- 1The Third Department of Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 2Hebei Key Laboratory of Precision Diagnosis and Comprehensive Treatment of Gastric Cancer, Shijiazhuang, China
- 3The Department of Computed Tomography (CT)/Magnetic Resonance Imaging (MRI), The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 4AMITA Health Saint Joseph Hospital Chicago, Chicago, IL, United States
- 5Newham University Hospital, London, United Kingdom
- 6College of Osteopathic Medicine, Kansas City University, Kansas City, MO, United States
Background: The relationship between sarcopenia and clinical outcomes during conversion therapy in patients with lavage cytology positive gastric cancer (GC-CY1) remains unclear. This study aimed to investigate the impact of sarcopenia and skeletal muscle loss on the efficacy of conversion therapy, tumour response and survival in GC-CY1 patients.
Methods: Retrospective analysis of data from a prospective trial of conversion therapy conducted between April 2018 and August 2019 in patients with GC-CY1 (NCT03718624). Skeletal muscle index (SMI) was measured at the level of the third lumbar (L3) vertebra and the sarcopenia was defined using published cut-off points in all patients. We defined ΔSMI (%)/50 days above 9.53% for men and ΔSMI (%)/50 days above 8.81% for women as significant muscle loss (SML) and analysed the changes in skeletal muscle during conversion therapy in relation to treatment efficacy, survival and tumour response.
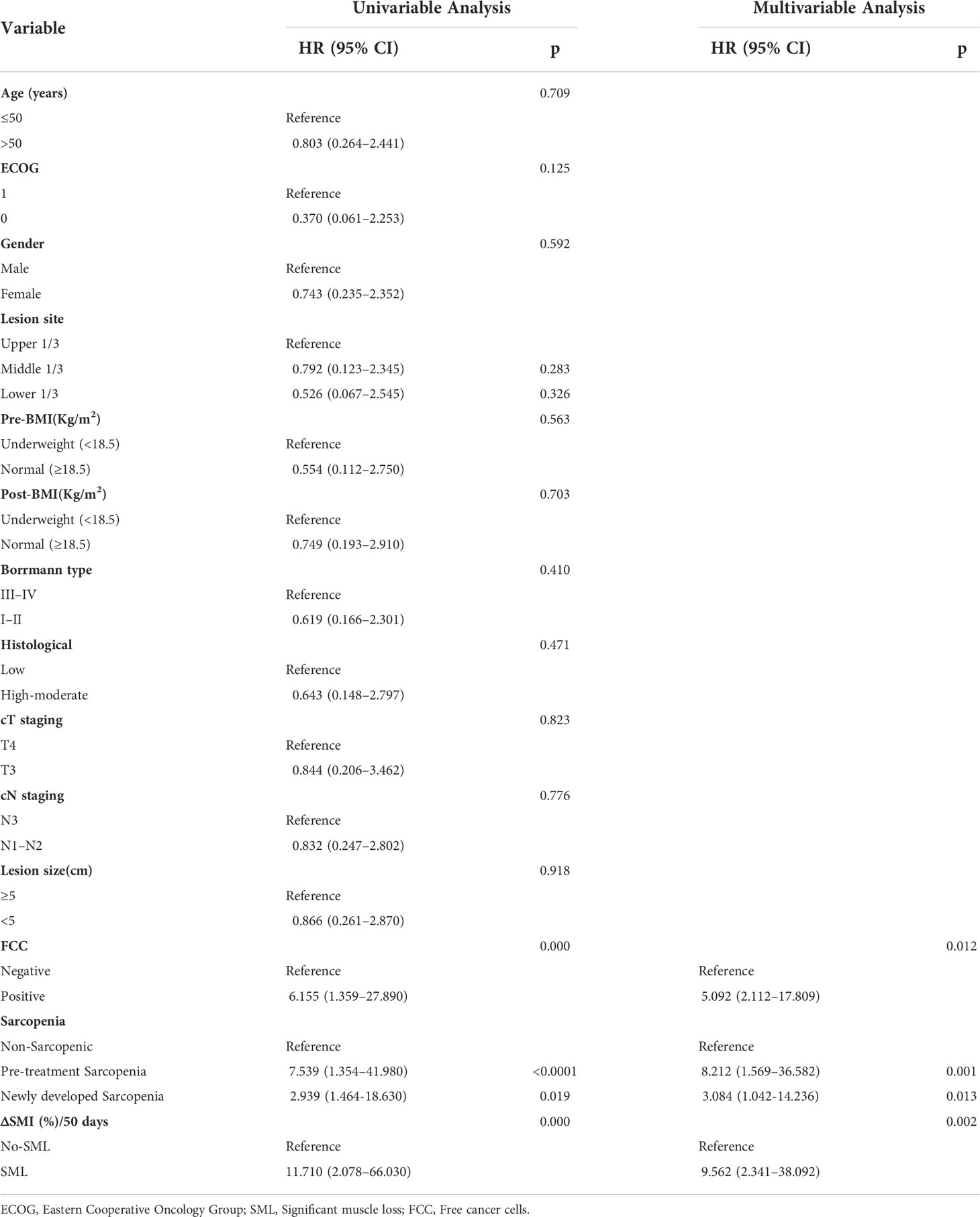
Results: Of the 36 patients, 7 patients (19.44%) developed sarcopenia before conversion therapy, 6 (16.67%) developed new sarcopenia after conversion therapy, and 8 (22.22%) developed SML during treatment. Multivariate analysis showed that sarcopenia before treatment [Odds Ratio (OR) =8.923, 95%CI: 1.341-25.321, p=0.002] and SML during treatment (OR=7.803, 95%CI: 1.106-16.189, p=0.001) had a negative impact on the success rate of conversion therapy. Cox multifactorial analysis found that pre-treatment sarcopenia [overall survival (OS): Hazard Ratio (HR) =6.341, 95%CI: 1.269-18.943, p=0.001; progression-free survival (PFS): HR=8.212, 95%CI: 1.569-36.582, p=0.001], newly developed sarcopenia after conversion therapy (OS: HR=3.189, 95%CI: 1.023-9.811, p=0.012; PFS: HR=3.084, 95%CI: 1.042-14.236, p=0.013) and the presence of SML during treatment (OS: HR=10.234, 95%CI: 2.532-54.231, p=0.002; PFS: HR=9.562, 95%CI: 2.341-38.092, p=0.002) were independent risk factor for OS and PFS in GC-CY1 patients.
Conclusion: Pre-treatment sarcopenia and the presence of SML during treatment are strongly correlated with the immediate and long-term outcomes of GC-CY1 patients and can be used as imaging markers to predict the treatment efficacy and prognosis of patients in clinical practice.
Introduction
In recent years, neoadjuvant intraperitoneal and systemic (NIPS) paclitaxel is the mainstay of treatment for GC-CY1 patients and is widely used in clinical practice (1, 2). Our previous prospective study also found that NIPS paclitaxel in combination with apatinib had a higher conversion success rate than conventional intravenous chemotherapy, which could improve the prognosis of GC-CY1 patients (3). However, the gastrointestinal toxicity induced by chemotherapy during conversion therapy may lead to malnutrition and weight loss in these patients. Moreover, the majority of GC-CY1 patients are malnourished at the time of their initial visit, and gastrointestinal adverse reactions during conversion therapy will further aggravate malnutrition.
Recent evidence suggests that the deterioration in nutritional status and weight loss in patients with gastric cancer will be directly reflected in the loss of skeletal muscle, which will further lead to sarcopenia (4–6). Up till now, numerous studies have demonstrated that sarcopenia is a predictor of poor prognosis for surgical complications and overall survival (OS) in patients with a variety of solid tumours (7–9). Otherwise, emerging evidence suggests that sarcopenia is also associated with poor prognosis and increased treatment-related toxicity in patients with advanced gastric cancer (10, 11). Nevertheless, there are rare reports of studies on the potential prognostic impact of sarcopenia during conversion therapy in GC-CY1 patients.
Abdominal computed tomography (CT) imaging is now widely used to assess the clinical staging of GC-CY1 patients with gastric cancer before and after conversion therapy (12, 13). The diagnosis of sarcopenia can be easily obtained from skeletal muscle measurements at the level of the L3 lumbar spine and several studies have confirmed that CT is the ‘gold standard’ for measuring muscle area (14, 15). Hence, changes in skeletal muscle area measured by pre- and post-treatment CT images during conversion therapy in GC-CY1 patients can be used as a potential imaging marker to predict immediate and long-term clinical outcomes. Previous studies on the prognosis of GC-CY1 patients have focused on the optimisation of treatment regimens and the specificity of the tumour (16, 17). However, there is still a lack of evidence regarding the impact of sarcopenic status before and after conversion therapy and changes in skeletal muscle during therapy on clinical outcomes in GC-CY1 patients.
