
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 29 August 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.954227
This article is part of the Research TopicNew Frontiers in Pancreatic Cancer Care: Multidisciplinary Approaches, the role of Pancreas Units, and their Organizational ImpactsView all 6 articles
Aim: The aim of this study was to compare the safety and overall effect of robotic distal pancreatectomy (RDP) to laparoscopic distal pancreatectomy (LDP) after the learning curve, especially in perioperative outcome and short-term oncological outcome.
Methods: A literature search was performed by two authors independently using PubMed, Embase, and Web of Science to identify any studies comparing the results of RDP versus LDP published until 5 January 2022. Only the studies where RDP was performed in more than 35 cases were included in this study. We performed a meta-analysis of operative time, blood loss, reoperation, readmission, hospital stay, overall complications, major complications, postoperative pancreatic fistula (POPF), blood transfusion, conversion to open surgery, spleen preservation, tumor size, R0 resection, and lymph node dissection.
Results: Our search identified 15 eligible studies, totaling 4,062 patients (1,413 RDP). It seems that the RDP group had a higher rate of smaller tumor size than the LDP group (MD: −0.15; 95% CI: −0.20 to −0.09; p < 0.00001). Furthermore, compared with LPD, RDP was associated with a higher spleen preservation rate (OR: 2.19; 95% CI: 1.36–3.54; p = 0.001) and lower rate of conversion to open surgery (OR: 0.43; 95% CI: 0.33–0.55; p < 0.00001). Our study revealed that there were no significant differences in operative time, overall complications, major complications, blood loss, blood transfusion, reoperation, readmission, POPF, and lymph node dissection between RDP and LDP.
Conclusions: RDP is safe and feasible for distal pancreatectomy compared with LDP, and it can reduce the rate of conversion to open surgery and increase the rate of spleen preservation, which needs to be further confirmed by quality comparative studies with large samples.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails.
Laparoscopic distal pancreatectomy (LDP) was firstly reported by Cuschieri in 1994 (1). In recent years, LDP was favored for being minimally invasive, reducing surgical morbidity and intraoperative blood loss, having a rapid postoperative recovery rate, and providing a high comfort level to patients (2–5). Robotic distal pancreatectomy (RDP) was first reported in 2003 (6), compared with the conventional laparoscopic procedures, and RDP overcomes some of the disadvantages (limited range of motion, reliance on two-dimensional imaging, reduced dexterity, fulcrum effect, natural tremors, poor surgeon ergonomics, and difficulty in vascular control), which made minimally invasive surgery popular in pancreas surgery (7). Although RDP has many advantages over LDP, overcoming this learning curve requires a relatively long training period for surgeons. It is well known that surgeons’ experience and performance play an important role in patient outcomes, which can lead to bias. Murtaza Shakir et al.’s study showed that the learning curve for RDP was 40 cases (8). However, Benrizi et al.’s study revealed that the learning curve was completed after 11 operations (9). Furthermore, when surgeons do not overcome the learning curve, surgical outcomes are often unsatisfactory, even at high-volume centers. As far as we know, there was no study comparing the perioperative and short-term oncological outcomes between RDP and LDP to avoid bias. Therefore, we conducted a systematic review and meta-analysis of studies that compare RDP and LDP after the learning curve by good quality articles.
This review was registered with PROSPERO (CRD42021268106) and reported with reference to the PRISMA guidelines (10).
An electronic search of the PubMed/MEDLINE, EMBASE, and Cochrane Library database for articles relating to RDP and LDP before 5 January 2022 was performed by three independent investigators (CC, QF, and MW). The search terms were the following: “robotic surgery” OR “robot-assisted” OR “robot” OR “robotic” OR “Da Vinci” AND “laparoscopic surgery” OR “laparoscope” AND “distal pancreatectomy”, either individually or in combination. The references of included articles were also screened manually for a comprehensive search.
Two independent researchers (CC and QF) independently reviewed current articles to check the eligibility for inclusion, and the third author (JH) participated in the evaluation of controversial articles. Retrospective and prospective cohort studies, cross-sectional studies, and randomized controlled trials with a reported RDP of greater than or equal to 35 cases were considered for inclusion. The latest study and PSM study were included to analysis, when the duplicate studies from the same institutions. Studies exclusion criteria: (I) non-English language articles; (II) no comparative analysis between RDP and LDP; (III) pediatric and pregnant women as participants; (IV) multicenter studies; and (V) outcomes of the following were not reported in the literature: reoperation, operation time, readmission, blood loss, hospital stay, tumor size, blood transfusion, R0 rate, conversion rate, lymph node harvested, overall complications, major complications, ROPF, and spleen preservation rate.
