methotrexate and re-induction therapy (3). Nevertheless, sufficient efficacy occurred temporarily due to disease relapse following treatment (4).
Interestingly, immunotherapy has been extensively investigated for decades in the treatment of almost all types of hematologic cancers (5). Chimeric antigen receptor (CAR) T cell therapy is one of the promising immunotherapy approaches (6, 7). CARs are genetically engineered proteins that manipulate the antigen-recognition ability of antibodies and the effector functions of T cells (8). A CAR is composed of three pivotal domains including an extracellular antigen recognition domain, a transmembrane domain, and an intracellular T cell activation domain (4, 6). T cells were collected from the patient or the donor, amplified in a bioreactor and modified to express a specific CAR before injection into the patient (9). Early clinical data have generated considerable promise, and it is reasonable to speculate that CAR-T based immunotherapy can fundamentally change the existing treatment paradigms of B-cell malignancies (10–14). Furthermore, CD19 directed CAR-T cell therapies namely tisagenlecleucel, axicabtagene ciloleucel, brexucabtagene autoleucel, and lisocabtagene maraleucel received the FDA approval in recent years (15–17). CAR T-cell therapy, however, is related to notable drawbacks that hinder its development and wide promotion: cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), B-cell regeneration disorders, and early and late infections (18–21). Of note, signs and symptoms of CRS can vary from mild to fatal (22). Albeit with worldwide exciting clinical data on anti-CD19 CAR-T therapy, relapse after this therapy is associated with poor prognosis and has become an urgent problem to be solved (7, 23). The recurrence of hematologic malignancies partly resulted from the loss of CD19 antigen expression on malignant cells after CD19 CAR-T cell treatment, thus CAR-T with new or dual targets on CD19 and CD22 may address the drug resistance (4).
CD22 CAR-T cell therapy, proposed as an alternative CAR-T method for treating primary or relapsed B-lymphoblastic leukemia (B-ALL), have been demonstrated to be effective in the treatment of patients with B-ALL who are not suitable for CD19 CAR-T cells therapy (24–27). Moreover, CD19 and CD22 bispecific CAR-T therapy via transduction of T cells with a bicistronic γ-retroviral vector encoding humanized anti-CD19 and CD22 CARs has also been investigated and revealed elicited clinical outcomes (28). Accumulated number of clinical trials with regard to CD22 single and CD19/CD22 bispecific CAR-T therapy in patients with hematologic malignancies has been performed, nevertheless, the sample sizes and outcomes of these studies are heterogeneous. The aim of this systematic review and meta-analysis was to assess the efficacy and safety of CD22 targeted and CD19/CD22 bispecific CAR-T cell therapies by summarizing the published data and to provide for clinicians with evidence-based references for clinical decision-making and scientific research.
The present meta-analysis was performed strictly based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (29). Informed consent was not acquired because the dataset used in this meta-analysis was derived from published literature.
Three online databases including PubMed, Embase, and Scopus were systematically searched by two independent authors from inception of the database to November 30, 2022 with articles in English language considered. The references of relevant reviews were manually screened for potentially eligible studies. The following terms and keywords were employed in literature search: CAR-T, chimeric antigen receptor -T cell, CD22, Hematologic Malignancies, Hematologic Neoplasms, Hematologic Neoplasm, Hematologic Neoplasia, Hematologic Neoplasias, Hematologic Tumor, Hematologic Cancer, and Hematologic Malignancy. Two investigators independently performed the study selection, disagreements were addressed through discussion.
Studies fulfilling the following inclusion criteria were enrolled:
1) Clinical studies investigating the efficacy and/or safety of anti-CD22 or anti-CD19/CD22 CAR-T cell therapy in the treatment of hematological malignancies.
2) Outcomes included complete response rate, partial response rate, overall response rate, minimal residual disease (MRD) negative response rate, progression-free survival, overall survival, cytokine release syndrome (CRS) rate and neurotoxicity rate.
3) Literature types included but not limited to research articles in case that complete data could be extracted.
Exclusion criteria were as follows:
1) Patients treated with combinations of CAR-T and other immunotherapies were excluded.
2) Case reports or case series, reviews, meta-analyses, abstracts or correspondence with unavailable data.
3) Citations not in the English language.
A predesigned table for the variables of data extraction was proposed. Parameters including name of first author, year of publication, country, number of patients, age of patients,percentage of the female, type of malignancies, type of CAR-T and information on outcomes regarding efficacy and safety aforementioned were retrieved by two reviewers, discrepancies were resolved through discussion. If two or more studies covered the same group/subgroup of patients, only the study with the largest sample size or the most complete data was enrolled to avoid duplicates. The Newcastle-Ottawa scale (NOS) was used to assess the quality of the included studies, this scale is a three-domain scale regarding the evaluation of selection, comparability, and outcome (30).
Statistical analyses were performed utilizing the R Foundation for Statistical Computing (Version 4.1.2, Vienna, Austria). Pooled estimates of response rates, adverse effects rates, survival rates with their respective 95% confidence intervals were calculated using the random effects methods considering that most of studies with regard to hematological malignancies were nonrandomized single arm studies. We conducted subgroup analyses to investigate the efficacy and safety according to the type of tumor and type of CAR-T therapies. The I2 value was used to assess the magnitude of heterogeneity between included studies. Meta-regression was utilized to evaluate the potential source of heterogeneity. Moreover, the Egger’s test of funnel plot asymmetry were performed to explore the potential publication bias (31). A p value < 0.05 was regarded as statistical significance.
