
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 02 September 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.951575
This article is part of the Research Topic 365 Days of Progress In Thoracic Oncology View all 6 articles
 Hyun Jung Yoon1,2†
Hyun Jung Yoon1,2† Jieun Choi3†
Jieun Choi3† Eunjin Kim4
Eunjin Kim4 Sang-Won Um5,6
Sang-Won Um5,6 Noeul Kang5,7
Noeul Kang5,7 Wook Kim1
Wook Kim1 Geena Kim1
Geena Kim1 Hyunjin Park8,9*
Hyunjin Park8,9* Ho Yun Lee1,6*
Ho Yun Lee1,6*Background: Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) showed potency as a non-invasive therapeutic approach in pure ground-glass opacity nodule (pGGN) lung adenocarcinoma. However, optimal methods of extracting information about EGFR mutation from pGGN lung adenocarcinoma images remain uncertain. We aimed to develop, validate, and evaluate the clinical utility of a deep learning model for predicting EGFR mutation status in lung adenocarcinoma manifesting as pGGN on computed tomography (CT).
Methods: We included 185 resected pGGN lung adenocarcinomas in the primary cohort. The patients were divided into training (n = 125), validation (n = 23), and test sets (n = 37). A preoperative CT-based deep learning model with clinical factors as well as clinical and radiomics models was constructed and applied to the test set. We evaluated the clinical utility of the deep learning model by applying it to 83 GGNs that received EGFR-TKI from an independent cohort (clinical validation set), and treatment response was regarded as the reference standard.
Results: The prediction efficiencies of each model were compared in terms of area under the curve (AUC). Among the 185 pGGN lung adenocarcinomas, 122 (65.9%) were EGFR-mutant and 63 (34.1%) were EGFR-wild type. The AUC of the clinical, radiomics, and deep learning with clinical models to predict EGFR mutations were 0.50, 0.64, and 0.85, respectively, for the test set. The AUC of deep learning with the clinical model in the validation set was 0.72.
Conclusions: Deep learning approach of CT images combined with clinical factors can predict EGFR mutations in patients with lung adenocarcinomas manifesting as pGGN, and its clinical utility was demonstrated in a real-world sample.
Detection of epidermal growth factor receptor (EGFR) mutations for lung adenocarcinoma is crucial since tyrosine kinase inhibitors (TKI) are tailored for treatments in lung adenocarcinoma with EGFR mutations (1–3). Approximately 80% of patients with EGFR‐mutant lung cancer respond to EGFR- TKIs therapy at initial treatment (4).
Due to the growing clinical use of low-dose computed tomography (CT) screening for lung cancer (5–7), pulmonary pure ground-glass opacity nodules (pGGN) are becoming clinically important in oncology especially for management given its diagnosis in practice is increasing, and the incidence of cancer in pGGN may be as high as 63% (8). In addition, around 20–30% of resected GGN were accompanied by multiple synchronous pGGNs (9), and there have been reports of developing metachronous pGGNs with an incidence of 2% after surgery in primary lung cancer (10). Therefore, there is a dilemma regarding how to deal with synchronous and metachronous pGGNs. Moreover, surgical therapy for pGGNs may be unfeasible for patients with poor pulmonary function or when lesions have central locations that make it difficult to perform repeated limited resection (11).
In terms of such a challenging condition, a few reports have shown the potency of molecular targeted therapy, EGFR-TKI as a novel strategy for the treatment of cases with multiple GGNs, and they helped provide a non-invasive therapeutic approach for EGFR-mutated lung adenocarcinoma manifesting as pGGN (12, 13). The authors used surgical resection for the major lesion which was the most invasive, and continued EGFR-TKI gefitinib treatment for unresectable GGNs (more than 10mm), and they achieved a complete response (12, 13). Additionally, there can be difficulty accessing tissue samples of pGGN through core biopsy due to the potential risk of complications and limitations in pathologic evaluation such as stromal invasion (14, 15). With such clinical conditions, EGFR mutation prediction using a noninvasive method such as imaging of lung adenocarcinoma manifesting as pGGN is desirable.
Models for predicting EGFR mutations on imaging have been developed using a radiomics approach (16–18), but these methods only reflect generalized adenocarcinomas and lack specificity for pGGN. Radiomics also rely on precise tumor boundary annotation, which requires manual labeling, and interobserver reproducibility and robustness of results are relatively unsatisfactory (19–21). In contrast, advanced artificial intelligence models can overcome these problems through self-learning strategies such as deep learning (22, 23). Deep learning models have shown promising performance in assisting lung cancer analysis (24–27). Nevertheless, deep learning models for the prediction of EGFR mutation in lung adenocarcinoma manifesting as pGGN have not been evaluated thus far. Besides, development and validation of models to deal with pGGN in particular are complicated and difficult due to need for copious data collection and image processing. Therefore, extraction of EGFR mutation information from lung adenocarcinoma manifesting as pGGN on images remains uncertain. Furthermore, there have been no attempts to evaluate the clinical utility of deep learning models by performance validation through the testing of clinically meaningful endpoints (28, 29).
Thus, we developed and validated a CT-based deep learning model with clinical factors for predicting EGFR mutation status in lung adenocarcinoma manifesting as pGGN. We demonstrated its clinical utility using an independent data set of patients who received EGFR-TKI and evaluated treatment response as the reference standard.