Therefore, our study aimed to retrospectively analyse GC-CY1 patients included in a prospective clinical trial (NCT03718624) to assess the impact of sarcopenia on the immediate and long-term clinical outcomes of GC-CY1 patients receiving NIPS paclitaxel in combination with apatinib conversion therapy by analysing the changes in skeletal muscle area at L3 level during treatment.
Materials and methods
Study design and participants
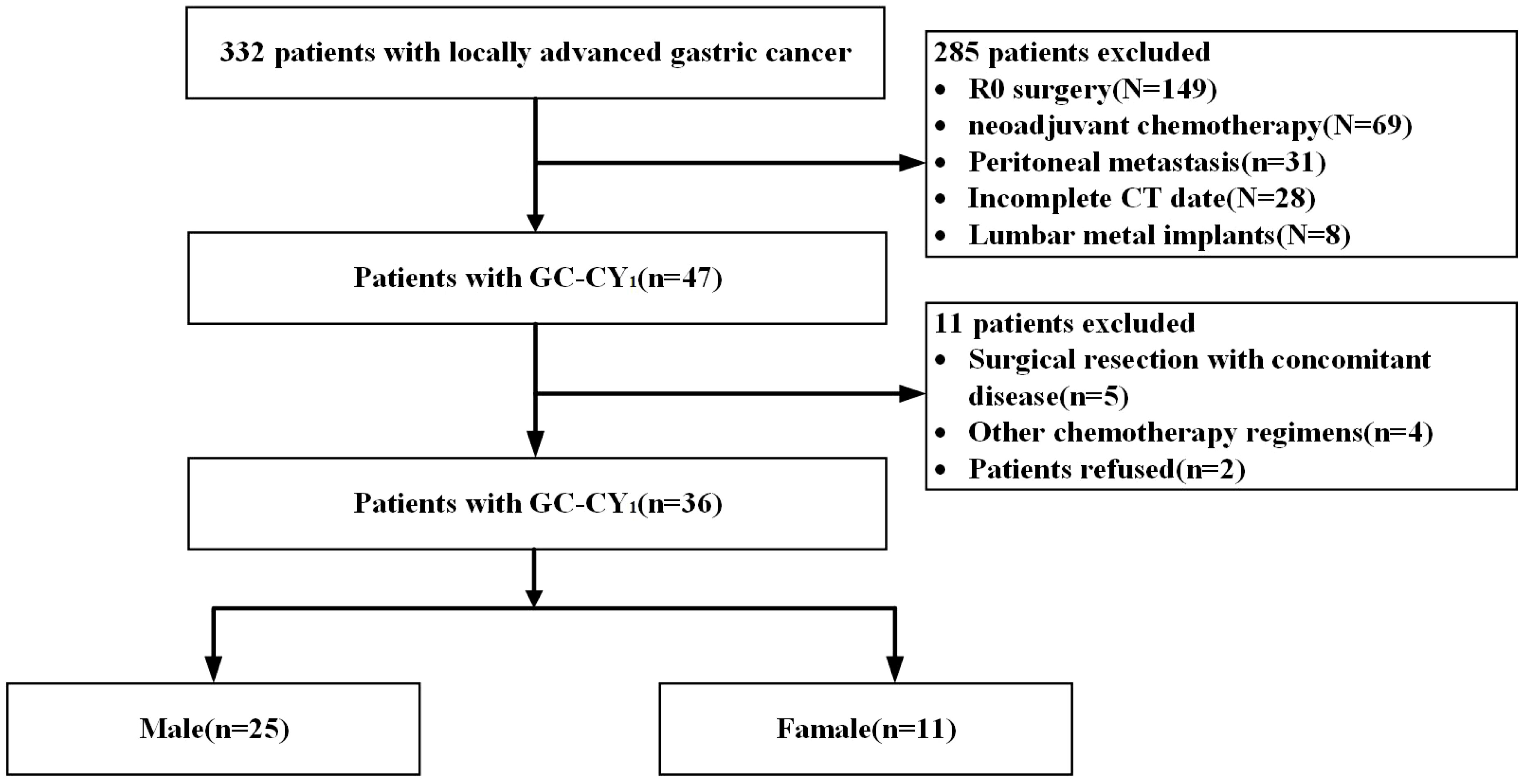
This study retrospectively analysed data from a prospective clinical study (NCT03718624) of 36 GC-CY1 patients who received NIPS paclitaxel in combination with apatinib conversion therapy from April 2018 to August 2019 at the Fourth Hospital of Hebei Medical University. The inclusion criteria were as follows: (1) all patients had gastric cancer confirmed histopathologically by gastroscopic biopsy and the human epidermal growth factor receptor 2 (HER2) tests were negative before the operation; (2) laparoscopic exploration and abdominal exfoliative cytology confirmed the presence of free cancer cells in the peritoneal cavity with the absence of peritoneal and distant organs metastases before conversion therapy; (3) age between 18 and 75 years; (4) organs with reserve functions were able to tolerate 3 cycles of NIPS paclitaxel and apatinib conversion therapy; (5) all patients underwent a repeat laparoscopic exploratory staging procedure after conversion therapy; (6) availability of complete hospitalisation data, including CT scans and follow-up data before and after conversion therapy. The exclusion criteria were as follows: (1) the coexistence of other malignancies; (2) organs with poor reserve function unable to tolerate surgery or patient refusal of surgical treatment, or unable to cooperate with treatment; (3) metal implants in the lumbar spine. This study was reviewed and approved by the ethics committee of the Fourth Hospital of Hebei Medical University (Ethics number: 2018088). All patients provided informed consent.
Conversion therapy
All patients underwent laparoscopic exploration and abdominal exfoliation cytology first, patients with intraoperative pathologically confirmed free cancer cells in the abdominal cavity were treated with NIPS paclitaxel in combination with apatinib. The detailed conversion treatment protocol was reported in the previous study (3). All patients were followed up with laparoscopic exploration and intra-abdominal free cancer detection after 3 cycles of conversion therapy. Patients were treated with the original regimen and radical surgical resection was performed if no free cancer cells (FCC) in abdominal cavity, otherwise, conversion therapy was continued.
Measurement and definition of body composition
We retrospectively analysed the CT images of all enrolled patients before and after conversion therapy. Previous studies have confirmed that the area of skeletal muscle and adipose tissue in CT cross-sectional images at the level of the L3 vertebral in the supine position correlates closely with whole-body muscle and fat mass (14, 15). Therefore, we chose to measure skeletal muscle area at the L3 vertebral level in patients with GC-CY1.
The 5-mm flat-scan images were uploaded to the Picture Archiving and Communication System (PACS, SIEMENS SOMATOM) after completing all image acquisition. Semi-automatic segmentation was performed by a board-certified radiologist (experience 5 years) and boundaries of the L3 vertebrae body were outlined along the inner edge of the abdominal wall subcutaneous fat. The Hounsfield unit (HU) threshold value was applied to skeletal muscle: attenuation range of -29 to 150 HU (18). The software evaluated and measured the pixel area of the corresponding skeletal muscle attenuation to obtain the skeletal muscle area (SMA). The cross-sectional area of skeletal muscle was divided by the square of height to obtain the skeletal muscle index (SMI), and all results were expressed in square meters (cm2/m2) and standardised for comparison.
Patients included in this study were Asian, so sarcopenia was defined using the results of Fujiwara et al. and Mardian et al. studies, which were conducted in Asian populations. Patients with SMI <36.2 cm2/m2 for men and SMI < 29.6 cm2/m2 for women were regarded as the presence of sarcopenia (19, 20). In addition, given the varying duration of conversion therapy with NIPS paclitaxel combined with apatinib in this cohort of patients, a standardised and uniform unit was used for comparison between groups. The median conversion treatment interval for the whole group of patients was 58 days. Therefore, the amount of change in SMI was calculated before and after conversion therapy[ΔSMI], the percentage of this value relative to the SMI before conversion therapy was recorded as ΔSMI [%] and it was then divided by the number of days apart and multiplied by 50 to represent the relative change over 50 days [ΔSMI[%]/50 days].
