- 1Department of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai Minimally Invasive Surgery Center, Shanghai, China
Background: Although neoadjvuant chemoradiotherapy (CRT) improves the local control rate of locally advanced rectal cancer (LARC), it fails to significantly improve disease-free survival (DFS) and overall survival (OS). We explored the efficacy of prolonged neoadjuvant chemotherapy (pNCT) without radiation and compared this schema with total neoadjuvant therapy (TNT).
Material and methods: Patients diagnosed with LARC and received TNT (4 cycles of induction CapeOX/FOLFOX followed with CRT) or pNCT (6~8 cycles of CapeOX/FOLFOX) between June 2016 and October 2021 were retrospective analyzed. All patients underwent total mesorectal excision (TME). A 1:1 propensity score match was performed to adjust baseline potential confounders. The tumor response, toxicity, recurrence-free survival (RFS) and OS were observed.
Results: A total of 184 patients with 92 patients in each group were finally enrolled. The median follow-up time was 35 months. TNT showed better pathological complete response (pCR) rate (25.0% vs 16.3%) and objective regression rate (73.9% vs 59.8%) than pNCT. TNT and pNCT produce similar 3-year RFS and OS rates in patients with mid-to-upper rectal cancer. TNT was associated with improved tumor responsiveness in all patients and improved 3-year RFS rates in those with low rectal cancer.
Conclusion: pNCT is an option for patients with mid-to-upper rectal cancer, but radiation is still necessary for low rectal cancer. To determine optimal schema for neoadjuvant therapy and patient selection, additional randomized controlled studies are needed.
1 Introduction
Neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME) and adjuvant systemic chemotherapy comprise the general paradigm of locally aggressive rectal cancer (LARC) treatment. Although the treatment strategy improves the local control of disease (1–4), it fails to significantly improve disease-free survival (DFS) and overall survival (OS) (5). Distant recurrence remains the leading cause of death for patients and is inadequately controlled by the current treatment mode (6, 7).
Recent evidence suggests that neoadjuvant chemotherapy may be used for controlling distant recurrence. Combined chemotherapy and chemoradiation, referred to as total neoadjuvant therapy (TNT), is used worldwide, and several high-quality trials showed that TNT improves oncological outcomes in two aspects (8–11). First, short-term recurrence rates decreased in those who underwent TNT, especially at 3-year follow up. Second, pathological complete response (pCR) rates increased after treatment with TNT, with the therapy nearly doubling pCR rates compared with CRT.
In recent years, use of neoadjuvant chemotherapy alone was reported to result in promising survival outcomes (12, 13). The FORWARC (13) study showed that 3-year DFS rates in those undergoing mFOLFOX6 with and without routine radiation did not significantly differ, and the elimination of radiation was unlikely to increase local recurrence risk after R0/1 resection. Especially in low-risk patients whose response to chemotherapy was good, the need for radiotherapy remains unclear. Findings of the trials have the potential to update clinical practice guidelines regarding the use of radiotherapy. To date, the optimal use of neoadjuvant chemotherapy and its target patient population remains controversial. The ideal way to maintain a balance between the benefits of TNT and overtreatment is of particular importance, and is likely to be debated well into the future.
In this study, we retrospectively reviewed records of 257 patients who were diagnosed with resectable LARC and treated with either TNT or prolonged neoadjuvant chemotherapy (pNCT) without radiation. We aimed to assess the safety, efficacy, and survival outcomes of pNCT versus TNT in patients with baseline resectable LARC.
2 Material and methods
2.1 Study population
This retrospective study utilized the data of consecutive patients who underwent TNT/pNCT followed with radical surgery for rectal cancer between June 2016 and October 2021 at Ruijin Hospital, Shanghai, China. Patients were randomly assigned to receive TNT or pNCT and were staged using preoperative imaging, including enhanced magnetic resonance imaging (MRI) and computed tomography (CT). Other clinical data were obtained from the patients’ medical history at Ruijin Hospital.
Patients meeting the following eligibility criteria were included: (I) aged between 18 and 80 years; (II) diagnosis of rectal adenocarcinoma via colonoscopy and a pathological examination (the lower tumor edge within 12 cm of the anal verge); (III) underwent TNT or pNCT, and (IV) postoperative pathological results showing R0 resection. Exclusion criteria were as follows: (I) the presence of concomitant malignant disease, (II) history of malignant disease, (III) failure to complete planned cycles of neoadjuvant or adjuvant treatment, and (IV) unresectable tumors or difficult to get R0 resection after neoadjuvant therapy.
2.2 Treatment
2.2.1 Neoadjuvant therapy and adjuvant therapy
All patients received oxaliplatin- and fluorouracil-based neoadjuvant chemotherapy (mFOLFOX6 or CapeOX). The mFOLFOX6 regimen consisted of an intravenous infusion of oxaliplatin (85 mg/ m2) followed by leucovorin (400 mg/m2), an intravenous bolus of 5-FU (400 mg/m2), and a continuous intravenous infusion of 5-FU (2,400 mg/m2) for 2 days. The CapeOX regimen consisted of intravenous infusion of oxaliplatin (130 mg/m2). Capecitabine (1,000 mg/m2) was orally administered twice daily for 14 days. The pNCT group received six to eight cycles of chemotherapy. After surgery, patients diagnosed with pathological stage III or high-risk stage II rectal adenocarcinoma received adjuvant therapy similar to the preoperative treatment for an additional four cycles. The high-risk factors included: CRM<1mm, ypN2/N1c, poor mesorectal quality, poor tumor differentiation.
2.2.2 Synchronous chemoradiotherapy
The TNT group received CRT after four cycles of induction chemotherapy. Patients received 50 Gy radiation throughout 5 weeks (2 Gy five times per week). During radiotherapy, continuous oral capecitabine was administered twice daily, on days 1–14 and 22–35. This procedure was performed by specific radiation oncologists.
2.3 Surgery and pathological examination
CT and enhanced rectal MRI imaging were repeated after preoperative treatment. Surgery was performed if the tumor was considered resectable. In the pNCT group, the median interval between the last treatment and surgery was 2 week (range, 2-3 weeks). In the TNT group, the median interval between CRT and surgery was 6 weeks (range, 4-6 weeks), with one cycle of chemotherapy during the interval. All included patients underwent laparoscopic TME with R0 resection. The surgical specimens were examined by pathologists from the Department of Pathology. Pathological features such as ypT stage, ypN stage, tumor differentiation, and tumor response were determined via routine methods, and a mismatch repair status (MMR) test was performed, if necessary. The radiological response to neoadjuvant therapy was evaluated based on response evaluation criteria for solid tumors (RECIST v1.1).
2.4 Post-treatment surveillance
All patients were followed-up every 3 months for the first year after surgery, and every 6 months for the next 4 years. Enhanced CT scans (chest, abdomen, and pelvis), serum tests for tumor markers (CEA, CA19-9, CA125, and CA242), and colonoscopies were performed every six months. Survival outcomes and the recurrence status of patients were also noted. Recurrence-free survival (RFS) was defined as the time between the end of treatment and date of recurrence. OS was defined as the time from surgery to the date of all-cause death.