Literature characteristics and patient characteristics (including operative time, mean age, blood loss, blood transfusion, tumor size, overall complication, major complication, hospital stay, R0 rate, blood transfusion, reoperation, readmission, POPF, and number of harvested lymph nodes) were extracted by two authors (CC and QF) into a unified datasheet. We consulted a third observer (MW) when there was an ambiguity in the study. A quality assessment of every included study was adopted using the Newcastle–Ottawa Scale (NOS), and NOS ≥6 was considered as being of high quality (11).
Statistical analyses were performed by Review Manager Software (RevMan5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). The 95% confidence interval (CI) and mean difference (MD) were used for continuous data. For dichotomous data, the pooled odds ratio (OR) with 95% CI was used. The method reported by Hozo et al. was used to convert medians and range values into means and standard deviations (12). Funnel plots and the I2 index were respectively used to assess potential publication bias and statistical heterogeneity. When heterogeneity was low or moderate (I2 < 50%), the fixed-effects model (FEM) was adopted. Meanwhile, for the study with high heterogeneity (I2 ≥ 50%), the random-effects model (REM) was considered.
In total, 1,827 relevant English articles were initially identified for evaluation. After scanning for inclusion criteria, a total of 4,602 patients [15 studies (7, 13–26)] were included this study; 1,413 and 3,189 patients underwent RDP and LDP, respectively. A flow diagram (Figure 1) shows our analysis scheme, and Table 1 reports the summary of the key characteristics and NOS for included articles.
To evaluate the perioperative outcomes, we compared the operative time, hospital stay, blood loss, blood transfusion, overall complication rates, major complications, postoperative pancreatic fistula, R0 rate, conversion to open surgery, spleen preservation, POPF, reoperation, and readmission.
Thirteen studies (7, 14–19, 21–26) (1,253 and 2,110 patients from the RDP group and LDP group, respectively) reported operative times. This meta-analysis revealed that there was no significant difference between the two groups (WMD: 17.42 min; 95% CI: −7.56–42.40; p = 0.17) with high heterogeneity (I2 = 98%; shown in Figure 2A).
All studies (7, 13–26) with a total of 4,602 patients (1,413 patients underwent RDP; 3,189 patients underwent LDP) investigated hospital stay. This meta-analysis showed no difference in hospital stay between the two groups (p = 0.30; 95% CI: −0.98 to 0.30; shown in Figure 2B).
Nine studies (14, 16, 18, 19, 21–24, 26) reported the estimated blood loss volume, and this meta-analysis revealed no difference in blood loss (MD: −42.67 ml; 95% CI: −87.85 to 2.50; p = 0.06) with high heterogeneity (I2 = 99%; shown in Figure 3A).
Blood transfusion data were available in nine studies (14, 16–19, 21, 22, 24, 26). This study revealed that blood transfusion rate was not different between RDP and LDP (OR: 0.90; 95% CI: 0.62–1.30; p = 0.56) with low heterogeneity (I2 = 21%; shown in Figure 3B).
Seven studies (14–16, 18, 19, 22, 25) (a total of 775 patients; 356 and 419 patients from the RDP group and LDP group, respectively) reported postoperative complications. Our study revealed that there was no significant difference in two groups (OR: 0.82; 95% CI: 0.61–1.11; p = 0.20) with no heterogeneity (I2 = 0%; Figure 4A).