Our institutional review board approved this retrospective study, and the requirement for informed consent was waived. We conducted a retrospective chart review and identified 2,851 patients who had undergone surgical resection for lung adenocarcinoma as initial curative resection from January 2014 to August 2019. Patients who met the following inclusion criteria were included in this study: 1) histologically confirmed primary lung adenocarcinoma; 2) pathological examination of tumor specimens carried out with proven records of EGFR mutation status; 3) pre-operative chest CT data obtained; and 4) CT findings of the tumor showed pGGN. Patients were excluded if 1) clinical data including age, sex, and smoking history were missing; or 2) CT findings of the tumor showed a large mass (>3 cm), part-solid lesion, or heterogeneous GGN. Finally, 185 pGGN adenocarcinomas of 179 patients of were included in the primary cohort (model training [n = 125 nodules of 120 patients], technical validation [n = 23 of 23 patients], and tests [n = 37 of 36 patients]). We randomly divided cohorts into training, validation, and test sets maintaining the ratio of EGFR-mutant and EGFR wild type (Figure 1).
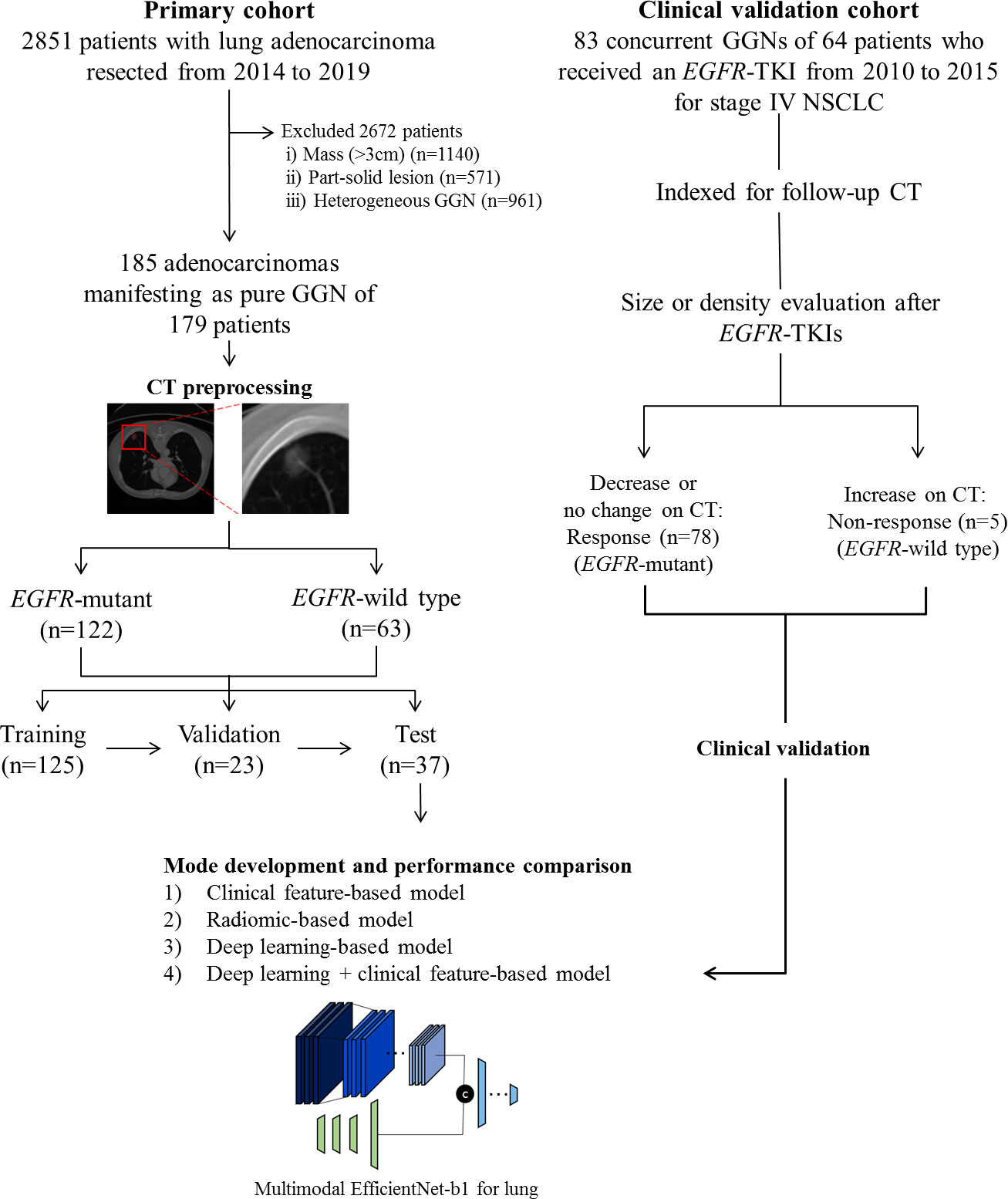
Figure 1 Flow diagram describing the development of the EGFR mutation prediction model in this study.
Clinical data were collected from electronic medical records at the time of diagnostic workup. Sex, age, smoking status, Union for International Cancer Control stage, and operation type were recorded. Histologic reports were also retrieved from electronic medical records with histological classifications based on the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society multidisciplinary classification of lung adenocarcinoma (30).
EGFR mutations for lung adenocarcinoma were identified using a PNA clamp kit or real-time polymerase chain reaction (31). Wild-type EGFR in this study referred to no mutations detected among those loci.
Heterogeneity in the imaging acquisition protocols was inevitable, as data were obtained retrospectively at a tertiary referral center. All patients underwent CT scans from the lung apex to the base at suspended maximum inspiration. Scans were performed at 120 kVp with mAs ranging at 150–200 mAs and detector collimation was 1.25 or 0.625 mm. CT scans were reconstructed with slice thickness less than or equal to 2.5 mm. Slice increments were equal to or less than the slice thickness. All CT scans included axial reconstruction and most CT scans also had coronal reformatted images. Most patients underwent contrast-enhanced CT scans at a scan delay of 60 s after contrast material injection. All helical CT images were obtained using a high-quality 16 or 64-channel multidetector CT scanner.
