
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 11 August 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.951387
 Naohiro Takeshita1
Naohiro Takeshita1 Tomohiro Enokida1
Tomohiro Enokida1 Susumu Okano1
Susumu Okano1 Takao Fujisawa1
Takao Fujisawa1 Akihisa Wada1
Akihisa Wada1 Masanobu Sato1
Masanobu Sato1 Hideki Tanaka1
Hideki Tanaka1 Nobukazu Tanaka1
Nobukazu Tanaka1 Atsushi Motegi2
Atsushi Motegi2 Sadamoto Zenda2
Sadamoto Zenda2 Tetsuo Akimoto2
Tetsuo Akimoto2 Makoto Tahara1*
Makoto Tahara1*Background: The addition of induction chemotherapy (IC) before chemoradiotherapy (CRT) has improved survival over CRT alone in locoregionally advanced nasopharyngeal cancer (LA-NPC). Nevertheless, this population would benefit from further development of a novel IC regimen with satisfactory efficacy and a more favorable safety profile.
Methods: We retrospectively assessed 29 LA-NPC patients who received the combination of paclitaxel (PTX), carboplatin (CBDCA), and cetuximab (Cmab) (PCE) as IC (IC-PCE) at the National Cancer Center Hospital East between March 2017 and April 2021. IC-PCE consisted of CBDCA area under the plasma concentration-time curve (AUC) = 1.5, PTX 80 mg/m2, and Cmab with an initial dose of 400 mg/m2 followed by 250 mg/m2 administered weekly for a maximum of eight weeks.
Results: Patient characteristics were as follows: median age, 59 years (range 24–75); 0, 1 performance status (PS), 25, 4 patients; and clinical stage III/IVA/IVB, 6/10/13. The median number of PCE cycles was 8(1-8). After IC-PCE, 26 patients received concurrent cisplatin and radiotherapy (CDDP-RT), one received concurrent carboplatin/5-fluorouracil and radiotherapy (CBDCA/5-FU-RT), and two received RT alone. The % completion of CDDP-RT was 88.5%. The response rate was 75.9% by IC and 100% at completion of CRT. The 3-year recurrence-free survival, locoregional failure-free survival, distant recurrence-free survival, and overall survival were 75.9%, 79.3%, 84.3%, and 96.3%, respectively. The incidence of adverse events of grade 3/4 was 34.5% during IC and 44.8% during CRT.
Conclusion: IC-PCE is feasible and effective for LA-NPC and may be a treatment option for this disease.
Worldwide, nasopharyngeal carcinoma (NPC) affected 133,354 patients and caused 80,008 deaths in 2020 (1). Prevalence is high in South China, Southeastern Asia, and North Africa. More than 70% of patients are diagnosed with locally advanced disease at presentation (2). Because of the anatomical location and high sensitivity of NPC to radiotherapy (RT) and chemotherapy, chemoradiotherapy (CRT) is the backbone of treatment. Moreover, the addition of chemotherapy as induction chemotherapy (IC) or adjuvant chemotherapy to CRT is now a standard treatment for this disease (3). Surprisingly, several prospective studies have shown that IC consistently results in higher response and exerts a pronounced effect on survival and distant metastasis (4–7). A meta-analysis also showed that the addition of IC to CRT improved progression-free survival (PFS) and overall survival (OS) (8–10). Based on these findings, platinum-based IC, herein the combination of docetaxel (DTX), cisplatin (CDDP), and fluorouracil (TPF) and gemcitabine (GEM)+CDDP (GP) followed by CRT, are now considered standard therapy over CRT alone for this patient population (4, 5).
However, GEM has not been approved for the treatment of NPC in several countries, including Japan, which hampers use of the drug as a part of IC. Further, IC-TPF as a CDDP-containing triplet regimen sometimes raises concerns about treatment-related toxicity, such as renal impairment related to repeated administration of CDDP over time and increased myelosuppression. Moreover, despite several studies suggesting that compliance with cisplatin during CRT is a critical factor in maximizing its efficacy (11–13), compliance with concomitant chemotherapy with RT following IC is generally impaired due to the adverse effects of the prior IC (9).
We previously tested the combination of paclitaxel (PTX), carboplatin (CBDCA), and cetuximab (Cmab) (PCE) as IC in a Japanese multicenter phase II trial in patients with unresectable locally advanced head and neck squamous cell carcinoma (LA-HNSCC) arising from the hypopharynx, oropharynx, and larynx (14). Results showed that PCE as IC(IC-PCE) was feasible and effective, with a response rate of 88.6% by IC, and had no effect on compliance with subsequent CRT with CDDP. Further, PCE has shown promising efficacy in recurrent or metastatic NPC (R/M NPC), represented by an overall response rate (ORR) of 58.3% (15).
Here, we investigated whether IC-PCE would also be an effective treatment option with a favorable toxicity profile for LA-NPC.
We retrospectively reviewed LA-NPC patients treated with IC-PCE from March 2017 to April 2021 at the National Cancer Center Hospital East, Japan. Inclusion criteria were as follows (1): pathologically proven NPC, (2) newly diagnosed non-distant metastatic stage III to IVB disease (except T3–4N0; 7th Union for International Cancer Control and American Joint Committee on Cancer), and (3) no other active malignant tumor during treatment. This study was approved by the Institutional Review Board of the National Cancer Center Hospital East.
