- 1National Engineering Laboratory for AIDS Vaccine, Key Laboratory for Molecular Enzymology and Engineering, The Ministry of Education, School of Life Sciences, Jilin University, Changchun, China
- 2Department of Thoracic Surgery, The First Hospital of Jilin University, Changchun, China
Sterol regulatory element binding protein-1 (SREBP-1), a transcription factor with a basic helix–loop–helix leucine zipper, has two isoforms, SREBP-1a and SREBP-1c, derived from the same gene for regulating the genes of lipogenesis, including acetyl-CoA carboxylase, fatty acid synthase, and stearoyl-CoA desaturase. Importantly, SREBP-1 participates in metabolic reprogramming of various cancers and has been a biomarker for the prognosis or drug efficacy for the patients with cancer. In this review, we first introduced the structure, activation, and key upstream signaling pathway of SREBP-1. Then, the potential targets and molecular mechanisms of SREBP-1-regulated lipogenesis in various types of cancer, such as colorectal, prostate, breast, and hepatocellular cancer, were summarized. We also discussed potential therapies targeting the SREBP-1-regulated pathway by small molecules, natural products, or the extracts of herbs against tumor progression. This review could provide new insights in understanding advanced findings about SREBP-1-mediated lipogenesis in cancer and its potential as a target for cancer therapeutics.
Introduction
Sterol regulatory element-binding proteins (SREBPs) are identified as a family of transcription factors with the domain of a basic helix–loop–helix leucine zipper (bHLH-LZ), which regulate genes involved in the pathways of lipid synthesis and uptake (1–3). In mammalian cells, three SREBP isoforms, SREBP-1a, SREBP-1c, and SREBP-2, from two genes, SREBF1 and SREBF2, have been identified, which have overlapping transcriptional programs for the synthesis of fatty acids and cholesterol (4, 5). SREBP-1a and SREBP-1c are derived from the same gene through alternative splicing at transcription start sites (6), but they have different regulations for downstream target genes (4, 7). A study conducted for about 30 years has confirmed that SREBP-1c is involved in regulating fatty acid synthesis and lipogenesis and SREBP-1a can be implicated in two pathways of SREBP-1c and SREBP-2 (specific to cholesterol metabolism) (8). Under pathological conditions, SREBP-1 activation can cause lipid dysfunction to contribute to various metabolic diseases, such as obesity, diabetes mellitus, non-alcoholic fatty liver disease, and cancer (9–12). Recently, more and more evidence has demonstrated that SREBP-1 participates in metabolic reprogramming in different cancer, such as prostate cancer (13), breast cancer (14), and glioblastoma (GBM) (15), which has been a potential target for cancer therapy. Moreover, several essential pathways, such as epidermal growth factor receptor (EGFR), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB, Akt)/mammalian target of rapamycin (mTOR), and Ras, can regulate SREBP-1 activation to mediate tumor growth and metastasis (15, 16). Importantly, multiple treatment strategies targeting the SREBP-1 signaling pathway, including small molecules or genetic inhibition, have been extensively studied and developed (17, 18). The preparation of the publications in this review was conducted as follows: 1) the articles in English were electronically searched from October 1993 to May 2022 in the databases of PubMed and the Web of Science. 2) “SREBP-1” or “sterol regulatory-element binding protein-1” and “cancer” were used as the search terms. 3) A secondary search was performed by checking the title and the abstract to collect published papers with the inclusion criteria. In this review, we summarized the key upstream signaling pathway and the function of SREBP-1-regulated lipogenesis in different cancer. We also discussed potential therapies targeting the SREBP-1-regulated pathway against tumor progression. This review could provide new insights in understanding advanced findings about SREBP-1-mediated lipid dysfunction in cancer and its potential as a target for cancer therapeutics.
SREBP-1 activation and its downstream targets in cancer
Human gene SREBF1 (26 kb, 22 exons, and 20 introns) at a chromosomal location of 17p11.2 was cloned and characterized in 1995, as a result of alternative splicing at both the 5′ and 3′ ends (19, 20). SREBP-1a and SREBP-1c differ in the extreme N-terminal acidic amino acids, which share a similar structure containing an NH2-terminal transcription factor domain (480 amino acids), a middle hydrophobic region (80 amino acids), and a COOH-terminal regulatory domain (590 amino acids) (3). SREBP-1a and SREBP-1c include 42 amino acids (12 acidic acids) and 24 amino acids (six acidic acids) in the acidic NH2-terminal domain, respectively. SREBP-1c has a much weaker effect in transcription activation than that of SREBP-1a, due to its shortened acidic domain (21). SREBP-1 is conserved from fission yeast to humans (22), and its two isoforms are found in the liver, adipose, and skeletal muscle tissues (20, 23, 24). SREBP-1c is more abundant than SREBP-1a in the liver (21, 25), and SREBP-1a is abundantly expressed in some tissues and cells, such as heart, macrophages, and dendritic cells from bone marrow (26). Recently, it has been revealed that SREBP-1 is obviously activated in different cancers, which is higher than that of non-tumor tissues (13, 27) and has been a biomarker for the prognosis or drug efficacy for patients with cancer (14, 28).
SREBP-1a and SREBP-1c are synthesized as 125-kDa precursors in endoplasmic reticulum membrane and cleaved into an NH2-terminal fragment (68 kDa) by site 1 and site 2 proteases to translocate into the nucleus for lipogenesis gene transcription, when kept in the environment from sterol depletion (29–31). SREBP-1 can form a complex with SREBP cleavage-activating protein (SCAP) to mediate its cleavage in sterol depletion or in the nutritional environment (10, 25). Insulin increase by carbohydrate ingestion can cause the decrease of Insig-2a, which leads to the release of the SREBP-1c/SCAP complex to stimulate SREBP-1c cleavage in the Golgi for the activation of lipogenic/glycolytic genes (25, 32). Moreover, insulin can increase SREBP-1c expression by activating the promoters containing the binding sites for the liver X receptor, Sp1, nuclear factor-Y, and SREBP-1c (33). In addition, the cleavage of SREBP-1c is regulated by polyunsaturated fatty acids, not as SREBP-2 by SCAP or Insig-1 (34). After the cleavage of SREBP-1, the nuclear form of SREBP-1 binds to sterol regulatory elements (SREs) to stimulate the transcriptions of lipogenic genes (25, 29). As reported, SREBP-1 can activate a series of genes for fatty acid synthesis, including ATP citrate lyase (ACLY), acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN), and stearoyl-CoA desaturase 1 and 2 (rodent)/5 (human) (SCD-1 and -2/5), and for lipid uptake, such as low-density lipoprotein receptor (LDLR) (4, 30–34). Furthermore, it has been reported that SREBP-1 activation can be involved in various cellular functions, such as cell proliferation, cell cycle, muscle cell differentiation, foam cell formation, and somatic cell reprogramming (35–39). Critically, the activation of SREBP-1 obviously stimulates de novo lipogenesis to regulate cell growth, cell cycle progression, and metastatic characteristics for cancer progression (12, 13, 40–42). Meanwhile, these genes regulated by SREBP-1, including ACLY, ACC, FASN, and SCD, are highly expressed in cancer tissues compared to adjacent non-tumor tissues, which play essential roles in the growth, metastasis, and survival of various cancers (43–46). Taken together, SREBP-1 and its target genes are essential in cancer progression, which have been potential targets for pharmacological or genetic therapy (Figures 1A, B).
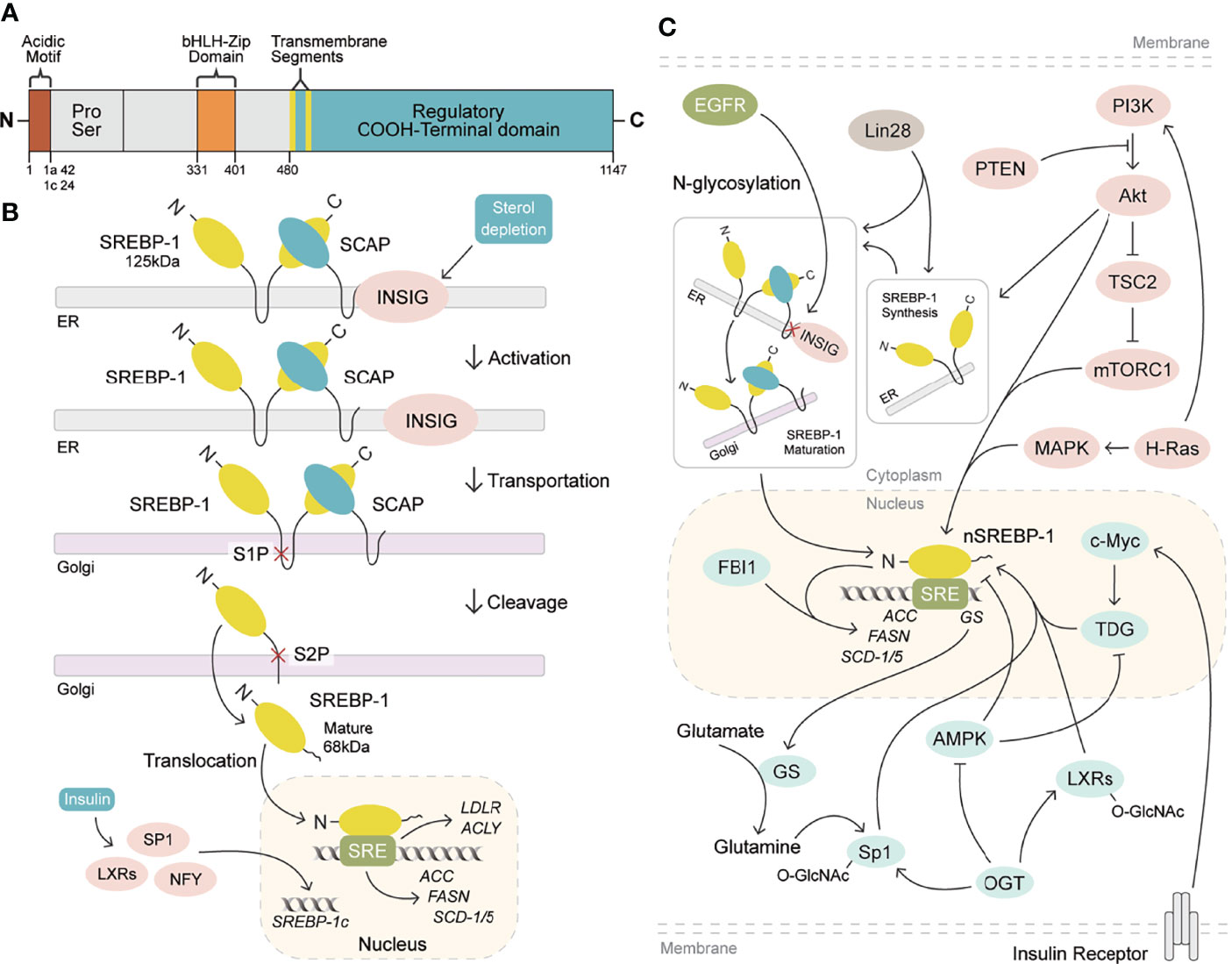
Figure 1 SREBP-1 structure, activation, and signaling pathways. (A) SREBP-1 structure and activation. SREBP-1 contains the NH2-terminal domain (the bHLH-Zip motif and an acidic motif), a middle hydrophilic region, and the COOH-terminal domain. (B) After INSIG dissociation from SCAP, SREBP-1 translocates to the Golgi apparatus and is cleaved by site 1 protease (S1P) and site 2 protease (S2P) to form a nuclear form (nSREBP-1) for activating the transcription of its downstream targets, such as FASN, ACC, SCD-1/5, ACLY, and LDLR. (C) Multiple signaling pathways regulate SREBP-1 expression, translocation, and maturation, including EGFR, PI3K/Akt/mTORC1, and others. OGT: O-GlcNAc transferase, GS: glutamate synthetase, TDG: thymine DNA glycosylase.
SREBP-1-regulated lipogenesis in cancers
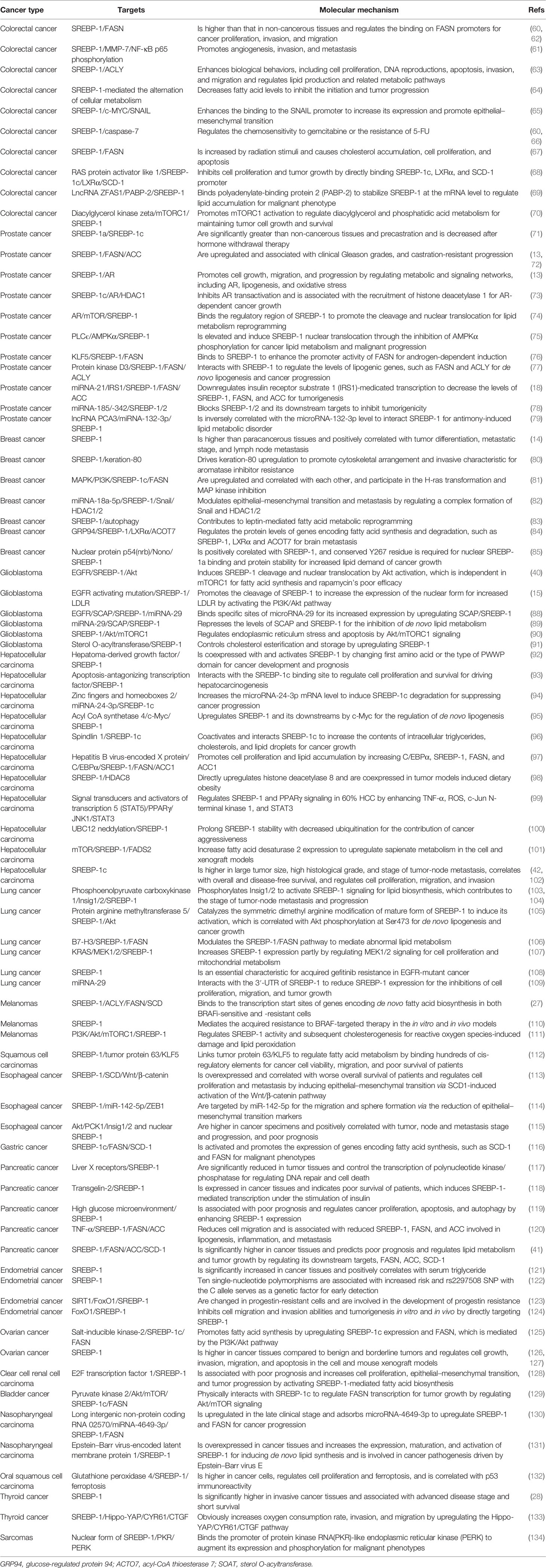
Currently, multiple signaling pathways control SREBP-1 activation to regulate the downstream genes for lipogenesis, including the EGFR, PI3K/Akt/mTOR, and p53 mutation pathways. EGFR signaling promotes N-glycosylation of SCAP to reduce the association with Insig-1, which consequently induces the proteolytic activation of SREBP-1 (47). Importantly, the hyperactive mutation of PI3K/Akt/mTOR signaling can inhibit oxidative stress and ferroptotic death by regulating SREBP-1/SCD-1-mediated lipogenesis (48). Akt activation can induce the synthesis of full-length SREBP-1 and cause the accumulation of nuclear SREBP-1 to increase the accumulation of intracellular lipids, such as fatty acids and phosphoglycerides, which is sterol sensitive for activating the FASN promoter (16). The mammalian/mechanistic target of rapamycin complex 1 (mTORC1) regulates the transcription and translation of SREBP-1 and SREBP-2 to promote de novo lipid biosynthesis in response to nutrients or insulin, which regulate cell proliferation, mitochondrial metabolism, or glycolytic capacity in cancer (49, 50). Similarly, insulin/glutamine deprivation activates SREBP-1 to bind to the promoter of glutamine synthetase (GS) for its transcription. GS results in the O-linked N-acetylglucosaminylation (O-GlcNAcylation) of specificity protein 1 (Sp1) for the induction of the SREBP-1/ACC1 pathway, which increases lipogenesis and lipid droplet accumulation in liver and breast cancer (51). Based on the key role of O-GlcNAcylation, it has been found that O-GlcNAc transferase (OGT) regulates SREBP-1 expression and its targets in a proteasomal and AMP-activated protein kinase (AMPK)-dependent manner (52). Meanwhile, OGT increases the O-GlcNAcylation of liver X receptors to induce the expression of secretory clusterin (sCLU), which is closely associated with SREBP-1c and its mediated activation of the CLU promoter for the contribution of cisplatin resistance (53). In addition, insulin can activate c-Myc to promote the transactivation of thymine DNA glycosylase (TDG), which decreases the abundance of 5-carboxylcytosine in the SREBP-1 promoter for the upregulation of SREBP-1 and ACC1. On the other hand, AMPK can inhibit DNA demethylation of the SREBP-1 promoter mediated by TDG, suggesting that TDG regulated by insulin or metformin is a potential therapeutic target for T2DM-related cancers (54). In the tumor immune microenvironment, Treg cells maintain the metabolic fitness and mitochondrial integrity of M2-like tumor-associated macrophages indirectly by repressing IFNγ production derived from CD8+ T cells, which is dependent on SREBP-1 and its mediated fatty acid synthesis (55). Under the conditions of serum starvation and hypoxia, cholesterol or O2 deficiency mediates the expression of the FVII gene encoding coagulation factor VII via the SREBP-1/glucocorticoid-induced leucine zipper, which leads to the production and shedding of procoagulant extracellular vesicles (56). A tumor gene, H-ras can upregulate mitogen-activated protein (MAP) kinase and PI3K signaling, which increases SREBP-1 and FASN levels to drive mammary epithelial cell malignant transformation and tumor virulence (57). A proto-oncogenic transcription factor, FBI-1 can directly interact with SREBP-1 via DNA-binding domains to synergistically activate FASN transcription for maintaining rapid cancer cell proliferation (58). An RNA-binding protein, Lin28 enhances SREBP-1 translation and maturation to promote cancer progression by directly binding to the mRNAs of both SREBP-1 and SCAP (59). Taken together, EGFR, PI3K/Akt, and mTOR signal pathways can control SREBP-1 expression and activation to regulate its downstream pathways in cancers (Figure 1C, Table 1).