The tumor region of interest (ROI) was automatically segmented for all patients in each dataset using commercial software (Aview, version 1.0.23, 2018; Coreline Soft, Seoul, Korea) to generate a volume of interest that included the entire target lesion (32). Additional manual correction was performed to exclude bronchovascular structures and the borders of ground-glass opacities by a thoracic radiologist (HYL, 15 years of experience). Since CT imaging resolution varied within and across the two cohorts, isotropic resampling to 1 mm x 1mm x 1mm was conducted. Resampling was performed with b-spline interpolation for CT images and with the nearest neighbor method for ROI. For deep learning methods, the center of the tumor was calculated as the centroid of the ROI and then we extracted one center slice and two additional slices positioned 3 mm below and above the center slice in the axial direction. The three slices were combined as a three-channel image mimicking the color red/green/blue channels of the natural image. We then cropped each image around the center of the tumor to a size of 64 mm. Finally, all tumor regions were represented in 64×64×3 image patches. The intensities were normalized with min-max scaling. As the clinical variable, sex was binarized with values 0 for female and 1 for male. Age was normalized between 0 and 1 with min-max scaling. Smoking status was also binarized with 0 representing never smokers and 1 representing others.
To predict EGFR mutation status in ground-glass opacity lung adenocarcinoma, we designed a deep learning method referred to as Multimodal EfficientNet-b1 for Lung (MENL). This method adopted Efficient as the backbone and is composed of an image feature extractor, clinical feature extractor, and classification network. Based on the mobile inverted bottleneck convolution (MBConv), the EfficientNet varies from b0 to b7 depending on the scaling factor (33, 34). We employed pre-trained EfficientNet-b1 as an image feature extractor. The following clinical factors were fed to the clinical feature extractor for predicting EGFR mutation status in pGGN: sex, age, and smoking status. The clinical feature extractor is a separate neural network that consists of four fully connected layers. We concatenated latent variables from the clinical feature extractor to the feature maps from the image feature extractor. Concatenated latent variables were fed into the classification network made of five fully connected layers for predicting EGFR mutation status. Finally, EGFR mutant probability was obtained by applying softmax to the two nodes in the last layer of the classification network. Figure 2 shows the details of the MENL.
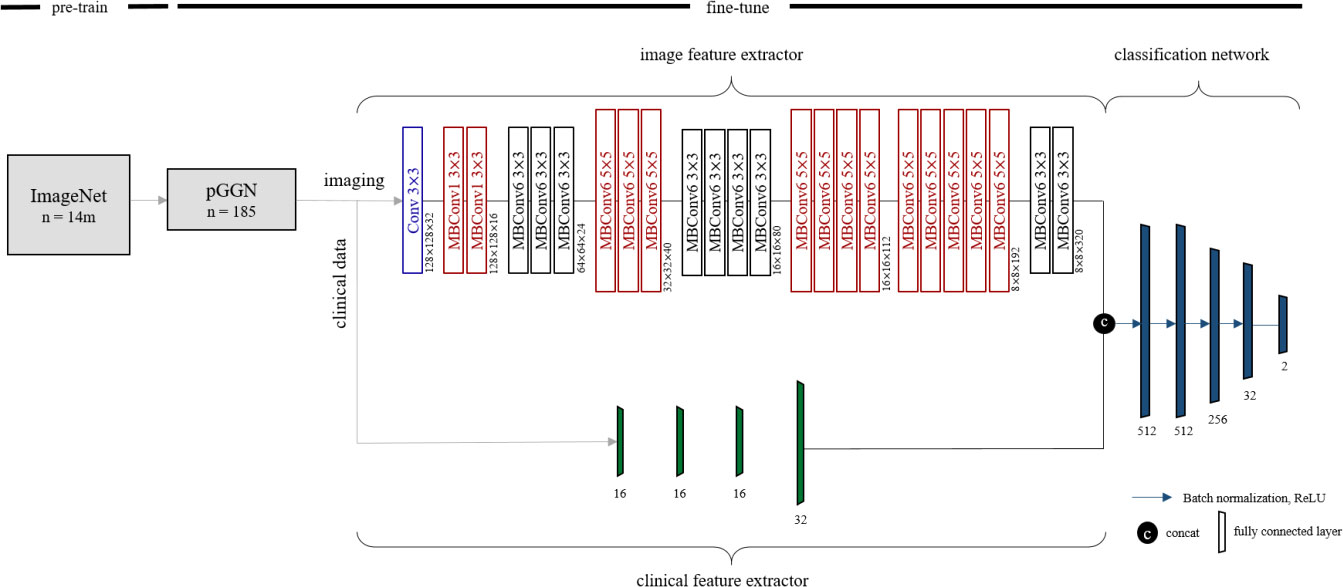
Figure 2 Details of Multimodal EfficientNet-b1 for Lung. Multimodal EfficientNet-b1 for Lung (MENL) consists of an image feature extractor, clinical feature extractor, and classification network. Pre-trained EfficientNet-b1 was used as an image feature extractor. For EfficientNet-b1, MBConv1 and MBConv6 were utilized as basic modules. MBConv1 was composed of depth-wise convolution, SENet (35), and 1×1 convolution. For MBConv6, 1×1 convolution was added before depth-wise convolution of MBConv1.
Clinical factors, sex, age, and smoking status were used as inputs to train a random forest classifier with five decision trees and a maximum depth of 16.