The induction PCE regimen consisted of CBDCA area under the plasma concentration-time curve (AUC) = 1.5, PTX 80 mg/m2, and Cmab with an initial dose of 400 mg/m2 followed by 250 mg/m2 administered weekly for eight weeks. Following IC, concurrent chemoradiotherapy was started. During CRT, cisplatin was administered intravenously at a dose of 80 mg/m2 every three weeks on days 1, 22, and 43; or at a dose of 20 mg/m2 on days 1-4, repeated three times at 3-week intervals. For one patient who received chemoradiotherapy consisting of RT plus the combination of CBDCA and 5-fluorouracil (5-FU), CBDCA (AUC = 5 on day 1) and 5-FU (500mg/m2 by 24-h continuous infusion on days 1-4) was administered every four weeks for up to two cycles. As with RT, all patients were treated with intensity-modulated radiation therapy (IMRT). The planned total radiation dose was 70 Gy in 35 fractions (2 Gy per day) or 69.96 Gy in 33 fractions (2.12 Gy per day), with prophylactic dose (56 - 63 Gy) irradiation to the elective neck. Toxicity during treatment was graded using the Common Toxicity Criteria for Adverse Events (CTCAE version 4.0).
Clinical tumor response to treatment was evaluated radiographically according to Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 using computerized tomography (CT) or magnetic resonance imaging (MRI) and [18F]-fluorodeoxyglucose positron-emission tomography (PET)/CT fusion imaging, as required. OS, Recurrence-free survival (RFS), locoregional failure-free survival (LFFS) and distant recurrence-free survival (DRFS) were calculated by the Kaplan–Meier product-limit method. OS was defined as the period from the first day of IC-PCE until death from any cause. RFS was calculated from the first day of IC-PCE until disease recurrence, disease progression or death from any cause. LFFS was calculated from the first day of IC-PCE until recurrence in the primary tumor or a local/regional lymph node, or death from any cause. DRFS was calculated from the first day of IC-PCE until distant metastasis, or death from any cause. For patients who were treated with CDDP plus RT following IC-PCE, the proportion of CRT completion (%CRT completion) was defined by (a) completion of cumulative CDDP dose ≥ 200 mg/m2; and (b) completion of radiotherapy within two weeks after the planned completion date. Patients who were lost to follow-up or remained alive without a specified event were censored at the date of the last follow-up. Differences in LFFS by compliance with CDDP during CRT, differences in DRFS by the number of IC-PCE cycles, and differences in RFS by the number of IC-PCE cycles and compliance with CDDP during CRT were assessed using stratified log-rank tests. Hazard ratios (HRs) were calculated by Cox regression analysis. All statistical analyses were performed with EZR (version.1.51; Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria; version.4.1.1). More precisely, it is a modified version of R commander which is designed to add statistical functions frequently used in biostatistics (16).
During the study period, 29 LA-NPC patients were included in the study. Baseline patient characteristics are presented in Table 1. Median age was 59 years (24−75 years), and all patients had an ECOG performance status (PS) of 0 to 1. Of the 29 patients, 22 (75.9%) were males, and 6 (20.7%), 10 (34.5%), and 13 (44.8%) were diagnosed with stage III, IVA and IVB disease, respectively (Supplementary Figure 1).
In this setting, in which the maximum number of PCE cycles is eight, the median number of administered PCE cycles reached 8 (range, 1-8). A total of 16 patients (55.2%) completed the eight cycles of planned IC. Reasons for discontinuation are described in Supplementary Table 1. Median time from the first visit to starting PCE was 11 days (range, 1-32). Among 29 patients receiving IC-PCE, 26 (89.7%) patients underwent CDDP+RT (Figure 1). One patient received CBDCA+5-FU+RT because of a decreased cardiac ejection fraction before initiating IC-PCE. Two patients received RT alone following IC-PCE, one with tumor infection and the second with repeated aspiration pneumonia after completion of IC. The objective response rate (ORR) after IC-PCE was 75.9%, including two patients (6.9%) with complete response (CR) and 20 (69.0%) with partial response (PR) (Table 2). Only one (3.4%) of 29 patients experienced tumor growth in size in the IC phase (Figure 2). When we focused on local therapy in the 26 patients who received CDDP+RT, RT with 70 Gy in 35 fractions and 69.96 Gy in 33 fractions was delivered to 10 and 16 patients, respectively. None required the temporary cessation of RT, resulting in a median length of RT of 48 days. %CRT completion in these 26 patients was 88.5% [95% confidence interval (CI), 70.2% - 96.8%] (Supplementary Table 3). After completing CRT or RT, ORR reached 100%, with CR in 28 patients (96.6%) and PR in one patient (3.4%) (Table 2).