2.5 Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics version 26 (IBM Corporation, Armonk, NY, USA) or R version 4.1.3. Propensity scores matching (PSM) at a ratio of 1:1 was completed using SPSS. The chi-square test was used to compare categorical variables, and continuous variables were analyzed using the Student’s t-test. Kaplan–Meier curves were used to analyze survival and recurrence. A Cox regression model was used to calculate hazard ratios of OS and RFS. Survival curves and forest plots were constructed using the R packages survminer, forestmodel and forestplot. P-values were two-sided, and P < 0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
A total of 257 patients with LARC who underwent TNT or pNCT from April 2016 to August 2021 were enrolled in this study (Figure 1). A total of 229 patients were included after screening, with 131 and 98 being treated with pNCT and TNT, respectively. Among the excluded patients, 4 and 2 patients in the pNCT and TNT groups (2.96% vs 1.72%), respectively, were excluded due to unsatisfied tumor response, and underwent additional treatment later (Supplementary Table1). Finally, 92 patients of each group were studied after a 1:1 PSM. Baseline clinical characteristics of patients before and after PSM are shown in Table 1. Overall, 176 of 184 patients (96%) had cT3 and cT4 tumors, and 174 of 184 patients (95%) had clinically involved lymph nodes. The mean distance to the anal verge was 5.95 cm, and 71 of 184 patients (39%) had low rectal cancer (within 5 cm from the anal verge).
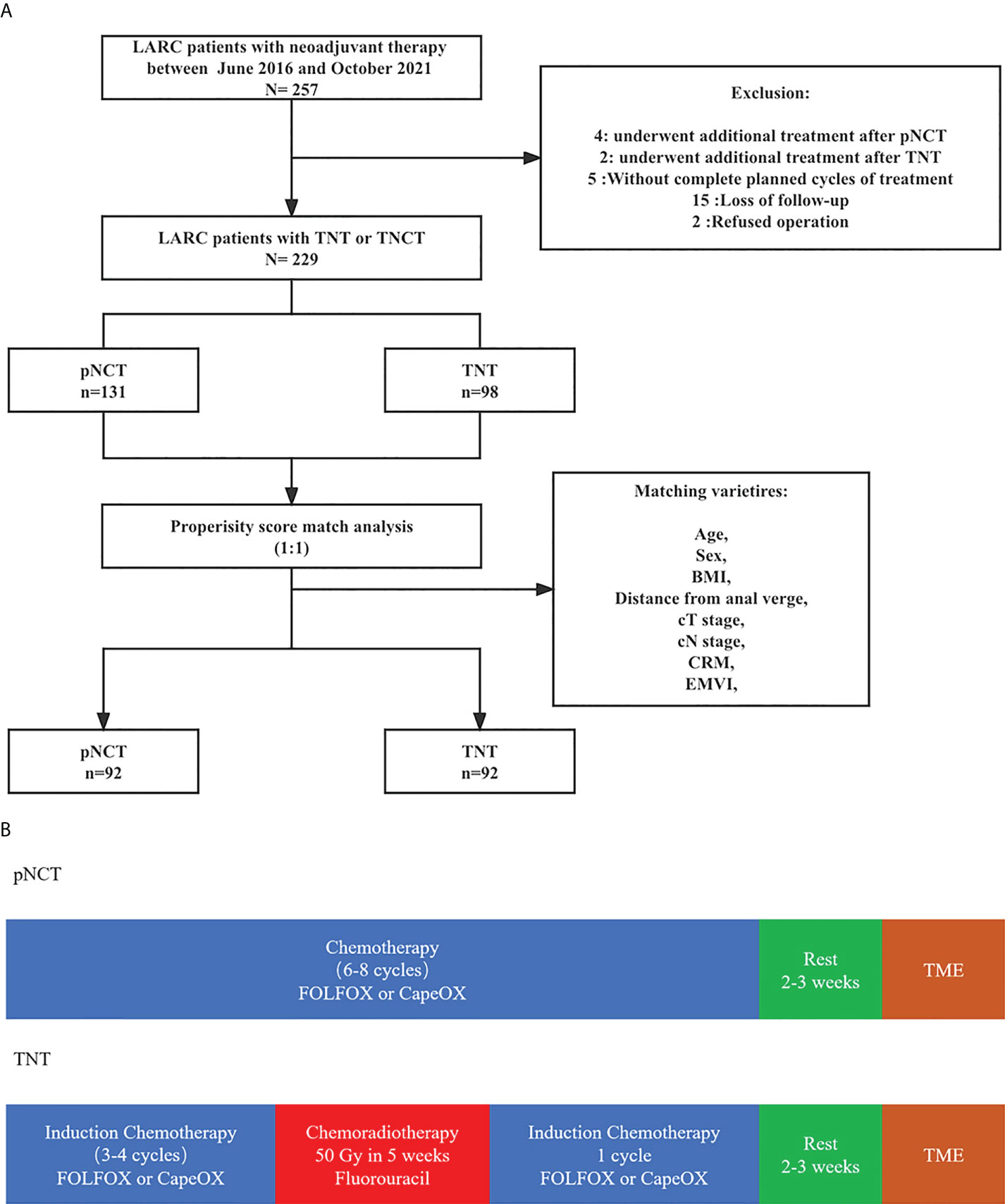
Figure 1 The diagram of total study (A) Neoadjuvant treatment; (B) Study population.The diagram of total study (A) Neoadjuvant treatment; (B) Study population. pNCT prolonged neoadjuvant chemotherapy; TNT, total neoadjuvant therapy; BMI, Body mass index; CRM, circumference resection margin; EMVI, extramural vascular invasion; TME, total mesorectal excision.