A total of 1943 records were retrieved from the initial database search. After removal of 698 duplicates, 30 case reports, 57 animal studies, 419 reviews, 269 abstracts, 425 topic irrelevant articles, 45 citations were screened in full text reading. Ten clinical studies with 194 patients with hematologic malignancies were eligible for inclusion after full text reading. Figure 1 shows the detailed flow of literature search. Five included studies were clinical trials investigating anti-CD22 CAR-T cell therapy for relapsed or refractory B-ALL. Five studies assessed the efficacy and safety of CD19/CD22 bispecific CAR-T cell therapy relapsed or refractory B-ALL. Table 1 reveals the details on characteristics of enrolled studies. The quality of included studies was regarded as moderate to high based on the NOS scale (Supplementary Table 1).
Nine studies reported the assessment of treatment complete responses. The overall complete response rates of CD22 and CD19/CD22 CAR-T cell therapies for relapsed or refractory B-ALL were 0.75 (95% CI: 0.60 - 0.88) and 0.87 (95% CI: 0.76 - 0.96), respectively (Figure 2). Six studies were evaluated for MRD negative responses, the pooled MRD negative response rates of CD22 and CD19/CD22 CAR-T cell therapies were 0.54 (95% CI: 0.42 - 0.66) and 0.91 (95% CI: 0.47 - 0.88) (Figure 3). Two studies reported overall response rates (ORRs), the ORR in the study of Spiegel et al. was 100%, as in the study of Tan et al., the ORR was 87.5%. Besides, results of Shah’s study demonstrated a median overall survival of 13.4 months (95% CI: 7.7 to 20.3 months) and a median relapse-free survival of 6.0 months (95% CI: 4.1 to 6.5 months) for anti-CD22 CAR T cell therapy. Kaplan-Meier survival analysis in Liu’s study manifested overall survival and event-free survival rates of 88.5% and 67.5% at both 12 months and 18 months.
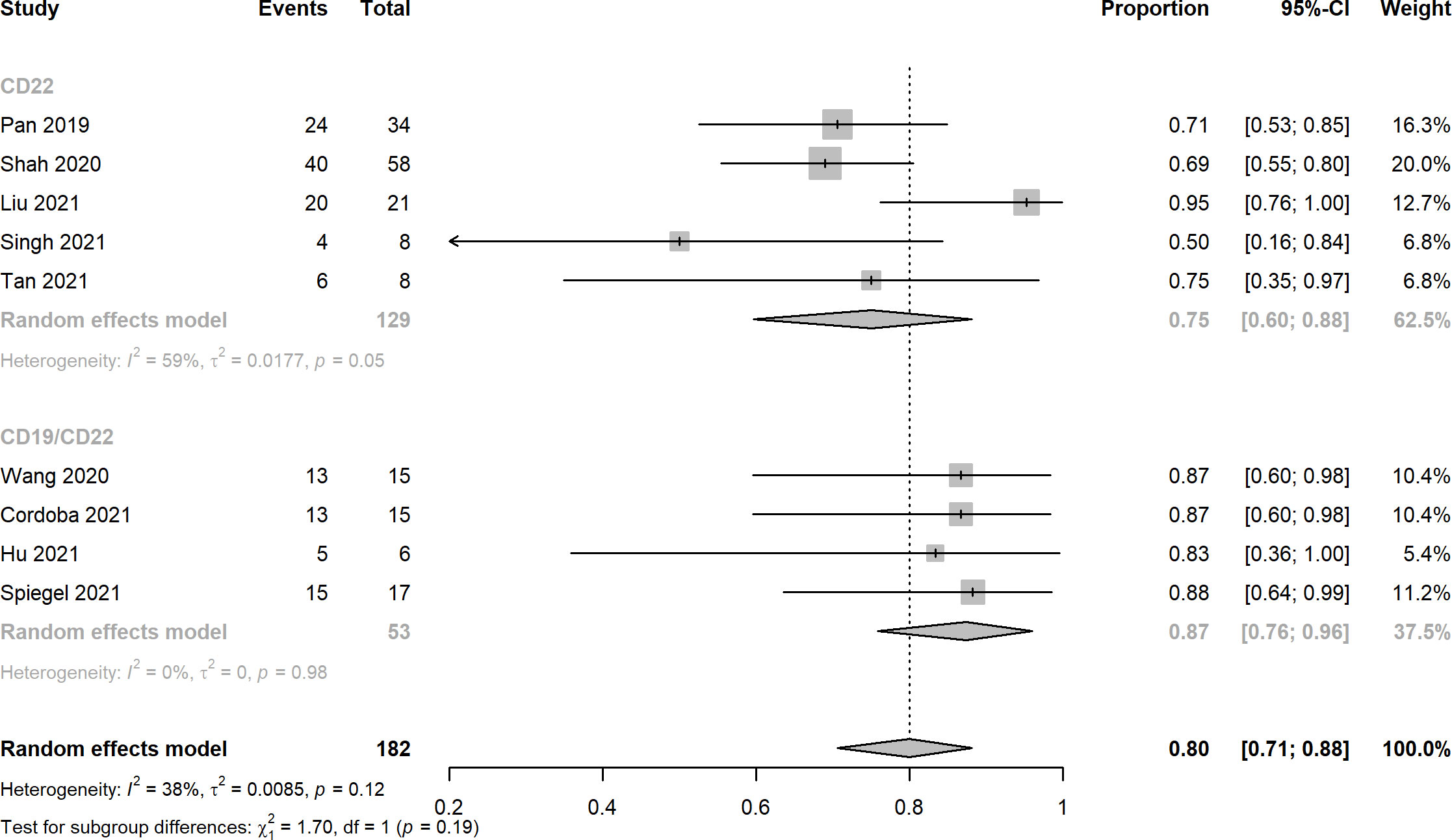
Figure 2 Forest plots of complete response rates of CD22 and CD19/CD22 targeted CAR-T cell therapies for relapsed or refractory B-ALL.