Outcome measures
The Response Evaluation Criteria for Solid Tumours (RECIST) version 1.1 was used to evaluate imaging outcomes after conversion therapy (21), including complete remission (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The percentage of patients with CR and PR is defined as the objective response rate (ORR), while the percentage of patients other than those with PD is defined as the preoperative disease control rate (DCR).
The pathological response is assessed and graded according to the TRG grading scale (AJCC/CAP criteria) using surgically resected specimens after conversion therapy (22). A TRG grade 0 is defined as no residual tumour cells, while sparse residual tumour cells can be seen microscopically as TRG grade 1, and more fibrosis than residual tumour cells as TRG grade 2. Conversely, a grade of TRG 3 is defined as having more residual tumour cells than fibrosis or no regressive changes. In this study, TRG grade 0 and TRG grade 1 were defined as major pathologic responses (MPR).
Meanwhile, the other endpoint observed in this study was overall survival (OS) and progression-free survival (PFS) at 2 years. OS was defined as the time interval from the initial date of conversion therapy to the date of last follow-up or death. In contrast, PFS was defined as the period from the start of conversion therapy to the date of disease progression, death or last follow-up, whichever occurred first. All patients were followed up every 3 months after starting treatment. In the meanwhile, enhanced CT scans were performed every 3 months for the first 3 years after surgery and then every 6 months for the next 2 years.
Statistical analysis
SPSS version 21.0 and GraphPad Prism 8.01 were used for statistical analyses. In this study, all continuous data median (interquartile spacing) or mean ± standard deviation were expressed and analysed for comparison using an independent t test or Mann-Whitney U-test. The categorical data were expressed as numbers and percentages and analysed using the chi-square test or Fisher’s exact test. The optimal cut-off value for ΔSMI (%)/50 days was used to classify patients with good or poor OS. The optimal cut-off value for ΔSMI (%)/50 days was obtained using the receiver operating characteristic (ROC) curve for the highest Youden’s index. Survival curves were plotted using the Kaplan-Meier method, and the log-rank test was used to compare survival rates between groups. Univariate and multivariate analyses were performed using Cox proportional risk regression models, and results were shown as hazard ratio (HR) with 95% confidence intervals (CI). A p < 0.05 was considered statistically significant.
Results
Changes in sarcopenia during neoadjuvant therapy
Based on inclusion and exclusion criteria, we reviewed data of 36 GC-CY1 patients who received NIPS paclitaxel in combination with apatinib conversion therapy (Figure 1). Seven (19.44%) GC-CY1 patients were already in sarcopenia before conversion therapy, which was slightly more common in males (4/7, 57.14%). The proportion of sarcopenia (13/36, 36.11%) increased after conversion therapy, with 6 cases of newly developed sarcopenia and the remaining 7 patients with persistent sarcopenia. The mean ΔSMI (%)/50 days during conversion therapy was -6.35 ± 3.57% for the whole group of patients. The optimal cut-off value for ΔSMI (%)/50 days was not clearly defined, so ROC curves were used for clarification. The different ROC curves based on gender showed an area under the curve (AUC) value of 0.960 (95%CI: 0.878-1.000, p=0.000) for men and 0.964 (95%CI: 0.859-1.000, p=0.014) for women (Figure 2). According to the Youden’s index, the optimal cut-off value of male ΔSMI (%)/50 days was 9.53%, and that of female was 8.81%. The patients were grouped according to this optimal cut-off value, with 8 (22.22%) in the significant muscle loss (SML, male: ΔSMI (%)/50 days ≥ 9.53%; female: ΔSMI (%)/50 days ≥ 8.81%) group and 5 (62.50%) in the non-SML group.
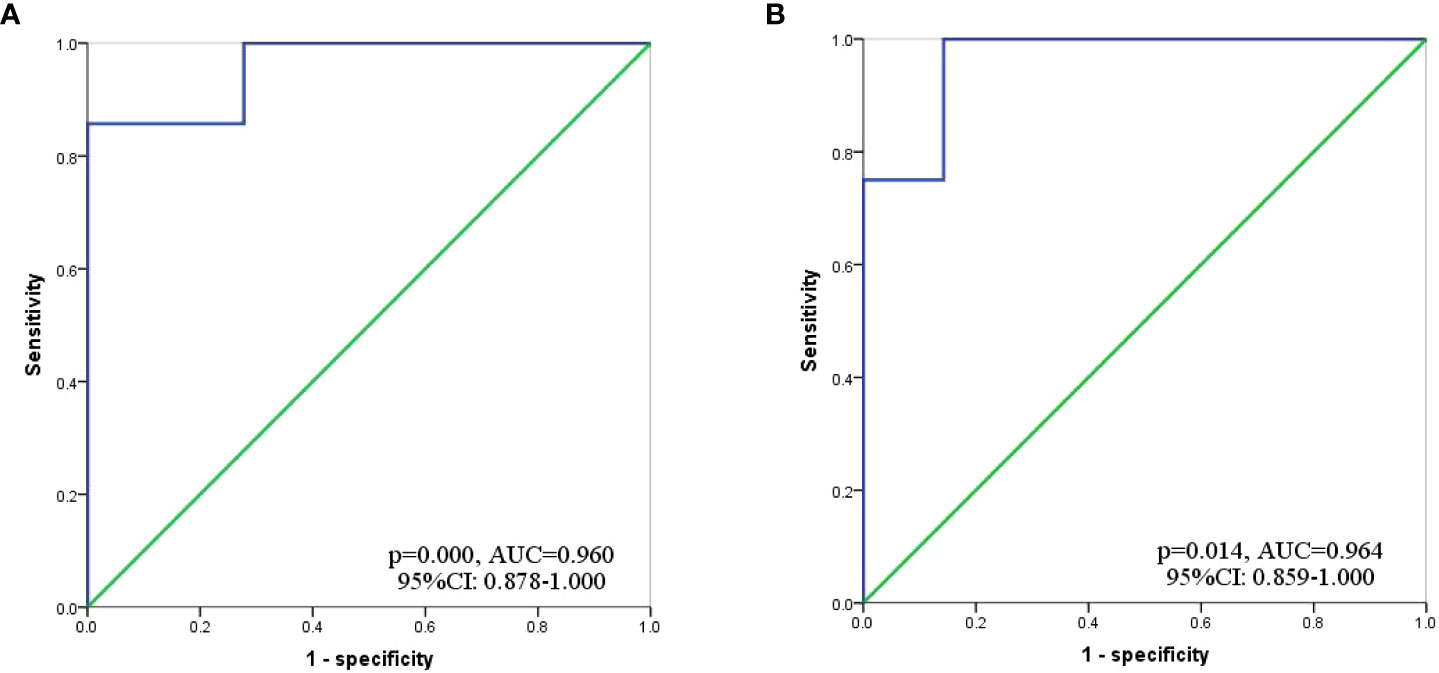
Figure 2 ROC curve to determine the optimal cut-off value for ΔSMI (%)/50 days. (A) Male; (B) Female.