3.2 Pathology staging and response to chemotherapy
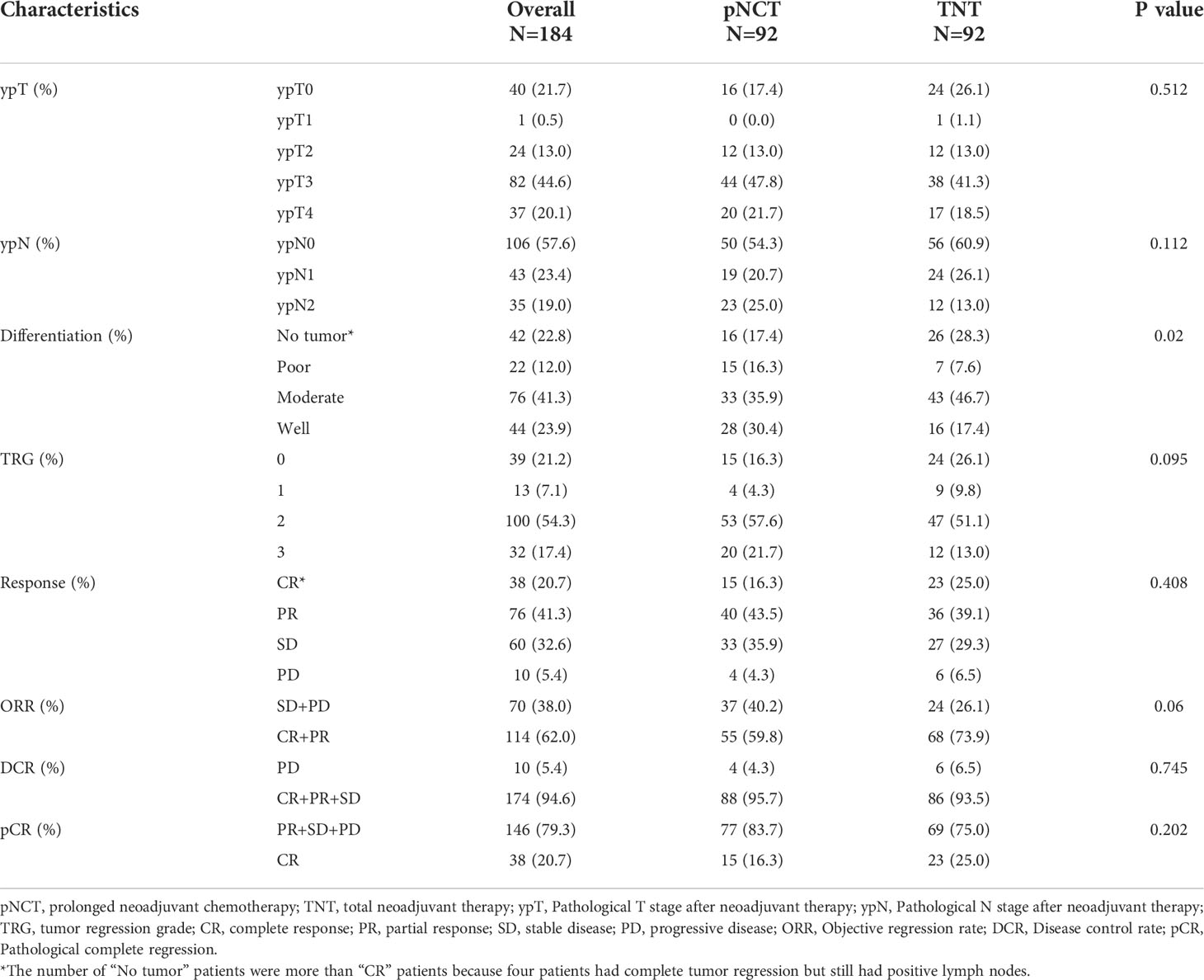
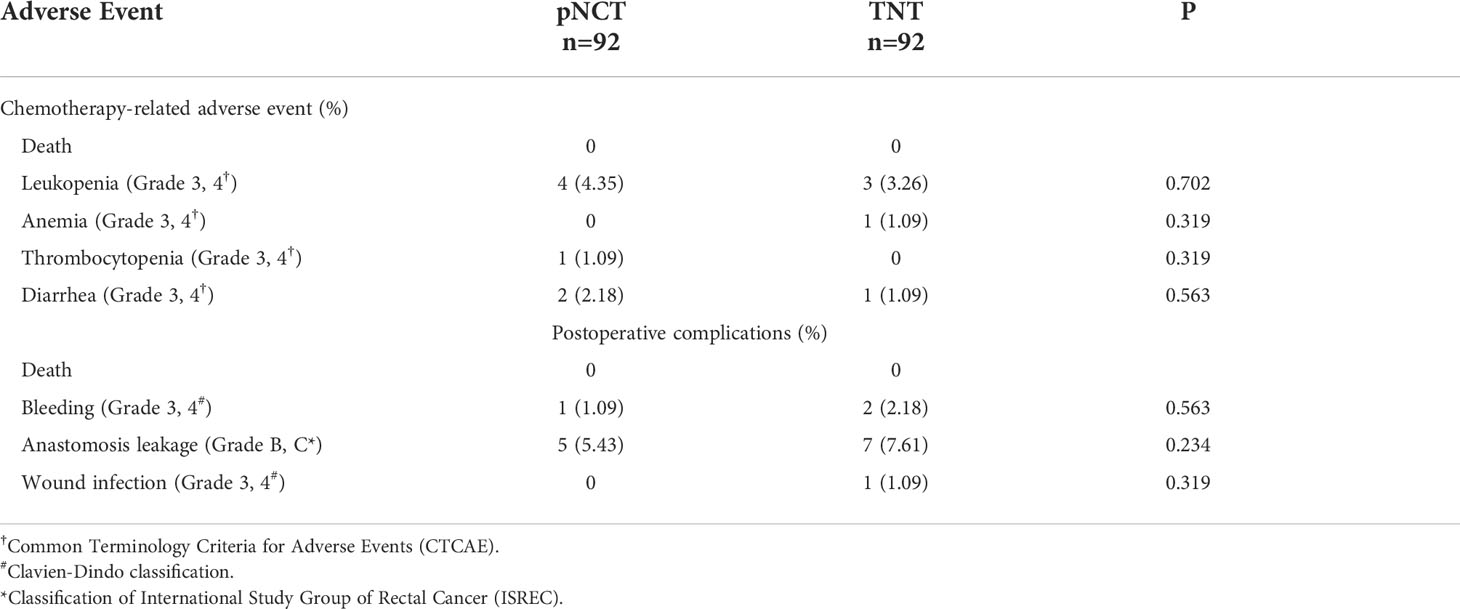
All patients underwent laparoscopic TME and a pathological examination to evaluate their responsiveness to treatment preoperatively. Similar ypT and ypN stages were observed after both TNT and pNCT. TRG findings revealed that the TNT group had a higher rate of TRG0 and TRG1 than the pNCT group; however, the difference was not statistically significant (p = 0.095, Table 2). Patients in the TNT group had higher pathological complete response (pCR; 25.0% and 16.3%, respectively) and objective regression rates (73.9% and 59.8%, respectively) than those of the pNCT group. TNT and pNCT groups showed promising disease control rates (93.5% and 95.7%, respectively). As for toxicity, no patient died of chemotherapy-related adverse events. Grade 3/4 adverse events in preoperative chemotherapy and postoperative hospitalization were rare in both groups (Table 3). The most common severe adverse event was leukopenia, which was similar between the two groups (4 in pNCT vs. 3 in TNT, p=0.702).
3.3 Surgical outcomes and survival
The permanent diversion rate was 20.65% (19/92) and 27.17% (25/92) in the pNCT and TNT group, and the temporary stoma rate was 29.35% (27/92) and 26.09% (24/92), respectively. Anastomosis bleeding and leakage were the most common short-term complications. Five patients in pNCT group and seven patients in TNT group suffered from grade 3/4 anastomosis leakage and showed no significant difference between two groups (5.43 vs. 7.61, p=0.234).
The median follow-up period was 35 months (5–64 months), with that for the pNCT and TNT groups being 34.5 months (9–61 months) and 36 months (5–64 months), respectively. As shown in Figure 2, 6 of 92 patients of the pNCT group (6.5%) and 8 (8.7%) of the TNT group died. Recurrence was reported in 40 (43.5%) and 29 (31.5%) patients of the pNCT and TNT groups, respectively (Table 4). Among these patients, local recurrence events were reported in 11 (12.0%) and 5 (5.4%) patients of the pNCT and TNT groups, respectively, and distant metastasis was reported in 30 (33.6%) and 26 (28.3%) patients.
