
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 28 September 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.954179
This article is part of the Research Topic Reviews in Breast Cancer View all 20 articles
Objectives: The primary purposes of this meta-analysis and systematic review were to evaluate the health-related quality of life (HRQoL) of Asian breast cancer (BC) patients to understand their holistic HRQoL level and provide medical and nursing recommendations to improve and preserve their quality of life.
Methods: A comprehensive literature search was conducted to find cross-sectional studies published in Chinese and English concerning HRQoL in BC patients from the inceptions of databases to 14 March 2022. The databases consulted were PubMed, Web of Science, Embase, Cochrane, PsyclNFO, CINAHL, and CNKI. Literature screening, data extraction, risk bias assessment, and data synthesis were independently carried out by two researchers. The Endnote X9 and Stata 15.0 software programs were used during the meta-analysis process.
Results: Out of the 8,563 studies identified, 23 cross-sectional studies involving 3,839 Asian BC patients were included in this meta-analysis. Two tools, namely, European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) and Quality of Life Questionnaire Breast Cancer module 23 (EORTC QLQ-BR23)—were used to evaluate the HRQoL of BC patients in Asia. The pooled mean of the global health status of Asian BC patients was 58.34 (95% confidence interval [CI]: 53.66–63.02). According to functional subscales of EORTC QLQ-C30 and EORTC QLQ-BR23, Asian BC patients suffered from the worst emotional functioning (pooled mean=66.38; 95% CI: 59.66–73.11) and sexual enjoyment (pooled mean=49.31; 95% CI: 31.97–63.36). In addition, fatigue (pooled mean=42.17; 95% CI: 34.46–49.88) and being upset by hair loss (pooled mean=48.38; 95% CI: 36.64–60.12) were the most obvious symptoms that Asian BC patients experienced according to the meta-analysis results of the EORTC QLQ-C30 and EORTC QLQ-BR23 symptom subscales.
Conclusion: Asian BC patients experience a relatively low HRQoL due to the prominent decline in their body functions, as well as the unpleasant experiences caused by their symptoms. It is suggested that timely, appropriate, and targeted intervention should be provided in relation to the physical, psychological, and social aspects of Asian BC patients’ lives to enhance their ability to function, relieve them of adverse symptoms, and improve their overall HRQoL.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022321165.
Breast cancer (BC), the most common malignant tumor, with the highest mortality among women globally, continues to threaten women’s lives, with an increasing number of cases yearly. The latest data pertaining to cancer worldwide in 2020 show that the number of new cases of BC has reached 2.26 million (11.7%), surpassing that of lung cancer, and so BC has become the world’s leading cancer (1, 2). Various factors may increase the likelihood of incidence of BC, and may also be influenced by socioeconomic developments, ethnicity, and lifestyle, among others (3–5). There are significant differences between Western countries and Asian countries in terms of clinical presentation, epidemiology, treatment methods, and prognosis of the disease. For instance, developing countries in Asia have a lower incidence of BC than Western countries but have a higher mortality rate (6). In addition, there are differences in the choice of BC treatment strategies between Asian and Western countries. To preserve their self-image and sexuality, young women with early-stage BC are more likely to choose breast-conserving surgery (BCS) as the primary treatment (7, 8). However, despite BC being diagnosed in Asian women at an earlier age approximately 10 years earlier than in Western countries, and more than 50% of Asian BC patients suffering from locally advanced cancers (9–11), findings from many studies have demonstrated that breast-conserving surgery is less common in Asia than in Europe and the United States (12, 13). The unique characteristics of BC in Asia and the different therapies adopted by Asian BC patients have led to a different health-related quality of life (HRQoL) compared to other continents.
HRQoL refers to an individual’s health status under the influence of illness and injury, medical intervention, aging, and changes in the social environment, as well as subjective satisfaction related to one’s economic and cultural backgrounds and values orientation (14). Owing to the complex treatment and prognosis of BC, patients usually experience chronic or prolonged diagnostic procedures and treatment processes, such as surgery, chemotherapy, radiotherapy, and hormonotherapy, which consequently lead to severe physical disorders, including breast removal, skin discoloration, hair loss, fatigue, sexual dysfunction, and distortions in body image (15, 16). However, as a chronic disease, BC usually affects more than just the body’s integrity (17). The treatment experiences and associated symptoms in BC patients also give rise to negative effects on mental health. Anxiety, depression, and distress in BC patients are found at high levels, even years after the acute phase or successful treatment in some cases (18–20). These negative effects on mental health can be caused by various factors, including, but not limited to, treatment-related side effects, interruption of the desire for childbearing (or even loss of fertility), and fear of cancer recurrence (FCR) (21, 22).
Impaired HRQoL is the most accurate indication of the gap between an individual’s actual functional level and the ideal standard (23). In Asia, a higher proportion of cancers are diagnosed at advanced stages due to a lack of early screening and insufficient understanding of the disease. Therefore, cancer patients in Asia tend to have more severe symptoms, such as pain, fatigue, insomnia, and anxiety, which are more likely to lower their HRQoL. There exists a consensus that the treatment of cancer patients should not only control their pathological reactions and relieve the discomfort caused by the disease and treatment but also reduce their psychological and emotional distress to improve their holistic HRQoL. However, in the context of the different types of economic and medical statuses, as well as cultural and habitual practices, such as the use of traditional medicine, there is a lack of overall understanding of the level of HRQoL of Asian BC patients (24, 25). Therefore, this meta-analysis aims to investigate the holistic HRQoL of Asian BC patients and provide conclusive evidence for developing more targeted measures to improve their quality of life.
This research was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (26). The current study’s protocol registration number in PROSPERO: International prospective register of systematic reviews is CRD42022321165.
Six English databases (PubMed, Web of Science, Embase, Cochrane, Psyclnfo, and CINAHL) and one Chinese database (CNKI) were searched to retrieve eligible articles from the inceptions of databases to 14 March 2022. The search terms “breast cancer,” “breast neoplasm,” “breast tumor,” “health-related quality of life,” “quality of life,” “Asia,” “Far East,” “Southeast Asia,” “South eastern Asia,” “Asia, Western,” “Middle East,” “China,” “Chine*,” “Hong Kong*,” “Macau,” “Tibet*,” “Taiwan*,” “Japan*,” “Korea*,” “Mongoli*,” “India*,” “Brunei*,” “Indonesia*,” “Lao*,” “Malay*,” “Myanmar,” “Burmese*,” “Philippines*,” “Singapore*,” “Thai*,” “Timor*,” “Vietnam*,” “Bangladesh,” “Bengal*,” “Bhutan*,” “India*,” “Nepal*,” “Pakistan*,” “Sri Lanka*,” “Kazakhstan,” “Tajikistan,” “Turkmenistan,” and “Borneo” were used in various combinations to ensure the capture of related literature. The asterisk (*) symbol is significant as it is the component of the search strategy. Appendix S1 in the Supplementary Materials reveals the search strategies used in the seven databases above. Furthermore, we checked the references listed in the selected articles for additional eligible resources.