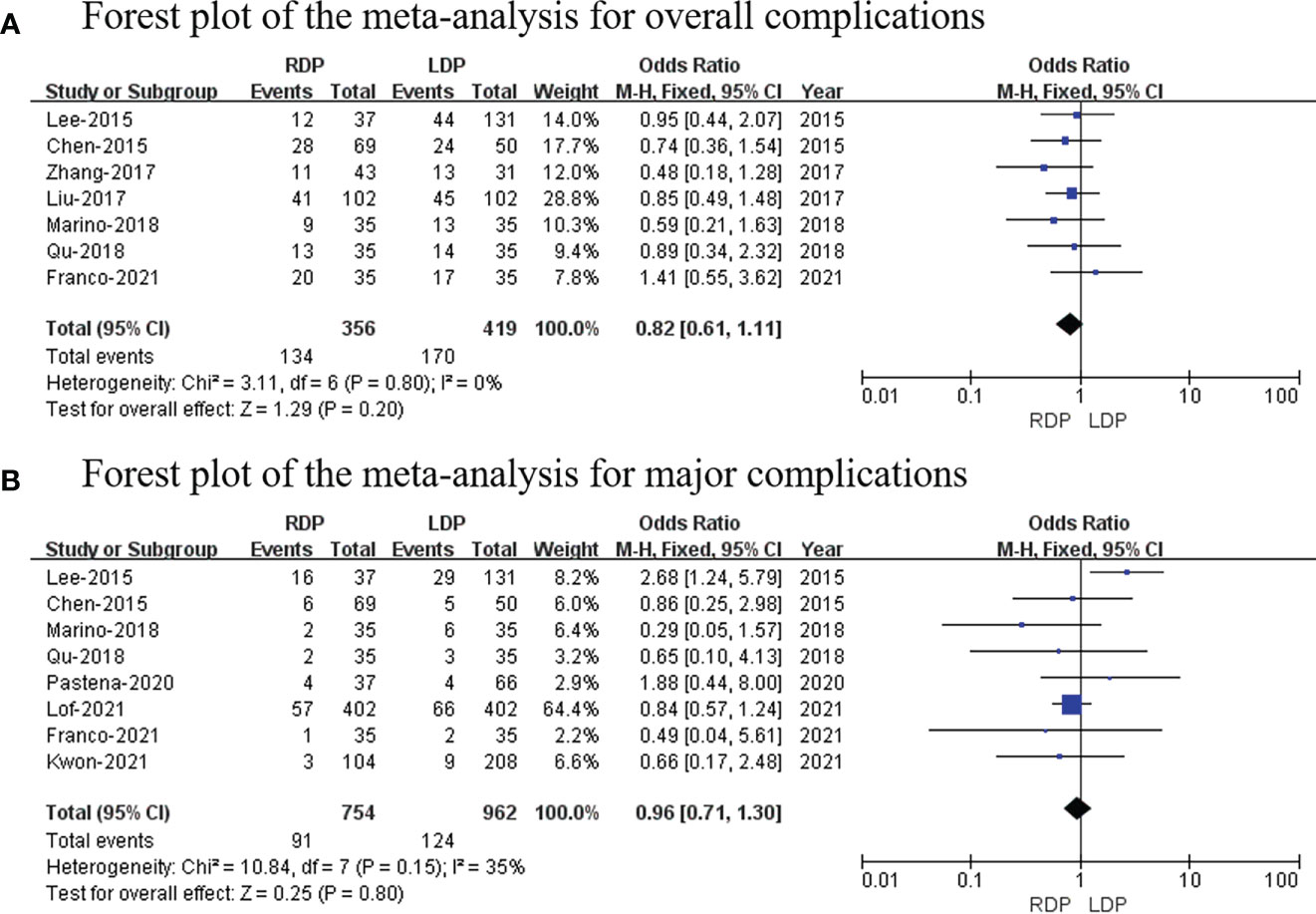
Figure 4 Forest plot of the meta-analysis for (A) overall complications and (B) major complications.
Eight studies (14, 15, 19, 22–26) (754 and 962 patients from the RDP group and LDP group, respectively) recorded major complications. Grade III to V complications based on the Clavien–Dindo classification were considered as major complications (27). No significant differences in major complications were observed between these two groups (OR: 0.96; 95% CI: 0.71 to 1.30; p = 0.80) with low heterogeneity (I2 = 35%; Figure 4B).
In total, 13 studies (3,363 patients) reported the incidence rate of POPF (7, 14–19, 21–26). Our study found that there was no significant difference in POPF rate between the two groups (OR: 0.93; 95% CI: 0.77 to 1.13; p = 0.46) with no heterogeneity (I2 = 0%) (Figure 5A).
In total, four studies (281 and 962 patients from the RDP group and LDP group, respectively) reported the R0 resection rate (18–20, 26). No significant differences in R0 resection rate were observed between the two groups (OR: 1.09; 95% CI: 0.67–1.77; p = 0.72) with no heterogeneity (I2 = 0%; Figure 5B).
