
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 23 September 2022
Sec. Cancer Molecular Targets and Therapeutics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.954026
The epidermal growth factor receptor (EGFR) typically contains an extracellular domain (ECD), a transmembrane (TM) domain, and an intracellular kinase (KD) domain. ECD mutations of EGFR in NSCLC may affect its normal function and intrinsic resistance to tyrosine kinase inhibitors (TKIs) and the effectiveness of drugs for these patients is unsatisfactory. Recently, we found an EGFR T263P mutation located at the ECD, which has never been reported in Chinese non-small cell lung cancer (NSCLC). Hence, we reported that a patient with advanced lung adenocarcinoma harboring the EGFR T263P mutation, L858R mutation and MET amplification was resistant to osimertinib but significantly benefited from erlotinib and capmatinib treatment. This patient achieved a partial response and had progression-free survival (PFS) for more than 19 months. In summary, we are the first researchers to report in detail on a Chinese patient carrying the T263P mutation and summarize all the ECD mutations in NSCLC. We believe this finding will enlighten us to treat patients with EGFR ECD mutations and more patients deserve further study.
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have been proven to be the most effective therapeutic option for advanced EGFR-mutant non-small cell lung cancer (NSCLC) patients (1). Patients with NSCLC harboring common mutations in the EGFR gene, such as L858R, have shown a remarkable response to TKIs (2). EGFR mutations in NSCLC patients mainly occur in the tyrosine kinase (TK) region and rarely in the extracellular domain (ECD) (3). Mutations in the ECD of EGFR have also been successively reported in NSCLC, but little is known about EGFR ECD mutations in lung cancer. EGFR ECD mutations are generally considered to have a poor response to TKIs. Few studies have reported on the treatment of ECD mutations in NSCLC, especially the efficacy of different ECD mutations to TKIs. Thus, we report a patient with lung adenocarcinoma harboring a rare EGFR mutation, EGFR exon 7 T263P, who was resistant to osimertinib but showed astonishing efficacy against erlotinib. Written informed consent was provided by the patient to use case details and the accompanying images for publication.
A 61-year-old female was admitted to our hospital with a severe headache in July 2020. Brain magnetic resonance imaging (MRI) showed space-occupying lesions in the right parietal lobe (Figures 1A, B). Further evaluation with computed tomography (CT) of the chest and whole-body bone scans revealed a lesion in the left lung (Figures 2A, B) and iliac bone metastasis. Fine needle aspiration biopsy of the left supraclavicular lymph nodes showed adenocarcinoma of the lung origin. To identify potentially actionable mutations in the patient, EGFR L858R mutations (allelic fraction, AF=3.37%) in exon 21 were identified by next generation sequencing (NGS, Illumina, NextSeq CN500, Suzhou, China) of the plasma in August 2020. The patient was diagnosed with stage IVB (T1bN2M1) NSCLC, harboring the EGFR the L858R mutation had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 3.