Radiomics features were calculated from ROIs. We used Python-based open-source software PyRadiomics (https://pyradiomics.readthedocs.io/) to extract 72 radiomics features in the following four categories: histogram (18 features), shape (14 features), gray-level co-occurrence matrix (GLCM) (24 features), and gray-level size-zone matrix (GLSZM) (16 features) (Supplementary Table S1). Additionally, MATLAB-based in-house software was used to calculate five marginal features (Appendix in the Supporting Information) (36). In total, we computed 77 radiomics features per ROI. Extracted features were normalized with z-score normalization. The least absolute shrinkage and selection operator (LASSO) was used to select the most useful predictive features for EGFR mutation status. Using the selected radiomics features, we applied random forest regression with five decision trees to construct the radiomics model.
We utilized gradient-weighted class activation mapping (Grad-CAM) (37) to compute an activation heat map of MENL. Grad-CAM uses gradient information to assign significant values to the feature map to determine where the model focus is when making the prediction. The last CNN layer of MENL was used to create the activation map. After overlaying the activation map and inputting CT image, we analyzed the outcomes of MENL using the four categories of true positive (activation map consistent with positive EGFR), true negative, false positive, and false negative cases for model interpretability.
We used Pytorch (version 1.8.0) for image analysis. Since EfficientNet-b1 has an input image size of 240, we resized the images from 64 to 256. Data augmentation was performed using horizontal and vertical flips with a probability of 0.5. Our MENL was trained on the training set of the primary cohort for 30 epochs. We adopted early-stopping where the model showed the highest accuracy in the technical validation set. Performance was computed on the test set. It took 30 secs to train MENL using the NVIDIA TITAN Xp graphics card.
For the independent clinical validation cohort, we included 64 consecutive patients who received an EGFR-TKI from January 2010 to December 2015 for stage IV non-small cell lung cancer (NSCLC) and had concurrent GGN(s) that overlapped with a previous study (38). We identified and indexed 83 concurrent GGNs of 64 patients for follow up and grouped these into a response group if concurrent GGN decreased in size or did not change in size, but decreased in density after EGFR-TKIs, or as a non-response group if concurrent GGN had an increase in size or density on the last follow-up chest CT (Figure 1). We applied MENL to the clinical validation dataset to assess the generalizability and clinical utility of our model and used EGFR-TKI treatment response as the reference standard for MENL. That is, the response group was regarded as the EGFR-mutant group and the non-response group was regarded as the EGFR-wild type group.
To compare clinical variables, ANOVA was conducted for continuous variables and chi-square tests were conducted for categorical variables. To assess the prediction performance of the proposed model, area under the curve (AUC), accuracy, sensitivity, and specificity were calculated to consider both majority and minority classes. All statistical analyses were performed with the statistics tools “scipy,” “statsmodels,” and “sklearn” in Python.
Among the 185 GGO lung adenocarcinomas, 122 (65.9%) were EGFR-mutant and 63 (34.1%) were EGFR-wild type. Demographic information and tumor characteristics of the primary cohort are listed in Table 1.
After feature selection processes, the 11 radiomics features that were selected were as follows: interquartile range, minimum, root mean squared, cluster shade, contrast, maximal correlation coefficient, gray level non-uniformity normalized, elongation, maximum 3D diameter, mean of the cumulative distribution function (CDF) slope, and standard deviation of the (CDF) slope (Table 2).
For the test set of the primary cohort (n = 37 of 36 patients), the AUC values of the clinical model (age, sex, and smoking history), radiomics model, and MENL to predict EGFR mutations were 0.50, 0.64, and 0.85, respectively (Table 3 and Figure 3).
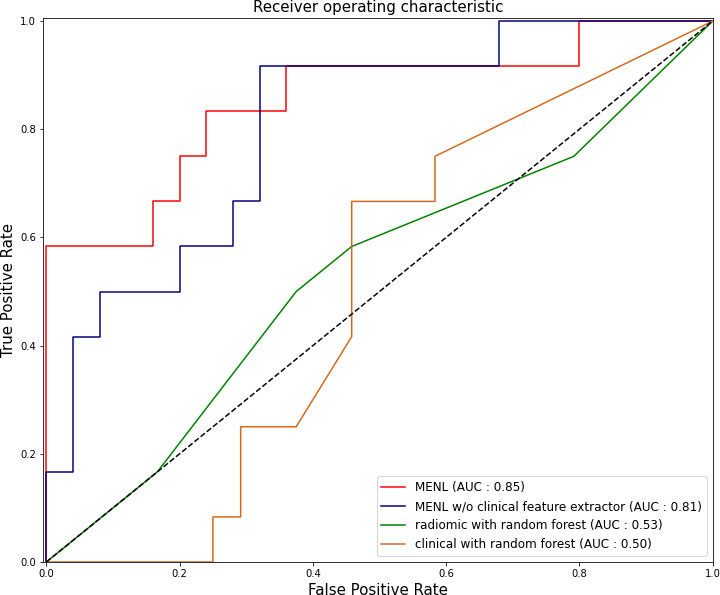
Figure 3 Receiver operating characteristic curves of the Multimodal EfficientNet-b1 for Lung (MENL), MENL without clinical feature extractor, radiomics-based model, and clinical feature-based model in the test set (n = 37) of the primary cohort.
For the test set (n = 37), the median EGFR-mutant probability was 0.58 (interquartile range [IQR], 0.57-0.59) in the EGFR-mutant group and 0.52 (IQR, 0.48-0.54) in the EGFR-wild type group. The discrimination performance of MENL was statistically significant (P < 0.001).
We added the clinical feature extractor to the existing EfficientNet-b1 to predict EGFR mutation status. The newly added clinical feature extractor receives three clinical factors as input and assists in predicting EGFR mutation status with CT images. To justify the effectiveness of this design, we deleted the clinical feature extractor in MENL. Thus, the model only consisted of the image feature extractor and classification network. As shown in Table 3, the ablation model without the clinical feature extractor showed poorer performance than the MENL.