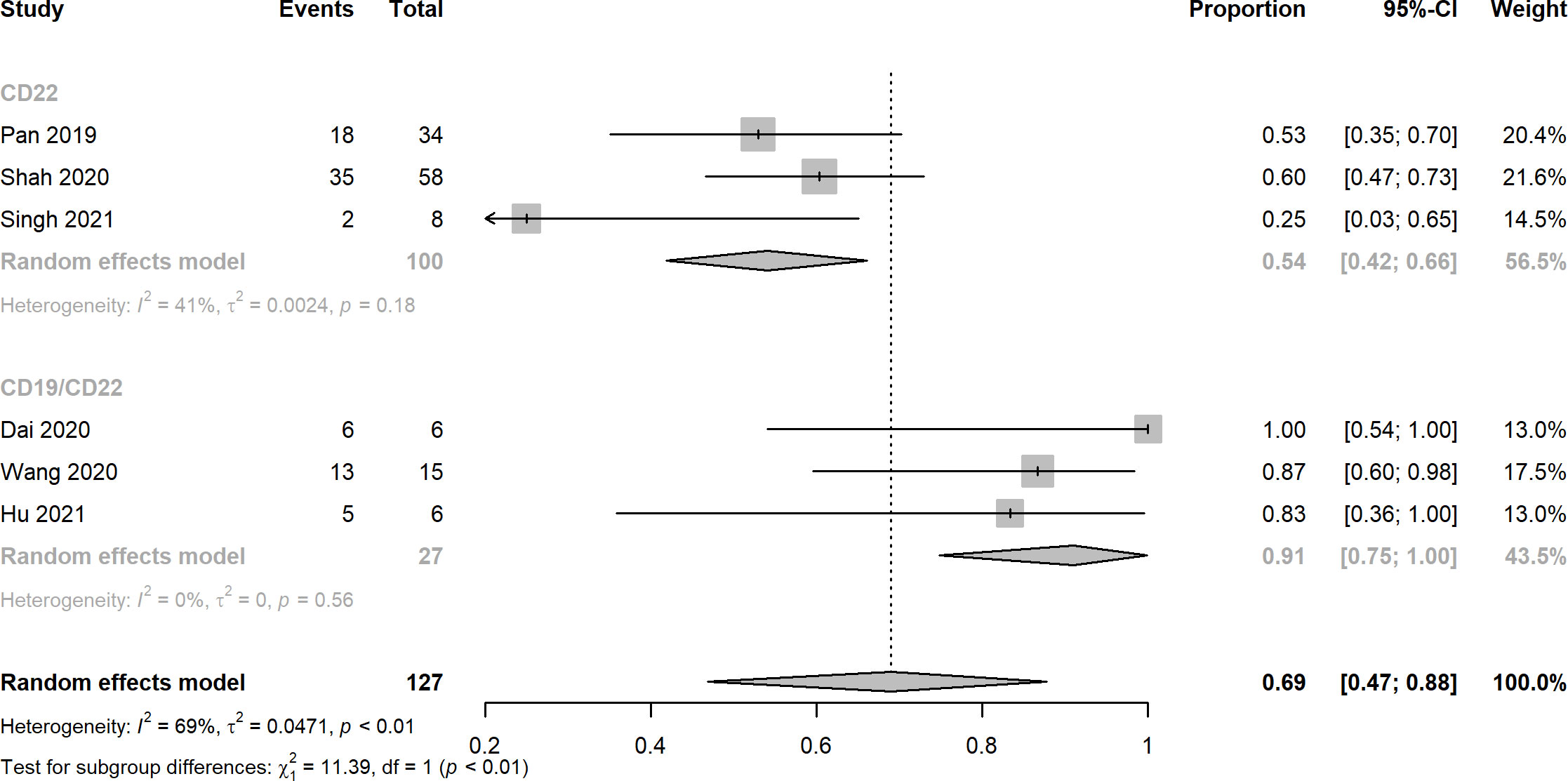
Figure 3 Forest plots of MRD negative rates of CD22 and CD19/CD22 targeted CAR-T cell therapies for relapsed or refractory B-ALL.
Ten records reported the rates of CRS, the pooled estimates of CRS rates of CD22 targeted and CD19/CD22 targeted CAR-T immunotherapy were 0.92 (95% CI: 0.82 - 0.98) and 0.94 (95% CI: 0.82 - 1.00), respectively (Figure 4). Overall rates of Grade 1 and 2 CRS for CD22 targeted and CD19/CD22 targeted therapies were 0.83 (95% CI: 0.60 - 0.98) and 0.77 (95% CI: 0.61 - 0.90), respectively. In the analysis of neurotoxicity, the pooled rates for anti-CD22 and anti-CD19/CD22 therapies were 0.83 (95% CI: 0.60 - 0.98) and 0.77 (95% CI: 0.71 - 0.83) (Figure 5). Low percentage of Grade 3 or above CRS was detected. In the studies of Dai and Cordoba no grade 3 or 4 CRS was observed in any of the treated patients. In Hu’s study, 1 of the 6 patients (16.7%) had grade 3 CRS with hypoxia and required facemask oxygen supplementation (15 L/minute). CRS ≥ Grade3 occurred in 30% (7/23) of patients in Liu’s study. Singh et al. reported one patient had Grade 3 CRS, including significant elevations in serum cytokines compared to the other patients, particularly notable for granulocyte colony-stimulating factor, interleukin-6 (IL-6) and monocyte chemoattractant protein 1. CRS Grade ≥ 3 occurred in 2 patients (5%) in Spiegel’s phase 1 trial. One patient with Grade 3 CRS (12.5%) was reported in Tan’s study. Furthermore, results of Cordoba’s study manifested that one patient 11 relapsed with CD19-negative disease with ongoing CAR T cell persistence > 1,000 copies per μg and B cell aplasia. Hu reported that 3 patients (50%) experienced infections with a severity ≥ grade 3, which included cytomegalovirus reactivation/infection (two cases), bacterial pneumonia (one case), and fungal sepsis (one case), 3 of the 6 patients (50%) experienced cytopenia lasting beyond day 28 after CD19/CD22-targeting CAR-T cells infusion.