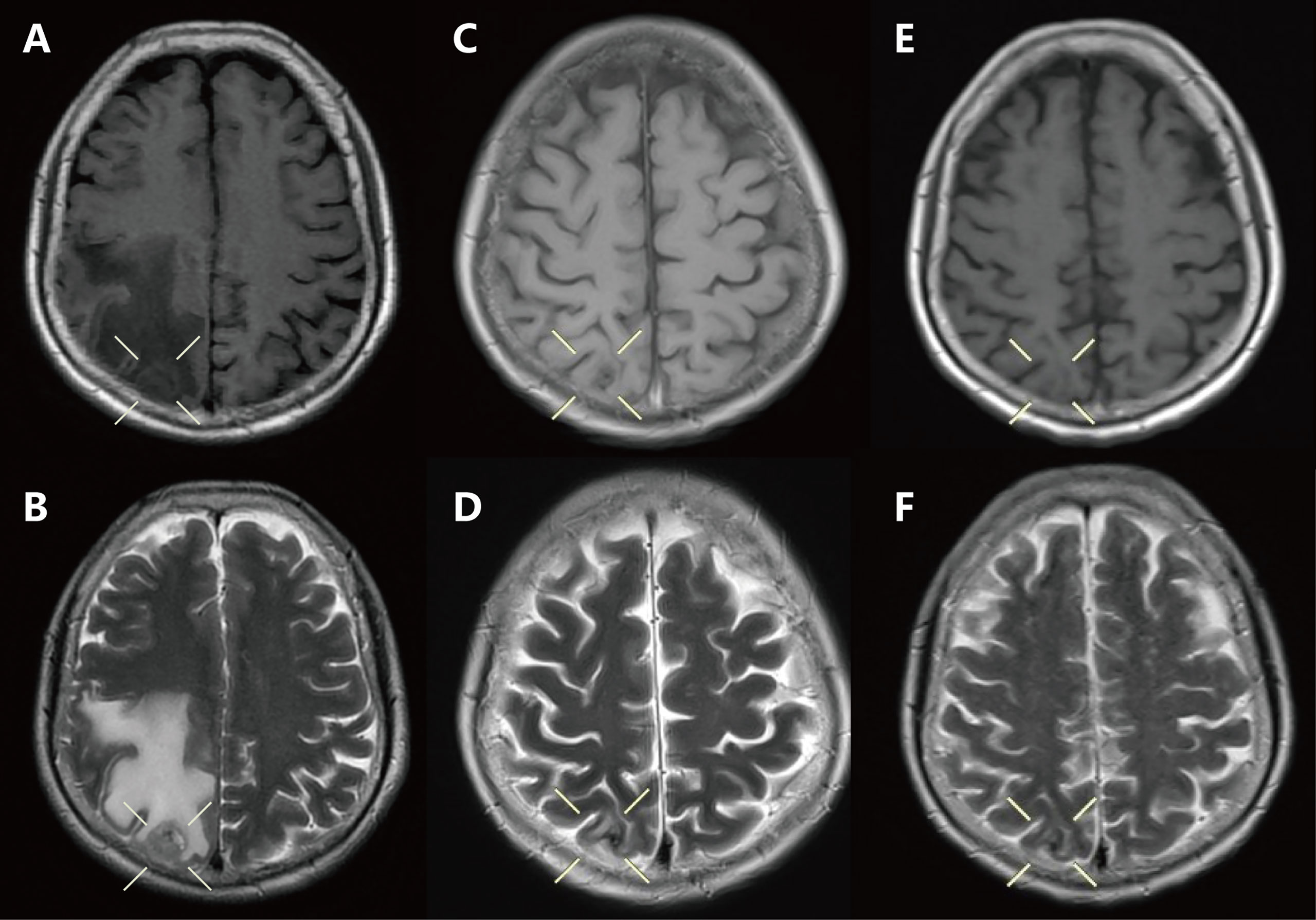
Figure 1 Craniocerebral magnetic resonance imaging of the brain showed: (A, B) Baseline: craniocerebral lesion before treatment. (C, D) Two months: after brain radiotherapy combined with osimertinib. (E, F) Seven months: after the use of erlotinib combined with capmatinib.