The relationship between sarcopenia and patient characteristics
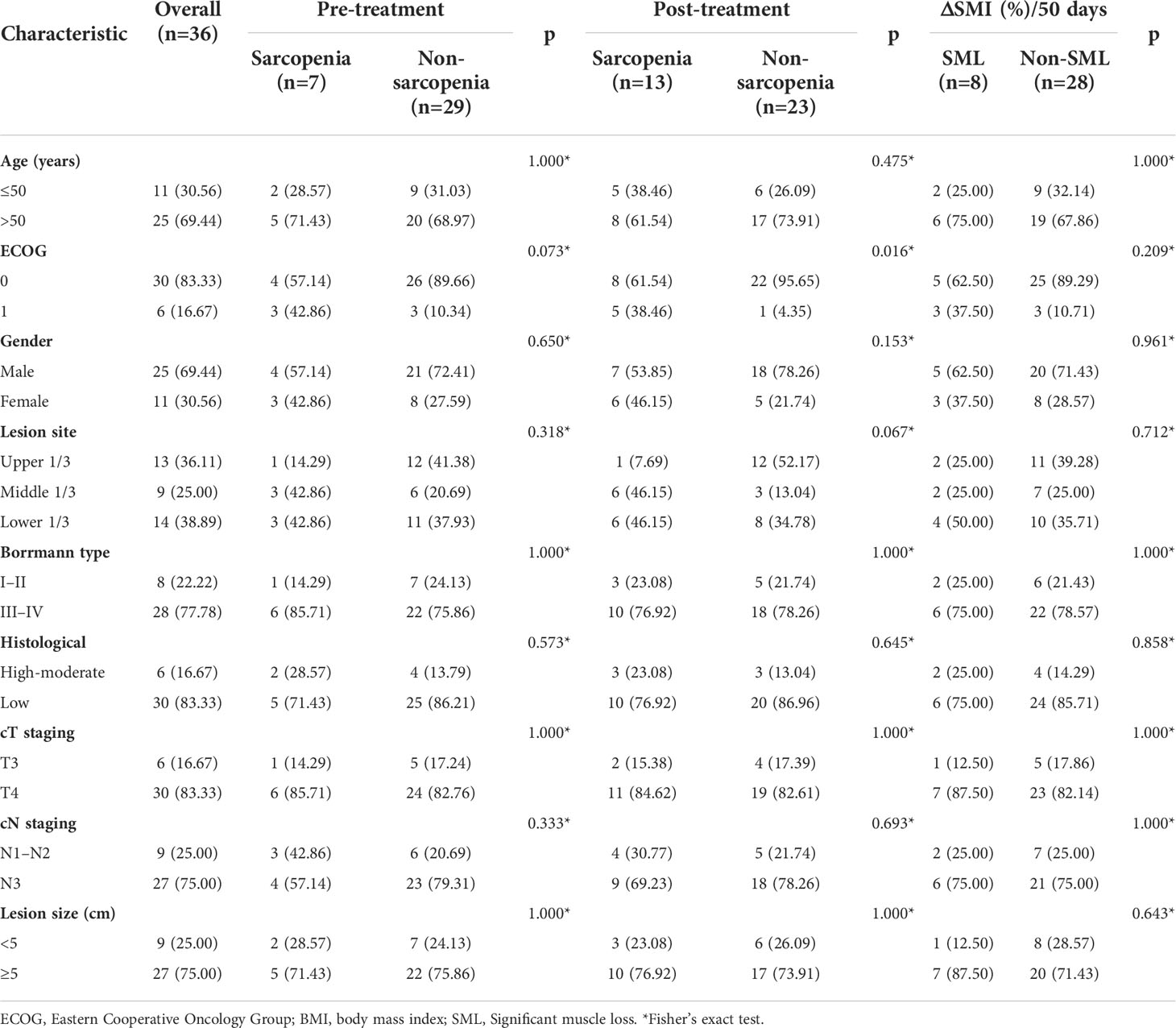
The mean age of the whole group of patients was 56.3 ± 10.0 years, of which 25 (69.44%) cases were male. The relationship between the status of sarcopenia and nutrition-related outcomes before and after patient conversion therapy is summarised in Figure 3. The difference in BMI, albumin, and prealbumin between sarcopenic patients and non-sarcopenic patients before and after conversion therapy was more (all p<0.05). Meanwhile, patients who developed SML during conversion therapy had significantly lower levels of BMI (pre-: 21.83 ± 2.38 versus 23.06 ± 3.62, p=0.012; post-: 21.23 ± 2.28 versus 22.47 ± 3.45, p=0.036), albumin (pre-: 43.00 ± 3.97 versus 46.25 ± 3.25, p=0.011; post-: 40.35 ± 3.71 versus 41.66 ± 2.51, p=0.042) and prealbumin (pre-: 208.88 ± 26.98 versus 224.70 ± 26.41, p=0.004; post-: 186.73 ± 21.93 versus 200.97 ± 25.45, p=0.009) than those without significant muscle loss (non-SML), both pre- and post-treatment. In addition, there was no significant difference in haemoglobin levels between the SML group and the non-SML group before and after conversion treatment (pre-: 124.34 ± 8.97 versus 123.70 ± 9.69, p=0.862; post-: 112.29 ± 10.45 versus 116.73 ± 8.68, p=0.281). The relationship between all other clinicopathological features and the patient’s sarcopenia before and after conversion therapy is shown in Table 1.
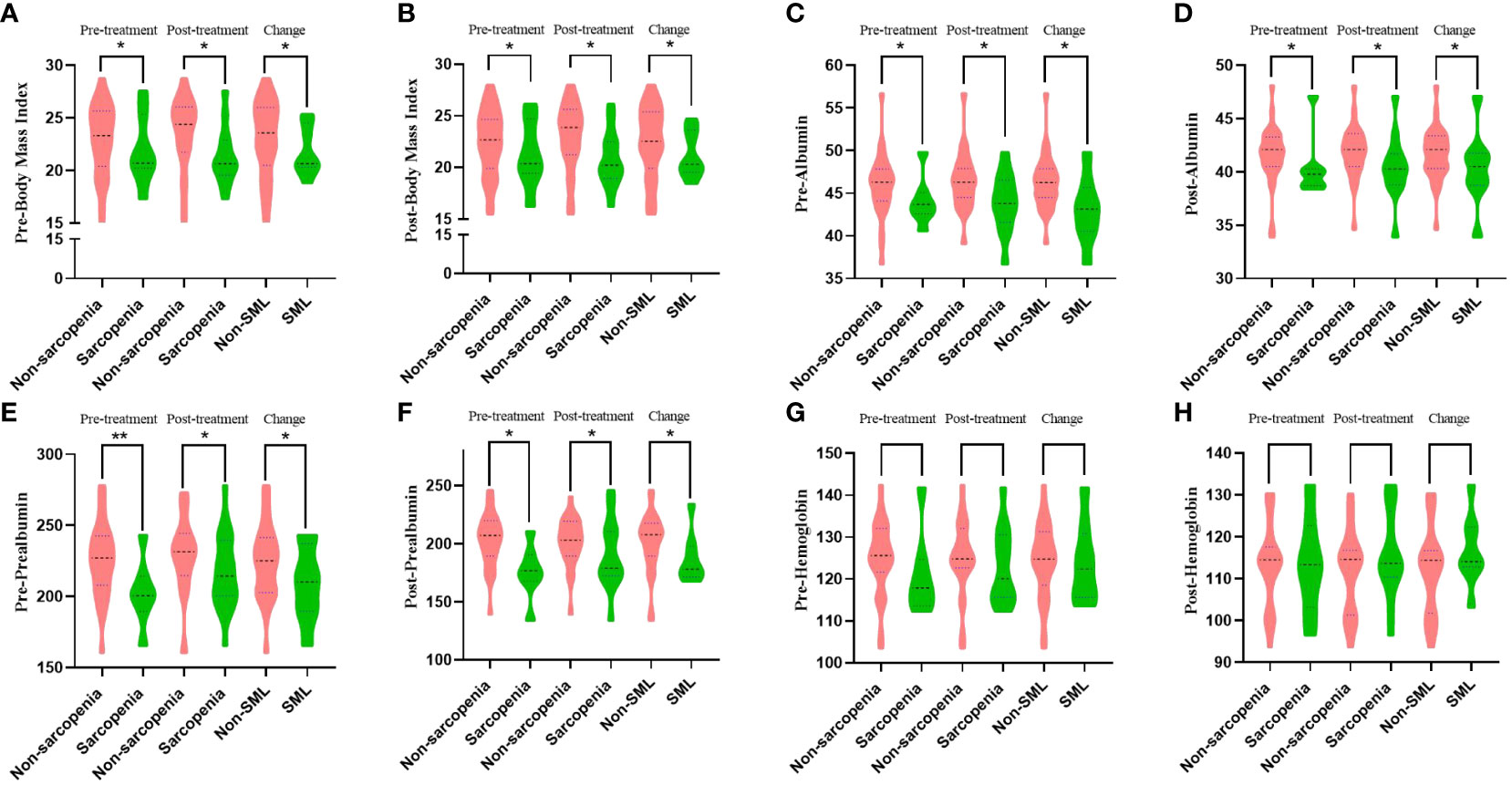
Figure 3 The relationship between sarcopenia and nutritional status outcomes before and after patient conversion therapy. SML: Significant muscle loss. (A, B) Relationship between sarcopenia during conversion therapy and body mass index before (A) and after (B) treatment. (C, D) Relationship between sarcopenia during conversion therapy and albumin before (C) and after (D) treatment. (E, F): Relationship between sarcopenia during conversion therapy and prealbumin before (E) and after (F) treatment. (G, H) Relationship between sarcopenia during conversion therapy and haemoglobin before (G) and after (H) treatment. *p < 0.05, **p < 0.01.