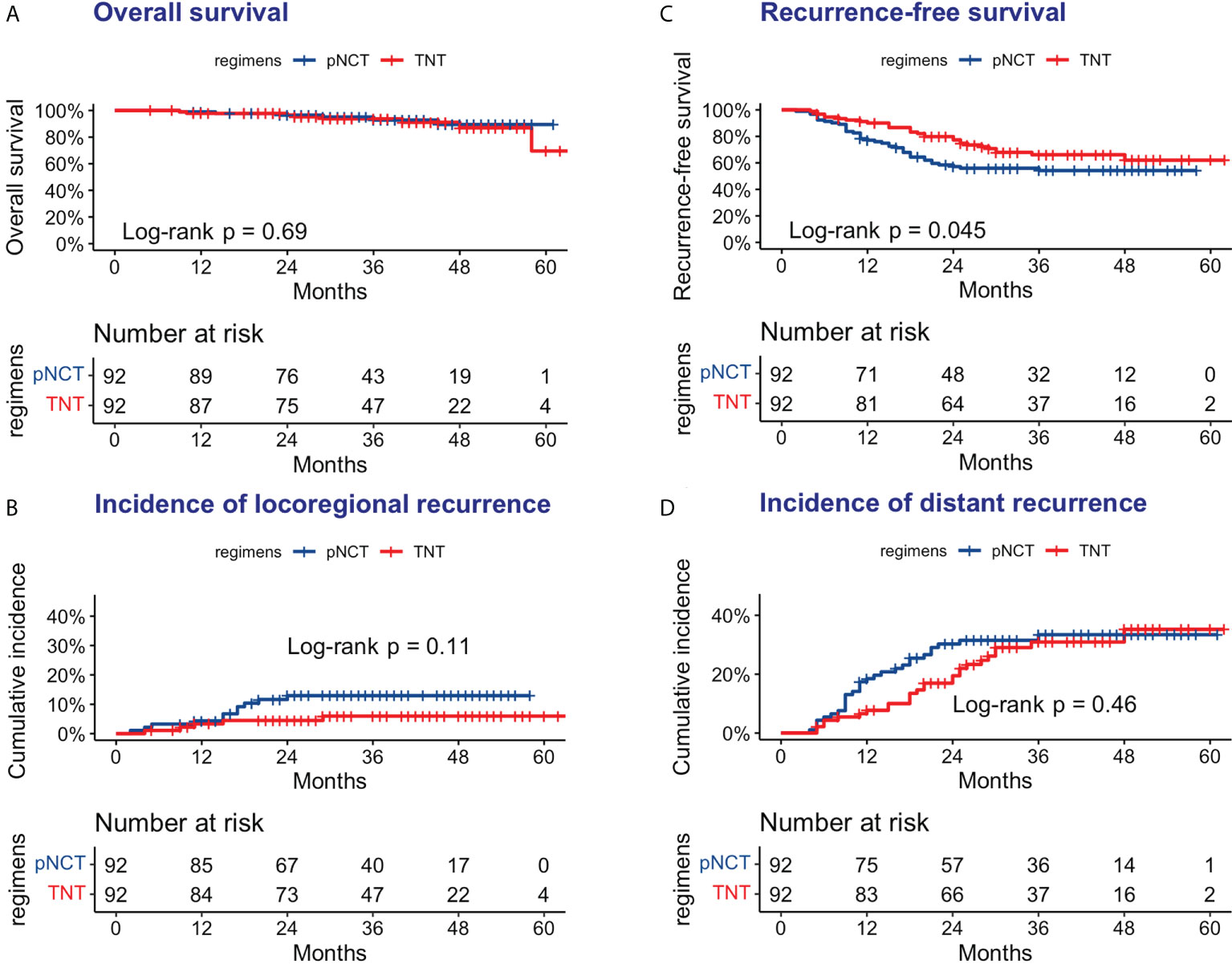
Figure 2 Oncologic outcomes of pNCT group and TNT group (A) Overall survival (B) Disease-free survival; (C) Cumulative incidence of locoregional recurrence; (D) Cumulative incidence of distant metastases. pNCT prolonged neoadjuvant chemotherapy; TNT total neoadjuvant therapy.
Subgroup analysis of survival outcomes showed that low rectal cancer (within 5 cm of the anal verge) was a strong indicator of TNT (P <0.01, Figure 3), while other factors assessed failed to show statistical significance. We further investigated survival outcomes of patients with and without low rectal cancer. Results showed that for patients with low rectal cancer, better RFS was associated with the use of TNT; however, this result was not observed in patients with mid-to-upper rectal cancer (Figure 4). In univariable and multivariable analysis, effects of these factors on RFS and OS were assessed (Table 5). Worsened RFS was associated with pNCT, cT4, ypT4, ypN2, and unfavorable tumor response, while ypT4 (HR, 2.00; CI,1.20–3.50) and ypN2 (HR,2.20; CI, 1.30–3.60) were independent risk factors.