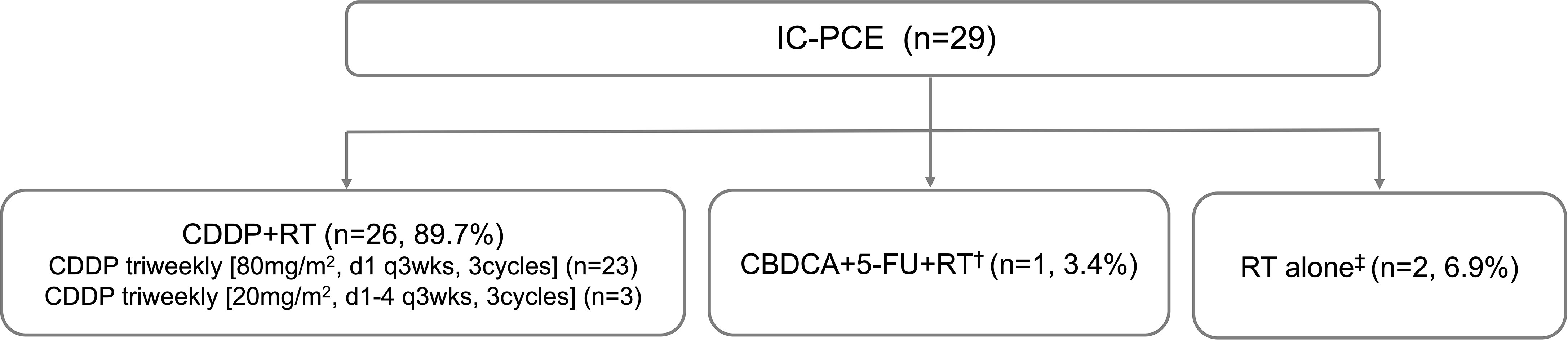
Figure 1 Patient flow diagram of treatment delivery. IC, induction chemotherapy; CDDP, cisplatin; RT, radiotherapy; CBDCA, carboplatin; FU, fluorouracil. †Ineligible for CDDP because of decreased ejection fraction. ‡Originally planned to receive CDDP+RT but CDDP could not be administered due to tumor infection or pneumonia.
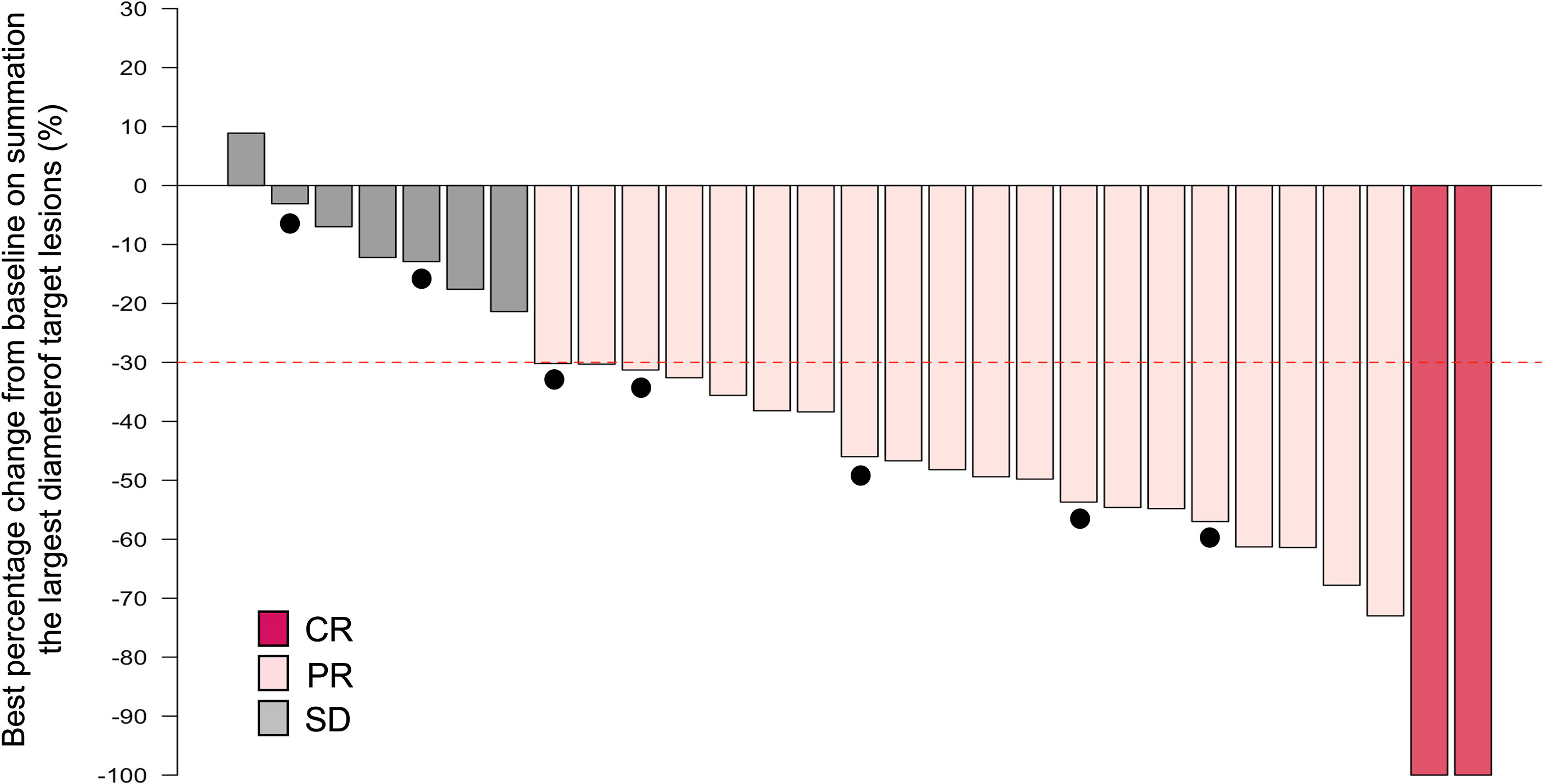
Figure 2 Waterfall plot of the maximum percentage change from baseline on summation of the largest diameter of target lesions by induction chemotherapy. Note that the dashed line indicates a 30% reduction in tumor burden in the target lesion and closed circles indicate cases with late recurrence. CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Median follow-up time was 26.9 months (range, 5.1–56.0 months). Details regarding patterns of relapse and subsequent therapies after recurrence are provided in Supplementary Figure 2. The 3-year RFS and OS were 75.9% (95% CI, 53.8-88.5) and 96.3% (95%CI, 76.5-99.5), respectively (Figures 3A, B). The 3-year LFFS and DRFS were 79.3% (95% CI, 56.8-91.0) and 84.3% (95%CI, 63.3-93.8), respectively (Figures 3C, D). In addition, we also observed a trend toward improved DRFS in patients with a higher number of IC-PCE cycles and a statistically