•Populations: Asian adults (age ≥ 18 years old) diagnosed with breast cancer at any stage of pathology
•Study type: cross-sectional studies that investigated HRQoL of BC patients
•Outcomes: HRQoL scores of BC patients were evaluated by related tools
•Patients with other diseases aside from breast cancer
•Patients who had cognitive impairment or any psychiatric disorder
•Studies not in English or Chinese, gray literature, and studies that are not original
•Incomplete information or studies without the full text available
•Unpublished data and presentations that did not provide data that were accurate and clear with respect to the research variables
Two researchers (XYC and CXW) screened all of the literature by reading the titles and abstracts, then they excluded the studies that did not clearly meet the inclusion criteria; the researchers then read the full text to determine which should be used in our study. The whole process of screening and reading was carried out by the two researchers independently, and a third reviewer (TTC) stepped in when there was any discrepancy between the first two researchers. Once the literature was chosen, both researchers (XYC and CXW) independently extracted the information regarding the authors, region of the country, year of publication, study design, sampling methods and settings, HRQoL instruments, etc. Finally, all the information was integrated and verified by the two researchers.
The quality of the eligible studies was assessed via the Joanna Briggs Institute tool for cross-sectional studies (JBI Critical Appraisal Checklist for Analytical Cross-Sectional Studies) (27). This tool includes eight aspects of every cross-sectional study, evaluated by “Yes,” “No,” and “Unclear.” The answers of “yes” ≥ 5, 3–4, and 0–2 times are considered to indicate high, moderate, and low methodological quality, respectively. Similarly, two researchers (XYC and CXW) conducted the quality evaluation procedure independently, and the third researcher (LLZ) was asked to address any divergence.
As recommended by the Cochrane Collaboration, we used the Risk of Bias in Non-Randomized Studies - of Intervention (ROBINS-I) tool to assess the risk of bias in the studies included (28) from the following seven domains: (i) bias in the selection of exposed and non-exposed cohorts; (ii) bias in the assessment of exposure; (iii) bias in the presence of outcome of interest at the start of study; (iv) bias in the control of prognostic variables (with matching or adjusting); (v) bias in the assessment of the presence or absence of prognostic factors; (vi) bias in the assessment of outcome; and (vii) bias in adequacy regarding follow-up of cohorts.
Stata 15.0 software was used to conduct the meta-analysis, and the pooled mean value was taken as the effect size (ES). The pooled mean of Asian BC patients’ HRQoL was estimated using a random effects model with a confidence interval of 95%, and the scores were from the “global health status” for Quality of Life Questionnaire C30 (QLQ-C30), functional scales, and symptom scales for both QLQ-C30 and Quality of Life Questionnaire Breast Cancer module 23 QLQ-BR23. Forest plots were used to present the pooled means (95% CI) of the studies included. Statistical heterogeneity was assessed with the I2 statistic, and I2 values above 50% were interpreted as heterogeneous. Meta-regression was then used to analyze the factors related to high heterogeneity. Publication bias was assessed via Begg’s test, and a p-value greater than 0.05 (p > 0.05) indicated no publication bias. If publication bias existed, the trim-and-fill method was employed to detect the effects of publication bias on the results. To assess the influence of each study on the pooled ES, sensitivity analysis was conducted by omitting an individual study each time and repeating the analysis.
After screening and removing duplicates, 23 cross-sectional studies (29–51) containing 3,839 Asian BC patients were included in this study from 8,563 retrieved records. The flow diagram of literature screening is displayed in Figure 1.
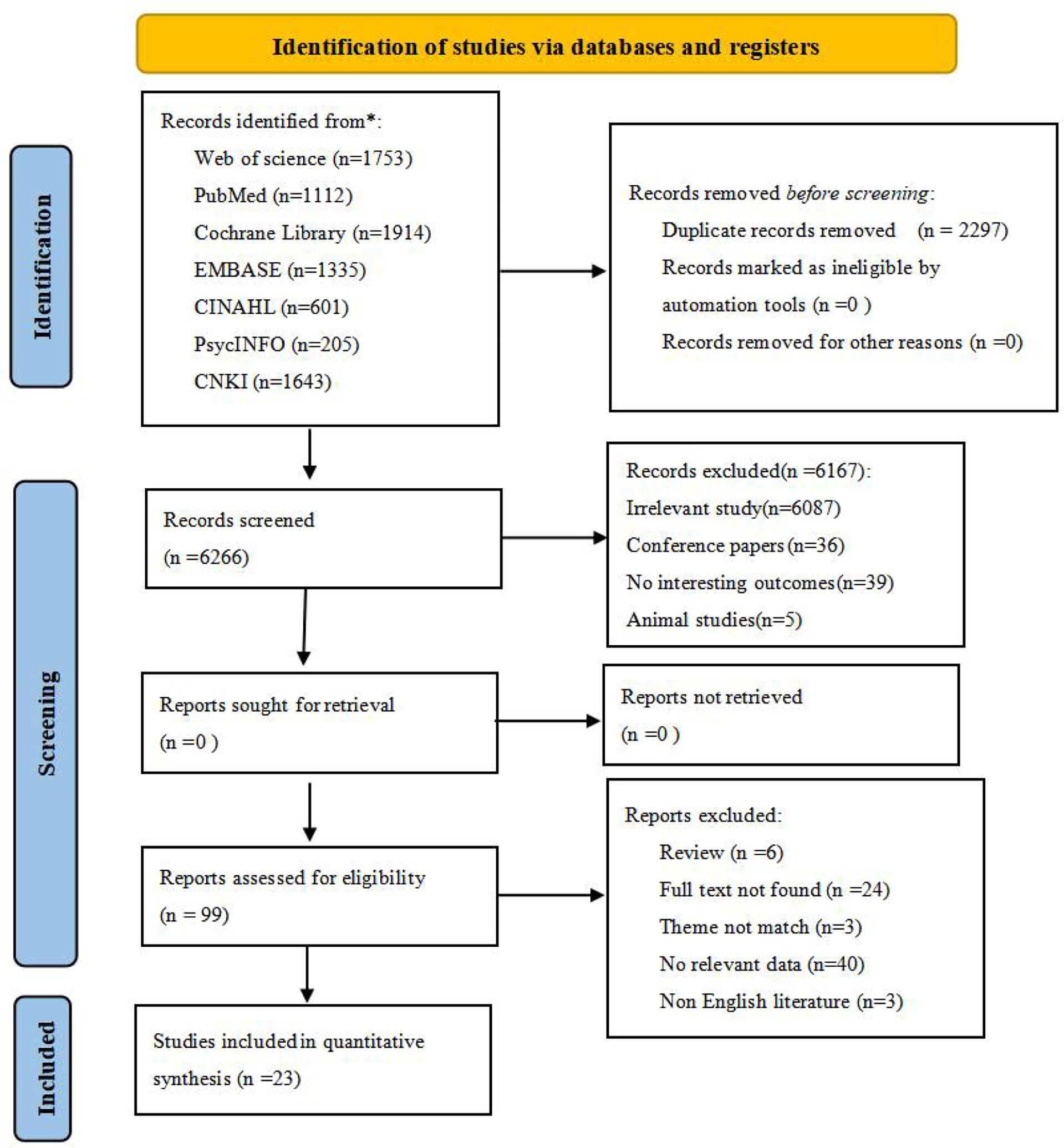
Figure 1 Flow diagram of the search strategy and study selection.From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71For more information, visit: http://www.prisma-statement.org/. * reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).