The relationship between sarcopenia and treatment efficacy
Figure 4 summarises the relationship between sarcopenia and the efficacy of conversion therapy for GC-CY1 patients. All 36 GC-CY1 patients underwent abdominal CT scans after 3 cycles of NIPS paclitaxel combined with apatinib conversion therapy. Of 36 patients enrolled, 5 patients (13.89%) achieved CR, 24 patients achieved (66.67%) PR, and 5 patients (13.89%) achieved SD, while 2 patients (5.56%) developed PD, based on the evaluation by RECIST (version 1.1). Patients in the sarcopenic group had a lower ORR than the non-sarcopenic group both before (42.86% versus 89.66%, p=0.016) and after (61.54% versus 91.30%, p=0.073) treatment, this difference was also present in patients who developed SML versus those who did not during conversion therapy (50.00% versus 89.29%, p=0.030), but there was no difference in DCR between the groups (all p>0.05). Logistic univariate and multifactorial analyses identified pre-conversion therapy sarcopenia (OR=7.891, 95%CI: 1.984-24.156, p=0.008), the presence of SML during conversion therapy (OR=10.642, 95%CI: 2.676-43.531, p=0.002) and cT staging (OR=0.074, 95% CI: 0.009-0.659, p=0.022) were statistically significantly associated with achieving CR and PR in GC-CY1 patients (Supplementary Table 1).
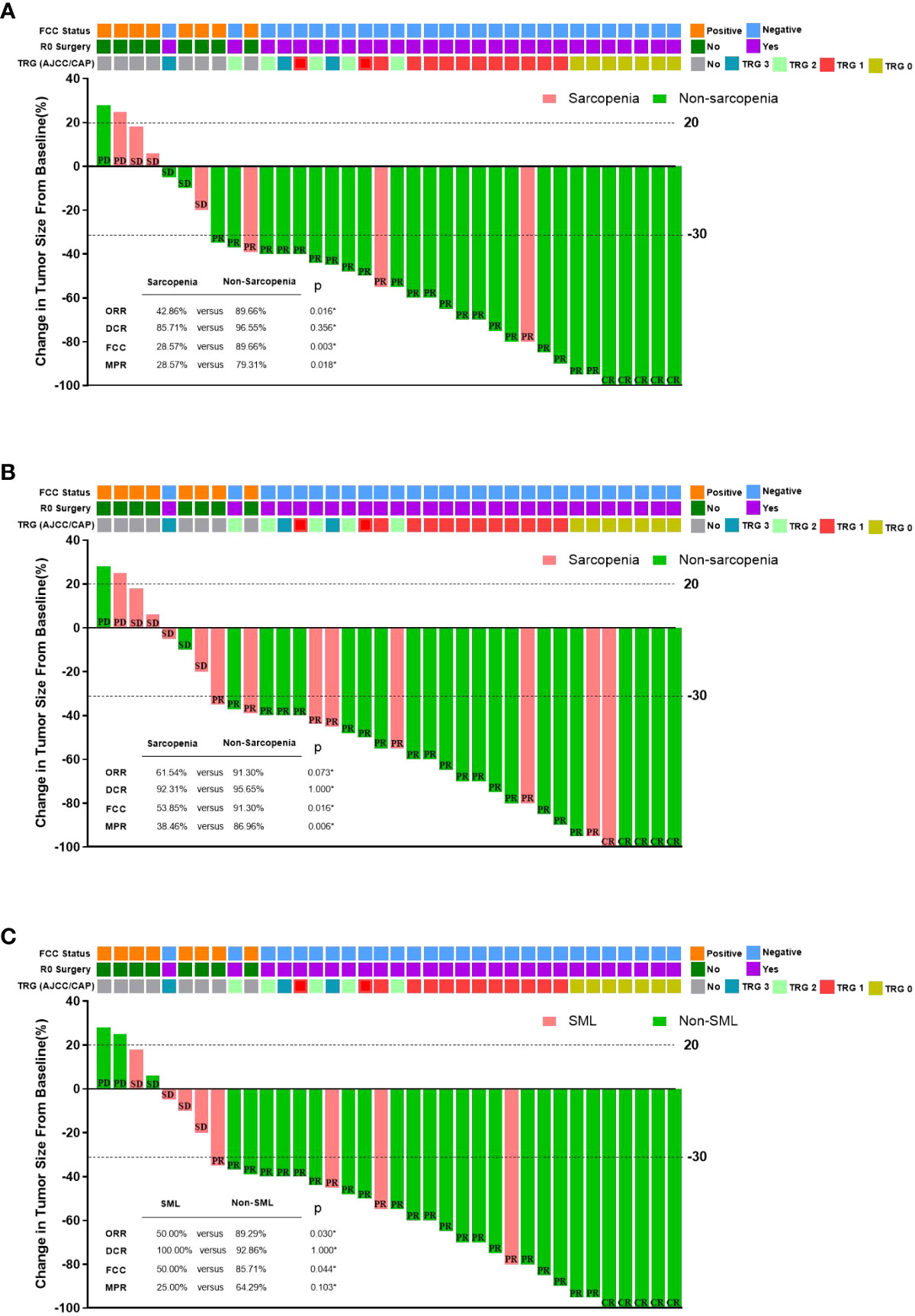
Figure 4 The relationship between sarcopenia and the efficacy of conversion therapy in GC-CY1 patients. (A) The relationship between pre-treatment sarcopenia and the efficacy of conversion therapy. (B) The relationship between post-treatment sarcopenia and the efficacy of conversion therapy. (C) The relationship between the presence of SML during treatment and the efficacy of conversion therapy. CR, complete remission; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate; MPR, major pathologic response; FCC, free cancer cells; *Fisher’s exact test.
All patients underwent laparoscopic exploration combined with abdominal exfoliative cytology, free cancer cells (FCC) were still present in the abdominal cavity in 8 patients (22.22%), while 28 patients (77.78%) who turned negative underwent R0 surgical resection. A subgroup analysis based on the presence or absence of sarcopenia before and after treatment found that all patients in the sarcopenic group had significantly lower rates of FCC conversion than those in the non-sarcopenic group (pre-: 28.57% versus 89.66%, p=0.003; post-: 53.85% versus 91.30%, p=0.016). In addition, patients who developed SML during conversion therapy had a lower rate of FCC conversion than those in the non-SML group (50.00% versus 85.71%, p=0.044). After univariate and multifactorial analyses, the presence of sarcopenia before conversion therapy (OR=8.923, 95%CI: 1.341-25.321, p=0.002) and the presence of SML during conversion therapy (OR=7.803, 95%CI: 1.106-16.189, p=0.001) were found to be the independent risk factors. (Supplementary Table 2).
According to the AJCC/CAP criteria for the assessment of pathological regression, seven (25.00%) of the 28 FCC-negative patients who underwent surgical resection achieved TRG grade 0, 13 patients (46.43%) achieved TRG grade 1, 5 patients (17.86%) were TRG grade 2, and 3 patients (10.71%) were TRG grade 3. We found that sarcopenia patients had lower MPR than normal groups before conversion therapy (28.57% vs. 79.31%, p=0.018) and those who developed sarcopenia after conversion therapy (38.46% vs. 86.96%, p=0.006), but there was no significant difference between patients with SML only during conversion therapy (25.00% vs. 64.29%, p=0.103) and patients without SML. Logistic univariate and multifactorial analyses revealed that the presence of non-Sarcopenic after conversion therapy (OR=0.007, 95%CI: 0.004-0.224, p=0.006) and histologically moderate to high differentiation (OR=0.014, 95%CI: 0.002-0.412, p=0.010) were independent protective factors influencing whether GC-CY1 patients were graded as TRG0 and TRG1 for pathological regression (Supplementary Table 3).
The relationship between sarcopenia and adverse effects
Among the 36 GC-CY1 patients who received conversion therapy, the incidence of hematological toxicity of grade III and above was 19.44%(7/36) (Table 2). We observed that patients with sarcopenia before treatment were more likely to have grade≥3 neutropenia(40.00% vs. 0, p=0.033) and nausea/vomiting(40.00% vs. 0, p=0.033) than patients without sarcopenia. This difference did not appear in patients with post-treatment sarcopenia and non-sarcopenia. More importantly, patients with SML experienced a significant increase in grade ≥3 toxicity during conversion therapy compared to patients without SML, particularly in leukopenia(33.33% vs. 0, p=0.044), neutropenia(33.33% vs. 0, p=0.044), and nausea/vomiting(33.33% vs. 0, p=0.044).