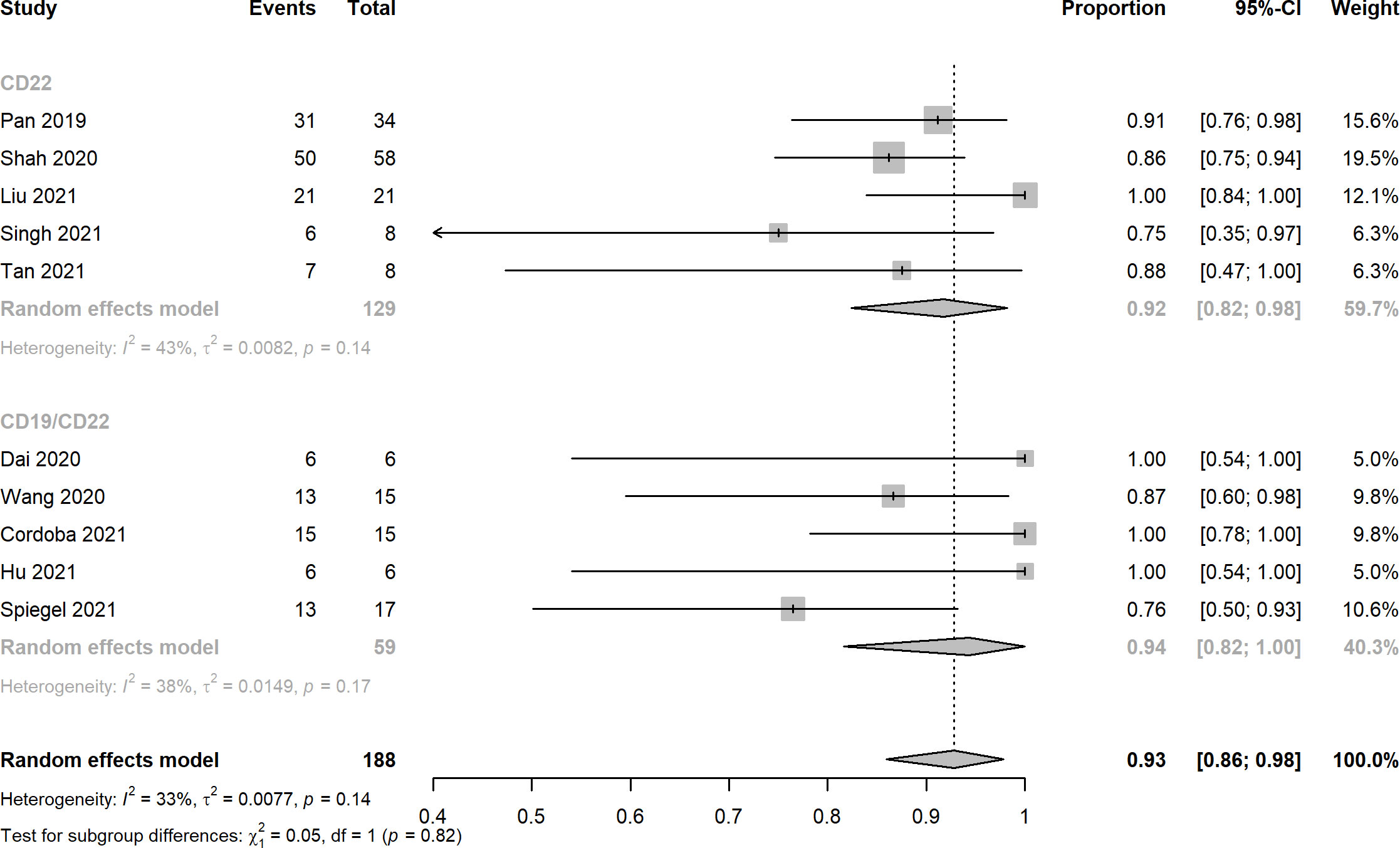
Figure 4 Forest plots of cytokine release syndrome rates of CD22 and CD19/CD22 targeted CAR-T cell therapies for relapsed or refractory B-ALL.