Grad-CAMs overlaid with CT images for the test set of the primary cohort varied in different tumors. However, a common pattern was that the MENL was highly focused its attention on the proximal bronchovascular bundle of the tumor with tumor inside for EGFR-mutant pGGN lung adenocarcinomas. For EGFR-wild type pGGN lung adenocarcinomas, a small portion of the tumor and its proximal bronchovascular bundle were activated (Figure 4 and Supplementary Figure S1).
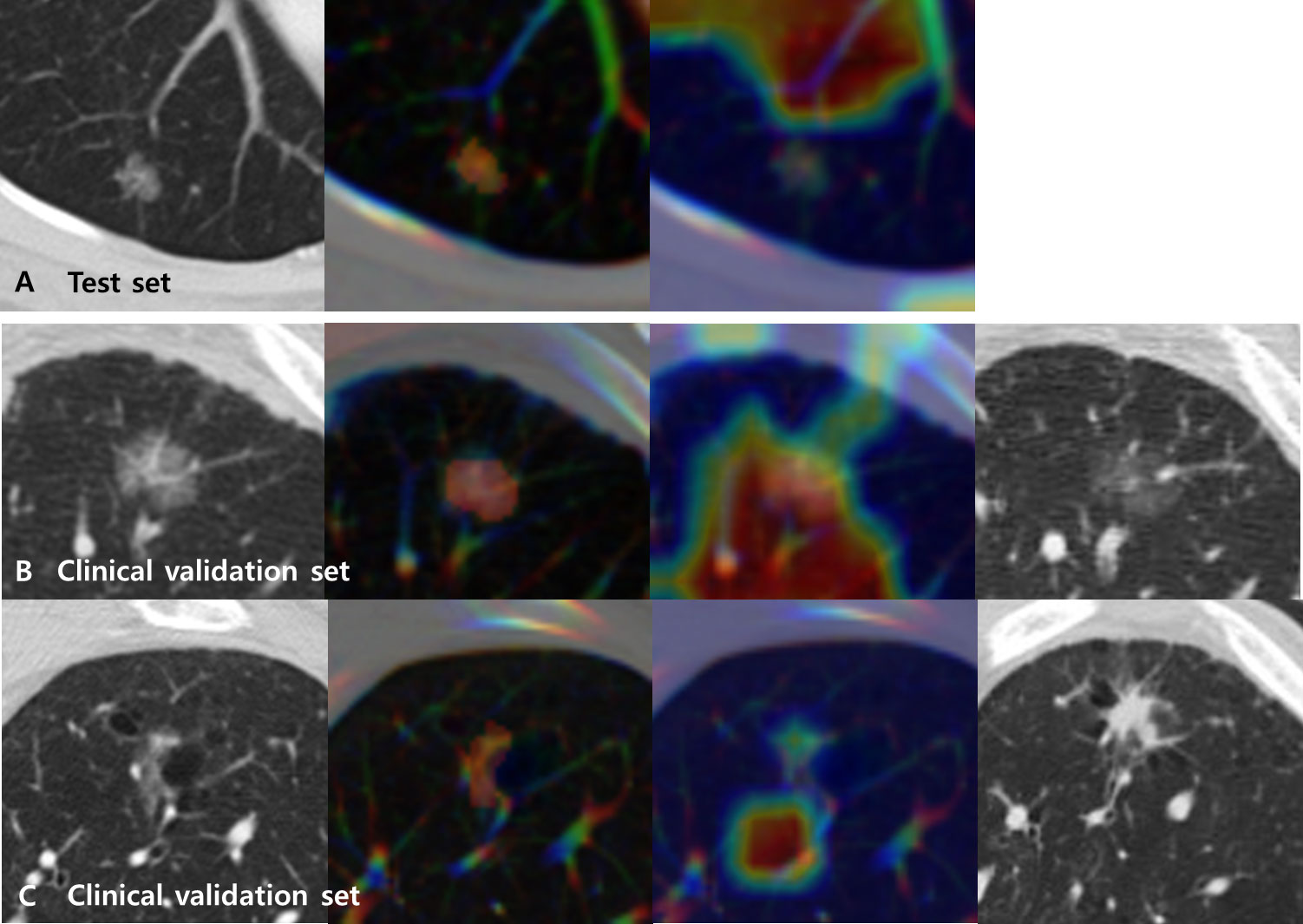
Figure 4 Representative CT images (first from the left) overlaid with regions of interest (ROIs) (second) and Grad-CAMs (third) for Multimodal EfficientNet-b1 for Lung (MENL) interpretation. (A) A EGFR-mutant correct case (probability 0.62) in the test set. (B) A EGFR-mutant (response) correct case (probability 0.69) in the clinical validation set. Compared to the baseline CT image (first), the last follow-up CT image after TKI (fourth) demonstrates a decrease in density. (C) A EGFR-wild type (non-response) correct case (probability 0.51) in the clinical validation set. Compared to the baseline CT image (first), the last follow-up CT image after TKI (fourth) demonstrates an increase in size and density. In all cases, the tumor and its proximal bronchovascular bundle are activated by the MENL.
The characteristics of the clinical validation cohort are presented in Supplementary Table S2. When our MENL was applied to an independent clinical validation set (n = 83 of 64 patients), the AUC was 0.72 (Table 4). For the clinical validation set, the median EGFR-mutant probability was 0.53 (IQR, 0.50-0.58) in the response group and 0.48 (IQR, 0.48-0.51) in the non-response group. However, discrimination performance was not statistically significant (P = 0.145) (Table 4). Grad-CAMs of the MENL in the clinical validation set showed similar patterns to those of the test set in the primary cohort (Figure 4).