Colorectal cancer
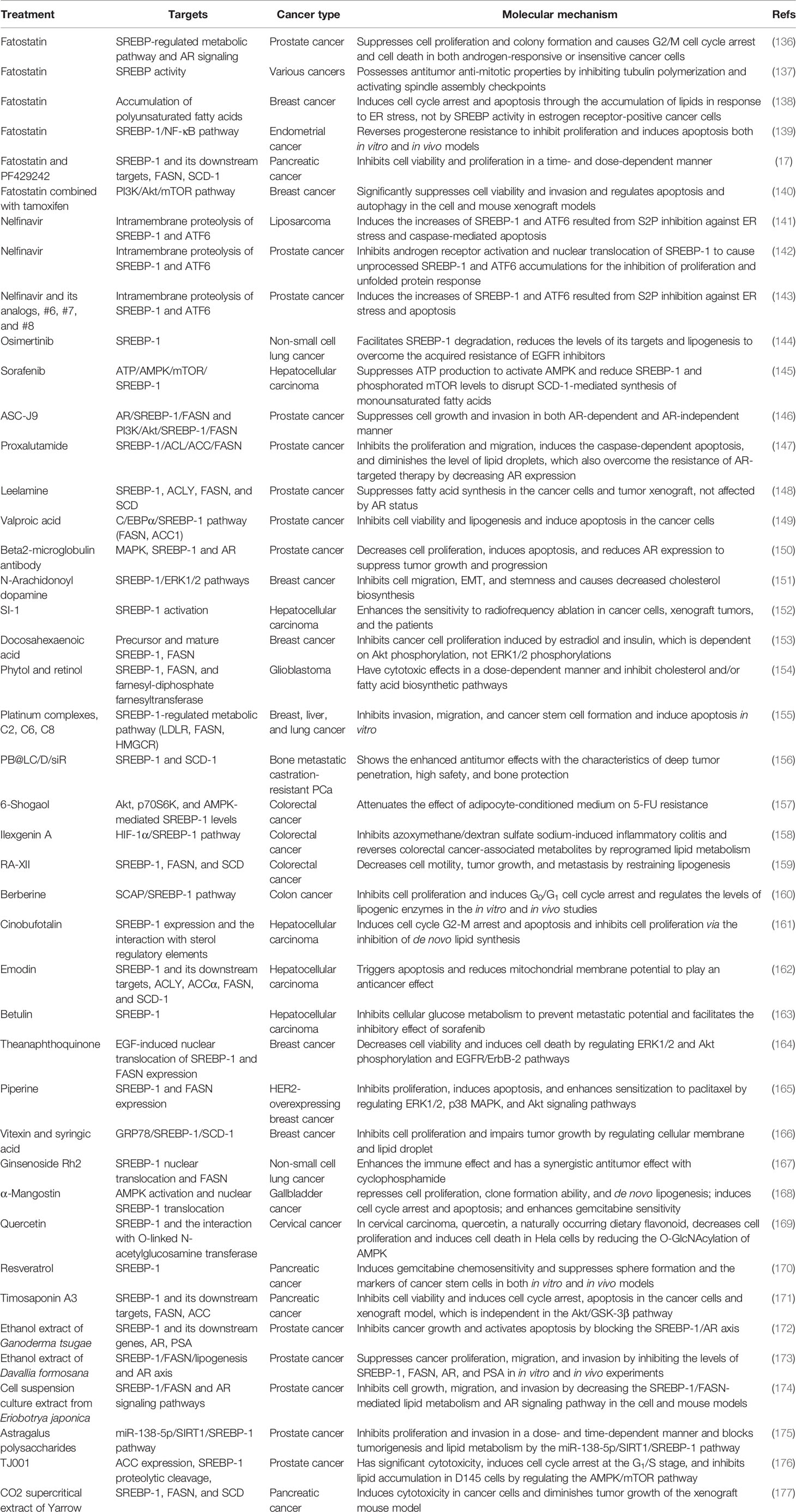
The SREBP-1 expression in colorectal cancer (CRC) tissues is significantly higher than that in non-cancerous or normal tissues (60, 61). The colocalization of SREBP-1 and FASN is found in CRC tissues, and the transcriptional regulation of SREBP-1 on the FASN promoter can respond to the deficiency in endogenous fatty acid synthesis in colorectal neoplasia (62). Moreover, the combination of SREBP-1 and ACLY can significantly promote CRC cell proliferation, migration, and invasion, which is mediated by lipid synthesis modulation (63). In addition, the overexpression of SREBP-1 promotes the angiogenesis and invasion of CRC cells by promoting MMP-7 expression related to NF-κB p65 phosphorylation (61). SREBP-1 silencing decreases the levels of fatty acid and SREBP-1 targets genes to inhibit the initiation and tumor progression of CRC, which might be associated with the alternation of cellular metabolism, including mitochondrial respiration, glycolysis, and fatty acid oxidation (64). SREBP-1 also can interact with c-MYC to enhance its binding ability to the snail family transcriptional repressor 1 (SNAIL) promoter to regulate its expression and promote epithelial–mesenchymal transition in CRC (65). For the resistance of chemotherapy in CRC, SREBP-1 is overexpressed in chemoresistant CRC samples and is correlated with poor survival. SREBP-1 can increase the chemosensitivity to gemcitabine through downregulation of caspase-7, which might be a new prognostic biomarker of CRC (66). Meanwhile, the overexpression of SREBP-1 enhances the resistance of 5-FU through regulation of caspase-7-dependent PARP1 cleavage in CRC (60). On the other hand, radiation stimuli can rapidly increase SREBP-1/FASN signaling to cause cholesterol accumulation, cell proliferation, and cell death, suggesting that targeting the SREBP-1/FASN/cholesterol axis is a potential strategy for CRC patients undergoing radiotherapy (67). Recently, results from colon cancer cells and xenograft tumor models have demonstrated that RAS protein activator-like 1 inhibits colon cancer cell proliferation by regulating LXRα/SREBP1c-mediated SCD1 activity (68). An lncRNA ZFAS1 can bind polyadenylate-binding protein 2 to stabilize and increase the levels of SREBP-1 and its targets, FASN and SCD1, for the promotion of lipid accumulation in CRC (69). In rapamycin-resistant colon cancer cells, diacylglycerol kinase zeta can promote mTORC1 activation and cell-cycle progression, which are essential for SREBP-1 expression (70). Together, these findings demonstrate that SREBP-1 participates in colon cancer growth, invasion, and the resistance of chemotherapy or radiation, which have been potential therapeutic targets for CRC treatment.
Prostate cancer
During the castrated progression of a prostate cancer xenograft model, SREBP-1a and SREBP-1c are significantly greater than precastrated levels. The staining for the clinical specimens shows a high level of SREBP-1 compared with non-cancerous prostate tissue and SREBP-1 is decreased after hormone withdrawal therapy for 6 weeks (71). SREBP-1 and its downstream effectors, FASN and ACC, are upregulated in a TCGA cohort of prostate cancer and late-stage transgenic adenocarcinoma of mouse prostate tumor (72). Furthermore, SREBP-1 expression is positively associated with the clinical Gleason grades and promotes prostate cancer cell growth, migration, and castration-resistant progression, which may be mediated by the alterations of metabolic signaling networks, including androgen receptor (AR), lipogenesis, and NAPDH oxidase 5-induced oxidative stress (13). More importantly, the results from overexpression or RNA interference of SREBP-1c demonstrate that SREBP-1c specifically inhibits AR transactivation by binding to the endogenous AR target promoter, which may be associated with the recruitment of histone deacetylase 1, suggesting that SREBP-1 plays an important role in regulating AR-dependent prostate cancer growth (73). On the other hand, AR and mTOR bind to the regulatory region of SREBP-1 to promote its cleavage and translocation to the nucleus. The synthetic androgen R1881 can induce SREBP-1 recruitment to regulatory regions of its targets, which is abrogated by the inhibition of the mTOR signaling pathway. These data suggest that the AR/mTOR complex can promote SREBP-1 expression and activity to activate its downstreams for reprogramming lipid metabolism (74). Additionally, phospholipase Cϵ, Kruppel-like factor 5 (KLF5), and protein kinase D3 can directly or indirectly interact with SREBP-1 to control lipid metabolism in the occurrence and progression of prostate cancer (75–77). Considering the key roles of miRNAs or lncRNAs, miRNA-21 transcriptionally regulates insulin receptor substrate 1/SREBP-1 signaling pathway to promote prostate cancer cell proliferation and tumorigenesis (18). Two other miRNAs, miRNA-185 and miRNA-342, can block SREBP-1/2 and its downregulated targets, FASN and hydroxy-3-methylglutaryl CoA reductase (HMGCR), to inhibit prostate cancer tumorigenicity (78). A long non-coding RNA molecule, PCA3 is inversely correlated with miRNA-132-3p to regulate the antimony-induced lipid metabolic imbalance, which is closely related to the SREBP-1 protein level, not influencing the SREBP-2 expression in mRNA and protein levels (79). Collectively, these data from cell and animal models or clinical specimens demonstrate that SREBP-1 plays a very important role in prostate cancer progression and that multiple signaling pathways, such as AR and miRNAs, can regulate SREBP-1 activation to mediate the dysfunction of lipid metabolism in prostate cancer.
Breast cancer
As the pattern in other cancers, the mRNA and protein levels of SREBP-1 are higher in tissues of breast cancer, compared with paracancerous tissues. A higher expression of SREBP-1 is positively correlated with tumor differentiation, metastatic stage, and metastasis in lymph nodes. Moreover, in vitro studies reveal that SREBP-1 inhibition can inhibit the cell migration and invasion of breast cancer cells, such as MDA-MB-231 and MCF-7 (14). In aromatase inhibitor-resistant breast cancer cells, SREBP-1 can drive Keratin-80 upregulation to directly promote cytoskeletal rearrangements, cellular stiffening, and cell invasion (80). In MCF-10a breast epithelial cells with H-ras transformation and inhibition of MAP kinase, SREBP-1c and FASN are upregulated and SREBP-1a shows less change. In a panel of samples with primary human breast cancer, SREBP-1c and FASN are increased and correlated with each other (81). miRNA expression profiling shows that miRNA-18a-5p is most evidently decreased in breast cancer cells, which can target SREBP-1 to repress E-cadherin expression. This suggests that the miR-18a-5p/SREBP-1 axis plays a crucial role in the epithelial–mesenchymal transition and metastasis of advanced breast cancer (82). A hormone derived from adipose tissue, leptin can induce ATP production from fatty acid oxidation and intracellular lipid accumulation in MCF-7 cells and tumor xenograft models, which is mediated by SREBP-1 induction and autophagy in breast cancer (83). Additionally, two key molecules, glucose-regulated protein 94 (GRP94) and nuclear protein p54(nrb)/Nono, can regulate the SREBP-1 signaling pathway in in vitro and in vivo models, which have been potential targets for breast cancer treatment (84, 85). GRP94 can upregulate genes for fatty acid synthesis and degradation, including SREBP-1, LXRα, and acyl-CoA thioesterase 7, to favor the progression of breast cancer metastasis (84). As shown in immunohistological staining for human breast cancer tissues, the level of p54(nrb) is positively correlated with SREBP-1a, and its conserved Y267 residue is required for the interaction with nuclear SREBP-1a. It suggests that p54(nrb) is a key regulator for the nuclear form of SREBP-1a, which might be a potential target for treating breast cancer (85). Taken together, SREBP-1 can favor breast cancer progression, which can be regulated by multiple signal molecules, such as miRNA-18a-5p, GRP94, and p54(nrb).
Glioblastoma
In clinic, the therapeutic efficacy of drugs on GBM might be closely related to EGFR amplification, its mutations, or PI3K hyperactivation (86). EGFR induces the SREBP-1 cleavage and nuclear translocation, which is mediated by Akt but is independent on mTORC1 (40). EGFR-activating mutation (EGFRvIII) can promote SREBP-1 cleavage and cause a higher expression of nuclear SREBP-1 to elevate LDLR expression in GBM patient samples, tumors cells, and mouse models, which is dependent on the PI3K/Akt pathway (15, 87). Moreover, EGFR signaling binds to specific sites of the enhanced miRNA-29 promoter to increase its expression in GBM cells by upregulating SCAP/SREBP-1 whereas miRNA-29 can suppress SCAP and SREBP-1 expression to inhibit lipid synthesis in GBM (88, 89). Additionally, it is found that SREBP-1 depletion can induce apoptosis and endoplasmic reticulum stress in U87 GBM cells and tumor xenograft models, which is the result of Akt/mTORC1 signaling-mediated cancer growth and survival (90). Except EGFR and Akt/mTORC1 signaling, sterol O-acyltransferase highly expressed in GBM can control the cholesterol esterification and storage as a key player via upregulation of SREBP-1 (91). Collectively, the oncogenic EGFR/PI3K/Akt pathway upregulates SREBP-1 activation to control lipid metabolism in GBM.