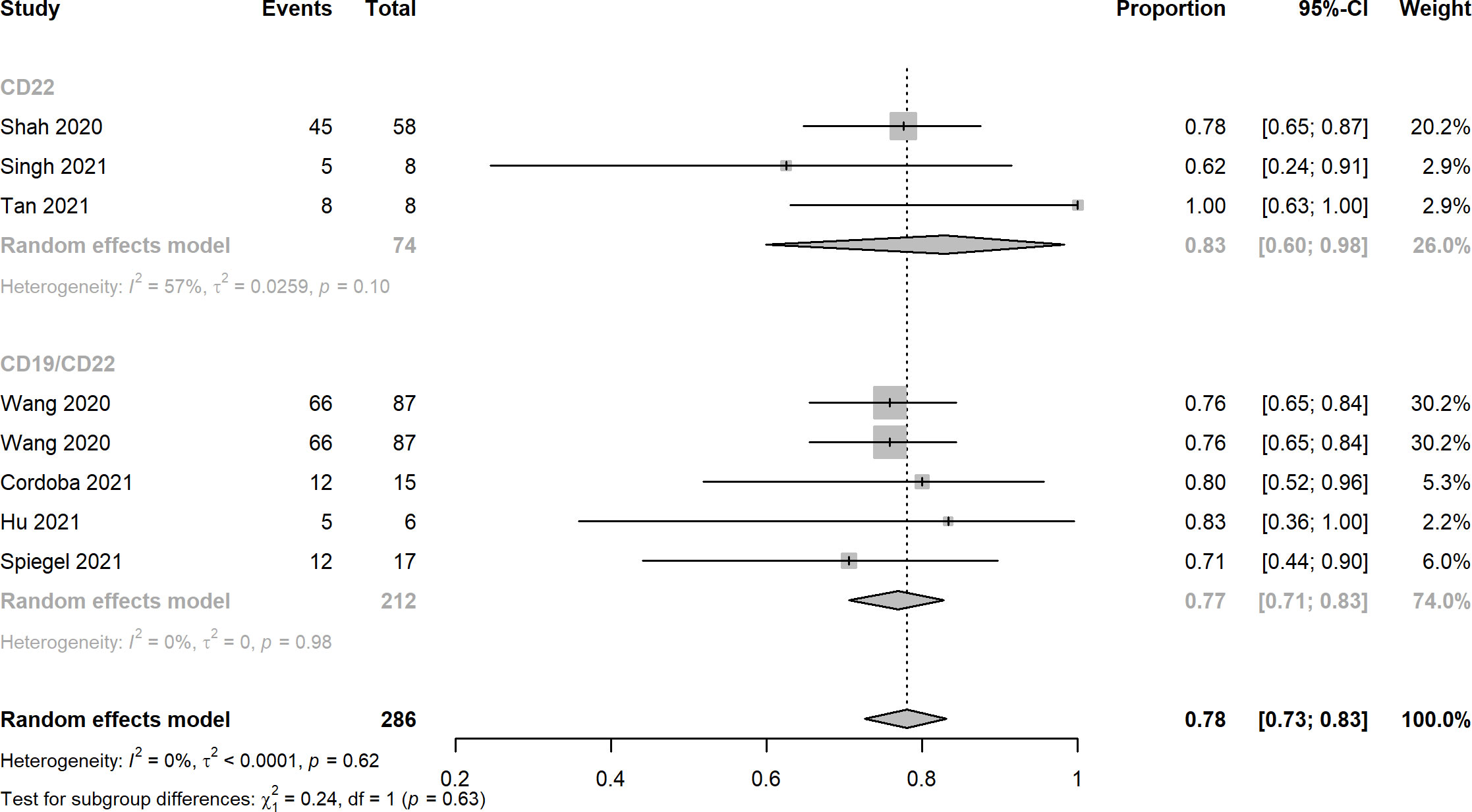
Figure 5 Forest plots of neurotoxicity rates of CD22 and CD19/CD22 targeted CAR-T cell therapies for relapsed or refractory B-ALL.
Results of Egger’s tests for publication bias revealed p values of 0.5568, 0.4480, 0.7306, and 0.0595 for CR, MRD, CRS and neurotoxicity which indicated the absence of significant publication bias in included studies. Funnel plots are displayed in Figure 6.
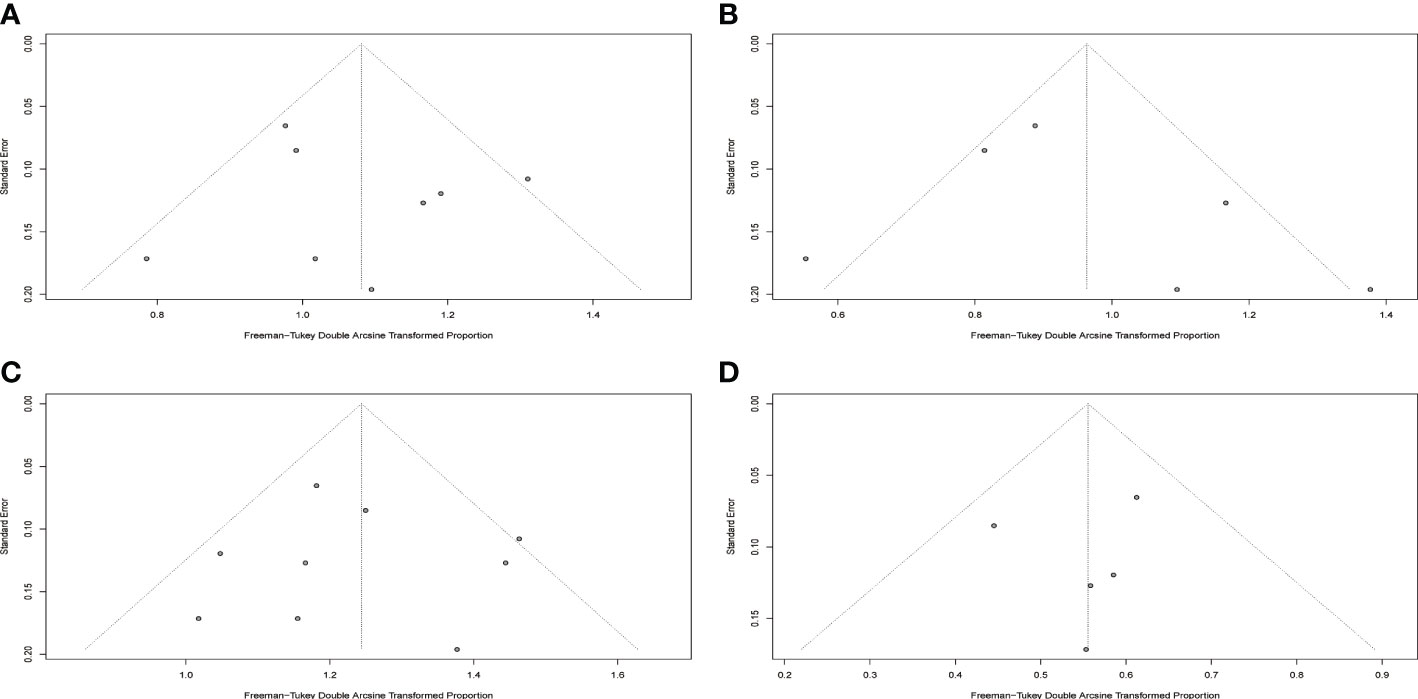
Figure 6 Funnel plots for included studies. (A) Funnel plot of complete response rate. (B) Funnel plot of MRD negative rate. (C) Funnel plot of cytokine release syndrome rate. (D) Funnel plot of neurotoxicity rate.