The characteristics of the 23 cross-sectional studies are given in Table 1. The details of quality assessment for the studies included are presented in Appendix S2 in the Supplementary Materials section, which shows that the scores of the studies included range from 5 to 8, all being of high methodological quality. Moreover, the assessment results for the risk of bias are given in Appendix S3 in the Supplementary Materials. There are 5 studies with a high risk of bias, 14 with a moderate risk of bias, and 4 with a low risk of bias.
Two types of standard tools are used alone or in combination with each other for the studies included: the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) (n=23) and the Quality of Life Questionnaire Breast Cancer module 23 (QLQ-BR23) (n=13). QLQ-BR23 is a supplement for the general cancer questionnaire QLQ-C30, and it aims to identify unique concerns of the BC patients (41). The detailed analysis of Asian BC patients’ HRQoL, evaluated by the two tools, is as follows.
EORTC QLQ-C30 was used in all studies included in this systematic review to assess HRQoL of Asian BC patients. The mean value of the global health status of Asian BC patients ranged from 31.2 to 79.43, and the pooled mean value of global health status was 58.34 (95%CI: 53.66–63.02) (I2 = 98.5%) by using the random effects model (Figure 2). Two subscales of QLQ-C30 also illustrate the HRQoL of BC patients with respect to their functions and symptoms. The functional subscales evaluate the functional status with respect to physical, role, emotional, cognitive, and social functioning. There exist 23 studies (29–51) that have reported the results of physical, role, emotional, and cognitive functioning status, while 22 (29–32, 34–51) have provided the results of social functioning. The pooled means of functional status from low to high are as follows: emotional functioning (pooled mean=66.38; 95% CI: 59.66–73.11), social functioning (pooled mean=71.26; 95% CI: 64.97–77.54), role functioning (pooled mean=72.70; 95% CI: 66.06–79.33), physical functioning (pooled mean=73.15; 95% CI: 66.84–79.46), and cognitive functioning (pooled mean=75.53; 95% CI: 70.58–80.49, Table 2). As for the symptom subscales, a total of nine symptoms are included. All 23 studies (29–51) reported the symptoms of fatigue, pain, insomnia, and appetite loss; 22 (30–51) reported nausea and vomiting, dyspnea, constipation, and diarrhea; and 21 (29–36, 38–48, 50, 51) reported financial difficulties. Among the nine symptoms, fatigue was the most common symptom suffered by Asian BC patients, with a pooled mean score of 42.17 (95% CI: 34.46–49.88), followed by financial difficulties (pooled mean=39.07; 95% CI: 39.07–49.07), insomnia (pooled mean=34.96; 95% CI: 26.93–42.99), pain (pooled mean=32.51; 95% CI:25.61–39.42), appetite loss (pooled mean=26.80; 95% CI: 18.50–35.10), dyspnea (pooled mean=24.46; 95% CI: 17.02–31.91), constipation (pooled mean=22.52; 95% CI: 15.24–29.81), nausea and vomiting (pooled mean=18.47; 95% CI: 14.26–22.67), and diarrhea (pooled mean=15.46; 95% CI: 8.60–22.33; Table 3).
There are 13 studies (29, 30, 32–34, 36, 38–40, 42, 43, 48, 50) that have used QLQ-BR23. The details of the results are displayed in Table 4. The QLQ-BR23 tool also has two subscales to assess the functions and symptoms of BC patients specifically. As can be seen in Table 4, all 13 studies reported the evaluated results of body image, future perspective, breast symptoms, and arm symptoms; and 12 reported the results of sexual functioning (29, 30, 32–34, 36, 38, 39, 42, 43, 48, 50), sexual enjoyment (29, 30, 32–34, 36, 38, 39, 42, 43, 48, 50), systemic side effects (29, 30, 32–34, 36, 38–40, 42, 48, 50), and upset by hair loss (30, 32–34, 36, 38–40, 42, 43, 48, 50). The pooled scores in the functional subscales ranged from 49 to 66: sexual enjoyment (pooled mean=49.31; 95% CI: 31.97–63.36), sexual functioning (pooled mean=50.77; 95% CI: 28.00–73.54), future perspective (pooled mean=53.81; 95% CI: 44.26–63.81), and body image (pooled mean=66.15; 95% CI: 61.08–71.21). Additionally, the pooled scores of the symptom subscale ranged from 29 to 49: breast symptoms (pooled mean=29.26; 95% CI: 20.12–38.40), arm symptoms (pooled mean=34.02; 95% CI: 24.91–43.13), systemic side effects (pooled mean=35.88; 95% CI: 25.76–46.00), and upset by hair loss (pooled mean=48.38; 95% CI: 36.64–60.12). The pooled results suggest that sexual enjoyment and being upset by hair loss are the most frequent problems faced by Asian BC patients.
As illustrated in Table 5, the heterogeneity between the studies included was high, with all I2 values above 90%. According to the results of Begg’s tests, there was no obvious publication bias for the meta-analysis, except for the results for nausea and vomiting (p=0.048), dyspnea (p=0.032), and diarrhea (p=0.011) in the QLQ-C30 tool (Table 5). Moreover, the outcomes of the trim-and-fill method showed that the publication bias may have exerted an influence on the results for nausea and vomiting (p=0.914), dyspnea (p=0.057), and diarrhea (p=0.361, Table 5). Moreover, the sensitivity-analysis results indicated that no single study essentially changed the pooled mean of all the outcomes, and we take the sensitivity analysis plot of global health status as an example (Figure 3).
We took the region of the country (in East Asia, Southeast Asia, South Asia, and in West Asia), the publication year (in 2008–2012, 2013–2017, and 2018–2022), the mean age of patients (age < 50; age ≥ 50), the sample size (1–200, 201–400, and 401–608), and the medical level worldwide (ranked below 50, ranked between 50 and 100,; and ranked above 100) (52) as covariates in the meta-regression. The results demonstrated that the five factors above could not explain the heterogeneity between studies of almost all the dimensions of HRQoL significantly through the univariate meta-regression method (p-value > 0.05), except the physical functioning dimension of the QLQ-C30, in which its high heterogeneity may be associated with the regions of countries (p=0.02, Table 6).