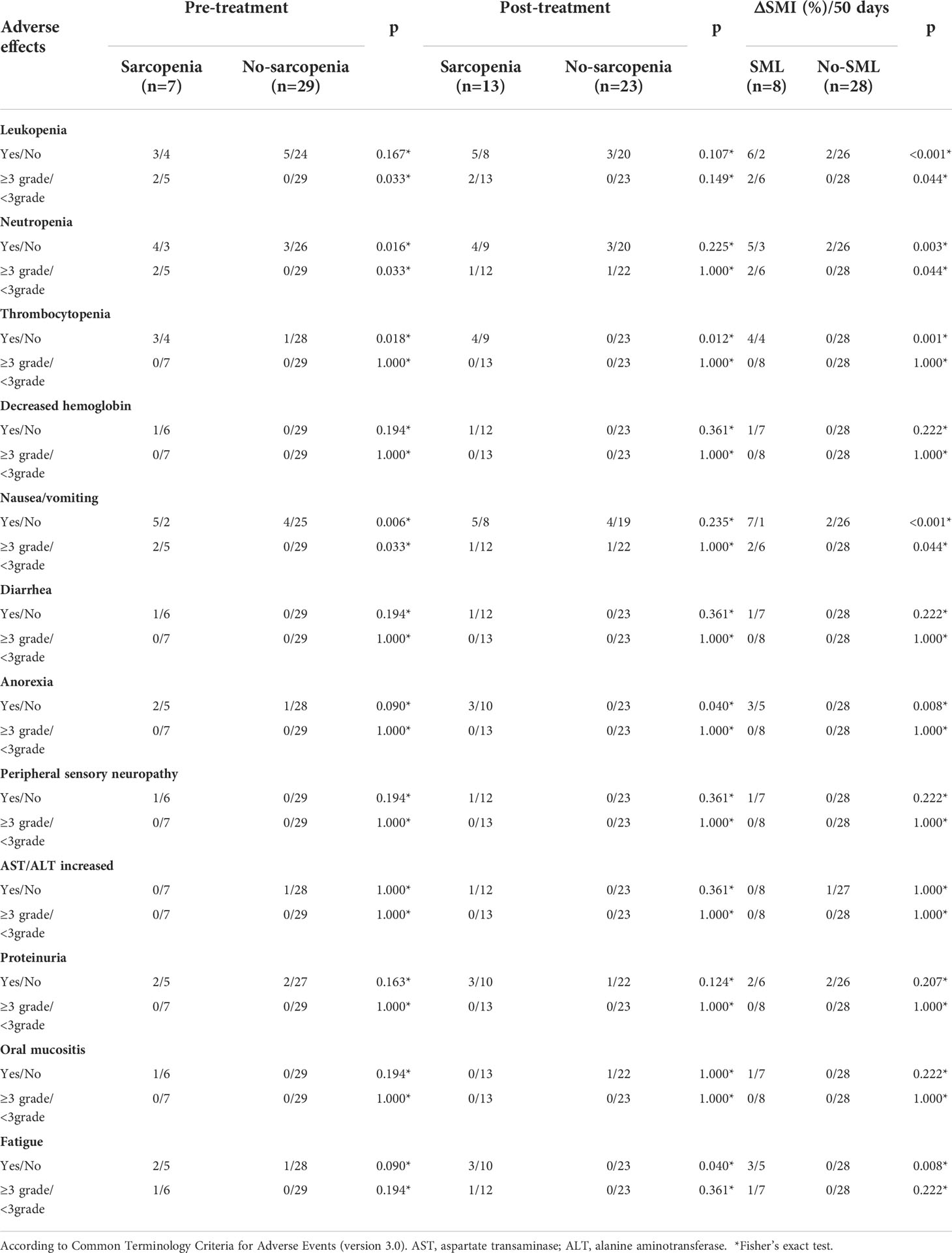
Table 2 Relationship between sarcopenia and adverse effects during conversion therapy in GC-CY1 patients.
The relationship between sarcopenia and prognosis
The median follow-up time for all patients was 25.5 (15.6-38.4) months, with 2-year OS and PFS rates of 69.44% and 58.33%, respectively. Figure 5 summarises the 2-year OS and PFS for GC-CY1 patients. The 2-year OS (pre: 28.57% versus 79.31%, p=0.002; post: 38.46% versus 86.96%, p=0.002) and PFS (pre: 14.29% versus 72.41%, p<0.001; post: 30.77% versus 78.26%, p=0.001) rates were significantly different between the pre- and post-treatment sarcopenia and non-sarcopenia groups. In addition, 2-year OS (0% versus 89.29%, p<0.001) and PFS (0% versus 78.57%, p<0.001) were significantly lower in the SML group than in the non-SML group during conversion therapy. Cox multifactorial analysis found that status of FCC (OS: HR=5.164, 95%CI: 2.146-14.709, p=0.011; PFS: HR=5.092, 95%CI: 2.112-17.809, p=0.012), pre-treatment sarcopenia (OS: HR=6.341, 95%CI: 1.269-18.943, p=0.001; PFS: HR=8.212, 95%CI: 1.569-36.582, p=0.001), newly developed sarcopenia after conversion therapy (OS: HR=3.189, 95%CI: 1.023-9.811, p=0.012; PFS: HR=3.084, 95%CI: 1.042-14.236, p=0.013) and the presence of SML during treatment (OS: HR=10.234, 95%CI: 2.532-54.231, p=0.002; PFS: HR=9.562, 95%CI: 2.341-38.092, p=0.002) were all independent risk factors for 2 year OS and PFS in GC-CY1 patients (Tables 3, 4).
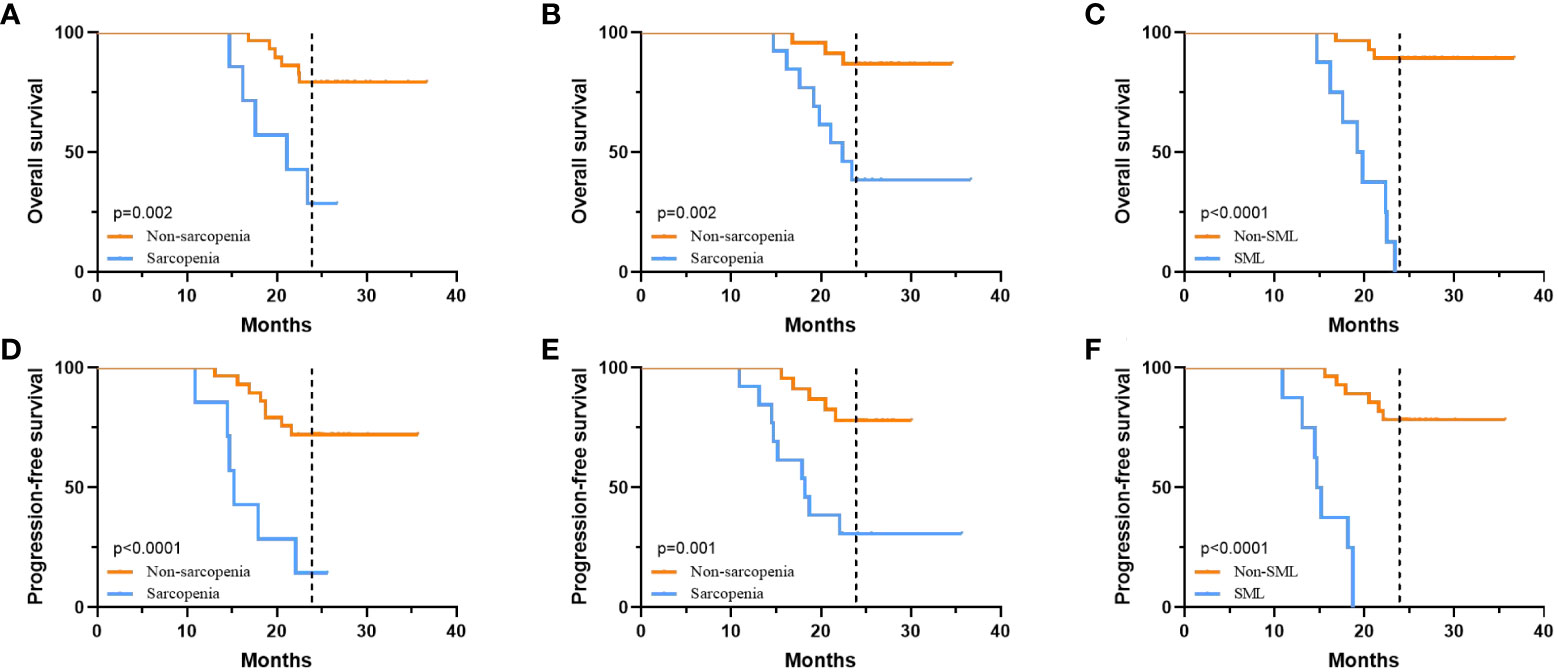
Figure 5 The relationship between sarcopenia and prognosis. (A) 2-year OS of patients in the pre-treatment sarcopenic group versus the non-sarcopenic group. (B) 2-year OS of patients in the post-treatment sarcopenic group versus the non-sarcopenic group. (C) 2-year OS for patients in the SML group versus the non-SML group who developed SML during treatment. (D) 2-year PFS of patients in the pre-treatment sarcopenic group versus the non-sarcopenic group. (E) 2-year PFS of patients in the post-treatment sarcopenic group versus the non-sarcopenic group. (F) 2-year PFS for patients in the SML group versus the non-SML group who developed SML during treatment. SML, significant muscle loss.