Hepatocellular carcinoma
Lipogenesis participates in the initiation and progression of HCC, one of the leading causes of cancer-related deaths worldwide. Multiple pathways can control SREBP-1 to regulate lipogenesis in HCC, such as hepatoma-derived growth factor (HDGF), apoptosis-antagonizing transcription factors (AATF), or acyl-CoA synthetases. HDGF is found to be closely associated with HCC prognosis, which is coexpressed with SREBP-1. The changes of the first amino acid or the type of PWWP domain are crucial for HDGF-mediated SREBP-1 activation for the lipogenesis promotion in HCC (92). In human non-alcoholic steatohepatitis, HCC tissues, and various human HCC cell lines, AATF expression is higher, which can be induced by TNF-α. Promoter analysis reveals that AATF can bind SREBP-1c to regulate HCC cell proliferation, migration, colony formation, and xenograft tumor growth and metastasis (93). As an HCC-associated tumor suppressor, zinc fingers and homeoboxes 2 (ZHX2) is negatively associated with SREBP-1c in HCC cell lines and human specimens. ZHX2 can increase miRNA-24-3p at the transcription level to induce SREBP-1c degradation for the suppression of HCC progression (94). A member of the acyl-CoA synthetase (ACS) family, acyl CoA synthetase 4 (ACSL4) has been identified as an oncogene and a novel marker of alpha-fetoprotein-high subtype HCC. ACSL4 upregulates SREBP-1 and its downstream lipogenic enzymes via c-Myc to modulate de novo lipogenesis during the progression of HCC (95). A tissue microarray analysis shows that 60 of 80 HCC tissues have a positive staining and high expression of spindlin 1 (SPIN1), which are positively associated with malignancy of HCC. SPIN1 coactivates and interacts with SREBP-1c to increase the levels of intracellular triglycerides, cholesterols, and lipid droplets, which can enhance HCC growth (96). Hepatitis B virus (HBV)-encoded X protein (HBx) significantly promotes HepG2 cell proliferation and lipid accumulation through increased protein levels of C/EBPα, SREBP-1, FASN, and ACC1 (97). Histone deacetylase 8 (HDAC8) is directly upregulated by SREBP-1 where it is coexpressed in dietary obesity models of HCC. HDAC8 can inhibit cell death mediated by p53/p21 and cell-cycle arrest at the G2/M phase and promote β-catenin-dependent cell proliferation by the interaction with chromatin modifier EZH2 to repress the Wnt antagonist in HCC (98). The deficiency of signal transducers and activators of transcription 5 (STAT5) causes the upregulation of SREBP-1 and peroxisome proliferator-activated receptor γ signaling. The combined loss of STAT5/glucocorticoid receptor can cause approximately 60% HCC at 12 months, which is associated with enhanced TNF-α and ROS and the activation of c-Jun N-terminal kinase 1 and STAT3 (99). Additionally, neddylation by UBC12 can prolong SREBP-1 stability with decreased ubiquitination. In human specimens of HCC and the Gene Expression Omnibus (GEO) database, the SREBP-1 level is positively correlated with UBC12 and contributes to HCC aggressiveness, which can be blocked by an inhibitor of NEDD8-activating enzyme E1, MLN4924 (100). Importantly, both mTOR signaling and SREBP-1 can increase fatty acid desaturase 2 expression to activate sapienate metabolism in the HCC cells and xenograft models (101). Certainly, SREBP-1 expression in HCC tissues is significantly higher than that in adjacent tissues, especially in large tumor sizes, high histological grade, and stage of tumor node metastasis (TNM). The level of SREBP-1 is correlated with a worse 3-year overall and disease-free survival of HCC patients, which is not dependent on the prediction for the prognosis. Moreover, SREBP-1 overexpression or knockdown can regulate cell proliferation, invasion, and migration of HCC cells (42). Especially, SREBP-1c, rather than SREBP-1a, is elevated and activated in HCC (102). Collectively, multiple upstream molecules, such as HDGF, AATF, ZHX2, ACSL4, SPIN, HBx, HDAC8, and STAT5, can regulate SREBP-1 expression, activation, and stability to promote HCC proliferation, invasion, and migration for tumor growth and metastasis.
Lung cancer
The phosphorylation of Akt-mediated phosphoenolpyruvate carboxykinase 1 (PCK1) at S90 can reduce its gluconeogenic activity and use GTP as a phosphate donor to phosphorylate Insig1/2 in the endoplasmic reticulum, which activates SREBP signaling for lipid synthesis in cancer (103). In non-small cell lung cancer (NSCLC), PCK1 can promote nuclear SREBP-1 activation by phosphorylating Insig1/2, which is associated with TNM stage and progression of NSCLC (104). The mature form of SREBP-1 is overexpressed and correlated with Akt phosphorylation at Ser473 for lipid synthesis, which may be reversed by protein arginine methyltransferase 5 (PTMI5) knockdown in lung adenocarcinoma (105). B7-H3, a glycoprotein, results in aberrant lipid metabolism in lung cancer, which is mediated by the SREBP-1/FASN signaling pathway (106). In mutant KRAS-expressing NSCLC, KRAS increases SREBP1 expression in part by MEK1/2 signaling and SREBP-1 knockdown significantly inhibits cell proliferation through regulating mitochondrial metabolism (107). In EGFR-mutant NSCLC cells, SREBP-1 is a key feature of the resistance to gefitinib, which might be a promising target for the treatment of EGFR mutation in lung cancer (108). As that in other cancers, miRNA-29 reduces lung cancer proliferation and migration through inhibition of SREBP-1 expression by interacting with the 3′-UTR of SREBP-1 (109). Taken together, Akt activation and its mediated PCK1, B7-H3, and KRAS can regulate SREBP-1 expression and activation in lung cancer progression.
Skin cancer
In melanomas, SREBP-1 predominantly binds to the transcription start sites of genes of de novo fatty acid biosynthesis (DNFA). The inhibition of DNFA or SREBP-1 by enzyme inhibitors or antisense oligonucleotides exerts obvious anticancer effects on both BRAFi-sensitive and -resistant melanoma cells (27). BRAF inhibition can induce SREBP-1 downregulation to inhibit lipogenesis, and SREBP-1 inhibition can overcome the acquired resistance to BRAF-targeted therapy in both in vitro and in vivo models (110). In addition, the PI3K/Akt/mTORC1 pathway, not by BRAF mutation, can regulate SREBP-1 activity and subsequent cholesterogenesis in melanoma (111). In another type of skin cancer, squamous cell carcinomas (SCCs), SREBP-1 can link tumor protein 63 (TP63)/KLF5 to regulate fatty acid metabolism, including fatty acid, sphingolipid, and glycerophospholipid biosynthesis, by binding to hundreds of cis-regulatory elements, which is associated with SCC viability, migration, and poor survival of patients (112). Thus, multiple signal pathways, such as BRAF, PI3K, and TP63/KLF5, can control SREBP-1 for the regulation of skin cancer progression.
Other cancers
In esophageal tumors, SREBP1 is overexpressed and associated with worse overall survival rate of patients. Overexpressing SREBP1 in OE33 cells leads to increased biological phenotypes, including colony formation, migration, and invasion, which is mediated by reduced mesenchymal markers (vimentin, zinc finger E-box binding homeobox 1 (ZEB1), N-cadherin) and increased epithelial markers (E-cadherin). Mechanistically, SREBP-1 can regulate T-cell factor 1/lymphoid enhancer factor 1 and their target proteins, such as CD44 and cyclin D1, and increase the levels of SCD-1, phosphorylated GSK-3β, and nuclear β-catenin for the proliferation and metastasis of esophageal carcinoma (113, 114). SREBP-1 and ZEB1 are potential targets of miRNA-142-5p, a tumor suppressor, and they are associated with tumor progression and poor prognosis (114). Moreover, the levels of PCK1 pS90, Insig1 pS207/Insig2 pS151, and SREBP-1 are associated with the tumor, metastasis stage, and progression of esophageal squamous cell carcinoma, suggesting PCK1 activity-regulated SREBP-1 as a potential target for the diagnosis and treatment of esophageal cancer (115). In gastric cancer tissues and cells, SREBP-1c is activated and promotes the expressions of FASN and SCD-1 to mediate malignant phenotypes, which has been identified as a potential target for the treatment of gastric cancer (116). In the poorest cancer of the digestive system, pancreatic cancer, the lipogenic liver X receptor (LXR)–SREBP-1 axis controls polynucleotide kinase/phosphatase (PNKP) transcription for regulating cancer cell DNA repair and apoptosis. Compared with the adjacent tissues, the levels of LXRs and SREBP-1 are significantly reduced in the tumor tissues, which are inhibited by a small molecule, triptonide, by activating p53 and DNA strand break for cell death (117). Insulin induces transgelin-2 via SREBP-1-mediated transcription, which has been a novel therapeutic target for diabetes-associated pancreatic ductal adenocarcinoma (118). High glucose can enhance the SREBP-1 level to promote tumor proliferation and suppress apoptosis and autophagy (119). A high expression of TNF-α is associated with significant reductions of SREBP-1, FASN, and ACC at the mRNA level in pancreatic ductal adenocarcinoma (120). Importantly, SREBP-1 is overexpressed in both pancreatic cancer tissues and cell lines, which is critical for cancer proliferation, apoptosis, tumor growth, and overall survival (41). Therefore, the SREBP-1 signaling axis has been a promising target for the prevention and treatment of pancreatic cancer.
Compared with controls, SREBP-1 is significantly increased and positively correlated with serum triglyceride in endometrial cancer (121). Ten single-nucleotide polymorphisms (SNPs) of SREBP-1 are associated with endometrial cancer, and rs2297508 SNP with the C allele accounts for 14%, which may serve as a genetic marker for early detection (122). In a progestin-resistant Ishikawa cell line, sirtuin 1 (SIRT1) and SREBP-1 at the mRNA and protein levels are upregulated, while progesterone receptor and forkhead transcription factor 1 (FoxO1) are downregulated, suggesting that the SIRT1/FoxO1/SREBP-1 pathway is involved in the progestin resistance of endometrial cancer (123). It is also found that FoxO1 can inhibit proliferative and invasive capacities by directly inhibiting SREBP-1 in this cancer (124). Salt-inducible kinase 2 can upregulate SREBP-1c to enhance fatty acid synthesis and SREBP-2 to promote cholesterol synthesis in in vitro and in vivo ovarian cancer models (125). In the obesity host, increased SREBP-1 is linked to ovarian cancer progression and metastasis (126) and meditates malignant characteristics, such as cell proliferation, migration, invasion, and tumor growth (127). In clear cell renal cell carcinoma (ccRCC) patients, high levels of E2F transcription factor 1 (E2F1) and SREBP-1 are associated with poor prognosis. E2F1 can increase ccRCC cell proliferation and epithelial–mesenchymal transition (EMT) and promote tumor progression of a mouse xenograft model by inhibiting SREBP-1-mediated aberrant lipid metabolism (128). In bladder cancer, PKM2 interacts with SREBP-1c through the regulation of the Akt/mTOR signaling pathway, which in turn activates FASN transcription for tumor growth (129). Collectively, SREBP-1-regulated lipid metabolism is essential for the cancer progression of the urinogenital system, including endometrial, ovarian, and bladder cancer and renal cell carcinomas. The results from cell lines and clinical tissues demonstrate that long intergenic non-protein-coding RNA 02570 can adsorb miRNA-4649-3p to upregulate SREBP-1 and FASN, which promotes the progression of nasopharyngeal carcinoma (NPC) (130). Epstein–Barr virus-encoded latent membrane protein 1 is expressed in NPC and increases the maturation and activation of SREBP-1 to induce de novo lipid synthesis in the tumor growth and metastasis, which can be inhibited by two inhibitors, luteolin and fatostatin (131). In oral squamous cell carcinoma, glutathione peroxidase 4 (GPX4) can reduce the ferroptosis-mediated cell number via downregulation of SREBP-1 (132). Compared with normal thyroid tissue or benign thyroid nodules, SREBP1 expression is significantly higher in invasive thyroid cancer, which is associated with extrathyroidal extension, advanced stage, and shorter survival of patients (28, 133). The in vitro results demonstrate that SREBP-1 can obviously increase the rate of oxygen consumption and the capacities of invasion and migration in thyroid cancer cells, which is mediated by the upregulation of the Hippo-YAP/CYR61/CTGF pathway (133). In addition, the nuclear form of SREBP-1 (nSREBP-1) can bind to the promoter of protein kinase RNA-like endoplasmic reticulum kinase (PERK) to augment its expression and phosphorylation, which might be the main reason that nSREBP-1 regulates cell proliferation, cell cycle arrest, apoptosis, and autophagy under endoplasmic reticulum (ER) stress in sarcomas (chondrosarcoma and osteosarcoma) (134). Together, SREBP-1 is upregulated and participates in the progression of NPC, oral squamous cell carcinoma, thyroid cancer, and sarcomas. Collectively, these findings indicate that the various key pathways that control SREBP-1 activation and SREBP-1-regulated lipogenesis significantly participate in tumor growth and progression and may be a promising target in multiple malignancies (Table 2, Figure 2).
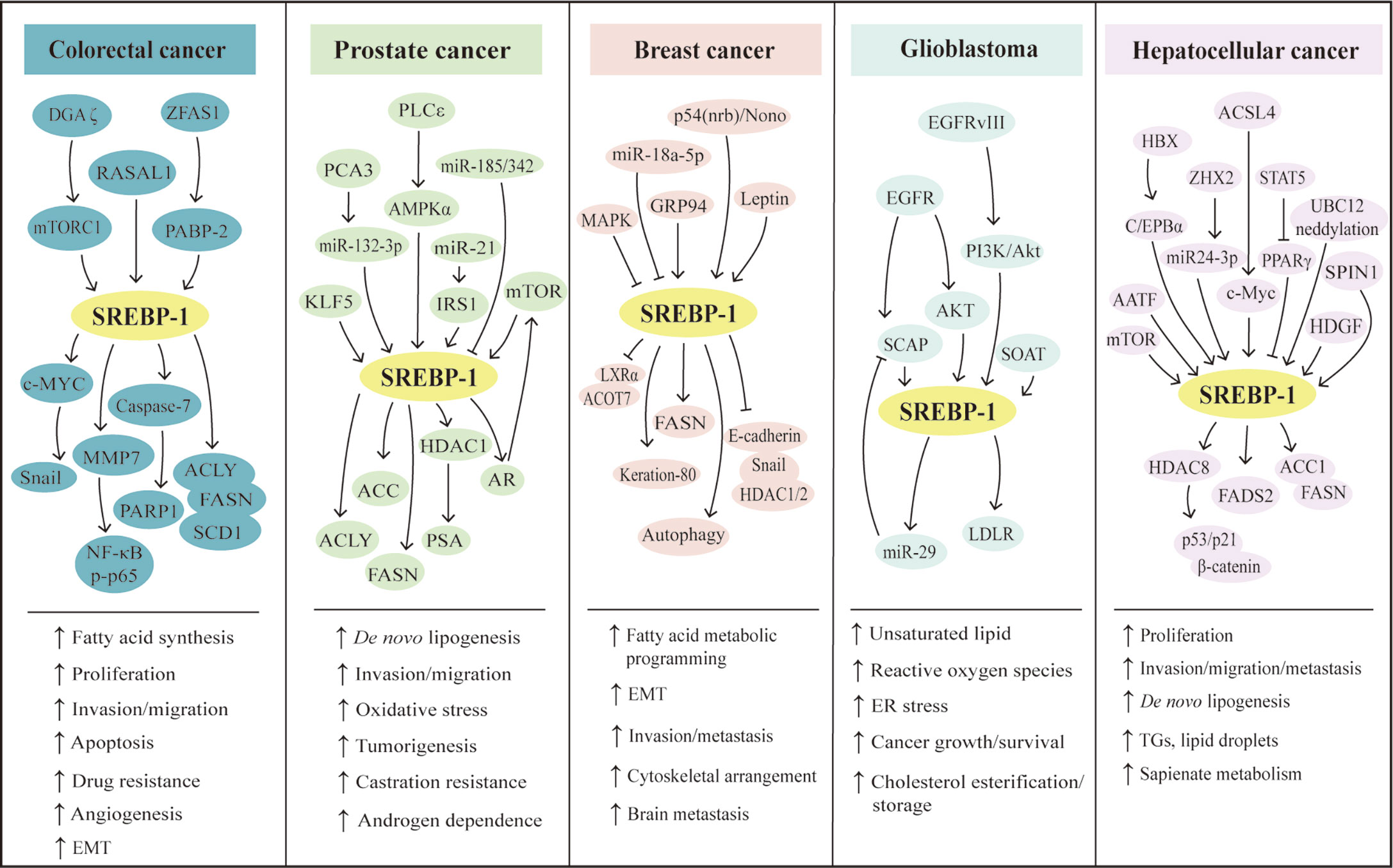
Figure 2 SREBP-1-mediated lipogenesis in the five types of cancers. Multiple pathways can regulate SREBP-1 and its downstream targets to mediate aggressive characteristics, including proliferation, invasion, migration, EMT, tumorigenesis, metastasis, angiogenesis, and drug resistance in colorectal, prostate, breast, hepatocellular cancer, and glioblastoma. Meanwhile, in these cancers, SREBP-1 activation can increase de novo lipogenesis and regulate fatty acid metabolic programming, lipid droplets, and sapienate metabolism. EMT: epithelial–mesenchymal transition, ER: endoplasmic reticulum, TGs: triglycerides.