This study shows that Asian BC patients have a global health status score of 58.34, similar to the results of research conducted by Hashemi (53) in the Middle East. However, compared with BC patients studied from other regions, Asian BC patients have a lower overall quality of life, especially when compared to those in Spain (54) or Germany (55). The literature review revealed that global health status is associated with many factors, such as the operation method (mastectomy or breast preservation) (29), presence of metastases (30), chemotherapy (56), and radiotherapy (57). In addition, the more comorbidities that BC patients have, the lower their quality of life. The survey conducted by Fu et al. (58) revealed that 20%–30% of BC patients present with comorbidities that were pre-existing or that developed after BC diagnosis, such as hypertension, arthritis, and diabetes (59). The research conducted by Miller et al., which addressed BC survivors in African-American and Latina groups, reached the same conclusion, i.e., that having fewer comorbidities means a better HRQoL (60). Therefore, it is necessary to help Asian BC patients to manage and control their comorbidities to enhance their HRQoL.
QLQ-C30 and QLQ-BR23 evaluate the functional status of BC patients from different perspectives. The meta-analysis demonstrated that for Asian BC patients, emotional functioning and sexual enjoyment were the most severely impaired elements of quality of life during the progression of BC. In terms of emotional functioning, Asian BC patients’ scores were lower than those of Brazilian (61) and Mexican patients (62). This may be attributed to the culture of collectivism in Asia, which encourages the suppression of emotions to preserve interpersonal harmony (63). With the understanding that “sharing personal problems with others are [sic] regarded as unacceptable since it may make inappropriate demands on the group,” Asian BC patients tend to restrain their emotional disclosure (64). This suppression of emotions, however, leads to more negative moods on the one hand, and on the other hand, decreases social functioning by discouraging patients from seeking help from family and society. Conversely, the differences in physical functioning, role functioning, and cognitive functioning are more significantly affected by treatment. Yue Li et al. once conducted a meta-analysis to compare patients’ HRQoL status between breast conservation treatment (BCT) and mastectomy, with the results showing significant differences in the levels of the physical, role, and cognitive functioning of patients who received different treatments (65). Moreover, Eman et al. (66) also demonstrated that hormonotherapy could maintain a better functional status for patients than could chemotherapy and radiotherapy.
Although sexual enjoyment presented the lowest score in our study, the score of sexual functioning was similar to that of sexual enjoyment, and both had a negative status, which is different from the studies conducted in Latin America and the Caribbean (67), where patients had different scores for sexual enjoyment and sexual functioning. The decline of the sexual functioning of BC patients could be caused by various treatments, such as the commonly used drugs tamoxifen and aromatase inhibitors in adjuvant endocrine therapy, whose side effects of vaginal dryness and dyspareunia are often reported in reviews (68). Research has also demonstrated that for cancer patients, conserving the breast or not has a significant impact on their sexual wellbeing and satisfaction since the breast is regarded as a secondary sexual characteristic (69). In addition, patients from different cultural backgrounds have quite different attitudes toward sexual knowledge and sexual life. Compared to Western patients, Asian patients treat their sexual life as a private topic and do not like to discuss it publicly; moreover, they even choose random answers when responding to questionnaires to avoid exposing their privacy (32, 40). Thus, healthcare professionals should consider the possibility of inconsistency between the outcomes evaluated and the actual HRQoL status when they provide medical and nursing care for Asian BC patients.
With respect to the assessment of body image, our study’s results are almost indistinguishable from those of Polish scholars (70): BC patients’ perception of their body image decreases after surgery. Montazeri et al. and Arora et al. stated that a deterioration in women’s perception of their body may worsen, even after their general physical condition has recovered and symptoms have been alleviated after surgery and subsequent systemic treatments (71, 72). Influenced by the negative emotion of anxiety, patients are usually pessimistic about their future, body image, and sexual functioning (73). Agnieszka showed that a higher level of emotional, cognitive, and social functioning cannot only help prevent negative assessment of scars in women but can also ensure a better perception of both their body image and future prospects (70). Therefore, paying long-term attention to the BC patients’ functional status and providing them with dynamic interventions according to updated psychological data are essential to integrally improving and maintaining their quality of life.
Along with a decline in functioning comes a battery of symptoms caused by drugs, surgeries, and other treatments that damage BC patients’ quality of life. Fatigue and being upset by hair loss are the common symptoms of BC patients in Asia. Cancer-related fatigue (CRF)—a persistent state of severe exhaustion—impairs BC patients’ quality of life. It not only makes patients’ functioning abnormal but also gives rise to an increase in BC incidence and mortality. Nevertheless, CRF is underestimated and underreported by physicians and patients (74–76). Currently, the pathophysiological mechanism of CRF remains unclear, except that surgery, chemotherapy, and hormonotherapy are associated with CRF. Also potentially contributing to different cancer outcomes are the following: less or no access to quality care, differences in tumor biology, and socioeconomic factors influencing treatment options that may be affected by racial differences (77, 78). These may help explain why, when we compared our results to those of Lucas, the degree of fatigue of patients in Asia is more severe than those in Latin America and the Caribbean (67). The etiology and pathogenesis of fatigue in BC patients are complicated and multicausal. Many studies have demonstrated that CRF and other symptom clusters, such as sleep disturbance, mood disorder, and pain, are simultaneous (79). The results of these studies are consistent with our conclusion that fatigue, pain, and insomnia affect BC patients’ quality of life to a similar extent. Moreover, inflammation may be the key biological mechanism underlying this symptom cluster since a prior study found that the coexistence of arthralgia, fatigue, and insomnia was associated with an increased level of inflammatory biomarkers among women on endocrine therapy (80).
As evaluated by the symptom scale of EORTC QLQ-C30, financial difficulty is also a serious problem that negatively impacts Asian patients’ quality of life. In comparison, Asian BC patients experience greater financial pressure than their American counterparts (67) but less than Egyptians (66). In addition to age, gender, marital status, monthly net income, educational level, and self-reported health status, national income level and health insurance coverage are also associated with financial hardship among BC patients (81–85), which suggests that with all relevant factors taken into consideration, designing a matching benefit package is essential to reducing the financial burden on patients.