Discussion
This study retrospectively analysed the effect of sarcopenia and skeletal muscle loss on clinical outcomes among 36 patients with GC-CY1, and they were included in a registered prospective clinical study (NCT03718624) during NIPS paclitaxel in combination with apatinib conversion therapy. Firstly, we observed that 19.44% of patients had sarcopenia before conversion therapy and this rose to 36.11% after conversion therapy. Secondly, SML was defined as above 9.53% of skeletal muscle loss during conversion therapy (ΔSMI (%)/50 days) for male patients and above 8.81% for females, with only 22.22% of all patients experiencing SML during conversion therapy. Thirdly, there was a strong correlation between sarcopenia and changes in laboratory indicators related to nutritional status during conversion therapy. Finally, sarcopenia before and after conversion therapy and the presence of SML during conversion therapy were significantly associated with both the patient’s immediate outcome and long-term prognosis.
In previous studies, BMI has been used to assess the occurrence of postoperative complications and the long-term prognosis of patients with malignancies (23). Nevertheless, growing evidence has found that using a single indicator of BMI alone for assessment is less effective because it does not take into account differences in gender and body composition, and in particular, it ignores changes in muscle (24). Presently, abdominal CT is widely used for GC-CY1 patients who require it before and after conversion therapy to assess treatment efficacy (17, 18). Moreover, there is growing attention to abdominal CT based body composition analysis, particularly at the L3 level (25, 26). Several studies have confirmed that skeletal muscle measurements at the L3 level have a good ability to predict postoperative complications, tumour response and prognosis in patients with malignancies (26, 27). A prospective study of 225 patients with foregut cancer found that 23.9% of patients had SML after preoperative neoadjuvant chemotherapy, with the highest proportion of patients with oesophageal and gastric cancers (28). As for GC-CY1 patients, our results found SML in 22.22% of patients, which is consistent with the results of previous studies (28, 29), it indicates that GC-CY1 patients also experience SML in conversion therapy.
A retrospective study involved 31 patients with oesophageal cancer who received neoadjuvant chemotherapy and underwent surgery found that pathological regression responses (grade 2 or higher) were more common in the non-muscle reduced group than in the muscle reduced group (53.3% vs. 25.0%) (30). However, a retrospective study by Takeda T et al. reached contradictory conclusions (31). In gastric cancer, few reports have evaluated the effect of sarcopenia on the efficacy of chemotherapy. For GC-CY1, our study revealed that sarcopenia before conversion therapy and SML development during therapy were strongly associated with local efficacy response and were independent risk factors for the success of conversion therapy. The reasons for the differences between the results of this study and previous studies may relate to treatment regimens, ethnicity of subjects, muscle mass measurements and the selection of optimal cut-off value. Therefore, a multicentre prospective study with large sample size is needed to validate the association between sarcopenia and treatment efficacy before and after conversion therapy treatment.
Currently, the association between sarcopenia and OS in patients with advanced gastric cancer are inconsistent, some studies report worse OS in patients with sarcopenia before or after treatment than in those without sarcopenia (25, 26), while others report that they do not have any association (26, 29). In this study, we performed Cox multivariate analysis of OS and PFS to explore the relationship between sarcopenia before and after conversion therapy and skeletal muscle changes during conversion therapy and prognosis in GC-CY1 patients. We found that pre-treatment sarcopenia, newly developed sarcopenia after conversion therapy and the presence of SML during treatment were independent risk factors for OS and PFS in GC-CY1 patients and were not associated with sarcopenia before treatment, which is consistent with previous findings on body composition (25, 32). The mechanisms by which sarcopenia affects the long-term prognosis of GC-CY1 patients are currently unknown and the reasons may be multifaceted. Firstly, gastric cancer is a highly aggressive malignancy, accompanied by higher catabolic and basal metabolic rates (33). The high metabolism aggravates systemic inflammatory response and causes severe muscle wastage, which ultimately leads to the development of sarcopenia (33). Furthermore, muscular sarcopenia exacerbates the inflammatory response of the body, which in turn is closely related to the long-term survival of the patient, resulting in a series of chain reactions leading to a poor prognosis (34).
Certain limitations of this study merit consideration. Firstly, this study is a single-centre, small sample prospective study and there may be some selection bias in the study population. Secondly, this study only examined the impact of skeletal muscle changes at two time points on treatment efficacy, pathological regression response and prognosis, without further in-depth analysis of skeletal muscle changes following later treatment. Thirdly, we did not investigate adverse effects during conversion therapy with NIPS paclitaxel plus apatinib. The presence of adverse effects, particularly gastrointestinal reactions, can affect patients’ nutritional status and further affect muscle loss. Therefore, the relationship between sarcopenia and adverse drug reactions during neoadjuvant therapy is required for further analyses in subsequent studies. Finally, further studies are needed to investigate the mechanisms of skeletal muscle loss and to find how to preserve skeletal muscle mass during conversion therapy to improve clinical outcomes.
Conclusions
In conclusion, although the average change in the SMI during conversion therapy in GC-CY1 patients was not significant, skeletal muscle loss(SML, male:ΔSMI (%)/50 days≥9.53%; female:ΔSMI(%)/50 days≥8.81%)was independently associated with poor OS and PFS. Meanwhile, pre-treatment sarcopenia and new sarcopenia after conversion therapy are also intimately associated with the success of conventional treatment and are independent risk factors for patient prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This trial was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (approval number: 2018088). The patients/participants provided their written informed consent to participate in this study.
Author contributions
(I) Conception and design: QZ; (II) Administrative support: QZ; (III) Provision of study materials or patients: P’AD, PY, YT, HG; (IV) Collection and assembly of data: P’AD, PY, YT, and HG; (V) Data analysis and interpretation: P’AD and CS; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Funding
This work was supported by the Cultivating Outstanding Talents Project of Hebei Provincial Government Fund (No.2019012); Hebei public health committee county-level public hospitals suitable health technology promotion and storage project (No.2019024); Hebei University Science and Technology Research Project (No.ZD2019139).