significantly favorable LFFS in patients with the completion of cumulative CDDP dose ≥ 200mg/m2 (Supplementary Figures 3A, B). Altogether, there were trends for improvement in RFS in patients with the completion of IC-PCE 8 cycles and the completion of cumulative CDDP dose ≥ 200mg/m2 (Supplementary Figure 3C). For the 26 patients who received CDDP+RT following IC-PCE, the 3-year RFS, OS, LFFS and DRFS were 81.7% (95% CI, 57.8-92.8), 95.8% (95%CI, 73.9-99.4), 86.6% (95% CI, 63.4-95.5) and 91.2% (95%CI, 69.0-97.7), respectively (Supplementary Figure 4). Furthermore, four patients developed local recurrence, three patients had local recurrence within the high-dose zone (cumulative RT dose: 70Gy), and the remaining patient developed recurrence within the prophylactic dose zone (cumulative RT dose: 63Gy) of their IMRT. While from the viewpoint of the use of the anti-tumor drug during RT, two patients who developed local recurrence were treated with RT alone without concurrent chemotherapy.
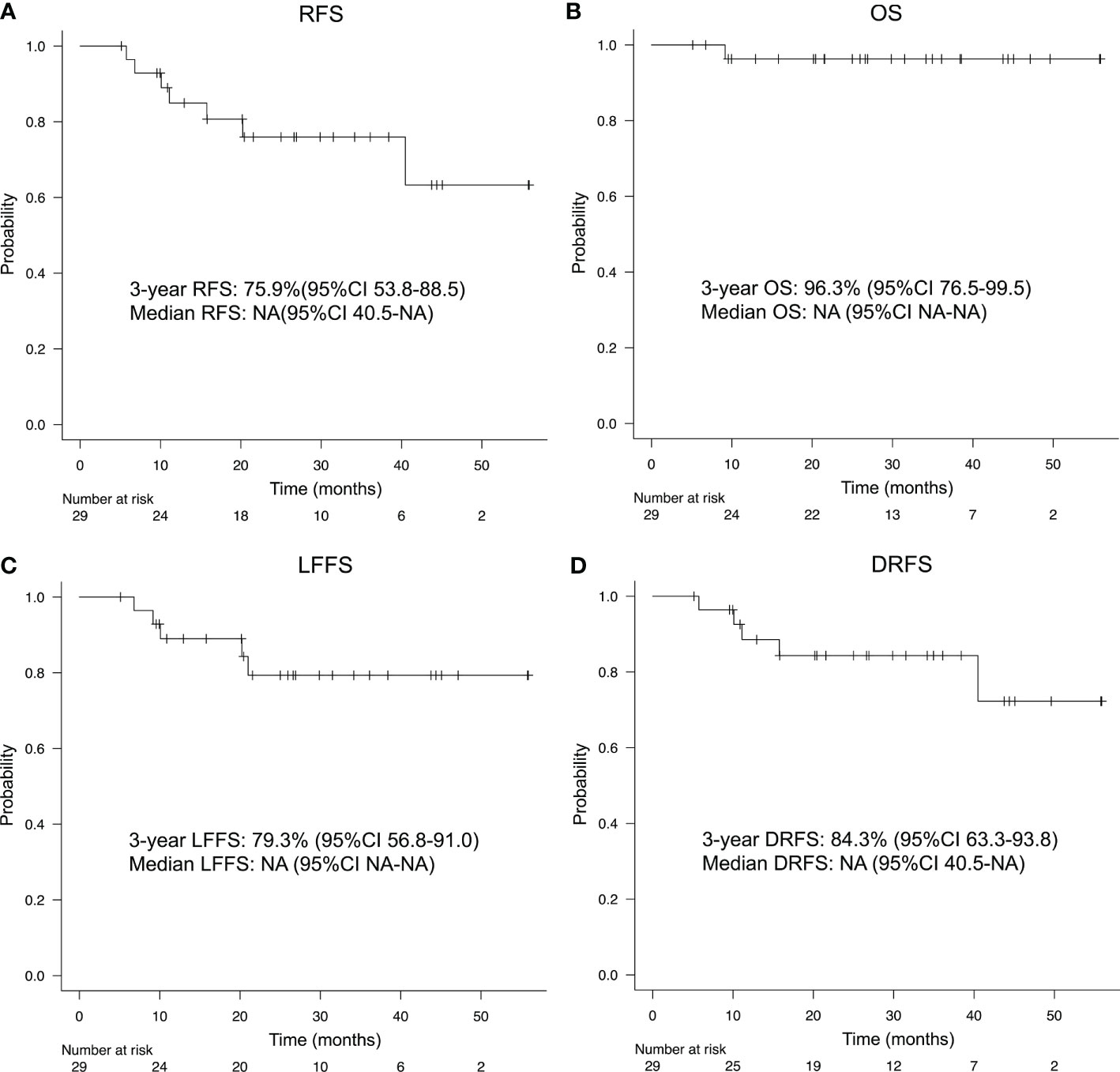
Figure 3 (A) Recurrence-free survival (RFS), (B) overall survival (OS), (C) locoregional failure-free survival (LFFS), and (D) distant recurrence-free survival (DRFS) of patients treated with IC-PCE. IC, induction chemotherapy; CI, confidence interval; NA, not available.