While tremendous strides have been made in the development of deep learning algorithms in oncology, as evidenced by the surge in publications and published datasets in recent years, there remains a large gap between the evidence for artificial intelligence (AI) performance and evidence for clinical impact (28, 29). There have been no studies demonstrating the clinical utility of deep learning models by applying the model to real-world cancer patients. In this study, we proposed a deep learning model using CT images to predict EGFR mutation status among patients with lung adenocarcinoma manifesting as pGGN and demonstrated its clinical utility using an independent cohort made up of patients who received EGFR-TKI (83 GGNs of 64 patients) and treatment response as the reference standard. The proposed model showed encouraging results in the primary cohort (AUC = 0.85) and achieved strong performance in the independent clinical validation cohort (AUC = 0.72). Thus, our results are valuable and can be distinguished from previous studies as the first attempt at bridging the AI translational gap between initial model development and routine clinical cancer care, and we demonstrated the clinical feasibility of our MENL model. Our design provides an alternative method to non-invasively assess EGFR information and to assist in decision-making when applying TKI as an initial treatment in inoperable or inappropriate situations for surgical treatment of lung adenocarcinoma manifesting as pGGN.
Although there have been studies of deep learning models demonstrating promising performance in assisting lung cancer analysis (24–27), our study is distinguished from prior studies by design and by the relative difficulty of the application. Because we extracted and collected examples of lung adenocarcinoma manifesting as pGGN only according to rigorous criteria not only to construct the primary cohort but also for clinical validation, we engaged in a long-term commitment to gather such patients and their pretreatment CT scans before surgery or TKI. By designing the deep learning model using image feature and clinical feature extractors, we were able to incorporate CT image and clinical factors simultaneously.
The Grad-CAM activation maps convey important regions of cues that dominate the prediction of EGFR mutation status. Since deep learning is an end-to-end prediction model that learns abstract mappings between tumor images and EGFR mutation status, it is important to explain the prediction process so that users can gain confidence in the prediction process. The activation map focused attention on the proximal bronchovascular bundle of the tumor with the tumor inside. These attention areas were inferred to be strongly related to EGFR mutation status by the deep learning model for a lung adenocarcinoma manifesting as a pGGN. Based on our observations, we hypothesized that our deep learning model used information from the proximal bronchovascular bundle of the tumor to make predictions.
In this study, the radiomics model achieved an AUC of 0.64, which was inferior to those of existing radiomics studies predicting EGFR mutation for lung adenocarcinoma (16–18). This shortcoming could be due to unique characteristics of pGGN such as extremely homogenous and negative CT density causing skewness of data, which makes it difficult to discriminate by morphology or radiomics. Thus, we believe deep learning methods can overcome such limitations in imaging prediction for specific subjects such as pGGN adenocarcinomas. In addition, previous studies used clinical factors to predict EGFR mutation status. For example, clinical factors such as age, sex, smoking status, tumor stage, and predominant subtype were used to build prediction models for EGFR mutation status. These studies achieved AUC ranging from 0.68-0.84 in different populations (39–41). In contrast, our clinical model (age, sex, and smoking history) was subpar with an AUC value of 0.5 (Table 3). One reason for this poor discrimination performance could be that our study subjects consisted of all pGGN adenocarcinomas. However, when the clinical model was added to the deep learning model, prediction performance improved from AUC 0.81 to AUC 0.85. Thus, clinical features (young age, female, and non-smoker) traditionally considered to be significant factors for predicting EGFR in lung adenocarcinoma retain important roles in pGGN adenocarcinoma.
In this study, our deep learning model demonstrated advantages since it can mine abstract features that are difficult to extract with conventional methods but are important for identifying EGFR mutation status. Compared with previously reported hand-crafted semantic or radiomics features, the deep learning model has additional advantages. First, the deep learning model extracts multi-level features from low-level visual characteristics to abstract features that are directly related to EGFR information through a hierarchical neural network structure. Second, the deep learning model does not require time-consuming tumor boundary annotation, which is a major advantage over the radiomics approach. Moreover, the microenvironment of tumors and the relationships between tumors and surrounding tissues such as lung parenchyma and bronchovascular structure are inherently considered in the deep learning model because the peripheral regions are typically included in the rectangular image patch. Third, the deep learning model is fast and easy to use, requires only the CT image as input, and predicts EGFR mutation status directly without further human input.
Despite the encouraging performance of our deep learning model, this study has several limitations. First, we only examined patients in an East Asian population. However, EGFR mutation rate can be affected by regional variation in humans. In future work, samples from multiple areas of the world will be necessary to test whether the deep learning model can be generalized to other populations. Second, although the deep learning model shows better performance than models using clinical features and radiomics, how to optimally combine these two models remains an open question. The predictive performance of our model may be improved if we adopt other advanced approaches to combine these two models. Third, our predictive model could not be applied for the predictive model with solid and solid dominant nodules for investigation of its scalability. In this study we focused on only pure GGNs because we were concerned with increasing cases of multiple pGGNs on screening chest CT and their early management strategy. However, application of our predictive model for solid and solid dominant nodules could be valuable and helpful especially on unresectable cases. Thus, we plan to expand cases with solid and solid dominant nodules and devise another cohort to validate our results for the next study. Finally, the number of patients in the clinical validation set was small and there were only five EGFR-wild type cases which made insufficient balance between ‘Response (EGFR-mutant) group (n = 78)’ and ‘Non-response (EGFR-wild type) group (n = 5)’. This might have limited the statistical power for validating the performances of the prediction models. Nevertheless, a total of 83 subjects could be meaningful because it is difficult to find such number of cases that have relatively long-term, serial follow-up CT scans. This data imbalance could be resolved in future work by using a larger number of non-response (EGFR-wild type) cases.