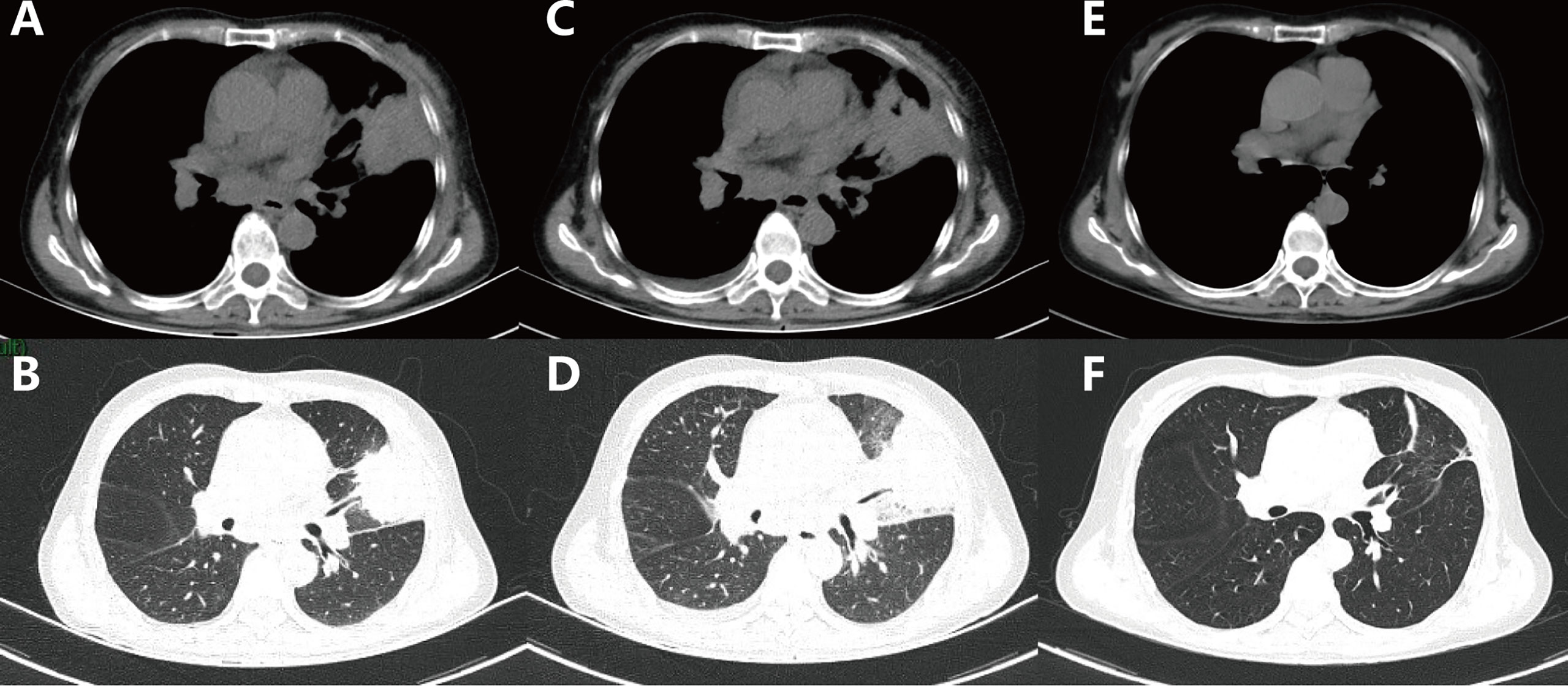
Figure 2 Computed tomography scans of the chest showed the following: (A, B) Baseline: lesion in the left lung before treatment. (C, D) One month after the use of osimertinib. (E, F) Thirteen months after the use of erlotinib combined with capmatinib.
Ten sessions of brain radiotherapy (30 Gy) were performed due to significant headache. At the same time, the patient was administered first-line osimertinib at 80 mg/d. One month later, the patient’s headache was significantly relieved after brain radiotherapy (Figures 1C, D) and the ECOG PS of the patient decreased to 1. Nevertheless, a re-examination of chest computed tomography (CT) showed that the lesion in the left upper lobe was enlarged (Figures 2C, D). Disease progression (PD) was thus considered. Then, the patient discontinued osimertinib and switched to twice traditional chemotherapy (pemetrexed 700 mg and lobaplatin 40 mg). Meanwhile, lung tumor biopsy was performed for the second genetic testing (Illumina, NextSeq 550Dx, Shanghai, China). In addition to the preserved EGFR L858R mutation, the report revealed a new EGFR exon 7 T263P mutation and MET amplification.