Acute toxicities experienced during IC-PCE and CRT are listed in Tables 3, 4, respectively. Common grade 3/4 adverse events during IC-PCE were neutropenia (24.1%), leukopenia (10.4%), and skin rash (6.9%). In contrast, mucositis (20.7%), leukopenia (17.2%), and neutropenia (17.2%) were the most frequently observed grade 3/4 adverse events during CRT. The total frequency of grade 3/4 toxicity in the IC and CRT phases was 34.5% and 44.8%, respectively. There was no treatment-related death throughout treatment.
This is the first study to indicate the feasibility and efficacy of PCE as IC for far-advanced LA-NPC. After completing locoregional therapy following IC-PCE, the ORR was 100%, and 3-year OS reached 96.3%.
Following the initial randomized phase II trial which found that the addition of two cycles of DTX and CDDP as induction therapy prior to CRT significantly improved 3-year OS (94% vs. 68%, HR, 0.24; 95% CI, 0.08 to 0.73) (6), reproduced evidence had shown that IC followed by CRT with cisplatin improves survival outcomes over CRT alone for LA-NPC (4, 5), with a positive effect primarily on distant control. However, these cisplatin-containing IC regimens can produce unacceptable toxicities such as severe renal impairment as well as myelosuppression which may cause treatment-related death, and sometimes compromise compliance with locoregional therapy following induction therapy, especially in daily practice. Thus, further development of a novel IC regimen with satisfactory efficacy and favorable safety profile has been warranted.
Given the frequency of epidermal growth factor receptor (EGFR) expression on NPC, in 73.3-84.1% of cases (17–20), several studies evaluated treatment with anti-EGFR therapy in collaboration with CRT for LA-NPC (21–23) and with chemotherapy for R/M NPC (15, 24–26), and reported promising clinical efficacy against these diseases. As an example of the former, Hao et al. revealed that adding the anti-EGFR monoclonal antibodies Cmab/nimotuzumab (NTZ) to IC was statistically significantly associated with improved OS compared with IC without these anti-EGFR drugs (IC vs. IC + Cmab/NTZ, HR,1.984; 95% CI, 1.023 to 3.848) (27). Ying et al. also showed that NTZ combined with cisplatin plus 5-fluorouracil as IC had a better lymph node response rate with milder adverse reactions than those in the TPF regimen (28). These suggest that Cmab-containing regimens may be worth testing in anticipation of an improved prognosis. Indeed, we recently reported that the PCE regimen was effective even in patients with R/M NPC (n=14), among whom 92% of evaluable patients experienced tumor shrinkage, with ORR and CR rates of 58.3% and 16.7%, respectively (15). However, no study has evaluated IC-PCE in the treatment of LA-NPC.
In the present study, anti-tumor efficacy and prognosis were equivalent or limited to slightly inferior to those in previous reports using IC-TPF (4) and -GP (5). This is notwithstanding the apparently far-advanced disease state in our cohort compared with these previous studies (eg. N-stage 3b: current study 44.8%, IC-TPF 11%, and IC-GP 6.2%; and cStage IVB: current study 44.8%, IC-TPF 16%, and IC-GP 11.2%), as shown in Supplementary Table 4. Further, as we mention above, a favorable toxicity profile during the IC phase is a key factor not only for patient safety but also for maximizing treatment efficacy by maintaining local therapy following IC. From this perspective, IC-PCE appears to offer several advantages over other IC-regimens: the rate of grade 3/4 adverse events during IC-PCE was low, at 34.5%, compared to another IC-regimen for LA-NPC at 42.3% in the TPF study (4) and 38.9% in the GP study (5), even though our present study included more patients aged over 60 years [52% in the current study vs. 0% in the TPF study (4)]. These safety data are similar to the contrasting results observed among the previous two prospective studies of IC-PCE (14) and IC-TPF (29) in unresectable LA-SCCHN, in which grade 3/4 toxicity was less frequent in the former study (14.2% vs. 79%). We believe that IC-PCE’s more feasible toxicity profile contributed to better compliance with CRT following IC compared with other IC regimens. Supporting these presumptions, our present study also provided an equivalent or better prognosis and relatively safer toxicity profile than a previous Japanese retrospective study for LA-NPC; patients in that study had similar clinical characteristics and were treated with IC-TPF followed CDDP plus RT (1y-PFS:73%, 1y-OS: 91%, G3/4AE during IC-TPF: 73%) (30). Together, these studies suggest the promise of IC-PCE in terms of both efficacy and safety in managing this population.