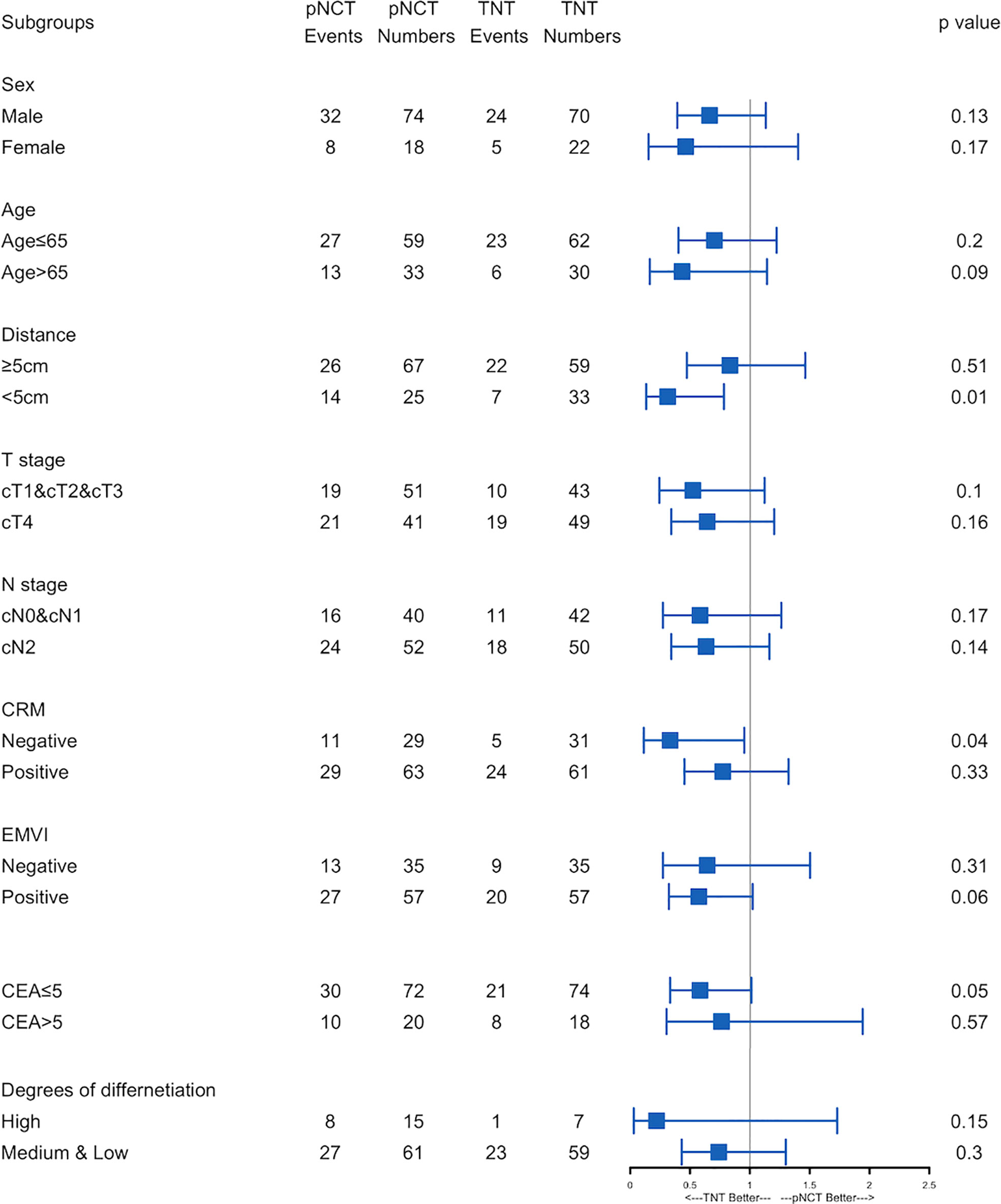
Figure 3 Forest plot of recurrence-free survival.Forest plot of recurrence-free survival. Subgroups analyses of recurrence-free survival was performed. pNCT, prolonged neoadjuvant chemotherapy; TNT, total neoadjuvant therapy; CRM, circumference resection margin; EMVI, extramural vascular invasion; ypT, Pathological T stage after neoadjuvant therapy; ypN, Pathological N stage after neoadjuvant therapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
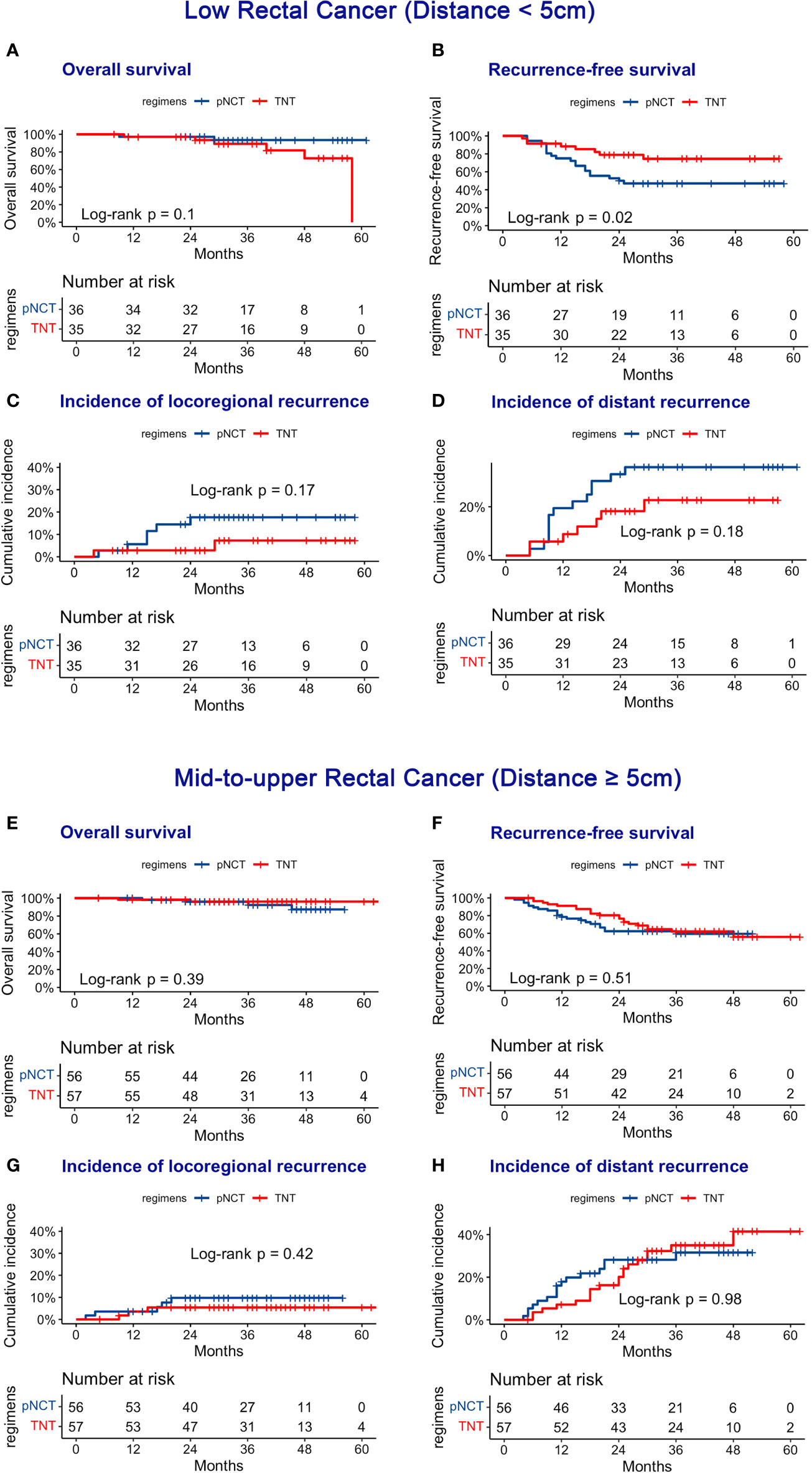
Figure 4 Oncologic Outcomes of pNCT and TNT in subgroup. Subgroup was divided according to the distance between tumor and anal verge. (A) Overall survival (B) Disease-free survival; (C) cumulative incidence of locoregional recurrence; (D) cumulative incidence of distant metastases in low group (distance <5 cm). E Overall survival (F) Disease-free survival; (G) cumulative incidence of locoregional recurrence; (H) cumulative incidence of distant metastases in mid-to-upper group (distance ≥5 cm).