Thirteen studies (14–26) (1,306 patients underwent RDP and 2,533 patients underwent LDP) reported conversion to open surgery, and this meta-analysis indicated that the higher conversion rate was observed in the RDP group (OR: 0.43; 95% CI: 0.33 to 0.55; p < 0.00001) with low heterogeneity (I2 = 30%; Figure 6A).
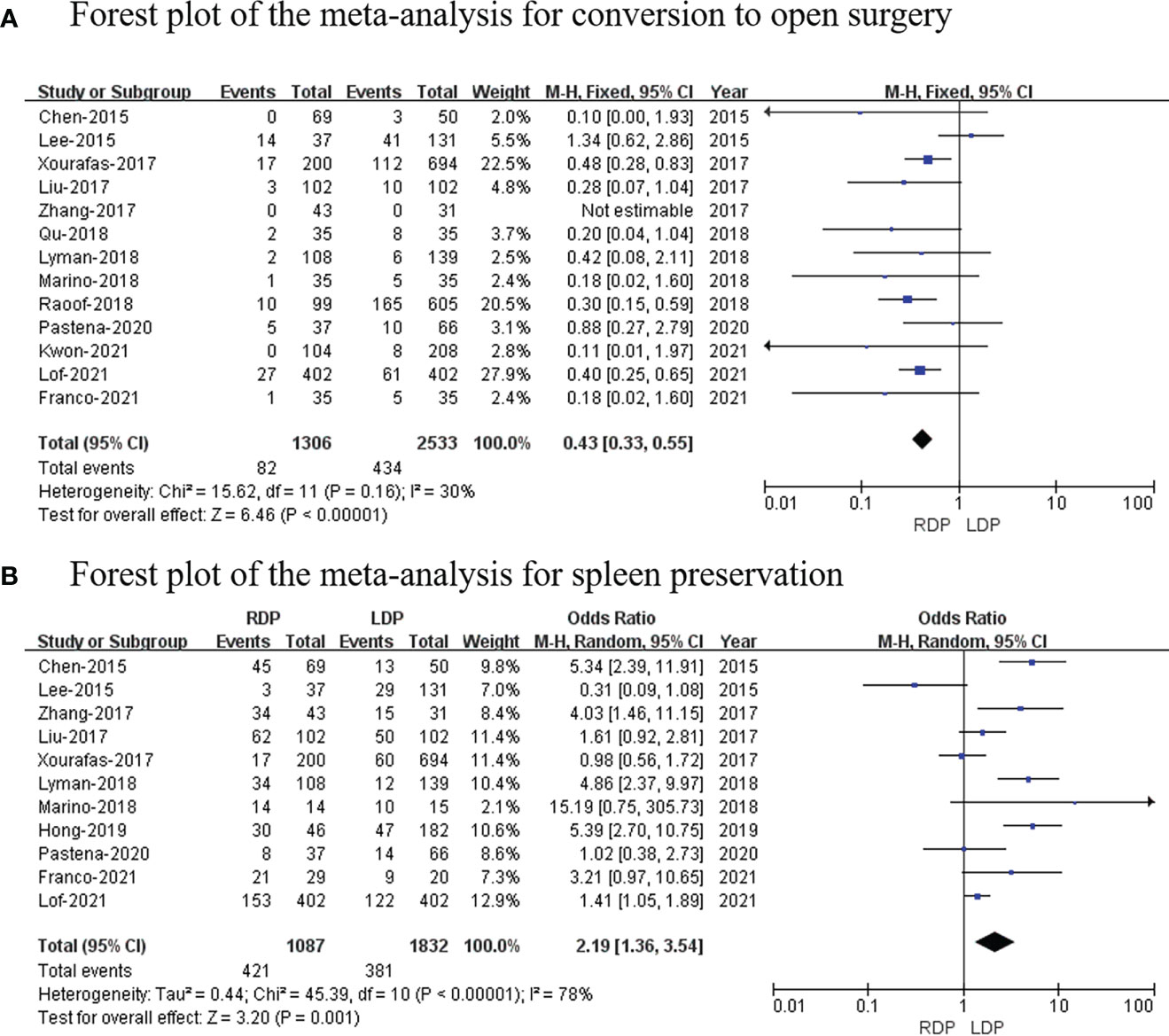
Figure 6 Forest plot of the meta-analysis for (A) conversion to open surgery and (B) spleen preservation.