We also obtained several suggestive findings from the present study regarding the potential meaning of IC and subsequent local therapy in controlling the disease in NPC. A meta-analysis showed a significant difference in completing all concomitant chemotherapy cycles in the CRT alone group compared to the IC group (9).
Specifically, a cumulative CDDP dose of 200 mg/m2 was enough to achieve significantly better locoregional control and survival than those without this dose (11–13). Based on this result, we initially set a target CDDP dose at CRT following IC of 240 mg/m2 (80 mg/m2, 3 cycles) as a reasonable and well-balanced threshold with regard to both efficacy and safety. Indeed, our present completion rate with a cumulative CDDP dose ≥ 200 mg/m2 during CRT reached 88.5%, which is closely similar to our previous phase II study (14); there was in fact a statistically significant difference in LFFS by compliance with CDDP during CRT (Supplementary Figure 3). We believe our initial attempt meets the purpose of treatment; however, at the same time, we expect that a higher dose (i.e., targeting 300mg/m2) of CDDP during CRT following IC-PCE can be given safely because of its favorable safety profile in the IC phase, and that it may further contribute to an improvement in patient prognosis, especially in patients with far-advanced disease. The point should be addressed in a prospective study with a larger patient cohort. Moreover, our study indicated that favorable compliance with IC was correlated with a reduced risk of distant failure (Supplementary Figure 3). This finding is also in accord with another meta-analysis showing a statistically significant 37% reduction in the hazard of developing distant metastases in favor of IC (8).
IC-PCE has several further advantages in addition to those described above. First, the regimen is safely delivered on an outpatient basis without such troublesome procedures as intravenous hydration, and can therefore be started immediately if needed. In this study, the median time from the first visit to starting PCE was as short as 11 days, with a range of 1-32. Considering that LA-NPC patients often experience disease-related symptoms, including severe pain as well as neurological manifestations, this ability to start treatment promptly can be of very substantial benefit to them. Second, thanks to its lower toxicity, the regimen can be used as a treatment option for fragile patients, including subjects who are ineligible for a cisplatin-containing IC regimen. Shirasu et al. demonstrated that 75% (18/24) of these patients completed IC-PCE with an ORR of 87% and equivalent incidence and severity of adverse events to that observed in studies for a cisplatin-eligible population with unresectable disease (31). Similarly, Rebecca et al. revealed that 86% (19/22) of frail or elderly patients with HNSCC successfully reached their endpoint with IC-PCE with Modified RECIST response rates (MRRR) of 64% (32). However, detailed exploration of the treatment flow diagram in the current study revealed two patients considered ineligible for CRT with CDDP due to infectious episodes in the IC phase; these patients were treated with RT alone, but both experienced late local recurrence (Supplementary Figure 2). Given that several alternative agents can be substituted for CDDP in CRT, such as carboplatin (33) and nedaplatin (34), which generally less frequently induce leucopenia/neutropenia - considered helpful in reducing the risk of infection - but which offer equivalent or non-inferior potency as RT sensitizers compared with CDDP, these drugs may be available when attempting to overcome treatment failure to achieve better local control. In contrast, for patients eligible for CDDP, adding Cmab to the CRT (herein CDDP + RT) may also be worth future evaluation, especially in IC-PCE responders, in whom Cmab can be a key drug (20, 23). By these means, the high feasibility and efficacy of IC-PCE should enable us to structure the most appropriate individualized treatment for each patient.
As a limitation, the present study was conducted under a retrospective design with a small number of patients at a single center, reducing its statistical power, particularly in the subgroup analyses. Indeed, although IC-PCE followed by locoregional therapy represented by CDDP plus RT is considered a promising treatment option for LA-NPC patients, no prospective study has yet compared this regimen with CRT alone, and clarifying its role in this field via a prospective randomized controlled trial with a larger sample size is therefore warranted. Accordingly, we are planning to conduct a study for both CDDP-eligible and -ineligible patients, and to comprehensively answer the questions raised but not concluded in the current study.
In conclusion, we demonstrated that IC-PCE is feasible and has promising efficacy in the treatment of far-advanced LA-NPC. In particular, 3-year RFS was 75.9% and 3-year OS was 96.3%. IC-PCE may be a suitable treatment option as IC for this population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was reviewed and approved by Institutional Review Board of the National Cancer Center Hospital East. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
NaT, TE and MT participated in the study concept and design, interpreted the data, and drafted the manuscript. All authors contributed to the article and approved the submitted version.