Being upset by hair loss is another serious symptom of Asian BC patients according to this meta-analysis. Hair loss is one of the distressing side effects for BC patients who undergo chemotherapy. Hence, we here allude to the term chemotherapy-induced alopecia (CIA), which is usually an unavoidable but transient side effect that can be dealt with by wearing wigs. BC patients are mostly women, and they regard hair as an integral part of their identity. CIA, however, affects their social life, bringing them physical and psychological distress. More seriously, some BC patients may bear the agony of hair loss even after 6 months of chemotherapy, leading to low self-esteem and lower HRQoL (86). Chemotherapy-induced irreversible alopecia (CIIA) usually occurs after high-dose chemotherapy, and married women seem to be more upset about hair loss than others (86, 87). Therefore, some preventive measures, such as scalp cooling or psychological intervention, should be implemented for high-risk CIIA patients to prevent or reduce the degree of their hair loss and emotional distress. Nausea and vomiting, dyspnea, loss of appetite, constipation, and breast and arm symptoms may still affect patients’ quality of life. However, the impact is much lighter than those symptoms mentioned above, perhaps because these symptoms usually appear during the acute phase of treatments and can be controlled by specific traditional medicines in Asia (88, 89).
This review used two tools to evaluate the HRQoL of BC patients, namely, EORTC QLQ-C30 and QLQ-BR23. Both tools represent patient-reported outcomes (PRO), which demonstrate the patients’ physical, psychological, and social response to disease and therapy from their own perspective but not from that of the physician or anyone else. The evaluation clearly shows that the patients’ functioning and symptoms interact with each other, jointly leading to the decline of HRQoL in BC patients. We therefore suggest that a holistic assessment of BC patients is necessary to provide targeted psychological and medical treatments and enhance their quality of life.
Nevertheless, there are still some limitations to this article. First, likely due to the specific methodological limitations of cross-sectional studies included in the meta-analysis, considerable heterogeneity was observed among the studies that evaluated the HRQoL of BC patients. Second, only studies published in English and Chinese were searched, and research projects that did not provide merged data were excluded. Third, gray literature was excluded from our review because of the difficulty of conducting a quality assessment without a detailed description of the methodology and peer review.
BC patients in Asia have a lower HRQoL under the high prevalence, growing incidence, and advanced breast cancer diagnosis at an earlier age. As assessed by EORTC QLQ-C30 and QLQ-BR23, Asian BC patients have different degrees of impairment in physical, social, sexual, and other functioning and suffer from various symptoms, including, but not limited to, fatigue, pain, insomnia, and the influence of financial difficulties, resulting to decline in their quality of life. Therefore, raising awareness of routine breast cancer screening in the Asian population is the first fundamental measure to curb BC deterioration and avoid s adverse impact on HRQoL. Since the symptoms of BC affect the body functions of BC patients, and serious symptoms of this illness can lead to psychological dilemma and mental disorder and vice versa, the mutual effects change over time; thus, and a dynamic assessment of BC patients and a corresponding treatment plan are essential and should be ensured. Psychological interventions with one-on-one psychotherapy and cognitive behavioral therapy (CBT) can also be introduced to relieve patients’ negative emotions caused by poor body image perceptions, such as embarrassment about hair loss and uneasiness about visible scarring. In addition, encouraging patients to seek help from their social networks could be an important approach to improving their psychological state. For pain, insomnia, arm dysfunction, and other symptoms, it is recommended that caregivers use traditional Asian medicine to help BC patients alleviate those symptoms in a cost-effective way. Finally, in view of the high prevalence of breast cancer in Asia, each country in Asia should do its best to increase the coverage of their healthcare systems so that BC patients’ financial difficulties may be alleviated.
The original contributions presented in the study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding authors.
All authors contributed to the article and approved the version submitted. JG and CH proposed the concept and monitored the progress of the work. CH provided the ideas for article revisions. CW and DB designed the work and provided solutions to the inconsistencies. XC, CW, and TC performed the literature search, study selection, and data extraction, and then wrote the manuscript. XC and CW undertook data analysis and interpretation. XC, CW, and DB wrote the manuscript and made the equal contributions to the article. Meanwhile, LZ and HL helped to review and check the manuscript. CW and DB contributed to the revision of the manuscript. All the authors approved the submitted version and agreed to be personally accountable to their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work are addressed.
This study was supported by grants from the Sichuan Federation of Social Science Associations (NO. SC22B149 and NO. SC22B150), the Health Commission of Sichuan Province (NO. 21PJ109), and the Sichuan Mental Health Education Research Center (NO. XLJKJY2203A).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.954179/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet (2014) 383(9935):2127–35. doi: 10.1016/S0140-6736(14)60488-8
3. Wong IO, Schooling CM, Cowling BJ, Leung GM. Breast cancer incidence and mortality in a transitioning Chinese population: Current and future trends. Br J Cancer. (2015) 112(1):167–70. doi: 10.1038/bjc.2014.532
4. Sung H, Rosenberg PS, Chen WQ, Hartman M, Lim WY, Chia KS, et al. Female breast cancer incidence among Asian and Western populations: More similar than expected. J Natl Cancer Inst (2015) 107(7):djv107. doi: 10.1093/jnci/djv107