Eleven studies (7, 14–19, 21–25) with a total of 2,919 patients reported the spleen preservation rate, and this study found that there was a significant higher spleen preservation rate in the RDP group (OR: 2.19; 95% CI: 1.36 to 3.54; p = 0.001) with high heterogeneity (I2 = 78%; Figure 6B).
The data of six studies (14, 16, 18, 22–24) (with a total of 1,374 patients) that assessed reoperation were pooled, and our analysis revealed no difference in reoperation (OR: 0.84; 95% CI: 0.51 to 1.40; p = 0.51) with no heterogeneity (I2 = 0%; Figure 7A).
Eight studies (7, 17, 20–24, 26) that included 3,362 patients assessed readmission. The study showed that there was no difference in readmission (OR: 1.20; 95% CI: 0.76–1.90; p = 0.74) with high heterogeneity (I2 = 62%; Figure 7B).
Twelve studies (7, 13, 14, 16, 18–21, 23–26) that included 3,470 patients reported the tumor size, and the results of the meta-analysis showed that RDP has a smaller tumor size than the LDP group (MD: −0.15; 95% CI: −0.20 to −0.09; p <0.00001) with high heterogeneity (I2 = 50%; Figure 8A).
Six studies (13, 19, 20, 23, 25, 26) including 1,861 patients reported lymph node dissection; meanwhile, this meta-analysis revealed that there was no difference in lymph node dissection (MD: −0.91; 95% CI: −2.94 to 1.12; p = 0.38) with high heterogeneity (I2 = 97%; Figure 8B).
Begg’s funnel plot was used to assess publication bias for each outcome. As shown in the funnel plots,conversion to open surgery (Figure 9A) and POPF (Figure 9B) all studies are within the 95% CI, indicating no publication bias.
The special anatomy, complicated vascular variations, and various postoperative complications make pancreatic resection a challenging surgical procedure (25). As minimally invasive surgery development, LDP and RDP have developed rapidly in recent years (28, 29). Previous studies have demonstrated that the LDP has the same safety and efficacy as open surgery (30, 31). Meanwhile, the LDP is more minimally invasive than traditional surgery (30, 31). Our study was designed to compare the clinical outcomes of RDP versus LDP after the learning curve. As we all know, the surgeons’ experience and performance play an important role in patient outcomes. The surgical results during the learning curve are not satisfactory, even at high-volume centers.
At present, some studies have reported the learning curve of RDP, but the heterogeneity of the outcome of these studies was significant (9, 32–35). A study by Murtaza Shakir et al. found the learning curve for RDP to be 40 cases (8). Additionally, two single-surgeon centers reported the learning curve for RDP: the RDP learning curve was reported to be five surgeries in a series of 43 by Takahashi et al., and the RDP learning curve was 10 surgeries by Napoli et al. (33, 36). However, two other multi-surgeon groups published reports in the literature: Benrizi et al. reported only 11 surgeries, while Shyr et al. reported 37 surgeries (9, 34). A study by Amr et al. determined that the RDP learning curve is 20–40 surgeries, with operating time being the most significant factor. In view of the above, we finally set RDP ≥ 35 as the criteria for passing the learning curve. Currently, the surgeons who performed RDP have gone through the LDP learning curve.
Finally, 15 retrospectives studies (4,602 patients) were incorporated into this study to compare the perioperative outcomes and oncologic outcomes between RDP and LPD after the learning curve. Our study found that RDP had a lower rate of conversion to open than LDP, which was consistent with the current mainstream clinical studies (17, 24, 28). Meanwhile, our study found a higher rate of spleen preservation in the RDP group than in the LDP group, which was consistent with the current mainstream publishing clinical studies (32, 37). We think that this may be explained by the fact that RDP has several technical advantages over laparoscopic techniques that make it potentially advantageous. These include a three-dimensional surgical view, motion scaling, tremor filtration, improved surgeon ergonomics, and the wide range of motion of the articulating instruments (7). These advantages lead to RDP having an upper hand in dealing with the special anatomy structure and complicated vascular variation, which reduced the rate of splenectomy (5, 34, 35). Undoubtedly, surgical technique and experience, as well as various patient factors (tumor types, tumor location, vascular invasion, surgical schedule, etc.), play a major role in spleen preservation (35).