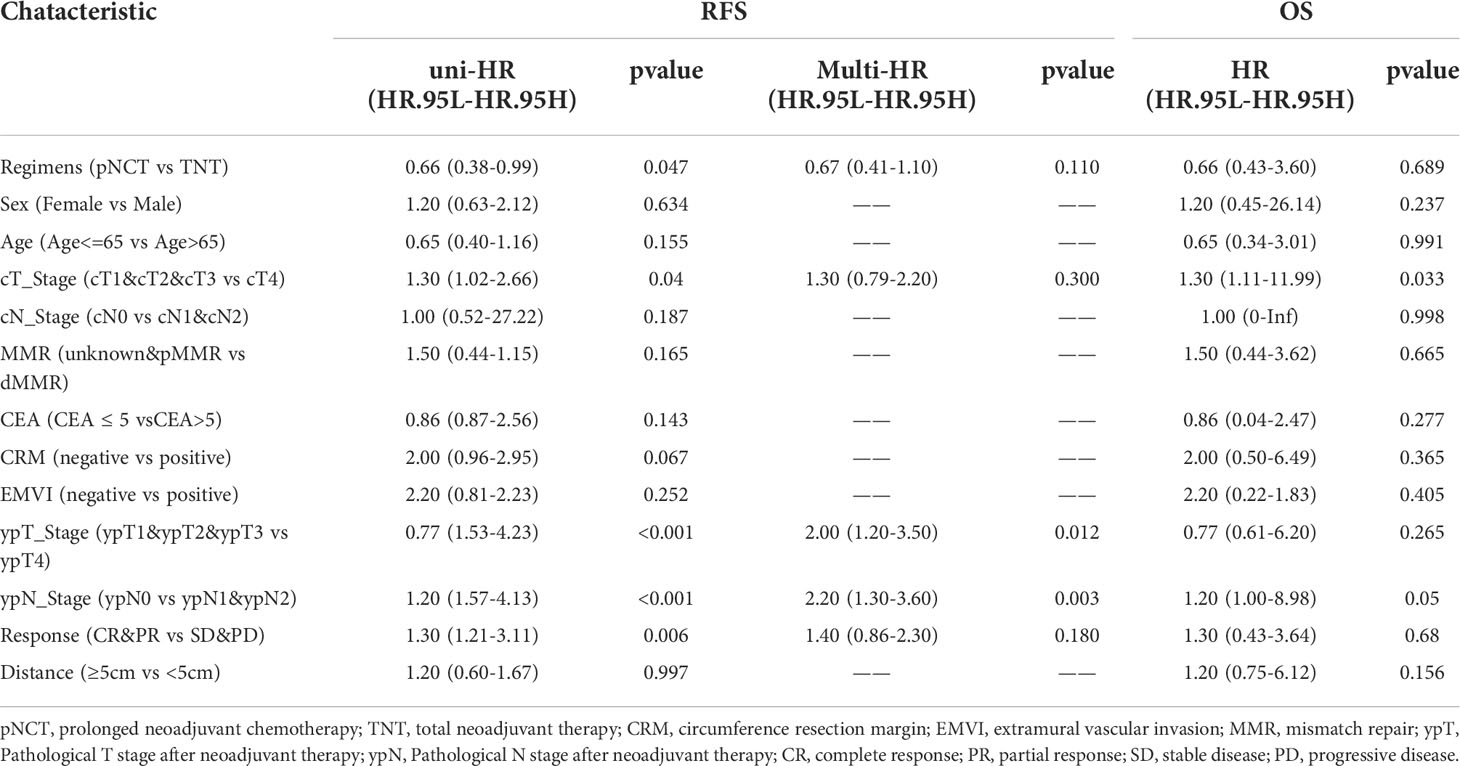
Table 5 Univariable and multivariable analysis of the effects of prognostic factors on recurrence-free survival and overall survival.
4 Discussion
Recently, TNT has been widely used to treat patients with LARC, which results in promising survival outcomes. One of the advantages of TNT is that it allows for the early use of systemic chemotherapy, which may improve the efficiency by which micro-metastases at early stages of tumor development are targeted (14, 15). However, with increasing focus on neoadjuvant chemotherapy, the value of preoperative radiation, especially in patients with initially resectable tumors that undergo high-quality TME surgery, is under question. Recently, several randomized studies have reported non-inferior survival outcomes in those receiving neoadjuvant chemotherapy without radiation (12, 13, 16, 17), which has prompted a reexamination of the significance of preoperative radiation.
In our study, we compared prolonged neoadjuvant chemotherapy without radiation to TNT composed of induction CapeOX/FOLFOX and CRT. All patients underwent R0 resection, as confirmed via a pathological examination. Results showed that the pCR and 3-year RFS rates of the TNT group were longer than those of the pNCT group; however, TNT failed to improve survival in those with middle and high rectal cancer. This result showed that TNT may related with improved tumor responsiveness and promising survival outcomes; however, preoperative radiation might not be necessary for initially resectable mid-to-upper rectal cancer.
Previous studies have reported that although neoadjuvant radiotherapy may improve the pCR rate, it fails to improve the prognosis. Moreover, radiation may damage normal tissue adjacent to the tumor, a process that may be related to several complications, including radiation-induced rectal injury, anastomosis leakage, sexual dysfunction, and bowel dysfunction (18, 19). Recently, several prospective clinical studies have assessed the effectiveness of radiotherapy-free regimens. The FOWARC trial compared the following treatment regimens: neoadjuvant mFOLFOX6 alone, fluorouracil plus radiotherapy, and mFOLFOX6 plus radiotherapy. Results showed that outcomes of those undergoing mFOLFOX6 with or without radiation and fluorouracil with radiation did not significantly differ (13). Deng et al. (16) assessed outcomes in those given neoadjuvant CapeOX alone, reporting that promising tumor response rates were observed. Zhang et al. (12) administered neoadjuvant chemotherapy alone with triplet regimens of mFOLFOXIRI, which also produced similar oncologic outcomes. On the other hand, it is still not clear whether all patients with LARC need chemoradiotherapy. The QuickSilver trial studied MRI-predicted good prognosis rectal patients, and suggested that CRT might not be necessary for stage II and III rectal cancer (20). The prospective multicenter OCUM trial compared surgery alone versus neoadjuvant CRT, and showed a better DFS and OS for high-risk patients (CRM+ or lower third rectal cancer) (21).These studies indicated that the application of radiotherapy needs more accurate selection of patients.
Our study shows that chemotherapy alone produces similar RFS and OS in patients with middle and high rectal cancer; however, chemoradiotherapy remained necessary in those undergoing low rectal cancer treatment. The following three explanations for this result are possible. First, the lower pelvic cavity is narrower than the upper pelvic cavity. Performing lower rectal cancer radical resection of the complete mesorectum with a sufficiently resection margin is difficult. In these cases, preoperative radiation could improve tumor downstaging more than chemotherapy alone, thereby limiting the risk of residual tumors. Second, the tumor location is a prognostic factor for assessing the treatment efficacy (22). Patients with lower rectal cancer may benefit from neoadjuvant radiation more than those undergoing upper rectal cancer therapy (23). Third, the increased rate of postoperative complications in low rectal cancer may delay treatment after surgery.