5. Porter P. Westernizing" women's risks? Breast cancer in lower-income countries. N Engl J Med (2008) 358(3):213–6. doi: 10.1056/NEJMp0708307
6. Hossain MS, Ferdous S, Karim-Kos HE. Breast cancer in south Asia: A Bangladeshi perspective. Cancer Epidemiol. (2014) 38(5):465–70. doi: 10.1016/j.canep.2014.08.004
7. Sinnadurai S, Kwong A, Hartman M, Tan EY, Bhoo-Pathy NT, Dahlui M, et al. Breast-conserving surgery versus mastectomy in young women with breast cancer in Asian settings. BJS Open (2018) 3(1):48–55. doi: 10.1002/bjs5.50111
8. Lazovich D, Solomon CC, Thomas DB, Moe RE, White E. Breast conservation therapy in the united states following the 1990 national institutes of health consensus development conference on the treatment of patients with early stage invasive breast carcinoma. Cancer (1999) 86(4):628–37. doi: 10.1002/(SICI)1097-0142(19990815)86:4<628::AID-CNCR11>3.0.CO;2-L
9. Pathy NB, Yip CH, Taib NA, Hartman M, Saxena N, Iau P, et al. Breast cancer in a multi-ethnic Asian setting: results from the Singapore-Malaysia hospital-based breast cancer registry. Breast (2011) 20 Suppl 2:S75–80. doi: 10.1016/j.breast.2011.01.015
10. Kwong A, Mang OW, Wong CH, Chau WW, Law SC, Hong Kong Breast Cancer Research Group. Breast cancer in Hong Kong, southern China: the first population-based analysis of epidemiological characteristics, stage-specific, cancer-specific, and disease-free survival in breast cancer patients: 1997-2001. Ann Surg Oncol (2011) 18(11):3072–8. doi: 10.1245/s10434-011-1960-4
11. Raina V, Bhutani M, Bedi R, Sharma A, Deo SV, Shukla NK, et al. Clinical features and prognostic factors of early breast cancer at a major cancer center in north India. Indian J Cancer. (2005) 42(1):40–5. doi: 10.4103/0019-509x.15099
12. Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg (2015) 150(1):9–16. doi: 10.1001/jamasurg.2014.2895
13. Garcia-Etienne CA, Tomatis M, Heil J, Friedrichs K, Kreienberg R, Denk A, et al. Mastectomy trends for early-stage breast cancer: A report from the EUSOMA multi-institutional European database. Eur J Cancer (2012) 48(13):1947–56. doi: 10.1016/j.ejca.2012.03.008
14. Kosmidis P. Quality of life as a new end point. Chest (1996) 109(5 Suppl):110S–2S. doi: 10.1378/chest.109.5_supplement.110s
15. Triberti S, Savioni L, Sebri V, Pravettoni G. eHealth for improving quality of life in breast cancer patients: A systematic review. Cancer Treat Rev (2019) 74:1–14. doi: 10.1016/j.ctrv.2019.01.003
16. Jacobs DHM, Charaghvandi RK, Horeweg N, Maduro JH, Speijer G, Roeloffzen EMA, et al. Health-related quality of life of early-stage breast cancer patients after different radiotherapy regimens. Breast Cancer Res Treat (2021) 189(2):387–98. doi: 10.1007/s10549-021-06314-4
17. Trusson D, Pilnick A, Roy S. A new normal?: women's experiences of biographical disruption and liminality following treatment for early stage breast cancer. Soc Sci Med (2016) 151:121–9. doi: 10.1016/j.socscimed.2016.01.011
18. Woertman L, van den Brink F. Body image and female sexual functioning and behavior: A review. J Sex Res (2012) 49(2-3):184–211. doi: 10.1080/00224499.2012.658586
19. Canada AL, Schover LR. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psychooncology (2012) 21(2):134–43. doi: 10.1002/pon.1875
20. Ljungman L, Ahlgren J, Petersson LM, Flynn KE, Weinfurt K, Gorman JR, et al. Sexual dysfunction and reproductive concerns in young women with breast cancer: Type, prevalence, and predictors of problems. Psychooncology (2018) 27(12):2770–7. doi: 10.1002/pon.4886
21. Bártolo A, Santos IM, Valério E, Monteiro S. Depression and health-related quality of life among young adult breast cancer patients: The mediating role of reproductive concerns. J Adolesc Young Adult Oncol (2020) 9(3):431–5. doi: 10.1089/jayao.2019.0144
22. McGinty HL, Small BJ, Laronga C, Jacobsen PB. Predictors and patterns of fear of cancer recurrence in breast cancer survivors. Health Psychol (2016) 35(1):1–9. doi: 10.1037/hea0000238
23. Cella DF. Quality of life: concepts and definition. J Pain Symptom. Manage (1994) 9(3):186–92. doi: 10.1016/0885-3924(94)90129-5
24. Chui PL, Abdullah KL, Wong LP, Taib NA. Quality of life in CAM and non-CAM users among breast cancer patients during chemotherapy in Malaysia. PloS One (2015) 10(10):e0139952. doi: 10.1371/journal.pone.0139952
25. Kang E, Yang EJ, Kim SM, Chung IY, Han SA, Ku DH, et al. Complementary and alternative medicine use and assessment of quality of life in Korean breast cancer patients: A descriptive study. Support Care Cancer. (2012) 20(3):461–73. doi: 10.1007/s00520-011-1094-z
26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ (2009) 339:b2535. doi: 10.1136/bmj
27. Peters M., Godfrey C., McInerney P., Soares C. B., Khalil H., Parker D. Methodology for JBI scoping reviews. In: The Joanna Briggs institute reviewers' manual 2015 (Joanna Briggs Institute) (2015).
28. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (2016) 12:355:i4919. doi: 10.1136/bmj.i4919
29. Dubashi B, Vidhubala E, Cyriac S, Sagar TG. Quality of life among younger women with breast cancer: study from a tertiary cancer institute in south India. Indian J Cancer. (2010) 47(2):142–7. doi: 10.4103/0019-509X.63005
30. Jassim GA, Whitford DL. Quality of life of bahraini women with breast cancer: A cross sectional study. BMC Cancer. (2013) 28:13:212. doi: 10.1186/1471-2407-13-212
31. Naung MT, Panza A. Quality of life and relationship between functioning and symptoms of female patients with breast cancer before chemotherapy in a cancer clinic At Yangon,Myanmar. J Ayub Med Coll Abbottabad. (2020) 32(4):540–5.
32. Chen Q, Li S, Wang M, Liu L, Chen G. Health-related quality of life among women breast cancer patients in Eastern China. BioMed Res Int (2018) 2018:1452635. doi: 10.1155/2018/1452635
33. Maharjan M, Thapa N, Adhikari RD, Petrini MA, Amatya KS. Quality of life of Nepalese women post mastectomy. Asian Pac J Cancer Prev (2018) 19(4):1005–12. doi: 10.22034/APJCP.2018.19.4.1005
34. Ou HT, Chung WP, Su PF, Lin TH, Lin JY, Wen YC, et al. Health-related quality of life associated with different cancer treatments in Chinese breast cancer survivors in Taiwan. Eur J Cancer Care (Engl). (2019) 28(4):e13069. doi: 10.1111/ecc.13069
35. Najafi F, Nedjat S, Zendehdel K, Mirzania M, Montazeri A. Self-reported versus proxy reported quality of life for breast cancer patients in the Islamic republic of Iran. East Mediterr. Health J (2017) 22(11):786–93. doi: 10.26719/2016.22.11.786