In conclusion, our preoperative CT-based deep learning model was able to predict EGFR mutations in patients with lung adenocarcinomas manifesting as pGGN. Our deep learning model outperformed the radiomics model in the detection of EGFR mutations. The combination of deep learning and clinical models showed further performance improvements in EGFR prediction and demonstrated its clinical utility in the real-world population. This deep learning model provides a non-invasive method to predict EGFR mutation status, can be used easily in routine CT diagnosis, and may facilitate clinical decision-making in the era of precision medicine.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Institutional Review Board of the Samsung Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HYL and HP takes full responsibility for the content of the manuscript, including data and analysis. HJY, JC, and HYL contributed to the study concept, design, interpretation and writing. EK, S-WU, NK, WK, and GK contributed to acquisition of data or analysis.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No.NRF-2022R1A2C1003999), the Future Medicine 20*30 Project of the Samsung Medical Center [#SMX1210781], the Institute for Basic Science (IBS-R015-D1), the National Research Foundation of Korea (NRF-2020M3E5D2A01084892), the Ministry of Science and ICT of Korea under the Information Technology Research Center program (IITP-2020-2018-0-01798), the AI Graduate School Program (2019-0-00421), the ICT Creative Consilience program (IITP-2020-0-01821), and the Artificial Intelligence Innovation Hub program (2021-0-02068).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.951575/full#supplementary-material
1. Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol (2013) 31(8):1039–49. doi: 10.1200/jco.2012.45.3753
2. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol (2011) 12(8):735–42. doi: 10.1016/s1470-2045(11)70184-x
3. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol (2013) 31(27):3327–34. doi: 10.1200/jco.2012.44.2806
4. Miller VA, Riely GJ, Zakowski MF, Li AR, Patel JD, Heelan RT, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol (2008) 26(9):1472–8. doi: 10.1200/jco.2007.13.0062
5. Pedersen JH, Saghir Z, Wille MM, Thomsen LH, Skov BG, Ashraf H. Ground-glass opacity lung nodules in the era of lung cancer CT screening: Radiology, pathology, and clinical management. Oncol (Williston Park) (2016) 30(3):266–74.
6. Silva M, Pastorino U, Sverzellati N. Lung cancer screening with low-dose CT in Europe: strength and weakness of diverse independent screening trials. Clin Radiol (2017) 72(5):389–400. doi: 10.1016/j.crad.2016.12.021
7. Ten Haaf K, Tammemägi MC, Bondy SJ, van der Aalst CM, Gu S, McGregor SE, et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: A microsimulation modeling analysis in Ontario, Canada. PloS Med (2017) 14(2):e1002225. doi: 10.1371/journal.pmed.1002225
8. Lin YH, Hsu HS. Ground glass opacity on chest CT scans from screening to treatment: A literature review. J Chin Med Assoc (2020) 83(10):887–90. doi: 10.1097/jcma.0000000000000394
9. Jiang G, Xie D. Early-stage lung cancer manifested as ground-glass opacity. Zhonghua Wai Ke Za Zhi (2015) 53(10):790–3. doi: 10.3760/cma.j.issn.0529-5815.2015.10.020
10. Wang Y, Yeung JC, Hanna WC, Allison F, Paul NS, Waddell TK, et al. Metachronous or synchronous primary lung cancer in the era of computed tomography surveillance. J Thorac Cardiovasc Surg (2019) 157(3):1196–202. doi: 10.1016/j.jtcvs.2018.09.052
11. Kim HK, Choi YS, Kim J, Shim YM, Lee KS, Kim K. Management of multiple pure ground-glass opacity lesions in patients with bronchioloalveolar carcinoma. J Thorac Oncol (2010) 5(2):206–10. doi: 10.1097/JTO.0b013e3181c422be
12. Ye C, Wang J, Li W, Chai Y. Novel strategy for synchronous multiple primary lung cancer displaying unique molecular profiles. Ann Thorac Surg (2016) 101(2):e45–7. doi: 10.1016/j.athoracsur.2015.06.042
13. Cheng B, Deng H, Zhao Y, Zhu F, Liang H, Li C, et al. Management for residual ground-glass opacity lesions after resection of main tumor in multifocal lung cancer: A case report and literature review. Cancer Manag Res (2021) 13:977–85. doi: 10.2147/cmar.s290830
14. Lu CH, Hsiao CH, Chang YC, Lee JM, Shih JY, Wu LA, et al. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J Thorac Oncol (2012) 7(1):143–50. doi: 10.1097/JTO.0b013e318233d7dd
15. Hu M, Liu L, Qian K, Li Y, Zhi X. Analysis of percutaneous biopsy of 41 small lung lesions. Zhongguo Fei Ai Za Zhi (2018) 21(9):682–5. doi: 10.3779/j.issn.1009-3419.2018.09.06
16. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun (2014) 5:4006. doi: 10.1038/ncomms5006
17. Liu Y, Kim J, Balagurunathan Y, Li Q, Garcia AL, Stringfield O, et al. Radiomic features are associated with EGFR mutation status in lung adenocarcinomas. Clin Lung Cancer (2016) 17(5):441–8.e6. doi: 10.1016/j.cllc.2016.02.001
18. Tu W, Sun G, Fan L, Wang Y, Xia Y, Guan Y, et al. Radiomics signature: A potential and incremental predictor for EGFR mutation status in NSCLC patients, comparison with CT morphology. Lung Cancer (2019) 132:28–35. doi: 10.1016/j.lungcan.2019.03.025