Targeting the SREBP-1 signaling pathway for cancer therapy
As reported, SREBP-1 has been a potential target and its inhibition by small molecules or natural products has been potential therapeutics for preventing and treating cancer (12). A specific inhibitor of SREBP activation, fatostatin is a diarylthiazole derivative and binds to SCAP to inhibit the translocations of SREBP-1 and SREBP-2 from the ER to Golgi (135, 136). In prostate cancer, fatostatin suppresses cell proliferation and colony formation in both androgen-responsive or -insensitive cancer cells and causes G2/M cell cycle arrest and cell death, which is mediated by the blockade of the SREBP-regulated metabolic pathway and AR signaling network (136). Fatostatin can inhibit SREBP activity to influence the assembly of mitotic microtubule spindle and cell division in various cancer cells (137). In breast cancer, fatostatin is more sensitive to estrogen receptor-positive cells in response with cell cycle arrest and apoptosis through accumulation of lipids under ER stress, such as polyunsaturated fatty acids (PUFAs), not the inhibition of SREBP activity (138). Moreover, fatostatin can reverse progesterone resistance through inhibition of the SREBP-1/NF-κB pathway in endometrial cancer (139). In pancreatic cancer, both fatostatin and PF429242 inhibit cell proliferation and the growth of xenograft tumor by reducing SREBP-1 and its downstream signaling cascades, such as FASN and SCD-1 (17). Additionally, the combination of fatostatin with tamoxifen has a synergistic effect on the inhibition of PI3K/Akt/mTOR signaling in ER-positive breast cancer (140). Together, fatostatin mainly regulates the activation of SREBP-1/2 to block different cancer progressions, while it also regulates the accumulations of PUFAs and the PI3K/Akt/mTOR pathway. Nelfinavir, a HIV protease inhibitor, induces ER stress and caspase-dependent apoptosis and increases SREBP-1 protein half-life by blocking intracellular trafficking of SREBP-1 and activating transcription factor 6 (ATF6) from site-2 protease (S2P) inhibition in liposarcoma cells and xenograft mouse models (141). In prostate cancer, nelfinavir inhibits AR activation and nuclear translocation of SREBP-1 and ATF6 by regulating S2P-mediated intramembrane proteolysis, which is associated with ER stress, inhibition of unfolded protein response, apoptosis, and autophagy for treating castration-resistant prostate cancer (142). In addition, nelfinavir and its analogs, #6, #7, and #8, have more potent effects on S2P cleavage than 1,10-phenanthroline, a metalloprotease-specific inhibitor. These molecules can block S2P cleavage activity to lead to the accumulations of precursor SREBP-1 and ATF6 against castration-resistant prostate cancer (143). These findings indicate that nelfinavir is a potential agent targeting S2P cleavage for cancer therapy. Currently, several targeted drugs targeting tyrosine kinase can regulate the SREBP-1 pathway to play anticancer effects. Osimertinib, the first-approved third-generation EGFR inhibitor, facilitates SREBP1 degradation and reduces the levels of its targets and lipogenesis in EGFR-mutant NSCLC cells and tumors, which suggests an effective strategy for overcoming acquired resistance of EGFR inhibitors by targeting SREBP-1 (144). Sorafenib, a multikinase inhibitor targeting RAS/MEK/ERK, VEGFR, and PDGFR, significantly affects SCD-1 expression to decrease the synthesis of monounsaturated fatty acids and suppresses ATP production to activate AMPK, which can reduce the levels of SREBP-1 and phosphorylate mTOR for the inhibition of liver cancer (145).
Furthermore, several small molecules or new dosage forms are reported as the regulators for SREBP-1-regulated lipogenesis. An AR degradation enhancer, ASC-J9 suppresses PCa cell growth and invasion by the AR/SREBP-1/FASN pathway in AR-positive cells and PI3K/Akt/SREBP-1/FASN signaling in AR-negative cells, which indicates that it mainly suppresses FASN-mediated PCa progression in both AR-dependent/independent manners (146). A newly developed AR antagonist, proxalutamide, significantly inhibits proliferation and migration, induces the caspase-dependent apoptosis, and diminishes the level of lipid droplets in PCa cells by regulating the levels of ACL, ACC, FASN, and SREBP-1. Moreover, proxalutamide can decrease AR expression in PCa cells, which may overcome the resistance of AR-targeted therapy (147). Leelamine, a pyruvate dehydrogenase kinase inhibitor, can downregulate the expressions of SREBP-1 and key fatty acid synthesis enzymes (ACLY, ACC1, FASN) at the mRNA and protein levels to suppress fatty acid synthesis against PCa progression (148). An HDAC inhibitor, valproic acid can inhibit prostate cancer cell viability and induce apoptosis by regulating the C/EBPα/SREBP-1 pathway based on the results of in vitro and in vivo experiments (149). A novel anti-beta2-microglobulin monoclonal antibody decreases cell proliferation, induces massive cell death, and decreases AR expression via inhibition of SREBP-1-mediated fatty acid synthesis in the models of multiple PCa cell lines and xenograft tumor (150). N-Arachidonoyl dopamine, a typical representative of N-acyl dopamines, can inhibit breast cancer cell migration, EMT, and stemness and cause decreased cholesterol biosynthesis by inhibiting SREBP-1, its key targets, and endoplasmic reticulum kinase 1/2 (ERK1/2) pathways (151). A novel small-molecule, SI-1, 1-(4-bromophenyl)-3-(pyridin-3-yl) urea inhibits aerobic glycolysis and enhances the antitumor effect of radiofrequency ablation in the HCC cells and xenograft tumors via inhibition of SREBP-1 activation (152). The treatment of docosahexaenoic acid, but neither n-6 PUFA arachidonic acid nor oleic acid, can inhibit the levels of the precursor of SREBP-1 and its mature form, and FASN is induced by estradiol and insulin to mediate breast cancer proliferation, which is the result from reduced phosphorylated Akt, not from ERK1/2 phosphorylation (153). In different GBM cells, phytol and retinol show cytotoxic effects at dose dependence, which might be mediated by the levels of SREBP-1, FASN, and farnesyl-diphosphate farnesyltransferase (FDFT1) to downregulate cholesterol and/or fatty acid biosynthetic pathways (154). In addition, it is reported that platinum complexes or micelles can regulate the SREBP-1 pathway to play a more effective function against cancer growth and progression. The pyridine co-ligand-functionalized cationic complexes, including C2, C6, and C8, can suppress cancer invasion, migration, and tumor spheroid formation through inhibition of SREBP-1-mediated lipid biogenesis (155). PB@LC/D/siR is synthesized by cross-linking for docetaxel and siSREBP1 delivery fused with PCa cell membranes and bone marrow mesenchymal stem cells, which show the enhanced antitumor effects in bone metastatic castration-resistant PCa, with the characteristics of deep tumor penetration, high safety, and bone protection via downregulation of SREBP-1 and SCD-1 at the mRNA level (156).
Importantly, natural products can regulate SREBP-1-regulated lipogenesis for the prevention and treatment of different cancers, including CRC, PCa, HCC, and breast cancer. In CRC, there are four compounds for regulating multiple signaling pathway-medicated SREBP-1 activation. One of ginger derivatives, 6-shogaol, can attenuate the adipocyte-conditioned medium effect by controlling the SREBP-1 level mediated by Akt, p70S6K, and AMPK signaling pathways in 5-FU-treated CRC (157). Ilexgenin A, the main bioactive compound from Ilex hainanensis Merr., significantly inhibits inflammatory colitis of mice induced by azoxymethane/dextran sulfate sodium and reverses the metabolites associated with colorectal cancer, which might be mediated by reprogramed lipid metabolism of the HIF-1α/SREBP-1 pathway (158). RA-XII, a natural cyclopeptide isolated from Rubia yunnanensis, decreases the motility of HCT 116 cells via inhibition of the β-catenin pathway and inhibits CRC growth and metastasis by downregulating the levels of SREBP-1, FASN, and SCD for restraining lipogenesis (159). Berberine inhibits colon cancer cell proliferation and induces cell cycle arrest of the G0/G1 phase via the Wnt/β-catenin pathway. Importantly, berberine blocks SREBP-1 activation and SCAP expression to downregulate the levels of lipogenic enzymes against tumor growth (160). The studies report that three compounds have effective anti-HCC effects by inhibiting SREBP-1 and its downstream targets. Cinobufotalin is extracted from the skin secretion of the giant toad and can effectively promote cell apoptosis, induce cell cycle G2/M arrest, and inhibit cell proliferation by downregulating SREBP-1 expression and the interaction with sterol regulatory elements for the inhibition of de novo lipid synthesis in HCC (161). Moreover, emodin, a main active component from Reynoutria multiflora (Thunb.) Moldenke, triggers apoptosis and reduces mitochondrial membrane potential by regulating intrinsic apoptosis signaling pathway in HCC cells. SREBP-1 and its downstream targets, such as ACLY, ACCα, FASN, and SCD-1, are inhibited to mediate the anticancer effect of emodin against HCC (162). Additionally, In HCC cells and xenograft tumors, betulin treatment inhibits cellular glucose metabolism to prevent metastatic potential and also facilitates the inhibitory effect of sorafenib on HCC through inhibition of SREBP-1 (163). In breast cancer cells, theanaphthoquinone, a member of the thearubigins generated by the oxidation of theaflavin, can block the EGF-induced nuclear translocation of SREBP-1 and also modulate the phosphorylation of ERK1/2, Akt, and EGFR/ErbB-2 induced by EGF, which can cause the blockade of FASN for the inhibition of cell viability and induction of cell death (164). Furthermore, in HER2-overexpressing breast cancer cells, piperineis strongly inhibits proliferation, induces apoptosis, and enhances paclitaxel sensitization through inhibition of SREBP-1/FASN signaling, which might be mediated by HER2 expression, ERK1/2, p38, and Akt signaling pathways (165). Additionally, vitexin and syringic acid from foxtail millet bran can inhibit breast cancer proliferation and block the conversion of saturated fatty acids to monounsaturated fatty acids, which is mediated by the decreases of glucose regulated protein 78 and SREBP-1, and its target, SCD-1 (166).
In NSCLC, ginsenoside Rh2 suppresses SREBP-1 expression and its nuclear translocation to disturb the interaction of SREBP-1 and FASN, which can enhance the immune effect and have a synergistic antitumor effect with cyclophosphamide (167). In gallbladder cancer, α-mangostin, a dietary xanthone, represses the proliferation, clone formation ability, and de novo lipogenesis; induces cell cycle arrest and apoptosis; and enhances gemcitabine sensitivity of gallbladder cancer cells, which might be mediated by AMPK activation and the inhibition of nuclear SREBP-1 translocation in cancer cells and tumor xenograft models (168). In cervical carcinoma, quercetin, a naturally occurring dietary flavonoid, decreases cell proliferation and induces cell death in Hela cells by reducing the O-GlcNAcylation of AMPK and the interaction of OGT and SREBP-1 (169). In both in vitro and in vivo models of pancreatic cancer, resveratrol induces gemcitabine chemosensitivity and suppresses sphere formation and markers of cancer stem cells by targeting SREBP-1, which indicates that resveratrol can be an effective sensitizer for chemotherapy (170). Timosaponin A3 can inhibit SREBP-1 and its targets, FASN and ACC, to reduce cell viability and increase cell cycle arrest and apoptosis of BxPC-3 cells and pancreatic cancer xenograft growth, which is independent in the Akt/GSK-3β pathway (171).
Besides these natural compounds, the extract, polysaccharides, or decoction from various herbs can also regulate the SREBP-1-mediated lipogenesis against PCa and pancreatic cancer growth and progression. In PCa, the ethanol extract of Ganoderma tsugae, a Chinese natural and herbal product, significantly inhibits the expressions of SREBP-1 and its downstream genes associated with lipogenesis and downregulates the levels of AR and PSA to block cancer growth and progression with androgen response and castration resistance (172). The results from the in vitro and in vivo experiments demonstrated that the ethanol extract of Davallia formosana can suppress proliferation, migration, and invasion in PCa cells by inhibiting the levels of SREBP-1, FASN, AR, and PSA, suggesting that SREBP-1/FASN/lipogenesis and the AR axis could be potential targets for the treatment of PCa (173). The cell suspension culture extract from Eriobotrya japonica significantly inhibits PCa cell growth, migration, and invasion by decreasing the SREBP-1/FASN-mediated lipid metabolism and AR signaling pathway in the cell and mouse models (174). Moreover, the polysaccharides from Astragalus membranaceus (APS) greatly inhibit the proliferation and invasion of PCa cells in a dose-dependent and time-dependent manner. Mechanistic studies demonstrate that APS treatment reduces the expressions of miR-138-5p, SIRT1, and SREBP-1 to block tumorigenesis and lipid metabolism in PCa (175). In addition, a traditional herbal decoction TJ001 has significant cytotoxicity, induces cell cycle arrest at the G1/S stage, and inhibits lipid accumulation in D145 and PCa cells with p53 mutation by regulating the ACC expression, SREBP-1 proteolytic cleavage, and the inhibition of AMPK/mTOR, which suggest that the combination of mutant p53 targeting and TJ001 can be considered as the potential strategy for PCa treatment (176). In pancreatic cancer, the CO2 supercritical extract of Yarrow (Achillea millefolium) can downregulate the levels of SREBP-1, FASN, and SCD to induce cytotoxicity in cancer cells and diminish tumor growth of xenograft mouse models, which can be developed as a complementary adjuvant or nutritional supplement (177). Taken together, the current findings of targeting SREBP-1-mediated lipogenesis by small molecules, natural products, or herb extracts are summarized in Table 3 and Figure 3.
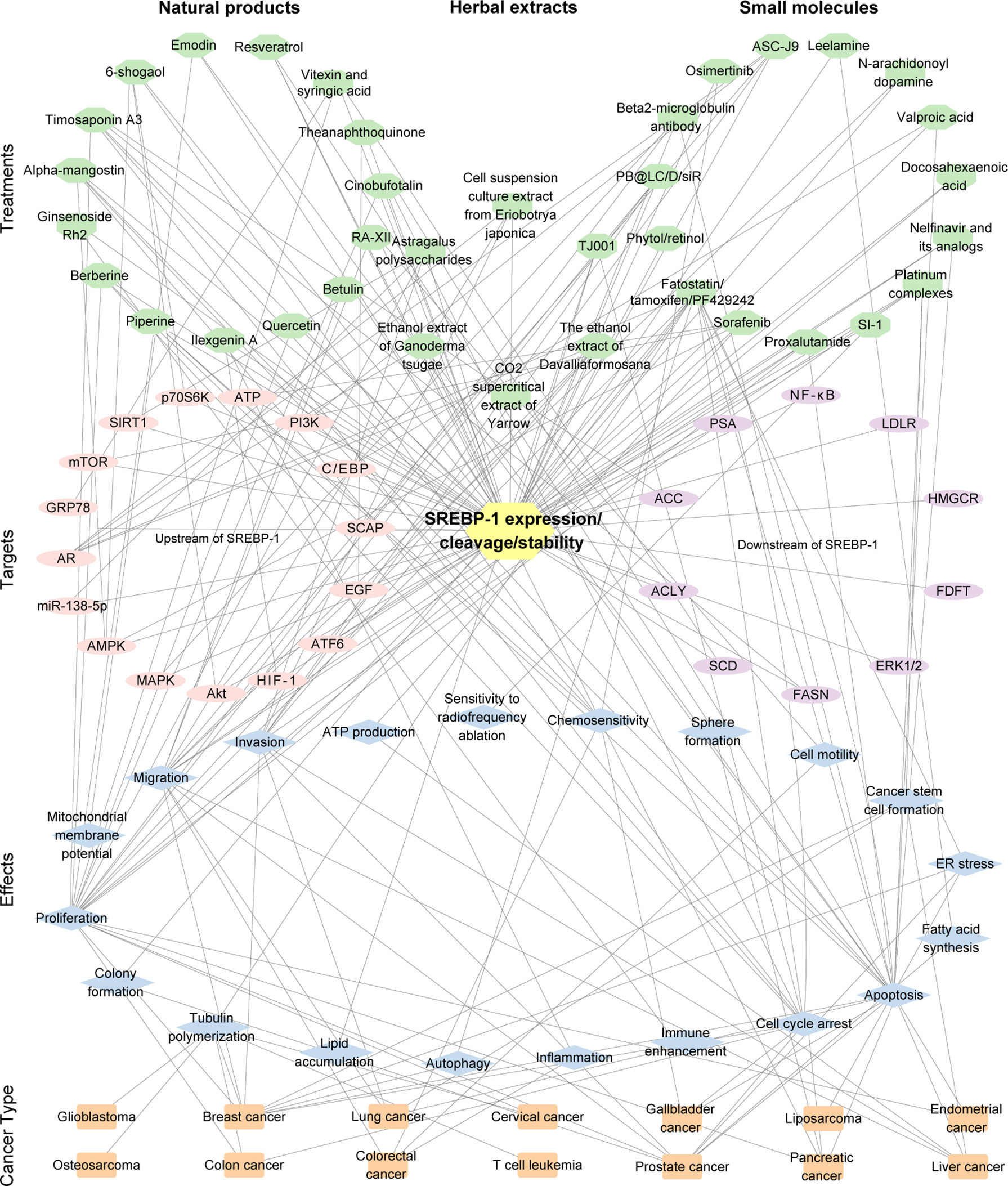
Figure 3 The interaction of the treatment by small molecules, natural products, or the extract of herbs, the targets (SREBP-1 expression/cleavage/stability), effects, and cancer types. The pink/purple circles represent upstream and downstream targets of SREBP-1. The blue diamonds represent the effects from the inhibition of the SREBP-1 pathway. The orange rectangles represent cancer types. The number of edges connected between the nodes in the network represents the count of their connections.