Clinical studies with CD19 CAR-T cell therapy have manifested > 70% CR rate in patients with relapsed/refractory B-ALL (14, 32, 33). In addition, a meta-analysis involving 2,172 patients with hematologic malignancies showed an overall response rate of nearly 70% or above. Nevertheless, it is estimated that approximately 50% of CR patients relapsed within 12 months after the primary treatment (7, 23). One of the predominant reasons for the relapse or treatment failure is the mutation or loss of CD19 (26). Another reason may be immune-mediated clearance of murine-derived CARs (34–36). CD22, an alternative candidate for anti-CD19 CAR T cells, which is also expressed on most B-ALL cells. Recently, CD22 CAR-T immunotherapy has achieved similar anti-leukemia effects in patients with r/r B-ALL, including those who previously received CD19 CAR-T cells and had dim/negative expression of CD19, resulting in a CR rate of 73 - 80% (26, 27). Evidence from preclinical models of solid tumors has shown that dual CAR-T cells may exhibit synergistic effects, permitting the optimization of response rates compared with those achieved by targeting a single antigen (37, 38). CAR T cells with dual targeting of CD19 and CD22 have demonstrated promising clinical outcomes in the treatment of hematological cancers with low CD19 relapse rate (39–41). We performed a meta-analysis by synthesizing the current evidence to assess the efficacy and safety of anti-CD22 and anti-CD19/CD22 CAR-T cell therapies for hematological tumors.
A total of 10 clinical trials with 194 r/r B-ALL patients met the inclusion criteria of this meta-analysis. The overall complete response rates of CD22 and CD19/CD22 CAR-T cell therapies for relapsed or refractory B-ALL were 0.75 and 0.87, the pooled estimate of CR rate of CD19/CD22 bispecific CAR-T cell therapy was higher than that of CD19 targeted CAR-T cell therapy as reported in Meng et al.’s meta-analysis. It is inferred that dual antigen targeting may prevent recurrence, given that a single leukemia stem cell is unlikely to downregulate both CD19 and CD22 at the same time (28). Notably, MRD negative rates of CD19/CD22 dual targeted CAR-T cell therapy was significantly greater than that of anti-CD22 CAR-T cell therapy (p < 0.01). The assessment of MRD has been widely used for the definition of deepness of treatment response and the main prognostic factor for different hematological malignancies (42, 43). However, due to limited data of follow-up, relapse after CD19/CD22 CAR-T cell treatment remains to be investigated in the upcoming clinical trials. CRS and neurotoxicity, also known as immune effector cell associated neurotoxicity syndrome, are considered to be the main obstacles to the widespread of CAR-T cell therapy (44). In this meta-analysis, the pooled CRS rates of CD22 targeted and CD19/CD22 targeted immunotherapy reached 0.92 and 0.94, a high fraction of the which was Grade 1 and 2 CRS. Likewise, mild or moderate neurotoxicity were observed in the studies included. In the investigation of Pan et al. Grade 2 neurotoxicity as manifested by brief generalized seizure occurred on day 7 after CAR T-cell treatment and was immediately managed with mannitol, furosemide, and dexamethasone, and was completely resolved within 3 days. Furthermore, low percentage of severe CRS (≥ Grade 3) was observed in several included studies. Prolonged cytopenia, B cell aplasia, and heightened infection risks were problems with dual CAR platforms that should be investigated and addressed in the future (45). In addition, the financial issue of CAR-T cell therapy could not be neglected in the clinical setting, in the United States, for example, with a price of $475000 for the pediatric CAR-T product, major payers have struggled to set adequate hospital reimbursement, which may lead to delays in care that could affect child health outcomes (46). It highlights the need to explicitly consider value when setting prices for treatments with CAR-T.
This is the most recent meta-analysis by far which investigated the efficacy and safety of CD22-specific and CD19/CD22-bispecific CAR-T cell therapy in patients with hematologic malignancies. This study was carried out under the guidance of PRISMA. Database search, study selection, data extraction, and quality assessment were performed by two independent reviewers to minimize potential bias. Heterogeneity and publication bias were appraised using statistical and graphical approaches. Mild to moderate heterogeneities and insignificant publication bias were detected in the current study.
There are limitations in this study. Although 10 studies were included in this study, the overall sample size remained small, the interpretation of the results in the meta-analysis should be with caution. Neither meta regression nor subgroup analysis was performed because of insufficient number of studies in each subgroup. Furthermore, due to limited number of studies, data on overall survival and progression-free survival in relapsed/refractory B-ALL patients treated with CD22 targeted or CD19/CD22 targeted CAR-T immunotherapies could not be synthesized at present. Thus, narrative depiction was proposed based on the available information retrieved from enrolled studies. Furthermore, in this study, adverse events regarding CRS and neurotoxicity were evaluated, other related adverse events including abnormal hemogram indices and infection were not pooled up which are actually of great clinical significance in the real-world practice. There is considerable heterogeneity as CRS may not be graded per a single criterion in all included studies, more well-designed clinical trials with larger sample sizes investigating the adverse events, especially on prolonged cytopenia, B cell aplasia, and heightened infection risks, are warranted. It’s reported that delayed immune reconstitution is the major issue behind diminished SARS-CoV-2 vaccine responses and increased infections, and this risk is potentially compounded by dual antigen targeting (47, 48).