In September 2020, the patient refused chemotherapy for severe adverse effects and started taking erlotinib plus capmatinib. One month later, the patient achieved partial response (PR) with significant tumor shrinkage after erlotinib plus capmatinb treatment. In September 2021, chest CT still showed a PR (Figures 2E, F) after 13 months of this treatment. No treatment-related adverse events led to discontinuation. The last follow-up was in February 2022, when the patient remained on the treatment, with a PFS of 19 months and counting (Figures 1E, F).
The advent of EGFR-TKIs has made epoch-making sense for treating patients with advanced NSCLC with sensitive EGFR mutations (4). In the present case, the initial detection of the patient’s blood specimen by NGS only revealed the presence of the EGFR L858R mutation. However, the patient had intrinsic resistance to osimertinib. One month later, an EGFR T263P mutation and MET amplification were detected from the patient’s primary tumor samples by the second NGS testing. To understand the actual EGFR mutation before osimertinib treatment, we tried NGS using the archived specimen. Unfortunately, it was not successful due to insufficient tissue in the supraclavicular lymph nodes. Interestingly, a prospective study observed only a 62.0% by variant concordance rate among primary tumor, metastatic lymph nodes, and plasma, suggesting that there is some deviation between the detection of plasma samples and the detection of primary tumor samples (5). Therefore, the genetic test results of the first blood sample do not reflect the actual mutation status of the patient. Besides, the second NGS platform was not limited to common mutations such as EGFR, MET and ALK, but also included rare mutation sites like EGFR extracellular domains. Combined with the fact that the patient received osimertinib for only 1 month, we speculated that the patient was not a single L858R mutation, but had the EGFR T263P mutation and MET amplification initially, which resulted in intrinsic resistance to osimertinib.
Meanwhile, MET amplification is a well-known mechanism of acquired resistance to EGFR-TKIs, which leads to EGFR-TKI resistance through activation of EGFR-independent ErbB3 phosphorylation and downstream activation of the PI3K/AKT pathway (6). MET and EGFR have overlapping and complementary growth and proliferation pathway activation. A phase I/II clinical study demonstrated that the combination of erlotinib and capmatinib is safe and effective and may help to treat NSCLC patients resistant to first-line therapy and increase the duration of response to TKI (7). The TATTON study also proved that combined EGFR and MET inhibition is reasonable to overcome resistance caused by simultaneous MET amplification (8). Given the patient’s financial situation and her physical condition, we finally chose erlotinib in combination with capmatinib and achieved good results, which also provides a valuable clinical reference for patients with similar compound mutations.
In this case, we report a rare EGFR mutation, EGFR T263P, a missense mutation located on ECD (domain II) that induces the conversion from Thr to Pro at position 263 encoded at EGFR exon 7. Previously, a series of missense mutations in the ECD of EGFR have been reported in cases of colorectal cancer, glioma and neuroblastoma. This is the first reported detailed clinical report of EGFR T263P mutations in patients with NSCLC. The majority of studies indicated that the T263P mutation might be sensitive to second-generation TKIs, such as afatinib, because afatinib is an irreversible ErbB family blocker(EGFR、HER-2、HER3 and HER4)that potently inhibits signaling from all ErbB family receptor homodimers and heterodimers (9, 10). Thanh et al. found that a Vietnamese NSCLC patient with the T263P mutation who received first-line afatinib treatment could achieved a mTTF of 10.8 months (11). However, Ba/F3 cells carrying the T263P variant in glioblastoma were also reported to be oncogenic and sensitive to the first-generation TKI, erlotinib (12). Similarly, the M277E mutation, which is also an EGFR extracellular region mutation, had dramatic antitumor responses to erlotinib treatment and could cause EGFR autophosphorylation in the absence of the EGFR ligand EGF, thus promoting tumor development (13).