Conclusions
This review mainly summarized the recent findings of SREBP-1-mediated lipogenesis in different cancer and as potential targets by small molecules, natural products, or the extracts of herbs against cancer growth and progression. Multiple signaling pathways, such as EGFR and PI3K/Akt/mTOR, control SREBP-1 expression and activation to regulate the transcription of multiple genes for fatty acid synthesis (ACLY, ACC, FASN, SCD-1/5) and lipid uptake (LDLR) in human cancer. Multiple signaling molecules can regulate SREBP-1 expression, activation, stability, and binding. Furthermore, SREBP-1 can regulate downstream signaling pathways to mediate cancer proliferation, apoptosis, endoplasmic reticulum stress, and epithelial–mesenchymal transition for tumor growth and metastasis of different cancers, including colon, prostate, breast, lung, and hepatocellular cancer. Additionally, many studies have demonstrated that fatostatin, nelfinavir, AR antagonist, natural compounds, or herbal extracts can target SREBP-1 and its downstream targets for the inhibition of lipid biosynthesis in tumor growth and progression of various cancers. This review could provide new insights into the critical function of the SREBP-1-regulated lipogenesis in different cancers and its potential for cancer therapy for targeting SREBP-1.
Critically, several issues and future perspectives are concerned, as in the following: 1) Targeting SREBP-1 can inhibit multiple enzymes in lipogenesis to significantly block cancer growth and aggressive progression, which is a promising strategy of multitarget therapeutics. 2) Current studies of targeting the SREBP-1 pathway against cancer are preclinical studies for investigating their effects and molecular mechanisms. It is necessary to conduct a clinical evaluation of SREBP-1-targeted therapy against cancer in future. 3) Except its role in cancer, SREBP-1 also plays a critical role in lipid metabolism of metabolic-related diseases. At present, no research is reported showing that those treatments targeting SREBP-1 have side effects, which might be related with the lower level of SREBP-1 in normal tissues, compared to that in cancer. The development of new and specific drugs targeting SREBP-1 with less or no toxic should be potential research directions.
Author Contributions
QZ, XL, and GW: conceptualization, writing for original preparation. XL and GW: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82100076). The funder had no role in the decision to publish or in the preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
SREBPs: sterol regulatory element-binding proteins
bHLH-LZ: basic helix–loop–helix leucine zipper
GBM: glioblastoma
EGFR: epidermal growth factor receptor
PI3K: phosphatidylinositol 3-kinase
PKB: protein kinase B
mTOR: mammalian target of rapamycin
SCAP: SREBP cleavage-activating protein
SREs: sterol regulatory elements
ACLY: ATP citrate lyase
ACC: acetyl-CoA carboxylase
FASN: fatty acid synthase
SCD-1/5: stearoyl-CoA desaturase 1 and 5
LDLR: low-density lipoprotein receptor
mTORC1: mammalian/mechanistic target of rapamycin complex 1
GS: glutamine synthetase
O-GlcNAcylation: O-linked N-acetylglucosaminylation
Sp1: specificity protein 1
OGT: O-GlcNAc transferase
AMPK: AMP-activated protein kinase
sCLU: secretory clusterin
TDG: thymine DNA glycosylase
MAP: mitogen-activated protein
CRC: colorectal cancer
SNAIL: snail family transcriptional repressor 1
AR: androgen receptor
KLF5: Kruppel-like factor 5
HMGCR: hydroxy-3-methylglutaryl CoA reductase
GRP94: glucose-regulated protein 94
EGFRvIII: EGFR-activating mutation
HDGF: hepatoma-derived growth factor
AATF: apoptosis-antagonizing transcription factors
ZHX2: zinc fingers and homeoboxes 2
ACS: acyl-CoA synthetases
ACSL4: acyl CoA synthetase 4
SPIN1: spindlin 1
HBV: hepatitis B virus
HBx: hepatitis B virus-encoded X protein
STAT5: signal transducers and activators of transcription 5
GEO: Gene Expression Omnibus
TNM: tumor-node metastasis
PCK1: phosphoenolpyruvate carboxykinase 1
NSCLC: non-small cell lung cancer
PTMI5: protein arginine methyltransferase 5
DNFA: de novo fatty acid biosynthesis
SCCs: squamous cell carcinomas
TP63: tumor protein 63
ZEB1: zinc finger E-box-binding homeobox 1
LXRs: liver X receptors
PNKP: polynucleotide kinase/phosphatase
SNPs: single-nucleotide polymorphisms
SIRT1: sirtuin 1
FoxO1: forkhead transcription factor 1
ccRCC: clear cell renal cell carcinoma
E2F1: E2F transcription factor 1
EMT: epithelial–mesenchymal transition
NPC: nasopharyngeal carcinoma
GPX4: glutathione peroxidase 4
nSREBP-1: nuclear form of SREBP-1
PERK: protein kinase RNA-like endoplasmic reticulum kinase
ER: endoplasmic reticulum
PUFAs: polyunsaturated fatty acids
ATF6: activating transcription factor 6
S2P: site-2 protease
ERK1/2: endoplasmic reticulum kinase 1/2
FDFT1: farnesyl-diphosphate farnesyltransferase
APS: polysaccharides from Astragalus membranaceus.
References
1. Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, et al. Srebp-1, a Basic-Helix-Loop-Helix-Leucine Zipper Protein That Controls Transcription of the Low Density Lipoprotein Receptor Gene. Cell (1993) 75(1):187–97. doi: 10.1016/S0092-8674(05)80095-9
2. Hua X, Yokoyama C, Wu J, Briggs MR, Brown MS, Goldstein JL, et al. Srebp-2, a Second Basic-Helix-Loop-Helix-Leucine Zipper Protein That Stimulates Transcription by Binding to a Sterol Regulatory Element. Proc Natl Acad Sci USA (1993) 90(24):11603–7. doi: 10.1073/pnas.90.24.11603
3. Brown MS, Goldstein JL. The Srebp Pathway: Regulation of Cholesterol Metabolism by Proteolysis of a Membrane-Bound Transcription Factor. Cell (1997) 89(3):331–40. doi: 10.1016/S0092-8674(00)80213-5
4. Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, et al. Transcriptional Activities of Nuclear Srebp-1a, -1c, and -2 to Different Target Promoters of Lipogenic and Cholesterogenic Genes. J Lipid Res (2002) 43(8):1220–35. doi: 10.1194/jlr.M100417-JLR200
5. Horton JD, Goldstein JL, Brown MS. Srebps: Activators of the Complete Program of Cholesterol and Fatty Acid Synthesis in the Liver. J Clin Invest (2002) 109(9):1125–31. doi: 10.1172/JCI15593
6. Harada N, Yonemoto H, Yoshida M, Yamamoto H, Yin Y, Miyamoto A, et al. Alternative Splicing Produces a Constitutively Active Form of Human Srebp-1. Biochem Biophys Res Commun (2008) 368(3):820–6. doi: 10.1016/j.bbrc.2008.02.004
7. Rome S, Lecomte V, Meugnier E, Rieusset J, Debard C, Euthine V, et al. Microarray Analyses of Srebp-1a and Srebp-1c Target Genes Identify New Regulatory Pathways in Muscle. Physiol Genomics (2008) 34(3):327–37. doi: 10.1152/physiolgenomics.90211.2008
8. Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. Srebp Transcription Factors: Master Regulators of Lipid Homeostasis. Biochimie (2004) 86(11):839–48. doi: 10.1016/j.biochi.2004.09.018
9. Shimano H, Sato R. Srebp-Regulated Lipid Metabolism: Convergent Physiology - Divergent Pathophysiology. Nat Rev Endocrinol (2017) 13(12):710–30. doi: 10.1038/nrendo.2017.91
10. Ferre P, Foufelle F. Srebp-1c Transcription Factor and Lipid Homeostasis: Clinical Perspective. Horm Res (2007) 68(2):72–82. doi: 10.1159/000100426
11. Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of Sterol Regulatory Element-Binding Protein 1 in Regulation of Renal Lipid Metabolism and Glomerulosclerosis in Diabetes Mellitus. J Biol Chem (2002) 277(21):18919–27. doi: 10.1074/jbc.M110650200
12. Guo D, Bell EH, Mischel P, Chakravarti A. Targeting Srebp-1-Driven Lipid Metabolism to Treat Cancer. Curr Pharm Design (2014) 20(15):2619–26. doi: 10.2174/13816128113199990486
13. Huang WC, Li X, Liu J, Lin J, Chung LW. Activation of Androgen Receptor, Lipogenesis, and Oxidative Stress Converged by Srebp-1 Is Responsible for Regulating Growth and Progression of Prostate Cancer Cells. Mol Cancer Res (2012) 10(1):133–42. doi: 10.1158/1541-7786.MCR-11-0206
14. Bao J, Zhu L, Zhu Q, Su J, Liu M, Huang W. Srebp-1 Is an Independent Prognostic Marker and Promotes Invasion and Migration in Breast Cancer. Oncol Lett (2016) 12(4):2409–16. doi: 10.3892/ol.2016.4988
15. Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D, et al. An Lxr Agonist Promotes Glioblastoma Cell Death Through Inhibition of an Egfr/Akt/Srebp-1/Ldlr-Dependent Pathway. Cancer Discovery (2011) 1(5):442–56. doi: 10.1158/2159-8290.CD-11-0102
16. Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, et al. Pkb/Akt Induces Transcription of Enzymes Involved in Cholesterol and Fatty Acid Biosynthesis Via Activation of Srebp. Oncogene (2005) 24(43):6465–81. doi: 10.1038/sjrnc.1208802
17. Siqingaowa, Sekar S, Gopalakrishnan V, Taghibiglou C. Sterol Regulatory Element-Binding Protein 1 Inhibitors Decrease Pancreatic Cancer Cell Viability and Proliferation. Biochem Biophys Res Commun (2017) 488(1):136–40. doi: 10.1016/j.bbrc.2017.05.023
18. Kanagasabai T, Li G, Shen TH, Gladoun N, Castillo-Martin M, Celada SI, et al. Microrna-21 Deficiency Suppresses Prostate Cancer Progression Through Downregulation of the Irs1-Srebp-1 Signaling Pathway. Cancer Lett (2022) 525:46–54. doi: 10.1016/j.canlet.2021.09.041
19. Hua X, Wu J, Goldstein JL, Brown MS, Hobbs HH. Structure of the Human Gene Encoding Sterol Regulatory Element Binding Protein-1 (Srebf1) and Localization of Srebf1 and Srebf2 to Chromosomes 17p11.2 and 22q13. Genomics (1995) 25(3):667–73. doi: 10.1016/0888-7543(95)80009-B
20. Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential Expression of Exons 1a and 1c in Mrnas for Sterol Regulatory Element Binding Protein-1 in Human and Mouse Organs and Cultured Cells. J Clin Invest (1997) 99(5):838–45. doi: 10.1172/JCI119247
21. Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of Sterol Regulatory Element Binding Protein Is Less Active Than Isoform 1a in Livers of Transgenic Mice and in Cultured Cells. J Clin Invest (1997) 99(5):846–54. doi: 10.1172/JCI119248
22. Hughes AL, Todd BL, Espenshade PJ. Srebp Pathway Responds to Sterols and Functions as an Oxygen Sensor in Fission Yeast. Cell (2005) 120(6):831–42. doi: 10.1016/j.cell.2005.01.012
23. Guillet-Deniau I, Mieulet V, Le Lay S, Achouri Y, Carre D, Girard J, et al. Sterol Regulatory Element Binding Protein-1c Expression and Action in Rat Muscles: Insulin-Like Effects on the Control of Glycolytic and Lipogenic Enzymes and Ucp3 Gene Expression. Diabetes (2002) 51(6):1722–8. doi: 10.2337/diabetes.51.6.1722
24. Ruiz R, Jideonwo V, Ahn M, Surendran S, Tagliabracci VS, Hou Y, et al. Sterol Regulatory Element-Binding Protein-1 (Srebp-1) Is Required to Regulate Glycogen Synthesis and Gluconeogenic Gene Expression in Mouse Liver. J Biol Chem (2014) 289(9):5510–7. doi: 10.1074/jbc.M113.541110
25. Ferre P, Phan F, Foufelle F. Srebp-1c and Lipogenesis in the Liver: An Update1. Biochem J (2021) 478(20):3723–39. doi: 10.1042/BCJ20210071
26. Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG, et al. Linking Lipid Metabolism to the Innate Immune Response in Macrophages Through Sterol Regulatory Element Binding Protein-1a. Cell Metab (2011) 13(5):540–9. doi: 10.1016/j.cmet.2011.04.001
27. Wu S, Naar AM. Srebp1-Dependent De Novo Fatty Acid Synthesis Gene Expression Is Elevated in Malignant Melanoma and Represents a Cellular Survival Trait. Sci Rep (2019) 9(1):10369. doi: 10.1038/s41598-019-46594-x
28. Li C, Peng X, Lv J, Zou H, Liu J, Zhang K, et al. Srebp1 as a Potential Biomarker Predicts Levothyroxine Efficacy of Differentiated Thyroid Cancer. Biomed Pharmacother = Biomed Pharmacotherapie (2020) 123:109791. doi: 10.1016/j.biopha.2019.109791
29. Wang X, Sato R, Brown MS, Hua X, Goldstein JL. Srebp-1, a Membrane-Bound Transcription Factor Released by Sterol-Regulated Proteolysis. Cell (1994) 77(1):53–62. doi: 10.1016/0092-8674(94)90234-8
30. DeBose-Boyd RA, Brown MS, Li WP, Nohturfft A, Goldstein JL, Espenshade PJ. Transport-Dependent Proteolysis of Srebp: Relocation of Site-1 Protease From Golgi to Er Obviates the Need for Srebp Transport to Golgi. Cell (1999) 99(7):703–12. doi: 10.1016/s0092-8674(00)81668-2
31. Goldstein JL, DeBose-Boyd RA, Brown MS. Protein Sensors for Membrane Sterols. Cell (2006) 124(1):35–46. doi: 10.1016/j.cell.2005.12.022