In our study, the RDP group had a smaller tumor size than the LDP group, which may be related to the RDP patients with benign or early-stage diseases. Surgeons tended to select LDP on patients with malignancy because it was more familiar to them than RDP, although Al Abbas et al. reported that RDP has a longer operative time and hospital stay (35). In our study, we found that there was no difference in operative time and length of hospital stay between RDP and LDP. This was easily explained in the Al Abbas et al. study that included literature with small samples and wherein the surgeons had not completed the learning stage. Recent studies have shown that the operative time and length of hospital stay were the same between RDP and LDP, as most clinical trials have shown (22, 24, 26). Distal pancreatectomy with negative margin and lymph node dissection are two important prognosis factors (7, 22, 38). Based on the tumor radical effect, our study showed that there was no difference in RDP and LDP lymph node dissections. Similar radical effects can be considered between RDP and LDP, which is consistent with major existing clinical studies (7, 22, 24, 25, 29, 38). Meanwhile, this study shows that no significant difference was observed in reoperation, blood transfusion, readmission, overall complication rates, POPF, and major complications (Clavien–Dindo≥3/4 grade) between the RDP group and LDP group, showing that RDP is as safe as LPD after the learning curve. Overall, a high rate of spleen preservation and low open conversion rates make RDP a safe and feasible alternative to LDP. Undoubtedly, the hospital cost of RDP was a crucial factor limiting the widespread use of RDP. However, the hospital cost was not described in detail in the included literature, and thus we could not perform further analysis. It is believed that with the development and modernization of robot technology and the many obvious advantages of RDP, including reduced cost, RDP will be widely used in the future.
This study included 15 studies to compare safety and efficiency following RDP and LDP. However, there are still several limitations to our study. Firstly, because our study included articles that were retrospective in nature, there may be inherent selection biases. In addition, addition, a short follow-up period in the included literature prevented the assessment of some long-term outcomes (overall survival and disease-free survival). Moreover, some studies had benign conditions included in them, which may affect patient prognosis. Therefore, in order to resolve this problem, we need to conduct larger prospective comparative studies and randomized clinical trials.
In summary, our meta-analysis found that RDP is a safe alternative to LDP as it is associated with a significant reduction in conversion to open surgery and increased spleen preservation. After the learning curve, RDP is a technically oncologically safe and feasible approach. Because RDP achieved similar outcomes to LDP, it should be the preferred choice. Future higher-quality large-scale comparative studies and long-term follow-up periods are necessary to confirm the safety and efficacy of RPD after the learning curve.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Conception and design: CWC, JH and MYW; Provision of study materials or patients: CWC, QBF and MYW; Collection and assembly of data: CWC, QBF and JH; Data analysis and interpretation: CWC, MYW, XJ Zhuo; Manuscript writing: CWC, MYW, JH and XJZ; Manuscript review: CWC, JH, MYW. Final approval of manuscript: All authors.
This work was supported by Model research and application demonstration of hierarchical coordination within health alliance based on a cloud platform (Project No.2020YFS0092).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Asbun HJ, Moekotte AL, Vissers FL, Kunzler F, Cipriani F, Alseidi A, et al. The miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg (2020) 271(1):1–14. doi: 10.1097/SLA.0000000000003590