According to a large retrospective study, ECD mutations account for only 0.36% (36/10100) of NSCLC and are more likely to co-occur with EGFR KD mutations, especially L858R (14). Thus, we summarized the ECD mutations that have been reported thus far in the literature on NSCLC (Figure 3) (15–18). The response of different ECD mutations to different EGFR-TKIs remains controversial. EGFR ECD mutations are generally considered to have a poor response to TKIs. Leilei et al. reviewed 21 patients resistant to TKI and found that one-third of NSCLC patients harbor ECD mutations (16). In contrast, some ECD mutations may also not affect the efficiency of the targeted therapy. The EGFR A289V mutation in NSCLC received the first-generation EGFR-TKIs (Icotinib) treatment and obtained the efficacy of PR for more than 5-month (19). Another patient in stage IV NSCLC with rare EGFR M277E mutation and high expression of PD-L1 had a 5 months PFS after first-line erlotinib and radiation treatment (20). Our case is the third real clinical case that demonstrates the effectiveness of EGFR ECD for first-generation TKI therapy.
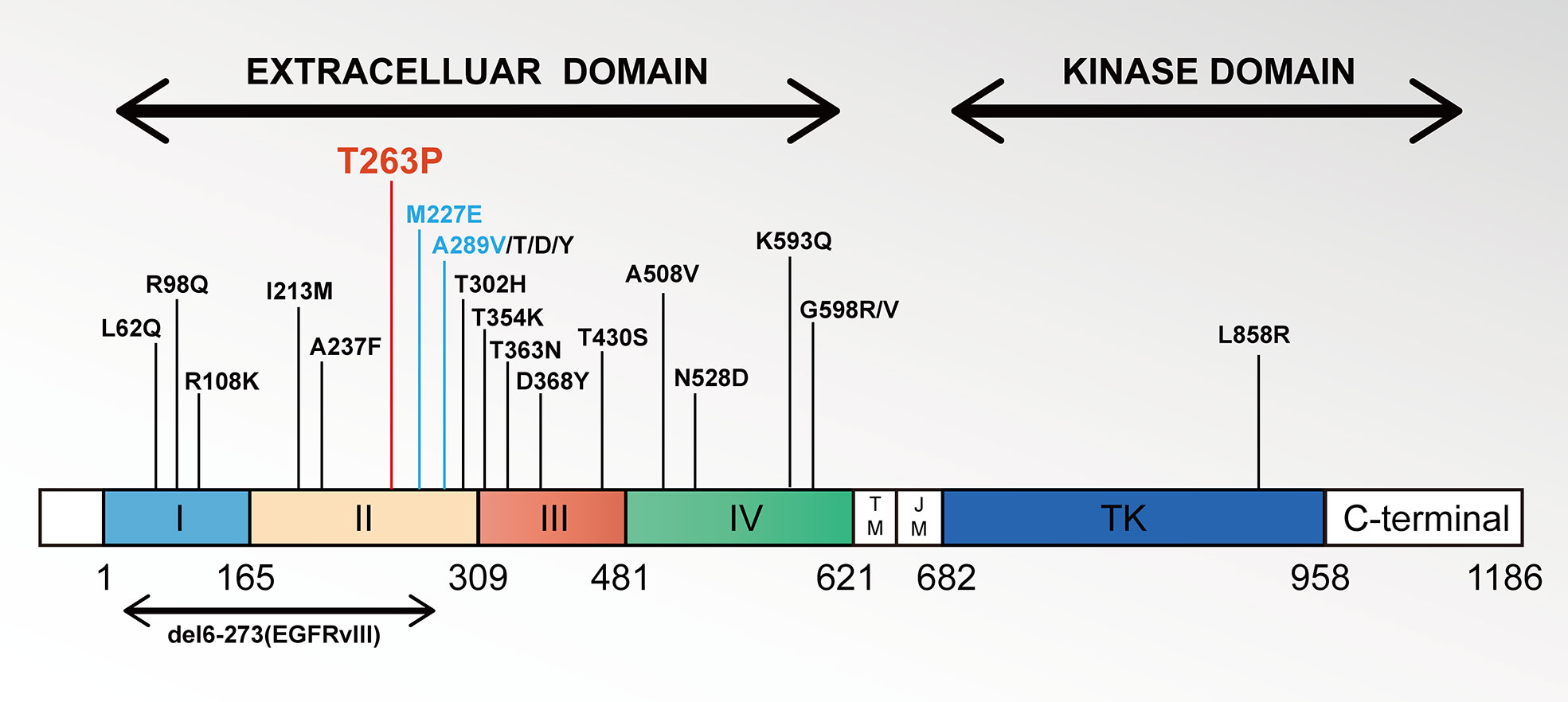
Figure 3 Schematic representations of EGFR gene extracellular domain mutations in NSCLC. Notes: Locations of ECD missense mutations are given within the EGFR gene found in the literature until now. Each vertical bar represents one sample harboring the indicated mutation.