36. Mirzaei F, Farshbaf-Khalili A, Nourizadeh R, Zamiri RE. Quality of life and its predictors in Iranian women with breast cancer undergoing chemotherapy and radiotherapy. Indian J Cancer. (2021) 58(1):76–83. doi: 10.4103/ijc.IJC_750_18
37. Safaee A, Moghimi-Dehkordi B, Zeighami B, Tabatabaee H, Pourhoseingholi M. Predictors of quality of life in breast cancer patients under chemotherapy. Indian J Cancer. (2008) 45(3):107–11. doi: 10.4103/0019-509x.44066
38. Almutairi KM, Mansour EA. Vinluan JM. A cross-sectional Assess Qual Life Breast Cancer patients Saudi Arabia. Public Health (2016) 136:117–25. doi: 10.1016/j.puhe.2016.03.008
39. Jafari N, Farajzadegan Z, Zamani A, Bahrami F, Emami H, Loghmani A. Spiritual well-being and quality of life in Iranian women with breast cancer undergoing radiation therapy. Support Care Cancer. (2013) 21(5):1219–25. doi: 10.1007/s00520-012-1650-1
40. Kshirsagar AS, Wani SK. Health-related quality of life in patients with breast cancer surgery and undergoing chemotherapy in ahmednagar district. J Cancer Res Ther (2021) 17(6):1335–8. doi: 10.4103/jcrt.JCRT_154_19
41. Ganesh S, Lye MS, Lau FN. Quality of life among breast cancer patients in Malaysia. Asian Pac J Cancer Prev (2016) 17(4):1677–84. doi: 10.7314/apjcp.2016.17.4.1677
42. Manandhar S, Shrestha DS, Taechaboonsermsk P, Siri S, Suparp J. Quality of life among breast cancer patients undergoing treatment in national cancer centers in Nepal. Asian Pac J Cancer Prev (2014) 15(22):9753–7. doi: 10.7314/apjcp.2014.15.22.9753
43. Yusuf A, Ahmad Z, Keng SL. Quality of life in Malay and Chinese women newly diagnosed with breast cancer in kelantan, Malaysia. Asian Pac J Cancer Prev (2013) 14(1):435–40. doi: 10.7314/apjcp.2013.14.1.435
44. Alacacioglu A, Yavuzsen T, Dirioz M, Yilmaz U. Quality of life, anxiety and depression in Turkish breast cancer patients and in their husbands. Med Oncol (2009) 26(4):415–9. doi: 10.1007/s12032-008-9138-z
45. Huang HY, Tsai WC, Chou WY, Hung YC, Liu LC, Huang KF, et al. Quality of life of breast and cervical cancer survivors. BMC Womens Health (2017) 17(1):30. doi: 10.1186/s12905-017-0387-x
46. Syed Alwi SM, Narayanan V, Mohd Taib NA, Che Din N. Predictors of health-related quality of life after completion of chemotherapy among Malaysian early-stage breast cancer survivors. Support Care Cancer. (2022) 30(3):2793–801. doi: 10.1007/s00520-021-06686-9
47. Abu-Saad Huijer H, Abboud S. Health-related quality of life among breast cancer patients in Lebanon. Eur J Oncol Nurs. (2012) 16(5):491–7. doi: 10.1016/j.ejon.2011.11.003
48. Alawadi SA, Ohaeri JU. Health - related quality of life of Kuwaiti women with breast cancer: A comparative study using the EORTC quality of life questionnaire. BMC Cancer. (2009) 9:222. doi: 10.1186/1471-2407-9-222
49. Homaee Shandiz F, Karimi FZ, Khosravi Anbaran Z, Abdollahi M, Rahimi N, Ghasemi M, et al. Investigating the quality of life and the related factors in Iranian women with breast cancer. Asian Pac J Cancer Prev (2017) 18(8):2089–92. doi: 10.22034/APJCP.2017.18.8.2089
50. Saleha SB, SHAKEEL A, Shumaila E, Shazia R., Rashid R., Ibaham M, et al. An assessment of quality of life in breast cancer patients using EORTC QLQ C30/+ Br23 questionnaire. International Journal Of Cancer Management (2010) 3:98–104. doi: 10.1186/1471-2288-11-56
51. Sehati Shafaee F, Mirghafourvand M, Harischi S, Esfahani A, Amirzehni J. Self-confidence and quality of life in women undergoing treatment for breast cancer. Asian Pac J Cancer Prev (2018) 19(3):733–40. doi: 10.22034/APJCP.2018.19.3.733
52. GBD 2016 Healthcare Access and Quality Collaborators. Measuring performance on the healthcare access and quality index for 195 countries and territories and selected subnational locations: A systematic analysis from the global burden of disease study 2016. Lancet (2018) 391(10136):2236–71. doi: 10.1016/S0140-6736(18)30994-2
53. Hashemi SM, Balouchi A, Al-Mawali A, Rafiemanesh H, Rezaie-Keikhaie K, Bouya S, et al. Health-related quality of life of breast cancer patients in the Eastern Mediterranean region: A systematic review and meta-analysis. Breast Cancer Res Treat (2019) 174(3):585–96. doi: 10.1007/s10549-019-05131-0
54. Villar RR, Fernández SP, Garea CC, Pillado MTS, Barreiro VB, Martín CG. Quality of life and anxiety in women with breast cancer before and after treatment. Rev Lat Am Enfermagem. (2017) 25:e2958. doi: 10.1590/1518-8345.2258.2958
55. Engel J, Schlesinger-Raab A, Emeny R, Hölzel D, Schubert-Fritschle G. Quality of life in women with localised breast cancer or malignant melanoma 2 years after initial treatment: A comparison. Int J Behav Med (2014) 21(3):478–86. doi: 10.1007/s12529-013-9334-x
56. Li Y, Zhou Z, Ni N, Luan Z, Peng X. Quality of life and hope of women in China receiving chemotherapy for breast cancer. Clin Nurs Res (2021) 14. doi: 10.1177/10547738211046737
57. Hauth F, De-Colle C, Weidner N, Heinrich V, Zips D, Gani C. Quality of life and fatigue before and after radiotherapy in breast cancer patients. Strahlenther. Onkol. (2021) 197(4):281–7. doi: 10.1007/s00066-020-01700-1
58. Fu MR, Axelrod D, Guth AA, et al. Comorbidities and quality of life among breast cancer survivors: A prospective study. J Pers Med (2015) 5(3):229–42. doi: 10.3390/jpm5030229
59. Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: A review. Clin Epidemiol. (2013) 5(Suppl 1):3–29. doi: 10.2147/CLEP.S47150
60. Miller AM, Ashing KT, Modeste NN, Herring RP, Sealy DA. Contextual factors influencing health-related quality of life in African American and latina breast cancer survivors. J Cancer Surviv. (2015) 9(3):441–9. doi: 10.1007/s11764-014-0420-0
61. Marcelo Castro E Silva I, Lúcia Penteado Lancellotti C. Health-related quality of life in women with breast cancer undergoing chemotherapy in Brazil. Int J Gen Med (2021) 14:10265–70. doi: 10.2147/IJGM.S343804
62. Cortés-Flores AO, Morgan-Villela G, Zuloaga-Fernández del Valle CJ, et al. Quality of life among women treated for breast cancer: A survey of three procedures in Mexico. Aesthetic Plast Surg (2014) 38(5):887–95. doi: 10.1007/s00266-014-0384-5
63. Wei M, Su JC, Carrera S, Lin SP, Yi F. Suppression and interpersonal harmony: A cross-cultural comparison between Chinese and European americans. J Couns Psychol (2013) 60(4):625–33. doi: 10.1037/a0033413