19. Balagurunathan Y, Gu Y, Wang H, Kumar V, Grove O, Hawkins S, et al. Reproducibility and prognosis of quantitative features extracted from CT images. Transl Oncol (2014) 7(1):72–87. doi: 10.1593/tlo.13844
20. Aerts HJ. The potential of radiomic-based phenotyping in precision medicine: A review. JAMA Oncol (2016) 2(12):1636–42. doi: 10.1001/jamaoncol.2016.2631
21. Kalpathy-Cramer J, Mamomov A, Zhao B, Lu L, Cherezov D, Napel S, et al. Radiomics of lung nodules: A multi-institutional study of robustness and agreement of quantitative imaging features. Tomography (2016) 2(4):430–7. doi: 10.18383/j.tom.2016.00235
22. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature (2015) 521(7553):436–44. doi: 10.1038/nature14539
23. Silver D, Schrittwieser J, Simonyan K, Antonoglou I, Huang A, Guez A, et al. Mastering the game of go without human knowledge. Nature (2017) 550(7676):354–9. doi: 10.1038/nature24270
24. Zhao W, Yang J, Ni B, Bi D, Sun Y, Xu M, et al. Toward automatic prediction of EGFR mutation status in pulmonary adenocarcinoma with 3D deep learning. Cancer Med (2019) 8(7):3532–43. doi: 10.1002/cam4.2233
25. Wang S, Shi J, Ye Z, Dong D, Yu D, Zhou M, et al. Predicting EGFR mutation status in lung adenocarcinoma on computed tomography image using deep learning. Eur Respir J (2019) 53(3):1800986. doi: 10.1183/13993003.00986-2018
26. Xiong JF, Jia TY, Li XY, Yu W, Xu ZY, Cai XW, et al. Identifying epidermal growth factor receptor mutation status in patients with lung adenocarcinoma by three-dimensional convolutional neural networks. Br J Radiol (2018) 91(1092):20180334. doi: 10.1259/bjr.20180334
27. Li XY, Xiong JF, Jia TY, Shen TL, Hou RP, Zhao J, et al. Detection of epithelial growth factor receptor (EGFR) mutations on CT images of patients with lung adenocarcinoma using radiomics and/or multi-level residual convolutionary neural networks. J Thorac Dis (2018) 10(12):6624–35. doi: 10.21037/jtd.2018.11.03
28. Kann BH, Hosny A, Aerts H. Artificial intelligence for clinical oncology. Cancer Cell (2021) 39(7):916–27. doi: 10.1016/j.ccell.2021.04.002
29. Park SH, Choi J, Byeon JS. Key principles of clinical validation, device approval, and insurance coverage decisions of artificial intelligence. Korean J Radiol (2021) 22(3):442–53. doi: 10.3348/kjr.2021.0048
30. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol (2011) 6(2):244–85. doi: 10.1097/JTO.0b013e318206a221
31. Cho JH, Jung HA, Lee SH, Ahn JS, Ahn MJ, Park K, et al. Impact of EGFR mutation on the clinical efficacy of PD-1 inhibitors in patients with pulmonary adenocarcinoma. J Cancer Res Clin Oncol (2019) 145(5):1341–9. doi: 10.1007/s00432-019-02889-0
32. Choe J, Lee SM, Do KH, Lee G, Lee JG, Lee SM, et al. Deep learning-based image conversion of CT reconstruction kernels improves radiomics reproducibility for pulmonary nodules or masses. Radiology (2019) 292(2):365–73. doi: 10.1148/radiol.2019181960
33. Sandler M, Howard A, Zhu M, Zhmoginov A, Chen LC. (2018). MobileNetV2: Inverted residuals and linear bottlenecks. Proceedings of the IEEE computer society conference on computer vision and pattern recognition; Piscataway, New Jersey, USA: IEEE (2018).
34. Tan M, Le QV. EfficientNet: Rethinking model scaling for convolutional neural networks (2019) (Accessed December 14, 2021).
35. Hu J, Shen L, Sun G. Squeeze-and-Excitation networks (2018). Available at: http://image-net.org/challenges/LSVRC/2017/results (Accessed December 14, 2021).
36. Cho HH, Lee G, Lee HY, Park H. Marginal radiomics features as imaging biomarkers for pathological invasion in lung adenocarcinoma. Eur Radiol (2020) 30(5):2984–94. doi: 10.1007/s00330-019-06581-2
37. Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D. Grad-CAM: Visual explanations from deep networks via gradient-based localization (2017). Available at: http://gradcam.cloudcv.org (Accessed December 14, 2021).
38. Kang N, Kim KH, Jeong BH, Lee K, Kim H, Kwon OJ, et al. The impact of EGFR tyrosine kinase inhibitor on the natural course of concurrent subsolid nodules in patients with non-small cell lung cancer. Cancer Res Treat (2021) 54(3):817–26. doi: 10.4143/crt.2021.822
39. Girard N, Sima CS, Jackman DM, Sequist LV, Chen H, Yang JC, et al. Nomogram to predict the presence of EGFR activating mutation in lung adenocarcinoma. Eur Respir J (2012) 39(2):366–72. doi: 10.1183/09031936.00010111
40. Liu Y, Kim J, Qu F, Liu S, Wang H, Balagurunathan Y, et al. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology (2016) 280(1):271–80. doi: 10.1148/radiol.2016151455
Keywords: lung adenocarcinoma, ground-glass opacity nodule, computed tomography, deep learning, epidermal growth factor receptor
Citation: Yoon HJ, Choi J, Kim E, Um S-W, Kang N, Kim W, Kim G, Park H and Lee HY (2022) Deep learning analysis to predict EGFR mutation status in lung adenocarcinoma manifesting as pure ground-glass opacity nodules on CT. Front. Oncol. 12:951575. doi: 10.3389/fonc.2022.951575
Received: 24 May 2022; Accepted: 15 August 2022;
Published: 02 September 2022.
Edited by:
Vamsi Velcheti, New York University, United StatesCopyright © 2022 Yoon, Choi, Kim, Um, Kang, Kim, Kim, Park and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ho Yun Lee, aG95dW5sZWU5NkBnbWFpbC5jb20=; Hyunjin Park, aHl1bmppbnBAc2trdS5lZHU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.