Anti-CD22 alone and CD19/CD22 bispecific targeted CAR-T cell immunotherapies displayed deep and durable responses and manifested manageable safety profiles in patients with relapsed/refractory B-ALL. Studies with larger sample sizes, and prolonged follow-up are warranted for further evaluation of the efficacy and safety of CAR-T cell immunotherapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
LL, LW and RX contributed to the conception and design of the study. LL and LW searched the database. LL and ZW extracted data. LW conducted data analysis. LL and LW wrote the manuscript. QL, YZ, and RX revised the manuscript. All authors contributed to the article and approved the submitted version.
Authors LW and YZ were employed by Precedo Pharmaceuticals Co. Ltd.
All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.954345/full#supplementary-material
1. Luo C, Wu G, Huang X, Zhang Y, Ma Y, Huang Y, et al. Efficacy of hematopoietic stem cell mobilization regimens in patients with hematological malignancies: A systematic review and network meta-analysis of randomized controlled trials. Stem Cell Res Ther (2022) 13(1):123. doi: 10.1186/s13287-022-02802-6
2. Stanchina M, Soong D, Zheng-Lin B, Watts JM, Taylor J. Advances in acute myeloid leukemia: Recently approved therapies and drugs in development. Cancers (Basel) (2020) 12(11):3225. doi: 10.3390/cancers12113225
3. Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int Off J Japan Pediatr Soc (2018) 60(1):4–12. doi: 10.1111/ped.13457
4. Noh JY, Seo H, Lee J, Jung H. Immunotherapy in hematologic malignancies: Emerging therapies and novel approaches. Int J Mol Sci (2020) 21(21):8000. doi: 10.3390/ijms21218000
5. Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: Past, present, and future. J Hematol Oncol (2017) 10(1):94. doi: 10.1186/s13045-017-0453-8
6. Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, et al. Chimeric antigen receptor (Car) T therapies for the treatment of hematologic malignancies: Clinical perspective and significance. J Immunother Cancer (2018) 6(1):137. doi: 10.1186/s40425-018-0460-5
7. Kenderian SS, Porter DL, Gill S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: How not to put the cart before the horse. Biol Blood Marrow Transplant (2017) 23(2):235–46. doi: 10.1016/j.bbmt.2016.09.002
8. Roselli E, Faramand R, Davila ML. Insight into next-generation car therapeutics: Designing car T cells to improve clinical outcomes. J Clin Invest (2021) 131(2):e142030. doi: 10.1172/jci142030
9. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: Immunomodulation, cars and combination immunotherapy. Nat Rev Clin Oncol (2016) 13(5):273–90. doi: 10.1038/nrclinonc.2016.25
10. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel car T-cell therapy in refractory Large b-cell lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
11. Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-Cd19 chimeric-Antigen-Receptor-Transduced T cells. Blood (2012) 119(12):2709–20. doi: 10.1182/blood-2011-10-384388
12. Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med (2011) 365(8):725–33. doi: 10.1056/NEJMoa1103849
13. Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T Cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med (2011) 3(95):95ra73. doi: 10.1126/scitranslmed.3002842
14. Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of b-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize Cd19. Blood (2010) 116(20):4099–102. doi: 10.1182/blood-2010-04-281931
15. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. Kte-X19 car T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med (2020) 382(14):1331–42. doi: 10.1056/NEJMoa1914347
16. Salmikangas P, Kinsella N, Chamberlain P. Chimeric antigen receptor T-cells (Car T-cells) for cancer immunotherapy - moving target for industry? Pharm Res (2018) 35(8):152. doi: 10.1007/s11095-018-2436-z
17. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory Large b-cell lymphomas (Transcend nhl 001): A multicentre seamless design study. Lancet (2020) 396(10254):839–52. doi: 10.1016/s0140-6736(20)31366-0
18. Cordeiro A, Bezerra ED, Hirayama AV, Hill JA, Wu QV, Voutsinas J, et al. Late events after treatment with Cd19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant (2020) 26(1):26–33. doi: 10.1016/j.bbmt.2019.08.003
19. Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of Cd19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood (2018) 131(1):121–30. doi: 10.1182/blood-2017-07-793760
20. Hirayama AV, Turtle CJ. Toxicities of Cd19 car-T cell immunotherapy. Am J Hematol (2019) 94(S1):S42–s9. doi: 10.1002/ajh.25445
21. Titov A, Petukhov A, Staliarova A, Motorin D, Bulatov E, Shuvalov O, et al. The biological basis and clinical symptoms of car-T therapy-associated toxicites. Cell Death Dis (2018) 9(9):897. doi: 10.1038/s41419-018-0918-x
22. Namuduri M, Brentjens RJ. Medical management of side effects related to car T cell therapy in hematologic malignancies. Expert Rev Hematol (2016) 9(6):511–3. doi: 10.1080/17474086.2016.1183479
23. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T Cells expressing Cd19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet (2015) 385(9967):517–28. doi: 10.1016/s0140-6736(14)61403-3
24. Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-Cd22-Chimeric antigen receptors targeting b-cell precursor acute lymphoblastic leukemia. Blood (2013) 121(7):1165–74. doi: 10.1182/blood-2012-06-438002