Although several clinical trials have focused on assessing the use of neoadjuvant treatment, the optimal treatment schema remains uncertain due to data heterogeneity among published studies. Most studies assessing TNT provided patients with fluorouracil- and oxaliplatin-based chemotherapy (10, 24, 25). In addition, irinotecan-based chemotherapy, including doublet regimen CAPIRI (26, 27) and triplet regimen FOFIRINOX (11), showed good toxicity, tumor response rates, and survival outcomes. Most studies that considered neoadjuvant chemotherapy alone provided patients with CapeOX/FOLFOX, with or without monoclonal antibodies. However, whether all patients are eligible for neoadjuvant chemotherapy alone remains debatable. Good tumor response rates were observed when Deng et al. (16) provided CapeOX alone to patients with low- and intermediate-risk LARC. However, the Japanese N-SOG 03 trial revealed that CapeOX plus bevacizumab was associated with a poorer local recurrence and OS rates in patients with cT4b LARC, indicating that chemotherapy alone might not be suitable for the cT4b population (17). In our study, we used CapeOX or mFOLFOX6 regimens for TNT and prolonged neoadjuvant chemotherapy. All patients had baseline resectable tumors, meaning that no cT4b patients were enrolled. We found that doublet regimen CapeOX/mFOLFOX6 was a safe and effective treatment as induction chemotherapy or pNCT alone, but the pCR rate of TNT patients in our study was lower than triplet regimen FOLFIRINOX reported in PRODIGE 23 trial (25% vs 28%) (11).
Chemotherapy cycles are also important. For postoperative chemotherapy, the IDEA study found that 6-months of adjuvant chemotherapy increased the cost and toxicity without improving survival outcomes when compared with 3-month chemotherapy. However, outcomes associated with neoadjuvant chemotherapy may differ from those of adjuvant chemotherapy. Patients who do not undergo surgery have improved the health status and compliance to chemotherapy. In our study, the pNCT group underwent two to four additional cycles of CapeOX/mFOLFOX6 instead of radiation. A comparison of our results with other published studies that assessed the use of neoadjuvant chemotherapy showed that pNCT failed to improve rates of pCR (16.3% versus 6.5%–21.0% for pNCT versus other neoadjuvant therapies, respectively) (13, 16, 17, 28–30). This difference may be explained by differences in the selection of patients and therapy regimens. To determine whether prolonged chemotherapy improves response rates, further prospective studies are needed.
The arrangement of chemoradiotherapy and chemotherapy regimens was another consideration. There are two major modes of TNT: induction chemotherapy plus chemoradiotherapy and chemoradiotherapy plus consolidation chemotherapy. The CAO/ARO/AIO-12 trial compared induction and consolidation chemotherapy in TNT (31, 32). Findings showed that chemoradiotherapy with consolidation chemotherapy was associated with higher pCR rates than induction chemotherapy (25% vs 17%). This can be explained by the longer interval between chemoradiotherapy and surgery. Nevertheless, improvement to pCR failed to improve survival outcomes according to final results of CAO/ARO/AIO-12. In our study, we applied the induction chemotherapy plus chemoradiotherapy followed with an extra cycle of chemotherapy, and achieved a pCR rate of 25% (23/92). Compared with relevant studies (32), our results showed that the additional chemotherapy could probably improve the pCR rate of induction chemotherapy mode. Moreover, based on our experience, induction chemotherapy is more suitable for a “neoadjuvant chemotherapy plus selective radiation” mode. When the scheme was used, patients underwent chemotherapy before their tumor response status was re-evaluated to determine whether additional radiation was needed.
Another consideration is the interval between the last treatment and surgery. The optimal radiotherapy fractionation and timing to surgery is still undetermined. The Stockholm III trial compared short interval (1 week before surgery) with long interval (4-8 weeks before surgery), and showed a comparable oncological outcome between the two groups (33). In a recent randomized study, Akgun et al. (34) compared outcomes of patients for whom intervals between surgery and chemotherapy were either less or more than 8 weeks. The results showed that patients with an interval of more than 8 weeks had improved disease regression and pCR rates compared to those with a surgery-to-chemotherapy interval of less than 8 weeks. Related systematic reviews also showed that surgical delay may improve pCR rates. However, the delayed surgery was not significantly associated with long-term prognosis. The timing to surgery and the best arrangement of neoadjuvant treatment and surgery worth more investigations.
We are aware that this study has some limitations. First, this was a retrospective study with a limited amount of patients. Although we found difference in RFS between the two groups, the study was likely not powered to detect the difference in terms of the recurrence patterns. Second, the functional outcomes of patients after surgery (sexual dysfunction, urinary dysfunction, etc.) were absent, and we failed to evaluate the functional complications between two groups. Third, all enrolled patients were diagnosed with baseline resectable LARC. The efficacy of pNCT for the conversion of unresectable tumors or lateral lymph node metastases was unable to be evaluated. Finally, since patients of both groups completed all cycles of neoadjuvant treatment, we were unable to compare the compliance differences between the two schemas.
In summary, the results of our study show that TNT and pNCT produce similar 3-year RFS and OS rates in patients with mid-to-upper rectal cancer. TNT was associated with improved tumor responsiveness in all patients and improved 3-year RFS rates in those with low rectal cancer. This result indicates that neoadjuvant chemotherapy without radiation might be an option for patients with mid-to-upper rectal cancer. More randomized controlled studies are needed to determine better schema for neoadjuvant therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of Shanghai Ruijin Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MZ and ZH contributed to conception and design of the study. JM, FD and LZ recruited the patients. XZ, PH and LYZ collected the database and performed the statistical analysis. XZ and PH wrote the first draft of the manuscript. MZ and ZH edited the draft. All authors contributed to manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No.82072614).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.953790/full#supplementary-material
References
1. Brændengen M, Tveit KM, Berglund Å, Birkemeyer E, Frykholm G, Påhlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol (2008) 26(22):3687–94. doi: 10.1200/jco.2007.15.3858
2. Gérard J-P, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol (2006) 24(28):4620–25. doi: 10.1200/jco.2006.06.7629
3. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. New Engl J Med (2004) 351(17):1731–40. doi: 10.1056/nejmoa040694