In conclusion, we described a Chinese NSCLC patient with a rare EGFR T263P mutation, L858R mutation and MET amplification who achieved good efficacy with erlotinib and capmatinib, with a PFS of more than 19 months. EGFR ECD mutations, such as the T263P mutation may contributing to resistance to osimertinib. Combined with the related literature, we considered erlotinib to be efficacious for the EGFR T263P mutation with the L858R mutation, but afatinib may also be a promising choice. This finding will enlighten us to treat patients with EGFR ECD mutations and further investigate treatment strategies.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Affiliated Hospital of Nanchang University. The patients/participants provided written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
(I) Conception and design: YL. (II) Administrative support: YW and XZ. (III) Provision of study materials or patients: CF and XQ. (IV) Collection and assembly of data: QW. All authors contributed to the article and approved the submitted version.
This research was supported by grants from the National Natural Science Foundation of China (No.81560379, 81460292, 81660315), and the Surface Project of the Natural Science Foundation of Jiangxi Province (No.20181BAB205046, No.20202BAB216031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
EGFR, Epidermal growth factor receptor; ECD, extracellular domain; TM, transmembrane; KD, intracellular kinase; TKIs, tyrosine kinase inhibitors; NSCLC, non-small cell lung cancer; PFS, progression-free survival.
1. Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement of the college of American Pathologists/International association for the study of lung Cancer/Association for molecular pathology clinical practice guideline update. J Clin Oncol (2018) 36(9):911–9. doi: 10.1200/JCO.2017.76.7293
2. Cho BC, Chewaskulyong B, Lee KH, Dechaphunkul A, Sriuranpong V, Imamura F, et al. Osimertinib versus standard of care EGFR TKI as first-line treatment in patients with EGFRm advanced NSCLC: FLAURA Asian subset. J Thorac Oncol (2019) 14(1):99–106. doi: 10.1016/j.jtho.2018.09.004
3. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer (2007) 7(3):169–81. doi: 10.1038/nrc2088
4. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol (2011) 12(8):735–42. doi: 10.1016/S1470-2045(11)70184-X
5. Tang Y, Liu X, Ou Z, He Z, Zhu Q, Wang Y, et al. Maximum allele frequency observed in plasma: A potential indicator of liquid biopsy sensitivity. Oncol Lett (2019) 18(2):2118–24. doi: 10.3892/ol.2019.10490
6. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med (2011) 3(75):26r–75r. doi: 10.1126/scitranslmed.3002003
7. McCoach CE, Yu A, Gandara DR, Riess JW, Vang DP, Li T, et al. Phase I/II study of capmatinib plus erlotinib in patients with MET-positive non-Small-Cell lung cancer. JCO Precis Oncol (2021) 1:177–90. doi: 10.1200/PO.20.00279
8. Sequist LV, Han JY, Ahn MJ, Cho BC, Yu H, Kim SW, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol (2020) 21(3):373–86. doi: 10.1016/S1470-2045(19)30785-5