TE, SO and MT receive honoraria from Merck Biopharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.951387/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys (2009) 73:1326–34. doi: 10.1016/j.ijrobp.2008.07.062
3. Chen YP, Ismaila N, Chua MLK, Colevas AD, Haddad R, Huang SH, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol (2021) 39:840–59. doi: 10.1200/JCO.20.03237
4. Sun Y, Li W-F, Chen N-Y, Zhang N, Hu G-Q, Xie F-Y, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol (2016) 17:1509–20. doi: 10.1016/s1470-2045(16)30410-7
5. Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med (2019) 381:1124–35. doi: 10.1056/NEJMoa1905287
6. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol (2009) 27:242–9. doi: 10.1200/JCO.2008.18.1545
7. Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, et al. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer (2019) 119:87–96. doi: 10.1016/j.ejca.2019.07.007
8. Tan TH, Soon YY, Cheo T, Ho F, Wong LC, Tey J, et al. Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: A systematic review and meta-analysis. Radiother Oncol (2018) 129:10–7. doi: 10.1016/j.radonc.2018.02.027
9. Mane M, Benkhaled S, Dragan T, Paesmans M, Beauvois S, Lalami Y, et al. Meta-analysis on induction chemotherapy in locally advanced nasopharyngeal carcinoma. Oncologist (2021) 26:e130–e41. doi: 10.1002/ONCO.13520
10. Xu G, Wang Q, Wu X, Lv C, Zeng G, Xue Z, et al. Comparison of induction chemotherapy plus concurrent chemoradiotherapy and concurrent chemoradiotherapy alone in locally advanced nasopharyngeal carcinoma. Technol Cancer Res Treat (2021) 20:1533033821990017. doi: 10.1177/1533033821990017
11. Peng H, Chen L, Zhang Y, Li WF, Mao YP, Zhang F, et al. Prognostic value of the cumulative cisplatin dose during concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: A secondary analysis of a prospective phase III clinical trial. Oncologist (2016) 21:1369–76. doi: 10.1634/theoncologist.2016-0105
12. Wen DW, Li ZX, Chen FP, Lin L, Peng BY, Kou J, et al. Individualized cumulative cisplatin dose for locoregionally-advanced nasopharyngeal carcinoma patients receiving induction chemotherapy and concurrent chemoradiotherapy. Oral Oncol (2020) 107:104675. doi: 10.1016/j.oraloncology.2020.104675
13. Jiang YT, Chen KH, Yang J, Liang ZG, Li L, Qu S, et al. Efficiency of high cumulative cisplatin dose in high- and low-risk patients with locoregionally advanced nasopharyngeal carcinoma. Cancer Med (2022) 11:715–27. doi: 10.1002/cam4.4477
14. Enokida T, Ogawa T, Homma A, Okami K, Minami S, Nakanome A, et al. A multicenter phase II trial of paclitaxel, carboplatin, and cetuximab followed by chemoradiotherapy in patients with unresectable locally advanced squamous cell carcinoma of the head and neck. Cancer Med (2020) 9:1671–82. doi: 10.1002/cam4.2852
15. Ueda Y, Enokida T, Okano S, Fujisawa T, Ito K, Tahara M. Combination treatment with paclitaxel, carboplatin, and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic nasopharyngeal carcinoma. Front Oncol (2020) 10:571304. doi: 10.3389/fonc.2020.571304
16. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transpl (2013) 48:452–8. doi: 10.1038/bmt.2012.244
17. Taheri-Kadkhoda Z, Magnusson B, Svensson M, Mercke C, Bjork-Eriksson T. Expression modes and clinical manifestations of latent membrane protein 1, ki-67, cyclin-B1, and epidermal growth factor receptor in nonendemic nasopharyngeal carcinoma. Head Neck (2009) 31:482–92. doi: 10.1002/hed.21002
18. Sheen TS, Huang YT, Chang YL, Ko JY, Wu CS, Yu YC, et al. Epstein-Barr Virus-encoded latent membrane protein 1 co-expresses with epidermal growth factor receptor in nasopharyngeal carcinoma. Jpn J Cancer Res (1999) 90:1285–92. doi: 10.1111/j.1349-7006.1999.tb00710.x
19. Leong JL, Loh KS, Putti TC, Goh BC, Tan LK. Epidermal growth factor receptor in undifferentiated carcinoma of the nasopharynx. Laryngoscope (2004) 114:153–7. doi: 10.1097/00005537-200401000-00029
20. Putti TC, To KF, Hsu HC, Chan AT, Lai GM, Tse G, et al. Expression of epidermal growth factor receptor in head and neck cancers correlates with clinical progression: a multicentre immunohistochemical study in the Asia-pacific region. Histopathology (2002) 41:144–51. doi: 10.1046/j.1365-2559.2002.01436.x
21. You R, Hua YJ, Liu YP, Yang Q, Zhang YN, Li JB, et al. Concurrent chemoradiotherapy with or without anti-EGFR-Targeted treatment for stage II-IVb nasopharyngeal carcinoma: Retrospective analysis with a Large cohort and long follow-up. Theranostics (2017) 7:2314–24. doi: 10.7150/thno.19710