32. Hegarty BD, Bobard A, Hainault I, Ferre P, Bossard P, Foufelle F. Distinct Roles of Insulin and Liver X Receptor in the Induction and Cleavage of Sterol Regulatory Element-Binding Protein-1c. Proc Natl Acad Sci United States America (2005) 102(3):791–6. doi: 10.1073/pnas.0405067102
33. Cagen LM, Deng X, Wilcox HG, Park EA, Raghow R, Elam MB. Insulin Activates the Rat Sterol-Regulatory-Element-Binding Protein 1c (Srebp-1c) Promoter Through the Combinatorial Actions of Srebp, Lxr, Sp-1 and Nf-Y Cis-Acting Elements. Biochem J (2005) 385(Pt 1):207–16. doi: 10.1042/BJ20040162
34. Nakakuki M, Kawano H, Notsu T, Imada K, Mizuguchi K, Shimano H. A Novel Processing System of Sterol Regulatory Element-Binding Protein-1c Regulated by Polyunsaturated Fatty Acid. J Biochem (2014) 155(5):301–13. doi: 10.1093/jb/mvu019
35. Nakakuki M, Shimano H, Inoue N, Tamura M, Matsuzaka T, Nakagawa Y, et al. A Transcription Factor of Lipid Synthesis, Sterol Regulatory Element-Binding Protein (Srebp)-1a Causes G(1) Cell-Cycle Arrest After Accumulation of Cyclin-Dependent Kinase (Cdk) Inhibitors. FEBS J (2007) 274(17):4440–52. doi: 10.1111/j.1742-4658.2007.05973.x
36. Motallebipour M, Enroth S, Punga T, Ameur A, Koch C, Dunham I, et al. Novel Genes in Cell Cycle Control and Lipid Metabolism With Dynamically Regulated Binding Sites for Sterol Regulatory Element-Binding Protein 1 and Rna Polymerase Ii in Hepg2 Cells Detected by Chromatin Immunoprecipitation With Microarray Detection. FEBS J (2009) 276(7):1878–90. doi: 10.1111/j.1742-4658.2009.06914.x
37. Lecomte V, Meugnier E, Euthine V, Durand C, Freyssenet D, Nemoz G, et al. A New Role for Sterol Regulatory Element Binding Protein 1 Transcription Factors in the Regulation of Muscle Mass and Muscle Cell Differentiation. Mol Cell Biol (2010) 30(5):1182–98. doi: 10.1128/MCB.00690-09
38. Wu Y, Chen K, Liu X, Huang L, Zhao D, Li L, et al. Srebp-1 Interacts With C-Myc to Enhance Somatic Cell Reprogramming. Stem Cells (2016) 34(1):83–92. doi: 10.1002/stem.2209
39. Varghese JF, Patel R, Yadav UCS. Sterol Regulatory Element Binding Protein (Srebp) -1 Mediates Oxidized Low-Density Lipoprotein (Oxldl) Induced Macrophage Foam Cell Formation Through Nlrp3 Inflammasome Activation. Cell signalling (2019) 53:316–26. doi: 10.1016/j.cellsig.2018.10.020
40. Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, et al. Egfr Signaling Through an Akt-Srebp-1-Dependent, Rapamycin-Resistant Pathway Sensitizes Glioblastomas to Antilipogenic Therapy. Sci Signaling (2009) 2(101):ra82. doi: 10.1126/scisignal.2000446
41. Sun Y, He W, Luo M, Zhou Y, Chang G, Ren W, et al. Srebp1 Regulates Tumorigenesis and Prognosis of Pancreatic Cancer Through Targeting Lipid Metabolism. Tumour Biol (2015) 36(6):4133–41. doi: 10.1007/s13277-015-3047-5
42. Li C, Yang W, Zhang J, Zheng X, Yao Y, Tu K, et al. Srebp-1 Has a Prognostic Role and Contributes to Invasion and Metastasis in Human Hepatocellular Carcinoma. Int J Mol Sci (2014) 15(5):7124–38. doi: 10.3390/ijms15057124
43. Kikuchi K, Tsukamoto H. Stearoyl-Coa Desaturase and Tumorigenesis. Chemico-biological Interact (2020) 316:108917. doi: 10.1016/j.cbi.2019.108917
44. Icard P, Wu Z, Fournel L, Coquerel A, Lincet H, Alifano M. Atp Citrate Lyase: A Central Metabolic Enzyme in Cancer. Cancer Lett (2020) 471:125–34. doi: 10.1016/j.canlet.2019.12.010
45. Fhu CW, Ali A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules (2020) 25(17):3935. doi: 10.3390/molecules25173935
46. Wang C, Ma J, Zhang N, Yang Q, Jin Y, Wang Y. The Acetyl-Coa Carboxylase Enzyme: A Target for Cancer Therapy? Expert Rev Anticancer Ther (2015) 15(6):667–76. doi: 10.1586/14737140.2015.1038246
47. Cheng C, Ru P, Geng F, Liu J, Yoo JY, Wu X, et al. Glucose-Mediated N-Glycosylation of Scap Is Essential for Srebp-1 Activation and Tumor Growth. Cancer Cell (2015) 28(5):569–81. doi: 10.1016/j.ccell.2015.09.021
48. Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic Activation of Pi3k-Akt-Mtor Signaling Suppresses Ferroptosis Via Srebp-Mediated Lipogenesis. Proc Natl Acad Sci United States America (2020) 117(49):31189–97. doi: 10.1073/pnas.2017152117
49. Bakan I, Laplante M. Connecting Mtorc1 Signaling to Srebp-1 Activation. Curr Opin Lipidology (2012) 23(3):226–34. doi: 10.1097/MOL.0b013e328352dd03
50. de la Cruz Lopez KG, Toledo Guzman ME, Sanchez EO, Garcia Carranca A. Mtorc1 as a Regulator of Mitochondrial Functions and a Therapeutic Target in Cancer. Front Oncol (2019) 9:1373. doi: 10.3389/fonc.2019.01373
51. Jhu JW, Yan JB, Lin ZH, Lin SC, Peng IC. Srebp1-Induced Glutamine Synthetase Triggers a Feedforward Loop to Upregulate Srebp1 Through Sp1 O-Glcnacylation and Augments Lipid Droplet Formation in Cancer Cells. Int J Mol Sci (2021) 22(18):9814. doi: 10.3390/ijms22189814
52. Sodi VL, Bacigalupa ZA, Ferrer CM, Lee JV, Gocal WA, Mukhopadhyay D, et al. Nutrient Sensor O-Glcnac Transferase Controls Cancer Lipid Metabolism Via Srebp-1 Regulation. Oncogene (2018) 37(7):924–34. doi: 10.1038/onc.2017.395
53. Kim MJ, Choi MY, Lee DH, Roh GS, Kim HJ, Kang SS, et al. O-Linked N-Acetylglucosamine Transferase Enhances Secretory Clusterin Expression Via Liver X Receptors and Sterol Response Element Binding Protein Regulation in Cervical Cancer. Oncotarget (2018) 9(4):4625–36. doi: 10.18632/oncotarget.23588
54. Yan JB, Lai CC, Jhu JW, Gongol B, Marin TL, Lin SC, et al. Insulin and Metformin Control Cell Proliferation by Regulating Tdg-Mediated DNA Demethylation in Liver and Breast Cancer Cells. Mol Ther Oncolytics (2020) 18:282–94. doi: 10.1016/j.omto.2020.06.010
55. Liu C, Chikina M, Deshpande R, Menk AV, Wang T, Tabib T, et al. Treg Cells Promote the Srebp1-Dependent Metabolic Fitness of Tumor-Promoting Macrophages Via Repression of Cd8(+) T Cell-Derived Interferon-Gamma. Immunity (2019) 51(2):381–97.e6. doi: 10.1016/j.immuni.2019.06.017
56. Koizume S, Takahashi T, Yoshihara M, Nakamura Y, Ruf W, Takenaka K, et al. Cholesterol Starvation and Hypoxia Activate the Fvii Gene Via the Srebp1-Gilz Pathway in Ovarian Cancer Cells to Produce Procoagulant Microvesicles. Thromb Haemostasis (2019) 119(7):1058–71. doi: 10.1055/s-0039-1687876
57. Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES. Activation of Fatty Acid Synthesis During Neoplastic Transformation: Role of Mitogen-Activated Protein Kinase and Phosphatidylinositol 3-Kinase. Exp Cell Res (2002) 279(1):80–90. doi: 10.1006/excr.2002.5600
58. Choi WI, Jeon BN, Park H, Yoo JY, Kim YS, Koh DI, et al. Proto-Oncogene Fbi-1 (Pokemon) and Srebp-1 Synergistically Activate Transcription of Fatty-Acid Synthase Gene (Fasn). J Biol Chem (2008) 283(43):29341–54. doi: 10.1074/jbc.M802477200
59. Zhang Y, Li C, Hu C, Wu Q, Cai Y, Xing S, et al. Lin28 Enhances De Novo Fatty Acid Synthesis to Promote Cancer Progression Via Srebp-1. EMBO Rep (2019) 20(10):e48115. doi: 10.15252/embr.201948115
60. Gao Y, Zhao Q, Mu X, Zhu H, Liu B, Yao B, et al. Srebp1 Promotes 5-Fu Resistance in Colorectal Cancer Cells by Inhibiting the Expression of Caspase7. Int J Clin Exp Pathol (2019) 12(3):1095–100.
61. Gao Y, Nan X, Shi X, Mu X, Liu B, Zhu H, et al. Srebp1 Promotes the Invasion of Colorectal Cancer Accompanied Upregulation of Mmp7 Expression and Nf-Kappab Pathway Activation. BMC Cancer (2019) 19(1):685. doi: 10.1186/s12885-019-5904-x
62. Li JN, Mahmoud MA, Han WF, Ripple M, Pizer ES. Sterol Regulatory Element-Binding Protein-1 Participates in the Regulation of Fatty Acid Synthase Expression in Colorectal Neoplasia. Exp Cell Res (2000) 261(1):159–65. doi: 10.1006/excr.2000.5054
63. Qiu Z, Deng W, Hong Y, Zhao L, Li M, Guan Y, et al. Biological Behavior and Lipid Metabolism of Colon Cancer Cells Are Regulated by a Combination of Sterol Regulatory Element-Binding Protein 1 and Atp Citrate Lyase. Onco Targets Ther (2021) 14:1531–42. doi: 10.2147/OTT.S282906
64. Wen YA, Xiong X, Zaytseva YY, Napier DL, Vallee E, Li AT, et al. Downregulation of Srebp Inhibits Tumor Growth and Initiation by Altering Cellular Metabolism in Colon Cancer. Cell Death Dis (2018) 9(3):265. doi: 10.1038/s41419-018-0330-6
65. Zhai D, Cui C, Xie L, Cai L, Yu J. Sterol Regulatory Element-Binding Protein 1 Cooperates With C-Myc to Promote Epithelial-Mesenchymal Transition in Colorectal Cancer. Oncol Lett (2018) 15(4):5959–65. doi: 10.3892/ol.2018.8058
66. Shen W, Xu T, Chen D, Tan X. Targeting Srebp1 Chemosensitizes Colorectal Cancer Cells to Gemcitabine by Caspase-7 Upregulation. Bioengineered (2019) 10(1):459–68. doi: 10.1080/21655979.2019.1676485
67. Jin Y, Chen Z, Dong J, Wang B, Fan S, Yang X, et al. Srebp1/Fasn/Cholesterol Axis Facilitates Radioresistance in Colorectal Cancer. FEBS Open Bio (2021) 11(5):1343–52. doi: 10.1002/2211-5463.13137
68. Wang G, Li Z, Li X, Zhang C, Peng L. Rasal1 Induces to Downregulate the Scd1, Leading to Suppression of Cell Proliferation in Colon Cancer Via Lxralpha/Srebp1c Pathway. Biol Res (2019) 52(1):60. doi: 10.1186/s40659-019-0268-x
69. Wang H, Chen Y, Liu Y, Li Q, Luo J, Wang L, et al. The Lncrna Zfas1 Regulates Lipogenesis in Colorectal Cancer by Binding Polyadenylate-Binding Protein 2 to Stabilize Srebp1 Mrna. Mol Ther Nucleic Acids (2022) 27:363–74. doi: 10.1016/j.omtn.2021.12.010
70. Torres-Ayuso P, Tello-Lafoz M, Merida I, Avila-Flores A. Diacylglycerol Kinase-Zeta Regulates Mtorc1 and Lipogenic Metabolism in Cancer Cells Through Srebp-1. Oncogenesis (2015) 4:e164. doi: 10.1038/oncsis.2015.22
71. Ettinger SL, Sobel R, Whitmore TG, Akbari M, Bradley DR, Gleave ME, et al. Dysregulation of Sterol Response Element-Binding Proteins and Downstream Effectors in Prostate Cancer During Progression to Androgen Independence. Cancer Res (2004) 64(6):2212–21. doi: 10.1158/0008-5472.can-2148-2
72. O'Malley J, Kumar R, Kuzmin AN, Pliss A, Yadav N, Balachandar S, et al. Lipid Quantification by Raman Microspectroscopy as a Potential Biomarker in Prostate Cancer. Cancer Lett (2017) 397:52–60. doi: 10.1016/j.canlet.2017.03.025
73. Suh JH, Gong EY, Kim JB, Lee IK, Choi HS, Lee K. Sterol Regulatory Element-Binding Protein-1c Represses the Transactivation of Androgen Receptor and Androgen-Dependent Growth of Prostatic Cells. Mol Cancer Res (2008) 6(2):314–24. doi: 10.1158/1541-7786.MCR-07-0354
74. Audet-Walsh E, Vernier M, Yee T, Laflamme C, Li S, Chen Y, et al. Srebf1 Activity Is Regulated by an Ar/Mtor Nuclear Axis in Prostate Cancer. Mol Cancer Res (2018) 16(9):1396–405. doi: 10.1158/1541-7786.MCR-17-0410
75. Zheng Y, Jin J, Gao Y, Luo C, Wu X, Liu J. Phospholipase Cepsilon Regulates Prostate Cancer Lipid Metabolism and Proliferation by Targeting Amp-Activated Protein Kinase (Ampk)/Sterol Regulatory Element-Binding Protein 1 (Srebp-1) Signaling Pathway. Med Sci Monitor (2020) 26:e924328. doi: 10.12659/MSM.924328
76. Lee MY, Moon JS, Park SW, Koh YK, Ahn YH, Kim KS. Klf5 Enhances Srebp-1 Action in Androgen-Dependent Induction of Fatty Acid Synthase in Prostate Cancer Cells. Biochem J (2009) 417(1):313–22. doi: 10.1042/BJ20080762
77. Li L, Hua L, Fan H, He Y, Xu W, Zhang L, et al. Interplay of Pkd3 With Srebp1 Promotes Cell Growth Via Upregulating Lipogenesis in Prostate Cancer Cells. J Cancer (2019) 10(25):6395–404. doi: 10.7150/jca.31254
78. Li X, Chen YT, Josson S, Mukhopadhyay NK, Kim J, Freeman MR, et al. Microrna-185 and 342 Inhibit Tumorigenicity and Induce Apoptosis Through Blockade of the Srebp Metabolic Pathway in Prostate Cancer Cells. PloS One (2013) 8(8):e70987. doi: 10.1371/journal.pone.0070987
79. Guo S, Zhang Y, Wang S, Yang T, Ma B, Li X, et al. Lncrna Pca3 Promotes Antimony-Induced Lipid Metabolic Disorder in Prostate Cancer by Targeting Mir-132-3 P/Srebp1 Signaling. Toxicol Lett (2021) 348:50–8. doi: 10.1016/j.toxlet.2021.05.006
80. Perone Y, Farrugia AJ, Rodriguez-Meira A, Gyorffy B, Ion C, Uggetti A, et al. Srebp1 Drives Keratin-80-Dependent Cytoskeletal Changes and Invasive Behavior in Endocrine-Resistant Eralpha Breast Cancer. Nat Commun (2019) 10(1):2115. doi: 10.1038/s41467-019-09676-y
81. Yang Y, Morin PJ, Han WF, Chen T, Bornman DM, Gabrielson EW, et al. Regulation of Fatty Acid Synthase Expression in Breast Cancer by Sterol Regulatory Element Binding Protein-1c. Exp Cell Res (2003) 282(2):132–7. doi: 10.1016/s0014-4827(02)00023-x