3. Sharpe SM, Talamonti MS, Wang E, Bentrem DJ, Roggin KK, Prinz RA, et al. The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. Am J Surg (2015) 209(3):557–63. doi: 10.1016/j.amjsurg.2014.11.001
4. Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Lee D, et al. A comparative study of laparoscopic vs open distal pancreatectomy for left-sided ductal adenocarcinoma: A propensity score-matched analysis. J Am Coll Surgeons (2015) 220(2):177–85. doi: 10.1016/j.jamcollsurg.2014.10.014
5. Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique a systematic review and meta-analysis. Ann Surg (2012) 255(6):1048–59. doi: 10.1097/SLA.0b013e318251ee09
6. Melvin WS, Needleman BJ, Krause KR, Ellison EC. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A (2003) 13(1):33–6. doi: 10.1089/109264203321235449
7. Hong S, Song KB, Madkhali AA, Hwang K, Yoo D, Lee JW, et al. Robotic versus laparoscopic distal pancreatectomy for left-sided pancreatic tumors: A single surgeon's experience of 228 consecutive cases. Surg Endosc (2020) 34(6):2465–73. doi: 10.1007/s00464-019-07047-8
8. Shakir M, Boone BA, Polanco PM, Zenati MS, Hogg ME, Tsung A, et al. The learning curve for robotic distal pancreatectomy: An analysis of outcomes of the first 100 consecutive cases at a high-volume pancreatic centre. Hpb (2015) 17(7):580–6. doi: 10.1111/hpb.12412
9. Benizri EI, Germain A, Ayav A, Bernard JL, Zarnegar R, Benchimol D, et al. Short-term perioperative outcomes after robot-assisted and laparoscopic distal pancreatectomy. J Robot Surg (2014) 8(2):125–32. doi: 10.1007/s11701-013-0438-8
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg (2010) 8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007
11. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: Comparing reviewers' to authors' assessments. BMC Med Res Methodol (2014) 14:45. doi: 10.1186/1471-2288-14-45
12. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
13. Adam MA, Choudhury K, Goffredo P, Reed SD, Blazer D 3rd, Roman SA, et al. Minimally Invasive Distal Pancreatectomy for Cancer: Short-Term Oncologic Outcomes in 1733 Patients. World J Surg (2015) 39(10):2564–72. doi: 10.1007/s00268-015-3138-x
14. Chen S, Zhan Q, Chen JZ, Jin JB, Deng XX, Chen H, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc (2015) 29(12):3507–18. doi: 10.1007/s00464-015-4101-5
15. Lee SY, Allen PJ, Sadot E, D'Angelica MI, DeMatteo RP, Fong Y, et al. Distal Pancreatectomy: A Single Institution's Experience in Open, Laparoscopic, and Robotic Approaches. J Am Coll Surgeons (2015) 220(1):18–27. doi: 10.1016/j.jamcollsurg.2014.10.004
16. Liu R, Liu Q, Zhao ZM, Tan XL, Gao YX, Zhao GD. Robotic versus laparoscopic distal pancreatectomy: A propensity score-matched study. J Surg Oncol (2017) 116(4):461–9. doi: 10.1002/jso.24676
17. Xourafas D, Ashley SW, Clancy TE. Comparison of perioperative outcomes between open, laparoscopic, and robotic distal pancreatectomy: An analysis of 1815 patients from the ACS-NSQIP procedure-targeted pancreatectomy database. J Gastrointest Surg (2017) 21(9):1442–52. doi: 10.1007/s11605-017-3463-5
18. Zhang JQ, Jin JB, Chen S, Gu J, Zhu Y, Qin K, et al. Minimally invasive distal pancreatectomy for PNETs: laparoscopic or robotic approach? Oncotarget (2017) 8(20):33872–83. doi: 10.18632/oncotarget.17513
19. Liu Q, Zhao ZM, Tan XL, Yuanxing G, Yong X, Rong L, et al. Short- and mid-term outcomes of robotic versus laparoscopic distal pancreatosplenectomy for pancreatic ductal adenocarcinoma: A retrospective propensity score-matched study. Int J Surg (2018) 55:81–6. doi: 10.1016/j.ijsu.2018.05.024
20. Raoof M, Nota CLMA, Melstrom LG, Warner SG, Woo Y, Singh G, et al. Oncologic outcomes after robot-assisted versus laparoscopic distal pancreatectomy: Analysis of the National Cancer Database. J Surg Oncol (2018) 118(4):651–6. doi: 10.1002/jso.25170
21. Lyman WB, Passeri M, Sastry A, Cochran A, Iannitti DA, Vrochides D, et al. Robotic-assisted versus laparoscopic left pancreatectomy at a high-volume, minimally invasive center. Surg Endosc (2019) 33(9):2991–3000. doi: 10.1007/s00464-018-6565-6