22. Ma BBY, Kam MKM, Leung SF, Hui EP, King AD, Chan SL, et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol (2012) 23:1287–92. doi: 10.1093/annonc/mdr401
23. Xia WX, Liang H, Lv X, Wang L, Qian CN, Ye YF, et al. Combining cetuximab with chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma: A propensity score analysis. Oral Oncol (2017) 67:167–74. doi: 10.1016/j.oraloncology.2017.02.026
24. Chan AT, Hsu MM, Goh BC, Hui EP, Liu TW, Millward MJ, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol (2005) 23:3568–76. doi: 10.1200/JCO.2005.02.147
25. Chen C, Zhou Y, Zhang X, Fu S, Lin Z, Fang W, et al. Anti-epidermal growth factor receptor monoclonal antibody plus palliative chemotherapy as a first-line treatment for recurrent or metastatic nasopharyngeal carcinoma. Cancer Med (2020) 9:1721–32. doi: 10.1002/cam4.2838
26. Zhang M, Huang H, Li X, Huang Y, Chen C, Fang X, et al. Long-term survival of patients with chemotherapy-naive metastatic nasopharyngeal carcinoma receiving cetuximab plus docetaxel and cisplatin regimen. Front Oncol (2020) 10:1011. doi: 10.3389/fonc.2020.01011
27. Peng H, Tang LL, Liu X, Chen L, Li WF, Mao YP, et al. Anti-epidermal growth factor receptor therapy concurrently with induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. Cancer Sci (2018) 109:1609–16. doi: 10.1111/cas.13589
28. Lu Y, Chen D, Liang J, Gao J, Luo Z, Wang R, et al. Administration of nimotuzumab combined with cisplatin plus 5-fluorouracil as induction therapy improves treatment response and tolerance in patients with locally advanced nasopharyngeal carcinoma receiving concurrent radiochemotherapy: a multicenter randomized controlled study. BMC Cancer (2019) 19:1262. doi: 10.1186/s12885-019-6459-6
29. Okano S, Enokida T, Onoe T, Ota Y, Motegi A, Zenda S, et al. Induction TPF chemotherapy followed by CRT with fractionated administration of cisplatin in patients with unresectable locally advanced head and neck cancer. Int J Clin Oncol (2019) 24:789–97. doi: 10.1007/s10147-019-01418-w
30. Takamizawa S, Honma Y, Murakami N, Mori T, Oka H, Yamamoto S, et al. Short-term outcomes of induction chemotherapy with docetaxel, cisplatin, and fluorouracil (TPF) in locally advanced nasopharyngeal carcinoma. Invest New Drugs (2021) 39:564–70. doi: 10.1007/s10637-020-00999-y
31. Shirasu H, Yokota T, Kawakami T, Hamauchi S, Onozawa Y, Ogawa H, et al. Efficacy and feasibility of induction chemotherapy with paclitaxel, carboplatin and cetuximab for locally advanced unresectable head and neck cancer patients ineligible for combination treatment with docetaxel, cisplatin, and 5-fluorouracil. Int J Clin Oncol (2020) 25:1914–20. doi: 10.1007/s10147-020-01742-6
32. Forman R, Deshpande H, Burtness B, Bhatia AK. Efficacy and toxicity of weekly paclitaxel, carboplatin, and cetuximab as induction chemotherapy or in cases of metastases or relapse for head and neck cancer with a focus on elderly or frail patients. Head Neck (2022) 43:1777–86. doi: 10.1002/hed.27077
33. Chitapanarux I, Lorvidhaya V, Kamnerdsupaphon P, Sumitsawan Y, Tharavichitkul E, Sukthomya V, et al. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial. Eur J Cancer (2007) 43:1399–406. doi: 10.1016/j.ejca.2007.03.022
34. Tang QN, Liu LT, Qi B, Guo SS, Luo DH, Sun R, et al. Effect of concurrent chemoradiotherapy with nedaplatin vs cisplatin on the long-term outcomes of survival and toxic effects among patients with stage II to IVB nasopharyngeal carcinoma: A 5-year follow-up secondary analysis of a randomized clinical trial. JAMA Netw Open (2021) 4:e2138470. doi: 10.1001/jamanetworkopen.2021.38470
Keywords: LA-NPC, induction chemotherapy, PCE, cetuximab, chemoradiotherapy
Citation: Takeshita N, Enokida T, Okano S, Fujisawa T, Wada A, Sato M, Tanaka H, Tanaka N, Motegi A, Zenda S, Akimoto T and Tahara M (2022) Induction chemotherapy with paclitaxel, carboplatin and cetuximab for locoregionally advanced nasopharyngeal carcinoma: A single-center, retrospective study. Front. Oncol. 12:951387. doi: 10.3389/fonc.2022.951387
Received: 23 May 2022; Accepted: 22 July 2022;
Published: 11 August 2022.
Edited by:
Liangru Ke, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Nobuhiko Oridate, Yokohama City University, JapanCopyright © 2022 Takeshita, Enokida, Okano, Fujisawa, Wada, Sato, Tanaka, Tanaka, Motegi, Zenda, Akimoto and Tahara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Makoto Tahara, bWF0YWhhcmFAZWFzdC5uY2MuZ28uanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.