82. Zhang N, Zhang H, Liu Y, Su P, Zhang J, Wang X, et al. Srebp1, Targeted by Mir-18a-5p, Modulates Epithelial-Mesenchymal Transition in Breast Cancer Via Forming a Co-Repressor Complex With Snail and Hdac1/2. Cell Death Differ (2019) 26(5):843–59. doi: 10.1038/s41418-018-0158-8
83. Pham DV, Tilija Pun N, Park PH. Autophagy Activation and Srebp-1 Induction Contribute to Fatty Acid Metabolic Reprogramming by Leptin in Breast Cancer Cells. Mol Oncol (2021) 15(2):657–78. doi: 10.1002/1878-0261.12860
84. Santana-Codina N, Marce-Grau A, Muixi L, Nieva C, Marro M, Sebastian D, et al. Grp94 Is Involved in the Lipid Phenotype of Brain Metastatic Cells. Int J Mol Sci (2019) 20(16):3883. doi: 10.3390/ijms20163883
85. Zhu Z, Zhao X, Zhao L, Yang H, Liu L, Li J, et al. P54(Nrb)/Nono Regulates Lipid Metabolism and Breast Cancer Growth Through Srebp-1a. Oncogene (2016) 35(11):1399–410. doi: 10.1038/onc.2015.197
86. Li X, Wu C, Chen N, Gu H, Yen A, Cao L, et al. Pi3k/Akt/Mtor Signaling Pathway and Targeted Therapy for Glioblastoma. Oncotarget (2016) 7(22):33440–50. doi: 10.18632/oncotarget.7961
87. Guo D, Bell EH, Chakravarti A. Lipid Metabolism Emerges as a Promising Target for Malignant Glioma Therapy. CNS Oncol (2013) 2(3):289–99. doi: 10.2217/cns.13.20
88. Ru P, Hu P, Geng F, Mo X, Cheng C, Yoo JY, et al. Feedback Loop Regulation of Scap/Srebp-1 by Mir-29 Modulates Egfr Signaling-Driven Glioblastoma Growth. Cell Rep (2016) 16(6):1527–35. doi: 10.1016/j.celrep.2016.07.017
89. Ru P, Guo D. Microrna-29 Mediates a Novel Negative Feedback Loop to Regulate Scap/Srebp-1 and Lipid Metabolism. RNA Dis (2017) 4(1):e1525. doi: 10.14800/rd.1525
90. Griffiths B, Lewis CA, Bensaad K, Ros S, Zhang Q, Ferber EC, et al. Sterol Regulatory Element Binding Protein-Dependent Regulation of Lipid Synthesis Supports Cell Survival and Tumor Growth. Cancer Metab (2013) 1(1):3. doi: 10.1186/2049-3002-1-3
91. Geng F, Cheng X, Wu X, Yoo JY, Cheng C, Guo JY, et al. Inhibition of Soat1 Suppresses Glioblastoma Growth Via Blocking Srebp-1-Mediated Lipogenesis. Clin Cancer Res (2016) 22(21):5337–48. doi: 10.1158/1078-0432.CCR-15-2973
92. Min X, Wen J, Zhao L, Wang K, Li Q, Huang G, et al. Role of Hepatoma-Derived Growth Factor in Promoting De Novo Lipogenesis and Tumorigenesis in Hepatocellular Carcinoma. Mol Oncol (2018) 12(9):1480–97. doi: 10.1002/1878-0261.12357
93. Kumar DP, Santhekadur PK, Seneshaw M, Mirshahi F, Uram-Tuculescu C, Sanyal AJ. A Regulatory Role of Apoptosis Antagonizing Transcription Factor in the Pathogenesis of Nonalcoholic Fatty Liver Disease and Hepatocellular Carcinoma. Hepatology (2019) 69(4):1520–34. doi: 10.1002/hep.30346
94. Yu X, Lin Q, Wu Z, Zhang Y, Wang T, Zhao S, et al. Zhx2 Inhibits Srebp1c-Mediated De Novo Lipogenesis in Hepatocellular Carcinoma Via Mir-24-3p. J Pathol (2020) 252(4):358–70. doi: 10.1002/path.5530
95. Chen J, Ding C, Chen Y, Hu W, Yu C, Peng C, et al. Acsl4 Reprograms Fatty Acid Metabolism in Hepatocellular Carcinoma Via C-Myc/Srebp1 Pathway. Cancer Lett (2021) 502:154–65. doi: 10.1016/j.canlet.2020.12.019
96. Zhao M, Bu Y, Feng J, Zhang H, Chen Y, Yang G, et al. Spin1 Triggers Abnormal Lipid Metabolism and Enhances Tumor Growth in Liver Cancer. Cancer Lett (2020) 470:54–63. doi: 10.1016/j.canlet.2019.11.032
97. Zhang X, Zhang X, Zhang JJ, Qiao L. [Hepatitis B Virus X Protein Regulates Lipid Metabolism and Promotes the Proliferation of Liver Cancer Cells Via the C/Ebpa/Srebp-1 Pathway]. Zhonghua gan zang bing za zhi = Zhonghua Ganzangbing zazhi = Chin J Hepatol (2020) 28(12):1036–41. doi: 10.3760/cma.j.cn501113-20190923-00346
98. Tian Y, Wong VW, Wong GL, Yang W, Sun H, Shen J, et al. Histone Deacetylase Hdac8 Promotes Insulin Resistance and Beta-Catenin Activation in Nafld-Associated Hepatocellular Carcinoma. Cancer Res (2015) 75(22):4803–16. doi: 10.1158/0008-5472.CAN-14-3786
99. Mueller KM, Kornfeld JW, Friedbichler K, Blaas L, Egger G, Esterbauer H, et al. Impairment of Hepatic Growth Hormone and Glucocorticoid Receptor Signaling Causes Steatosis and Hepatocellular Carcinoma in Mice. Hepatology (2011) 54(4):1398–409. doi: 10.1002/hep.24509
100. Heo MJ, Kang SH, Kim YS, Lee JM, Yu J, Kim HR, et al. Ubc12-Mediated Srebp-1 Neddylation Worsens Metastatic Tumor Prognosis. Int J Cancer (2020) 147(9):2550–63. doi: 10.1002/ijc.33113
101. Triki M, Rinaldi G, Planque M, Broekaert D, Winkelkotte AM, Maier CR, et al. Mtor Signaling and Srebp Activity Increase Fads2 Expression and Can Activate Sapienate Biosynthesis. Cell Rep (2020) 31(12):107806. doi: 10.1016/j.celrep.2020.107806
102. Yahagi N, Shimano H, Hasegawa K, Ohashi K, Matsuzaka T, Najima Y, et al. Co-Ordinate Activation of Lipogenic Enzymes in Hepatocellular Carcinoma. Eur J Cancer (2005) 41(9):1316–22. doi: 10.1016/j.ejca.2004.12.037
103. Jiang H, Zhu L, Xu D, Lu Z. A Newly Discovered Role of Metabolic Enzyme Pck1 as a Protein Kinase to Promote Cancer Lipogenesis. Cancer Commun (2020) 40(9):389–94. doi: 10.1002/cac2.12084
104. Shao F, Bian X, Wang J, Xu D, Guo W, Jiang H, et al. Prognostic Impact of Pck1 Protein Kinase Activity-Dependent Nuclear Srebp1 Activation in Non-Small-Cell Lung Carcinoma. Front Oncol (2021) 11:561247. doi: 10.3389/fonc.2021.561247
105. Liu L, Yan H, Ruan M, Yang H, Wang L, Lei B, et al. An Akt/Prmt5/Srebp1 Axis in Lung Adenocarcinoma Regulates De Novo Lipogenesis and Tumor Growth. Cancer Sci (2021) 112(8):3083–98. doi: 10.1111/cas.14988
106. Luo D, Xiao H, Dong J, Li Y, Feng G, Cui M, et al. B7-H3 Regulates Lipid Metabolism of Lung Cancer Through Srebp1-Mediated Expression of Fasn. Biochem Biophys Res Commun (2017) 482(4):1246–51. doi: 10.1016/j.bbrc.2016.12.021
107. Ruiz CF, Montal ED, Haley JA, Bott AJ, Haley JD. Srebp1 Regulates Mitochondrial Metabolism in Oncogenic Kras Expressing Nsclc. FASEB J (2020) 34(8):10574–89. doi: 10.1096/fj.202000052R
108. Xu C, Zhang L, Wang D, Jiang S, Cao D, Zhao Z, et al. Lipidomics Reveals That Sustained Srebp-1-Dependent Lipogenesis Is a Key Mediator of Gefitinib-Acquired Resistance in Egfr-Mutant Lung Cancer. Cell Death Discovery (2021) 7(1):353. doi: 10.1038/s41420-021-00744-1
109. Lin L, Bao YX, Tian M, Ren Q, Zhang W. Mir-29 Family Inhibited the Proliferation and Migration of Lung Cancer Cells by Targeting Srebp-1. Mol Cell Toxicol (2022) 18(2):165–75. doi: 10.1007/s13273-021-00180-3
110. Talebi A, Dehairs J, Rambow F, Rogiers A, Nittner D, Derua R, et al. Sustained Srebp-1-Dependent Lipogenesis as a Key Mediator of Resistance to Braf-Targeted Therapy. Nat Commun (2018) 9(1):2500. doi: 10.1038/s41467-018-04664-0
111. Yamauchi Y, Furukawa K, Hamamura K, Furukawa K. Positive Feedback Loop Between Pi3k-Akt-Mtorc1 Signaling and the Lipogenic Pathway Boosts Akt Signaling: Induction of the Lipogenic Pathway by a Melanoma Antigen. Cancer Res (2011) 71(14):4989–97. doi: 10.1158/0008-5472.CAN-10-4108
112. Li LY, Yang Q, Jiang YY, Yang W, Jiang Y, Li X, et al. Interplay and Cooperation Between Srebf1 and Master Transcription Factors Regulate Lipid Metabolism and Tumor-Promoting Pathways in Squamous Cancer. Nat Commun (2021) 12(1):4362. doi: 10.1038/s41467-021-24656-x
113. Wang J, Ling R, Zhou Y, Gao X, Yang Y, Mao C, et al. Srebp1 Silencing Inhibits the Proliferation and Motility of Human Esophageal Squamous Carcinoma Cells Via the Wnt/Beta-Catenin Signaling Pathway. Oncol Lett (2020) 20(3):2855–69. doi: 10.3892/ol.2020.11853
114. Huang CM, Huang CS, Hsu TN, Huang MS, Fong IH, Lee WH, et al. Disruption of Cancer Metabolic Srebp1/Mir-142-5p Suppresses Epithelial-Mesenchymal Transition and Stemness in Esophageal Carcinoma. Cells (2019) 9(1):7. doi: 10.3390/cells9010007
115. Shao F, Bian X, Jiang H, Zhao G, Zhu L, Xu D, et al. Association of Phosphoenolpyruvate Carboxykinase 1 Protein Kinase Activity-Dependent Sterol Regulatory Element-Binding Protein 1 Activation With Prognosis of Oesophageal Carcinoma. Eur J Cancer (2021) 142:123–31. doi: 10.1016/j.ejca.2020.09.040
116. Sun Q, Yu X, Peng C, Liu N, Chen W, Xu H, et al. Activation of Srebp-1c Alters Lipogenesis and Promotes Tumor Growth and Metastasis in Gastric Cancer. Biomed Pharmacother = Biomed Pharmacotherapie (2020) 128:110274. doi: 10.1016/j.biopha.2020.110274
117. Yang B, Zhang B, Cao Z, Xu X, Huo Z, Zhang P, et al. The Lipogenic Lxr-Srebf1 Signaling Pathway Controls Cancer Cell DNA Repair and Apoptosis and Is a Vulnerable Point of Malignant Tumors for Cancer Therapy. Cell Death Differ (2020) 27(8):2433–50. doi: 10.1038/s41418-020-0514-3
118. Sun Y, He W, Luo M, Zhou Y, Chang G, Ren W, et al. Role of Transgelin-2 in Diabetes-Associated Pancreatic Ductal Adenocarcinoma. Oncotarget (2017) 8(30):49592–604. doi: 10.18632/oncotarget.17519
119. Zhou C, Qian W, Li J, Ma J, Chen X, Jiang Z, et al. High Glucose Microenvironment Accelerates Tumor Growth Via Srebp1-Autophagy Axis in Pancreatic Cancer. J Exp Clin Cancer Res (2019) 38(1):302. doi: 10.1186/s13046-019-1288-7
120. Al-Zoubi M, Chipitsyna G, Saxena S, Sarosiek K, Gandhi A, Kang CY, et al. Overexpressing Tnf-Alpha in Pancreatic Ductal Adenocarcinoma Cells and Fibroblasts Modifies Cell Survival and Reduces Fatty Acid Synthesis Via Downregulation of Sterol Regulatory Element Binding Protein-1 and Activation of Acetyl Coa Carboxylase. J Gastrointestinal Surg (2014) 18(2):257–68. doi: 10.1007/s11605-013-2370-7
121. Shafiee MN, Mongan N, Seedhouse C, Chapman C, Deen S, Abu J, et al. Sterol Regulatory Element Binding Protein-1 (Srebp1) Gene Expression Is Similarly Increased in Polycystic Ovary Syndrome and Endometrial Cancer. Acta Obstetricia Gynecologica Scandinavica (2017) 96(5):556–62. doi: 10.1111/aogs.13106
122. Qiu CP, Lv QT, Dongol S, Wang C, Jiang J. Single Nucleotide Polymorphism of Srebf-1 Gene Associated With an Increased Risk of Endometrial Cancer in Chinese Women. PloS One (2014) 9(3):e90491. doi: 10.1371/journal.pone.0090491
123. Wang Y, Zhang L, Che X, Li W, Liu Z, Jiang J. Roles of Sirt1/Foxo1/Srebp-1 in the Development of Progestin Resistance in Endometrial Cancer. Arch Gynecol Obstetrics (2018) 298(5):961–9. doi: 10.1007/s00404-018-4893-3
124. Zhang Y, Zhang L, Sun H, Lv Q, Qiu C, Che X, et al. Forkhead Transcription Factor 1 Inhibits Endometrial Cancer Cell Proliferation Via Sterol Regulatory Element-Binding Protein 1. Oncol Lett (2017) 13(2):731–7. doi: 10.3892/ol.2016.5480
125. Zhao J, Zhang X, Gao T, Wang S, Hou Y, Yuan P, et al. Sik2 Enhances Synthesis of Fatty Acid and Cholesterol in Ovarian Cancer Cells and Tumor Growth Through Pi3k/Akt Signaling Pathway. Cell Death Dis (2020) 11(1):25. doi: 10.1038/s41419-019-2221-x
126. Yang J, Stack MS. Lipid Regulatory Proteins as Potential Therapeutic Targets for Ovarian Cancer in Obese Women. Cancers (2020) 12(11):3469. doi: 10.3390/cancers12113469
127. Nie LY, Lu QT, Li WH, Yang N, Dongol S, Zhang X, et al. Sterol Regulatory Element-Binding Protein 1 Is Required for Ovarian Tumor Growth. Oncol Rep (2013) 30(3):1346–54. doi: 10.3892/or.2013.2575
128. Shen D, Gao Y, Huang Q, Xuan Y, Yao Y, Gu L, et al. E2f1 Promotes Proliferation and Metastasis of Clear Cell Renal Cell Carcinoma Via Activation of Srebp1-Dependent Fatty Acid Biosynthesis. Cancer Lett (2021) 514:48–62. doi: 10.1016/j.canlet.2021.05.012
129. Tao T, Su Q, Xu S, Deng J, Zhou S, Zhuang Y, et al. Down-Regulation of Pkm2 Decreases Fasn Expression in Bladder Cancer Cells Through Akt/Mtor/Srebp-1c Axis. J Cell Physiol (2019) 234(3):3088–104. doi: 10.1002/jcp.27129
130. Liu F, Wei J, Hao Y, Lan J, Li W, Weng J, et al. Long Intergenic Non-Protein Coding Rna 02570 Promotes Nasopharyngeal Carcinoma Progression by Adsorbing Microrna Mir-4649-3p Thereby Upregulating Both Sterol Regulatory Element Binding Protein 1, and Fatty Acid Synthase. Bioengineered (2021) 12(1):7119–30. doi: 10.1080/21655979.2021.1979317
131. Lo AK, Lung RW, Dawson CW, Young LS, Ko CW, Yeung WW, et al. Activation of Sterol Regulatory Element-Binding Protein 1 (Srebp1)-Mediated Lipogenesis by the Epstein-Barr Virus-Encoded Latent Membrane Protein 1 (Lmp1) Promotes Cell Proliferation and Progression of Nasopharyngeal Carcinoma. J Pathol (2018) 246(2):180–90. doi: 10.1002/path.5130