22. Marino MV, Mirabella A, Gomez Ruiz M, Komorowski AL. Robotic-assisted versus laparoscopic distal pancreatectomy: The results of a case-matched analysis from a tertiary care center. Dig Surg (2020) 37(3):229–39. doi: 10.1159/000501428
23. De Pastena M, Esposito A, Paiella S, Surci N, Montagnini G, Marchegiani G, et al. Cost-effectiveness and quality of life analysis of laparoscopic and robotic distal pancreatectomy: A propensity score-matched study. Surg Endosc (2021) 35(3):1420–8. doi: 10.1007/s00464-020-07528-1
24. Lof S, van der Heijde N, Abuawwad M, Al-Sarireh B, Boggi U, Butturini G, et al. Robotic versus laparoscopic distal pancreatectomy: Multicentre analysis. Brit J Surg (2021) 108(2):188–95. doi: 10.1093/bjs/znaa039
25. Di Franco G, Peri A, Lorenzoni V, Palmeri M, Furbetta N, Guadagni S, et al. Minimally invasive distal pancreatectomy: A case-matched cost-analysis between robot-assisted surgery and direct manual laparoscopy. Surg Endosc (2022) 36(1):651–62. doi: 10.1007/s00464-021-08332-1
26. Kwon J, Lee JH, Park SY, Park Y, Lee W, Song KB, et al. A comparison of robotic versus laparoscopic distal pancreatectomy: Propensity score matching analysis. Int J Med Robot. (2022) 18(2):e2347. doi: 10.1002/rcs.2347
27. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
28. Kamarajah SK, Sutandi N, Robinson SR, French JJ, White SA. Robotic versus conventional laparoscopic distal pancreatic resection: A systematic review and meta-analysis. HPB (Oxford) (2019) 21(9):1107–18. doi: 10.1016/j.hpb.2019.02.020
29. Magistri P, Boggi U, Esposito A, Carrano FM, Pesi B, Ballarin R, et al. Robotic vs open distal pancreatectomy: A multi-institutional matched comparison analysis. J Hepatobiliary Pancreat Sci (2021) 28(12):1098–106. doi: 10.1002/jhbp.881
30. Wang M, Zhang H, Wu Z, Zhang Z, Peng B. Laparoscopic pancreaticoduodenectomy: single-surgeon experience. Surg Endosc (2015) 29(12):3783–94. doi: 10.1007/s00464-015-4154-5
31. Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: Oncologic advantages over open approaches? Ann Surg (2014) 260(4):633–8. doi: 10.1097/SLA.0000000000000937
32. Huang B, Feng L, Zhao J. Systematic review and meta-analysis of robotic versus laparoscopic distal pancreatectomy for benign and malignant pancreatic lesions. Surg Endosc (2016) 30(9):4078–85. doi: 10.1007/s00464-015-4723-7
33. Napoli N, Kauffmann EF, Perrone VG, Miccoli M, Brozzetti S, Boggi U. The learning curve in robotic distal pancreatectomy. Updates Surg (2015) 67(3):257–64. doi: 10.1007/s13304-015-0299-y
34. Shyr BU, Chen SC, Shyr YM, Wang SE. Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Med (Baltimore) (2018) 97(45):e13000. doi: 10.1097/MD.0000000000013000
35. Al Abbas AI, Wang C, Hamad AB, Knab LM, Rice MK, Moser AJ, et al. Mentorship and formal robotic proficiency skills curriculum improve subsequent generations' learning curve for the robotic distal pancreatectomy. HPB (Oxford) (2021) 23(12):1849–55. doi: 10.1016/j.hpb.2021.04.022
36. Takahashi C, Shridhar R, Huston J, Meredith K. Outcomes associated with robotic approach to pancreatic resections. J Gastrointest Oncol (2018) 9(5):936–41. doi: 10.21037/jgo.2018.08.04
37. Guerrini GP, Lauretta A, Belluco C, Olivieri M, Forlin M, Basso S, et al. Robotic versus laparoscopic distal pancreatectomy: An up-to-date meta-analysis. BMC Surg (2017) 17(1):105. doi: 10.1186/s12893-017-0301-3
Keywords: minimally invasive surgery, robotic distal pancreatectomy, laparoscopic distal pancreatectomy, Da Vinci, meta-analysis
Citation: Chen C, Hu J, Yang H, Zhuo X, Ren Q, Feng Q and Wang M (2022) Is robotic distal pancreatectomy better than laparoscopic distal pancreatectomy after the learning curve? A systematic review and meta-analysis. Front. Oncol. 12:954227. doi: 10.3389/fonc.2022.954227
Received: 27 May 2022; Accepted: 20 July 2022;
Published: 29 August 2022.
Edited by:
Lorenzo Cobianchi, University of Pavia, ItalyCopyright © 2022 Chen, Hu, Yang, Zhuo, Ren, Feng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miye Wang, OTE4ODgyMEBxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.