9. Lee CK, Kim S, Lee JS, Lee JE, Kim SM, Yang IS, et al. Next-generation sequencing reveals novel resistance mechanisms and molecular heterogeneity in EGFR-mutant non-small cell lung cancer with acquired resistance to EGFR-TKIs. Lung Cancer (2017) 113:106–14. doi: 10.1016/j.lungcan.2017.09.005
10. Hirsh V. Next-generation covalent irreversible kinase inhibitors in NSCLC: Focus on afatinib. Biodrugs (2015) 29(3):167–83. doi: 10.1007/s40259-015-0130-9
11. Vu TH, Nguyen H, Dao LK, Duong CK, Nguyen CV, Doan TT, et al. Effectiveness and tolerability of first-line afatinib for advanced EGFR-mutant non-small cell lung cancer in Vietnam. Asian Pac J Cancer Prev (2021) 22(5):1581–90. doi: 10.31557/APJCP.2021.22.5.1581
12. Lee JC, Vivanco I, Beroukhim R, Huang JH, Feng WL, DeBiasi RM, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PloS Med (2006) 3(12):e485. doi: 10.1371/journal.pmed.0030485
13. Yu S, Zhang Y, Pan Y, Cheng C, Sun Y, Chen H. The non-small cell lung cancer EGFR extracellular domain mutation, M277E, is oncogenic and drug-sensitive. Onco Targets Ther (2017) 10:4507–15. doi: 10.2147/OTT.S131999
14. Gu. D, Zhao. J, Guo R. EGFR extracellular domain mutation in patients with lung cancer. J Clin Oncol (2019) 37(15_suppl):e20532. doi: 10.1200/JCO.2019.37.15_suppl.e20532.
15. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results from a phase II basket trial. J Clin Oncol (2018) 36(24):2532–7. doi: 10.1200/JCO.2018.77.9777
16. Lei L, Wang WX, Zhu YC, Li JL, Fang Y, Wang H, et al. Potential mechanism of primary resistance to icotinib in patients with advanced non-small cell lung cancer harboring uncommon mutant epidermal growth factor receptor: A multi-center study. Cancer Sci (2020) 111(2):679–86. doi: 10.1111/cas.14277
17. Illei PB, Belchis D, Tseng LH, Nguyen D, De Marchi F, Haley L, et al. Clinical mutational profiling of 1006 lung cancers by next generation sequencing. Oncotarget (2017) 8(57):96684–96. doi: 10.18632/oncotarget.18042
18. Stein MK, Morris L, Sullivan JL, Fenton M, VanderWalde A, Schwartzberg LS, et al. Expanding the search for significant EGFR mutations in NSCLC outside of the tyrosine kinase domain with next-generation sequencing. Med Oncol (2017) 34(7):126. doi: 10.1007/s12032-017-0985-3
19. Dai L, Su X, Lu L, Lv D. Nonsmall cell lung cancer with rare exon 7 p.A289V mutation in the EGFR gene responds to icotinib treatment: A case report. Med (Baltimore) (2018) 97(51):e13809. doi: 10.1097/MD.0000000000013809
Keywords: EGFR extracellular domain mutations, exon 7 T263P mutation, tyrosine kinase inhibitors, erlotinib (ELTN), adenocarcinoma of the lung
Citation: Wang Q, Wang Y, Zhang X, Fang C, Qian X and Li Y (2022) Efficacy of erlotinib in NSCLC harboring rare EGFR extracellular domain mutation (T263P) and common mutations: Case report and literature review. Front. Oncol. 12:954026. doi: 10.3389/fonc.2022.954026
Received: 26 May 2022; Accepted: 29 August 2022;
Published: 23 September 2022.
Edited by:
Nobuyuki Koyama, Saitama Medical Center, JapanReviewed by:
Hiroyuki Kyoyama, Saitama Medical University, JapanCopyright © 2022 Wang, Wang, Zhang, Fang, Qian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Li, bGl5b25nY3Njb0BlbWFpbC5uY3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.