132. Fukuda M, Ogasawara Y, Hayashi H, Okuyama A, Shiono J, Inoue K, et al. Down-Regulation of Glutathione Peroxidase 4 in Oral Cancer Inhibits Tumor Growth Through Srebp1 Signaling. Anticancer Res (2021) 41(4):1785–92. doi: 10.21873/anticanres.14944
133. Kuo CY, Chang YC, Chien MN, Jhuang JY, Hsu YC, Huang SY, et al. Srebp1 Promotes Invasive Phenotypes by Upregulating Cyr61/Ctgf Via the Hippo-Yap Pathway. Endocr Relat Cancer (2021) 29(2):47–58. doi: 10.1530/ERC-21-0256
134. Hu Q, Mao Y, Liu M, Luo R, Jiang R, Guo F. The Active Nuclear Form of Srebp1 Amplifies Er Stress and Autophagy Via Regulation of Perk. FEBS J (2020) 287(11):2348–66. doi: 10.1111/febs.15144
135. Shao W, Machamer CE, Espenshade PJ. Fatostatin Blocks Er Exit of Scap But Inhibits Cell Growth in a Scap-Independent Manner. J Lipid Res (2016) 57(8):1564–73. doi: 10.1194/jlr.M069583
136. Li X, Chen YT, Hu P, Huang WC. Fatostatin Displays High Antitumor Activity in Prostate Cancer by Blocking Srebp-Regulated Metabolic Pathways and Androgen Receptor Signaling. Mol Cancer Ther (2014) 13(4):855–66. doi: 10.1158/1535-7163.MCT-13-0797
137. Gholkar AA, Cheung K, Williams KJ, Lo YC, Hamideh SA, Nnebe C, et al. Fatostatin Inhibits Cancer Cell Proliferation by Affecting Mitotic Microtubule Spindle Assembly and Cell Division. J Biol Chem (2016) 291(33):17001–8. doi: 10.1074/jbc.C116.737346
138. Brovkovych V, Izhar Y, Danes JM, Dubrovskyi O, Sakallioglu IT, Morrow LM, et al. Fatostatin Induces Pro- and Anti-Apoptotic Lipid Accumulation in Breast Cancer. Oncogenesis (2018) 7(8):66. doi: 10.1038/s41389-018-0076-0
139. Ma X, Zhao T, Yan H, Guo K, Liu Z, Wei L, et al. Fatostatin Reverses Progesterone Resistance by Inhibiting the Srebp1-Nf-Kappab Pathway in Endometrial Carcinoma. Cell Death Dis (2021) 12(6):544. doi: 10.1038/s41419-021-03762-0
140. Liu Y, Zhang N, Zhang H, Wang L, Duan Y, Wang X, et al. Fatostatin in Combination With Tamoxifen Induces Synergistic Inhibition in Er-Positive Breast Cancer. Drug Des Devel Ther (2020) 14:3535–45. doi: 10.2147/DDDT.S253876
141. Guan M, Fousek K, Jiang C, Guo S, Synold T, Xi B, et al. Nelfinavir Induces Liposarcoma Apoptosis Through Inhibition of Regulated Intramembrane Proteolysis of Srebp-1 and Atf6. Clin Cancer Res (2011) 17(7):1796–806. doi: 10.1158/1078-0432.CCR-10-3216
142. Guan M, Fousek K, Chow WA. Nelfinavir Inhibits Regulated Intramembrane Proteolysis of Sterol Regulatory Element Binding Protein-1 and Activating Transcription Factor 6 in Castration-Resistant Prostate Cancer. FEBS J (2012) 279(13):2399–411. doi: 10.1111/j.1742-4658.2012.08619.x
143. Guan M, Su L, Yuan YC, Li H, Chow WA. Nelfinavir and Nelfinavir Analogs Block Site-2 Protease Cleavage to Inhibit Castration-Resistant Prostate Cancer. Sci Rep (2015) 5:9698. doi: 10.1038/srep09698
144. Chen Z, Yu D, Owonikoko TK, Ramalingam SS, Sun SY. Induction of Srebp1 Degradation Coupled With Suppression of Srebp1-Mediated Lipogenesis Impacts the Response of Egfr Mutant Nsclc Cells to Osimertinib. Oncogene (2021) 40(49):6653–65. doi: 10.1038/s41388-021-02057-0
145. Liu G, Kuang S, Cao R, Wang J, Peng Q, Sun C. Sorafenib Kills Liver Cancer Cells by Disrupting Scd1-Mediated Synthesis of Monounsaturated Fatty Acids Via the Atp-Ampk-Mtor-Srebp1 Signaling Pathway. FASEB J (2019) 33(9):10089–103. doi: 10.1096/fj.201802619RR
146. Wen S, Niu Y, Lee SO, Yeh S, Shang Z, Gao H, et al. Targeting Fatty Acid Synthase With Asc-J9 Suppresses Proliferation and Invasion of Prostate Cancer Cells. Mol Carcinog (2016) 55(12):2278–90. doi: 10.1002/mc.22468
147. Gu Y, Xue M, Wang Q, Hong X, Wang X, Zhou F, et al. Novel Strategy of Proxalutamide for the Treatment of Prostate Cancer Through Coordinated Blockade of Lipogenesis and Androgen Receptor Axis. Int J Mol Sci (2021) 22(24):13222. doi: 10.3390/ijms222413222
148. Singh KB, Hahm ER, Pore SK, Singh SV. Leelamine Is a Novel Lipogenesis Inhibitor in Prostate Cancer Cells in Vitro and in Vivo. Mol Cancer Ther (2019) 18(10):1800–10. doi: 10.1158/1535-7163.MCT-19-0046
149. Pang B, Zhang J, Zhang X, Yuan J, Shi Y, Qiao L. Inhibition of Lipogenesis and Induction of Apoptosis by Valproic Acid in Prostate Cancer Cells Via the C/Ebpalpha/Srebp-1 Pathway. Acta Biochim Biophys Sin (Shanghai) (2021) 53(3):354–64. doi: 10.1093/abbs/gmab002
150. Huang WC, Zhau HE, Chung LW. Androgen Receptor Survival Signaling Is Blocked by Anti-Beta2-Microglobulin Monoclonal Antibody Via a Mapk/Lipogenic Pathway in Human Prostate Cancer Cells. J Biol Chem (2010) 285(11):7947–56. doi: 10.1074/jbc.M109.092759
151. Bandyopadhayaya S, Akimov MG, Verma R, Sharma A, Sharma D, Kundu GC, et al. N-Arachidonoyl Dopamine Inhibits Epithelial-Mesenchymal Transition of Breast Cancer Cells Through Erk Signaling and Decreasing the Cellular Cholesterol. J Biochem Mol Toxicol (2021) 35(4):e22693. doi: 10.1002/jbt.22693
152. Zou XZ, Hao JF, Zhou XH. Inhibition of Srebp-1 Activation by a Novel Small-Molecule Inhibitor Enhances the Sensitivity of Hepatocellular Carcinoma Tissue to Radiofrequency Ablation. Front Oncol (2021) 11:796152. doi: 10.3389/fonc.2021.796152
153. Huang LH, Chung HY, Su HM. Docosahexaenoic Acid Reduces Sterol Regulatory Element Binding Protein-1 and Fatty Acid Synthase Expression and Inhibits Cell Proliferation by Inhibiting Pakt Signaling in a Human Breast Cancer Mcf-7 Cell Line. BMC Cancer (2017) 17(1):890. doi: 10.1186/s12885-017-3936-7
154. Facchini G, Ignarro RS, Rodrigues-Silva E, Vieira AS, Lopes-Cendes I, Castilho RF, et al. Toxic Effects of Phytol and Retinol on Human Glioblastoma Cells Are Associated With Modulation of Cholesterol and Fatty Acid Biosynthetic Pathways. J Neuro-Oncol (2018) 136(3):435–43. doi: 10.1007/s11060-017-2672-9
155. Bai X, Ali A, Wang N, Liu Z, Lv Z, Zhang Z, et al. Inhibition of Srebp-Mediated Lipid Biosynthesis and Activation of Multiple Anticancer Mechanisms by Platinum Complexes: Ascribe Possibilities of New Antitumor Strategies. Eur J Med Chem (2022) 227:113920. doi: 10.1016/j.ejmech.2021.113920
156. Chen J, Wu Z, Ding W, Xiao C, Zhang Y, Gao S, et al. Srebp1 Sirna Enhance the Docetaxel Effect Based on a Bone-Cancer Dual-Targeting Biomimetic Nanosystem Against Bone Metastatic Castration-Resistant Prostate Cancer. Theranostics (2020) 10(4):1619–32. doi: 10.7150/thno.40489
157. Lee KC, Wu KL, Yen CK, Chen CN, Chang SF, Huang WS. 6-Shogaol Antagonizes the Adipocyte-Conditioned Medium-Initiated 5-Fluorouracil Resistance in Human Colorectal Cancer Cells Through Controlling the Srebp-1 Level. Life (Basel) (2021) 11(10):1067. doi: 10.3390/life11101067
158. Zhang L, Qiao X, Chen M, Li P, Wen X, Sun M, et al. Ilexgenin a Prevents Early Colonic Carcinogenesis and Reprogramed Lipid Metabolism Through Hif1alpha/Srebp-1. Phytomedicine (2019) 63:153011. doi: 10.1016/j.phymed.2019.153011
159. Wang Y, Guo D, He J, Song L, Chen H, Zhang Z, et al. Inhibition of Fatty Acid Synthesis Arrests Colorectal Neoplasm Growth and Metastasis: Anti-Cancer Therapeutical Effects of Natural Cyclopeptide Ra-Xii. Biochem Biophys Res Commun (2019) 512(4):819–24. doi: 10.1016/j.bbrc.2019.03.088
160. Liu Y, Hua W, Li Y, Xian X, Zhao Z, Liu C, et al. Berberine Suppresses Colon Cancer Cell Proliferation by Inhibiting the Scap/Srebp-1 Signaling Pathway-Mediated Lipogenesis. Biochem Pharmacol (2020) 174:113776. doi: 10.1016/j.bcp.2019.113776
161. Meng H, Shen M, Li J, Zhang R, Li X, Zhao L, et al. Novel Srebp1 Inhibitor Cinobufotalin Suppresses Proliferation of Hepatocellular Carcinoma by Targeting Lipogenesis. Eur J Pharmacol (2021) 906:174280. doi: 10.1016/j.ejphar.2021.174280
162. Yang N, Li C, Li H, Liu M, Cai X, Cao F, et al. Emodin Induced Srebp1-Dependent and Srebp1-Independent Apoptosis in Hepatocellular Carcinoma Cells. Front Pharmacol (2019) 10:709. doi: 10.3389/fphar.2019.00709
163. Yin F, Feng F, Wang L, Wang X, Li Z, Cao Y. Srebp-1 Inhibitor Betulin Enhances the Antitumor Effect of Sorafenib on Hepatocellular Carcinoma Via Restricting Cellular Glycolytic Activity. Cell Death Dis (2019) 10(9):672. doi: 10.1038/s41419-019-1884-7
164. Weng MS, Ho CT, Ho YS, Lin JK. Theanaphthoquinone Inhibits Fatty Acid Synthase Expression in Egf-Stimulated Human Breast Cancer Cells Via the Regulation of Egfr/Erbb-2 Signaling. Toxicol Appl Pharmacol (2007) 218(2):107–18. doi: 10.1016/j.taap.2006.10.021
165. Do MT, Kim HG, Choi JH, Khanal T, Park BH, Tran TP, et al. Antitumor Efficacy of Piperine in the Treatment of Human Her2-Overexpressing Breast Cancer Cells. Food Chem (2013) 141(3):2591–9. doi: 10.1016/j.foodchem.2013.04.125
166. Zhang LC, La XQ, Tian JM, Li HQ, Li AP, Liu YZ, et al. The Phytochemical Vitexin and Syringic Acid Derived From Foxtail Fillet Bran Inhibit Breast Cancer Cells Proliferation Via Grp78/Srebp-1/Scd1 Signaling Axis. J Funct Foods (2021) 85(26):104620. doi: 10.1016/j.jff.2021.104620
167. Qian Y, Huang R, Li S, Xie R, Qian B, Zhang Z, et al. Ginsenoside Rh2 Reverses Cyclophosphamide-Induced Immune Deficiency by Regulating Fatty Acid Metabolism. J Leukoc Biol (2019) 106(5):1089–100. doi: 10.1002/JLB.2A0419-117R
168. Shi Y, Fan Y, Hu Y, Jing J, Wang C, Wu Y, et al. Alpha-Mangostin Suppresses the De Novo Lipogenesis and Enhances the Chemotherapeutic Response to Gemcitabine in Gallbladder Carcinoma Cells Via Targeting the Ampk/Srebp1 Cascades. J Cell Mol Med (2020) 24(1):760–71. doi: 10.1111/jcmm.14785
169. Ali A, Kim MJ, Kim MY, Lee HJ, Roh GS, Kim HJ, et al. Quercetin Induces Cell Death in Cervical Cancer by Reducing O-Glcnacylation of Adenosine Monophosphate-Activated Protein Kinase. Anat Cell Biol (2018) 51(4):274–83. doi: 10.5115/acb.2018.51.4.274
170. Zhou C, Qian W, Ma J, Cheng L, Jiang Z, Yan B, et al. Resveratrol Enhances the Chemotherapeutic Response and Reverses the Stemness Induced by Gemcitabine in Pancreatic Cancer Cells Via Targeting Srebp1. Cell Prolif (2019) 52(1):e12514. doi: 10.1111/cpr.12514
171. Kim Y, Jee W, An EJ, Ko HM, Jung JH, Na YC, et al. Timosaponin A3 Inhibits Palmitate and Stearate Through Suppression of Srebp-1 in Pancreatic Cancer. Pharmaceutics (2022) 14(5):945. doi: 10.3390/pharmaceutics14050945
172. Huang SY, Huang GJ, Wu HC, Kao MC, Huang WC. Ganoderma Tsugae Inhibits the Srebp-1/Ar Axis Leading to Suppression of Cell Growth and Activation of Apoptosis in Prostate Cancer Cells. Molecules (2018) 23(10):2539. doi: 10.3390/molecules23102539
173. Hsieh PF, Jiang WP, Huang SY, Basavaraj P, Wu JB, Ho HY, et al. Emerging Therapeutic Activity of Davallia Formosana on Prostate Cancer Cells Through Coordinated Blockade of Lipogenesis and Androgen Receptor Expression. Cancers (2020) 12(4):914. doi: 10.3390/cancers12040914
174. Hsieh PF, Jiang WP, Basavaraj P, Huang SY, Ruangsai P, Wu JB, et al. Cell Suspension Culture Extract of Eriobotrya Japonica Attenuates Growth and Induces Apoptosis in Prostate Cancer Cells Via Targeting Srebp-1/Fasn-Driven Metabolism and Ar. Phytomedicine (2021) 93:153806. doi: 10.1016/j.phymed.2021.153806
175. Guo S, Ma B, Jiang X, Li X, Jia Y. Astragalus Polysaccharides Inhibits Tumorigenesis and Lipid Metabolism Through Mir-138-5p/Sirt1/Srebp1 Pathway in Prostate Cancer. Front Pharmacol (2020) 11:598. doi: 10.3389/fphar.2020.00598
176. Kang S, Kim HI, Choi YJ, Lee SK, Kim JH, Cheon C, et al. Traditional Herbal Formula Taeeumjowi-Tang (Tj001) Inhibits P53-Mutant Prostate Cancer Cells Growth by Activating Ampk-Dependent Pathway. Evidence-Based Complementary Altern Med (2019) 2019:2460353. doi: 10.1155/2019/2460353
Keywords: SREBP-1, fatty acid synthase, fatty acids, lipogenesis, cancer therapy
Citation: Zhao Q, Lin X and Wang G (2022) Targeting SREBP-1-Mediated Lipogenesis as Potential Strategies for Cancer. Front. Oncol. 12:952371. doi: 10.3389/fonc.2022.952371
Received: 25 May 2022; Accepted: 22 June 2022;
Published: 14 July 2022.
Edited by:
Maoshan Chen, Faculty of Medicine, Nursing & Health Sciences, Monash University, AustraliaReviewed by:
Peter Espenshade, Johns Hopkins University, United StatesMing-Hsien Chan, Academia Sinica, Taiwan
Copyright © 2022 Zhao, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingyu Lin, bGluMjAyMEBqbHUuZWR1LmNu; Guan Wang, d2cxMEBqbHUuZWR1LmNu
 Qiushi Zhao1
Qiushi Zhao1 Guan Wang
Guan Wang