Conflict of interest
All authors have completed the ICMJE uniform disclosure form.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.949511/full#supplementary-material
References
1. Gong Y, Wang P, Zhu Z, Zhang J, Huang J, Wang T, et al. Benefits of surgery after neoadjuvant intraperitoneal and systemic chemotherapy for gastric cancer patients with peritoneal metastasis: A meta-analysis. J Surg Res (2020) 245:234–43. doi: 10.1016/j.jss.2019.07.044
2. Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imano M, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus s-1 versus cisplatin plus s-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol (2018) 36(19):1922–9. doi: 10.1200/JCO.2018.77.8613
3. Ding P, Yang P, Tian Y, Guo H, Liu Y, Zhang Z, et al. Neoadjuvant intraperitoneal and systemic paclitaxel combined with apatinib and s-1 chemotherapy for conversion therapy in gastric cancer patients with positive exfoliative cytology: a prospective study. J Gastrointest Oncol (2021) 12(4):1416–27. doi: 10.21037/jgo-21-375
4. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol (2011) 12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7
5. Peixoto da Silva S, Santos JMO, Costa E Silva MP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle (2020) 11(3):619–35. doi: 10.1002/jcsm.12528
6. Ryan AM, Prado CM, Sullivan ES, Power DG, Daly LE. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition. (2019) 67-68:110539. doi: 10.1016/j.nut.2019.06.020
7. Schneider M, Hübner M, Becce F, Koerfer J, Collinot JA, Demartines N, et al. Sarcopenia and major complications in patients undergoing oncologic colon surgery. J Cachexia Sarcopenia Muscle (2021) 12(6):1757–63. doi: 10.1002/jcsm.12771
8. Rutten IJ, Ubachs J, Kruitwagen RF, van Dijk DP, Beets-Tan RG, Massuger LF, et al. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur J Surg Oncol (2017) 43(4):717–24. doi: 10.1016/j.ejso.2016.12.016
9. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: A meta-analysis. Ann Surg (2018) 268(1):58–69. doi: 10.1097/SLA.0000000000002679
10. Chen F, Chi J, Liu Y, Fan L, Hu K. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: A meta-analysis of cohort studies. Arch Gerontol Geriatr (2022) 98:104534. doi: 10.1016/j.archger.2021.104534
11. Kawamura T, Makuuchi R, Tokunaga M, Tanizawa Y, Bando E, Yasui H, et al. Long-term outcomes of gastric cancer patients with preoperative sarcopenia. Ann Surg Oncol (2018) 25(6):1625–32. doi: 10.1245/s10434-018-6452-3
12. Jeong O, Jung MR, Kang JH, Ryu SY. Prognostic performance of preoperative staging: Assessed by using multidetector computed tomography-between the new clinical classification and the pathological classification in the eighth American joint committee on cancer classification for gastric carcinoma. Ann Surg Oncol (2020) 27(2):545–51. doi: 10.1245/s10434-019-07845-3
13. Dong D, Tang L, Li ZY, Fang MJ, Gao JB, Shan XH, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol (2019) 30(3):431–8. doi: 10.1093/annonc/mdz001
14. Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Optimal body size adjustment of L3 CT skeletal muscle area for sarcopenia assessment. Sci Rep (2021) 11(1):279. doi: 10.1038/s41598-020-79471-z
15. Muresan BT, Sánchez Juan C, Artero A, Hernández Machancoses A, Almendros-Blanco P, Montoro A, et al. Measurement of body composition in cancer patients using CT planning scan at the third lumbar vertebra. Nutr Hosp (2019) 36(6):1307–14. doi: 10.20960/nh.2435
16. Kobayashi H, Honda M, Kawamura H, Takiguchi K, Muto A, Yamazaki S, et al. Clinical impact of gastrectomy for gastric cancer patients with positive lavage cytology without gross peritoneal dissemination. J Surg Oncol (2022) 125(4):615–20. doi: 10.1002/jso.26770
17. Yamaguchi T, Takashima A, Nagashima K, Terashima M, Aizawa M, Ohashi M, et al. Impact of preoperative chemotherapy as initial treatment for advanced gastric cancer with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) or localized peritoneal metastasis (P1a): a multi-institutional retrospective study. Gastric Cancer (2021) 24(3):701–9. doi: 10.1007/s10120-020-01137-6
18. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) (1998) 85(1):115–22. doi: 10.1152/jappl.1998.85.1.115
19. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol (2015) 63(1):131–40. doi: 10.1016/j.jhep.2015.02.031
20. Mardian Y, Yano Y, Ratnasari N, Choridah L, Wasityastuti W, Setyawan NH, et al. Sarcopenia and intramuscular fat deposition are associated with poor survival in Indonesian patients with hepatocellular carcinoma: a retrospective study. BMC Gastroenterol (2019) 19(1):229. doi: 10.1186/s12876-019-1152-4
21. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
22. Shi CJ, Berlin J, Branton PA, et al. Protocol for the examination of specimens from patients with carcinoma of the stomach[EB/OL]. [2018–12–03]. Available at: https://cap.objects.frb.
23. Jun DH, Kim BJ, Park JH, Kim JG, Chi KC, Park JM, et al. Preoperative body mass index may determine the prognosis of advanced gastric cancer. Nutr Cancer. (2016) 68(8):1295–300. doi: 10.1080/01635581.2016.1224363
24. Dong QT, Cai HY, Zhang Z, Zou HB, Dong WX, Wang WB, et al. Influence of body composition, muscle strength, and physical performance on the postoperative complications and survival after radical gastrectomy for gastric cancer: A comprehensive analysis from a large-scale prospective study. Clin Nutr (2021) 40(5):3360–9. doi: 10.1016/j.clnu.2020.11.007
25. Sugiyama K, Narita Y, Mitani S, Honda K, Masuishi T, Taniguchi H, et al. Baseline sarcopenia and skeletal muscle loss during chemotherapy affect survival outcomes in metastatic gastric cancer. Anticancer Res (2018) 38(10):5859–66. doi: 10.21873/anticanres.12928
26. Tan S, Zhuang Q, Zhang Z, Li S, Xu J, Wang J, et al. Postoperative loss of skeletal muscle mass predicts poor survival after gastric cancer surgery. Front Nutr (2022) 9:794576. doi: 10.3389/fnut.2022.794576
27. Lee J, Chang CL, Lin JB, Wu MH, Sun FJ, Jan YT, et al. Skeletal muscle loss is an imaging biomarker of outcome after definitive chemoradiotherapy for locally advanced cervical cancer. Clin Cancer Res (2018) 24(20):5028–36. doi: 10.1158/1078-0432.CCR-18-0788
28. Daly LE, Ní Bhuachalla ÉB, Power DG, Cushen SJ, James K, Ryan AM. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle. (2018) 9(2):315–25. doi: 10.1002/jcsm.12267
29. Yoon HG, Oh D, Ahn YC, Noh JM, Pyo H, Cho WK, et al. Prognostic impact of sarcopenia and skeletal muscle loss during neoadjuvant chemoradiotherapy in esophageal cancer. Cancers (Basel) (2020) 12(4):925. doi: 10.3390/cancers12040925
30. Ota T, Ishikawa T, Endo Y, Matsumura S, Yoshida J, Yasuda T, et al. Skeletal muscle mass as a predictor of the response to neo-adjuvant chemotherapy in locally advanced esophageal cancer. Med Oncol (2019) 36(2):15. doi: 10.1007/s12032-018-1242-0
31. Takeda T, Sasaki T, Mie T, Furukawa T, Yamada Y, Kasuga A, et al. The impact of body composition on short-term outcomes of neoadjuvant chemotherapy with gemcitabine plus s-1 in patients with resectable pancreatic cancer. Jpn J Clin Oncol (2021) 51(4):604–11. doi: 10.1093/jjco/hyaa247
32. Mirkin KA, Luke FE, Gangi A, Pimiento JM, Jeong D, Hollenbeak CS, et al. Sarcopenia related to neoadjuvant chemotherapy and perioperative outcomes in resected gastric cancer: A multi-institutional analysis. J Gastrointest Oncol (2017) 8(3):589–95. doi: 10.21037/jgo.2017.03.02
33. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med (2011) 62:265–79. doi: 10.1146/annurev-med-061509-131248
Keywords: abdominal lavage cytology positive, gastric cancer, skeletal muscle loss, sarcopenia, conversion therapy, significant muscle loss
Citation: Ding P, Yang P, Yang L, Sun C, Chen S, Li M, Lowe S, Guo H, Tian Y, Liu Y and Zhao Q (2022) Impact of skeletal muscle loss during conversion therapy on clinical outcomes in lavage cytology positive patients with gastric cancer. Front. Oncol. 12:949511. doi: 10.3389/fonc.2022.949511
Received: 23 May 2022; Accepted: 06 September 2022;
Published: 23 September 2022.
Edited by:
Hiroki Tanabe, Asahikawa Medical University, JapanReviewed by:
Yan Mardian, Indonesia Research Partnership on Infectious Disease (INA-RESPOND), IndonesiaSa-Hong Min, University of Ulsan, South Korea
Copyright © 2022 Ding, Yang, Yang, Sun, Chen, Li, Lowe, Guo, Tian, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Zhao, emhhb3F1bkBoZWJtdS5lZHUuY24=
†These authors have contributed equally to this work
 Ping’an Ding
Ping’an Ding Peigang Yang
Peigang Yang Li Yang3†
Li Yang3† Chenyu Sun
Chenyu Sun Scott Lowe
Scott Lowe Qun Zhao
Qun Zhao