4. Bosset J-F, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. New Engl J Med (2006) 355(11):1114–23. doi: 10.1056/nejmoa060829
5. Rahbari NN, Elbers H, Askoxylakis V, Motschall E, Bork U, Büchler MW, et al. Neoadjuvant radiotherapy for rectal cancer: Meta-analysis of randomized controlled trials. Ann Surg Oncol (2013) 20(13):4169–82. doi: 10.1245/s10434-013-3198-9
6. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet (2009) 373(9666):811–20. doi: 10.1016/s0140-6736(09)60484-0
7. Gollins S, Sebag-Montefiore D. Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol (2016) 28(2):146–51. doi: 10.1016/j.clon.2015.11.003
8. Garant A, Kavan P, Martin A-G, Azoulay L, Vendrely V, Lavoie C, et al. Optimizing treatment sequencing of chemotherapy for patients with rectal cancer: The KIR randomized phase II trial. Radiotherapy Oncol (2021) 155:237–45. doi: 10.1016/j.radonc.2020.11.008
9. Moore J, Price T, Carruthers S, Selva-Nayagam S, Luck A, et al. Prospective randomized trial of neoadjuvant chemotherapy during the ‘wait period’ following preoperative chemoradiotherapy for rectal cancer: Results of the WAIT trial. Colorectal Dis (2017) 19(11):973–79. doi: 10.1111/codi.13724
10. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(1):29–42. doi: 10.1016/s1470-2045(20)30555-6
11. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(5):702–15. doi: 10.1016/s1470-2045(21)00079-6
12. Zhang J, Huang M, Cai Y, Wang L, Xiao J, Lan P, et al. Neoadjuvant chemotherapy with mFOLFOXIRI without routine use of radiotherapy for locally advanced rectal cancer. Clin Colorectal Canc (2019) 18(4):238–44. doi: 10.1016/j.clcc.2019.07.001
13. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: Final results of the Chinese FOWARC trial. J Clin Oncol (2019) 37(34):3223–33. doi: 10.1200/jco.18.02309
14. Giunta EF, Bregni G, Pretta A, Deleporte A, Liberale G, Bali AM, et al. Total neoadjuvant therapy for rectal cancer: Making sense of the results from the RAPIDO and PRODIGE 23 trials. Cancer Treat Rev (2021) 96:102177. doi: 10.1016/j.ctrv.2021.102177
15. Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin oncology: Off J Am Soc Clin Oncol (2014) 32(6):513–18. doi: 10.1200/JCO.2013.51.7904
16. Deng X, Wu Q, Bi L, Yu Y, Huang S, He D, et al. Early response to upfront neoadjuvant chemotherapy (CAPOX) alone in low- and intermediate-risk rectal cancer: A single-arm phase II trial. Br J Surg (2021) 109(1):121–28. doi: 10.1093/bjs/znab388
17. Tomida A, Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Okada Y, et al. Neoadjuvant CAPOX and bevacizumab alone for locally advanced rectal cancer: Long-term results from the n-SOG 03 trial. Int J Clin Oncol (2019) 24(4):403–10. doi: 10.1007/s10147-018-1372-6
18. Sun W, Dou R, Chen J, Lai S, Zhang C, Ruan L, et al. Impact of long-course neoadjuvant radiation on postoperative low anterior resection syndrome and quality of life in rectal cancer: Post hoc analysis of a randomized controlled trial. Ann Surg Oncol (2019) 26(3):746–55. doi: 10.1245/s10434-018-07096-8
19. Kumagai T, Rahman F, Smith AM. The microbiome and radiation induced-bowel injury: Evidence for potential mechanistic role in disease pathogenesis. Nutrients (2018) 10(10):1405. doi: 10.3390/nu10101405
20. Kennedy ED, Simunovic M, Jhaveri K, Kirsch R, Brierley J, Drolet S, et al. Safety and feasibility of using magnetic resonance imaging criteria to identify patients with “Good prognosis” rectal cancer eligible for primary surgery: The phase 2 nonrandomized QuickSilver clinical trial. JAMA Oncol (2019) 5(7):961–66. doi: 10.1001/jamaoncol.2019.0186
21. Ruppert R, Junginger T, Ptok H, Strassburg J, Maurer CA, Brosi P, et al. Oncological outcome after MRI-based selection for neoadjuvant chemoradiotherapy in the OCUM rectal cancer trial. Br J Surg (2018) 105(11):1519–29. doi: 10.1002/bjs.10879
22. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Suppl 4):iv263. doi: 10.1093/annonc/mdy161
23. Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, et al. A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci (2016) 12(8):1022–31. doi: 10.7150/ijbs.15438
24. Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol (2016) 27(5):834–42. doi: 10.1093/annonc/mdw062
25. Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol (2022), 40(15):Jco2101667. doi: 10.1200/jco.21.01667
26. Zhu J, Liu A, Sun X, Liu L, Zhu Y, Zhang T, et al. Multicenter, randomized, phase III trial of neoadjuvant chemoradiation with capecitabine and irinotecan guided by UGT1A1 status in patients with locally advanced rectal cancer. J Clin Oncol (2020) 38(36):4231–39. doi: 10.1200/jco.20.01932
27. Wang J, Fan J, Li C, Yang L, Wan J, Zhang H, et al. The impact of chemotherapy completion on the efficacy of irinotecan in the preoperative chemoradiotherapy of locally advanced rectal cancer: An expanded analysis of the CinClare phase III trial. Clin Colorectal Cancer (2020) 19(2):e58–69. doi: 10.1016/j.clcc.2020.01.004
28. Kamiya T, Uehara K, Nakayama G, Ishigure K, Kobayashi S, Hiramatsu K, et al. Early results of multicenter phase II trial of perioperative oxaliplatin and capecitabine without radiotherapy for high-risk rectal cancer: CORONA I study. Eur J Surg Oncol (2016) 42(6):829–35. doi: 10.1016/j.ejso.2016.02.014
29. Oshiro T, Uehara K, Aiba T, Mukai T, Ebata T, Nagino M. Impact of RAS/BRAF mutation status in locally advanced rectal cancer treated with preoperative chemotherapy. Int J Clin Oncol (2018) 23(4):681–88. doi: 10.1007/s10147-018-1253-z
30. Nishimura J, Hasegawa J, Kato T, Yoshioka S, Noura S, Kagawa Y, et al. Phase II trial of capecitabine plus oxaliplatin (CAPOX) as perioperative therapy for locally advanced rectal cancer. Cancer Chemother Pharmacol (2018) 82(4):707–16. doi: 10.1007/s00280-018-3663-z
31. Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol (2019) 37(34):3212–22. doi: 10.1200/jco.19.00308
32. Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: Long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol (2022) 8(1):e215445. doi: 10.1001/jamaoncol.2021.5445
33. Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol (2017) 18(3):336–46. doi: 10.1016/s1470-2045(17)30086-4
Keywords: chemoradiotherapy, rectal cancer, neoadjuvant chemotherapy, recurrence, propensity score
Citation: Zhao X, Han P, Zhang L, Ma J, Dong F, Zang L, He Z and Zheng M (2022) Prolonged neoadjuvant chemotherapy without radiation versus total neoadjuvant therapy for locally advanced rectal cancer: A propensity score matched study. Front. Oncol. 12:953790. doi: 10.3389/fonc.2022.953790
Received: 26 May 2022; Accepted: 25 August 2022;
Published: 16 September 2022.
Edited by:
Xinxiang Li, Fudan University, ChinaReviewed by:
J. Joshua Smith, Memorial Sloan Kettering Cancer Center, United StatesRi Na Yoo, The Catholic University of Korea, South Korea
Charles Ternent, American Society of Colon and Rectal Surgeons, United States
Copyright © 2022 Zhao, Han, Zhang, Ma, Dong, Zang, He and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minhua Zheng, emhlbmdtaHJqaG9zcGl0YWxAMTYzLmNvbQ==; Zirui He, aGV6aXJ1aUBhbGl5dW4uY29t
†These authors have contributed equally to this work and share first authorship
 Xuan Zhao
Xuan Zhao Peiyi Han
Peiyi Han Luyang Zhang
Luyang Zhang Junjun Ma
Junjun Ma Feng Dong1,2
Feng Dong1,2 Lu Zang
Lu Zang Zirui He
Zirui He Minhua Zheng
Minhua Zheng