25. Tan Y, Cai H, Li C, Deng B, Song W, Ling Z, et al. A novel full-human Cd22-car T cell therapy with potent activity against Cd22(Low) b-all. Blood Cancer J (2021) 11(4):71. doi: 10.1038/s41408-021-00465-9
26. Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, et al. Cd22 car T-cell therapy in refractory or relapsed b acute lymphoblastic leukemia. Leukemia (2019) 33(12):2854–66. doi: 10.1038/s41375-019-0488-7
27. Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. Cd22-targeted car T cells induce remission in b-all that is naive or resistant to Cd19-targeted car immunotherapy. Nat Med (2018) 24(1):20–8. doi: 10.1038/nm.4441
28. Cordoba S, Onuoha S, Thomas S, Pignataro DS, Hough R, Ghorashian S, et al. Car T Cells with dual targeting of Cd19 and Cd22 in pediatric and young adult patients with relapsed or refractory B Cell acute lymphoblastic leukemia: A phase 1 trial. Nat Med (2021) 27(10):1797–805. doi: 10.1038/s41591-021-01497-1
29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
30. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
32. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med (2014) 371(16):1507–17. doi: 10.1056/NEJMoa1407222
33. Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X, Lin YH, et al. High efficacy and safety of low-dose Cd19-directed car-T cell therapy in 51 refractory or relapsed b acute lymphoblastic leukemia patients. Leukemia (2017) 31(12):2587–93. doi: 10.1038/leu.2017.145
34. Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. Cd19 car-T cells of defined Cd4+:Cd8+ composition in adult b cell all patients. J Clin Invest (2016) 126(6):2123–38. doi: 10.1172/jci85309
35. Cao J, Wang G, Cheng H, Wei C, Qi K, Sang W, et al. Potent anti-leukemia activities of humanized Cd19-targeted chimeric antigen receptor T (Car-T) cells in patients with Relapsed/Refractory acute lymphoblastic leukemia. Am J Hematol (2018) 93(7):851–8. doi: 10.1002/ajh.25108
36. Jain MD, Davila ML. Concise review: Emerging principles from the clinical application of chimeric antigen receptor T cell therapies for b cell malignancies. Stem Cells (2018) 36(1):36–44. doi: 10.1002/stem.2715
37. Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, et al. Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res an Off J Am Assoc Cancer Res (2017) 23(22):6982–92. doi: 10.1158/1078-0432.Ccr-17-0867
38. Wu X, Li J, Connolly EM, Liao X, Ouyang J, Giobbie-Hurder A, et al. Combined anti-vegf and anti-Ctla-4 therapy elicits humoral immunity to galectin-1 which is associated with favorable clinical outcomes. Cancer Immunol Res (2017) 5(6):446–54. doi: 10.1158/2326-6066.Cir-16-0385
39. Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific car-T cells targeting both Cd19 and Cd22 for therapy of adults with relapsed or refractory b cell acute lymphoblastic leukemia. J Hematol Oncol (2020) 13(1):30. doi: 10.1186/s13045-020-00856-8
40. Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, et al. Cd4/Cd8 T-cell selection affects chimeric antigen receptor (Car) T-cell potency and toxicity: Updated results from a phase I anti-Cd22 car T-cell trial. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(17):1938–50. doi: 10.1200/jco.19.03279
41. Liu S, Deng B, Yin Z, Lin Y, An L, Liu D, et al. Combination of Cd19 and Cd22 car-T cell therapy in relapsed b-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol (2021) 96(6):671–9. doi: 10.1002/ajh.26160
42. Riva G, Nasillo V, Ottomano AM, Bergonzini G, Paolini A, Forghieri F, et al. Multiparametric flow cytometry for mrd monitoring in hematologic malignancies: Clinical applications and new challenges. Cancers (Basel) (2021) 13(18):4582. doi: 10.3390/cancers13184582
43. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of aml in adults: 2017 eln recommendations from an international expert panel. Blood (2017) 129(4):424–47. doi: 10.1182/blood-2016-08-733196
44. Gill S, Brudno JN. Car T-cell therapy in hematologic malignancies: Clinical role, toxicity, and unanswered questions. Am Soc Clin Oncol Educ Book (2021) 41:1–20. doi: 10.1200/edbk_320085
45. Meir J, Abid MA, Abid MB. State of the car-T: Risk of infections with chimeric antigen receptor T-cell therapy and determinants of SARS-CoV-2 vaccine responses. Transplant Cell Ther (2021) 27(12):973–87. doi: 10.1016/j.jtct.2021.09.016
46. Leech AA, Dusetzina SB. Cost-effective but unaffordable: The CAR-T conundrum. J Natl Cancer Inst (2019) 111(7):644–5. doi: 10.1093/jnci/djy195
47. Abid MA, Abid MB. SARS-CoV-2 vaccine response in CAR T-cell therapy recipients: A systematic review and preliminary observations. Hematol Oncol (2022) 40(2):287–91. doi: 10.1002/hon.2957
Keywords: immunotherapy, chimeric antigen receptor t-cell therapy, hematologic malignancy, CD22, CD19/CD22-bispecific, meta-analysis
Citation: Li L, Wang L, Liu Q, Wu Z, Zhang Y and Xia R (2022) Efficacy and safety of CD22-specific and CD19/CD22-bispecific CAR-T cell therapy in patients with hematologic malignancies: A systematic review and meta-analysis. Front. Oncol. 12:954345. doi: 10.3389/fonc.2022.954345
Received: 10 June 2022; Accepted: 13 December 2022;
Published: 29 December 2022.
Edited by:
Jose-Maria Ribera, Germans Trias i Pujol Health Science Research Institute (IGTP), SpainReviewed by:
Tibor Bakacs, Alfred Renyi Institute of Mathematics, HungaryCopyright © 2022 Li, Wang, Liu, Wu, Zhang and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulong Zhang, eWx6aGFuZ0BwcmVjZWRvLmNu; Ruixiang Xia, eHJ4MjA0MUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.