64. Chu Q, Wong CCY, Lu Q. Social constraints and PTSD among Chinese American breast cancer survivors: not all kinds of social support provide relief. J Behav Med (2021) 44(1):29–37. doi: 10.1007/s10865-020-00165-y
65. Li Y, Guo J, Sui Y, Chen B, Li D, Jiang J. Quality of life in patients with breast cancer following breast conservation surgery: A systematic review and meta-analysis. J Healthc. Eng. (2022) 2022:3877984. doi: 10.1155/2022/3877984
66. Mortada EM, Salem RA, Elseifi OS, Khalil OM. Comparing health-related quality of life among breast cancer patients receiving different plans of treatment, Egypt. J Community Health (2018) 43(6):1183–91. doi: 10.1007/s10900-018-0538-5
67. Gonzalez L, Bardach A, Palacios A, Peckaitis C, Ciapponi A, Pichón-Riviere A, et al. Health-related quality of life in patients with breast cancer in Latin America and the Caribbean: A systematic review and meta-analysis. Oncologist (2021) 26(5):e794–806. doi: 10.1002/onco.13709
68. Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat (2008) 107(2):167–80. doi: 10.1007/s10549-007-9548-1
69. Stein MJ, Karir A, Arnaout A, Roberts A, Cordeiro E, Zhang T, et al. Quality-of-Life and surgical outcomes for breast cancer patients treated with therapeutic reduction mammoplasty versus mastectomy with immediate reconstruction. Ann Surg Oncol (2020) 27(11):4502–12. doi: 10.1245/s10434-020-08574-8
70. Słowik AJ, Jabłoński MJ, Michałowska-Kaczmarczyk AM, Jach R. Evaluation of quality of life in women with breast cancer, with particular emphasis on sexual satisfaction, future perspectives and body image, depending on the method of surgery. Psychiatr Pol (2017) 51(5):871–88. doi: 10.12740/PP/OnlineFirst/63787
71. Montazeri A, Vahdaninia M, Harirchi I. Quality of life in patients with breast cancer before and after diagnosis: An eighteen months follow-up study. BMC Cancer. (2008) 8:330. doi: 10.1186/1471-2407-8-330
72. Arora NK, Gustafson DH, Hawkins RP, McTavish F, Cella DF, Pingree S, et al. Impact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma: A prospective study. Cancer (2001) 92(5):1288–98. doi: 10.1002/1097-0142(20010901)92:5<1288::aid-cncr1450>3.0.co;2-e
73. Van Esch L, Roukema JA, van der Steeg AF, De Vries J. Trait anxiety predicts disease-specific health status in early-stage breast cancer patients. Qual. Life Res (2011) 20(6):865–73. doi: 10.1007/s11136-010-9830-2
74. Wang XS, Woodruff JF. Cancer-related and treatment-related fatigue. Gynecol. Oncol (2015) 136(3):446–52. doi: 10.1016/j.ygyno.2014.10.013
75. Ebede CC, Jang Y, Escalante CP. Cancer-related fatigue in cancer survivorship. Med Clin North Am (2017) 101(6):1085–97. doi: 10.1016/j.mcna.2017.06.007
76. Mohandas H, Jaganathan SK, Mani MP, Ayyar M, Rohini Thevi GV. Cancer-related fatigue treatment: An overview. J Cancer Res Ther (2017) 13(6):916–29. doi: 10.4103/jcrt.JCRT_50_17
77. Grimison PS, Stockler MR. Quality of life and adjuvant systemic therapy for early-stage breast cancer. Expert Rev Anticancer Ther (2007) 7(8):1123–34. doi: 10.1586/14737140.7.8.1123
78. Coughlin SS, Yoo W, Whitehead MS, Smith SA. Advancing breast cancer survivorship among African-American women. Breast Cancer Res Treat (2015) 153(2):253–61. doi: 10.1007/s10549-015-3548-3
79. Kenne Sarenmalm E, Browall M, Gaston-Johansson F. Symptom burden clusters: A challenge for targeted symptom management. a longitudinal study examining symptom burden clusters in breast cancer. J Pain Symptom. Manage (2014) 47(4):731–41. doi: 10.1016/j.jpainsymman.2013.05.012
80. Bauml J, Chen L, Chen J, Boyer J, Kalos M, Li SQ, et al. Arthralgia among women taking aromatase inhibitors: is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res (2015) 17(1):89. doi: 10.1186/s13058-015-0599-7
81. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: A population-based exploratory analysis. J Clin Oncol (2012) 30(14):1608–14. doi: 10.1200/JCO.2011.37.9511
82. Dee EC, Nipp RD, Muralidhar V, Yu Z, Butler SS, Mahal BA, et al. Financial worry and psychological distress among cancer survivors in the united states, 2013-2018. Support Care Cancer. (2021) 29(9):5523–35. doi: 10.1007/s00520-021-06084-1
83. Knight TG, Deal AM, Dusetzina SB, Muss HB, Choi SK, Bensen JT, et al. Financial toxicity in adults with cancer: Adverse outcomes and noncompliance. J Oncol Pract (2018) 24:JOP1800120. doi: 10.1200/JOP.18.00120
84. Yabroff KR, Dowling EC, Guy GP Jr, Banegas MP, Davidoff A, Han X, et al. Financial hardship associated with cancer in the united states: Findings from a population-based sample of adult cancer survivors. J Clin Oncol (2016) 34(3):259–67. doi: 10.1200/JCO.2015.62.0468
85. Kong YC, Wong LP, Ng CW, Taib NA, Bhoo-Pathy NT, Yusof MM, et al. Understanding the financial needs following diagnosis of breast cancer in a setting with universal health coverage. Oncologist (2020) 25(6):497–504. doi: 10.1634/theoncologist.2019-0426
86. Kim GM, Kim S, Park HS, Kim JY, Nam S, Park S, et al. Chemotherapy-induced irreversible alopecia in early breast cancer patients. Breast Cancer Res Treat (2017) 163(3):527–33. doi: 10.1007/s10549-017-4204-x
87. Konieczny M, Cipora E, Sygit K, Fal A. Quality of life of women with breast cancer and socio-demographic factors. Asian Pac J Cancer Prev (2020) 21(1):185–93. doi: 10.31557/APJCP.2020.21.1.185
88. Shi G, Yu D, Wu J, Liu Y, Huang R, Zhang CS. A systematic review and meta-analysis of traditional Chinese medicine with chemotherapy in breast cancer. Gland Surg (2021) 10(5):1744–55. doi: 10.21037/gs-21-284
Keywords: Asia, breast cancer, health-related quality of life, meta-analysis, systematic review
Citation: Chen X, Wu C, Bai D, Gao J, Hou C, Chen T, Zhang L and Luo H (2022) Health-related quality of life in breast cancer patients in Asia: A meta-analysis and systematic review. Front. Oncol. 12:954179. doi: 10.3389/fonc.2022.954179
Received: 27 May 2022; Accepted: 29 August 2022;
Published: 28 September 2022.
Edited by:
Claudia Mello-Thoms, The University of Iowa, United StatesReviewed by:
Phuong D (Yun) Trieu, The University of Sydney, AustraliaCopyright © 2022 Chen, Wu, Bai, Gao, Hou, Chen, Zhang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Gao, MTk5NDIwMjFAY2R1dGNtLmVkdS5jbg==; Chaoming Hou, OTgzNzI5NDg0QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.